- 1Department of Neurology, Beijing Tiantan Hospital, Capital Medical University, Beijing, China
- 2Department of Neurology, Beijing Haidian Hospital, Beijing, China
- 3China National Clinical Research Center for Neurological Diseases, Beijing, China
- 4Center of Stroke, Beijing Institute for Brain Disorders, Beijing, China
- 5Beijing Key Laboratory of Translational Medicine for Cerebrovascular Disease, Beijing, China
Background: Low heart rate variability (HRV) is known to be associated with increased all-cause, cardiovascular, and cerebrovascular mortality but its association with clinical outcomes in patients with transient ischemic attack (TIA) or minor stroke is unclear.
Methods: We selected TIA and minor stroke patients from a prospective registration study. From each continuous electrocardiograph (ECG) record, each QRS complex was detected and normal-to-normal (N-N) intervals were determined. The standard deviation of all N-N intervals (SDNN) and the square root of the mean squared differences of successive N-N intervals (RMSSD) were calculated. Logistic regression analysis and Cox regression analysis were performed to assess the outcomes of patients at 90 days, and the odds and risk ratios (OR/HR) of each index quartile were compared.
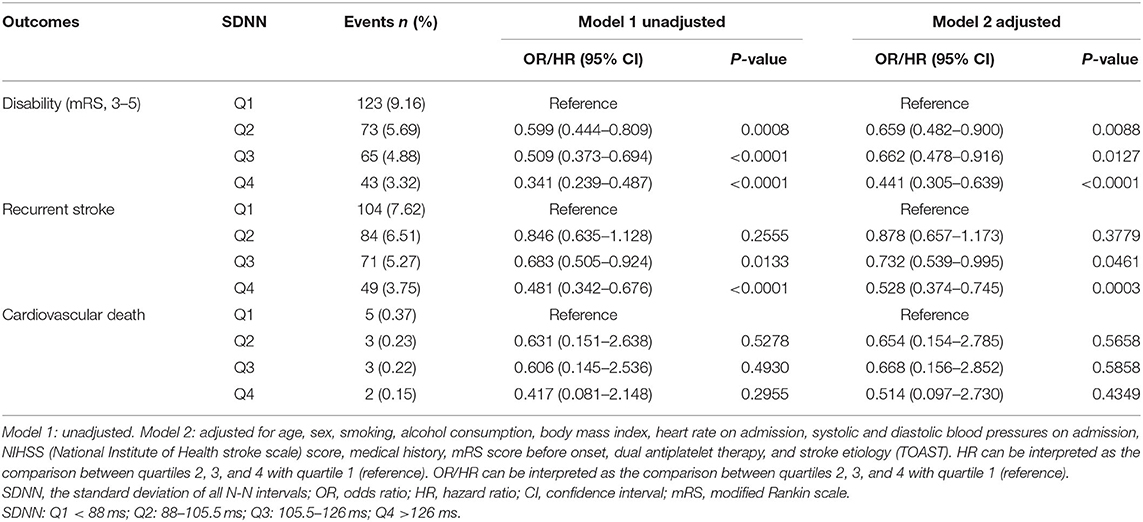
Results: Compared with SDNN patients in the lowest quartile, neurological disability was significantly reduced in other quartile groups at 90 days, with significant differences [OR of group Q2 was 0.659; 95% confidence interval (CI), 0.482–0.900; p = 0.0088; OR of group Q3 was 0.662; 95% CI, 0.478–0.916; p = 0.0127; OR of group Q4 was 0.441; 95% CI, 0.305–0.639; p <0.0001]. Compared with the lowest quartile, the recurrence rate of TIA or minor stroke in patients of the two higher quartiles (Q3 and Q4) of SDNN was significantly reduced at 90 days (HR of Q3 group was 0.732; 95% CI, 0.539–0.995; p = 0.0461; HR of Q4 group was 0.528; 95% CI, 0.374–0.745; p = 0.0003).
Conclusions: Based on our findings, autonomic dysfunction is an adverse indicator for neurological function prognosis and stroke recurrence 90 days after TIA or minor stroke.
Introduction
Stroke is the second leading cause of death worldwide (1) and the leading cause of mortality and disability in China (2). About 40% of stroke survivors are disabled [modified Rankin Scale (mRS) score 3–5] between 1 month and 5 years after stroke (3). Depending on the circumstances of treatment, the rate of stroke recurrence 90 days after the first ischemic event ranges from 3.7 to 20% (4–6). Approximately 40% of recurrent stroke events are fatal within 30 days, which is nearly twice the 30-day case fatality of a first-ever stroke (7). According to the data of the Third China National Stroke Registry (CNSR-III), TIA and minor stroke (an National Institutes of Health Stroke Scale (NIHSS) score ≤ 5) account for about 73% of acute ischemic stroke cases. Both TIA and minor stroke are characterized by a high risk of early stroke recurrence (8). Currently, assessment tools have limitations in predicting the early recurrence of stroke (9–12). It is still challenging to stratify the risk and identify high-risk patients accurately in the early treatment stage of stroke.
Heart rate variability (HRV) is a commonly used quantitative marker for measuring autonomic nerve system (13). HRV is easy to obtain. It quantifies sympathetic-vagus regulation at the sinus level as a tool (14) for assessing overall heart health and autonomic nerve system function (13, 15). Dysfunction of the autonomic nerve system after stroke increases the risk of stroke recurrence and death (16–18). Therefore, exploitation of the predictive function of HRV in risk stratification tools has become an important measure to identify high-risk populations. Although correlation between autonomic nerve system function and stroke prognosis has been studied previously, the sample sizes were small (19, 20).
To date, no study has been done to evaluate how HRV is related to a comprehensive 90-day prognosis in patients with TIA or minor stroke. Using the CNSR-III database, this study focused on the correlation between HRV and 90-day outcomes in patients with TIA and minor stroke including neurological disability, stroke recurrence, and cardiovascular death.
Methods
Study Population
The CNSR-III database is a nationwide prospective clinical registry of ischemic stroke or TIA in China based on etiology, imaging, and biology markers. The detailed study design of the CNSR-III trial has been described elsewhere (21). Briefly, between August 2015 and March 2018, the CNSR-III recruited consecutive patients with ischemic stroke or TIA from 201 hospitals that covered 22 provinces and four municipalities in China. Informed consent received from the patient or legally authorized representative (primarily spouse, parents, adult children, otherwise indicated). Clinical data were collected prospectively using an electronic data capture system by face-to-face interviews. Brain imaging, including brain magnetic resonance imaging (MRI) and computed tomography (CT), were completed at baseline. Blood samples were collected and biomarkers were tested at baseline. Face-to-face follow-up was conducted at 3 months, and telephone follow-up was conducted at 6 months and 1–5 years.
The registry recruited consecutive patients who met the following criteria: age >18 years; ischemic stroke or TIA; within 7 days from the onset of symptoms to enrolment; Acute ischemic stroke was diagnosed according to the World Health Organization (WHO) criteria (22) and confirmed by MRI or brain CT. Patients who had silent cerebral infarction with no manifestation of symptoms and signs or who refused to participate in the registry were excluded. The protocol of the CNSR-III trial was approved by the ethics committee at Beijing Tiantan Hospital affiliated to Capital Medical University (IRB Approval Number: KY2015-001-01) and all participating centers. In this study, minor stroke was defined as an NIHSS score ≤ 5.
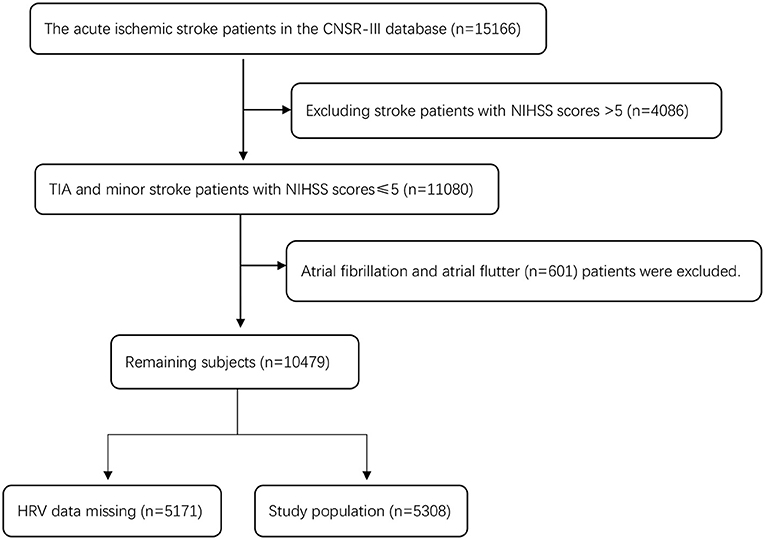
There were 15,166 patients in CNSR-III, and 4,086 patients with an NIHSS score >5 were excluded. There were 11,080 patients with TIA and minor stroke (an NIHSS score ≤ 5). Six hundred and one patients with atrial fibrillation and atrial flutter (n = 601) or those with missing HRV data (n = 5,171) were excluded (including no 24-h ECG examination or HRV data generated during 24-h ECG examination). A total of 5,308 patients were eligible for the study. Figure 1 shows a detailed flow chart for the study population selection from CNSR-III.
Baseline Variables
Age, sex, smoking history (never, occasionally, current, and past), drinking history (never, occasionally, current, and past), body mass index (BMI), heart rate on admission, blood pressure on admission, NIHSS (National Institute of Health stroke scale), medical history (including stroke, heart disease, hypertension, diabetes mellitus, and hyperlipidemia), mRS score before onset, medication history, secondary prevention treatment, and stroke etiology were all collected at the baseline.
During hospitalization, the patient received 24-h ECG, from which the SDNN and RMSSD were automatically obtained. SDNN is a global index of HRV, and reflects the standard deviation of the normal R-R intervals (N-N intervals) (23). RMSSD is the square root of the mean squared differences of successive N-N intervals. It is thought to reflect the activity of the parasympathetic nervous system (13).
Outcome Measures
Neurological function prognosis, stroke recurrence, and cardiovascular death were recorded 90 day after TIA or minor stroke. Disability after stroke was defined as an mRS score ≥ 3. Recurrent stroke was defined as new ischemic and recurrent hemorrhagic strokes (intracerebral and subarachnoid hemorrhages). Cardiovascular death was defined as ischemic stroke, hemorrhagic stroke, sudden cardiac death, acute myocardial infarction, death directly caused by heart failure, and other cardiovascular death [including cardiac arrhythmias unrelated to sudden cardiac death, pulmonary embolism, cardiovascular intervention (unrelated to acute myocardial infarction), aortic aneurysm rupture, and peripheral arterial disease]. Each case fatality was either confirmed on a death certificate from the attending hospital or local citizen registry.
Statistical Analysis
Continuous variables are expressed as the mean ± standard deviation, and classification variables are expressed as a percentage. A quartile classification method was used for SDNN and RMSSD, with the lowest quartile as a reference for all comparisons. Baseline variables between different quartile groups were compared using a chi-square test for classification variables and Kruskal-wallis-test for continuous variables. Logistic regression analysis and Cox regression analysis were used to calculate the odds and risk ratios (OR/HR) and 95% confidence intervals (CIs). The adjusted clinical covariables included age, sex, smoking, alcohol consumption, previous stroke history, heart disease, hypertension, diabetes mellitus, lipid metabolic disorders, and other variables with a p < 0.1. A two-sided significance level of P < 0.05 was determined. All analyses were performed using SAS 9.4 software.
Results
A total of 5,308 patients (mean age, 61.13 ± 10.81 years) were enrolled in the study: 69.11% males (mean age, 60.2 ± 10.8 years) and 30.89% females (mean age, 63.2 ± 10.4 years). SDNN was 108.32 ± 30.55 ms and RMSSD was 30.79 ± 15.05 ms. Among the patients, the prevalence of previous stroke/TIA, coronary heart disease, hypertension, diabetes mellitus, and dyslipidemia were 22.89% (1,215 cases), 10.70% (568 cases), 62.08% (3,296 cases), 22.92% (1,217 cases), and 8.16% (433 cases), respectively. The proportion of anti-platelet, anticoagulation, stains, antioxidant, hypoglycemic and antihypertensive drugs in secondary prevention were 97.98% (5,201 cases), 5.58% (296 cases), 95.65% (5,077 cases), 15.79% (838 cases), 24.89% (1,321 cases), 47.14% (2,502 cases), respectively.
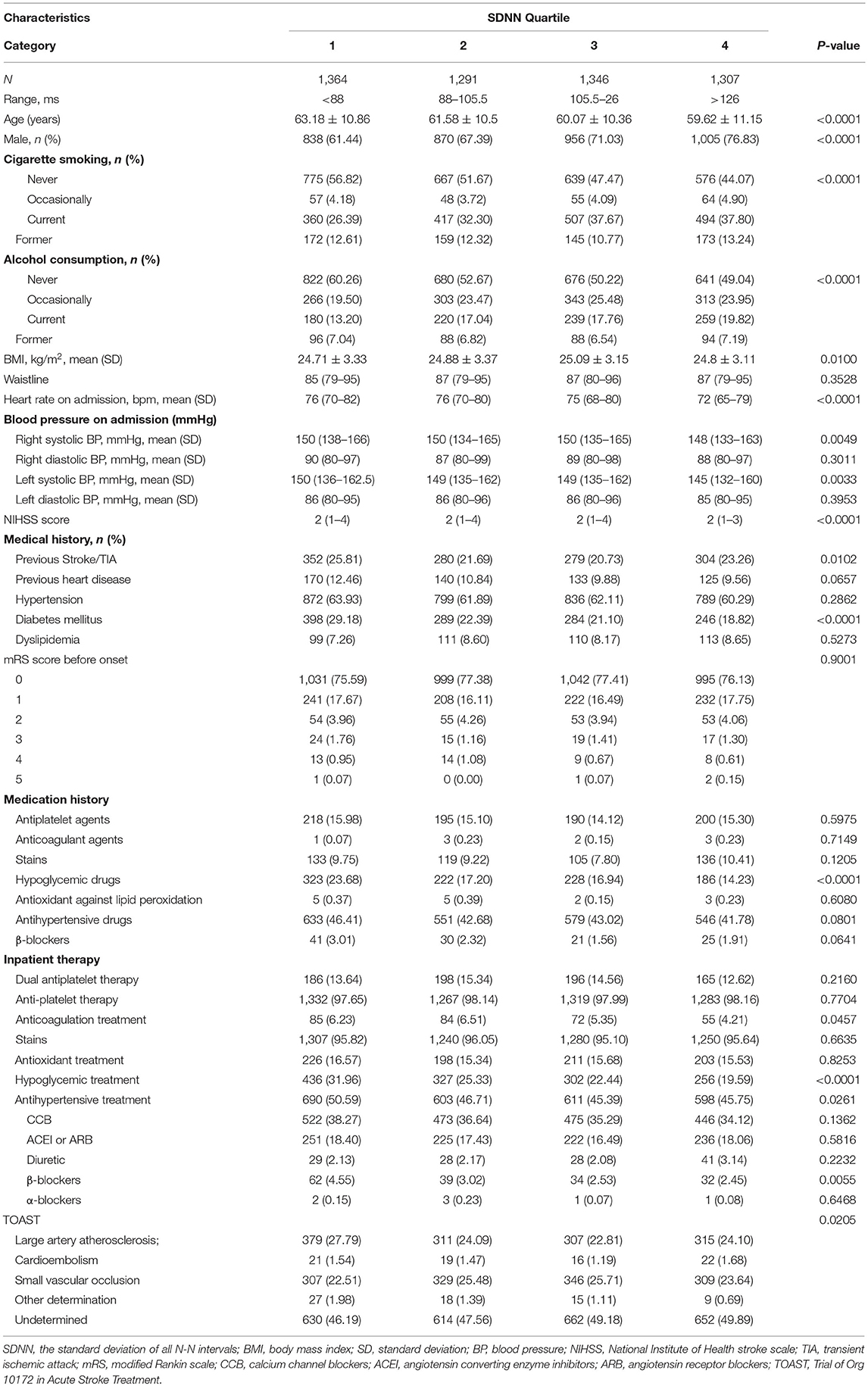
Table 1 showed the descriptive statistics for the baseline variables in terms of SDNN quartiles. The group with a lower SDNN value tended to be older, with an increased proportion of women, faster heart rate, and higher systolic blood pressure at first admission. In the medical history, the prevalence of diabetes mellitus increased significantly in the group with a low SDNN value, and there was a significant difference between groups (p < 0.0001).
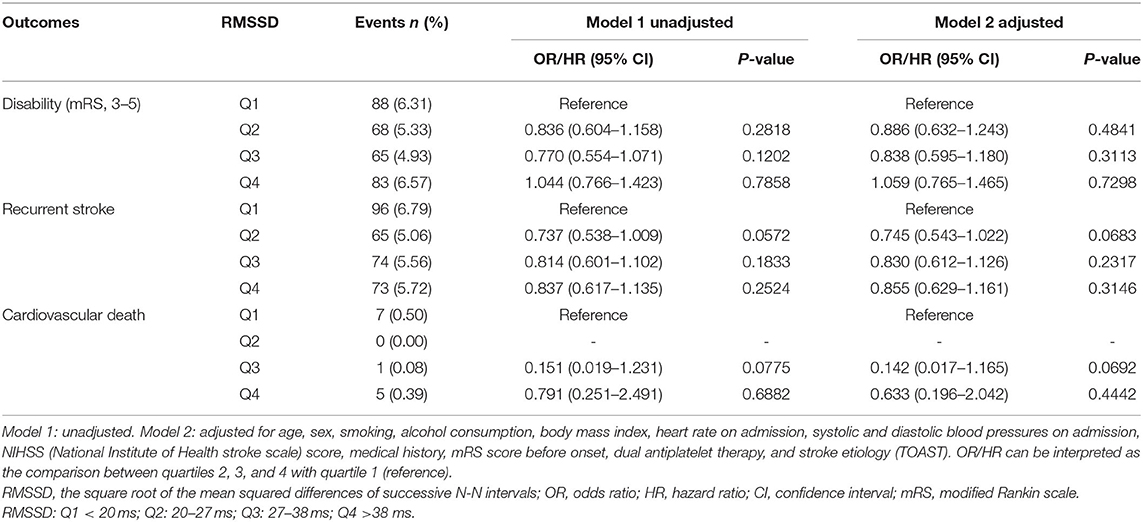
With a gradual increase of SDNN, the rate of neurological disability in patients with TIA or minor stroke decreased significantly 90 days after stroke. Compared with the lowest SDNN quartile, findings for the other three groups were as follows, [OR in group Q2 after correction, 0.659; 95% confidence interval (CI), 0.482–0.900; p = 0.0088; OR of group Q3, 0.662; 95% CI, 0.478–0.916; p = 0.0127; OR of group Q4, 0.441; 95% CI, 0.305–0.639; p < 0.0001]. With an increasing SDNN, stroke recurrence showed a decreasing trend. The recurrence rate of patients of the two higher quartiles of SDNN was significantly reduced at 90 days (HR of Q3 group after correction, 0.732; 95% CI, 0.539–0.995; p = 0.0461; HR of Q4 group after correction, 0.528; 95% CI, 0.374–0.745; p = 0.0003). No clear association was found between SDNN and cardiovascular death (Table 2). In addition, no association was found between RMSSD and 90-day neurological disability, recurrent stroke, or cardiovascular death (Table 3).
Discussion
In the time domain measurement of HRV, SDNN reflects the overall condition of the autonomic nerve system, and a decrease of the SDNN usually indicates a relative superiority of the sympathetic nerve in the autonomic nervous system (24). Aging (25), female sex (25, 26), increased blood pressure, and diabetes mellitus (26, 27) all showed the characteristics of relative sympathetic nerve superiority in the autonomic nervous system, and thus with a decreased SDNN. This is consistent with our baseline data analysis results (Table 1). In this study, we found that with an increasing SDNN, the rate of neurological dysfunction in patients at 90 days after ischemic stroke decreased significantly. The recurrence rate of 90-day stroke was significantly reduced for participants in the two higher quartiles of SDNN.
Previous studies have shown that age, diabetes mellitus, and NIHSS scores are predictors of 90-day neurological disability (28, 29). But in this study, after correcting for confounding factors, we found that with an increasing SDNN, the rate of neurological dysfunction in patients 90 days after ischemic stroke decreased significantly. After the acute phase, patients with autonomic nerve system dysfunction need more help in their daily rehabilitation tasks (30). Poor adaptability of the cardiac autonomic nerve system in different rehabilitation training activities and poor rehabilitation dependence impact the rehabilitation effects (30, 31). At the same time, autonomic nerve system dysfunction is not only associated with overall cognitive function, processing speed, executive function, and poor retrospective memory performance in patients (32, 33) but also associated with post-stroke depression (34). All of these might have a negative impact on the patients' positive initiative in rehabilitation training and their ability to follow the rehabilitation regimens, resulting in unsatisfactory rehabilitation results.
According to previous studies, age, blood pressure and diabetes mellitus were all risk factors for recurrence of TIA and minor stroke (35, 36). However, after adjusting for risk factors such as age, blood pressure, and diabetes mellitus, SDNN was still significantly correlated with stroke recurrence, suggesting that autonomic dysregulation was associated with stroke recurrence. There is a balance between the sympathetic and parasympathetic nervous systems, which is important for regulating cerebral blood flow. Dysfunction of the autonomic nervous system after stroke aggravated secondary brain injury through changes in hemodynamics (37) and non-hemodynamic factors. Changes in hemodynamics, such as increased blood pressure variability, impaired brain autoregulation, and cardiovascular complications, lead to secondary brain injury. Non-hemodynamic factors such as the production of inflammatory factors (38), hyperglycemia, and increased blood-brain barrier permeability (39), coagulation factor activation, and platelet activation (40, 41) also cause secondary brain damage. These all increase the risk of further vascular events, such as myocardial infarction, recurrent stroke, and deep vein thrombosis (40). In animal experiments, chronic stress increased sympathetic nerve activity to increase the heart rate of mice. It was found that vascular endothelial function was damaged and oxidative stress in the blood vessels and brain as well as the susceptibility to cerebral ischemia were increased (42), consequently increasing the area of brain injury. Lowering the heart rate can restore vascular endothelial function, reduce oxidative stress, increase capillary density and collateral circulation (43), protect ischemic brain injury (43), and reduce stroke volume (44). The above mechanisms may explain our findings, that is, sympathetic hyperexcitation leads to poor neurological outcomes and stroke recurrence, while sympathetic suppression leads to favorable neurological outcomes and a reduction in stroke recurrence.
Previous evidence had shown that 24-h SDNN was strongly associated with all-cause mortality (45). Low HRV predicted increased mortality, and the association could not be attributed to cardiovascular risk factors or underlying disease (23). The cardiac complications resulting from autonomic dysfunction in stroke patients were 2–6% of the total mortality rate 90 days after acute ischemic stroke (46). In our study, both SDNN and RMSSD were not associated with 90-day vascular death in patients. This is different from previous studies, which may be due to the study population difference. The study population we selected were TIA and minor stroke patients with an NIHSS score ≤5, with mild clinical symptoms, and a total mortality rate of 0.24%.
SDNN reflects the overall autonomic function, including sympathetic and parasympathetic activity, while RMSSD only reflects parasympathetic activity (13). The parasympathetic effect is transient, while there is a long period of sympathetic excitation after ischemic stroke (47). Therefore, considering that 90-day neurological dysfunction and stroke recurrence are both associated with sympathetic hyperexcitability after ischemic stroke, reducing sympathetic activity may improve the prognosis of ischemic stroke after 90 days. Vagal nerve stimulation (VNS) was also shown to reduce infarct volume and improve neurological outcome at 1 day after acute ischemic stroke in middle cerebral artery occlusion rats (48). The mechanism of protection with VNS may involve a reduction in extracellular glutamate and reduced excitotoxicity during cerebral ischemia, and/or a reduction in inflammation and release of norepinephrine. Parasympathetic activation also increases cerebral blood flow and enhance neurogenesis. However, parasympathetic activation is an invasive technique, which limits its use in acute stroke treatment (49). Beta blockade (50), statin (51), external counter pulsation (52), tele-acupuncture (53) have all been reported to modulate autonomic nervous dysfunction although more research is needed to confirm these findings.
Limitations
Our research has some limitations. Firstly, CNSR-III is a prospective clinical registry study of ischemic stroke or TIA nationwide based on etiological classification, imaging, and biological markers. It is not a specific study on the correlation between HRV and stroke prognosis. Second, patients received 24-h Holter during the acute period of hospitalization for stroke, and we did not conduct statistics on the interval between the examination and stroke occurrence. Third, in this study, we mainly analyzed the correlation between autonomic nerve system function and short-term prognosis of patients with TIA and minor ischemic stroke. Considering that TIA has no lesions and the lesions of minor stroke are relatively small, the localization of the lesions has not been evaluated yet. The relationship between stroke location and HRV need be investigated.
Conclusion
This study shows that autonomic nerve system dysfunction (sympathetic hyperexcitability and/or decreased parasympathetic activity) is an adverse factor for 90-day neurological prognosis and stroke recurrence after TIA and minor stroke. Regulating autonomic nerve system function may be a potential new target for improving the 90-day prognosis in these patients and is worthy to be further investigated.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by Beijing Tiantan Hospital affiliated to Capital Medical University (IRB Approval Number: KY2015-001-01) and all participating centers. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
CL conceived the study and wrote the first draft of the paper. YP and MW analyzed the data. XM and ZL critically edited the manuscript. YW supervised the study. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by National Key R&D Program of China, Code (2016YFC0901002, 2018YFC1312903), Beijing Municipal Science and Technology Commission, Code (D171100003017002) and National Science and Technology Major Project, code (2017ZX09304018).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are grateful to Yong Jiang and Xinying Huang (from the China National Clinical Research Center for Neurological Diseases) for the data collection.
References
1. Collaborators GBDCoD. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. (2018) 392:1736–88. doi: 10.1016/S0140-6736(18)32203-7
2. Zhou M, Wang H, Zhu J, Chen W, Wang L, Liu S, et al. Cause-specific mortality for 240 causes in China during 1990-2013: a systematic subnational analysis for the Global Burden of Disease Study 2013. Lancet. (2016) 387:251–72. doi: 10.1016/S0140-6736(15)00551-6
3. Luengo-Fernandez R, Paul NL, Gray AM, Pendlebury ST, Bull LM, Welch SJ, et al. Population-based study of disability and institutionalization after transient ischemic attack and stroke: 10-year results of the Oxford Vascular Study. Stroke. (2013) 44:2854–61. doi: 10.1161/STROKEAHA.113.001584
4. Amarenco P, Lavallee PC, Labreuche J, Albers GW, Bornstein NM, Canhao P, et al. One-year risk of stroke after transient ischemic attack or minor stroke. N Engl J Med. (2016) 374:1533–42. doi: 10.1056/NEJMoa1412981
5. Lovett JK, Dennis MS, Sandercock PA, Bamford J, Warlow CP, Rothwell PM. Very early risk of stroke after a first transient ischemic attack. Stroke. (2003) 34:e138–40. doi: 10.1161/01.STR.0000080935.01264.91
6. Johnston SC, Gress DR, Browner WS, Sidney S. Short-term prognosis after emergency department diagnosis of TIA. JAMA. (2000) 284:2901–6. doi: 10.1001/jama.284.22.2901
7. Hardie K, Hankey GJ, Jamrozik K, Broadhurst RJ, Anderson C. Ten-year risk of first recurrent stroke and disability after first-ever stroke in the Perth community stroke study. Stroke. (2004) 35:731–5. doi: 10.1161/01.STR.0000116183.50167.D9
8. Coull AJ, Lovett JK, Rothwell PM, Oxford Vascular Study. Population based study of early risk of stroke after transient ischaemic attack or minor stroke: implications for public education and organisation of services. BMJ. (2004) 328:326. doi: 10.1136/bmj.37991.635266.44
9. Chandratheva A, Geraghty OC, Rothwell PM. Poor performance of current prognostic scores for early risk of recurrence after minor stroke. Stroke. (2011) 42:632–7. doi: 10.1161/STROKEAHA.110.593301
10. Diener HC, Ringleb PA, Savi P. Clopidogrel for the secondary prevention of stroke. Expert Opin Pharmacother. (2005) 6:755–64. doi: 10.1517/14656566.6.5.755
11. Committee CS. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). CAPRIE Steering Committee. Lancet. (1996) 348:1329–39. doi: 10.1016/S0140-6736(96)09457-3
12. Wardlaw JM, Brazzelli M, Chappell FM, Miranda H, Shuler K, Sandercock PA, et al. ABCD2 score and secondary stroke prevention: meta-analysis and effect per 1,000 patients triaged. Neurology. (2015) 85:373–80. doi: 10.1212/WNL.0000000000001780
13. Heart rate variability: standards of measurement physiological interpretation and clinical use. Task force of the European society of cardiology and the North American society of pacing and electrophysiology. Circulation. (1996) 93:1043–65.
14. Sztajzel J. Heart rate variability: a noninvasive electrocardiographic method to measure the autonomic nervous system. Swiss Med Wkly. (2004) 134:514–22.
15. Rajendra Acharya U, Paul Joseph K, Kannathal N, Lim CM, Suri JS. Heart rate variability: a review. Med Biol Eng Comput. (2006) 44:1031–51. doi: 10.1007/s11517-006-0119-0
16. De Raedt S, De Vos A, De Keyser J. Autonomic dysfunction in acute ischemic stroke: an underexplored therapeutic area? J Neurol Sci. (2015) 348:24–34. doi: 10.1016/j.jns.2014.12.007
17. Lees T, Shad-Kaneez F, Simpson AM, Nassif NT, Lin Y, Lal S. Heart rate variability as a biomarker for predicting stroke, post-stroke complications and functionality. Biomark Insights. (2018) 13:1177271918786931. doi: 10.1177/1177271918786931
18. Zhao M, Guan L, Wang Y. The association of autonomic nervous system function with ischemic stroke, and treatment strategies. Front Neurol. (2019) 10:1411. doi: 10.3389/fneur.2019.01411
19. Graff B, Gasecki D, Rojek A, Boutouyrie P, Nyka W, Laurent S, et al. Heart rate variability and functional outcome in ischemic stroke: a multiparameter approach. J Hypertens. (2013) 31:1629–36. doi: 10.1097/HJH.0b013e328361e48b
20. Mo J, Huang L, Peng J, Ocak U, Zhang J, Zhang JH. Autonomic disturbances in acute cerebrovascular disease. Neurosci Bull. (2019) 35:133–44. doi: 10.1007/s12264-018-0299-2
21. Wang Y, Jing J, Meng X, Pan Y, Wang Y, Zhao X, et al. The third China national stroke registry (CNSR-III) for patients with acute ischaemic stroke or transient ischaemic attack: design, rationale and baseline patient characteristics. Stroke Vasc Neurol. (2019) 4:158–64. doi: 10.1136/svn-2019-000242
22. Stroke−1989. Recommendations on stroke prevention, diagnosis, and therapy. Report of the WHO task force on stroke and other cerebrovascular disorders. Stroke. (1989) 20:1407–31. doi: 10.1161/01.STR.20.10.1407
23. Dekker JM, Crow RS, Folsom AR, Hannan PJ, Liao D, Swenne CA, et al. Low heart rate variability in a 2-minute rhythm strip predicts risk of coronary heart disease and mortality from several causes: the ARIC Study. Atherosclerosis Risk In Communities. Circulation. (2000) 102:1239–44. doi: 10.1161/01.CIR.102.11.1239
24. Lombardi F. Clinical implications of present physiological understanding of HRV components. Card Electrophysiol Rev. (2002) 6:245–9. doi: 10.1023/a:1016329008921
25. Bonnemeier H, Richardt G, Potratz J, Wiegand UK, Brandes A, Kluge N, et al. Circadian profile of cardiac autonomic nervous modulation in healthy subjects: differing effects of aging and gender on heart rate variability. J Cardiovasc Electrophysiol. (2003) 14:791–9. doi: 10.1046/j.1540-8167.2003.03078.x
26. Ramaekers D, Ector H, Aubert AE, Rubens A, Van de Werf F. Heart rate variability and heart rate in healthy volunteers. Is the female autonomic nervous system cardioprotective? Eur Heart J. (1998) 19:1334–41. doi: 10.1053/euhj.1998.1084
27. Shah AS, El Ghormli L, Vajravelu ME, Bacha F, Farrell RM, Gidding SS, et al. Heart rate variability and cardiac autonomic dysfunction: prevalence, risk factors, and relationship to arterial stiffness in the treatment options for type 2 diabetes in adolescents and youth (TODAY) study. Diabetes Care. (2019) 42:2143–50. doi: 10.2337/dc19-0993
28. Cucchiara B, George DK, Kasner SE, Knutsson M, Denison H, Ladenvall P, et al. Disability after minor stroke and TIA: a secondary analysis of the SOCRATES trial. Neurology. (2019) 93:e708–16. doi: 10.1212/WNL.0000000000007936
29. Coutts SB, Modi J, Patel SK, Aram H, Demchuk AM, Goyal M, et al. What causes disability after transient ischemic attack and minor stroke? Results from the CT and MRI in the triage of TIA and minor cerebrovascular events to identify high risk patients (CATCH) study. Stroke. (2012) 43:3018–22. doi: 10.1161/STROKEAHA.112.665141
30. Bassi A, Colivicchi F, Santini M, Caltagirone C. Cardiac autonomic dysfunction and functional outcome after ischaemic stroke. Eur J Neurol. (2007) 14:917–22. doi: 10.1111/j.1468-1331.2007.01875.x
31. Raphaely Beer N, Soroker N, Bornstein NM, Katz-Leurer M. The cardiac autonomic nervous system response to different daily demands among patients at the sub-acute phase post ischemic stroke and healthy controls. NeuroRehabilitation. (2018) 42:391–6. doi: 10.3233/NRE-172295
32. Zeki Al Hazzouri A, Haan MN, Deng Y, Neuhaus J, Yaffe K. Reduced heart rate variability is associated with worse cognitive performance in elderly Mexican Americans. Hypertension. (2014) 63:181–7. doi: 10.1161/HYPERTENSIONAHA.113.01888
33. Zeki Al Hazzouri A, Elfassy T, Carnethon MR, Lloyd-Jones DM, Yaffe K. Heart rate variability and cognitive function in middle-age adults: the coronary artery risk development in young adults. Am J Hypertens. (2017) 31:27–34. doi: 10.1093/ajh/hpx125
34. Robinson RG, Spalletta G, Jorge RE, Bassi A, Colivicchi F, Ripa A, et al. Decreased heart rate variability is associated with poststroke depression. Am J Geriatr Psychiatry. (2008) 16:867–73. doi: 10.1097/JGP.0b013e318180057d
35. Merwick A, Albers GW, Amarenco P, Arsava EM, Ay H, Calvet D, et al. Addition of brain and carotid imaging to the ABCD(2) score to identify patients at early risk of stroke after transient ischaemic attack: a multicentre observational study. Lancet Neurol. (2010) 9:1060–9. doi: 10.1016/S1474-4422(10)70240-4
36. Mayer L, Ferrari J, Krebs S, Boehme C, Toell T, Matosevic B, et al. ABCD3-I score and the risk of early or 3-month stroke recurrence in tissue- and time-based definitions of TIA and minor stroke. J Neurol. (2018) 265:530–4. doi: 10.1007/s00415-017-8720-8
37. Mortara A, La Rovere MT, Pinna GD, Prpa A, Maestri R, Febo O, et al. Arterial baroreflex modulation of heart rate in chronic heart failure: clinical and hemodynamic correlates and prognostic implications. Circulation. (1997) 96:3450–8. doi: 10.1161/01.CIR.96.10.3450
38. Wang YY, Lin SY, Chuang YH, Chen CJ, Tung KC, Sheu WH. Adipose proinflammatory cytokine expression through sympathetic system is associated with hyperglycemia and insulin resistance in a rat ischemic stroke model. Am J Physiol Endocrinol Metab. (2011) 300:E155–63. doi: 10.1152/ajpendo.00301.2010
39. Watanabe M, Tomiyama-Miyaji C, Kainuma E, Inoue M, Kuwano Y, Ren H, et al. Role of alpha-adrenergic stimulus in stress-induced modulation of body temperature, blood glucose and innate immunity. Immunol Lett. (2008) 115:43–9. doi: 10.1016/j.imlet.2007.09.010
40. von Kanel R, Dimsdale JE. Effects of sympathetic activation by adrenergic infusions on hemostasis in vivo. Eur J Haematol. (2000) 65:357–69. doi: 10.1034/j.1600-0609.2000.065006357.x
41. Stampfli SF, Camici GG, Keller S, Rozenberg I, Arras M, Schuler B, et al. Restraint stress enhances arterial thrombosis in vivo–role of the sympathetic nervous system. Stress. (2014) 17:126–32. doi: 10.3109/10253890.2013.862616
42. Balkaya M, Prinz V, Custodis F, Gertz K, Kronenberg G, Kroeber J, et al. Stress worsens endothelial function and ischemic stroke via glucocorticoids. Stroke. (2011) 42:3258–64. doi: 10.1161/STROKEAHA.110.607705
43. Custodis F, Gertz K, Balkaya M, Prinz V, Mathar I, Stamm C, et al. Heart rate contributes to the vascular effects of chronic mental stress: effects on endothelial function and ischemic brain injury in mice. Stroke. (2011) 42:1742–9. doi: 10.1161/STROKEAHA.110.598607
44. Bohm M, Cotton D, Foster L, Custodis F, Laufs U, Sacco R, et al. Impact of resting heart rate on mortality, disability and cognitive decline in patients after ischaemic stroke. Eur Heart J. (2012) 33:2804–12. doi: 10.1093/eurheartj/ehs250
45. Binici Z, Mouridsen MR, Kober L, Sajadieh A. Decreased nighttime heart rate variability is associated with increased stroke risk. Stroke. (2011) 42:3196–201. doi: 10.1161/STROKEAHA.110.607697
46. Prosser J, MacGregor L, Lees KR, Diener HC, Hacke W, Davis S, et al. Predictors of early cardiac morbidity and mortality after ischemic stroke. Stroke. (2007) 38:2295–302. doi: 10.1161/STROKEAHA.106.471813
47. Sander D, Winbeck K, Klingelhofer J, Etgen T, Conrad B. Prognostic relevance of pathological sympathetic activation after acute thromboembolic stroke. Neurology. (2001) 57:833–8. doi: 10.1212/WNL.57.5.833
48. Ay I, Sorensen AG, Ay H. Vagus nerve stimulation reduces infarct size in rat focal cerebral ischemia: an unlikely role for cerebral blood flow. Brain Res. (2011) 1392:110–5. doi: 10.1016/j.brainres.2011.03.060
49. Cheyuo C, Jacob A, Wu R, Zhou M, Coppa GF, Wang P. The parasympathetic nervous system in the quest for stroke therapeutics. J Cereb Blood Flow Metab. (2011) 31:1187–95. doi: 10.1038/jcbfm.2011.24
50. Bieber M, Werner RA, Tanai E, Hofmann U, Higuchi T, Schuh K, et al. Stroke-induced chronic systolic dysfunction driven by sympathetic overactivity. Ann Neurol. (2017) 82:729–43. doi: 10.1002/ana.25073
51. Wang D, Liu T, Shi S, Li R, Shan Y, Huang Y, et al. Chronic administration of catestatin improves autonomic function and exerts cardioprotective effects in myocardial infarction rats. J Cardiovasc Pharmacol Ther. (2016) 21:526–35. doi: 10.1177/1074248416628676
52. Xiong L, Tian G, Wang L, Lin W, Chen X, Leung TWH, et al. External counterpulsation increases beat-to-beat heart rate variability in patients with ischemic stroke. J Stroke Cerebrovasc Dis. (2017) 26:1487–92. doi: 10.1016/j.jstrokecerebrovasdis.2017.03.007
Keywords: neurological function, prognosis, stroke, transient ischemic attack, heart rate variability, stroke recurrence
Citation: Li C, Meng X, Pan Y, Li Z, Wang M and Wang Y (2021) The Association Between Heart Rate Variability and 90-Day Prognosis in Patients With Transient Ischemic Attack and Minor Stroke. Front. Neurol. 12:636474. doi: 10.3389/fneur.2021.636474
Received: 12 January 2021; Accepted: 04 May 2021;
Published: 28 May 2021.
Edited by:
Mirjam R. Heldner, University Hospital Bern, SwitzerlandReviewed by:
Adrien Ter Schiphorst, Centre Hospitalier Universitaire de Montpellier, FrancePierre Seners, Fondation Ophtalmologique Adolphe de Rothschild, France
Copyright © 2021 Li, Meng, Pan, Li, Wang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yongjun Wang, eW9uZ2p1bndhbmdAbmNyY25kLm9yZy5jbg==
 Changhong Li
Changhong Li Xia Meng
Xia Meng Yuesong Pan3,4,5
Yuesong Pan3,4,5 Zixiao Li
Zixiao Li Yongjun Wang
Yongjun Wang