- 1Department of Neurology, Tong Ren Hospital Shanghai Jiaotong University School of Medicine, Shanghai, China
- 2Department of Interventional Neuroradiology, Beijing Tiantan Hospital, Beijing, China
- 3Department of Neurology, Beijing Anzhen Hospital, Beijing, China
- 4Department of Neurology, Hebei General Hospital, Shijiazhuang, China
- 5Department of Neurology, Shanxi Provincial People's Hospital, Taiyuan, China
- 6Department of Neurology, Taiyuan Central Hospital, Taiyuan, China
- 7Department of Neurology, Liangxiang Teaching Hospital, Beijing, China
- 8Department of Neurology, ORDOS Central Hospital, Ordos, China
- 9Department of Neurology, TongLiao City Hospital, Tongliao, China
- 10Department of Neurology, Tai'an Hospital of Traditional Chinese Medicine, Tai'an, China
- 11Department of Interventional Neurology, Beijing You'anmen Hospital, Beijing, China
- 12Department of Neurology, Handan Central Hospital, Handan, China
- 13Department of Neurology, Dalian Municipal Central Hospital, Dalian, China
- 14Department of Neurology, Jingjiang People's Hospital, Taizhou, China
- 15Department of Neurology, Taizhou First People's Hospital, Taizhou, China
Background: The management of patients with symptomatic non-acute intracranial artery occlusion (sNA-ICAO), which is a special subset with high morbidity and a high probability of recurrent serious ischemic events despite standard medical therapy (SMT), has been clinically challenging. A number of small-sample clinical studies have also discussed endovascular recanalization (ER) for sNA-ICAO; however, there is currently a lack of evidence from multicenter, prospective, large-sample cohort trials. The purpose of our present study was to evaluate the technical feasibility and safety of ER for sNA-ICAO.
Methods: Our group is currently undertaking a multisite, non-randomized cohort, prospective registry study enrolling consecutive patients presenting with sNA-ICAO at 15 centers in China between January 1, 2020 and December 31, 2022. A cohort of patients who received SMT and a cohort of similar patients who received ER plus SMT were constructed and followed up for 2 years. The primary outcome is any stroke from enrollment to 2 years of follow-up. The secondary outcomes are all-cause mortality, mRS score, NIHSS score and cognitive function from enrollment to 30 days, 3 months, 8 months, 12 months, 18 months, and 2 years of follow-up. Descriptive statistics and linear/logistic multiple regression models will be generated. Clinical relevance will be measured as relative risk reduction, absolute risk reduction and the number needed to treat.
Discussion: The management of patients with sNA-ICAO has been clinically challenging. The current protocol aims to evaluate the technical feasibility and safety of ER for sNA-ICAO.
Trial Registration Number: www.ClinicalTrials.gov, identifier: NCT04864691.
Background
Large intracranial artery occlusion is a major cause of stroke and is associated with a high risk of stroke recurrence and poor stroke outcome, especially in China (1, 2). For symptomatic non-acute intracranial artery occlusion (sNA-ICAO) (within 24 h to 6 months), some patients continue to be symptomatic despite standard medical therapy (SMT) (3–5). Extracranial-intracranial (EC-IC) artery bypass surgery fails to show benefits in preventing ischemic attacks or ischemic stroke when performed for sNA-ICAO (6, 7). The optimal treatment for patients with sNA-ICAO disease remains undefined. Currently, SMT, including an antiplatelet regimen and risk factor management, has been used to treat patients with sNA-ICAO disease. Unfortunately, the natural course of this condition shows that these patients often experience recurrent symptoms despite SMT. Recently, a series of small-sample clinical studies have reported that endovascular recanalization (ER) is feasible for sNA-ICAO (8–13). However, most of the previous studies are based on small-sample, single-center retrospective analyses, and there is no high-level evidence from large multicenter samples or prospective studies to indicate the effectiveness and safety of ER for sNA-ICAO.
Therefore, we launched a prospective registry study of patients with sNA-ICAO from 15 centers in China to test whether ER combined with SMT is superior to SMT alone in the primary prevention of stroke in patients with symptomatic non-acute cerebral artery occlusion.
Methods And Design
Study Design and Setting
The trial was retrospectively registered on ClinicalTrials.gov on April 25, 2021, with reference number NCT(04864691). The study is a multicenter, prospective registry, non-randomized cohort study sponsored by professor Feng Gao of Beijing Tiantan hospital to assess patients affected by sNA-ICAO undergoing ER and SMT. This protocol was developed according to the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) Statement. Fifteen centers across China will participate in the study and provide data. All centers have a similar perioperative pathway and use enhanced recovery after surgery (ERAS) protocols. The participating centers are as follows: Department of Interventional Neuroradiology, Beijing Tiantan Hospital; Department of Neurology, Tong Ren Hospital Shanghai Jiaotong University School of Medicine; Department of Interventional Neurology, Beijing You 'anmen Hospital; Department of Neurology, Beijing Anzhen Hospital; Department of Neurology, Hebei General Hospital; Department of Neurology, Shanxi General Hospital; Department of Neurology, Taiyuan Central Hospital; Department of Neurology, Liangxiang Hospital; Department of Neurology, ORDOS Central Hospital; Department of Neurology, TongLiao City Hospital; Department of Neurology, Tai'an Hospital of Traditional Chinese Medicine; Department of Neurology, Handan Central Hospital; Department of Neurology, Dalian Municipal Central Hospital; Department of Neurology, Jingjiang people's Hospital; Department of Neurology, Taizhou first people's Hospital.
Participants
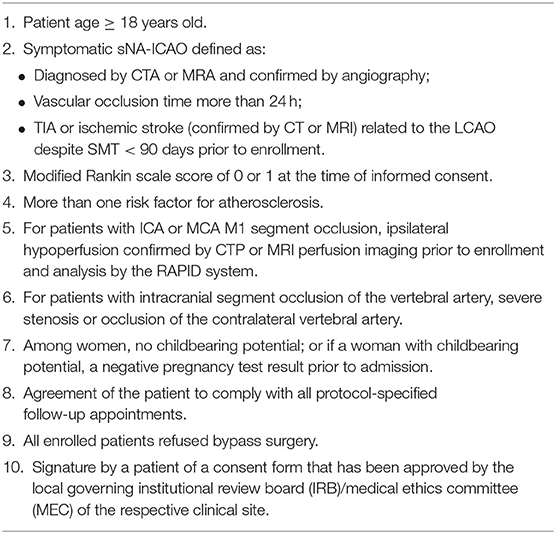
We will include patients with imaging (MRA/CTA/DSA) and clinical diagnosis of sNA-ICAO (Figure 1) between January 1, 2020 and December 31, 2022 in the participating centers. Eligibility screening will be performed by the principal investigator in accordance with the inclusion/exclusion criteria (Tables 1, 2). Based on the patient's previous history, imaging features of the lesion and the attitudes of the patient and family members, the local investigative team in each center will determine whether to give SMT plus SMZ or SMT alone. Both groups of patients share general inclusion/exclusion criteria and primary and secondary endpoints.
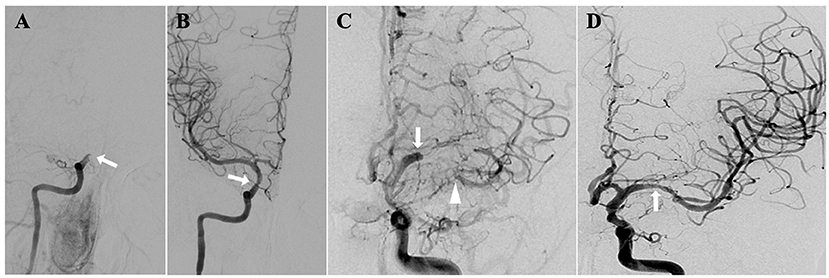
Figure 1. sNA-ICAO diagnosed by DSA. (A) Illustrations of non-acute occlusion of intracranial segment of internal carotid artery; (B) recanalization after endovascular treatment; (C) illustrations of non-acute occlusion of middle cerebral artery; (D) recanalization after endovascular treatment.
Ethical Issues
Data collection will be performed according to the World Medical Association Declaration of Helsinki. All the patients gave written informed consent to participate. Ethical permission was received from Beijing Tiantan Hospital, the Capital Medical University Medical Ethics Committee (number: KY2020-114-02), and the institutional review boards of all partner sites. The standard of care for patients participating in this study will remain the same.
SMT
Sites implemented SMT for all patients with guidance from the Medical Management Core. Patients in SMT group will take aspirin (100 mg/day) and clopidogrel (75 mg/day) for 90 days followed by lifelong aspirin or clopidogrel monotherapy thereafter. The primary risk factors of cerebrovascular disease including systolic blood pressure and LDL cholesterol, will be controlled in line with protocols. Systolic blood pressure will be controlled below 140 mmHg or 130 mmHg in patients with diabetes and LDL will be controlled below 70 mg/dl with Atorvastatin (14). At each follow-up visit, blood pressure and LDL will be tested, and if the standard is not met, the medication will be adjusted based on the measurements. Management of secondary risk factors such as diabetes, non-HDL cholesterol, smoking, weight and physical activity will be coordinated with the patient's primary physician or other consultant as needed. A lifestyle modification program, INTERVENT, will be provided to each patient.
ER Protocol
A dual antiplatelet regimen with acetylsalicylic acid (100 mg) and clopidogrel (75 mg) is started at least 3 days before the procedure. All procedures are performed under general anesthesia by an experienced interventional neuroradiologist. After placement of sheath introducers, heparin is given intravenously to maintain the coagulation time between 200 and 300 s. The 6- or 8-French guiding catheter is located distal to the occluded artery as much as possible. Under the route map, the micro guidewire in combination with a microcatheter and the microcatheter are used to carefully pass through the occluded segment. Angiography with the microcatheter should confirm that the guidewire is in the true lumen. The exchange micro guidewire is then sent into the micro catheter, and the microcatheter is exchanged out. The balloon catheter is advanced smoothly into the occluded segment along the exchange micro guidewire. After the occluded segment is dilated with the balloon, angiography with a guiding catheter is performed. Stents are deployed in cases of residual severe stenosis, vascular dissection and failure to maintain forward flow (according to the judgment of the neuroradiologist to select the stent). If one stent cannot completely cover the lesion, multiple stents can be implanted. Successful revascularization is defined as a modified TICI grade 2b or 3 and residual stenosis <50%.
For patients with ICA or MCA M1 segment occlusion, ipsilateral hypoperfusion should be confirmed by CTP or MRI perfusion imaging prior to enrollment according to a previous study (15). Moreover, the non-contrast and perfusion scans are additionally transferred to the Rapid Processing of Perfusion and Diffusion (RAPID) system, providing analysis of perfusion source images with respect to the DEFUSE 3 criteria (16).
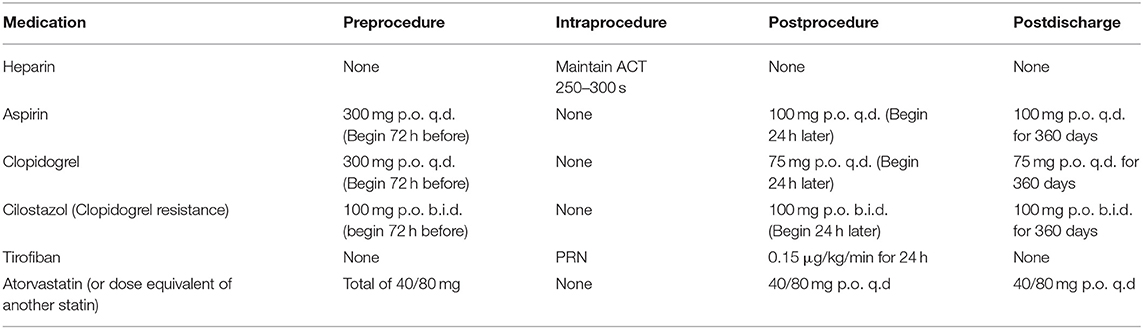
Periprocedural drug therapy is shown in Table 3. After the procedure, if there are no hemorrhagic complications on the head CT scan, intravenous anticoagulation or antiplatelet therapy is continued for at least 24-48 h. Then, dual antiplatelet therapy is maintained for 3-6 months followed by lifelong aspirin or clopidogrel monotherapy thereafter.
Data Design and Management
Data design and management is the responsibility of the Scientific Committee of Capital Medical University experts. They will keep watch on the database and propose amendments at any time to achieve the purpose of the study. We will collect patient information in a confidential manner in line with China privacy laws. Each center will be in charge of the personal data collected related to the study. Then each patient will be assigned an anonymous identification code. In each center, a responsible physician will registered the information of every enrolled patients on the internet-based data storage file. Each center has its own account and password, and each center can only see patient information uploaded by their own center when they access the web database; if a center research investigator wants to see information on all patients enrolled in their study, they need to request it from the Scientific Committee. All the data will be analyzed anonymously by a statistician.
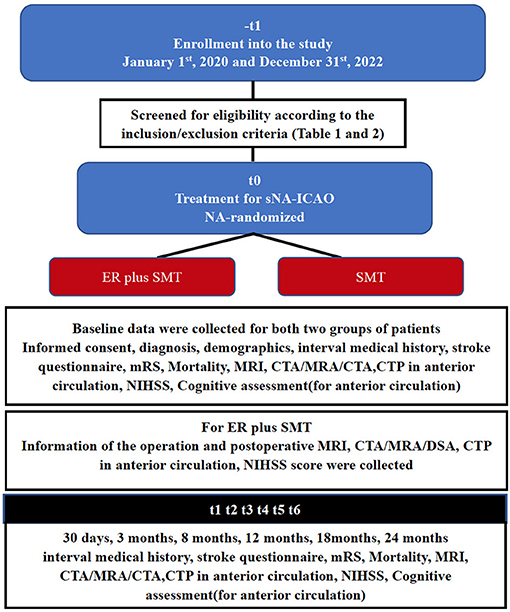
Information of baseline including demographics, vascular risk factors (such as diabetes mellitus, arterial blood pressure, hyperlipidemia, cardiac disease, and smoking) and stroke symptoms [with the Questionnaire for Verifying Stroke-free Status (QVSS) (17), the modified Rankin Scale (mRS) (18) and the National Institutes of Health Stroke Scale (NIHSS) (19)], including morphology occlusion stump, occlusion to recanalization, last symptom to recanalization and cognitive testing (in anterior circulation) were collected (Figure 2).
For patients with ICA or MCA M1 segment occlusion, Cognitive function assessment related to vascular cognitive status, is performed at baseline, 30 days, 3 months, 8 months, 12 months, 18 months, and 24 months. The assessment consists of five tests covering the following four domains of cognitive function: the word list learning test and the delayed recall test from the Chinese version of the Alzheimer's Disease Assessment Scale-cognitive subscale (ADAS-Cog) (20), executive function/processing speed (animal naming and letter fluency), and attention/working memory (digit span) (Figure 2).
Figure 2 illustrates the follow-up schedule. If the patient is unable to come to the hospital for a face-to-face follow-up visit, a telephone follow-up visit will be conducted if possible and will include a brief medication history, any ischemic events, daily functioning and changes in cognitive function.
Primary and Secondary Outcomes
In this trial, the primary outcome is any stroke from enrollment to 2 years of follow-up. The secondary outcomes are all-cause mortality, mRS score, NIHSS score and cognitive function from enrollment to 30 days, 3 months, 8 months, 12 months, 18months, and 2 years of follow-up. Stroke will be defined as rapidly developing clinical signs of focal disturbance of cerebral function lasting more than 24 h with no apparent cause other than that of vascular origin according to the World Health Organization (21). Outcome will be determined by an adjudication committee that is unaware of the trial design and grouping.
Major stroke is defined as the NIHSS score is ≥6 at least 30 days after the date of stroke onset, mild stroke is defined as deterioration in the NIHSS score is ≤ 4 points and bright spots appear on the brain DWI or as determined by the Stroke Adjudication Committee according to clinical data.
Statistical Analysis
Based on data from previous studies (6, 9), the 2-year incidence rates of ipsilateral ischemic stroke in ER plus SMT and SMT alone are approximately 10 and 20%, respectively. Assuming a two-sided significance level of 5%, a power of 80%, non-participating rate of 20% and dropout rate of 20%, the requirement for the ER plus SMT and SMT alone group was calculated to be 160 and 320 patients, respectively (1:2 allocation). Reviewing previous studies (8–13), we took the preoperative complication (arterial dissection, arterial perforation, thrombus translocation, subacute stent thrombosis, hemorrhage, and died) rate >15% as the termination criteria.
Prior to statistical analysis, the statistician will collate the data. If he finds any missing data, he will contact the responsible doctor and ask him to check the medical records of the patient visits and follow-up visits to clarify whether the missing information is in the data sheet. If the missing data cannot be obtained, we will conduct multiple imputation under a multivariate normal distribution to impute missing outcome data in the primary analysis of all outcomes, with a sensitivity analysis on complete cases only. Continuous variables are expressed as medians and interquartile ranges (IQRs) and as absolute numbers and percentages, while categorical variables are expressed as the means and standard deviations (SDs). Shapiro–Wilk test, histogram, and QQ chart were used to confirm normal distribution of data. We use chi-squared test, t-tests and Mann–Whitney U-test to compare categorical variables, continuous variables and scores, respectively. Using cox proportional regression models with 95% confidence intervals to test the risk of mortality or Ischemic events. Adjusted estimates of outcome (common odds ratio, odds ratio, and β) will be calculated by taking the following variables into account: age, baseline NIHSS and mRS score, baseline cognitive function, sex, medical history, ischemic stroke, duration from last neurologic event and occlusion site. For propensity score matching analysis, we will perform 1:1 matching based on the nearest-neighbor matching algorithm with a caliper width of 0.2 of the propensity score with age, baseline NIHSS and mRS score, baseline cognitive function, location of occlusion and medical history questionnaire. All statistical analyses will be performed with SPASS 25.0, and P < 0.05 will be considered significant.
The Responsibilities of the Scientific and Steering Committees
The responsibilities of the Scientific Committee is to supervise the publication and presentation of the final research results on the academic symposium, in consultation with the Steering Committee. The Committee will make sure that all publications adhere to authorship guidelines. Members are Xuan Sun, and Miao Zhongrong.
The Steering Committee will be responsible for the planning and implementation of the registry. Specifically, they: approve the participating centers and the corresponding doctors in each center; perform quality control of the data; direct and propose amendments at any time to achieve the purpose of the study; analyze and revise the final results for submission to the congress and publication in a scientific papers. Members are Feng Gao; Xu Guo; Chao Wen; Hui-Jun Zhang.
Discussion
Summary
The management of patients with sNA-ICAO, which is a unique subset with high morbidity and a high probability of recurrent serious ischemic events despite maximal medical therapy, has been clinically challenging. Some small-sample clinical studies have also discussed endovascular recanalization for sNA-ICAO; however, there is no evidence from multicenter large-sample trials. The aim of our present study is to evaluate the technical feasibility and safety of non-acute intracranial artery occlusion. We will perform subgroup analysis according to the angiographic classification of sNA-ICAO proposed by our previous studies (9, 13), stump morphology, duration from occlusion confirmed by imaging and clot characteristics evaluated by High Resolution MRI (optional examine).
Limitations
Non-randomized of the treatment arms is the main limitation of the present research. Clinical reasoning behind treatment choice may affect conclusions, but the extensive data collection of numerous potentially relevant factors will allow us to adjust for potential confounders. Comparative effectiveness trials such as this are valuable because both physicians and patients have a complex range of factors and decisions that affect their treatment options, which cannot be assessed in RCTs. Another limitation of the study design is that our present study population is limited to the Chinese patients which restrains generalizability of the results to other populations/ethnicities. For this limitation, we will perform our further studies which will include patients from other countries.
Strengths and Relevance
Our present study have several advantages except for non-randomization. Current studies on endovascular treatment of ICAS are mainly from a number of single-center, small-sample, single-arm retrospective analyses. First, our study is prospectively designed and will contain the largest sample size to date. Second, our study is a multicenter design across 15 provinces of the country, thus minimizing selection bias; finally, we will also compare the results of ER combined with SMT and SMT alone for sNA-ICAO. Importantly, we planned a long-term follow-up of 24 months, which will allow us to examine both short-term and long-term outcomes of patients.
Ethics Statement
We will follow the World Medical Association Declaration of Helsinki to collect data and all potential participants will be required to sign an informed consent form. Ethical permission was received from the Institutional Review Board of Beijing Tiantan Hospital (number: KY2020-114-02).
Author Contributions
FG, XG, CWe, and HZ are on the Scientific Committee for the current project. XS and ZM are on the steering committee for the current project. HZ, XS, FG, XG, GX, YS, CWe, CWa, YW, YX, YJ, SZ, CL, DL, YL, and CX will provide patient information for the multicenter trial. HZ, XS, and FG wrote the draft. All authors were involved in the design of the protocol, revised the draft, and approved the final manuscript.
Funding
This study was funded by National Key R&D Program (2018AAA0102600).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
sNA-ICAO, symptomatic non-acute intracranial artery occlusion; SMT, standard medical therapy; ER, endovascular recanalization; EC-IC, Extracranial-intracranial; SPIRIT, Recommendations for Interventional Trials; ERAS, enhanced recovery after surgery; IRB, institutional review board; MEC, medical ethics committee; RAPID, Rapid Processing of Perfusion and Diffusion; ADAS-Cog, Alzheimer's Disease Assessment Scale-cognitive subscale.
References
1. Gorelick PB, Wong KS, Bae HJ, Pandey DK. Large artery intracranial occlusive disease: a large worldwide burden but a relatively neglected frontier. Stroke. (2008) 39 8:2396-9. doi: 10.1161/STROKEAHA.107.505776
2. Wong LK. Global burden of intracranial atherosclerosis. Int J Stroke. (2006) 1 3:158-9. doi: 10.1111/j.1747-4949.2006.00045.x
3. Yamauchi H, Higashi T, Kagawa S, Kishibe Y, Takahashi M. Chronic hemodynamic compromise and cerebral ischemic events in asymptomatic or remote symptomatic large-artery intracranial occlusive disease. AJNR Am J Neuroradiol. (2013) 34 9:1704-10. doi: 10.3174/ajnr.A3491
4. Kuroda S, Houkin K, Kamiyama H, Mitsumori K, Iwasaki Y, Abe H. Long-term prognosis of medically treated patients with internal carotid or middle cerebral artery occlusion: can acetazolamide test predict it? Stroke. (2001) 32 9:2110-6. doi: 10.1161/hs0901.095692
5. Yamauchi H, Fukuyama H, Nagahama Y, Nabatame H, Nakamura K, Yamamoto Y, et al. Evidence of misery perfusion and risk for recurrent stroke in major cerebral arterial occlusive diseases from PET. J Neurol Neurosurg Psychiatry. (1996) 61 1:18-25. doi: 10.1136/jnnp.61.1.18
6. Group EIBS. Failure of extracranial-intracranial arterial bypass to reduce the risk of ischemic stroke. Results of an international randomized trial. N Engl J Med. (1985) 313:1191-200. doi: 10.1056/NEJM198511073131904
7. Powers WJ, Clarke WR, Grubb RL, Videen TO, Adams HP, Derdeyn CP, et al. Extracranial-intracranial bypass surgery for stroke prevention in hemodynamic cerebral ischemia: the Carotid Occlusion Surgery Study randomized trial. JAMA. (2011) 306:1983-92. doi: 10.1001/jama.2011.1610
8. Kao HL, Lin MS, Wang CS, Lin YH, Lin LC, Chao CL, et al. Feasibility of endovascular recanalization for symptomatic cervical internal carotid artery occlusion. J Am Coll Cardiol. (2007) 49:765-71. doi: 10.1016/j.jacc.2006.11.029
9. Gao F, Guo X, Han J, Sun X, Zhou Z, Miao Z. Endovascular recanalization for symptomatic non-acute middle cerebral artery occlusion: proposal of a new angiographic classification. J Neurointerv Surg. (2020) 900–5. doi: 10.1136/neurintsurg-2020-016692
10. Yao YD, Liu AF, Qiu HC, Zhou J, Li C, Wang Q, et al. Outcomes of late endovascular recanalization for symptomatic non-acute atherosclerotic intracranial large artery occlusion. Clin Neurol Neurosurg. (2019) 187:105567. doi: 10.1016/j.clineuro.2019.105567
11. Chen L, Jiang Y, Hu F, He L, Zheng H. Endovascular revascularization of nonacute symptomatic proximal extracranial vertebral artery occlusion. World Neurosurg. (2020) 134:39-44. doi: 10.1016/j.wneu.2019.10.059
12. Zhao W, Zhang J, Song Y, Sun L, Zheng M, Yin H, et al. Endovascular recanalization for symptomatic subacute to chronic atherosclerotic basilar artery occlusion. Front Neurol. (2019) 10:1290. doi: 10.3389/fneur.2019.01290
13. Gao F, Sun X, Zhang H, Ma N, Mo D, Miao Z. Endovascular recanalization for nonacute intracranial vertebral artery occlusion according to a new classification. Stroke. (2020) 51:3340-3. doi: 10.1161/STROKEAHA.120.030440
14. Chimowitz MI, Lynn MJ, Derdeyn CP, Turan TN, Fiorella D, Lane BF, et al. Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl J Med. (2011) 365:993-1003. doi: 10.1056/NEJMoa1105335
15. Murphy BD, Fox AJ, Lee DH, Sahlas DJ, Black SE, Hogan MJ, et al. Identification of penumbra and infarct in acute ischemic stroke using computed tomography perfusion-derived blood flow and blood volume measurements. Stroke. (2006) 37:1771-7. doi: 10.1161/01.STR.0000227243.96808.53
16. Albers GW, Marks MP, Kemp S, Christensen S, Tsai JP, Ortega-Gutierrez S, et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med. (2018) 378:708-18. doi: 10.1056/NEJMoa1713973
17. Meschia JF, Lojacono MA, Miller MJ, Brott TG, Atkinson EJ, O'Brien PC. Reliability of the questionnaire for verifying stroke-free status. Cerebrovasc Dis. (2004) 17:218-23. doi: 10.1159/000075794
18. Kasner SE. Clinical interpretation and use of stroke scales. Lancet Neurol. (2006) 5:603-12. doi: 10.1016/S1474-4422(06)70495-1
19. Lyden P, Lu M, Jackson C, Marler J, Kothari R, Brott T, et al. Underlying structure of the National Institutes of Health Stroke Scale: results of a factor analysis. NINDS tPA Stroke Trial Investigators. Stroke. (1999) 30:2347-54. doi: 10.1161/01.STR.30.11.2347
20. Chu LW, Chiu KC, Hui SL, Yu GK, Tsui WJ, Lee PW. The reliability and validity of the Alzheimer's Disease Assessment Scale Cognitive Subscale (ADAS-Cog) among the elderly Chinese in Hong Kong. Ann Acad Med Singap. (2000) 29:474-85. doi: 10.1016/S0197-4580(00)83371-0
Keywords: symptomatic non-acute intracranial artery occlusion, standard medical therapy, endovascular recanalization, major and mild stroke, primary and secondary outcomes
Citation: Zhang H, Han J, Sun X, Miao Z, Guo X, Xu G, Sun Y, Wen C, Wang C, Wu Y, Xu Y, Jiang Y, Zhang S, Liu C, Li D, Liu Y, Xu C and Gao F (2021) Endovascular Recanalization and Standard Medical Management for Symptomatic Non-acute Intracranial Artery Occlusion: Study Protocol for a Non-randomized, 24-Month, Multicenter Study. Front. Neurol. 12:729534. doi: 10.3389/fneur.2021.729534
Received: 23 June 2021; Accepted: 31 August 2021;
Published: 28 September 2021.
Edited by:
Peter Sporns, University Hospital of Basel, SwitzerlandReviewed by:
Nikoloz Tsiskaridze, Pineo Medical Ecosystem, GeorgiaFrieder Schlunk, Charité-Universitätsmedizin Berlin, Germany
Copyright © 2021 Zhang, Han, Sun, Miao, Guo, Xu, Sun, Wen, Wang, Wu, Xu, Jiang, Zhang, Liu, Li, Liu, Xu and Gao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Feng Gao, ZmVuZ2dhbzEyMTJAMTI2LmNvbQ==
†These authors have contributed equally to this work
 Huijun Zhang
Huijun Zhang Jianjia Han2†
Jianjia Han2† Xuan Sun
Xuan Sun Zhongrong Miao
Zhongrong Miao Feng Gao
Feng Gao