- Department of Interventional Neuroradiology, Beijing Neurosurgical Institute, Beijing Tian Tan Hospital, Capital Medical University, Beijing, China
Spontaneous vertebral artery dissecting aneurysm has been increasingly attributed as a major cause of focal neurological deficits due to vertebrobasilar artery ischemia or subarachnoid hemorrhage (SAH). Although the development of spontaneous vertebral artery dissecting aneurysm (VADA) is rare, de novo VADA after treatment of contralateral vertebral artery (VA) is more less frequently observed. There are only a few reports related to de novo VADA after treatment of the contralateral VA in the medical literature. The mechanisms responsible for de novo dissection after treatment of unilateral VADA are still not clearly understood. In this manuscript, we report an unusual case of a patient with a de novo VADA after placement of a pipeline embolization device (PED) stent on the contralateral VA along with a thorough review of the literature. A 42-years old male patient was referred to the hospital with sudden onset of dizziness, nausea, and vomiting. Initial digital subtraction angiography (DSA) images demonstrated a VADA in the fourth segment of the left VA without the involvement of the posterior inferior cerebellar artery (PICA). There were no significant abnormalities found in the right vertebral artery. He underwent an endovascular pipeline embolization to treat the dissecting aneurysm (DA). Surprisingly, follow-up DSA imaging 14 months after the initial treatment showed a segmental dilatation and narrowing of the right VA, which suggested a de novo VADA on the right side that had occurred postoperatively. This was followed by a tent-assisted coil embolization therapy for occluding this de novo VADA. This patient showed an uneventful postoperative course with no neurological abnormalities. In addition to hemodynamic stress changes, the unique clinicopathological features of dissecting aneurysms may contribute significantly to the pathogenesis of de novo VA dissection. Given that VA in VADA patients may be vulnerable on both sides, it is important to consider the risk of de novo dissection after initial aneurysm treatment. The bilateral vertebral artery has to be carefully observed when treating any VADA patient to prevent any complications.
Introduction
With a significant improvement in the understanding of the disease entity and angiographic appearance, the vertebral artery dissecting aneurysm (VADA) is considered rare, but has been increasingly reported as a fairly common cause of subarachnoid hemorrhage (SAH) or brain stem ischemia (1). In cases with SAH, previous studies have reported a high incidence of rebleeding with a high mortality rate during the time of recurrent bleeding (2, 3) thereby underscoring the necessity of early interventions. The development of spontaneous vertebral artery (VA) dissecting aneurysm is of rare occurrence, and de novo VADA after treatment of contralateral VA has been even less commonly observed. Mechanisms underlying de novo dissection after treatment of unilateral VADA have not been completely deciphered. In this manuscript, we report an unusual case of a patient with a de novo VADA after placement of a PED stent on the contralateral VA followed by an exhaustive literature review.
Case Description
A 42-years old male patient was referred to a local hospital with a sudden onset of dizziness, nausea, as well as vomiting, and MRI revealed a partially thrombosed aneurysm adjacent to the left portion of the medulla (Figure 1A). The patient was admitted to our hospital without any major symptoms. An initial DSA image demonstrated dilatation at the fourth segment of the left VA, thus indicating a VADA without the involvement of the posterior inferior cerebellar artery (PICA) (Figure 1B). The right vertebral and basilar artery showed no major abnormalities. He had a medical history of hypertension and hyperlipidemia, but no previously reported head trauma and family history of aneurysm. The patient had a history of smoking 20 cigarettes a day for 20 years, which was ceased just at the time of this admission.
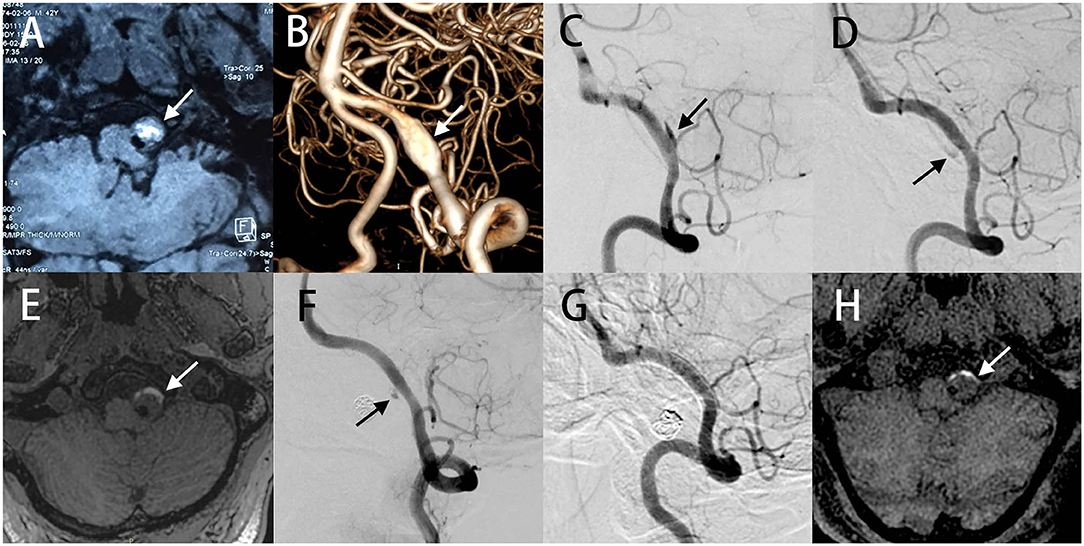
Figure 1. MR image detected a partially thrombosed aneurysm adjacent to the left part of medulla (A). 3D DSA image demonstrated a VADA in the fourth segment of the left VA (B). Angiography at 5 months follow-up revealed that the stent were patent with partial aneurysm residues (C). Partial residual aneurysm of the left VADA was observed on 14 months (D) and 2 years (F) follow-up angiography, and complete occlusion on 3 years (G). The volume of the aneurysm was not significantly changed from 14 months (E) and 2 years follow-up MR images (H).
We treated the left VADA using endovascular pipeline embolization for preserving the normal blood flow. In addition, a dual antiplatelet therapy, comprising 300 mg aspirin and 300 mg clopidogrel were administered 5 days before the surgery. Under general anesthesia, a pipeline embolization device (PED) was successfully implanted with satisfactory adherence between the PED and vessel wall. No intraoperative complications were encountered, and the right VA was preserved. He was discharged home 1 week after the operation and prescribed dual antiplatelet therapy (aspirin 100 mg/day and clopidogrel l00 mg/ day) for 6 months. Angiography conducted at 5 months after initial treatment revealed the patency of the VA and partial aneurysm residues (Figure 1C). This residual of the left VADA persisted on 14 months (Figure 1D) and 2 years (Figure 1F), and completely occluded at 3 years angiography follow-up (Figure 1G). The volume of the aneurysm did not significantly alter from 14 months (Figure 1E) and as noted in 2 years of follow-up MR images (Figure 1H), and the patient did not display any adverse symptoms after the surgery.
In addition, no major abnormalities were found in the right VA at 5 months after initial treatment (Figure 2A). Surprisingly, follow-up imaging 14 months postoperatively showed a segmental dilatation and narrowing of the right VA (Figure 2B), which suggested the formation of a de novo VADA. Stent-assisted coiling was performed for this de novo VADA. Under general anesthesia, Guglielmi detachable coils were positioned in the dissecting aneurysms after placing an LVIS stent in the true lumen of the VA (Figure 2C). Dual antiplatelet therapy was prescribed to him as done before. The patient had an uneventful postoperative progression with no observation of any occurrence of neurological deficits. Moreover, a 20 months angiography follow-up revealed complete occlusion of the aneurysm (Figure 2D).
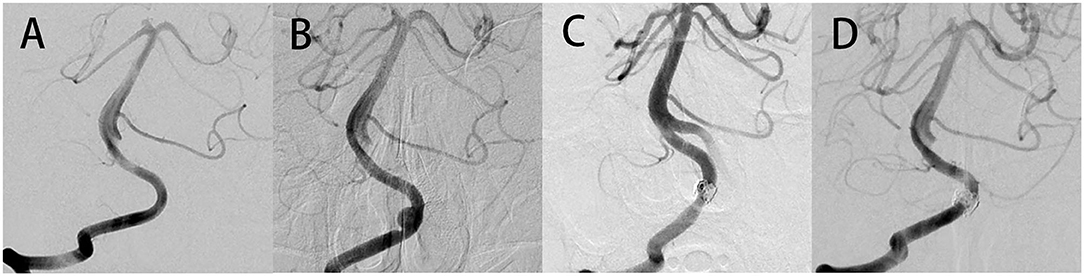
Figure 2. No abnormalities were found in the right VA at the time of 5 months after initial treatment (A). Follow-up imaging 14 months postoperatively showed a segmental dilatation and narrowing of the right VA, which suggested that a de novo VADA occurred (B). Stent-assisted coil embolization therapy was performed to occlude this de novo VADA (C). Angiography at 20 months follow-up after surgery revealed a complete occlusion of the aneurysm (D).
Discussion
Dissecting aneurysms of the intracranial vertebral arteries are observed rarely, and either present themselves as ischemic symptoms of the brain stem or subarachnoid hemorrhage (SAH) (4–6). Unruptured dissecting aneurysms typically may have a benign course, and conservative measures such as antiplatelet or anticoagulation therapy are often recommended (6). However, once ruptured, vertebrobasilar aneurysms may have a poor prognosis with a mortality rate of approximately 50%, and recurrent hemorrhage can account for between 24 and 70% (4–6). Hence, appropriate treatment modalities are needed to avoid serious complications. When treating a VADA, a suitable consideration should be given to angioarchitecture including VA dominancy, location of the PICA origin, and anterior spinal artery involvement. Given its minimally invasive characteristics, endovascular treatment of VADA has become one of the most commonly used method, including internal trapping and stenting. Although internal trapping was the previously preferred treatment, with the advent of the appropriate use of antiplatelet agents and newly developed flow diverters, stenting has also shown favorable safety and efficacy in the management of VADA. Therefore, stent implantation was performed on the left VADA to maintain normal blood flow. The aneurysm reached complete occlusion at 3 years follow-up angiography. Numerous studies (7, 8) have revealed shrinkage of aneurysms following PED placement in cerebral aneurysms, however, the volume of the aneurysm in the present case was not significantly altered from follow-up MR images. The presence of substantial prior thrombosis appears to compromise the reduction of aneurysm volume after FD treatment.
Surprisingly, a follow-up angiography at 14 months after the initial treatment revealed a de novo VADA in the right vertebral artery, which is a very interesting observation. However, there is a paucity of data related to the de novo aneurysm formation rates in different patients with unruptured aneurysms. Moreover, in a systematic review and meta-analysis involving nearly 15,000 patients, the incidence of de novo aneurysms in patients with unruptured aneurysms was observed to be around 3% (9). In addition, history of smoking, hypertension, family history, and female gender are considered as high-risk factors for the development of de novo aneurysms (9, 10).
There are only a few reports about the de novo VADA after treatment of the contralateral VA in the existing literature. Previously reported cases are summarized in Table 1 (11–17). Most initial aneurysms appear on the left side and manifest as SAH or infarction, and can be treated by trapping or occlusion of VA. The interval between the initial dissection and the discovery of de novo contralateral dissection varies from patient to patient. The mechanism responsible for de novo dissection after treatment of unilateral VADA has not been well-defined. It is however possible, that the unique clinicopathological features of dissecting aneurysms and changes in hemodynamic stress may significantly contribute to the pathogenesis of de novo VA dissection.
A few other studies suggest that sudden changes in hemodynamic stress may be the major causal factor behind the development of VA dissecting aneurysms. Two different cases have reported that the diameter of the VA increased after trapping of the contralateral VA (14, 18). Kono et al. performed the computational fluid dynamics (CFD) simulations of bilateral VADA and found that trapping of unilateral VA increased the wall shear stress in the dome surface of the contralateral aneurysm (19). Abrupt changes in hemodynamic stress after occlusion of unilateral VA may play an important role in the occurrence of contralateral VADA. However, as compared to the previously reported cases, our case retains the normal blood flow of unilateral VA, which may greatly alleviate the impact on hemodynamic changes. Furthermore, we noted that our patient had an uneventful postoperative with no neurological deficits and displayed good blood pressure control. Hemodynamic analysis by CFD can also aid in evaluating the formation and growth of aneurysms (20, 21), but there are few data available related to the correlation between hemodynamic changes after stenting and the occurrence of contralateral VADA (19). To the best of our knowledge, this is the first case of development of a de novo VADA after stent placing of the contralateral VA while the contralateral VAs blood flow was maintained in a normal manner.
The clinicopathological features associated with the intracranial dissecting aneurysms have been discussed in detail previously (22–24). The characteristic pathological features include defect or fragmentation of the internal elastic lamina, intimal thickening, and medial degeneration, which can lead to the formation of an aneurysm with or without relevant narrowing of the arterial lumen (24, 25). Generally, the main mechanism associated with intracranial arterial dissection is the diversion of the arterial stream into a weakened arterial wall. An important factor in this process is the development of multiple intramural hemorrhages, which are usually isolated and non-contiguous in the walls of the VA (22, 23, 26). However, these small intramural hemorrhages may be closely related to the disruption of vasa vasorum or new vessels. Although the pathogenesis and clinical manifestations of vertebral artery dissection and carotid artery dissection have not been fully explained, it is reported that patients with spontaneous intracranial artery dissection involve multiple arteries, and the incidence of spontaneous multivessel dissection has been found to be between 10 and 15% (27, 28). For example, Aronov et al. (29) reported a case of acute three-vessel carotid artery occlusion due to spontaneous quadruple carotid dissection occurring 1 week after cesarean section. Ro et al. (30) conducted a detailed pathological investigation of bilateral vertebral arteries in patients who died of SAH due to VADA. They found that 25 of the 58 patients had a latent previous dissection at a different location from the rupture point, with small disruption in the internal elastic lamina covered by an intimal thickening. Besides, they observed that the latent previous dissection had a tendency to occur as bilateral multiple lesions, thereby suggesting that the VA of patients with VADA may be vulnerable on both sides. It is unclear whether the de novo VADA, in this case, developed because of an extension of a latent previous dissection or by the occurrence of a possible new dissection. Therefore, the bilateral vertebral artery needs to be carefully observed when treating any VADA patient.
Many studies have suggested that smoking is a major risk factor for the formation of de novo aneurysms due to its propensity to result in an elastase/alpha antitrypsin imbalance, which may exacerbate the effect of hemodynamic stress on the aneurysm wall (31). Moreover, the other authors have speculated that hypertension may be a risk factor because the interval between identifying newly formed aneurysms has been noted to be significantly shorter in patients with hypertension (32). Furthermore, both smoking and hypertension may contribute to the degradation of the vessel wall and can lead to the development of de novo aneurysms as found in the present case.
It is worth mentioning that a recent Japanese survey of spontaneous cerebral arterial dissection showed that intracranial VA dissection can occur more frequently in Japan (33). This is completely different from the findings among the American population, which displayed a higher incidence of cervical internal carotid artery dissection (34). Actually, all previous case reports about de novo VA dissection were collected from Japan. The reason for this difference has not been clearly elucidated so far and may be possibly related to the variation in genes and the environment.
Conclusions
Endovascular treatment with stent placement can often preserve the normal blood flow of the VA and thereby reduce the changes observed in postoperative hemodynamic stress, but there is still a substantial risk of de novo dissection. In addition to hemodynamic stress changes, the unique clinicopathological features of dissecting aneurysms may significantly contribute to the pathogenesis of de novo VA dissection. As VA in VADA patients may be at risk on both sides, the bilateral vertebral artery needs to be carefully monitored while treating VADA patients.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Author Contributions
WY wrote the manuscript and edited the figure and the table of the article. Together with QL, XL, and JF performed the revision of the current literature. WY and JL collection and interpretation of patient data. YL, YJ, and PL conceived and designed the research. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the National Key Research and Development Program of China (Grant no. 2017YFB1304400).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
SAH, subarachnoid hemorrhage; VA, Vetebral artery; DA, dissecting aneurysm; VADA, vertebral artery dissecting aneurysm; PED, pipeline embolization device; PICA, posterior inferior cerebellar artery; DSA, digital subtraction angiography; CFD, computational fluid dynamics.
References
1. Fusco MR, Harrigan MR. Cerebrovascular dissections–a review part I: spontaneous dissections. Neurosurgery. (2011) 68:242–57; discussion 257. doi: 10.1227/NEU.0b013e3182012323
2. Kemp WJ III, Fulkerson DH, Payner TD, Leipzig TJ, Horner TG, Palmer EL, et al. Risk of hemorrhage from de novo cerebral aneurysms. J Neurosurg. (2013) 118:58–62. doi: 10.3171/2012.9.JNS111512
3. Yoon W, Seo JJ, Kim TS, Do HM, Jayaraman MV, Marks MP. Dissection of the V4 segment of the vertebral artery: clinicoradiologic manifestations and endovascular treatment. Eur Radiol. (2007) 17:983–93. doi: 10.1007/s00330-006-0272-8
4. Rabinov JD, Hellinger FR, Morris PP, Ogilvy CS, Putman CM. Endovascular management of vertebrobasilar dissecting aneurysms. AJNR Am J Neuroradiol. (2003) 24:1421–8.
5. Yamaura A, Watanabe Y, Saeki N. Dissecting aneurysms of the intracranial vertebral artery. J Neurosurg. (1990) 72:183–8. doi: 10.3171/jns.1990.72.2.0183
6. Guan J, Li G, Kong X, He C, Long J, Qin H, et al. Endovascular treatment for ruptured and unruptured vertebral artery dissecting aneurysms: a meta-analysis. J Neurointerv Surg. (2017) 9:558–63. doi: 10.1136/neurintsurg-2016-012309
7. Miyachi S, Hiramatsu R, Ohnishi H, Yagi R, Kuroiwa T. Usefulness of the pipeline embolic device for large and giant carotid cavernous aneurysms. Neurointervention. (2017) 12:83–90. doi: 10.5469/neuroint.2017.12.2.83
8. Carneiro, Rane N, Kuker W, Cellerini M, Corkill R, Byrne JV. Volume changes of extremely large and giant intracranial aneurysms after treatment with flow diverter stents. Neuroradiology. (2014) 56:51–8. doi: 10.1007/s00234-013-1304-0
9. Giordan E, Lanzino G, Rangel-Castilla L, Murad MH, Brinjikji W. Risk of de novo aneurysm formation in patients with a prior diagnosis of ruptured or unruptured aneurysm: systematic review and meta-analysis. J Neurosurg. (2018) 131:14–24. doi: 10.3171/2018.1.JNS172450
10. Martinez-Perez R, Pelz DM, Lownie SP. De novo giant posterior cerebral artery aneurysm developing 25 years after basilar bifurcation aneurysm treatment using a Drake tourniquet: case report and implications for aneurysm follow-up. J Neurosurg. (2018) 128:1028–31. doi: 10.3171/2016.11.JNS161740
11. Kubo Y, Miura K, Suzuki M, Tsuiki K, Kuwata N, Kubo N, et al. Development of a dissecting aneurysm on the vertebral artery immediately after occlusion of the contralateral vertebral artery: a case report. Neurosurg Rev. (1998) 21:177–80. doi: 10.1007/BF02389328
12. Dissecting Aneurysms of the BilateralVertebral Arteries with SubarachnoidHemorrhage: Report of Three Cases (2002).
13. Otawara Y, Ogasawara K, Ogawa A, Kogure T. Dissecting aneurysms of the bilateral vertebral arteries with subarachnoid hemorrhage: report of three cases. Neurosurgery. (2002). 50, 1372–75. doi: 10.1097/00006123-200206000-00033
14. Inui Y, Oiwa Y, Terada T, Nakakita K, Kamei I, Hayashi S. De novo vertebral artery dissecting aneurysm after contralateral vertebral artery occlusion–two case reports. Neurol Med Chir. (2006) 46:32–6. doi: 10.2176/nmc.46.32
15. Katsuno M, Mizunari T, Kobayashi S, Takahashi H, Teramoto A. Rupture of a vertebral artery dissecting aneurysm developing immediately after trapping of a dissecting aneurysm on the contralateral vertebral artery: case report. Neurol Med Chir. (2009) 49:468–70. doi: 10.2176/nmc.49.468
16. Tsuji K, Watanabe A, Nakagawa N, Kato A. A case of unilateral vertebral artery dissection progressing in a short time period to bilateral vertebral artery dissection. Surg Neurol Int. (2019) 10:126. doi: 10.25259/SNI-78-2019
17. Kidani N, Sugiu K, Hishikawa T, Hiramatsu M, Haruma J, Nishihiro S, et al. De novo vertebral artery dissecting aneurysm after internal trapping of the contralateral vertebral artery. Acta Neurochir. (2017) 159:1329–33. doi: 10.1007/s00701-017-3204-2
18. Yasui T, Sakamoto H, Kishi H, Komiyama M, Iwai Y, Yamanaka K, et al. Bilateral dissecting aneurysms of the vertebral arteries resulting in subarachnoid hemorrhage: case report. Neurosurgery. (1998) 42:162–4; discussion 165. doi: 10.1097/00006123-199801000-00035
19. Kono K, Shintani A, Fujimoto T, Terada T. Stent-assisted coil embolization and computational fluid dynamics simulations of bilateral vertebral artery dissecting aneurysms presenting with subarachnoid hemorrhage: case report. Neurosurgery. (2012) 71:E1192–200; discussion E1200–1. doi: 10.1227/NEU.0b013e318270603a
20. Meng H, Wang Z, Hoi Y, Gao L, Metaxa E, Swartz DD, et al. Complex hemodynamics at the apex of an arterial bifurcation induces vascular remodeling resembling cerebral aneurysm initiation. Stroke. (2007) 38:1924–31. doi: 10.1161/STROKEAHA.106.481234
21. Xiang J, Natarajan SK, Tremmel M, Ma D, Mocco J, Hopkins LN, et al. Hemodynamic-morphologic discriminants for intracranial aneurysm rupture. Stroke. (2011) 42:144–52. doi: 10.1161/STROKEAHA.110.592923
22. Endo S, Nishijima M, Nomura H, Takaku A, Okada E. A pathological study of intracranial posterior circulation dissecting aneurysms with subarachnoid hemorrhage: report of three autopsied cases and review of the literature. Neurosurgery. (1993) 33:732–8. doi: 10.1227/00006123-199310000-00026
23. Mizutani T, Kojima H, Asamoto S, Miki Y. Pathological mechanism and three-dimensional structure of cerebral dissecting aneurysms. J Neurosurg. (2001) 94:712–7. doi: 10.3171/jns.2001.94.5.0712
24. Mizutani T, Miki Y, Kojima H, Suzuki H. Proposed classification of nonatherosclerotic cerebral fusiform and dissecting aneurysms. Neurosurgery. (1999) 45:253–9; discussion 259–60. doi: 10.1097/00006123-199908000-00010
25. Sasaki O, Ogawa H, Koike T, Koizumi T, Tanaka R. A clinicopathological study of dissecting aneurysms of the intracranial vertebral artery. J Neurosurg. (1991) 75:874–82. doi: 10.3171/jns.1991.75.6.0874
26. Yasui T, Komiyama M, Nishikawa M, Nakajima H, Kobayashi Y, Inoue T. Fusiform vertebral artery aneurysms as a cause of dissecting aneurysms. Report of two autopsy cases and a review of the literature. J Neurosurg. (1999) 91:139–44. doi: 10.3171/jns.1999.91.1.0139
27. Hassan AE, Zacharatos H, Mohammad YM, Tariq N, Vazquez G, Rodriguez GJ, et al. Comparison of single versus multiple spontaneous extra- and/or intracranial arterial dissection. J Stroke Cerebrovasc Dis. (2013) 22:42–8. doi: 10.1016/j.jstrokecerebrovasdis.2011.06.004
28. Campos CR, Evaristo EF, Yamamoto FI, Puglia P Jr, Lucato LT, Scaff M. [Spontaneous cervical carotid and vertebral arteries dissection: study of 48 patients]. Arq Neuropsiquiatr. (2004) 62:492–8. doi: 10.1590/S0004-282X2004000300021
29. Aronov M, Shevchenko NS, Amosova NA, Kotenko KV. Acute three-vessel cervical arterial occlusion due to spontaneous quadruple cervical artery dissection. BMJ Case Rep. (2014) 2014:bcr2014203725. doi: 10.1136/bcr-2014-203725
30. Ro A, Kageyama N, Abe N, Takatsu A, Fukunaga T. Intracranial vertebral artery dissection resulting in fatal subarachnoid hemorrhage: clinical and histopathological investigations from a medicolegal perspective. J Neurosurg. (2009) 110:948–54. doi: 10.3171/2008.11.JNS08951
31. Burkhardt JK, Chua MHJ, Weiss M, Do ASS, Winkler EA, Lawton MT. Risk of aneurysm residual regrowth, recurrence, and de novo aneurysm formation after microsurgical clip occlusion based on follow-up with catheter angiography. World Neurosurg. (2017) 106:74–84. doi: 10.1016/j.wneu.2017.06.110
32. Tonn J, Hoffmann O, Hofmann E, Schlake HP, Sorensen N, Roosen K. “De novo” formation of intracranial aneurysms: who is at risk? Neuroradiology. (1999) 41:674–9. doi: 10.1007/s002340050823
33. Tsukahara T, Minematsu K. Overview of spontaneous cervicocephalic arterial dissection in Japan. Acta Neurochir Suppl. (2010) 107:35–40. doi: 10.1007/978-3-211-99373-6_5
Keywords: vertebral artery dissecting aneurysm, de novo aneurysm, bilateral vertebral artery dissection, endovascular embolization, pipeline embolization device
Citation: You W, Feng J, Liu Q, Liu X, Lv J, Jiang Y, Liu P and Li Y (2021) Case Report: De novo Vertebral Artery Dissection After Intravascular Stenting of the Contralateral Unruptured Vertebral Artery Aneurysm. Front. Neurol. 12:599197. doi: 10.3389/fneur.2021.599197
Received: 26 August 2020; Accepted: 15 March 2021;
Published: 23 April 2021.
Edited by:
Osama O. Zaidat, Northeast Ohio Medical University, United StatesReviewed by:
Moisey Aronov, A.I. Burnazyan Federal Medical and Biophysical Center, Federal Medical and Biological Agency, RussiaAdam A. Dmytriw, Brigham and Women’s Hospital and Harvard Medical School, United States
Copyright © 2021 You, Feng, Liu, Liu, Lv, Jiang, Liu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Peng Liu, c2tlbGV0b25saXVAc2luYS5jb20=; Youxiang Li, eW91eGlhbmcubGlAZ21haWwuY29t
 Wei You
Wei You Junqiang Feng
Junqiang Feng Xinke Liu
Xinke Liu Peng Liu
Peng Liu