- 1Ear Institute, University College London, London, United Kingdom
- 2National Institute for Health Research, University College London Hospitals Biomedical Research Centre (Deafness and Hearing Problems Theme), London, United Kingdom
- 3Department of Neuro-Otology, Royal National Throat, Nose and Ear Hospital, London, United Kingdom
- 4Lysholm Department of Neuroradiology, National Hospital for Neurology and Neurosurgery, London, United Kingdom
- 5Department of Brain Repair and Rehabilitation, Stroke Research Centre, Institute of Neurology, University College London, London, United Kingdom
- 6Department of Neurology, National Hospital for Neurology and Neurosurgery, London, United Kingdom
Hearing and balance impairment are the most frequently reported features of infratentorial (classical) superficial siderosis (iSS). There are few comprehensive descriptions of audiovestibular function in iSS and therefore limited understanding of the affected segment(s) of the audiovestibular pathway. In addition, monitoring disease progression and response to treatment is challenging and currently mainly guided by subjective patient reports and magnetic resonance imaging. To the best of our knowledge, there have been no previous reports assessing central auditory function in iSS. We describe such findings in a patient with iSS in an attempt to precisely localize the site of the audiovestibular dysfunction, determine its severity and functional impact. We confirm the presence of (asymmetrical) auditory neuropathy and identify central auditory processing deficits, suggesting involvement of the central auditory pathway beyond the brainstem. We correlate the audiological and vestibular findings with self-report measures and the siderosis appearances on brain magnetic resonance images.
Introduction
Infratentorial (classical) superficial siderosis (iSS) is a rare but increasingly recognized disabling neurological condition (1–3). It is characterized by haemosiderin deposition on the surfaces of the brain, cerebellum, brainstem and spinal cord due to chronic continuous or intermittent low volume and low pressure bleeding into the subarachnoid space (1). The most commonly identified cause of iSS is a dural defect, usually due to previous trauma or neurosurgery; the bleeding may originate from damaged capillaries at the dural breach margins (1, 2).
Clinically, iSS is characterized by a triad of hearing loss (most frequent symptom), imbalance (ataxia) and myelopathy. Hearing loss is usually described as high-frequency sensorineural, bilateral and often asymmetrical, ranging from mild-moderate to severe-profound (1, 3). It may resemble age-related hearing loss (ARHL) (1, 3, 4). The choice of audiological (and vestibular) tests is guided by the patient's signs and symptoms and the overall clinical presentation (4, 5). Reports of the auditory brainstem responses and stapedial reflexes findings are variable, with some also reporting cochlear involvement (3, 4). Balance dysfunction in iSS can be of both central (cerebellar) and peripheral vestibular origin (4, 5). Comprehensive systematic analysis of the audiovestibular function in iSS is lacking and it is difficult to ascertain the exact site of lesion (4). Central auditory function in iSS may be affected since haemosiderin is frequently deposited in the surfaces of key auditory processing areas including temporal cortices but there are no detailed studies (2, 4).
We report findings of central auditory dysfunction and bilateral (asymmetrical) auditory neuropathy in a patient with iSS and correlate these with the self-report measures and the brain magnetic resonance images (MRI). To our knowledge this is the first case-study to report central auditory processing testing in iSS in combination with structural neuroimaging and self-reports.
Case Description
A 58 year-old male was referred with a radiologically confirmed diagnosis of iSS, likely from dural ectasia of the lumbosacral region. The patient reported a 4-year history of increasing difficulty hearing in noisy environments and a 3-year history of episodes of vertigo when standing or walking, a tendency to veer from the midline when walking and progressive imbalance when going uphill or on uneven surfaces. He had Marfan's syndrome, hypertension and was on warfarin for aortic valve replacement. There was no history of ear disease, noise exposure, head trauma, central nervous system tumors or surgery, and no family history of balance or hearing disorders.
Otoscopy was normal bilaterally. There was left-sided primary position esotropia (present since childhood) and reduced upgaze and medial gaze eye movements, no nystagmus, normal smooth pursuit, saccades and finger-nose test. The patient had a broad-based gait, mild heel-shin dysmetria and was unable to perform tandem walk, Romberg's or Unterberger's-tests.
Audiological Testing
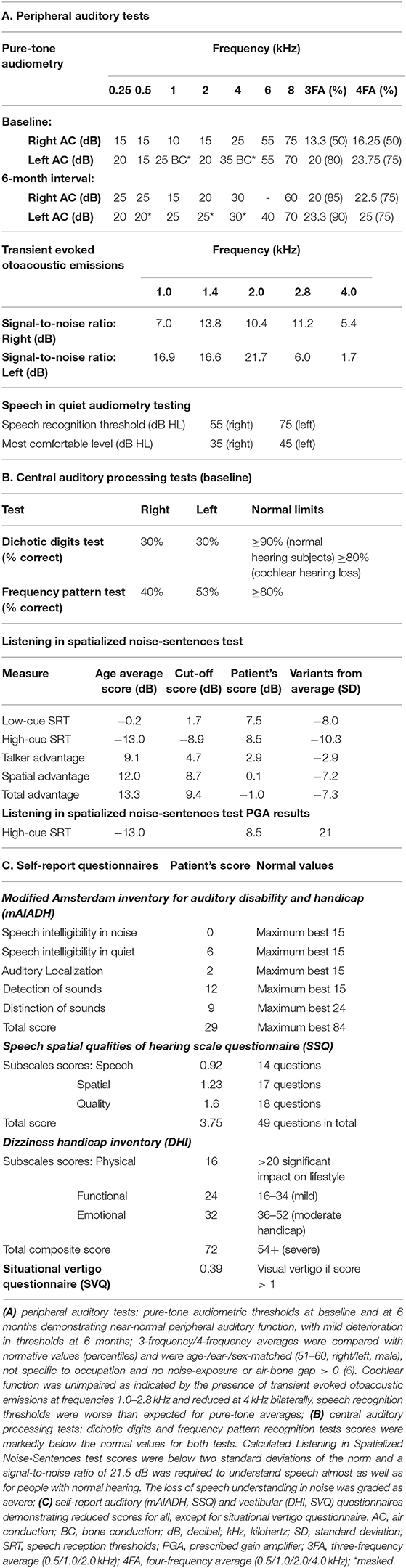
Pure-tone audiometry, speech recognition tests, transient evoked otoacoustic emissions, and auditory brainstem responses were performed to assess auditory function up to the brainstem (Figure 1 and Table 1). Middle ear involvement was ruled out with normal tympanometry. Pure-tone audiometry showed mild-to-moderate high-frequency sensorineural hearing loss attributable to age-related changes (6). Transient evoked otoacoustic emissions were reduced at 4 kHz bilaterally consistent with pure-tone audiometry findings. Speech recognition thresholds were elevated compared to pure-tone thresholds, indicating bilateral auditory neuropathy.
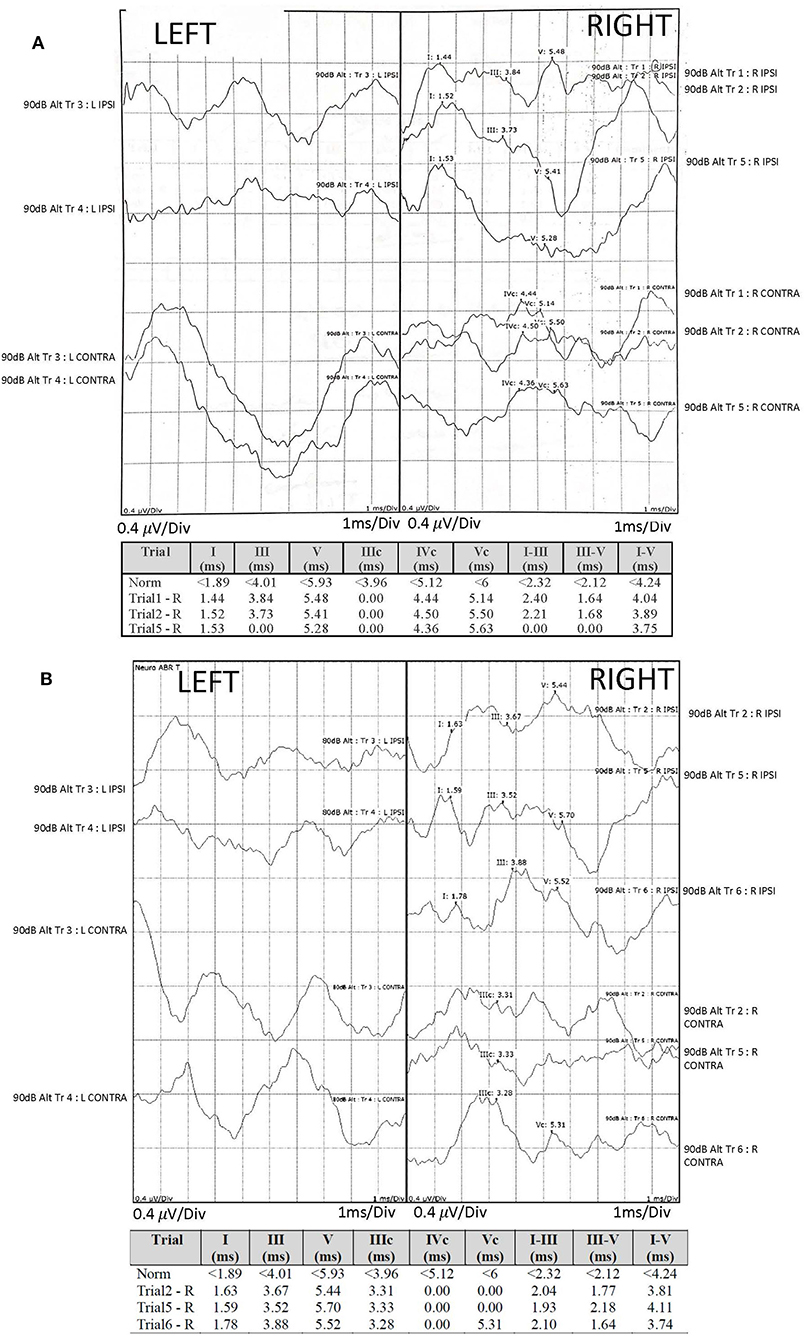
Figure 1. Audiological assessment: auditory brainstem responses (ABR) were recorded using TDH-39 headphones and monaurally presented alternating polarity click stimuli of 100 μs duration, 11.33 Hz repetition rate and intensity of 90 dB nHL (normalized hearing level). The electrodes were mounted on center forehead (common); A1 left mastoid (active), A2 right mastoid (active) and high center forehead (reference). The responses were compared with our institutional normative values (provided) and demonstrated reproducible right waves I-V of degraded morphology, yet normal amplitude and latency and absent left responses at baseline (A) and at a 6-month interval (B), with additional findings of poorly reproducible right waves I-V and poor wave I morphology at a 6-month interval (B). Due to retrospective nature of the case report, it was impossible to separate the recordings into condensation and rarefaction buffers and to comment on the cochlear microphonic potentials.
Auditory brainstem responses (compared against our normative values) demonstrated reproducible right waves I-V of degraded morphology, yet normal amplitude and latency and absent left responses. Absent left responses persisted on 6-month interval testing, with additional findings of poorly reproducible right waves I-V and poor wave I morphology, suggesting progressive right auditory nerve involvement.
Measures of central auditory function included dichotic digits test, (monaurally presented) frequency pattern test and Listening in Spatialized Noise-Sentences test. Dichotic digits test score was calculated as percent correct of sets of four digits presented simultaneously (two digits on each side). Frequency pattern test score was calculated as percent correct of the recognized sequences of three tone bursts of high and low frequency. The stimuli for both tests were presented at 50 dB SL (sensation-level) above the threshold level. Listening in Spatialized Noise-Sentences test scores were calculated by the software based on correct recognition of stimuli (sentences) presented simultaneously with competing sentences presented at 55 dB SPL (sound-pressure-level) of the same and different speaker-voice and at azimuth and 90 degrees to the stimuli sentences at a stimulus presentation level determined by the software. The scores were adjusted for mild peripheral hearing loss and were markedly low (Table 1).
Vestibular Testing
Peripheral vestibular tests included cervical and ocular vestibular evoked myogenic potentials and video head impulse test, whereas videonystagmography was performed to distinguish between the peripheral and central vestibular involvement and to assess severity of the dysfunction (Figure 2). Video head impulse testing identified reduced mean vestibulo-ocular reflex gains for all six canals (Figure 2A). Left ocular vestibular evoked myogenic potentials were not detected (Figure 2B). Cervical vestibular evoked myogenic potentials were within normal limits (Figure 2C). Videonystagmography showed impaired gaze holding to the left and minimally impaired smooth pursuit at 0.4 Hz only, indicating mild central vestibular involvement (7–10). Vestibular test values were compared against our normative values where available.
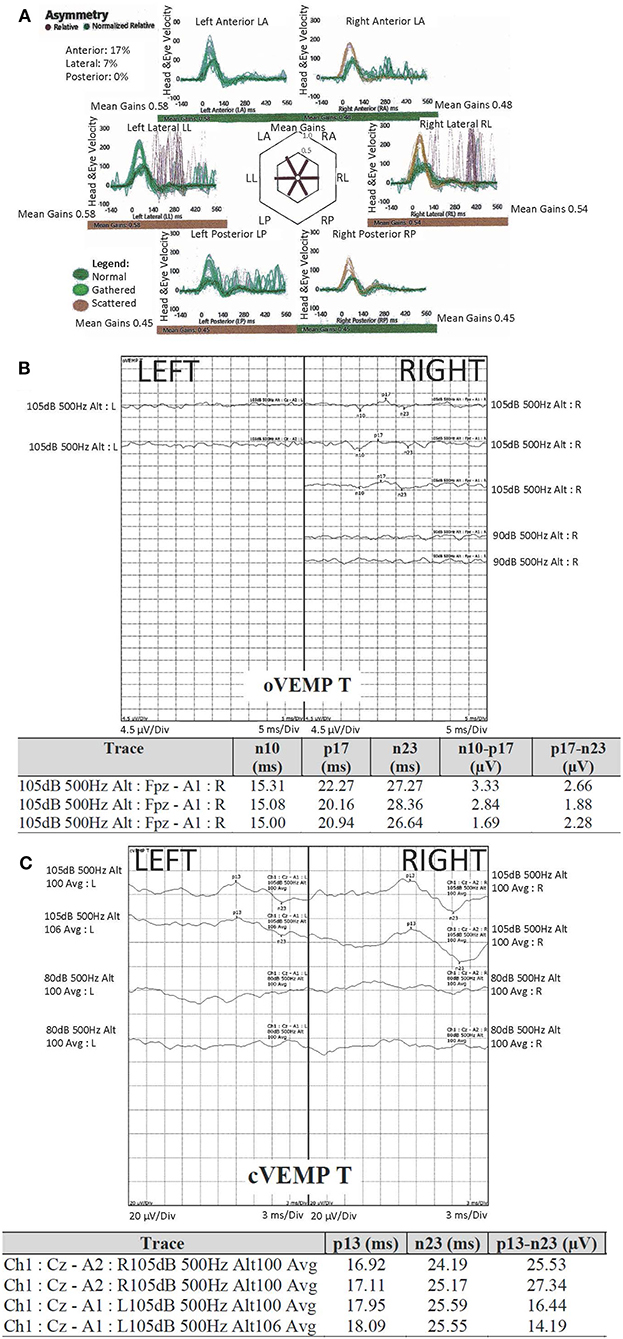
Figure 2. Peripheral vestibular assessment: (A) video head impulse Test (vHIT), demonstrated reduced gain in all six canals, more marked in the right anterior (asymmetry 17%) and both posterior canals; (B) ocular vestibular evoked myogenic potentials (oVEMP) were not detected on the left; (C) cervical vestibular evoked myogenic potentials (cVEMP) were compared to our normative values and were within normal limits. Tone burst stimuli of 500 Hz was used for both oVEMP and cVEMP, of alternating polarity (specific to the unit's equipment) and 2:1:2 cycle. The scale was 5 ms per division (oVEMP) and 3 ms per division (cVEMP). For oVEMP, the electrodes were placed on forehead (common), A1 centrally below the eye; REF (reference) electrode on the cheek 1 cm below (but not touching) A1. For cVEMP, the electrodes were placed on forehead (common), sternoclavicular joint (inverting/negative), sternocleidomastoid muscle belly (non-inverting/positive).
Neuro-Psychological Assessment and Self-Report Measures
The patient completed validated hearing- and balance-specific questionnaires. Modified Amsterdam Inventory for Auditory Disability and Handicap (28 items) and Speech, Spatial and Qualities of Hearing Scale (49 items), were used to assess hearing difficulties attributable to everyday situations in five domains (sound recognition, detection and localization, and speech intelligibility in quiet and in noise) (11, 12), and in the domains of speech, spatial and qualities of hearing, respectively (13). Dizziness Handicap Inventory (25 items) was used to assess functional, emotional and physical impact of imbalance and vertigo on the patient's quality of life (14), whereas Situational Vertigo Questionnaire (19 items) assessed severity of vestibular symptoms in visually disorienting situations (15).
The self-report measures scores were consistent with severe disability, except for normal Situational Vertigo Questionnaire score (Table 1). Neuro-psychological assessment was performed and the scores were within low average range except for visual recognition and phonemic fluency impairment and minimal attentional and executive inefficiency.
Imaging
The brain and spine MRI were performed with MAGNETOM SKYRA 3T (Siemens, UK). Susceptibility weighted imaging sequences (SWI-MRI, 3D-T2* GRE, 1.5 mm) identified appearances of haemosiderin deposits consistent with the radiological diagnostic criteria for iSS (2). It demonstrated hypointense regions along the cerebellar folia and superior vermis, midbrain, pons and both vestibulocochlear nerves (more marked on the left), medulla and cranio-cervical junction. There was additional involvement of supratentorial structures, with severe and widespread siderosis affecting the surfaces of temporal lobes, including Sylvian fissures and insular areas, frontal and occipital lobes, but sparing the vertex (Figure 3).
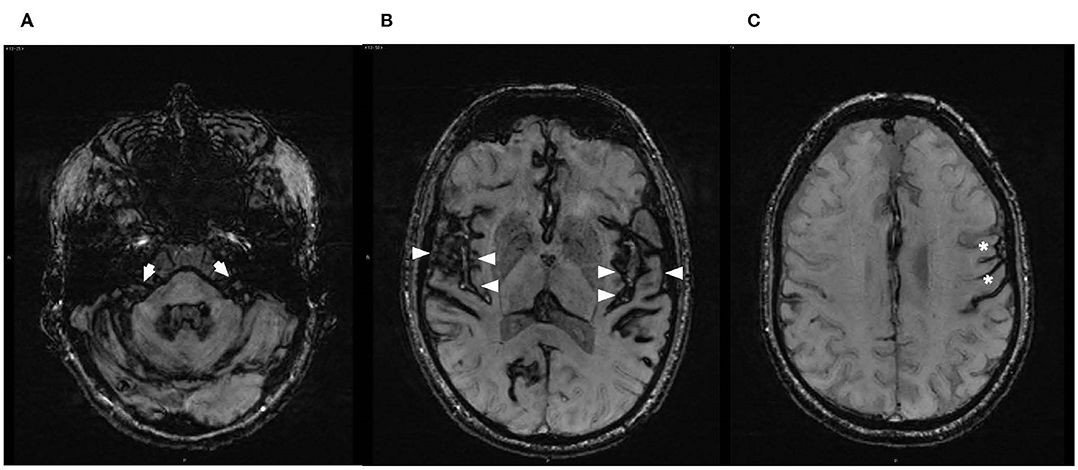
Figure 3. Axial susceptibility-weighted magnetic resonance images (SW-MRI) at the level of (A) brainstem/internal auditory canals, demonstrating haemosiderin deposition over the pons and vestibulocochlear nerves bilaterally with slightly thicker hypointense rim of hemosiderin on the left (arrows); (B) superior temporal gyri, demonstrating supratentorial haemosiderin deposition along the surfaces of the temporal lobes, involving insular areas and particularly Sylvian fissures and Heschl's gyri (arrowheads); and (C) asymmetry in the supratentorial appearance of haemosiderin deposition on the left (asterisks).
Management
Following the Siderosis Multidisciplinary Team meeting, the patient was commenced on Deferiprone, as there was no clear neurosurgical target for dural repair. The patient was aware of the risks associated with Deferiprone and the need for regular blood monitoring for neutropenia. He was referred for hearing therapy as a rehabilitative measure in view of central auditory deficits with near-normal peripheral auditory function, and for neuro-vestibular physiotherapy to address mixed (central/peripheral) vestibular deficits. There were no adverse events, such as neutropenic sepsis. The treatment was suspended in view of COVID19 risks. He has a regular follow-up with Neurology, Neuro-otology and Hematology teams.
Discussion
A significant finding in this case report is the presence of central auditory processing deficits. It is important not only for diagnosis and understanding of the clinical spectrum of iSS but also for the potential approach to treatment.
Central auditory involvement was indicated by the bilaterally reduced scores for frequency pattern and dichotic digits tests (Table 1). These could not be attributed to auditory neuropathy alone as they previously showed correlation with cortical lesions (16, 17) and are known to be robust against mild-to-moderate cochlear hearing loss (16, 18). Cerebral involvement was further indicated by reduced scores for Listening in Spatialized Noise-Sentences test, particularly in low and high cues and spatial advantage domains which could not be attributed to auditory neuropathy alone (Table 1) (19, 20). The findings of difficulty integrating spatial auditory information and impairment of temporal processing were previously reported in individuals with auditory neuropathy in Friedreich's Ataxia (FRDA) and Charcot Marie Tooth (CMT) disease (Type 1A) (20). Yet, in our own FRDA cohort (21), we found abnormal spatial advantage on Listening in Spatialized Noise-Sentences test even in cases with normal auditory brainstem responses and conversely a case with abnormal responses yet normal spatial advantage. This is possibly consistent with the reports of cerebral cortical atrophy in the auditory brain areas in FRDA (22). Thus, the abnormal Listening in Spatialized Noise-Sentences test findings are likely to be congruent with central auditory involvement, and in particular the antero-lateral aspect of Heschl's gyrus (23), rather than the fronto-temporal and fronto-parietal cortical network as our patient's memory functions were intact.
Our case highlights the shortcomings of pure-tone audiometry as a single tool for hearing assessment. Marked hearing difficulties reported by our patient were not consistent with mildly elevated (likely age-related) thresholds (24, 25) as further testing including speech recognition thresholds identified asymmetrical auditory neuropathy and central auditory processing deficits (20, 26). Comprehensive test battery should be performed for patients with auditory symptoms that are more marked than their peripheral auditory test results. This is in line with the current audiological guidelines (27, 28) and the recommended neurological work-up for patients with other neurodegenerative disorders such as FRDA and CMT (20, 29).
Our findings also build on the previous reports of mixed vestibular involvement (8–10). ISS should be considered as a differential diagnosis in patients with chronic combined vestibulopathy (30). It is plausible that the vestibular hypofunction may inform an iSS-specific pattern for vestibular involvement, with preferentially lower gains for posterior semi-circular canals on video head impulse testing, as seen in our patient (9, 31, 32). Although the interpretation of ocular vestibular evoked myogenic potentials should be done with caution—due to the patient's restricted upward gaze—the responses were not detected on the left which may be consistent with the involvement of the left superior vestibular nerve. This finding may be further commensurate with the absent left ABR (33).
In a case-series of five patients with superficial siderosis, the superior vestibular nerve and utricle involvement were identified in all five cases (34). Three patients had a protracted course and showed additional involvement of the inferior vestibular nerve and saccule. It was hypothesized that the superior vestibular nerve involvement may occur in earlier stages of iSS, with gradual disease progression leading to the inferior vestibular nerve involvement (5).
Although the identified vestibulopathy may be associated with vestibular nerve involvement and sparing of saccule and utricle (35, 36), vestibulocochlear end-organ damage was previously reported in iSS (5, 34, 37). In the same case series, the authors suggested that the vestibular loss in superficial siderosis was likely to be due to impaired blood flow to the vestibulocochlear apparatus rather than damage to the vestibular nerves. This cannot be supported by the presence of otoacoustic emissions in our patient, as outer hair cells are very susceptible to hypoxia (38–41).
Involvement of vestibulocochlear apparatus in iSS cannot be supported by the single report of temporal bone histology in a patient with superficial siderosis (42). The authors described atrophy of the strial ganglia, absence of hair cells only in the cochlear basal turn bilaterally, iron deposits in stria vascularis and spiral ligament and the subepithelial layers of macula, and a marked atrophy of the vestibulocochlear nerve (42). It is, difficult to conclude that those histological findings could be solely siderosis-related, but perhaps due to several pathological processes in the inner ear and along the auditory pathway, likely from the reported noise exposure and identified otosclerosis (42). Stria vascularis and spiral ligament are highly vascularized structures, and the presence of ferritin in stria vascularis (with a proposed possible function of stria vascularis for iron storage) was reported (43, 44).
Cochlear aqueduct patency was proposed as a mechanism for hearing loss in iSS (37) which would imply damage to the inner ear apparatus through altered biochemical composition of perilymph or direct effect of haemosiderin and iron by-products. Damage to the inner ear sensory and neural apparatus was not evident in a study of 12 temporal bones of patients who died from subarachnoid hemorrhage (45).
Equally, it is important to consider hearing loss in the setting of Marfan's syndrome which has been described, albeit infrequently, as predominantly conductive, associated with otitis media, Eustachian tube dysfunction and cranio-facial abnormalities (46). Sensorineural hearing loss in this group may be due to hypertension, thus resulting in cochlear vascular damage and sensory hearing loss (46–48). This is in contrast to the findings in our patient as presence of otoacoustic emissions indicated near-normal cochlear function and normal middle ear conduction. It is possible that the vestibulocochlear apparatus damage, described in other studies, might be due to age-related changes, presence of other risk factors for hearing and balance impairment, and possibly the anterograde progression of auditory and vestibular dysfunction.
The MRI appearance of hypointense regions along the course of both vestibulocochlear nerves (slightly more marked on the left) was consistent with the clinical findings of asymmetrical auditory neuropathy and concurrent vestibulopathy (Figure 3). Severe auditory processing deficits in our patient may be commensurate with the cerebral abnormalities involving the auditory cortex as evidenced by the MRI appearance of hypointensities over the cortical surfaces involving Sylvian fissures, superior temporal gyri and the insulae (Figure 3).
Scores of dedicated self-report measures were consistent with the identified auditory and vestibular deficits (Table 1). The total score for modified Amsterdam Inventory for Auditory Disability and Handicap questionnaire was below the reported scores for patients with mildly elevated mean audiometric thresholds, for those undergoing tympanoplasty (11, 49) and for patients with auditory processing disorder (50). The overall Speech Spatial and Qualities of Hearing Scale score was similar to the scores reported for hearing-impaired individuals (51, 52). These two self-report measures were previously shown to correlate with auditory processing deficits in adults with normal hearing thresholds, although not in a neurological population (50). The total Dizziness Handicap Inventory score of 72 (severe) (14) was comparable to scores for individuals with benign paroxysmal positional vertigo (BPPV) yet worse than in central, bilateral peripheral or mixed vestibular dysfunction and traumatic brain injury (7, 53–55). In contrast, the Situational Vertigo Questionnaire score of 0.39 was similar to the scores for normal individuals (56), most probably indicating little-to-no impact of visual stimuli on our patient's vestibular symptoms.
Management of auditory processing disorder and ataxia in complex neurological patients requires individualized approach and is deficits- and needs-specific. Listening strategies, use of assistive listening devices, auditory training, hearing aids and cochlear implants (where fitting the criteria) should be considered. Reported outcomes of cochlear implantation in iSS patients are variable (57, 58) but may improve with meticulous auditory evaluation and precise site-of-lesion identification. Dedicated neuro-vestibular physiotherapy previously demonstrated benefits (59) and should be prescribed for patients with complex vestibular impairment to address their functional deficits based on the lesion location.
There are several learning points in our case-study. We identified central auditory processing deficits in iSS, which correlate with the MRI findings and scores for self-report measures. Our case-study provides clear evidence of auditory and (concurrently) vestibular neuropathy, without confounding risk factors for end-organ involvement. It is possible that the anterograde progression of audiovestibular dysfunction may result in end-organ involvement. We highlight the need for comprehensive audiovestibular assessments in patients with neurodegenerative conditions to identify the site of lesion and provide patient- and deficit-specific management strategies.
The limitations of this case-study are in its level/strength of evidence. Further dedicated prospective studies are needed to investigate whether the findings are patient-specific or are characteristic for iSS. Although there was stark asymmetry between right and left ocular vestibular evoked myogenic potentials, the results should be interpreted with caution in view of patient's restricted upgaze and previous reports of absent potentials in normal individuals (60).
Patient's perspective: the patient's referral to our tertiary center, dedicated investigations and multidisciplinary input helped his understanding of the condition and of his symptoms. While he perceived little benefit from physiotherapy, with further gradual deterioration in balance, he reported benefit from management of his hearing deficits.
Conclusion
There have been no previously documented central auditory function assessments for patients with iSS and correlation with the scores of self-report measures and MRI findings. Our case-study provides clear evidence of auditory and (concurrently) vestibular neuropathy, without confounding risk factors for end-organ involvement. It is possible that the end-organ involvement may be the result of anterograde progression of audiovestibular dysfunction. The importance of comprehensive audiovestibular assessments to identify the site of lesion in patients with neurodegenerative conditions is highlighted. Due to progressive, and possibly irreversible, nature and significant morbidity, it is necessary to determine the features that differentiate iSS-related and age-related hearing loss for timely diagnosis and to provide needs- and deficit-specific management strategies, with further efforts to halt disease progression by means of prompt surgical repair (61, 62), or by using iron chelating agents (1, 63, 64) and ultimately, may inform research on novel therapeutic agents. Dedicated and longitudinal studies that correlate the audiovestibular assessments, patients' symptoms and the degree of functional impairment with the respective imaging would help establish MRI usefulness in functional assessment of patients with iSS. It is possible that MRI may lack sensitivity in determining subtle changes associated with disease progression or treatment response. It is plausible that serial audiovestibular testing, alongside self-report measures, may be more useful in identifying such changes and appropriate in the setting of neuro-otology clinic without invasive testing or MR imaging.
Data Availability Statement
The original contributions generated in the study are included in the article/supplementary materials, further inquiries can be directed to the corresponding author.
Ethics Statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article. Formal written consent was sought from the patient prior to the write up and submission of the manuscript, including consent for the publication of any potentially identifiable images or data included in this article.
Author Contributions
NK wrote the manuscript. D-EB contributed to content and manuscript writing. DJW and PC reviewed the manuscript and provided comments. All authors reviewed the final version of the manuscript.
Funding
This work was funded by the NIHR UCLH BRC Deafness and Hearing Problems Theme. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health. Co-author NK was in direct receipt of the funding by the NIHR UCLH BRC Deafness and Hearing Problems Theme doctoral studentship grant (BRC-1215-20016-546624). Additional support was provided by the Bernice Bibby Research Charity grant (UK Registered Charity Number 1058703).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank the patient and his family for allowing us to report on his case. We would like to particularly thank Dr. Fan Liu and Mr. Jay Patel for their help with audiovestibular testing interpretation and providing guidance with the methodology section, as well as Dr. Nehzat Koohi and Mr. Harry Akram, for performing the audiovestibular tests, Dr. Edgar Chan for performing the neuro-psychological assessment, and Professor G. M. Halmagyi for his insights on the interpretation of the audiovestibular results.
References
1. Fearnley JM, Stevens JM, Rudge P. Superficial siderosis of the central nervous system. Brain. (1995) 118:1051–66. doi: 10.1093/brain/118.4.1051
2. Wilson D, Chatterjee F, Farmer SF, Rudge P, McCarron MO, Cowley P, et al. Infratentorial superficial siderosis: classification, diagnostic criteria, and rational investigation pathway. Ann Neurol. (2017) 81:333–43. doi: 10.1002/ana.24850
3. Sydlowski SA, Cevette MJ, Shallop J. Superficial siderosis of the central nervous system: phenotype and implications for audiology and otology. Otol Neurotol. (2011) 32:900–8. doi: 10.1097/MAO.0b013e31822558a9
4. Yoo A, Jou J, Klopfenstein JD, Kattah JC. Focused neuro-otological review of superficial siderosis of the central nervous system. Front Neurol. (2018) 9:358. doi: 10.3389/fneur.2018.00358
5. Takeda T, Kawashima Y, Hirai C, Makabe A, Ito T, Fujikawa T, et al. Vestibular dysfunction in patients with superficial siderosis of the central nervous system. Otol Neurotol. (2018) 39:e468–74. doi: 10.1097/MAO.0000000000001844
6. Davis AC. Hearing in Adults: The Prevalence and Distribution of Hearing Impairment and Reported Hearing Disability. London: Whurr (1995). p. xv,1011.
7. Hermann R, Ionescu EC, Dumas O, Tringali S, Truy E, Tilikete C. Bilateral vestibulopathy: vestibular function, dynamic visual acuity and functional impact. Front Neurol. (2018) 9:555. doi: 10.3389/fneur.2018.00555
8. Strupp M, Kim JS, Murofushi T, Straumann D, Jen JC, Rosengren SM, et al. Bilateral vestibulopathy: diagnostic criteria consensus document of the classification committee of the barany society. J Vestib Res. (2017) 27:177–89. doi: 10.3233/VES-170619
9. Halmagyi GM, Chen L, MacDougall HG, Weber KP, McGarvie LA, Curthoys IS. The video head impulse test. Front Neurol. (2017) 8:258. doi: 10.3389/fneur.2017.00258
10. Walker MF, Zee DS. Cerebellar disease alters the axis of the high-acceleration vestibuloocular reflex. J Neurophysiol. (2005) 94:3417–29. doi: 10.1152/jn.00375.2005
11. Meijer AG, Wit HP, TenVergert EM, Albers FW, Muller Kobold JE. Reliability and validity of the (modified) Amsterdam inventory for auditory disability and handicap. Int J Audiol. (2003) 42:220–6. doi: 10.3109/14992020309101317
12. Kramer SE, Kapteyn TS, Festen JM, Tobi H. Factors in subjective hearing disability. Audiology. (1995) 34:311–20. doi: 10.3109/00206099509071921
13. Gatehouse S, Noble W. The speech, spatial and qualities of hearing scale (SSQ). Int J Audiol. (2004) 43:85–99. doi: 10.1080/14992020400050014
14. Jacobson GP, Newman CW. The development of the dizziness handicap inventory. Arch Otolaryngol Head Neck Surg. (1990) 116:424–7. doi: 10.1001/archotol.1990.01870040046011
15. Jacob RG, Lilienfeld SO, Furman JMR, Durrant JD, Turner SM. Panic disorder with vestibular dysfunction: further clinical observations and description of space and motion phobic stimuli. J Anxiety Disord. (1989) 3:117–30. doi: 10.1016/0887-6185(89)90006-6
16. Musiek FE, Pinheiro ML. Frequency patterns in cochlear, brainstem, and cerebral lesions. Audiology. (1987) 26:79–88. doi: 10.3109/00206098709078409
18. Musiek FE. Assessment of central auditory dysfunction—the dichotic digit test revisited. Ear Hear. (1983) 4:79–83. doi: 10.1097/00003446-198303000-00002
19. Cameron S, Dillon H. Spatial hearing deficits as a major cause of auditory processing disorders: diagnosis with the LiSN-S and management options. Seewald R, Bamford J, editors. Staefa: Phonak AG (2007). p. 235–41.
20. Rance G, Ryan MM, Carew P, Corben LA, Yiu E, Tan J, et al. Binaural speech processing in individuals with auditory neuropathy. Neuroscience. (2012) 226:227–35. doi: 10.1016/j.neuroscience.2012.08.054
21. Koohi N, Thomas-Black G, Giunti P, Bamiou D-E. Auditory Phenotypic Variability in Friedreich's Ataxia Patients. (Under review).
22. Selvadurai LP, Harding IH, Corben LA, Stagnitti MR, Storey E, Egan GF, et al. Cerebral and cerebellar grey matter atrophy in Friedreich ataxia: the IMAGE-FRDA study. J Neurol. (2016) 263:2215–23. doi: 10.1007/s00415-016-8252-7
23. Gutschalk A, Uppenkamp S. Sustained responses for pitch and vowels map to similar sites in human auditory cortex. Neuroimage. (2011) 56:1578–87. doi: 10.1016/j.neuroimage.2011.02.026
24. Davis AMIoHR. Hearing in Adults: The Prevalence and Distribution of Hearing Impairment and Reported Hearing Disability in the MRC Institute of Hearing Research's National Study of Hearing. London: Whurr Publishers (1995).
25. Musiek FE, Shinn J, Chermak GD, Bamiou DE. Perspectives on the pure-tone audiogram. J Am Acad Audiol. (2017) 28:655–71. doi: 10.3766/jaaa.16061
26. Starr A, Picton TW, Sininger Y, Hood LJ, Berlin CI. Auditory neuropathy. Brain. (1996) 119:741–53. doi: 10.1093/brain/119.3.741
27. Clinical Practice Guideline: Diagnosis, Treatment and Management of Children and Adults with Central Auditory Processing Disorder. American Academy of Audiology. Reston, VA: Americal Academy of Audiology (2010). p. 3–51.
28. British Society of Audiology. Position Statement and Practice Guidance. Auditory Processing Disorder (APD). Bathgate: British Society of Audiology (2018).
29. Rance G, Fava R, Baldock H, Chong A, Barker E, Corben L, et al. Speech perception ability in individuals with Friedreich ataxia. Brain. (2008) 131:2002–12. doi: 10.1093/brain/awn104
30. Szmulewicz DJ. Combined central and peripheral degenerative vestibular disorders: CANVAS, idiopathic cerebellar ataxia with bilateral vestibulopathy (CABV) and other differential diagnoses of the CABV phenotype. Curr Otorhinolaryngol Rep. (2017) 5:167–74. doi: 10.1007/s40136-017-0161-5
31. Kremmyda O, Kirchner H, Glasauer S, Brandt T, Jahn K, Strupp M. False-positive head-impulse test in cerebellar ataxia. Front Neurol. (2012) 3:162. doi: 10.3389/fneur.2012.00162
32. Lee SH, Park SH, Kim JS, Kim HJ, Yunusov F, Zee DS. Isolated unilateral infarction of the cerebellar tonsil: ocular motor findings. Ann Neurol. (2014) 75:429–34. doi: 10.1002/ana.24094
33. Fujikawa S, Starr A. Vestibular neuropathy accompanying auditory and peripheral neuropathies. Arch Otolaryngol. (2000) 126:1453–6. doi: 10.1001/archotol.126.12.1453
34. Miwa T, Minoda R, Matsuyoshi H. Vestibular function in superficial siderosis. BMC Ear Nose Throat Disord. (2013) 13:5. doi: 10.1186/1472-6815-13-5
35. Agrawal Y, Bremova T, Kremmyda O, Strupp M. Semicircular canal, saccular and utricular function in patients with bilateral vestibulopathy: analysis based on etiology. J Neurol. (2013) 260:876–83. doi: 10.1007/s00415-012-6724-y
36. Zingler VC, Weintz E, Jahn K, Botzel K, Wagner J, Huppert D, et al. Saccular function less affected than canal function in bilateral vestibulopathy. J Neurol. (2008) 255:1332–6. doi: 10.1007/s00415-008-0887-6
37. Sydlowski SA, Levy M, Hanks WD, Clark MD, Ackley RS. Auditory profile in superficial siderosis of the central nervous system: a prospective study. Otol Neurotol. (2013) 34:611–9. doi: 10.1097/MAO.0b013e3182908c5a
38. Kim JS, Lopez I, DiPatre PL, Liu F, Ishiyama A, Baloh RW. Internal auditory artery infarction: clinicopathologic correlation. Neurology. (1999) 52:40–4. doi: 10.1212/WNL.52.1.40
39. Telischi FF, Stagner B, Widick MP, Balkany TJ, Lonsbury-Martin BL. Distortion-product otoacoustic emission monitoring of cochlear blood flow. Laryngoscope. (1998) 108:837–42. doi: 10.1097/00005537-199806000-00011
40. Tsuji S, Tabuchi K, Hara A, Kusakari J. Long-term observations on the reversibility of cochlear dysfunction after transient ischemia. Hear Res. (2002) 166:72–81. doi: 10.1016/S0378-5955(02)00299-X
41. Tabuchi K, Tsuji S, Fujihira K, Oikawa K, Hara A, Kusakari J. Outer hair cells functionally and structurally deteriorate during reperfusion. Hear Res. (2002) 173:153–63. doi: 10.1016/S0378-5955(02)00349-0
42. Nadol JB Jr., Adams JC, O'Malley JT. Temporal bone histopathology in a case of sensorineural hearing loss caused by superficial siderosis of the central nervous system and treated by cochlear implantation. Otol Neurotol. (2011) 32:748–55. doi: 10.1097/MAO.0b013e31820e7195
43. Santos-Sacchi J, Marovitz WF. An evaluation of normal strial capillary transport using the electron-opaque tracers ferritin and iron dextran. Acta Otolaryngol. (1980) 89:12–26. doi: 10.3109/00016488009127103
44. Santos-Sacchi J, Marovitz WF. A ferritin-containing cell type in the stria vascularis of the mouse inner ear. Acta Otolaryngol. (1985) 100:26–32. doi: 10.3109/00016488509108583
45. Holden HB, Schuknecht HF. Distribution pattern of blood in the inner ear following spontaneous subarachnoid haemorrhage. J Laryngol Otol. (1968) 82:321–9. doi: 10.1017/S0022215100068833
46. Hamberis AO, Mehta CH, Valente TA, Dornhoffer JR, Nguyen SA, Meyer TA. The pattern and progression of hearing loss in Marfan Syndrome: a study of children and young adults. Int J Pediatr Otorhinolaryngol. (2020) 138:110207. doi: 10.1016/j.ijporl.2020.110207
47. Gates GA, Cobb JL, D'Agostino RB, Wolf PA. The relation of hearing in the elderly to the presence of cardiovascular disease and cardiovascular risk factors. Arch Otolaryngol Head Neck Surg. (1993) 119:156–61. doi: 10.1001/archotol.1993.01880140038006
48. Trune DR, Nguyen-Huynh A. Vascular pathophysiology in hearing disorders. Semin Hear. (2012) 33:242–50. doi: 10.1055/s-0032-1315723
49. Meijer AG, Wit HP, Albers FW. Relation between change of hearing and (modified) Amsterdam inventory for auditory disability and handicap score. Clin Otolaryngol Allied Sci. (2004) 29:565–70. doi: 10.1111/j.1365-2273.2004.00844.x
50. Bamiou DE, Iliadou VV, Zanchetta S, Spyridakou C. What can we learn about auditory processing from adult hearing questionnaires? J Am Acad Audiol. (2015) 26:824–37. doi: 10.3766/jaaa.15009
51. Noble W, Gatehouse S. Interaural asymmetry of hearing loss, speech, spatial and qualities of hearing scale (SSQ) disabilities, and handicap. Int J Audiol. (2004) 43:100–14. doi: 10.1080/14992020400050015
52. Lenarz T, Muller L, Czerniejewska-Wolska H, Valles Varela H, Orus Dotu C, Durko M, et al. Patient-related benefits for adults with cochlear implantation: a multicultural longitudinal observational study. Audiol Neurootol. (2017) 22:61–73. doi: 10.1159/000477533
53. Saxena A, Prabhakar MC. Performance of DHI score as a predictor of benign paroxysmal positional vertigo in geriatric patients with dizziness/vertigo: a cross-sectional study. PLoS ONE. (2013) 8:e58106. doi: 10.1371/journal.pone.0058106
54. Kaufman KR, Brey RH, Chou LS, Rabatin A, Brown AW, Basford JR. Comparison of subjective and objective measurements of balance disorders following traumatic brain injury. Med Eng Phys. (2006) 28:234–9. doi: 10.1016/j.medengphy.2005.05.005
55. Whitney SL, Hudak MT, Marchetti GF. The activities-specific balance confidence scale and the dizziness handicap inventory: a comparison. J Vestib Res. (1999) 9:253–9.
56. Guerraz M, Yardley L, Bertholon P, Pollak L, Rudge P, Gresty MA, et al. Visual vertigo: symptom assessment, spatial orientation and postural control. Brain. (2001) 124:1646–56. doi: 10.1093/brain/124.8.1646
57. Modest MC, Carlson ML, Wanna GB, Driscoll CL. Cochlear implantation in patients with superficial siderosis: seven cases and systematic review of the literature. Otol Neurotol. (2015) 36:1191–6. doi: 10.1097/MAO.0000000000000792
58. Tyler GK, Martin TP, Baguley DM. Systematic review of outcome of cochlear implantation in superficial siderosis. Otol Neurotol. (2012) 33:976–82. doi: 10.1097/MAO.0b013e3182565a46
59. Brown KE, Whitney SL, Marchetti GF, Wrisley DM, Furman JM. Physical therapy for central vestibular dysfunction. Arch Phys Med Rehabil. (2006) 87:76–81. doi: 10.1016/j.apmr.2005.08.003
60. Piker EG, Jacobson GP, Burkard RF, McCaslin DL, Hood LJ. Effects of age on the tuning of the cVEMP and oVEMP. Ear Hear. (2013) 34:e65–73. doi: 10.1097/AUD.0b013e31828fc9f2
61. Posti JP, Juvela S, Parkkola R, Roine S. Three cases of superficial siderosis of the central nervous system and review of the literature. Acta Neurochir (Wien). (2011) 153:2067–73. doi: 10.1007/s00701-011-1116-0
62. Kumar R, Jacob JT, Welker KM, Cutrer FM, Link MJ, Atkinson JL, et al. Superficial siderosis of the central nervous system associated with incomplete dural closure following posterior fossa surgery: report of 3 cases. J Neurosurg. (2015) 123:1326–30. doi: 10.3171/2014.12.JNS141920
63. Cummins G, Crundwell G, Baguley D, Lennox G. Treatment of superficial siderosis with iron chelation therapy. BMJ Case Rep. (2013) 2013:bcr2013003316. doi: 10.1136/bcr-2013-009916
Keywords: infratentorial superficial siderosis, central auditory deficits, auditory neuropathy, MRI, case report
Citation: Kharytaniuk N, Cowley P, Werring DJ and Bamiou D-E (2021) Case Report: Auditory Neuropathy and Central Auditory Processing Deficits in a Neuro-Otological Case-Study of Infratentorial Superficial Siderosis. Front. Neurol. 11:610819. doi: 10.3389/fneur.2020.610819
Received: 27 September 2020; Accepted: 15 December 2020;
Published: 14 January 2021.
Edited by:
Sergio Carmona, INEBA Institute of Neurosciences Buenos Aires, ArgentinaReviewed by:
Takeshi Tsutsumi, Tokyo Medical and Dental University, JapanRachael L. Taylor, The University of Auckland, New Zealand
Copyright © 2021 Kharytaniuk, Cowley, Werring and Bamiou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Natallia Kharytaniuk, bi5raGFyeXRhbml1a0B1Y2wuYWMudWs=
 Natallia Kharytaniuk
Natallia Kharytaniuk Peter Cowley4
Peter Cowley4 David J. Werring
David J. Werring Doris-Eva Bamiou
Doris-Eva Bamiou