- 1Department of Anatomical and Histological Sciences, Legal Medicine and Orthopedics, Sapienza University of Rome, Rome, Italy
- 2Department of Medical and Oral Sciences and Biotechnologies, University G. d'Annunzio Chieti-Pescara, Chieti, Italy
- 3Complex Operational Unit (UOC) Physical Medicine and Rehabilitation, Santa Caterina Novella Hospital, Galatina, Italy
Fatigue is a multidimensional symptom with both physical and cognitive aspects, which can affect the quality of daily and working life activities. Motor Imagery (MI) represents an important resource for use during the rehabilitation processes, useful, among others, for job integration/reintegration, of neurological pathologies, such as Multiple Sclerosis (MS). To define the effective rehabilitation protocols that integrate MI for the reduction of fatigue in patients with MS (PwMS), a literary review was performed through August 2020. Five articles were included in the qualitative synthesis, including two feasibility pilot randomized control trials (RCTs) and 3 RCTs with good quality according to the PEDro score and a low risk of bias according to the Cochrane Collaboration tool. The literature suggested that MI, in association with rhythmic-auditory cues, may be an effective rehabilitation resource for reducing fatigue. Positive effects were observed on perceived cognitive and psychological fatigue. PwMS require greater compensatory strategies than healthy individuals, and the use of rhythmic-auditory cues may be useful for optimizing the cognitive processing of MI, which acts as an internal stimulus that is enhanced and made more vivid by outside cues. These findings provide evidence that MI is a promising rehabilitation tool for reducing fatigue in PwMS and return to work strategies.
Introduction
Fatigue affects more than 80% of patients with multiple sclerosis (PwMS), among whom 55% report fatigue as being one of the worst symptoms that is experienced, often independently of the level of disability (1). Patients describe fatigue as a feeling of weakness that worsens with exercise or as the day progresses or as an abnormal, constant, and persistent sense of tiredness (2). Fatigue in Multiple Sclerosis (MS) could be a direct effect of the pathological process on the central nervous system (CNS) or secondary to weakness, stiffness, tremor, sleep disturbances, or depression (3, 4). Fatigue management is challenging, and physiotherapy treatment represents a valid resource of fatigue support to complement pharmacological treatment (5, 6). The literature indicates that therapeutic exercise is considered a safe and effective form of rehabilitation for the reduction of fatigue among PwMS and that individualized exercise programs should be designed to address each patient's chief complaint (7). Specifically, endurance and progressive resistance training (PRT) may reduce self-reported fatigue (8, 9). However, in a study by Hameau and colleagues, after a short, intensive, combined rehabilitation program among PwMS, fatigue decreased, but fatigability appeared to increase (10). Fatigue is a multidimensional symptom that involves both physical and cognitive aspects which can affect the quality of daily and working life activities. Often, endurance and aerobic training rehabilitation protocols are not easily applied or well-tolerated among PwMS with medium-to-high levels of disability, such as those patients who require walking or balance aids (11). Some studies focusing on rehabilitation in MS have demonstrated a transitory positive effect on the reduction in fatigue symptoms (7–9, 12); however, other studies that examined the efficacy of various specific rehabilitation programs showed no significant effects on fatigue compared with placebo (13, 14). Novel approaches to physiotherapy in MS include Motor Imagery (MI) and Rhythmic Auditory Stimulation (RAS), which have been shown to improve walking in PwMS, accompanied by reductions in fatigue. Other authors, such as Hanson et al., have suggested that a neurocognitive rehabilitation approach—specifically, the use of MI could represent an important resource for reducing fatigue, because MI involves motor planning and mild exercise execution (15–17). In PwMS, fatigue involves the dysfunction of the circuits connecting the thalamus, basal ganglia, and frontal cortex, which require a specific balance to enable motor and executive motor planning (18–20). MI is the mental rehearsal of movements without actual execution, which involves similar spatial and temporal characteristics, activates the same brain areas that are executed during actual movements (21), and can be performed with or without verbal guidance and additional visual or auditory cues (15, 22). Several studies have investigated the relationship between MS and return-to-work trying to highlight the elements or symptoms that most negatively impact on it, such as fatigue (23). MI represents an important resource for use during the rehabilitation processes, useful, among others, for job integration/reintegration, of neurological pathologies, such as MS (23–25). Several studies have suggested that the connections between rhythmic auditory and motor processing, which reflects sensorimotor synchronization with RAS, may also apply to MI, which involves the mental execution of movements without performing any actual movements (26). The performance of MI has obvious advantages over actual movement practice, including the lack of motor fatigue and reducing the risk of falls, because MI can be realized in a sitting position. In people with other types of neurologic disorders, such as stroke, MI has been shown to improve motor performance (27), with moderate effect sizes (28).
Given the connection between return-to-work and fatigue, and the effects of MI on the latter, the purpose of this mini systematic review was to investigate the effects of rehabilitation protocols that integrate MI to decrease symptoms of fatigue, and therefore, favor the return-to-work, in PwMS.
Materials and Methods
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) was used to guide this review (29).
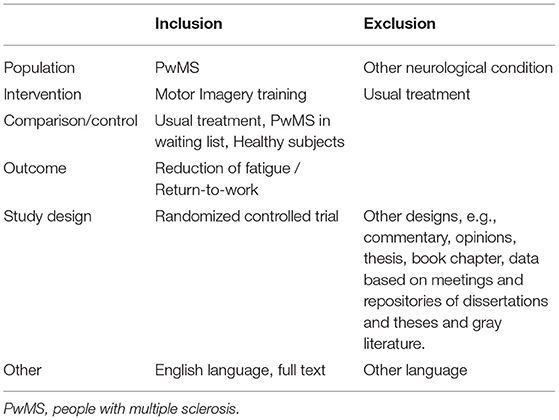
Data Sources and Search Strategy
The literature research was performed (PubMed, Scopus, PEDro, PsychINFO and Google Scholar) through August 2020 (22), using the following keywords: Job integration/reintegration OR return-to-work AND Multiple sclerosis AND Motor imagery; Multiple sclerosis AND Motor imagery; Multiple sclerosis AND fatigue; Motor imagery AND fatigue; and Multiple sclerosis AND Motor imagery AND fatigue. Two independent reviewers searched each database using the same strategy to ensure proper cross-checking of the results. Table 1 shows the eligibility criteria that were used to determine the inclusion of studies in the review and the algorithm that was developed, based on PICO (patients, intervention, comparison, outcome) (30). The authors evaluated the studies identified by the database searches based on the established inclusion and exclusion criteria (Table 1). The authors independently screened the titles, abstracts, and full texts of all eligible studies. The reference lists of the most relevant studies were scanned for additional citations. Data including the country, author, affiliated institutions, and enrollment periods were extracted and reviewed to identify and exclude duplicate publications using the same cohort. Any disagreements regarding the acceptance of full-text articles were resolved by discussion until a consensus was reached.
Quality and Risk of Bias Assessment
The methodological quality of each RCT was assessed using the Physiotherapy Evidence Database (PEDro) scale (31). Two researchers independently applied the scale to each considered study. We considered trials with scores equal to or <9 to be “excellent,” studies, that ranged from 6–8, were considered “good,” trials, that scored 4–5, were deemed to be “fair” quality, and studies, with scores of ≤4, were categorized as “poor” quality (32).
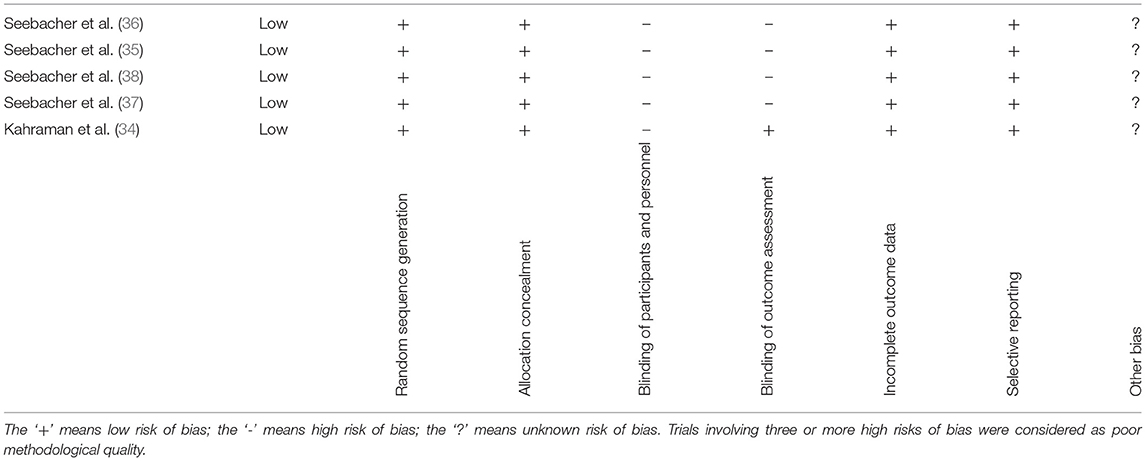
Furthermore, the risk of bias was assessed independently for each study by two authors according to the Cochrane Collaboration's domain-based evaluation framework (33). Main domains were assessed in the following sequence: (1) selection bias (randomized sequence generation and allocation concealment); (2) performance bias (blinding of participants and personnel); (3) detection bias (blinding of outcome assessment); (4) attrition bias (incomplete outcome data, such as that due to dropouts); (5) reporting bias (selective reporting); and (6) other sources of bias. The scores for each bias domain and the final score for the risk of systematic bias were graded as low, high, or unclear risk.
Results
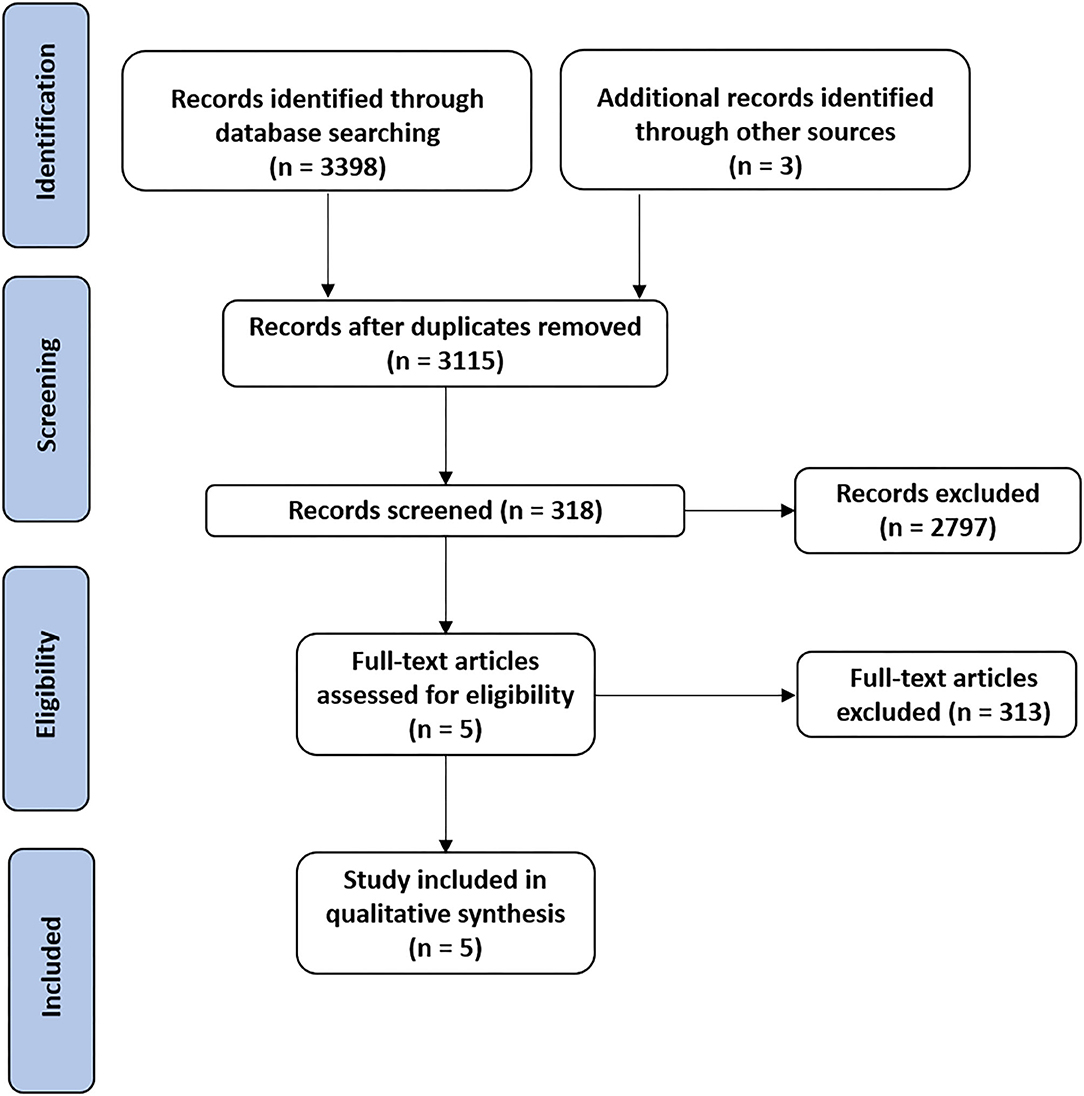
Search Results
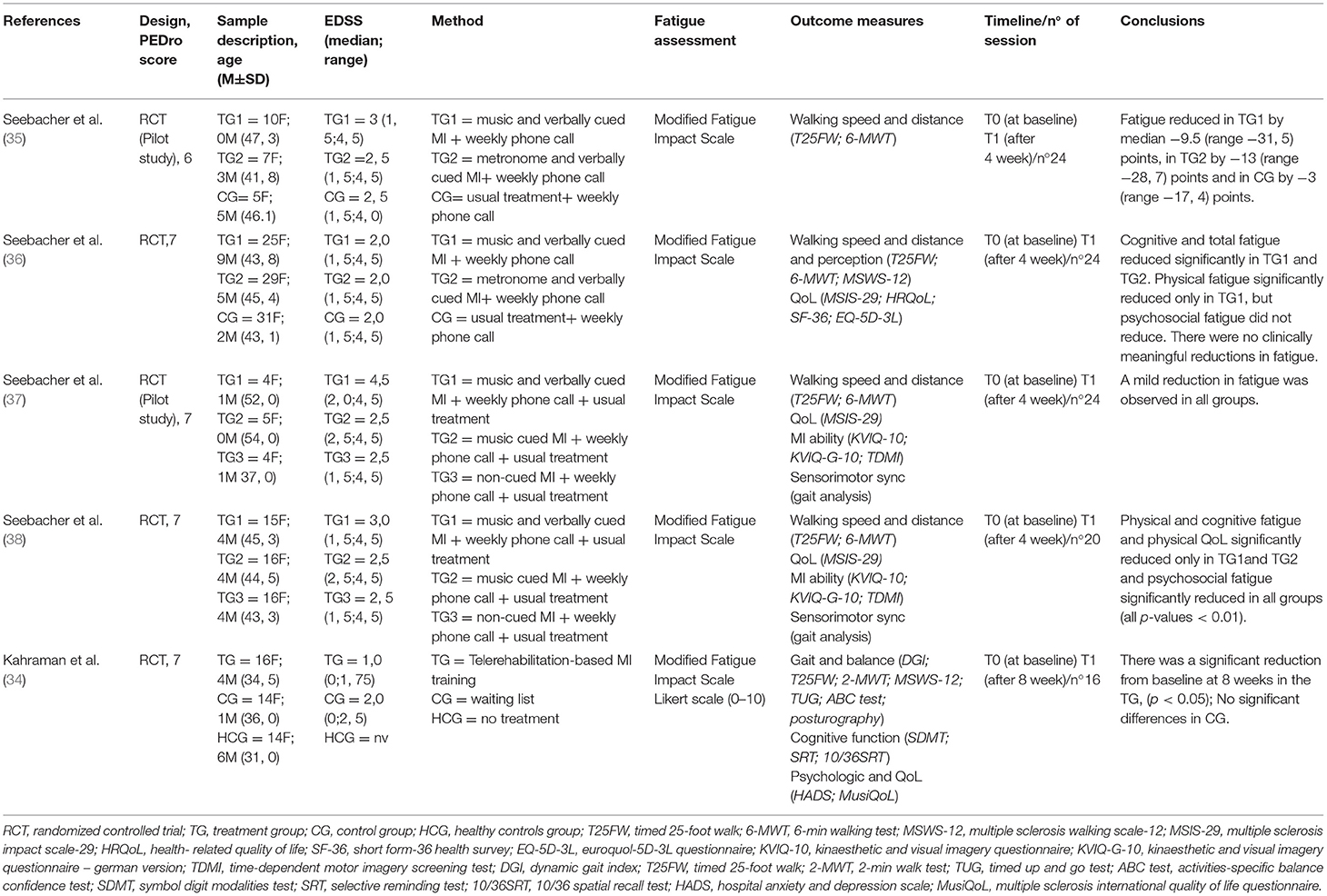
The findings are presented in narrative form, including tables and figures, to present the data in a format that is structured around the assessment, sample characteristics, and results. Our initial literature search identified 4,001 records. After removing duplicates, 3,115 records were assessed for eligibility. Following the application of inclusion and exclusion criteria and verifying the full-text articles for eligibility, a total of five articles (34–38) were included in the qualitative synthesis, including two feasibility pilot RCTs and 3 RCTs, as shown in the study flowchart (Figure 1). The mean methodological quality of the five included RCTs, according to the PEDro scale, was 6.8/10 (Table 2), indicating the good overall quality of the included studies. Table 2 also describes the protocols used, the outcomes measured and the times and number of sessions. The risk of bias was considered low for all five studies (Table 3). The most frequent source of potential bias was performance bias, related to the assessments of the blinding of participants and personnel and the blinding of the outcome.
Participants
A total of 261 participants were analyzed in the included studies (50 men/211 women) with a median age of 43.55 years. All included studies evaluated a mixed-sex sample, with an Expanded Disability Status Scale (EDSS) score of 2.5, indicating only mild impairments. Fatigue was evaluated using the Modified Fatigue Impact Scale (MFIS) in all included studies (39–41).
Interventions
Most of the included interventions consisted of home-based, rhythmic, cued MI training (using instrumental music, a metronome, or verbal cueing) (35–38), in which the patients were instructed in the concept of MI and its rehabilitation applications and effects. The patients learned how attention and perception are fundamental components in the planning and controlling of movement before execution. The patients were asked to imagine themselves walking in various manners, accompanied by music and beat, as described by a recent publication (42). Three studies (34, 37, 38) for MI standardization followed the PETTLEP (physical, environmental, task, timing, learning, emotional, and perspective) approach, which may serve as a viable tool to enhance the effectiveness of an intervention. The PETTLEP model is based on neuroscientific findings, developed by Holmes and Collins, and includes a 7-point checklist of guidelines to follow when devising an imagery intervention (43). The durations and intensities of the rehabilitation interventions varied: in 4 studies (35–38), the patients practiced MI for 17 min, 6 times each week for 4 weeks at home. In contrast, Kahraman et al. (34) reported that patients engaged in twice-a-week, 20–30-min sessions for 8 weeks.
Included Articles
Seebacher et al. (35), with the aim of evaluating changes in fatigue caused by rhythmic motor images, enrolled thirty adults with MS and randomly assigned them into three groups: 17 min of motor imagery, six times a week, for 4 weeks, with music (A) or metronome cues (B) and controls (C). Primary outcomes were recruitment rates, retention, compliance, adverse events, and fatigue (Modified Fatigue Impact Scale). Secondary outcomes were walking speed (25-foot walking time) and distance traveled (6-min walking). The authors concluded that preliminary improvements in walking speed, distance walked, and fatigue of group A need to be confirmed in a larger process.
Seebacher et al. (36), in order to investigate the effect of motor imagery combined with rhythmic cues on walking, fatigue and quality of life in people with MS, enrolled 101 individuals with MS and randomized them into three groups: 17 min of motor imagery, six times a week, for 4 weeks, with musical cues (A) or metronome (B), both with verbal cues, and controls (C). The primary outcomes were walking speed (25-foot timed walk) and distance (6-min walk test). Secondary outcomes were Multiple Sclerosis Walking Scale-12, Modified Fatigue Impact Scale and QoL (Short Form-36 Health Survey, Multiple Sclerosis Impact Scale-29, Euroquol-5D-3L Questionnaire). The authors concluded that rhythm-guided motor images improve walking, fatigue and quality of life in people with MS, while music-guided motor images are more effective.
Seebacher et al. (37), with the aim to obtain preliminary information of changes in walking, fatigue, quality of life (QoL) and MI ability following cued and non-cued MI in pwMS, they enrolled 55 adults with MS and randomized them to three groups: 24 sessions of 17 min of MI with music and verbal cueing (MVMI), with music alone (MMI), or non-cued (MI). Primary outcomes were walking speed (Timed 25-Foot Walk) and walking distance (6-Min Walk Test). Secondary outcomes were recruitment rate, retention, adherence, acceptability, adverse events, MI ability (Kinaesthetic and Visual Imagery Questionnaire, Time-Dependent MI test), fatigue (Modified Fatigue Impact Scale) and quality of life (Multiple Sclerosis Impact Scale-29). The authors concluded that their study suggest that cued and non-cued MI are valuable interventions in patients with MS who were able to imagine movements.
Seebacher et al. (38), with the aim of studying the effects and mechanisms of differently cued and non-cued MI on walking, fatigue and quality of life in patients with MS, enrolled 59 patients with mild to moderate disability and randomized them to music- and verbally cued MI (MVMI), music-cued MI (MMI) or MI. Participants practiced guided or unguided MI of walking for 17 min, six times a week for 4 weeks at home. The primary outcomes were walking speed (timed 25-foot walk) and distance traveled (6-min walk test). The authors concluded that all interventions significantly improved walking. MVMI was superior in improving walking, fatigue and quality of life. The results suggest that MI and sensorimotor synchronization were mechanisms of action.
Kahraman et al. (34), with the aim to investigate the effects of telerehabilitation-based motor imaging training (Tele-MIT) on gait, balance, and cognitive and psychosocial outcomes in people with multiple sclerosis, have created a randomized, controlled pilot trial included people with MS and healthy individuals. People with MS were randomly divided into two groups (intervention and control). The intervention group received Tele-MIT (2/week for 8 weeks). The control group was a wait-list group without any additional specific treatment. Healthy participants served as a baseline comparison. The Dynamic Gait Index, used to assess dynamic balance during walking, was the primary outcome. Secondary outcomes included assessments of walking speed, endurance and perceived ability, balance performance assessed by a computerized posturography device, balance confidence, cognitive functions, fatigue, anxiety, depression, and quality of life. The authors concluded that Tele-MIT is a novel method that proved feasible and effective in improving dynamic balance during walking, walking speed and perceived walking ability, balance confidence, cognitive functions, fatigue, anxiety, depression, and quality of life in people with MS.
Discussion
The literature reports that MI could represent a rehabilitation resource for relieving symptoms, with the aim of adequate social reintegration and return to work. Evidence suggests that neurocognitive rehabilitation can be used to help patients overcome pain, and MI has been shown to facilitate learning more efficient movement execution strategies to make return to work faster and more manageable by the patients. In PwMS, fatigue represents one of the most disabling symptoms, from a neuromotor point of view, and limiting the execution of activities of daily life and not allowing the patient a complete and timely return to work. This aspect also has consequences from a psychological point of view that led the patient to completely abandon his or her work, no longer feeling able to carry it out. As demonstrated by (44), interventions aimed at reducing fatigue decrease the number of days away from work. The studies that were included in this review showed encouraging results. Catalan et al. have suggested that an MI program could be effective for reducing fatigue in PwMS, with a mean EDSS of 2.5 ± 1.29. The authors observed that patients who were guided by a physiotherapist to correctly perceive kinesthetic information (over a period of 5 weeks of treatment, performed twice a week) learned new motor planning strategies, which might persist up to 6 months after treatment. Seebacher et al. (35–38), in various studies, have reported that MI is an effective rehabilitation resource for decreasing the symptoms of fatigue. The authors used MI in rehabilitation protocols, associated with music and verbal cues, metronomes and verbal cues, or no cues (35–38). Cues are defined as any external stimuli, either temporal or spatial in nature, that are associated with the facilitation of motor activity in PwMS (45). The physical execution of movement and the imagination of movement both involve the activation of similar brain regions (primary motor cortex, supplementary motor area, premotor area, somatosensory area, prefrontal cortex, parietal lobule, cingulate area, basal ganglia, and cerebellum) (46), and various cueing strategies have been associated with improvements in motor performance. The use of cues that are associated with MI can facilitate the process of learning a movement in individuals who present with attention deficits, which is typical of some neurological disorders, including MS (47). The results of (38) are certainly the most interesting as they showed that cued and non-cued MI improved walking speed and walking distance in PwMS, but music- and verbally cued MI were more effective than MI in improve walking, subjective fatigue and QoL (38). In this study, music-cued MI but not MI alone improved fatigue and quality of life while music- and verbally cued MI was more effective, suggesting that these findings are related to the effects of music and verbal cues (38). These results are likely associated with the two important dimensions of fatigue: the perception of fatigue and performance fatigability (48, 49). Differences in these two aspects may explain the discrepancies reported for some rehabilitation approaches to fatigue in MS, in which some authors report increased fatigue after exercise (10, 13, 14), such as the observable decrease in performance during a cognitive or motor task. The subjective perception of fatigue requires a cognitive perspective involving interoception and metacognition (48, 50, 51). The use of music during therapy for neurological diseases may affect cognitive functions, such as increasing verbal memory, in addition to improving motor performance (52–54) and providing benefits for the psycho-emotional sphere (55). The rehabilitative effects of music during therapy for neurological disorders appear to be associated with brain neuroplasticity and neural activation changes; however, the specific mechanisms remain unknown (56). Seebacher et al. (35–38) suggested a 4-week rehabilitation program and identified the specific characteristics of the music cues: the music style and beat were selected based on published summaries of practical guidelines for RAS and other relevant publications (53). The selected music was in 2/4 or 4/4 time, with strong ON and OFF beat patterns, such that every first beat or every first and third beat was stressed. The beat was emphasized by rhythmic verbal cues from the researcher (e.g., rhythmic speech, such as “step-step,” “toe-off”). The music-cued MI synchronizes the motor response, and patients unconsciously adapt their movements to the external rhythm (56), which has been shown to be well-suited for improving gait during rehabilitative protocols, as reported by Seebacher. The patients enrolled in these studies reported the perception that the treatment was safe and convenient, and even those enrolled in non-cued-MI arms reported satisfaction with the intervention, especially in terms of the focus on body awareness, without distraction (37). Generally, the studies by Seebacher and colleagues on the use of MI combined with rhythmic-auditory cues have suggested that this approach resulted in positive effects on perceived cognitive fatigue and various aspects of walking among PwMS. The synchronization between external rhythmic signals and movement showed positive effects compared with the isolated use of MI during rehabilitation (35–38). The study by Hereman et al. (57) showed that visual stimuli improved the spatial accuracy of movements during MI, whereas auditory stimuli improved temporal precision, both of which had positive effects on the vividness of the images. This finding suggested that cues related to movement may facilitate the generation of MI, and the use of external stimuli to provide the spatial and temporal components of the movement appeared to improve the efficacy of MI. PwMS require compensatory strategies to overcome their movement dysfunction, and the use of cues has been shown to be useful for optimizing the cognitive processing required for MI (58), which acts as an internal stimulus that is enhanced and made more vivid by outside cues. Moumdjian et al. (59, 60) compared the abilities of PwMS with those of healthy controls (HC) for sustaining synchronization of a 12-min period of walking accompanied by music and a metronome. They analyzed physical and cognitive fatigue, motivation, and gait compared with walking in silence. PwMS could walk for 12 min of uninterrupted walking under all tested conditions; however, improved synchronization, reduced perception of cognitive fatigue, and high motivation were observed when external cues were used. Listening to music instead of a metronome might be more pleasurable and may increase adherence to the MI rehabilitation process, which is important for home-based interventions. Moreover, music may be an interesting form of diversifying the training (61) and could have positive effects on fatigue during therapeutic treatment with MI. The study by (34) described training in tele-motor imagery (MIT), conducted by an expert physiotherapist. At the beginning of the session, the authors proposed relaxation exercises, including 5 min of free breathing, followed by deep breathing and awareness exercises. To evoke MI, the physiotherapist used auditory, visual, tactile, and olfactory cues that were easily available within the patient's home context. Authors used multimodal cues for enhancing the motor imagery vividness. In contrast to the studies from Seebacher et al. (35–38), these cues were not real but imagined. Patients in the MIT-treated group reported functional improvements in fatigue. Telerehabilitation was reported to be effective for the treatment of various neurological conditions, including MS. Telerehabilitation reflects a new approach to facilitate the delivery of rehabilitation programs in the patient's home, using new technologies (62). However, a Cochrane review highlighted the limitations and the paucity of high-quality studies conducted in PwMS to date. MS is a complex and challenging condition requiring individualized and integrated multidisciplinary care, and telerehabilitation interventions are difficult to standardize (63, 64). Several studies have demonstrated that mental practice through MI can result in motor improvements, indicating that MI represents a potential tool for motor learning, relearning, and rehabilitation, especially among people with physical disabilities (63). Mental practice with MI offers the opportunity to improve motor skills through safe and self-paced training among people with severe disabilities, such as PwMS, and the association of MI with auditory cues appears to improve outcomes. The evidence currently present in the literature on the use of MI of PwMS to reduce fatigue, although not numerous, suggests how this method can be effective not only for an improvement in the quality of life and autonomy in the activities of daily life, but also in conclusion, for a better return to work, not only by imagining work tasks (as a kind of imaginary occupational therapy), but also because patients can do it at home even after working.
Strengths and Limitations
According to our knowledge, this is the first review on the use of MI, for the reduction of fatigue in PwMS, aimed at return to work. This certainly represents a current and extremely important issue today. Our work is not free from limitations such as certainly the low number of works included which is secondary to the lack of study and scientific evidence present in scientific literature today.
Conclusion
Fatigue in PwMS is a complex clinical problem, with a lack of currently effective treatments and it represents one of the most severe restrictions on return to work in PwMS. Therefore, when establishing a rehabilitation plan, particular attention should be paid to the most convenient techniques, aimed at a better and faster restitutio ad integrum of the patient and a more effective return to work. MI could be a promising rehabilitation tool, which has been shown to be effective for decreasing the symptoms of fatigue and improving motivation. These findings provide evidence that MI is a promising rehabilitation tool for reducing fatigue in PwMS and return to work strategies. Given the potential benefits of MI for neurological rehabilitation, we recommend future studies to explore the motor representations in PwMS to improve the provision of effective and tailored rehabilitative treatments.
Author Contributions
FA, LP, and TP: conceptualization. MP and RS: methodology. MM: software, data curation, and project administration. MP and AB: validation. CA and TP: formal analysis. FA: investigation. TP and AB: resources. FA, LP, and MP: writing—original draft preparation. AB: writing—review and editing. FA: visualization. RS and TP: supervision. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer GC declared a shared affiliation, with no collaboration, with the authors FA, MP, RI, AB, and MM at the time of the review.
Acknowledgments
We thank Lisa Giles, from Blue Pencil Science (http://www.bluepencilscience.com/) for editing an English draft of this manuscript.
References
1. Paolucci T, Bernetti A, Sbardella S, La Russa C, Murgia M, Salom, è A, et al. Straighten your back! self-correction posture and postural balance in “non rehabilitative instructed” multiple sclerosis patients. Neuro Rehabil. (2020) 46:333–41. doi: 10.3233/NRE-192987
2. Bernetti A, Agostini F, de Sire A, Mangone M, Tognolo L, Di Cesare A, et al. Neuropathic pain and rehabilitation: a systematic review of international guidelines. Diagnostics. (2021) 11:74. doi: 10.3390/diagnostics11010074
3. Penner IK, Paul F. Fatigue as a symptom or comorbidity of neurological diseases. Nat Rev. Neurology. (2017) 13:662–75. doi: 10.1038/nrneurol.2017.117
4. Rottoli M, La Gioia S, Frigeni B, Barcella V. Pathophysiology, assessment and management of multiple sclerosis fatigue: an update. Exp Rev Neurother. (2017) 17:373–9. doi: 10.1080/14737175.2017.1247695
5. Mangone M, Paoloni M, Procopio S, Venditto T, Zucchi B, Santilli V, et al. Sagittal spinal alignment in patients with ankylosing spondylitis by rasterstereographic back shape analysis: an observational retrospective study. Euro J Phys Rehabil Med. (2020) 56:191–6. doi: 10.23736/S1973-9087.20.05993-6
6. Seccia R, Boresta M, Fusco F, Tronci E, Di Gemma E, Palagi L, et al. Data of patients undergoing rehabilitation programs. Data Brief. (2020) 30:105419. doi: 10.1016/j.dib.2020.105419
7. Halabchi F, Alizadeh Z, Sahraian MA, Abolhasani M. Exercise prescription for patients with multiple sclerosis; potential benefits and practical recommendations. BMC Neurol. (2017) 17:185. doi: 10.1186/s12883-017-0960-9
8. Kjølhede T, Vissing K, Dalgas U. Multiple sclerosis and progressive resistance training: a systematic review. Mult Scler. (2012) 18:1215–28. doi: 10.1177/1352458512437418
9. Heine M, van de Port I, Rietberg MB, van Wegen EE, Kwakkel G. Exercise therapy for fatigue in multiple sclerosis. Cochr Datab Syst Rev. (2015) CD009956. doi: 10.1002/14651858.CD009956.pub2
10. Hameau S, Bensmail D, Roche N, Zory R. Adaptations of fatigue and fatigability after a short intensive, combined rehabilitation program in patients with multiple sclerosis. J Rehabil Med. (2018) 50:59–66. doi: 10.2340/16501977-2277
11. Damiani C, Mangone M, Paoloni M, Goffredo M, Franceschini M, Servidio M, et al. Trade-offs with rehabilitation effectiveness (REs) and efficiency (REy) in a sample of Italian disabled persons in a in post-acuity rehabilitation unit. Ann Ig. (2020) 32:327–35. doi: 10.7416/ai.2020.2356
12. Wiles CM, Newcombe RG, Fuller KJ, Shaw S, Furnival-Doran J, Pickersgill TP, et al. Controlled randomised crossover trial of the effects of physiotherapy on mobility in chronic multiple sclerosis. J Neurol Neuro Psychiatry. (2001) 70:174–9. doi: 10.1136/jnnp.70.2.174
13. Rasova K, Havrdova E, Brandejsky P, Zálisová M, Foubikova B, Martinkova P. Comparison of the influence of different rehabilitation programmes on clinical, spirometric and spiroergometric parameters in patients with multiple sclerosis. Mult Scler. (2006) 12:227–34. doi: 10.1191/135248506ms1248oa
14. Kos D, Duportail M, D'hooghe M, Nagels G, Kerckhofs E. Multidisciplinary fatigue management programme in multiple sclerosis: a randomized clinical trial. Mult Scler. (2007) 13:996–1003. doi: 10.1177/1352458507078392
15. Catalan M, De Michiel A, Bratina A, Mezzarobba S, Pellegrini L, Marcovich R, et al. Treatment of fatigue in multiple sclerosis patients: a neurocognitive approach. Rehabil Res Pract. (2011) 2011:670537. doi: 10.1155/2011/670537
16. Hanson M, Concialdi M. Motor imagery in multiple sclerosis: exploring applications in therapeutic treatment. J Neurophysiol. (2019) 121:347–9. doi: 10.1152/jn.00291.2018
17. Paolucci T, Cardarola A, Colonnelli P, Ferracuti G, Gonnella R, Murgia M, et al. Give me a kiss! an integrative rehabilitative training program with motor imagery and mirror therapy for recovery of facial palsy. Eur J Phys Rehabil Med. (2020) 56:58–67. doi: 10.23736/S1973-9087.19.05757-5
18. Leocani L, Colombo B, Magnani G, Martinelli-Boneschi F, Cursi M, Rossi P, et al. Fatigue in multiple sclerosis is associated with abnormal cortical activation to voluntary movement–EEG evidence. NeuroImage. (2001) 13(6 Pt 1):1186–92. doi: 10.1006/nimg.2001.0759
19. Filippi M, Rocca MA, Colombo B, Falini A, Codella M, Scotti G, et al. Functional magnetic resonance imaging correlates of fatigue in multiple sclerosis. NeuroImage. (2002) 15:559–67. doi: 10.1006/nimg.2001.1011
20. Téllez N, Alonso J, Río J, Tintor, é M, Nos C, Montalban X, et al. The basal ganglia: a substrate for fatigue in multiple sclerosis. Neuroradiology. (2008) 50:17–23. doi: 10.1007/s00234-007-0304-3
21. Jeannerod M. The representing brain: neural correlates of motor intention and imagery. Behav Brain Sci. (1994) 17:187–202. doi: 10.1017/S0140525X00034026
22. Schuster C, Hilfiker R, Amft O, Scheidhauer A, Andrews B, Butler J, et al. Best practice for motor imagery: a systematic literature review on motor imagery training elements in five different disciplines. BMC Med. (2011) 9:75. doi: 10.1186/1741-7015-9-75
23. Persechino B, Fontana L, Buresti G, Fortuna G, Valenti A, Iavicoli S. Improving the job-retention strategies in multiple sclerosis workers: the role of occupational physicians. Ind Health. (2019) 57:52–69. doi: 10.2486/indhealth.2017-0214
24. Ranavolo A, Serrao M, Varrecchia T, Casali C, Filla A, Roca A, et al. The working life of people with degenerative cerebellar ataxia. Cerebellum. (2019) 18:910–921. doi: 10.1007/s12311-019-01065-x
25. Ghanbari Ghoshchi S, De Angelis S, Morone G, Panigazzi M, Persechino B, Tramontano M, et al. Return to work and quality of life after stroke in Italy: a study on the efficacy of technologically assisted neurorehabilitation. Int J Environ Res Public Health. (2020) 17:5233. doi: 10.3390/ijerph17145233
26. Decety J. Do imagined and executed actions share the same neural substrate? Brain Res Cogn Brain Res. (1996) 3:87–93. doi: 10.1016/0926-6410(95)00033-X
27. Cho HY, Kim JS, Lee GC. Effects of motor imagery training on balance and gait abilities in post-stroke patients: a randomized controlled trial. Clin Rehabil. (2013) 27:675–80. doi: 10.1177/0269215512464702
28. Schuster C, Butler J, Andrews B, Kischka U, Ettlin T. Comparison of embedded and added motor imagery training in patients after stroke: results of a randomised controlled pilot trial. Trials. (2012) 13:11. doi: 10.1186/1745-6215-13-11
29. Moher D, Liberati A, Tetzlaff J, Altman DG, Group PRISMA. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
30. van Loveren C, Aartman IH. De PICO-vraag [The PICO (Patient-Intervention-Comparison-Outcome) question]. Ned Tijdschr Tandheelkd. (2007) 114:172–8.
31. De Morton NA. The PEDro scale is a valid measure of the methodological quality of clinical trials: a demographic study. Aust J Physiother. (2009) 55:129–33. doi: 10.1016/S0004-9514(09)70043-1
32. Maher CG, Sherrington C, Herbert RD, Moseley AM, Elkins M. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys Ther. (2003) 83:713–21. doi: 10.1093/ptj/83.8.713
33. Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. Chapter 8.5. Oxford (2011).
34. Kahraman T, Savci S, Ozdogar AT, Gedik Z, Idiman E. Physical, cognitive and psychosocial effects of telerehabilitation-based motor imagery training in people with multiple sclerosis: a randomized controlled pilot trial. J Telemed Telecare. (2020) 26:251–60. doi: 10.1177/1357633X18822355
35. Seebacher B, Kuisma R, Glynn A, Berger T. Rhythmic cued motor imagery and walking in people with multiple sclerosis: a randomised controlled feasibility study. Pilot Feasibil Stud. (2015) 1:25. doi: 10.1186/s40814-015-0021-3
36. Seebacher B, Kuisma R, Glynn A, Berger T. The effect of rhythmic-cued motor imagery on walking, fatigue and quality of life in people with multiple sclerosis: a randomised controlled trial. Mult Sclero J. (2017) 23:286–96. doi: 10.1177/1352458516644058
37. Seebacher B, Kuisma R, Glynn A, Berger T. Exploring cued and non-cued motor imagery interventions in people with multiple sclerosis: a randomised feasibility trial and reliability study. Arch Physiother. (2018) 8:6. doi: 10.1186/s40945-018-0045-0
38. Seebacher B, Kuisma R, Glynn A, Berger T. Effects and mechanisms of differently cued and non-cued motor imagery in people with multiple sclerosis: a randomised controlled trial. Mult Sclero J. (2019) 25:1593–604. doi: 10.1177/1352458518795332
39. Brunier G, Graydon J. A comparison of two methods of measuring fatigue in patients on chronic haemodialysis: visual analogue vs Likert scale. Int J Nurs Stud. (1996) 33:338–48. doi: 10.1016/0020-7489(95)00065-8
40. Kos D, Kerckhofs E, Carrea I, Verza R, Ramos M, Jansa J. Evaluation of the modified fatigue impact scale in four different European countries. Mult Sclero. (2005) 11:76–80. doi: 10.1191/1352458505ms1117oa
41. Téllez N, Río J, Tintor, é M, Nos C, Galán I, Montalban X. Does the modified fatigue impact scale offer a more comprehensive assessment of fatigue in MS?. Mult Sclero J. (2005) 11:198–202. doi: 10.1191/1352458505ms1148oa
42. Thaut MH, Thaut M. Rhythm, Music, and the Brain: Scientific Foundations and Clinical Applications. New York, NY: Routledge (2005).
43. Holmes PS, Collins DJ. The PETTLEP approach to motor imagery: a functional equivalence model for sport psychologists. J Appl Sport Psychol. (2001) 13:60–83. doi: 10.1080/10413200109339004
44. Hasanpour Dehkordi A. Influence of yoga and aerobics exercise on fatigue, pain and psychosocial status in patients with multiple sclerosis: a randomized trial. J Sports Med Phys Fitness. (2016) 56:1417–22.
45. Harrison SL, Laver KE, Ninnis K, Rowett C, Lannin NA, Crotty M. Effectiveness of external cues to facilitate task performance in people with neurological disorders: a systematic review and meta-analysis. Disab Rehabil. (2019) 41:1874–81. doi: 10.1080/09638288.2018.1448465
46. Bunno Y. The application of motor imagery to neurorehabilitation. In: Larrivee D, editor. Evolving BCI TherapyEngaging Brain State Dynamics. Kansai: Intech (2018). p. 53–71.
47. Amato MP, Prestipino E, Bellinvia A. Identifying risk factors for cognitive issues in multiple sclerosis. Exp Rev Neurother. (2019) 19:333–47. doi: 10.1080/14737175.2019.1590199
48. Kluger BM, Krupp LB, Enoka RM. Fatigue and fatigability in neurologic illnesses: proposal for a unified taxonomy. Neurology. (2013) 80:409–16. doi: 10.1212/WNL.0b013e31827f07be
49. Manjaly ZM, Harrison NA, Critchley HD, Do CT, Stefanics G, Wenderoth N, et al. Pathophysiological and cognitive mechanisms of fatigue in multiple sclerosis. J Neurol Neuro Psychiatry. (2019) 90:642–51. doi: 10.1136/jnnp-2018-320050
50. Stephan KE, Manjaly ZM, Mathys CD, Weber LA, Paliwal S, Gard T, et al. Allostatic self-efficacy: a metacognitive theory of dyshomeostasis-induced fatigue and depression. Front Human Neurosci. (2016) 10:550. doi: 10.3389/fnhum.2016.00550
51. Kuppuswamy A. The fatigue conundrum. Brain J Neurol. (2017) 140:2240–5. doi: 10.1093/brain/awx153
52. Moore KS, Peterson DA, O'Shea G, McIntosh GC, Thaut MH. The effectiveness of music as a mnemonic device on recognition memory for people with multiple sclerosis. J Music Ther. (2008) 45:307–29. doi: 10.1093/jmt/45.3.307
53. Thaut MH, Peterson DA, McIntosh GC, Hoemberg V. Music mnemonics aid verbal memory and induce learning - related brain plasticity in multiple sclerosis. Front Human Neurosci. (2014) 8:395. doi: 10.3389/fnhum.2014.00395
54. Moumdjian L, Sarkamo T, Leone C, Leman M, Feys P. Effectiveness of music-based interventions on motricity or cognitive functioning in neurological populations: a systematic review. Eur J Phys Rehabil Med. (2017) 53:466–82. doi: 10.23736/S1973-9087.16.04429-4
55. Vinciguerra C, De Stefano N, Federico A. Exploring the role of music therapy in multiple sclerosis: brief updates from research to clinical practice. Neurol Sci. (2019) 40:2277–85. doi: 10.1007/s10072-019-04007-x
56. Sihvonen AJ, Särkämö T, Leo V, Tervaniemi M, Altenmüller E, Soinila S. Music-based interventions in neurological rehabilitation. Lancet Neurol. (2017) 16:648–60. doi: 10.1016/S1474-4422(17)30168-0
57. Heremans E, Helsen WF, De Poel HJ, Alaerts K, Meyns P, Feys P. Facilitation of motor imagery through movement-related cueing. Brain Res. (2009) 1278:50–8. doi: 10.1016/j.brainres.2009.04.041
58. Heremans E, Nieuwboer A, Spildooren J, De Bondt S, D'hooge AM, Helsen W, et al. Cued motor imagery in patients with multiple sclerosis. Neuroscience. (2012) 206:115–21. doi: 10.1016/j.neuroscience.2011.12.060
59. Moumdjian L, Moens B, Maes PJ, Van Geel F, Ilsbroukx S, Borgers S, et al. Continuous 12 min walking to music, metronomes and in silence: auditory-motor coupling and its effects on perceived fatigue, motivation and gait in persons with multiple sclerosis. Mult Scler Relat Disord. (2019) 35:92–99. doi: 10.1016/j.msard.2019.07.014
60. Moumdjian L, Moens B, Maes PJ, Van Nieuwenhoven J, Van Wijmeersch B, Leman M, et al. Walking to music and metronome at various tempi in persons with multiple sclerosis: a basis for rehabilitation. Neurorehabil Neural Rep. (2019) 33:464–75. doi: 10.1177/1545968319847962
61. Van Geel F, Van Asch P, Veldkamp R, Feys P. Effects of a 10-week multimodal dance and art intervention program leading to a public performance in persons with multiple sclerosis-a controlled pilot-trial. Mult Scler Relat Disord. (2020) 44:102256. doi: 10.1016/j.msard.2020.102256
62. Galea MD. Telemedicine in rehabilitation. Physical Medicine and Rehabilitation Clinics. (2019) 30:473–483. doi: 10.1016/j.pmr.2018.12.002
63. Malouin F, Richards CL. Mental practice for relearning locomotor skills. Phys Ter. (2010) 90:240–51. doi: 10.2522/ptj.20090029
Keywords: exercise, neurocognitive, cues, rehabilitation, balance
Citation: Agostini F, Pezzi L, Paoloni M, Insabella R, Attanasi C, Bernetti A, Saggini R, Mangone M and Paolucci T (2021) Motor Imagery: A Resource in the Fatigue Rehabilitation for Return-to-Work in Multiple Sclerosis Patients—A Mini Systematic Review. Front. Neurol. 12:696276. doi: 10.3389/fneur.2021.696276
Received: 16 April 2021; Accepted: 11 June 2021;
Published: 05 July 2021.
Edited by:
Alberto Ranavolo, National Institute for Insurance Against Accidents at Work (INAIL), ItalyReviewed by:
Giorgia Chini, Sapienza University of Rome, ItalyBarbara Seebacher, Dr. Barbara Seebacher, Austria
Copyright © 2021 Agostini, Pezzi, Paoloni, Insabella, Attanasi, Bernetti, Saggini, Mangone and Paolucci. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Massimiliano Mangone, bWFzc2ltaWxpYW5vLm1hbmdvbmVAdW5pcm9tYTEuaXQ=
 Francesco Agostini
Francesco Agostini Letizia Pezzi
Letizia Pezzi Marco Paoloni
Marco Paoloni Roberta Insabella1
Roberta Insabella1 Carmine Attanasi
Carmine Attanasi Andrea Bernetti
Andrea Bernetti Massimiliano Mangone
Massimiliano Mangone Teresa Paolucci
Teresa Paolucci