- 1Department of Neurology, Kaohsiung Medical University Hospital, Kaohsiung Medical University, Kaohsiung, Taiwan
- 2Department of Neurology, Faculty of Medicine, College of Medicine, Kaohsiung Medical University, Kaohsiung, Taiwan
- 3Department of Neurology, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan
- 4Department of Neurology, Stroke Center, Linkou Chang Gung Memorial Hospital, Taoyuan, Taiwan
- 5College of Medicine, Chang Gung University, Taoyuan, Taiwan
- 6Department of Neurology, National Defense Medical Center, Tri-Service General Hospital, Taipei, Taiwan
- 7Department of Neurology, Tainan Municipal Hospital (Managed by Show Chwan Medical Care Corporation), Tainan, Taiwan
- 8Department of Neurology, Kuang Tien General Hospital, Taichung, Taiwan
- 9Department of Nutrition, Huang-Kuang University, Taichung, Taiwan
- 10Department of Neurology, Tungs' Taichung Metro Harbor Hospital, Taichung, Taiwan
- 11Department of Neurology, Stroke Center, MacKay Memorial Hospital, Taipei, Taiwan
- 12Department of Neurology, National Taiwan University Hospital Yunlin Branch, Yunlin, Taiwan
- 13Department of Neurology, Sin-Lau Hospital, The Presbyterian Church of Taiwan, Tainan, Taiwan
- 14Department of Neurology of Changhua Christian Hospital, Changhua, Taiwan
- 15Department of Neurology, Taipei Medical University-Shaung Ho Hospital, Taipei, Taiwan
- 16Beijing Tiantan Hospital, Capital Medical University, Beijing, China
- 17Advanced Innovation Center for Human Brain Protection, Capital Medical University, Beijing, China
Background: Breakthrough strokes during treatment with aspirin, termed clinical aspirin treatment failure (ATF), is common in clinical practice. The burden of cerebral small vessel disease (SVD) is associated with an increased recurrent ischemic stroke risk. However, the association between SVD and ATF remains unclear. This study investigated the prevalence and clinical characteristics of SVD in stroke patients with ATF.
Methods: Data from a prospective, and multicenter stroke with ATF registry established in 2018 in Taiwan were used, and 300 patients who developed ischemic stroke concurrent with regular use of aspirin were enrolled. White matter lesions (WMLs) and cerebral microbleeds (CMBs) were identified using the Fazekas scale and Microbleed Anatomical Rating Scale, respectively. Demographic data, cardiovascular comorbidities, and index stroke characteristics of patients with different WML and CMB severities were compared. Logistic regression analyses were performed to explore the factors independently associated with outcomes after ATF.
Results: The mean patient age was 69.5 ± 11.8 years, and 70.0% of patients were men. Among all patients, periventricular WML (PVWML), deep WML (DWML), and CMB prevalence was 93.3, 90.0, and 52.5%, respectively. Furthermore, 46.0% of the index strokes were small vessel occlusions. Severe PVWMLs and DWMLs were significantly associated with high CMB burdens. Patients with moderate-to-severe PVWMLs and DWMLs were significantly older and had higher cardiovascular comorbidity prevalence than did patients with no or mild WMLs. Moreover, patients with favorable outcomes exhibited significantly low prevalence of severe PVWMLs (p = 0.001) and DWMLs (p = 0.001). After logistic regression was applied, severe WMLs predicted less favorable outcomes independently, compared with those with no to moderate PVWMLs and DWMLs [odds ratio (OR), 0.47; 95% confidence interval (CI), 0.25–0.87 for severe PVWMLs; OR, 0.40; 95% CI, 0.21–0.79 for severe DWMLs].
Conclusions: SVD is common in stroke patients with ATF. PVWMLs and DWMLs are independently associated with functional outcomes in stroke patients with ATF. The burden of SVD should be considered in future antiplatelet strategies for stroke patients after ATF.
Introduction
Stroke is the second leading cause of death worldwide and results in disability in a large proportion of survivors (1). Ischemic strokes account for ~80% of strokes (2). Cerebral small vessel disease (SVD) refers to a group of pathological processes involving the small arteries, arterioles, venules, and capillaries of the brain, such as lacunar infarcts, white matter lesions (WMLs), and cerebral microbleeds (CMBs) (3). SVD plays a critical role in stroke. The total burden of SVD was reported to be positively associated with the risk of recurrent ischemic strokes and intracerebral hemorrhage (ICH) in patients with a prior transient ischemic attack (TIA) or ischemic stroke (4).
Antiplatelet therapy is recommended for non-cardioembolic ischemic stroke treatment and prevention (5). Aspirin is the most commonly used antiplatelet agent for the secondary prevention of ischemic stroke because of its low cost and availability (6). Aspirin was determined to reduce the risk of ischemic stroke by 22% in secondary prevention (7). However, patients who develop an ischemic stroke during aspirin treatment, termed clinical aspirin treatment failure (ATF), represent 30–40% and 40–50% of patients with stroke in clinical trials (8–10), and in clinical practice (11–13), respectively.
ATF has been reported to be associated with old age, high prevalence of comorbidities, history of cardiovascular disease, and prior symptomatic cerebrovascular disease (10, 13). However, the characteristics and effect of SVD in ATF remain unclear. SVD is associated with an increased risk of recurrent ischemic stroke (4). In addition, SVD is associated with cerebral endothelium dysfunction (14) and impairment of brain network connectivity (15). Therefore, we hypothesized that SVD is associated with, and could affect the functional outcome of stroke with ATF. In this research, the prevalence and clinical characteristics of WMLs and CMBs were investigated in stroke patients with ATF.
Materials and Methods
Participants
This study was conducted using data from a prospective, and multicenter registry of specific stroke patients with ATF, established in 2018 in Taiwan. Eligible patients were older than 20 years, developed an ischemic stroke (the index stroke) within 60 days before recruitment, and had a regular use of aspirin 7 days before the index stroke. The indication of aspirin was primary or secondary prevention of cardiovascular or cerebrovascular events, and the dose of aspirin was 50 to 100 mg per day according to the prescription of the physician. Patients who received intravenous thrombolysis and endovascular thrombectomy for the index stroke could be included. Exclusion criteria were a history of antiplatelet agents use other than aspirin, a known or high risk of cardioembolism, a history of anticoagulant use, an impaired medical adherence and contraindication to magnetic resonance imaging (MRI) examination.
A comprehensive evaluation, including demographic and medical history data collection, physical and neurological examination, blood biochemistry examination, carotid artery ultrasonography, transcranial color Doppler, and cerebral MRI with magnetic resonance angiography, was performed for each patient. The hypertension referred to either a diastolic and systolic blood pressure of ≥90 and ≥140 mmHg, respectively, or the use of antihypertensive medications. The hyperlipidemia referred to a total cholesterol ≥160 mg/dl or a low-density lipoprotein ≥100 mg/dl. The Essen Stroke Risk Score (ESRS), a 10-point scale derived from Clopidogrel vs. Aspirin in Patients at Risk of Ischemic Events (CAPRIE) trial (16), was applied to predict the risk of recurrent ischemic stroke after the index stroke, based on the cardiovascular comorbidities (17, 18). The diagnosis of ischemic stroke was confirmed by a neurologist based on the clinical symptoms of stroke and a cerebral MRI. Stroke severity was evaluated using the National Institutes of Health Stroke Scale (NIHSS) score, and functional outcomes were determined using the modified Rankin scale (mRS). Neurological deterioration was defined based on the increase in NIHSS scores during hospitalization or at discharge, compared with the initial NHISS. A favorable functional outcome was defined as an mRS score of 0–2 at discharge. The stroke subtype was classified according to the TOAST criteria (19).
WML and CMB Grading
All MRIs were performed following a standardized MRI protocol, including axial diffusion-weighted imaging, axial T2-weighted fluid-attenuated inversion recovery imaging (FLAIR), and gradient recalled echo (GRE) T2*-weighted imaging or susceptibility-weighted imaging (SWI) with whole-brain coverage. Four experienced stroke neurologists, blinded to the clinical course and outcome of the patients, assessed the WMLs and CMBs of the cerebral MRI by using FLAIR and GRE or SWI, respectively.
WMLs were visually determined to be periventricular WMLs (PVWMLs) and deep WMLs (DWMLs) using the modified Fazekas scale (20, 21), which is the most frequently used assessment tool for WMLs. The Fazekas scale is a 4-point scale: 0 (no WML), 1 (mild WML), 2 (moderate WML), and 3 (severe WML). The definition, location, and number of CMBs were determined using a validated visual rating scale, the Microbleed Anatomical Rating Scale (MARS) (22). Patients were divided into two subgroups: with and without CMBs.
Ethical Statements
All centers were required to receive approval from their Institutional Review Board before initiation of this study. All patients, or their legal representatives, provided written informed consent before inclusion.
Statistical Analysis
Statistical analysis was performed using SPSS (version 22.0). All statistical tests were two-tailed, and an α ≥ 0.05 was considered significant. Analysis of variance or independent student t-tests were used for continuous variables and chi-squared test was used for categorical variables to evaluate the differences and trends in demographic data, cardiovascular comorbidities, and severities and subtypes of the index stroke based on WML severity and CMB presence and burden.
Independent student t and the chi-square tests were performed to assess the differences in WMLs and CMBs between patients with and without a neurological deterioration and favorable outcome. Furthermore, a multivariable logistic regression analysis was conducted, in which a neurological deterioration and favorable outcome were used as dependent variables to compare the effect of WMLs and CMBs on the stroke outcome after ATF, after adjustment for the effects of age, sex, ESRS scores, and TOAST classification of the index stroke.
Results
Study Participants
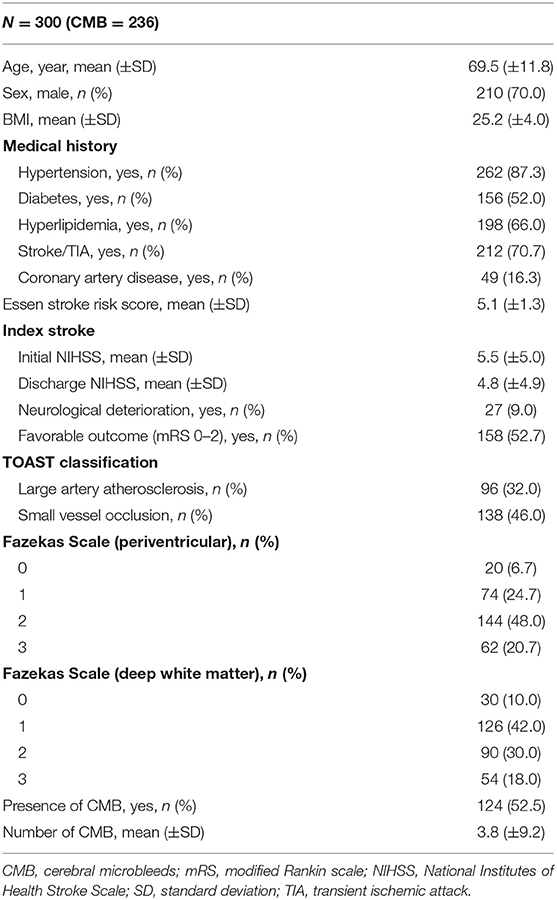
The cohort comprised 300 patients who developed an acute ischemic stroke during aspirin treatment, confirmed based on clinical symptoms and cerebral MRI. The mean age of the patients was 69.5 ± 11.8 years, and 70.0% of the patients were men. The mean ESRS score of the patients was 5.1 ± 1.3. For the index stroke, the mean initial and discharge NIHSS scores of the patients was 5.5 ± 5.0 and 4.8 ± 4.9, respectively. In the TOAST classification, 32.0% of strokes were large artery atherosclerosis and 46.0% were small vessel occlusions. The demographic characteristics, prevalence of vascular risk factors, severities of the index stroke, and presence of SVD are displayed in Table 1.
WMLs
Among all patients, 93.3% had PVWMLs, and 90.0% had DWMLs. Furthermore, 74 (24.7%), 144 (48.0%), and 62 (20.7%) patients displayed mild, moderate, and severe PVWMLs, respectively; 126 (42.0%), 90 (30.0%), and 54 (18.0%) patients had mild, moderate, and severe DWMLs, respectively. The relationships between severities of WMLs, vascular risk factors, and stroke index are presented in Tables 2, 3. The age differed significantly between patients with different severities of PVWMLs (ptrend < 0.001) and DWMLs (ptrend < 0.001). PVWML and DWML severities increased along with age. Furthermore, PVWMLs and DWMLs were significantly associated with numbers of CMBs (ptrend = 0.004 for PVWMLs, and ptrend < 0.001 for DWMLs). Patients with severe PVWMLs and DWMLs had significantly high CMB burdens. The ESRS scores varied significantly between PVWMLs and DWMLs severity groups (ptrend < 0.001 for both). Patients with no or mild PVWMLs and DWMLs had significantly low ESRS scores. Patients with severe PVWMLs and DWMLs showed a trend toward a high prevalence of hypertension (ptrend = 0.005 for PVWMLs and ptrend = 0.110 for DWMLs). Patients with severe PVWMLs and DWMLs had a trend toward an increase in initial and discharge NIHSS scores. Patients with moderate-to-severe PVWMLs had significantly high rates of stroke caused by small vessel occlusion, whereas the distribution of TOAST stroke subtype did not differ significantly based on DWML severity.
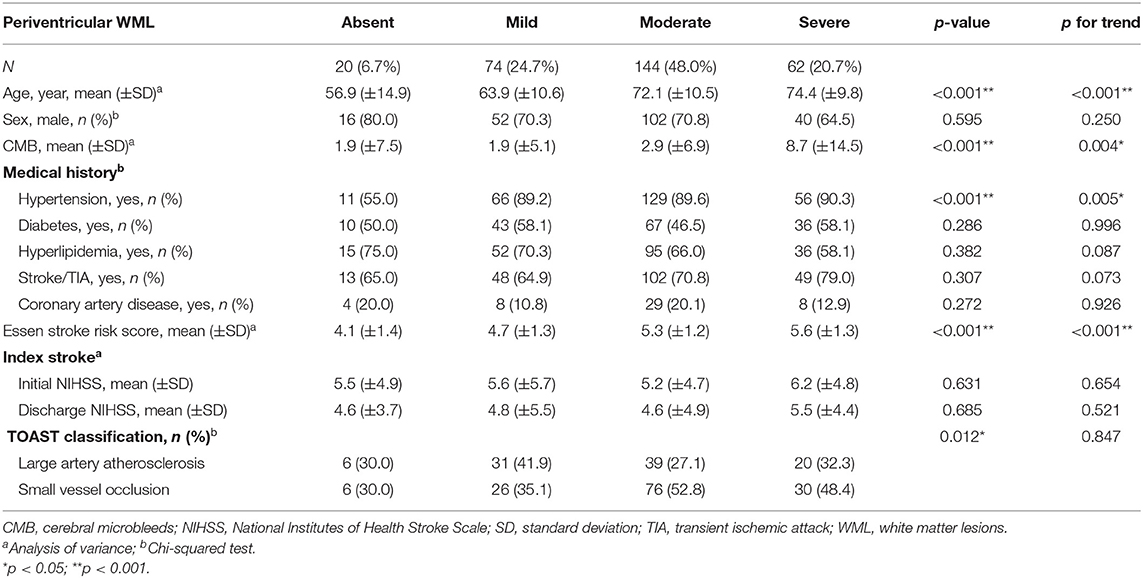
Table 2. Relationship between periventricular white matter lesions and characteristics of stroke patients with aspirin treatment failure.
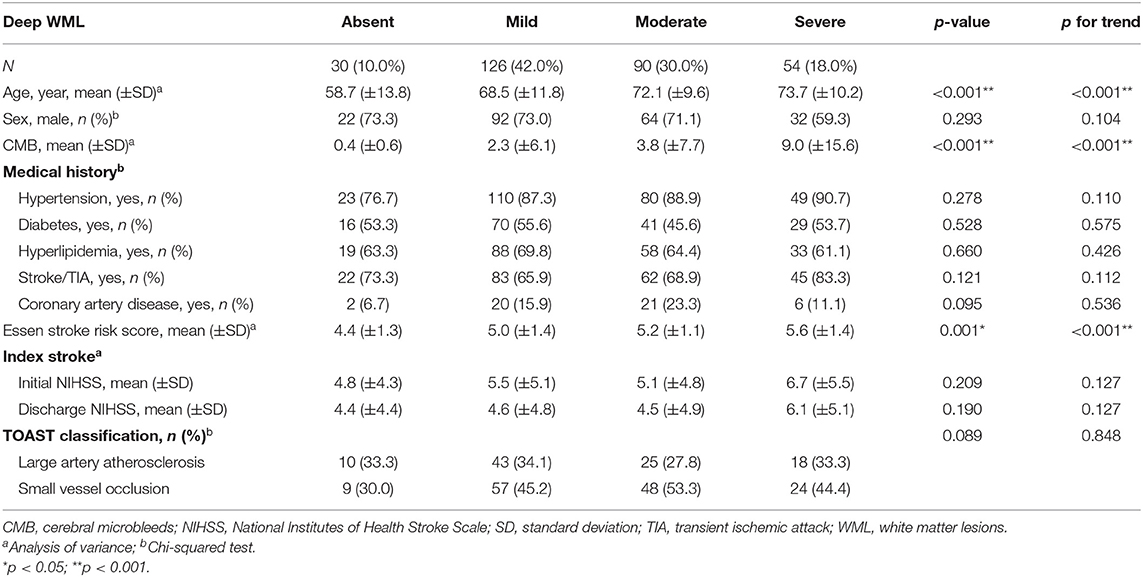
Table 3. Relationship between deep white matter lesions and characteristics of stroke patients with aspirin treatment failure.
CMBs
In total, 236 patients were evaluated for CMBs. CMBs were identified in 124 (52.5%) patients; the mean number of CMBs was 3.8 ± 9.2. Among patients with CMBs, 41 (33.1%) patients had a single lesion, 39 (31.5%) had 2–4 CMBs, and 44 (35.5%) patients had ≥5 CMBs. The comparisons of demographic characteristics and detailed information for the index stroke between the dichotomous and burdens of CMBs are presented in Table 4.
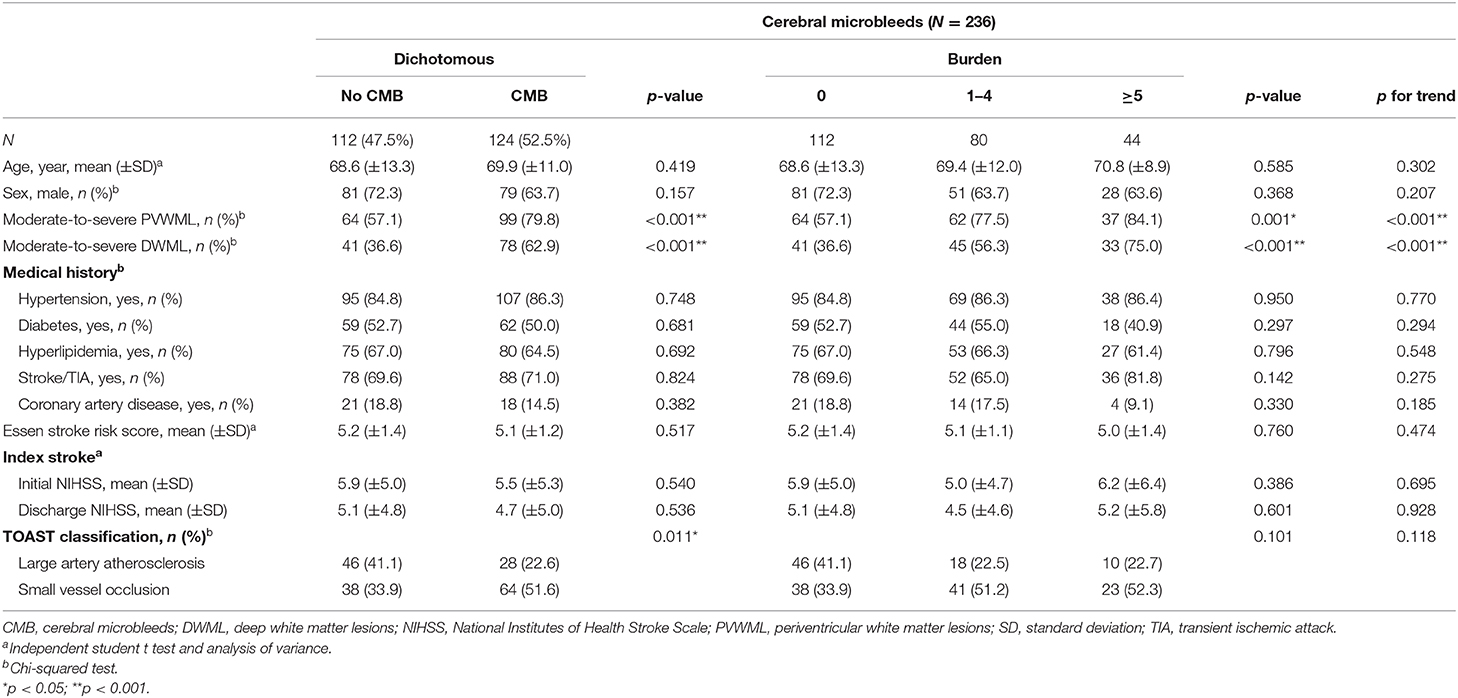
Table 4. Relationship between cerebral microbleeds and characteristics of stroke patients with aspirin treatment failure.
For associations between CMBs and other parameters, no significant differences in age, sex, vascular risk factors, and ESRS scores were observed between patients with and without CMBs. The presence of CMBs was associated with moderate-to-severe PVWMLs (p < 0.001) and DWMLs (p < 0.001). There were increasing prevalence of moderate-to-severe PVWMLs and DWMLs along with increasing CMBs burden (ptrend < 0.001 for both). No association was observed between the presence and burden of CMBs and the severity of the index stroke, indicated by initial and discharge NIHSS scores. The distribution of the TOAST stroke subtype differed significantly between patients with and without CMBs (p = 0.011). A high prevalence of stroke due to small vessel occlusion was observed in patients with CMBs.
Index Stroke Outcomes
For the outcome of the index stroke, 27 (9.0%) patients experienced a neurological deterioration, and 158 (52.7%) patients had a favorable functional outcome. The relationships between neurological deterioration, favorable functional outcomes, and SVD severity are presented in Table 5. Neurological deterioration was not associated with age, sex, presence of WMLs and CMBs, and TOAST stroke subtype. Patients with favorable outcomes were significantly younger (p < 0.001), and a larger proportion of patients was male (p = 0.009) and with low cardiovascular comorbidities (p < 0.001), compared to patients with unfavorable outcomes. Furthermore, PVWML and DWML severities were associated with functional outcomes. Patients with severe PVWMLs and DWMLs had less favorable outcomes compared with those with no to moderate PVWMLs and DWMLs (p = 0.001 for both). The presence and number of CMBs were not associated with functional outcomes.
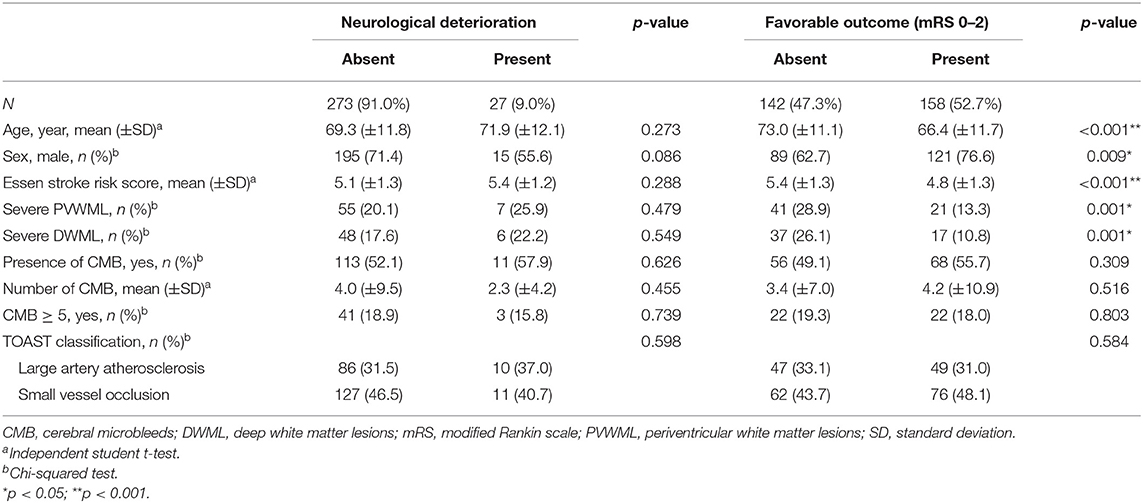
Table 5. Risk factors and predictors for neurological deterioration and favorable outcome in stroke patients with aspirin treatment failure.
After logistic regression was applied, the severe PVWMLs and DWMLs were associated with non-significant risks of increased neurological deterioration. Severe WMLs predicted significantly less favorable outcomes independently, compared with those with no to moderate PVWMLs and DWMLs [odds ratio (OR), 0.47; 95% confidence interval (CI), 0.25–0.87 for severe PVWMLs; OR, 0.40; 95% CI, 0.21–0.79 for severe DWMLs]. The burden of CMBs was not associated with a neurological deterioration and functional outcome (Table 6).
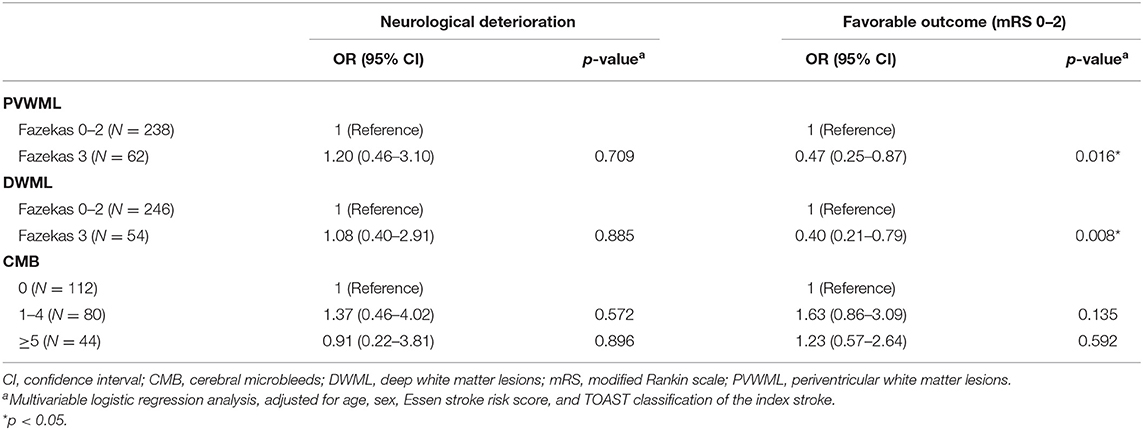
Table 6. A multivariable logistic regression analysis to estimate the odds ratio (OR) of burdens of white matter lesions and cerebral microbleeds for neurological deterioration and favorable outcome in stroke patients with aspirin treatment failure.
Discussion
The present study reported the prevalence and clinical characteristics of SVD in ATF. The prevalence of PVWMLs, DWMLs and CMBs were 93.3, 90.0, and 52.5%, respectively. Age and cardiovascular comorbidities, particularly hypertension, were major risks for both PVWMLs and DWMLs in ATF. The severity of PVWMLs and DWMLs increased with age. Small vessel occlusion contributed to the most of ischemic stroke with ATF, compared with other TOAST classifications. Both PVWMLs and DWMLs were associated with functional outcomes after stroke. Furthermore, the extent of WMLs was associated with the CMB burden. However, CMBs were not significantly associated with stroke severity, neurological deterioration, or functional outcomes.
ATF is associated with increased cardiovascular event and mortality risks (23). Therefore, patients with ATF require a comprehensive investigation to identify the contributing factors. Different categories of ATF can be distinguished in stroke patients. ATF may exceed functional resistance to aspirin, and could be associated with multiple factors, including advanced age, non-compliance, pharmacodynamic interaction, comorbidities, and undetected cardioembolic sources or atherosclerotic disease (13, 23, 24). In addition, ischemic stroke with ATF could be associated with prior ischemic stroke, particularly lacunar stroke (10, 12, 25). However, to the best of our knowledge, the burden of SVD in stroke patients with ATF has not been well-established.
The prevalence of moderate-to-severe PVWMLs and DWMLs in Asian stroke patients are estimated at 40–53%, and 29–41%, respectively (26, 27). CMBs are present in 20–40% of stroke patients (28). The cohort of stroke with ATF in this study has numerically higher prevalence of WMLs and CMBs than general stroke patients. In addition, a positive relationship between WMLs severity and CMBs burden was demonstrated. The presence and burden of WMLs and CMBs indicate endothelial dysfunction (14), which contribute to platelet activation (29), and then decrease platelet response to aspirin. In addition, the severity of WMLs was positively correlated with cardiovascular comorbidities, which could contribute to aspirin resistance via cyclooxygenase (COX)-1-independent mechanisms (30). The small vessel occlusions of the TOAST classification contributed to 46.0% of stroke after ATF in this study, a higher incidence compared to that of other Asian countries (31–33). Therefore, the high SVD burden could be associated with ATF in stroke patients.
The presence of severe PVWMLs and DWMLs was independently associated with an unfavorable short-term functional outcome in stroke patients with ATF, which was in line with reports regarding outcomes of general stroke patients with WMLs (34, 35). Recovery from stroke involves widespread neural network to compensate for the damaged neural connections (36). SVD are associated with impairment of neural connectivity, which supports the finding that WMLs could contribute to poor functional outcome in stroke patients with ATF.
The optimal antiplatelet regimen for patients with stroke and ATF remains undetermined. Moreover, the safety should be considered when determining antiplatelet regimens in patients with ATF. A meta-analysis suggested that adding to or replacing aspirin with another antiplatelet agent is associated with fewer recurrent stroke and cardiovascular events than maintaining aspirin monotherapy (37). Clopidogrel is the most used alternative antiplatelet agent in ATF. However, the therapeutic efficacy was heterogeneous. Two cohort studies in Asia indicated that dual antiplatelet therapy (DAPT; clopidogrel plus aspirin) or clopidogrel monotherapy was associated with reduced recurrent vascular events in patients with ATF than was aspirin monotherapy (38, 39). However, the Secondary Prevention of Small Subcortical Strokes Trial (SPS3) cohort reported that the addition of clopidogrel to aspirin therapy did not reduce vascular events compared with aspirin monotherapy in patients with a lacunar stroke with ATF (10).
Ticagrelor, a direct-acting antiplatelet agent that reversibly binds and inhibits the P2Y12 receptor on platelets, has been investigated for secondary vascular events prevention after acute ischemic stroke. In ischemic stroke with ATF, treatment with ticagrelor could reduce the risk of recurrent ischemic stroke, but increase combination of major or minor bleedings (40). However, combination of ticagrelor and aspirin does not decrease the incidence of composite of stroke or death and disabling stroke compared with aspirin after ischemic stroke with ATF (41, 42).
Cilostazol was the second most commonly used antiplatelet agent after ATF to add to or replace aspirin in an Asian cohort (39). The cilostazol monotherapy reduced recurrent, ischemic and hemorrhagic stroke compared with aspirin, and cilostazol combination with aspirin or clopidogrel did not increase hemorrhagic stroke prevalence in patients with non-cardioembolic stroke (43).
The burden of SVD has a predictive value for ICH after TIA or ischemic stroke (4), particularly among those with multiple CMBs receiving treatment with antiplatelet agents (44). Therefore, the optimal antiplatelet strategy after ATF warrants further consideration, given the SVD burden. Cilostazol reduced hemorrhagic stroke prevalence in patients with multiple CMBs and reduced total stroke in patients with mild-to-moderate WMLs compared with aspirin (45). However, evidence on the safety of antiplatelet regimens in patients combined with ATF and SVD is scarce.
The novelty of this study is that the clinical association between SVD and ATF was well-presented. The association between WMLs and poor functional outcome in stroke patients with ATF was shown to be significant. However, there are several limitations to our study. First, this study adapted a cross-sectional and observational design, and there is no control group in this registry. Therefore, it limited the interpretation of causal association of SVD on ATF. In addition, we could not determine the long-term effect of SVD on stroke recurrence and cognition after adjustment of antiplatelet regimens. Second, all patients in this study were Taiwanese, limiting the generalizability to other populations. Third, the registry enrolled stroke patients taking aspirin regularly 7 days before the index stroke. However, the precise duration of aspirin treatment before the index stroke was not recorded.
In conclusion, WMLs severity is highly correlated with age, cardiovascular comorbidities and CMBs burden. PVWMLs and DWMLs are significantly associated with functional outcomes in stroke patients with ATF. Further antiplatelet strategy after ATF considering the burden of SVD should be warranted.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Institutional Review Board of all centers in this study. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
H-HH and A-CC: study concept, design, and supervision. P-SC, P-SS, C-HLiu, Y-FS, R-CT, C-HLie, HP, S-CH, Y-TT, T-SC, and S-LW: acquisition and integrity of data. P-SC, P-SS, C-HLiu, and Y-FS: analysis and interpretation of data, statistical analysis and drafting the article. All authors contributed to the critical revision of the article for important intellectual content, agreed to the final version of the manuscript.
Funding
This study was supported by grants from Kaohsiung Medical University (KMU-Q108031) and Kaohsiung Medical University Hospital (KMUH 109-9R72).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank our colleagues, including Kang-Po Lee from National Cheng Kung University Hospital, Tsong-Hai Lee, Ting-Yu Chang, Chien-Hung Chang and Yeu-Jhy Chang from Linkou Chang Gung Memorial Hospital, Ching-Tsu Wang from Tainan Municipal Hospital, Chen-Wen Fang, Yu-Jen Hsiao, and Chih-Hung Tsai from National Taiwan University Hospital Yunlin Branch, Cheng-Yang Hsieh from Sin-Lau Hospital, and Chia-Ju Lee and Chu-Ling Chen from Changhua Christian Hospital, for their contribution to and collaboration for this study.
References
1. Katan M, Luft A. Global burden of stroke. Semin Neurol. (2018) 38:208–11. doi: 10.1055/s-0038-1649503
2. Donkor ES. Stroke in the 21(st) century: a snapshot of the burden, epidemiology, and quality of life. Stroke Res Treat. (2018) 2018:3238165. doi: 10.1155/2018/3238165
3. Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. (2010) 9:689–701. doi: 10.1016/S1474-4422(10)70104-6
4. Lau KK, Li L, Schulz U, Simoni M, Chan KH, Ho SL, et al. Total small vessel disease score and risk of recurrent stroke: Validation in 2 large cohorts. Neurology. (2017) 88:2260–7. doi: 10.1212/WNL.0000000000004042
5. Kernan WN, Ovbiagele B, Black HR, Bravata DM, Chimowitz MI, Ezekowitz MD, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American heart association/American stroke association. Stroke. (2014) 45:2160–236. doi: 10.1161/STR.0000000000000024
6. Guzik A, Bushnell C. Stroke epidemiology and risk factor management. Continuum. (2017) 23:15–39. doi: 10.1212/CON.0000000000000416
7. Baigent C, Blackwell L, Collins R, Emberson J, Godwin J, Peto R, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet. (2009) 373:1849–60. doi: 10.1016/S0140-6736(09)60503-1
8. National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. (1995) 333:1581–7. doi: 10.1056/NEJM199512143332401
9. The Publications Committee for the Trial of ORG 10172 in Acute Stroke Treatment (TOAST) Investigators. Low molecular weight heparinoid, ORG 10172 (danaparoid), and outcome after acute ischemic stroke: a randomized controlled trial. JAMA. (1998) 279:1265–72. doi: 10.1001/jama.279.16.1265
10. Cote R, Zhang Y, Hart RG, McClure LA, Anderson DC, Talbert RL, et al. ASA failure: does the combination ASA/clopidogrel confer better long-term vascular protection? Neurology. (2014) 82:382–9. doi: 10.1212/WNL.0000000000000076
11. Qureshi AI, Kirmani JF, Safdar A, Ahmed S, Sayed MA, Pande RU, et al. High prevalence of previous antiplatelet drug use in patients with new or recurrent ischemic stroke: buffalo metropolitan area and Erie county stroke study. Pharmacotherapy. (2006) 26:493–8. doi: 10.1592/phco.26.4.493
12. Pujol Lereis VA, Ameriso S, Povedano GP, Ameriso SF. Ischemic stroke in patients receiving aspirin. J Stroke Cerebrovasc Dis. (2012) 21:868–72. doi: 10.1016/j.jstrokecerebrovasdis.2011.05.009
13. Gallo A, Galliazzo S, Grazioli S, Guasti L, Ageno W, Squizzato A. Epidemiology and secondary prevention of ischemic stroke in patients on antiplatelet drug: a retrospective cohort study. J Thromb Thrombolysis. (2019) 48:336–44. doi: 10.1007/s11239-019-01893-y
14. Quick S, Moss J, Rajani RM, Williams A. A vessel for change: endothelial dysfunction in cerebral small vessel disease. Trends Neurosci. (2020) 44:289–305. doi: 10.1016/j.tins.2020.11.003
15. Petersen M, Frey BM, Schlemm E, Mayer C, Hanning U, Engelke K, et al. Network localisation of white matter damage in cerebral small vessel disease. Sci Rep. (2020) 10:9210. doi: 10.1038/s41598-020-66013-w
16. CAPRIE Steering Committee. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). CAPRIE Steering Committee. Lancet. (1996) 348:1329–39. doi: 10.1016/S0140-6736(96)09457-3
17. Weimar C, Benemann J, Michalski D, Muller M, Luckner K, Katsarava Z, et al. Prediction of recurrent stroke and vascular death in patients with transient ischemic attack or nondisabling stroke: a prospective comparison of validated prognostic scores. Stroke. (2010) 41:487–93. doi: 10.1161/STROKEAHA.109.562157
18. Fitzek S, Leistritz L, Witte OW, Heuschmann PU, Fitzek C. The essen stroke risk score in one-year follow-up acute ischemic stroke patients. Cerebrovasc Dis. (2011) 31:400–7. doi: 10.1159/000323226
19. Adams HP Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. trial of Org 10172 in acute stroke treatment. Stroke. (1993) 24:35–41. doi: 10.1161/01.STR.24.1.35
20. Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA. MR signal abnormalities at 1.5 T in alzheimer's dementia and normal aging. AJR Am J Roentgenol. (1987) 149:351–6. doi: 10.2214/ajr.149.2.351
21. Pantoni L, Basile AM, Pracucci G, Asplund K, Bogousslavsky J, Chabriat H, et al. Impact of age-related cerebral white matter changes on the transition to disability – the LADIS study: rationale, design and methodology. Neuroepidemiology. (2005) 24:51–62. doi: 10.1159/000081050
22. Gregoire SM, Chaudhary UJ, Brown MM, Yousry TA, Kallis C, Jager HR, et al. The microbleed anatomical rating scale (MARS): reliability of a tool to map brain microbleeds. Neurology. (2009) 73:1759–66. doi: 10.1212/WNL.0b013e3181c34a7d
23. Maree AO, Fitzgerald DJ. Variable platelet response to aspirin and clopidogrel in atherothrombotic disease. Circulation. (2007) 115:2196–207. doi: 10.1161/CIRCULATIONAHA.106.675991
24. Hankey GJ, Eikelboom JW. Aspirin resistance. Lancet. (2006) 367:606–17. doi: 10.1016/S0140-6736(06)68040-9
25. Englyst NA, Horsfield G, Kwan J, Byrne CD. Aspirin resistance is more common in lacunar strokes than embolic strokes and is related to stroke severity. J Cereb Blood Flow Metab. (2008) 28:1196–203. doi: 10.1038/jcbfm.2008.9
26. Chou PS, Chen CH, Wu MN, Lin YH, Lai CL, Lin RT, et al. Determinants of cerebral white matter changes in patients with stroke. Intern Med J. (2015) 45:390–5. doi: 10.1111/imj.12704
27. Xu YY, Zong LX, Zhang CQ, Pan YS, Jing J, Meng X, et al. The association of white matter hyperintensities with stroke outcomes and antiplatelet therapy in minor stroke patients. Ann Transl Med. (2020) 8:331. doi: 10.21037/atm.2020.02.137
28. Kim BJ, Lee SH. Prognostic impact of cerebral small vessel disease on stroke outcome. J Stroke. (2015) 17:101–110. doi: 10.5853/jos.2015.17.2.101
29. Hamilos M, Petousis S, Parthenakis F. Interaction between platelets and endothelium: from pathophysiology to new therapeutic options. Cardiovasc Diagn Ther. (2018) 8:568–80. doi: 10.21037/cdt.2018.07.01
30. Feher G, Feher A, Pusch G, Koltai K, Tibold A, Gasztonyi B, et al. Clinical importance of aspirin and clopidogrel resistance. World J Cardiol. (2010) 2:171–86. doi: 10.4330/wjc.v2.i7.171
31. Jung KH, Lee SH, Kim BJ, Yu KH, Hong KS, Lee BC, et al. Secular trends in ischemic stroke characteristics in a rapidly developed country: results from the Korean stroke registry study (secular trends in Korean stroke). Circ Cardiovasc Qual Outcomes. (2012) 5:327–34. doi: 10.1161/CIRCOUTCOMES.111.963736
32. Hsieh FI, Chiou HY. Stroke: morbidity, risk factors, and care in taiwan. J Stroke. (2014) 16:59–64. doi: 10.5853/jos.2014.16.2.59
33. Tian D, Yang Q, Dong Q, Li N, Yan B, Fan D. Trends in stroke subtypes and vascular risk factors in a stroke center in China over 10 years. Sci Rep. (2018) 8:5037. doi: 10.1038/s41598-018-23356-9
34. Liou LM, Chen CF, Guo YC, Cheng HL, Lee HL, Hsu JS, et al. Cerebral white matter hyperintensities predict functional stroke outcome. Cerebrovasc Dis. (2010) 29:22–7. doi: 10.1159/000255970
35. Griessenauer CJ, McPherson D, Berger A, Cuiper P, Sofoluke N, Adams MD, et al. Effects of white matter hyperintensities on 90-day functional outcome after large vessel and non-large vessel stroke. Cerebrovasc Dis. (2020) 49:419–26. doi: 10.1159/000509071
36. Di Filippo M, Tozzi A, Costa C, Belcastro V, Tantucci M, Picconi B, et al. Plasticity and repair in the post-ischemic brain. Neuropharmacology. (2008) 55:353–62. doi: 10.1016/j.neuropharm.2008.01.012
37. Lee M, Saver JL, Hong KS, Rao NM, Wu YL, Ovbiagele B. Antiplatelet regimen for patients with breakthrough strokes while on aspirin: a systematic review and meta-analysis. Stroke. (2017) 48:2610–3. doi: 10.1161/STROKEAHA.117.017895
38. Lee M, Wu YL, Saver JL, Lee HC, Lee JD, Chang KC, et al. Is clopidogrel better than aspirin following breakthrough strokes while on aspirin? A retrospective cohort study. BMJ Open. (2014) 4:e006672. doi: 10.1136/bmjopen-2014-006672
39. Kim JT, Park MS, Choi KH, Cho KH, Kim BJ, Han MK, et al. Different antiplatelet strategies in patients with new ischemic stroke while taking aspirin. Stroke. (2016) 47:128–34. doi: 10.1161/STROKEAHA.115.011595
40. Wong KSL, Amarenco P, Albers GW, Denison H, Easton JD, Evans SR, et al. Efficacy and safety of ticagrelor in relation to aspirin use within the week before randomization in the SOCRATES trial. Stroke. (2018) 49:1678–85. doi: 10.1161/STROKEAHA.118.020553
41. Johnston SC, Amarenco P, Denison H, Evans SR, Himmelmann A, James S, et al. Ticagrelor and aspirin or aspirin alone in acute ischemic stroke or TIA. N Engl J Med. (2020) 383:207–17. doi: 10.1056/NEJMoa1916870
42. Amarenco P, Denison H, Evans SR, Himmelmann A, James S, Knutsson M, et al. Ticagrelor added to aspirin in acute ischemic stroke or transient ischemic attack in prevention of disabling stroke: a randomized clinical trial. JAMA Neurol. (2020) 78:1–9. doi: 10.1001/jamaneurol.2020.4396
43. Kim SM, Jung JM, Kim BJ, Lee JS, Kwon SU. Cilostazol mono and combination treatments in ischemic stroke: an updated systematic review and meta-analysis. Stroke. (2019) 50:3503–11. doi: 10.1161/STROKEAHA.119.026655
44. Lau KK, Lovelock CE, Li L, Simoni M, Gutnikov S, Kuker W, et al. Antiplatelet treatment after transient ischemic attack and ischemic stroke in patients with cerebral microbleeds in 2 large cohorts and an updated systematic review. Stroke. (2018) 49:1434–42. doi: 10.1161/STROKEAHA.117.020104
Keywords: aspirin treatment failure, cerebral small vessel disease, microbleed, stroke, white matter lesion
Citation: Chou P-S, Sung P-S, Liu C-H, Sung Y-F, Tzeng R-C, Yang C-P, Lien C-H, Po HL, Ho S-C, Tsai Y-T, Chen T-S, Wu S-L, Hu H-H and Chao A-C (2021) Prevalence and Effect of Cerebral Small Vessel Disease in Stroke Patients With Aspirin Treatment Failure–A Hospital-Based Stroke Secondary Prevention Registry. Front. Neurol. 12:645444. doi: 10.3389/fneur.2021.645444
Received: 23 December 2020; Accepted: 18 March 2021;
Published: 13 April 2021.
Edited by:
Zhengqi Lu, Third Affiliated Hospital of Sun Yat-sen University, ChinaReviewed by:
Ozge Altintas Kadirhan, Kirklareli University, TurkeyGeoffrey Cloud, Monash University, Australia
Copyright © 2021 Chou, Sung, Liu, Sung, Tzeng, Yang, Lien, Po, Ho, Tsai, Chen, Wu, Hu and Chao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Han-Hwa Hu, aGFuaHdhQGhvdG1haWwuY29t; A-Ching Chao, YWNoY2hAY2Mua211LmVkdS50dw==
 Ping-Song Chou
Ping-Song Chou Pi-Shan Sung
Pi-Shan Sung Chi-Hung Liu4,5
Chi-Hung Liu4,5 Helen L. Po
Helen L. Po Tsang-Shan Chen
Tsang-Shan Chen Han-Hwa Hu
Han-Hwa Hu A-Ching Chao
A-Ching Chao