- 1Department of Neurointervention, Beijing Tiantan Hospital, Capital Medical University, Beijing, China
- 2Beijing Neurosurgical Institute, Beijing Tiantan Hospital, Capital Medical University, Beijing, China
- 3Department of Neurosurgery, Beijing Tiantan Hospital, Capital Medical University, Beijing, China
Objective: To investigate the safety and efficacy of Neuroform Atlas stent-assisted coiling for the treatment of tiny wide-necked intracranial aneurysms and evaluate risk factors associated with procedure-related complications.
Methods: We retrospectively examined 46 patients with 46 tiny wide-necked aneurysms who were treated using Atlas stent-assisted coiling at our institution from August 2020 to May 2022. Patient and aneurysm characteristics, procedural details, procedure-related complications, and angiographic and clinical outcomes were analyzed.
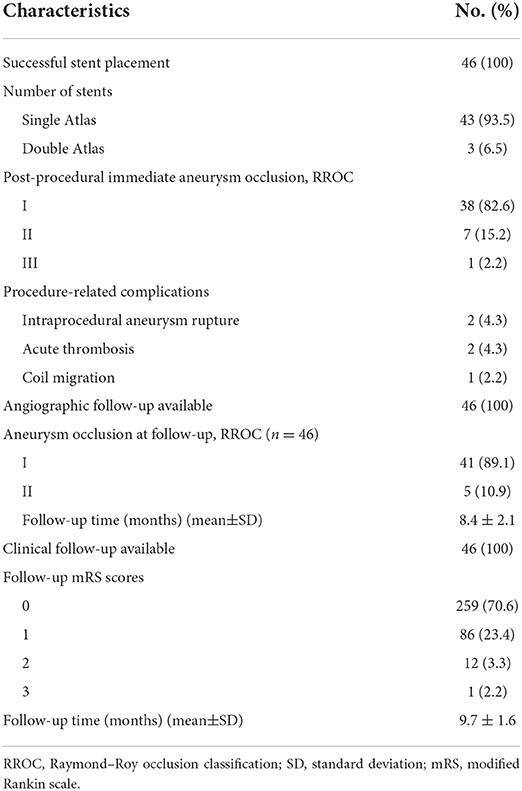
Results: A total of 10 patients presented with aneurysmal rupture. Atlas stent placement was successful in all patients. Angiography immediately after the procedure showed complete occlusion in 38 patients (82.6%), neck remnant in 7 (15.2%), and partial occlusion in 1 (2.2%). The mean angiographic follow-up was 8.4 months (range, 6–16). At the last follow-up, angiography showed complete occlusion in 41 patients (89.1%) and neck remnant in 5 (10.9%). No aneurysm recurrence or in-stent stenosis occurred. Incidence of procedure-related complications was 10.8% (intraprocedural aneurysm rupture, two cases; acute thrombosis, two cases; and coil migration, one case); only one patient (2.2%) experienced procedural neurological morbidity. The mean clinical follow-up was 9.7 months. A favorable outcome was achieved in 45 patients (97.8%). In univariate logistic regression analysis, aneurysm size (odds ratio, 4.538; P = 0.045) was significantly associated with procedure-related complications. However, multivariate analysis found no independent risk factors.
Conclusion: Atlas stent-assisted coiling of tiny wide-necked intracranial aneurysms is feasible and effective. Outcomes and occlusion rates are favorable and morbidity is low. The complication rate may be higher in larger tiny aneurysms.
Introduction
Aneurysms with a maximum diameter of ≤ 3 mm are defined as tiny (1–3). A greater number of tiny aneurysms are being diagnosed owing to recent advances in neuroradiological techniques and improvements in imaging resolution (4). Treatment of these aneurysms is particularly challenging because they are characterized by thin fragile walls. When surgically clipping a tiny aneurysm, partial clipping of the parent artery is frequently required to prevent the clip from falling off (5). When performing endovascular embolization, coil stabilization within the sac is difficult, particularly in wide-necked aneurysms; in addition, subarachnoid hemorrhage (SAH) may result from a catheter or coil piercing the fragile aneurysm wall (6).
Management of tiny wide-necked intracranial aneurysms is controversial. Reported rupture rates are low, ranging from 0 to 0.4% (7). However, tiny aneurysms account for 15% of all ruptured aneurysms, which suggests that rupture of tiny aneurysms is not uncommon and that they should be treated (8).
In May 2020, the Neuroform Atlas stent (Stryker Neurovascular, Fremont, CA, USA) was introduced in China. Since then, it has been widely used to treat wide-necked aneurysms. The Atlas stent is a self-expanding laser-cut nitinol stent that is delivered using a low-profile microcatheter (size, 0.0165–0.017 inch). Its novel mixed open-cell/closed-cell design enhances stability within the vessel, provides high flexibility, and promotes proper apposition to the vessel wall (9). These design innovations, along with the development of smaller coils, may result in fewer complications and better efficacy when treating tiny aneurysms. Although previous studies have confirmed Atlas stent safety and efficacy (10–12), they included few patients with tiny wide-necked aneurysms. This study aimed to report our preliminary experience with Atlas stent-assisted coiling of tiny wide-necked aneurysms. We also examined safety and efficacy and evaluated risk factors for procedure-related complications.
Materials and methods
Patient population and data collection
We performed a retrospective analysis of consecutive adult patients who underwent Atlas stent-assisted coiling of tiny wide-necked intracranial aneurysms between August 2020 and May 2022 in our institution. All aneurysms were diagnosed using digital subtraction angiography (DSA). Tiny was defined as a maximum diameter of ≤ 3 mm. Wide-necked was defined as a dome-to-neck ratio < 2. Patients with ruptured and unruptured saccular aneurysms were included. Those with blood blister-like aneurysms, which have a broad base, lack an identifiable neck, and originate from a nonbranching portion of an artery (13), were excluded. We also excluded patients who did not have postoperative imaging follow-up. The study was approved by the institutional review board of Beijing Tiantan Hospital. All patients provided written informed consent.
The following baseline clinical data were recorded: age, sex, cigarette smoking, alcohol intake, hypertension, diabetes mellitus, and clinical presentation. Imaging data were analyzed to record aneurysm size, shape, location, rupture status, and dome-to-neck ratio. Hunt and Hess's grade was recorded in patients who presented with aneurysmal rupture.
Endovascular treatment and antiplatelet regimen
Patients with unruptured aneurysms were premedicated with aspirin 100 mg and clopidogrel 75 mg daily for at least 5 days. Patients with ruptured aneurysms received 300 mg loading doses of both aspirin and clopidogrel 4 h before the procedure. All procedures were performed via the femoral approach under general anesthesia and full anticoagulation with heparin (targeted activated clotting time was two to three times above the patient's baseline value). A triaxial guide-catheter system using a 6-Fr Cook (Cook Medical, Bloomington, IN, USA) or 6-Fr Neuron MAX (Penumbra, Alameda, California, USA) long sheath, 5-Fr or 6-Fr Navien (Covidien, Irvine, California, USA) intermediate support catheter, and Excelsior SL-10 or XT-17 microcatheter (Stryker Neurovascular) was used to deploy the stent. Aneurysm morphology and parent arterial structure were assessed using a three-dimensional rotational angiography and the proper working projection was selected. An Echelon-10 microcatheter (Medtronic, Dublin, Ireland) was then placed into the aneurysm lumen. An Excelsior SL-10 or XT-17 microcatheter was placed into the parent artery under microguidewire guidance. Aneurysm coiling was performed using the jailing technique; if this failed, the trans-cell technique (through the struts) was performed. All endovascular procedures were performed by neurointerventionalists with more than 10 years of experience. Aspirin 100 mg and clopidogrel 75 mg daily were continued for at least 3 months after the procedure, and then aspirin alone for 6 months or life.
Postoperative evaluation and follow-up
Procedure-related complications were categorized as hemorrhagic, ischemic, or other. Hemorrhagic complications were defined as visualization of contrast leakage from the aneurysm or ruptured vessel during the procedure or visualization of intracranial hemorrhage on an imaging study performed after the procedure. Ischemic complications were defined as thromboembolic events associated with re-treatment, namely persistent focal neurological deficit, transient ischemic attack, or cerebral infarction.
Clinical outcome was evaluated immediately after the procedure and 3, 6, 12, and 24 months after the procedure via outpatient clinic visits or phone calls using the modified Rankin Scale (mRS). Clinical outcome was classified as favorable (mRS score 0–2) or poor (mRS score 3–6). Morbidity was defined as any procedure-related neurological deterioration that caused an increase in the mRS score. Imaging follow-up (DSA or computed tomography angiography) was performed 6, 12, and 24 months after the procedure and was evaluated using the Raymond–Roy (RR) occlusion classification system: grade I, complete occlusion; grade II, residual neck; and grade III, residual aneurysm (14).
Statistical analysis
Statistical analyses were performed using SPSS software version 24.0 (IBM Corp., Armonk, NY, USA). Continuous variables with a normal distribution are expressed as means with standard deviation (SD); those with a skewed distribution are expressed as medians with an interquartile range. Categorical variables are expressed as numbers with percentages. Continuous variables were compared using the Student's t-test or Wilcoxon rank-sum test, as appropriate. Categorical variables were compared using the chi-square or Fisher's exact test as appropriate. Variables with P < 0.1 in univariate logistic regression analyses were included in the multivariate analysis to determine independent risk factors for procedure-related complications. P < 0.05 was considered significant.
Results
Patient and aneurysm characteristics
A total of 46 tiny wide-necked intracranial aneurysms in 46 patients (32 women, 14 men) were included for analysis. The mean patient age was 56.1 years (range, 39–74). A total of 10 patients presented with a ruptured aneurysm. The Hunt-Hess grade was I in five patients, II in three, and III in two. All patients with an unruptured aneurysm had an mRS score < 2 on admission. Of the 46 aneurysms, the location was anterior circulation in 37 (80.4%) and posterior circulation in 9 (19.6%). The mean aneurysm size was 2.5 ± 0.3 mm (range, 1.9–3.0). Mean dome-to-neck ratio was 1.0 ± 0.4 (range, 0.4–2.0). Patient and aneurysm characteristics are shown in Table 1.
Postprocedural angiographic and clinical outcomes
Atlas stent deployment was successful in all patients (100% technical success rate). A single stent was placed in 43 cases (93.5%) and two stents were placed in 3 (6.5%). Immediate postprocedural angiography showed complete occlusion (RR grade I) in 38 patients (82.6%), neck remnant (RR grade II) in 7 (15.2%), and partial occlusion (RR grade III) in 1 (2.2%). Complete occlusion was achieved in all 10 patients with a ruptured aneurysm. Figures 1, 2 show representative cases. Postprocedural angiographic and clinical outcomes are shown in Table 2.
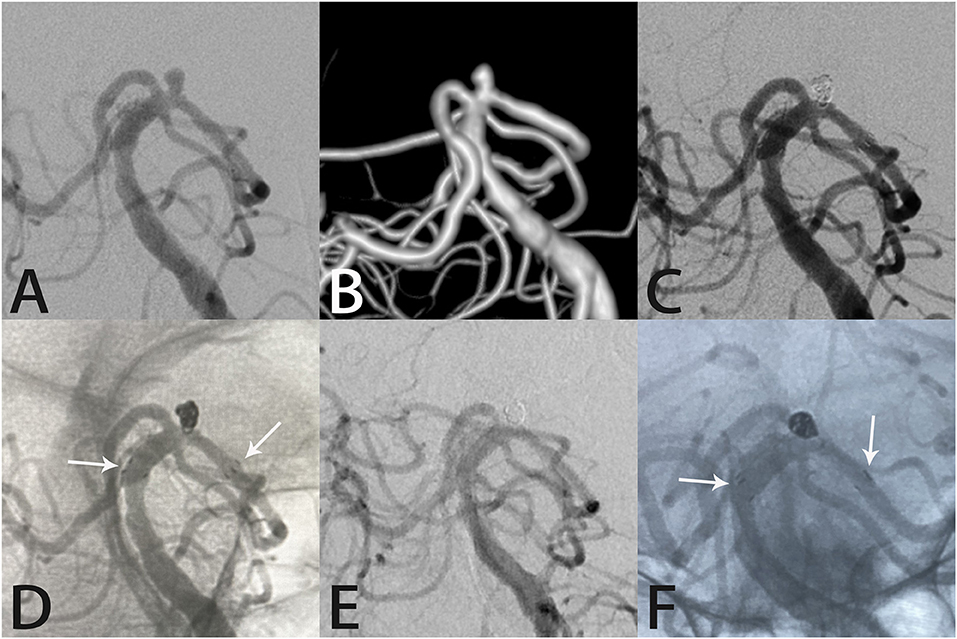
Figure 1. Images from a 66-year-old woman with a tiny left posterior cerebral artery aneurysm. Digital subtraction angiography with three-dimensional reconstruction images demonstrated a left posterior cerebral artery aneurysm (A,B). Angiography immediately after Atlas stent-assisted coiling showed complete aneurysm occlusion (C). An unsubtracted image shows the three radiopaque markers (white arrows) at the proximal and distal ends of the Atlas stent (4.0 × 15 mm) and the coils within the aneurysm sac (D). Follow-up angiography 6 months later showed complete aneurysm occlusion, parent artery patency, stability of the Atlas stent (white arrows indicate the ends of the stent), and the coils densely packed within the aneurysm sac (E,F).
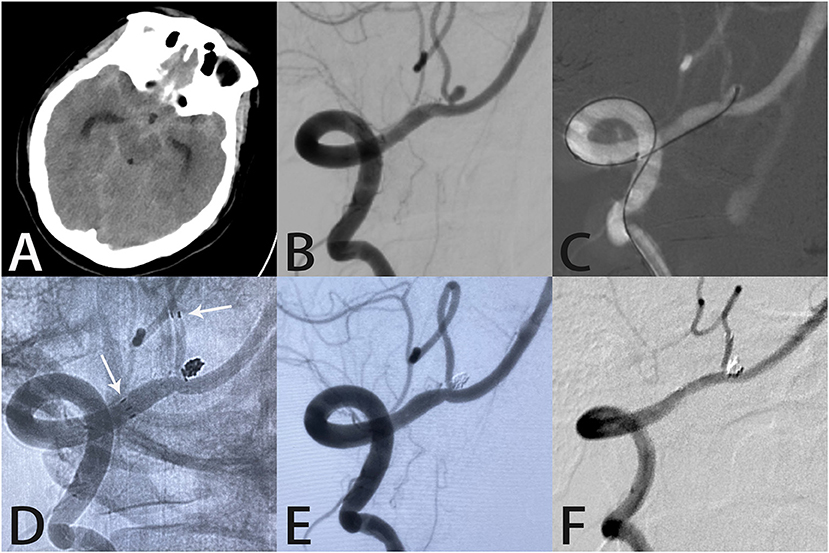
Figure 2. Images from a 45-year-old woman with a tiny ruptured right posterior inferior cerebellar artery aneurysm. Computed tomography showed subarachnoid hemorrhage in the lateral fissure and basal cisterns (A). Angiography demonstrated a right posterior inferior cerebellar artery aneurysm (B). A microcatheter was delivered into the aneurysm sac (C). An unsubtracted image showed the distal end of the Atlas stent (3.0 × 15 mm) was placed in the right posterior inferior cerebellar artery with the proximal end in the right vertebral artery (white arrows indicate the ends of the stent) (D). Angiography immediately after the procedure showed complete aneurysm occlusion and unobstructed blood flow in the posterior inferior cerebellar artery (E). Follow-up angiography 7 months later showed complete aneurysm occlusion with the coils densely packed within the aneurysm sac (F).
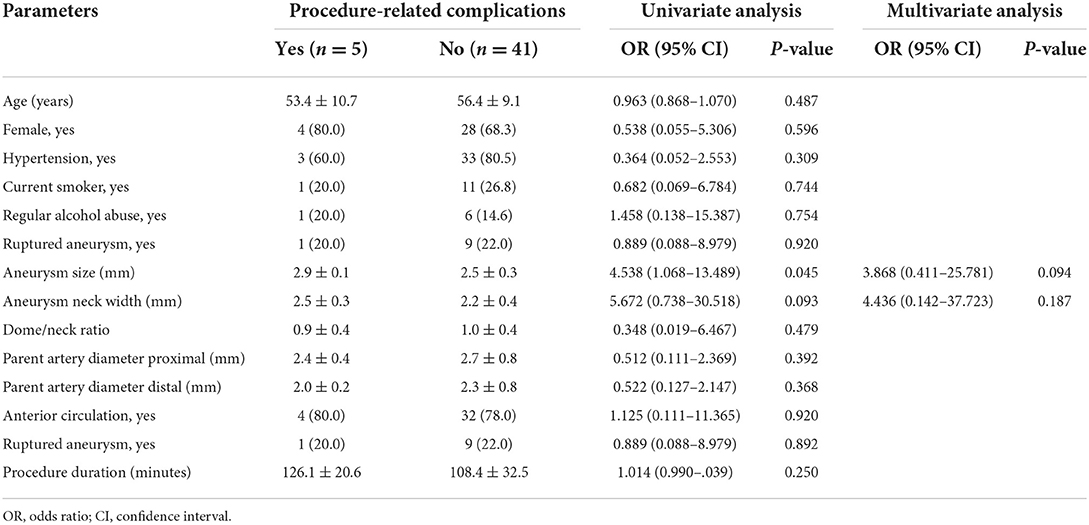
Procedure-related complications occurred in five patients (10.8%), including two intraprocedural aneurysm ruptures, two acute thromboses, and one case of coil migration. No procedure-related death occurred. Both cases of intraprocedural rupture occurred in anterior communicating artery aneurysms. In one, aneurysm perforation occurred during coil placement. Although the rapid deployment of additional coils successfully controlled the hemorrhage, the redundant coils protruded into the parent artery and slowed blood flow in the A2 segment of the right anterior cerebral artery. Therefore, we placed another Atlas stent such that the two stents formed a “Y-shape.” Angiography immediately after the procedure showed complete aneurysm occlusion and unobstructed A2 segment blood flow. The patient experienced transient headaches following the procedure and computed tomography showed only a small amount of SAH (Figure 3). On the other, the patient developed hemiparesis from an intracranial hematoma caused by intraprocedural re-rupture of a ruptured aneurysm; the mRS score was 4 at discharge, which improved to 3 at the last follow-up. Two patients developed in-stent thrombosis during the procedure and were immediately treated with intra-arterial thrombolysis using tirofiban. Recanalization was achieved in both without any clinical sequelae. Coil migration occurred in one patient during coiling, which was treated using Atlas stent deployment to prevent coil protrusion into the parent artery. This patient remained neurologically intact throughout the follow-up. Procedural morbidity and mortality rates were 2.2% and 0, respectively.
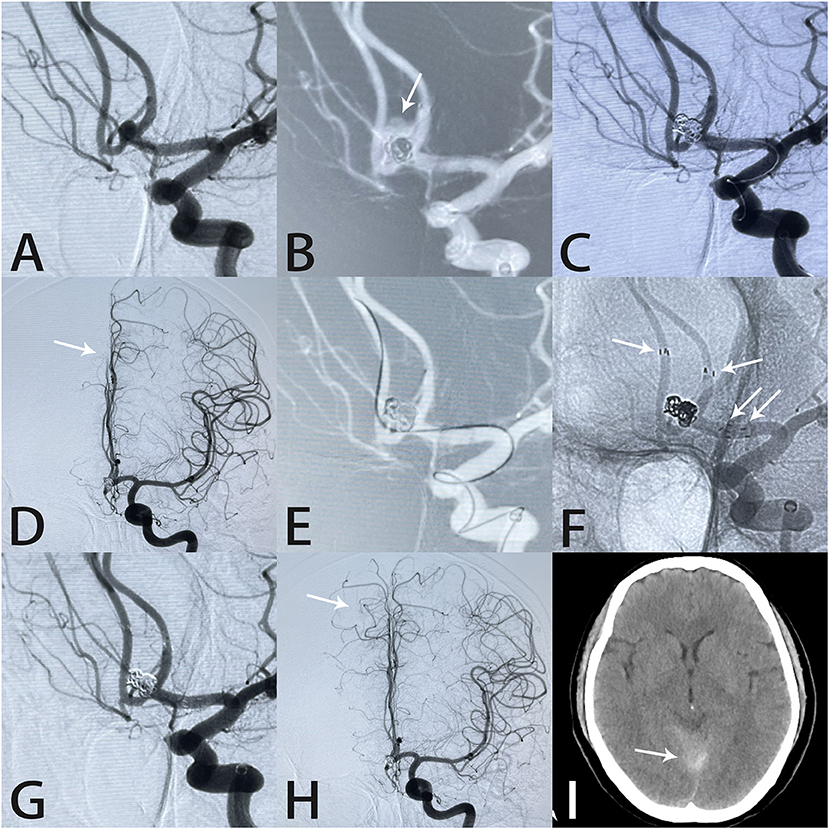
Figure 3. Images from a 56-year-old woman with a tiny anterior communicating artery aneurysm. Angiography demonstrated an anterior communicating artery aneurysm (A). The aneurysm (white arrow) ruptured during coil placement (B). Rapid deployment of additional coils controlled the hemorrhage (C). Intraprocedural angiography showed slow blood flow in the A2 segment of the right anterior cerebral artery (white arrow) (D). An Echelon-10 microcatheter was navigated into the right anterior cerebral artery in preparation for Atlas stent deployment (E). Two Atlas stents formed a “Y-shape” (white arrows) (F). Angiography immediately after the procedure showed complete aneurysm occlusion and unobstructed blood flow in the A2 segment (white arrow) (G,H). Postprocedural computed tomography showed subarachnoid hemorrhage in the tentorium cerebelli (I).
Follow-up angiographic and clinical outcomes
The mean angiographic follow-up was 8.4 ± 2.1 months (range, 6–16). Complete occlusion (RR grade I) was achieved in 41 patients (89.1%) and a neck remnant (RR grade II) was present in 5 (10.9%). No aneurysms recurred and no in-stent stenosis developed during follow-up, even in the three patients treated with the Y-stenting technique.
The mean clinical follow-up was 9.7 ± 1.6 months (range, 8–18). Clinical outcome was favorable in 45 patients (97.8%) and poor in 1 (2.2%). The mRS score was 3 in the single patient with poor clinical outcomes. Follow-up angiographic and clinical outcomes are shown in Table 2.
Risk factors for procedure-related complications
Univariate logistic regression showed that aneurysm size (odds ratio [OR] 4.538; 95% confidence interval [CI], 1.068–13.489; P = 0.045) was significantly associated with procedure-related complications. However, none of the variables examined was an independent risk factor for procedure-related complications in multivariate analysis (Table 3).
Discussion
Although guidelines for the treatment of tiny intracranial aneurysms are lacking, commonly applied strategies include surgical clipping and endovascular embolization. Clipping is not ideal for treating tiny aneurysms because it usually causes parent artery narrowing and is associated with a high incidence of complications (15). In a series of 32 tiny aneurysms (≤ 3 mm) that were surgically clipped, Rahmanian et al. reported a 30.8% intraoperative aneurysm rupture rate and 11.5% mortality (16). Considering the high rates of morbidity and mortality associated with neurosurgical clipping, safer and more effective treatment methods are needed for tiny aneurysms, especially those that have a wide neck. Endovascular embolization can achieve permanent occlusion in up to 85% of intracranial aneurysms and has a lower procedure-related hemorrhage rate (17, 18). Since the publication of the International Subarachnoid Aneurysm Trial, the treatment paradigm for intracranial aneurysms has shifted from surgical clipping to endovascular embolization (19). Moreover, recent technological advances, such as smaller coils and smoother microcatheters, and accumulated operator experience have improved the safety and efficacy of endovascular treatment of tiny aneurysms (20). In a meta-analysis that included 1,105 tiny aneurysms (844 ruptured and 261 unruptured), immediate and long-term complete occlusion rates were 85 and 91%, respectively, and rates of intraprocedural rupture and thromboembolism were 7 and 4%, respectively (3).
Since the introduction of the initial Neuroform stent (Stryker Neurovascular) in 2002, various commercially available intracranial stents have followed. The Atlas stent is the successor to the Neuroform stent and has a structure that is compatible with a 0.0165-inch microcatheter, which enables safer and easier treatment of tiny aneurysms on small vessels. Several studies have demonstrated the safety and effectiveness of the Atlas stent for treating both unruptured and ruptured intracranial aneurysms (10, 11). Compared with the LVIS Jr stent (MicroVention, Inc., Aliso Viejo, CA, USA), the Atlas stent is associated with a higher occlusion rate and lower in-stent stenosis rate (21). However, safety and efficacy data regarding Atlas stent-assisted coiling for treating tiny aneurysms is lacking.
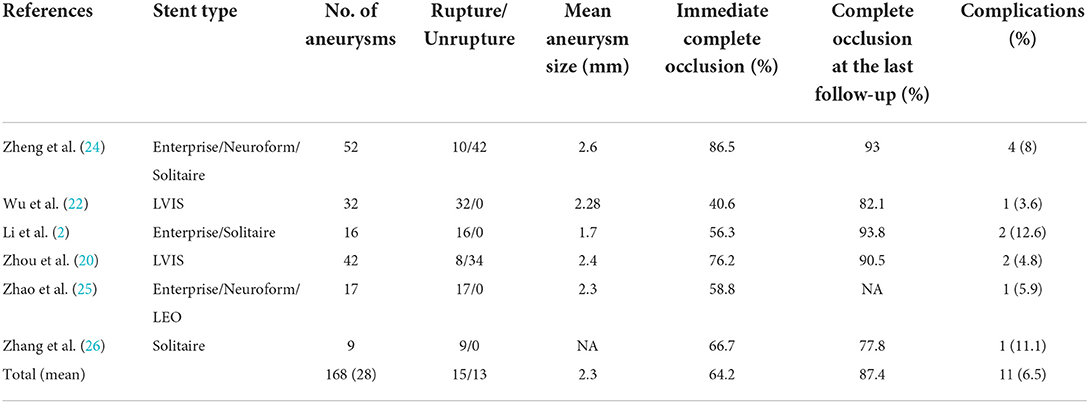
Stent assistance during coiling assists with coil placement improves coverage of the aneurysm neck and increases coil packing density. In addition, the presence of stent wires across the aneurysm neck may provide a structural basis for endothelialization and improve hemodynamic conditions. In our series, Atlas stent deployment was successful in all patients, similar to the results of two other studies (22, 23). Table 4 summarizes the results of six studies that examined stent-assisted coil embolization of tiny aneurysms published within the past 10 years. Collectively, the complete occlusion rates immediately after the procedure and at the last follow-up were 64.2 and 87.4%, respectively. In our study, the corresponding rates were 82.6 and 89.1%, respectively. Corresponding rates for the LVIS stent (MicroVention, Inc.) were 40.6 and 82.1%, respectively, while those for conventional stents were 37.8 and 80%, respectively (22, 27). A meta-analysis of small intracranial aneurysms (≤ 3 mm) treated using endovascular techniques reported postoperative and follow-up complete occlusion rates of 85% and 91%, respectively (3).
The Atlas stent may be associated with a higher rate of complete occlusion than other stents because its design allows for a lower profile delivery, improved trackability, and higher conformability to the vessel wall. Available diameters range from 3.0 to 4.5 mm and lengths range between 15 and 30 mm; therefore, the Atlas stent can be used to treat tiny aneurysms located on small distal vessels. Furthermore, dense coil packing was performed in all aneurysms in our study, regardless of rupture status, and the jailing technique was used in most cases. This technique, in which the coil delivery catheter is placed into the aneurysm before stent deployment (Figure 4), may be safer than the trans-cell coiling technique for treating tiny wide-necked aneurysms. With the trans-cell technique, the stent is deployed first and then the coil delivery catheter is delivered into the aneurysm through the stent interstices; however, catheter navigation can be difficult and any unexpected movement may pierce the aneurysm wall and cause hemorrhage.
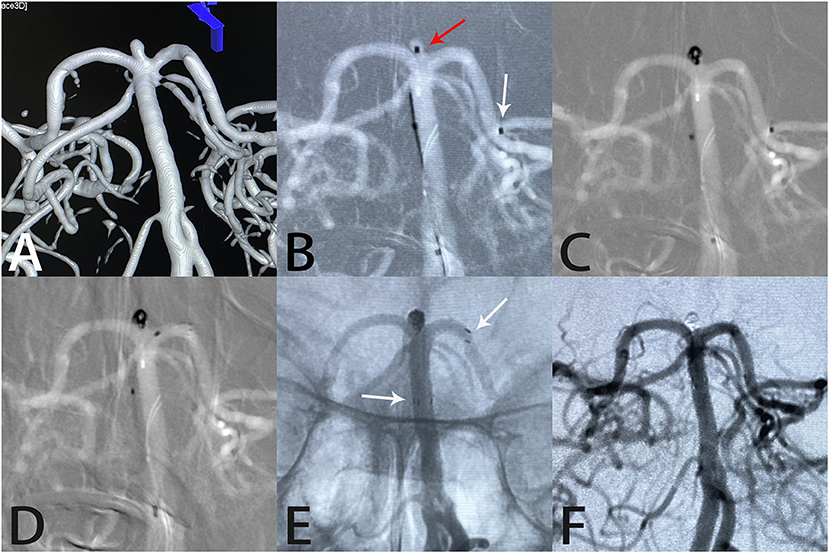
Figure 4. Images from a 65-year-old woman with a tiny basilar artery aneurysm. Three-dimensional digital reconstruction angiography demonstrated a basilar artery aneurysm (A). An Echelon-10 microcatheter was first navigated into the left posterior cerebral artery in preparation for deployment of the Atlas stent (white arrow); then, another Echelon-10 microcatheter was placed into the aneurysm sac (red arrow) for coil placement (B, C). An Atlas stent (3.0 × 15 mm) was deployed through another microcatheter to cover the aneurysm neck (D). An unsubtracted image showed that the coils were packed densely within the sac and the Atlas stent was fitted well against the vessel wall (white arrows indicate the ends of the stent) (E). Angiography immediately after the procedure showed complete aneurysm occlusion and unobstructed blood flow in the left posterior cerebral artery (F).
Intraprocedural rupture during endovascular aneurysm treatment usually leads to poor clinical outcomes. Several studies have shown that the risk of intraprocedural aneurysm rupture appears to be higher for aneurysms < 3 mm in diameter (28, 29). In addition, a 2016 meta-analysis of aneurysms < 3 mm in diameter reported a 7% intraprocedural rupture rate (3), which is higher than the 4.3% rate in our series. However, we excluded patients with blood blister-like aneurysms, which have a higher risk of intraprocedural rupture.
Intraprocedural rupture prevention is critical for success when treating tiny aneurysms. We prefer to use coils that are ultra-soft and slightly smaller than the maximum diameter of the aneurysm, which enables a high packing density. In addition, we place the microwire tip near the neck of the aneurysm rather than inside it to avoid piercing the aneurysm. Furthermore, to achieve adequate maneuverability, the shape of the tip should be adjusted based on the angle between the parent artery and the aneurysm. Delicate maneuvering contributes to lowering the risk of intraprocedural rupture. Li et al. (2) developed the stent-assisted coil-jailing technique, which staples the redundant coil tail between the stent and the parent artery wall, which lowers the risk of intraprocedural rupture and coil displacement.
The Atlas stent may be associated with an increased risk of intraprocedural thrombosis. Intraprocedural thrombosis is mainly associated with poor stent–vessel wall apposition, inadequate antiplatelet therapy, longer procedure time, and thrombogenicity of endovascular embolization materials (30, 31). In our study, in-stent thrombosis occurred in two patients (4.3%), which is slightly higher than the rate reported by Ioanniaid et al. (3.1%) (32). Fortunately, the tirofiban administration resulted in recanalization in both.
Our overall complication rate of 10.8% was higher than the collective rate compiled from our literature review (6.5%). Although aneurysm size was associated with procedure-related complications in our univariate analysis, the multivariate analysis did not identify any independent risk factors.
Most neurointerventionalists are reluctant to place stents in patients with a ruptured aneurysm because they are in a hypercoagulable state and at risk for in-stent thrombosis and associated ischemic complications. In addition, the administration of antiplatelet drugs may cause intracranial hemorrhage again. However, we found no significant difference in procedure-related complications between ruptured and unruptured aneurysms.
Limitations
Our study has several limitations. First, it was retrospective in design and was conducted in a single center. Therefore, selection bias may have been introduced. Second, the mean angiographic follow-up was only 8.4 months, which is too short to determine the true final complete occlusion rate. Third, we did not compare the results of stent-assisted coiling and coiling alone. This comparison would better demonstrate the effect of stenting on outcomes in endovascular treatment of tiny aneurysms.
Conclusion
Atlas stent-assisted coiling of tiny wide-necked intracranial aneurysms is safe and effective. Outcomes and occlusion rates are favorable and morbidity is low; however, the complication rate may be higher in larger tiny aneurysms. Prospective multicenter studies with long-term follow-ups are warranted.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
This study was reviewed and approved by the Ethics Committee of Beijing Tiantan Hospital. Written informed consent to participate in this study was provided by the patients or their legal guardian/next of kin.
Author contributions
LD and XC collected the clinical data, performed the statistical analysis, and wrote the manuscript. LZ, ZZ, and QP helped to collect the clinical data. PL and ML helped revise the manuscript, designed the research, and handled funding and supervision. ML approved the final version on behalf of all authors. All authors read and approved the final manuscript.
Funding
This study was supported by the Youth Program of National Natural Science Foundation of China (Grant no. 81901197).
Acknowledgments
We thank Liwen Bianji (Edanz) (https://www.liwenbianji.cn) for editing the language of a draft of this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Wu X, Matouk CC, Mangla R, Forman HP, Gandhi D, Sanelli P, et al. Cost-Effectiveness of Computed Tomography Angiography in Management of Tiny Unruptured Intracranial Aneurysms in the United States. Stroke. (2019) 50:2396–403. doi: 10.1161/STROKEAHA.119.025600
2. Li CH, Su XH, Zhang B, Han YF, Zhang EW, Yang L, et al. The stent-assisted coil-jailing technique facilitates efficient embolization of tiny cerebral aneurysms. Korean J Radiol. (2014) 15:850–57. doi: 10.3348/kjr.2014.15.6.850
3. Yamaki VN, Brinjikji W, Murad MH, Lanzino G. Endovascular Treatment of Very Small Intracranial Aneurysms: Meta-Analysis. AJNR Am J Neuroradiol. (2016) 37:862–67. doi: 10.3174/ajnr.A4651
4. Van Rooij WJ, Sprengers ME, Gast AN, Peluso JP, Sluzewski M. 3D rotational angiography: the new gold standard in the detection of additional intracranial aneurysms. AJNR Am J Neuroradiol. (2008) 29:976–79. doi: 10.3174/ajnr.A0964
5. Li Y, Bai P, Li J, Xiang S, Geng X, Zou Y, et al. Single coil endovascular embolization of very tiny (≤ 2 mm) intracranial aneurysms: one center's experience. J Integr Neurosci. (2022) 21:27. doi: 10.31083/j.jin2101027
6. Lu J, Liu JC, Wang LJ Qi P, Wang DM. Tiny intracranial aneurysms: endovascular treatment by coil embolisation or sole stent deployment. Eur J Radiol. (2012) 81:1276–81. doi: 10.1016/j.ejrad.2011.03.005
7. Malhotra A, Wu X, Forman HP, Grossetta Nardini HK, Matouk CC, Gandhi D, et al. Growth and Rupture Risk of Small Unruptured Intracranial Aneurysms: A Systematic Review. Ann Intern Med. (2017) 167:26–33. doi: 10.7326/M17-0246
8. Van Rooij WJ, Keeren GJ, Peluso JP, Sluzewski M. Clinical and angiographic results of coiling of 196 very small (< or = 3 mm) intracranial aneurysms. AJNR Am J Neuroradiol. (2009) 30:835–39. doi: 10.3174/ajnr.A1429
9. Caragliano AA, Papa R, Pitrone A, Limbucci N, Nappini S, Ruggiero M, et al. The low-profile Neuroform Atlas stent in the treatment of wide-necked intracranial aneurysms - immediate and midterm results: An Italian multicenter registry. J Neuroradiol. (2020) 47:421–27. doi: 10.1016/j.neurad.2019.03.005
10. Zaidat OO, Hanel RA, Sauvageau EA, et al. Pivotal Trial of the Neuroform Atlas Stent for Treatment of Anterior Circulation Aneurysms: One-Year Outcomes. Stroke. (2020) 51:2087–94.
11. Jankowitz BT, Jadhav AP, Gross B, Jovin TG, Alhajeri AA, Fraser JF, et al. Pivotal trial of the Neuroform Atlas stent for treatment of posterior circulation aneurysms: one-year outcomes. J Neurointerv Surg. (2022) 14:143–48. doi: 10.1136/neurintsurg-2020-017115
12. Hanel RA, Yoon N, Sauvageau E, Aghaebrahim A, Lin E, Jadhav AP, et al. Neuroform Atlas Stent for Treatment of Middle Cerebral Artery Aneurysms: 1-Year Outcomes From Neuroform Atlas Stent Pivotal Trial. Neurosurgery. (2021) 89:102–08. doi: 10.1093/neuros/nyab090
13. Lim YC, Kim BM, Suh SH, Jeon P, Kim SH, Ihn YK, et al. Reconstructive treatment of ruptured blood blister-like aneurysms with stent and coil. Neurosurgery. (2013) 73:480–88. doi: 10.1227/NEU.0000000000000005
14. Roy D, Milot G, Raymond J. Endovascular treatment of unruptured aneurysms. Stroke. (2001) 32:1998–2004. doi: 10.1161/hs0901.095600
15. Qin F, Liu J, Zhao X, Wu D, Lai N, Zhang Z, et al. Endovascular Treatment of Ruptured Very Small Intracranial Aneurysms: Complications, Recurrence Rate, and Clinical Outcomes. Front Neurol. (2021) 12:767649. doi: 10.3389/fneur.2021.767649
16. Rahmanian A, Ghaffarpasand F, Alibai E, Choque-Velasquez J, Jahromi BR, Hernesniemi J, et al. Surgical Outcome of Very Small Intracranial Aneurysms Utilizing the Double Clip Technique. World Neurosurg. (2018) 110:e605–e11. doi: 10.1016/j.wneu.2017.11.060
17. Lin N, Cahill KS, Frerichs KU, Friedlander RM, Claus EB. Treatment of ruptured and unruptured cerebral aneurysms in the USA: a paradigm shift. J Neurointerv Surg. (2018) 10:i69–76. doi: 10.1136/jnis.2011.004978.rep
18. Qureshi AI, Vazquez G, Tariq N, Suri MF, Lakshminarayan K, Lanzino G. Impact of International Subarachnoid Aneurysm Trial results on treatment of ruptured intracranial aneurysms in the United States. Clin Article J Neurosurg. (2011) 114:834–41. doi: 10.3171/2010.6.JNS091486
19. Molyneux AJ, Kerr RSC Yu L-M. International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet. (2005) 366:809–17. doi: 10.1016/S0140-6736(05)67214-5
20. Zhou Y, Peng Q, Wu X, Zhang Y, Liu J, Yang X, et al. Endovascular Treatment of Tiny Aneurysms With Low-Profile Visualized Intraluminal Support Devices Using a “Compressed” Stent Technique. Front Neurol. (2020) 11:610126. doi: 10.3389/fneur.2020.610126
21. Gross BA, Ares WJ, Ducruet AF, Jadhav AP, Jovin TG, Jankowitz BT, et al. clinical comparison of Atlas and LVIS Jr stent-assisted aneurysm coiling. J Neurointerv Surg. (2019) 11:171–74. doi: 10.1136/neurintsurg-2018-014208
22. Wu P, Ocak PE, Wang D, Ocak U, Xu S, Li Y, et al. Endovascular Treatment of Ruptured Tiny Intracranial Aneurysms with Low-Profile Visualized Intraluminal Support Device. J Stroke Cerebrovasc Dis. (2019) 28:330–37. doi: 10.1016/j.jstrokecerebrovasdis.2018.09.052
23. Jin Y, Guo X, Quan T, Chen Z, Liu C, Guan S. Safety and efficacy of endovascular treatment for tiny ruptured intracranial aneurysms with low-profile visualized intraluminal support stents. Interv Neuroradiol. (2022) 11:15910199221079967. doi: 10.1177/15910199221079967
24. Zheng Y, Song Y, Liu D, Liu Y, Xu Q, Tian Y, et al. Stent-assisted coiling embolization of tiny, wide-necked intracranial aneurysms. Acta Neurochir. (2017) 159:93–100. doi: 10.1007/s00701-016-3022-y
25. Zhao R, Shen J, Huang QH, Nie JH, Xu Y, Hong B, et al. Endovascular treatment of ruptured tiny, wide-necked posterior communicating artery aneurysms using a modified stent-assisted coiling technique. J Clin Neurosci. (2013) 20:1377–81. doi: 10.1016/j.jocn.2012.12.012
26. Zhang J, Wang D, Li X. Solitaire AB stent-assisted coiling embolization for the treatment of ruptured very small intracranial aneurysms. Exp Ther Med. (2015) 10:2239–44. doi: 10.3892/etm.2015.2826
27. Liu Y, Wang F, Fu X, Liu Y, Zhang G, Xu K. Clinical and angiographic outcomes following endovascular treatment of very small (3 mm or smaller) intracranial aneurysm: A single-center experience. Medicine. (2017) 96:e7457. doi: 10.1097/MD.0000000000007457
28. Nguyen TN, Raymond J, Guilbert F, Roy D, Bérubé MD, Mahmoud M, et al. Association of endovascular therapy of very small ruptured aneurysms with higher rates of procedure-related rupture. J Neurosurg. (2008) 108:1088–92. doi: 10.3171/JNS/2008/108/6/1088
29. Pierot L, Barbe C, Spelle L. Endovascular treatment of very small unruptured aneurysms: rate of procedural complications, clinical outcome, and anatomical results. Stroke. (2010) 41:2855–59. doi: 10.1161/STROKEAHA.110.588830
30. Kim JS, Nah HW, Park SM, Kim SK, Cho KH, Lee J, et al. Risk factors and stroke mechanisms in atherosclerotic stroke: intracranial compared with extracranial and anterior compared with posterior circulation disease. Stroke. (2012) 43:3313–18. doi: 10.1161/STROKEAHA.112.658500
31. Peret A, Mine B, Bonnet T, Ligot N, Bouziotis J, Lubicz B. Safety and efficacy of a pre-treatment antiplatelet regimen of unruptured intracranial aneurysms: a single-center experience. Neuroradiology. (2020) 62:1029–41. doi: 10.1007/s00234-020-02387-y
Keywords: Neuroform atlas stent, tiny intracranial aneurysms, wide-necked, stent-assisted coiling, endovascular treatment
Citation: Dong L, Chen X, Wang J, Zhang L, Zhao Z, Peng Q, Liu P and Lv M (2022) Neuroform atlas stent-assisted coiling of tiny wide-necked intracranial aneurysms. Front. Neurol. 13:1020785. doi: 10.3389/fneur.2022.1020785
Received: 16 August 2022; Accepted: 14 October 2022;
Published: 10 November 2022.
Edited by:
Jianmin Liu, Second Military Medical University, ChinaReviewed by:
Yingkun He, Henan Provincial People's Hospital, ChinaYang Wang, Beijing Chaoyang Hospital, Capital Medical University, China
Copyright © 2022 Dong, Chen, Wang, Zhang, Zhao, Peng, Liu and Lv. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Peng Liu, c2tlbGV0b25saXUxOTg3QDE2My5jb20=; Ming Lv, ZHJhZ29udGlnZXJAMTYzLmNvbQ==
†These authors share first authorship
 Linggen Dong
Linggen Dong Xiheng Chen
Xiheng Chen Jiejun Wang
Jiejun Wang Longhui Zhang
Longhui Zhang Zhiqiang Zhao1,2
Zhiqiang Zhao1,2 Qichen Peng
Qichen Peng Peng Liu
Peng Liu Ming Lv
Ming Lv