- 1Eli Lilly and Company, Indianapolis, IN, United States
- 2Evidera, Bethesda, MD, United States
Migraine is a highly prevalent neurological disease of varying attack frequency. Headache attacks that are accompanied by a combination of impact on daily activities, photophobia and/or nausea are most commonly migraine. The headache phase of a migraine attack has attracted more research, assessment tools and treatment goals than any other feature, characteristic, or phase of migraine. However, the migraine attack may encompass up to 4 phases: the prodrome, aura, headache phase and postdrome. There is growing recognition that the burden of migraine, including symptoms associated with the headache phase of the attack, may persist between migraine attacks, sometimes referred to as the “interictal phase.” These include allodynia, hypersensitivity, photophobia, phonophobia, osmophobia, visual/vestibular disturbances and motion sickness. Subtle interictal clinical manifestations and a patient's trepidation to make plans or commitments due to the unpredictability of migraine attacks may contribute to poorer quality of life. However, there are only a few tools available to assess the interictal burden. Herein, we examine the recent advances in the recognition, description, and assessment of the interictal burden of migraine. We also highlight the value in patients feeling comfortable discussing the symptoms and overall burden of migraine when discussing migraine treatment needs with their provider.
Introduction
Migraine is an especially common disorder, with a prevalence exceeding that of diabetes, epilepsy and asthma combined, affecting as much as 15% of the population of the United States (US) (1, 2). Migraine is the second leading cause of years lived with disability and the leading cause among adult women less than age 50 (3) and can place significant burden on an individual's ability to function at their best at work, home, and social activities. In spite of its prevalence, recognition of migraine as an important disabling public health concern has been slow in coming, and it was not included in the Global Burden of Diseases, Injuries, and Risk Factors (GBD) studies prior to 2000 (4).
Although migraine has been traditionally regarded as a paroxysmal disorder characterized by headache attacks separated by normal intervals, patients are often affected during headache-free phases (5). Data on hypersensitivity to external stimuli outside attacks and the migraine interictal impact on quality of life became available around 3–4 decades ago (6–9). Guidelines for clinical trials evaluating the benefit of migraine preventive treatments make either change in migraine days, moderate-severe headache days, or responder rate, the recommended primary endpoint (10, 11). Although a focus on the ictal symptoms and frequency is helpful for diagnosing migraine and evaluating the benefit of a treatment and evaluating a standard outcome, migraine involves much more than the headache attack. Guidelines for evaluating migraine preventive treatments also recommend including a measure of health-related quality of life (QoL) and/or disability as a secondary outcome and yet a recent evaluation of clinical trials for migraine and other headache found that only 40.3% included a patient reported outcome measure of disability/impact/HRQoL (12). Thus, it appears that many clinical interactions for migraine focus on symptoms and/or counting monthly migraine days and may be missing a substantial part of the picture by overlooking the impact of migraine on QoL during and also in between the pain phase of migraine attacks.
In addition to the headache phase, migraine can be accompanied by a constellation of other manifestations in varying combinations apart from the symptomatology listed in the diagnostic criteria, including visual disturbances, osmophobia, allodynia (i.e., as the normally non-noxious stimulus from light touch or brushing of the skin causing pain or discomfort), pain on movement, motion sickness, vestibular dysfunctions, cognitive symptoms, and cranial autonomic symptoms (Figure 1). Patients with migraine may experience many of these symptoms even in the interictal phase, although generally at a reduced frequency and/or intensity (13–20).
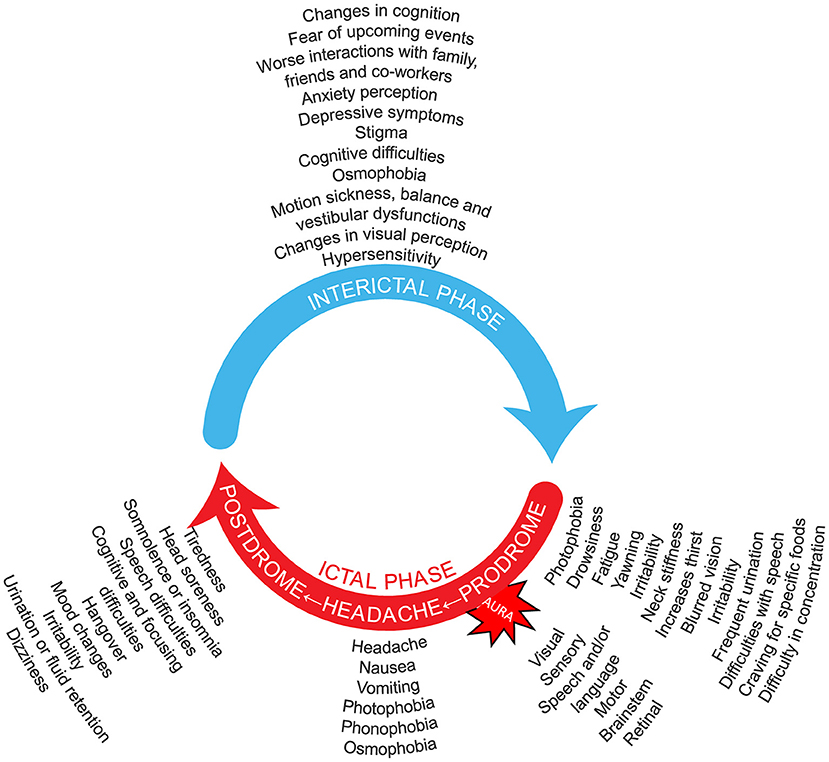
Figure 1. Migraine is an episodic, cycling neurologic condition, where the patient with migraine goes through cycles of relative quiescence (interictal period) that are punctuated by occasional attacks that include the migraine headache. Thus, patients with migraine cycle through an interictal period, that leads to the premonitory phase, then the full-blown migraine attack that includes the severe migraine headache, that is in turn followed by a postdrome phase of waning symptoms and fatigue. Symptoms listed for the interictal (5, 13–26), prodromal (27–32), headache (27, 29, 30, 32–35), and postdromal (27, 29, 32–38) phases, as well as the migraine aura symptoms (27), are suggestions not based on frequency and do not include all possibilities. It should be noted that no phase is obligatory in the migraine wheel; and not all possible symptomatology is depicted. Many symptoms may be present around the entire cycle. One may imagine that the speed with which the migraine wheel rotates distinguishes episodic from chronic migraine. Preventive treatment is a break that reduces this rotation speed. Acute treatment does not reduce that speed but hides one of its components.
Often, some of these symptoms can impact quality of life (QoL) during and in between attacks. Between attacks patients may be fearful, anxious, or worried about when their next one might occur or reflect a patient's concerns about how a future attack can affect plans or activities (21, 39, 40). The impact produced by these phenomena has been described as Interictal Burden (IIB) (5). Moreover, patients with migraine may be subjected to social stigma, which inhibits their seeking treatment and adds to the emotional burden of the disease (41). These non-headache aspects of migraine can be disabling in their own right. We suggest that simply measuring a change in the number of days with a headache may be an inadequate measure to gauge the true impact of migraine, as well as the success of a novel treatment, and that a more holistic approach should be added to future investigations to fully capture the potential benefit to a patient's overall wellbeing.
The need for objective data, such as monthly headache days, by third-party payers in order to approve access to certain therapies, in addition to hesitancies in patient-provider communication or dialogue may be partially responsible for QoL not receiving the attention that it may warrant. It is possible that closed-ended questions constrain discussion of how or why a patient is presenting and what they are currently doing and/or taking that is not working due to the impact it has on their life. If IIB is discussed or brought up by the patient, then that would help identify a need for initiating or modifying treatment. For instance, in a study of doctor-patient interactions, the investigators found that “characteristically, after a brief period of time (mean, 18 s), and most often after the expression of a single stated concern, the physicians in our study took control of the visit by asking increasingly specific, closed-ended questions that effectively halted the spontaneous flow of information from the patient” (42). This is unfortunate because migraine is a condition in which provider-patient communication is paramount especially since the clinical and/or neurological examinations of patients with migraine are typically normal and providers are unable to rely upon biomarkers to aid in diagnosis or tracking of disease progression. This underscores the importance of the patient's narrative which should be explored along with their ideas, feelings, and expectations, which would provide new insights into the illness as the patient is experiencing it (43). Exploring interictal burden necessitates encouraging patients to talk about the entire migraine experience. The use of open-ended questions and an “ask-tell-ask” strategy can yield important information about IIB and QoL and may also leads to shorter office visits, more frequent discussions of preventive therapy, and higher levels of satisfaction for both patient and health-care provider (39).
Migraine frequency
Migraine can be classified based on headache frequency. Chronic migraine (CM) is defined by a patient having ≥15 headache days/month for >3 months with at least 8 of which fulfill diagnostic criteria for migraine (27). There is a proposed definition of episodic migraine (EM) as being “Headache occurring on <15 days a month over the last 3 months, which on some days is migraine” (44). Attempts are being made to identify high-frequency episodic migraine (HFEM) and low-frequency episodic migraine (LFEM), in order to understand how migraine frequency may affect potential responses to medications, and to help in the development of treatment guidelines, as well as to better understand the influence of frequency on QoL (45–50). Although QoL is underutilized as an indicator of disease severity and response to treatment, we are certainly not the first to underscore its importance in making treatment decisions. The American Migraine Prevalence and Prevention (AMPP) Advisory Group proposed that preventive treatment be offered to patients with ≥6 monthly headache days regardless of impairment, those with ≥4 monthly headache days with “some impairment,” or ≥3 monthly headache days with severe impairment or bed rest (51). It was also proposed that preventive treatment be considered for patients with 4 or 5 monthly headache days and no impairment, 3 monthly headache days and some impairment, or 2 monthly headache days and severe impairment, whereas it is not indicated for patients with <4 monthly headache days and no impairment, or ≤1 monthly headache days regardless of impairment (51). These recommendations were also made within the most recent version of the American Headache Society's consensus statement (52). Data suggests that IIB is an important metric for organizations to add to future guidelines, consensus statements, and recommendations for initiating treatment and evaluating response to treatment. Using MHDs and IIB, as part of overall QoL considerations, to guide preventive treatment decisions, might not only improve the patient's overall QoL, but may help reduce the risk of a patient progressing from EM to CM (33, 53, 54).
Burden of migraine–Migraine attacks
The burden of migraine extends beyond disability-adjusted life years (DALYs) and years lost to disability (YLDs), as defined by the Global Burden of Disease (55). The debilitating symptoms can affect daily functioning not just during a migraine attack, but extend from the prodrome phase through the postdrome phase (56). In a recent cross-sectional, multi-country, online survey of participants who self-reported a migraine diagnosis, 54% reported severe disability related to migraine, and 30% reported that they restricted their activity for 1–2 days during an attack (56).
The headache phase of a migraine attack is but a part of the overall migraine experience, as illustrated in Figure 1. The headache phase can be preceded by up to 72 h by a prodrome phase, also known as premonitory phase, that may consist of some combination of fatigue, difficulty in concentrating, neck stiffness, sensitivity to light and/or sound, nausea, blurred vision, yawning, and pallor, among other phenomena (1). The early onset of these premonitory symptoms before a migraine headache phase suggests that changes in CNS activity precede the onset of the migraine headache phase (57). A substantial proportion of patients can predict a migraine headache from the presence of prodromes with a reasonable degree of accuracy, even hours before the headache phase onset (58). Most of the premonitory symptoms continue, and may intensify, in the headache phase, suggesting that the premonitory phase symptoms may signal an increase in the neurophysiologic changes preceding the pain phase of an attack (58).
The headache phase (4–72 h) may be followed by a postdrome (i.e., postictal) phase that can last up to 24 h (59). This phase has not been well-studied (27, 36). The most commonly cited symptoms of the postdrome phase are sleepiness/weariness or feeling tired, stiff neck, difficulty concentrating, and mild residual head discomfort (27, 36, 60). The postdrome phase is not trivial, as many patients report that they are “somewhat limited,” and a majority (63%) of patients with chronic migraine are “very/extremely limited,” in completing daily activities (36, 56). The interictal phase constitutes the time between the attacks. Allodynia, is an important symptom associated with migraine, yet not mentioned in the International Headache Society International Classification of Headache Disorders, 3rd edition (IHS ICHD-3) classification of migraine (27). This symptom can manifest as discomfort when combing or shaving, or when wearing glasses, contact lenses, earrings, a hat, or even tight clothing, and thus can impact activities of daily living (34). Many studies have addressed allodynia in migraine with respect to the ictal phase (33, 61–64). Whereas, interictal allodynia and hypersensitivity have been documented (16, 17), a concerted effort is needed to better understand the extent to which this is happening and whether it contributes to the burden between attacks. Studies bringing this issue to a better level of evidence are lacking. Here is exactly where the importance of narrative medicine becomes apparent.
Burden of migraine–Interictal period
Patients with migraine have a burden of disease that likely extends into the interictal phases, impacting quality of life even between migraine attacks (65). Moreover, many symptoms that are associated with the ictal phase of migraine can still be detected interictally, although, in general, less frequently and with less intensity than during an active migraine attack. However, this phase of migraine has received scant attention until recently. The IIB in people with migraine impacts overall activity, with lower levels of mobility, as well as a greater level of sleepiness and reduced vigor when compared to those who do not have migraine (65). A cross-sectional study of patients who had migraine without aura found that there was an association between executive disturbances and the duration and intensity of migraine headache as well as evidence of mild executive dysfunction during the interictal phase (40). Emerging literature suggests interictal symptoms may involve both emotional and non-emotional (“neurological”) symptoms (13, 14, 39, 64, 66).
Functional impact
A prospective, longitudinal, Web-based survey of 13,064 respondents with migraine [Chronic Migraine Epidemiology and Outcomes (CaMEO) Study] found that migraine had a significant impact on many important aspects of life that reaches beyond the individual attacks such as marital, parenting, romantic and family relationships, career/financial achievement and stability, and overall health. The reported burden was consistently greater among patients with chronic migraine compared to those with episodic migraine, and there were few sex differences (66). The ObserVational survey of the Epidemiology, tReatment and Care of MigrainE (OVERCOME) study found, in both the US and Japan, headache frequency was associated with increased disability and/or absenteeism, and that regardless of frequency, patients with migraine experienced substantial impacts on productivity and QoL (67).
MIBS-4
Lipton, Buse and colleagues developed the Migraine Interictal Burden Scale (MIBS)-4 to quantify the interictal burden over the past 4 weeks on days without a headache. This self-administered questionnaire consists of 4 items measuring impairment in work or school, impairment in family and social life, difficulty making plans or commitments, and emotional/affective and cognitive distress (39, 68). Each question was scored by the patient to give a total MIBS-4 score (score range 0–12; 0 = none and ≥5 = severe). Moderate correlation validity was observed between MIBS-4 and health-related QoL, lost productivity and psychological disorders, but also ictal disability (39). Though, further development of interictal scales may be desirable, the MIBS-4 has shown usefulness in both real-world evidence (RWE) studies and as an additional tool in pharmaceutical migraine studies. When MIBS-4 was applied in a cross-sectional, observational, population-based web survey (OVERCOME-Japan) of Japanese people with migraine, 41.5% of respondents experienced moderate-to-severe interictal burden that worsened with increasing frequency (67). A cross-sectional survey of 10 European Union countries (Eurolite survey) revealed that patients with migraine suffered from interictal anxiety that increased with headache frequency and intensity, as well as interictal avoidance behavior (69). Patients reported that they felt that they had done less well in their education, careers, or earnings because of migraine. About 10% worried about their next headache and felt that family and friends did not understand their burden. A recent pharmaceutical study reported that treatment with the calcitonin gene-related peptide (CGRP) monoclonal antibody galcanezumab significantly reduced the interictal burden of migraine as measured by MIBS-4 (70, 71). Interestingly, among its sample of more than 60,000 individuals with migraine, OVERCOME (US) found that, in a study that utilized machine learning to determine what factors, among more than 50 sociodemographic, clinical, and migraine-related factors were most associated with seeking care for migraine, that higher IIB was the factor most associated with differentiating those who did/did not seek care for migraine (72). According to the Clinicaltrials.gov website 4 clinical trials for migraine are either ongoing (1) or recently ended (3) that included a consideration of IIB. Thus, the MIBS-4 is a useful scale for the assessment of the interictal burden in migraine that should be more widely used. Moreover, it is the authors' hope that additional, and better, instruments be developed for assessment of the interictal burden in migraine, both in practice and as a research tool.
Non-emotional (“neurological”) symptoms: Physiologic and neuroimaging changes
It has been well-appreciated that patients with migraine can experience hypersensitivity to light, sound, and odors during the interictal phase. In a study that examined discomfort threshold levels to auditory (13, 14) or visual (14) stimuli, patients with migraine were found to have significantly greater sensitivity to light and sound during the interictal phase when compared to healthy control subjects. Studies assessing auditory and visual evoked potentials indicated enhanced interictal activation of the brainstem and visual cortex in patients with migraine (73, 74). Patients with migraine who show interictal photosensitivity were found to have thicker cortical regions (i.e., right lingual, isthmus cingulate and pericalcarine regions, and the left precentral, postcentral and supramarginal regions) (75). In addition to photophobia, patients with migraine may have persistent, continuous visual disturbances, such as “visual snow” (20). A study was conducted where patients with migraine were presented with visual stimuli and subjected to functional magnetic resonance imaging (fMRI) (76). This study found that patients with migraine had enhanced cortical responsiveness to visual cues during the interictal period. Other studies found that patients with migraine may have interictal osmophobia, and that higher olfactory sensitization may be associated with a higher burden of disease (18, 19). Patients with episodic or chronic migraine may also have enhanced levels of cortical excitability during the interictal phase compared to normal control subjects that contributes to sensory hypersensitivities (77, 78), as well as interictal autonomic abnormalities (22).
Patients with migraine also show interictal vestibular symptoms of dizziness and vertigo (23). Participants with migraine underwent fMRI while watching customized forward self-motion roller coaster videos on a screen, and rating their perceptions of dizziness and motion sickness during the interictal phase (23). Changes in activity of brain regions (inferior and superior occipital gyrus, middle frontal gyrus, pontine nuclei, and cerebellar lobules V, VI, and VIIb) correlated with motion sickness and disability scores, suggesting an increased susceptibility to dizziness and motion sickness (23). It has been suggested that there are common mechanisms and neurologic pathways that contribute to symptoms of motion sickness and of migraine (24).
The concept of interictal allodynia has been proposed and examples of interictal allodynia do exist (16), as do reports of enhanced sensitivity to pain (17, 64, 79). However, a high-level evidence is not yet available, and this has not been rigorously studied. If interictal allodynia were to be found to indeed exist, though, it would be a significant contributor to IIB much as ictal allodynia is a contributor to the burden of the migraine attack. This symptom calls for additional attention.
The concept that patients with migraine have a hyperexcitable, or sensitized, cortex brings to mind some features of migraine which are similar to those of epilepsy. Both conditions are episodic, disorders where a susceptible brain is hyperexcitable and may be associated with abnormal neuronal activity (80). It is an incorrect presumption that patients with epilepsy are only affected during seizures and “normal” in between; rather, many individuals with epilepsy are not truly “normal” interictally, even if seizures are controlled. Likewise, what differentiates migraine patients from individuals without migraine is the permanent susceptibility to an attack, independently from the presence of triggers. Thus, like patients with epilepsy, those with migraine are likewise impacted during the interictal phase.
Some studies have shown that patients with episodic migraine (81, 82) or chronic migraine (83, 84) have elevated blood or saliva levels of CGRP interictally. In one study, the elevation in CGRP levels in patients with chronic migraine was not significantly different from levels in control patients, and the elevations in CGRP levels of patients with CM were significantly greater than those of patients with EM (83). Moreover, in one study, the interictal levels of CGRP of patients with chronic migraine are significantly elevated relative to those of patients with episodic migraine, whereas those levels, though elevated relative to control individuals, were not significantly so (83). Moreover, patients with chronic migraine who were responsive to treatment with onabotulinumtoxin A had reduced interictal CGRP blood levels relative to those who were not responsive to the treatment (85, 86).
“Objective” interictal findings
Few studies have assessed potential changes in the neurophysiology during the interictal phase. One study using fMRI in 32 patients with migraine during the interictal phase found altered global sensory processing in the pain-free state, providing a neurophysiological basis for a potentially altered auditory, gustatory, motor and somatosensory processing (87). A proton magnetic resonance spectroscopy (1H-MRS) and fMRI daily in one patient for 21 days identified interictal abnormalities that could suggest an increased susceptibility to excitatory migraine triggers (88).
The IIB of migraine can also include reduced overall activity, lower levels of mobility, as well as a greater level of sleepiness and reduced vigor when compared to the control subjects (65). Patients with migraine with aura showed executive dysfunction in the interictal phase, and an association between executive disturbances and the duration and intensity of migraine headache (40).
Emotional and psychological co-morbidities
While the symptoms of migraine are attracting serious research and treatment attention, there are other aspects that are less obvious, and thus less studied. It is becoming clear that the diagnosis of migraine is stigmatizing, which adds to the emotional burden of the patient, and may inhibit seeking treatment (89). For example, historically, migraine had come to be associated with a “sensitive” or “nervous,” personality (25). Even now, people with migraine are often portrayed in media as being lazy, hypochondriac, hysterical, and unable to deal with stress (25). In a study using the validated stigma scale for chronic illness (SSCI), it was found that chronic migraine was as stigmatizing as epilepsy, whereas episodic was less so, and was most highly correlated with ability to work (41). According to an epidemiologic survey that included 9,999 respondents without migraine, 31% believed that patients with migraine use migraine as a way to get out of work or school, 45% believe that migraine is easily treatable, and 36% believe that migraine is a result of unhealthy behaviors (90). Fear of being stigmatized leads to patients being hesitant to seek diagnosis, or to engage in treatment, and adds to the emotional burden of the patient (25). Patients with migraine struggle with the feeling of having an invisible disorder and of being doubted (91). Many patients feel that their migraine is under-recognized and not well-managed due to it being largely attributed to psychological disease (92). It is worth noting that these stigma, descriptors, and misperceptions of people with migraine do not occur only during the migraine attack. These are descriptions that these individuals carry with them at all times, thus adding to IIB.
Along with the stigma associated with migraine come increased risks of psychiatric comorbidities, especially of anxiety and depression, although the causal relationship between these comorbidities and migraine is unclear (26). In a survey conducted in France of patients with migraine and age-matched control subjects, both men and women with migraine showed significantly elevated scores for stress, anxiety, and depression (26). Depressive disorders appear to be the most common psychiatric comorbidity that occurs with migraine, and patients with migraine have ~2–4 times greater odds of developing a depressive disorder sometime during their lifetime when compared to patients without migraine (93). An analysis of results from the large, prospective Women's Health Study found that, among middle-aged women, migraine and non-migraine headache were both associated with an increased risk of incident depression compared to patients who had no history of headache, and that increased headache frequency was associated with a higher risk for developing incident depression (94). In a study that applied polygenic (genetic risk) score analysis, it was found that migraine and major depressive disorder (MDD) are genetically distinct disorders, but that in the subset of subset of migraine patients with MDD, migraine could be either a symptom or consequence of MDD (95). It has also been suggested that shared neurobiology and neurotransmitters may account in part for the association between depression and migraine (92).
In general, it appears that depressive symptoms may accompany migraine episodes; it is reported that they may be preceded by a sense of anxiety (96). Anxiety affects quality of life of patients with migraine, in part because the interictal period is spent in fear, anticipating the next attack (26). Neurophysiologic data supports the association between emotional factors and migraine. For example, dysregulation of the limbic system by the hypothalamus was found in patients with migraine (97), providing a neurologic substrate for the role of stress in migraine (98). In an imaging study of patients with migraine, performed during the interictal period, negative, but not neutral or positive, emotional cues caused the activation of brain regions associated with emotional processing (99). Moreover, there was overlap between regions involved in nociceptive and emotional processing (i.e., posterior cingulate, caudate, amygdala, and thalamus) (99).
Discussion
The burden suffered by people with migraine encompasses not only the period immediately surrounding the migraine attack but extends throughout the interictal periods. Although symptoms experienced during these phases may be more subtle, they nevertheless impact the quality of life. It is important that patients be encouraged to discuss comfortably their feelings and symptoms even when they may not seem to be directly related to a migraine headache, and for their healthcare providers to actively listen to all patients' concerns.
There is a growing realization that increasing our understanding of IIB as being an important component of migraine deserves considerable attention, as QoL is affected. Moreover, recent studies have now found that not only does the phase between migraine attacks have the potential to have a significant emotional impact on the patient, but it may also include allodynia and hypersensitivity, changes in taste or smell, sensitivity to light or sound, changes in visual perception, and vestibular dysfunctions affecting balance and motion sickness. Collectively, these symptoms contribute to the overall burden experienced by patients with migraine and deserve consideration in research, patient-provider dialogue, and treatment.
The headache burden of migraine is well-documented, and reduction in migraine headache days is a common primary outcome measure that is used in the evaluation of novel preventive therapeutics for migraine. However, as our understanding of the constellation of symptoms associated with migraine throughout the cycle from prodrome through the next prodrome expands, we can appreciate that migraine is much more than the headache phase. Rather, it is a cycling syndrome that exacts a considerable burden on the patient, even independent of the headache itself. Thus, the burden of migraine continues throughout not only the well-examined headache phase, but through the interictal phase as well. The importance of the interictal burden is beginning to receive the attention it requires and is increasingly being included as an outcome measure in clinical trials. There is emerging evidence that suggests that preventive treatments can be beneficial in reducing the interictal burden of migraine, which strengthens the argument for paying more attention to this phase of migraine. However, most studies look only at metrics that pertain to the ictal phases of migraine, notably, numbers of monthly headache days (migraine or otherwise). Consequently, patients who obtain an interictal benefit from treatment may be overlooked. The AHS consensus statement and other publications outline the importance of QoL measures in supporting and continuing treatment of migraine. The IIB is a measurable and important issue that is related to QoL. We propose that all patients should have QoL and IIB assessed in some fashion to make informed treatment decisions and tracking migraine headache days alone does not give an adequate insight into the overall patient journey. Given the importance of the interictal burden for the wellbeing of people with migraine, it is unfortunate that headache diaries fail in capturing this facet of the migraine disease. We hope the greater recognition of IIB as a key component in migraine symptomatology may stimulate further research leading to its incorporation in headache diaries. It is important that patients feel comfortable in expressing their concerns, even when they do not directly relate to the migraine headache. In doing so, they may reveal symptoms that may not be readily apparent in a short interview.
Author contributions
RAN, MHO, MV, LV, and BBV contributed to the conception of the work, interpretation of data for the work, drafting of the manuscript, and critical revision of the manuscript for intellectual content. All authors agree to be accountable for the content of the work.
Funding
This work was supported by Eli Lilly and Company.
Acknowledgments
Eli Lilly and Company contracted Evidera for medical writing and editorial services.
Conflict of interest
Authors BBV, LV, MV, and RAN were employed by Eli Lilly and Company and may own some Lilly stock. Author MHO was employed by Evidera.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Vos T, Abajobir AA, Abate KH, Abbafati C, Abbas KM, Abd-Allah F, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. (2017) 390:1211–59. doi: 10.1016/S0140-6736(17)32154-2
2. Burch R, Rizzoli P, Loder E. The prevalence and impact of migraine and severe headache in the United States: figures and trends from government health studies. Headache. (2018) 58:496–505. doi: 10.1111/head.13281
3. Steiner TJ, Stovner LJ, Jensen R, Uluduz D, Katsarava Z. Migraine remains second among the world's causes of disability, and first among young women: findings from GBD2019. J Headache Pain. (2020) 21:137. doi: 10.1186/s10194-020-01208-0
4. Stovner LJ, Nichols E, Steiner TJ, Abd-Allah F, Abdelalim A, Al-Raddadi RM, et al. Global, regional, and national burden of migraine and tension-type headache, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. (2018) 17:954–76. doi: 10.1016/S1474-4422(18)30322-3
5. Brandes JL. The migraine cycle: patient burden of migraine during and between migraine attacks. Headache. (2008) 48:430–41. doi: 10.1111/j.1526-4610.2007.01004.x
6. Graham JR. The migraine connection. Headache. (1981) 21:243–50. doi: 10.1111/j.1526-4610.1981.hed2106243.x
7. Amery WK, Waelkens J, Vandenbergh V. The sensorium of the migraineur. Ital J Neurol Sci. (1988) 9:539–45. doi: 10.1007/BF02337006
8. Stewart WF, Lipton RB. The economic and social impact of migraine. Eur Neurol. (1994) 34(Suppl. 2):12–7. doi: 10.1159/000119527
9. Dahlöf CG, Dimenäs E. Migraine patients experience poorer subjective well-being/quality of life even between attacks. Cephalalgia. (1995) 15:31–6. doi: 10.1046/j.1468-2982.1995.1501031.x
10. Diener HC, Tassorelli C, Dodick DW, Silberstein SD, Lipton RB, Ashina M, et al. Guidelines of the international headache society for controlled trials of acute treatment of migraine attacks in adults: fourth edition. Cephalalgia. (2019) 39:687–710. doi: 10.1177/0333102419828967
11. Tassorelli C, Diener HC, Dodick DW, Silberstein SD, Lipton RB, Ashina M, et al. Guidelines of the international headache society for controlled trials of preventive treatment of chronic migraine in adults. Cephalalgia. (2018) 38:815–32. doi: 10.1177/0333102418758283
12. McGinley JS, Houts CR, Nishida TK, Buse DC, Lipton RB, Goadsby PJ, et al. Systematic review of outcomes and endpoints in preventive migraine clinical trials. Headache. (2021) 61:253–62. doi: 10.1111/head.14069
13. Ashkenazi A, Mushtaq A, Yang I, Oshinsky ML. Ictal and interictal phonophobia in migraine-a quantitative controlled study. Cephalalgia. (2009) 29:1042–8. doi: 10.1111/j.1468-2982.2008.01834.x
14. Main A, Dowson A, Gross M. Photophobia and phonophobia in migraineurs between attacks. Headache. (1997) 37:492–5. doi: 10.1046/j.1526-4610.1997.3708492.x
15. Chu MK, Im HJ, Chung CS, Oh K. Interictal pattern-induced visual discomfort and ictal photophobia in episodic migraineurs: an association of interictal and ictal photophobia. Headache. (2011) 51:1461–7. doi: 10.1111/j.1526-4610.2011.02010.x
16. Lovati C, D'Amico D, Bertora P, Rosa S, Suardelli M, Mailland E, et al. Acute and interictal allodynia in patients with different headache forms: an Italian pilot study. Headache. (2008) 48:272–7. doi: 10.1111/j.1526-4610.2007.00998.x
17. Toriyama T, Horiuchi T, Hongo K. Characterization of migraineurs presenting interictal widespread pressure hyperalgesia identified using a tender point count: a cross-sectional study. J Headache Pain. (2017) 18:117. doi: 10.1186/s10194-017-0824-0
18. Gossrau G, Frost M, Klimova A, Koch T, Sabatowski R, Mignot C, et al. Interictal osmophobia is associated with longer migraine disease duration. J Headache Pain. (2022) 23:81. doi: 10.1186/s10194-022-01451-7
19. Demarquay G, Royet JP, Giraud P, Chazot G, Valade D, Ryvlin P. Rating of olfactory judgements in migraine patients. Cephalalgia. (2006) 26:1123–30. doi: 10.1111/j.1468-2982.2006.01174.x
20. Schankin CJ, Viana M, Goadsby PJ. Persistent and repetitive visual disturbances in migraine: a review. Headache. (2017) 57:1–16. doi: 10.1111/head.12946
21. Vuralli D, Ayata C, Bolay H. Cognitive dysfunction and migraine. J Headache Pain. (2018) 19:109. doi: 10.1186/s10194-018-0933-4
22. Cambron M, Maertens H, Paemeleire K, Crevits L. Autonomic function in migraine patients: ictal and interictal pupillometry. Headache. (2014) 54:655–62. doi: 10.1111/head.12139
23. Carvalho GF, Mehnert J, Basedau H, Luedtke K, May A. Brain processing of visual self-motion stimuli in patients with migraine. An fMRI study. Neurology. (2021) 97:e996–1006. doi: 10.1212/WNL.0000000000012443
24. Cuomo-Granston A, Drummond PD. Migraine and motion sickness: what is the link? Prog Neurobiol. (2010) 91:300–12. doi: 10.1016/j.pneurobio.2010.04.001
25. Parikh SK, Kempner J, Young WB. Stigma and migraine: developing effective interventions. Curr Pain Headache Rep. (2021) 25:75. doi: 10.1007/s11916-021-00982-z
26. Wacogne C, Lacoste JP, Guillibert E, Hugues FC, Le Jeunne C. Stress, anxiety, depression and migraine. Cephalalgia. (2003) 23:451–5. doi: 10.1046/j.1468-2982.2003.00550.x
27. Headache Classification Committee of the International Headache Society (IHS). The international classification of headache disorders, 3rd edition. Cephalalgia. (2018) 38:1–211. doi: 10.1177/0333102417738202
28. Kelman L. The premonitory symptoms (prodrome): a tertiary care study of 893 migraineurs. Headache. (2004) 44:865–72. doi: 10.1111/j.1526-4610.2004.04168.x
29. Goadsby PJ, Holland PR, Martins-Oliveira M, Hoffmann J, Schankin C, Akerman S. Pathophysiology of migraine: a disorder of sensory processing. Physiol Rev. (2017) 97:553–622. doi: 10.1152/physrev.00034.2015
30. Schwartz D, Dodick DW. Defining the migraine phenotype. In:Borsook D, May A, Goadsby JP, Hargreaves R, , editors. The Migraine Brain: Imaging Structure and Function. New York, NY: Oxford University Press (2012).
31. Schwedt TJ, Peplinski J, Garcia-Filion P, Berisha V. Altered speech with migraine attacks: a prospective, longitudinal study of episodic migraine without aura. Cephalalgia. (2019) 39:722–31. doi: 10.1177/0333102418815505
32. Burstein R, Noseda R, Borsook D. Migraine: multiple processes, complex pathophysiology. J Neurosci. (2015) 35:6619–29. doi: 10.1523/JNEUROSCI.0373-15.2015
33. Burstein R, Collins B, Jakubowski M. Defeating migraine pain with triptans: a race against the development of cutaneous allodynia. Ann Neurol. (2004) 55:19–26. doi: 10.1002/ana.10786
34. Baykan B, Ekizoglu E, Karli N, Kocasoy-Orhan E, Zarifoglu M, Saip S, et al. Characterization of migraineurs having allodynia: results of a large population-based study. Clin J Pain. (2016) 32:631–5. doi: 10.1097/AJP.0000000000000301
35. Lipton RB, Bigal ME, Ashina S, Burstein R, Silberstein S, Reed ML, et al. Cutaneous allodynia in the migraine population. Ann Neurol. (2008) 63:148–58. doi: 10.1002/ana.21211
36. Giffin NJ, Lipton RB, Silberstein SD, Olesen J, Goadsby PJ. The migraine postdrome: an electronic diary study. Neurology. (2016) 87:309–13. doi: 10.1212/WNL.0000000000002789
37. Kelman L. The postdrome of the acute migraine attack. Cephalalgia. (2006) 26:214–20. doi: 10.1111/j.1468-2982.2005.01026.x
38. Ng-Mak DS, Fitzgerald KA, Norquist JM, Banderas BF, Nelsen LM, Evans CJ, et al. Key concepts of migraine postdrome: a qualitative study to develop a post-migraine questionnaire. Headache. (2011) 51:105–17. doi: 10.1111/j.1526-4610.2010.01817.x
39. Buse DC, Rupnow MFT, Lipton RB. Assessing and managing all aspects of migraine: Migraine attacks, migraine-related functional impairment, common comorbidities, and quality of life. Mayo Clinic Proceedings. (2009) 84:422–35. doi: 10.1016/S0025-6196(11)60561-2
40. Camarda C, Monastero R, Pipia C, Recca D, Camarda R. Interictal executive dysfunction in migraineurs without aura: relationship with duration and intensity of attacks. Cephalalgia. (2007) 27:1094–100. doi: 10.1111/j.1468-2982.2007.01394.x
41. Young WB, Park JE, Tian IX, Kempner J. The stigma of migraine. PLoS ONE. (2013) 8:e54074. doi: 10.1371/journal.pone.0054074
42. Beckman HB, Frankel RM. The effect of physician behavior on the collection of data. Ann Intern Med. (1984) 101:692–6. doi: 10.7326/0003-4819-101-5-692
44. Goadsby PJ, Evers S. International classification of headache disorders - ICHD-4 alpha. Cephalalgia. (2020) 40:887–8. doi: 10.1177/0333102420919098
45. Katsarava Z, Manack A, Yoon MS, Obermann M, Becker H, Dommes P, et al. Chronic migraine: classification and comparisons. Cephalalgia. (2011) 31:520–9. doi: 10.1177/0333102410383590
46. Maleki N, Becerra L, Brawn J, Bigal M, Burstein R, Borsook D. Concurrent functional and structural cortical alterations in migraine. Cephalalgia. (2012) 32:607–20. doi: 10.1177/0333102412445622
47. Torres-Ferrús M, Quintana M, Fernandez-Morales J, Alvarez-Sabin J, Pozo-Rosich P. When does chronic migraine strike? A clinical comparison of migraine according to the headache days suffered per month. Cephalalgia. (2017) 37:104–13. doi: 10.1177/0333102416636055
48. Silberstein SD, Stauffer VL, Day KA, Lipsius S, Wilson MC. Galcanezumab in episodic migraine: subgroup analyses of efficacy by high versus low frequency of migraine headaches in phase 3 studies (EVOLVE-1 & EVOLVE-2). J Headache Pain. (2019) 20:75. doi: 10.1186/s10194-019-1024-x
49. Jedynak J, Eross E, Gendolla A, Rettiganti M, Stauffer VL. Shift from high-frequency to low-frequency episodic migraine in patients treated with Galcanezumab: results from two global randomized clinical trials. J Headache Pain. (2021) 22:48. doi: 10.1186/s10194-021-01222-w
50. Silberstein SD. Practice parameter: evidence-based guidelines for migraine headache (an evidence-based review): report of the quality standards subcommittee of the American academy of neurology. Neurology. (2000) 55:754–62. doi: 10.1212/WNL.55.6.754
51. Lipton RB, Bigal ME, Diamond M, Freitag F, Reed ML, Stewart WF, et al. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology. (2007) 68:343–9. doi: 10.1212/01.wnl.0000252808.97649.21
52. Ailani J, Burch RC, Robbins MS. The American headache society consensus statement: update on integrating new migraine treatments into clinical practice. Headache. (2021) 61:1021–39. doi: 10.1111/head.14153
53. Charles JA, Peterlin BL, Rapoport AM, Linder SL, Kabbouche MA, Sheftell FD. Favorable outcome of early treatment of new onset child and adolescent migraine-implications for disease modification. J Headache Pain. (2009) 10:227–33. doi: 10.1007/s10194-009-0133-3
54. Lipton RB, Fanning KM, Serrano D, Reed ML, Cady R, Buse DC. Ineffective acute treatment of episodic migraine is associated with new-onset chronic migraine. Neurology. (2015) 84:688–95. doi: 10.1212/WNL.0000000000001256
55. Murray CJ Lopez AD World Health Organization. The Global Burden of Disease: A Comprehensive Assessment of Mortality and Disability from Diseases, Injuries, and Risk Factors in 1990 and Projected to 2020: Summary. World Health Organization (1996).
56. Gibbs SN, Shah S, Deshpande CG, Bensink ME, Broder MS, Dumas PK, et al. United States patients' perspective of living with migraine: country-specific results from the global “my migraine voice” survey. Headache. (2020) 60:1351–64. doi: 10.1111/head.13829
57. May A, Burstein R. Hypothalamic regulation of headache and migraine. Cephalalgia. (2019) 39:1710–9. doi: 10.1177/0333102419867280
58. Giffin NJ, Ruggiero L, Lipton RB, Silberstein SD, Tvedskov JF, Olesen J, et al. Premonitory symptoms in migraine: an electronic diary study. Neurology. (2003) 60:935–40. doi: 10.1212/01.WNL.0000052998.58526.A9
59. Peng KP, May A. Redefining migraine phases - a suggestion based on clinical, physiological, and functional imaging evidence. Cephalalgia. (2020) 40:866–70. doi: 10.1177/0333102419898868
60. Blau JN. Resolution of migraine attacks: sleep and the recovery phase. J Neurol Neurosurg Psychiatry. (1982) 45:223–6. doi: 10.1136/jnnp.45.3.223
61. Louter MA, Bosker JE, van Oosterhout WP, van Zwet EW, Zitman FG, Ferrari MD, et al. Cutaneous allodynia as a predictor of migraine chronification. Brain. (2013) 136:3489–96. doi: 10.1093/brain/awt251
62. Mínguez-Olaondo A, Quintas S, Morollón Sánchez-Mateos N, López-Bravo A, Vila-Pueyo M, Grozeva V, et al. Cutaneous allodynia in migraine: a narrative review. Front Neurol. (2021) 12:831035. doi: 10.3389/fneur.2021.831035
63. Benatto MT, Florencio LL, Carvalho GF, Dach F, Bigal ME, Chaves TC, et al. Cutaneous allodynia is more frequent in chronic migraine, and its presence and severity seems to be more associated with the duration of the disease. Arq Neuropsiquiatr. (2017) 75:153–9. doi: 10.1590/0004-282x20170015
64. Maleki N, Szabo E, Becerra L, Moulton E, Scrivani SJ, Burstein R, et al. Ictal and interictal brain activation in episodic migraine: neural basis for extent of allodynia. PLoS ONE. (2021) 16:e0244320. doi: 10.1371/journal.pone.0244320
65. Stronks DL, Tulen JH, Bussmann JB, Mulder LJ, Passchier J. Interictal daily functioning in migraine. Cephalalgia. (2004) 24:271–9. doi: 10.1111/j.1468-2982.2004.00661.x
66. Buse DC, Fanning KM, Reed ML, Murray S, Dumas PK, Adams AM, et al. Life with migraine: effects on relationships, career, and finances from the chronic migraine epidemiology and outcomes (CaMEO) study. Headache. (2019) 59:1286–99. doi: 10.1111/head.13613
67. Matsumori Y, Ueda K, Komori M, Zagar AJ, Kim Y, Jaffe DH, et al. Burden of migraine in japan: results of the observational survey of the epidemiology, treatment, and care of migraine (OVERCOME [Japan]) study. Neurol Ther. (2022) 11:205–22. doi: 10.1007/s40120-021-00305-9
68. Buse DC, Bigal M, Rupnow M. Development and validation of the Migraine Interictal Burden Scale (MIBS): a self-administered instrument for measuring the burden of migraine between attacks [abstract S05003]. Neurology. (2007) 68:A89.
69. Lampl C, Thomas H, Stovner LJ, Tassorelli C, Katsarava Z, Lainez JM, et al. Interictal burden attributable to episodic headache: findings from the Eurolight project. J Headache Pain. (2016) 17:9. doi: 10.1186/s10194-016-0599-8
70. Sandoe C, Lipton R, Buse D, Ford J, Hand A, Jedynak J, et al. Interictal burden of migraine: correlations with other measures of migraine burden and effects of galcanezumab migraine-preventive treatment. Cephalalgia. (2020) 96.
71. Lipton RB, Buse DC, Sandoe C, Ford J, Hand A, Jedynak J, et al. Changes in migraine interictal burden following treatment with galcanezumab: results from a phase 3 randomized, placebo-controlled study. Headache. (2022).
72. Reed ML, Johnston E, Nicholson RA, Buse DC, Zagar A, Ashina S, et al. Unmet treatment needs in migraine among those seeking care from neurologists or headache specialists: results of the OVERCOME US study. Neurology. (2022) 98(18 Supp.):3213.
73. Aurora S, Wilkinson F. The brain is hyperexcitable in migraine. Cephalalgia. (2007) 27:1442–53. doi: 10.1111/j.1468-2982.2007.01502.x
74. Boulloche N, Denuelle M, Payoux P, Fabre N, Trotter Y, Geraud G. Photophobia in migraine: an interictal PET study of cortical hyperexcitability and its modulation by pain. J Neurol Neurosurg Psychiatry. (2010) 81:978–84. doi: 10.1136/jnnp.2009.190223
75. Chong CD, Starling AJ, Schwedt TJ. Interictal photosensitivity associates with altered brain structure in patients with episodic migraine. Cephalalgia. (2016) 36:526–33. doi: 10.1177/0333102415606080
76. Vincent M, Pedra E, Mourao-Miranda J, Bramati IE, Henrique AR, Moll J. Enhanced interictal responsiveness of the migraineous visual cortex to incongruent bar stimulation: a functional MRI visual activation study. Cephalalgia. (2003) 23:860–8. doi: 10.1046/j.1468-2982.2003.00609.x
77. Mulleners WM, Chronicle EP, Palmer JE, Koehler PJ, Vredeveld JW. Suppression of perception in migraine. Evidence for reduced inhibition in the visual cortex. Neurology. (2001) 56:178–83. doi: 10.1212/WNL.56.2.178
78. Aurora SK, Barrodale PM, Tipton RL, Khodavirdi A. Brainstem dysfunction in chronic migraine as evidenced by neurophysiological and positron emission tomography studies. Headache. (2007) 47:996–1003. doi: 10.1111/j.1526-4610.2007.00853.x
79. Pan L-LH, Treede R-D, Wang S-J. Mechanical punctate pain thresholds in patients with migraine across different migraine phases: a narrative review. Front Neurol. (2022) 12:801437. doi: 10.3389/fneur.2021.801437
80. Rogawski MA. Migraine and epilepsy—shared mechanisms within the family of episodic disorders. In:Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV, , editors. Jasper's Basic Mechanisms of the Epilepsies. 4th ed Bethesda, MD: National Center for Biotechnology Information (2012).
81. Cady RK, Vause CV, Ho TW, Bigal ME, Durham PL. Elevated saliva calcitonin gene-related peptide levels during acute migraine predict therapeutic response to rizatriptan. Headache. (2009) 49:1258–66. doi: 10.1111/j.1526-4610.2009.01523.x
82. Fusayasu E, Kowa H, Takeshima T, Nakaso K, Nakashima K. Increased plasma substance P and CGRP levels, and high ACE activity in migraineurs during headache-free periods. Pain. (2007) 128:209–14. doi: 10.1016/j.pain.2006.09.017
83. Cernuda-Morollón E, Larrosa D, Ramón C, Vega J, Martínez-Camblor P, Pascual J. Interictal increase of CGRP levels in peripheral blood as a biomarker for chronic migraine. Neurology. (2013) 81:1191–6. doi: 10.1212/WNL.0b013e3182a6cb72
84. Jang MU, Park JW, Kho HS, Chung SC, Chung JW. Plasma and saliva levels of nerve growth factor and neuropeptides in chronic migraine patients. Oral Dis. (2011) 17:187–93. doi: 10.1111/j.1601-0825.2010.01717.x
85. Cernuda-Morollón E, Martínez-Camblor P, Ramón C, Larrosa D, Serrano-Pertierra E, Pascual J, et al. and VIP levels as predictors of efficacy of Onabotulinumtoxin type A in chronic migraine. Headache. (2014) 54:987–95. doi: 10.1111/head.12372
86. Cernuda-Morollón E, Ramón C, Martínez-Camblor P, Serrano-Pertierra E, Larrosa D, Pascual J. OnabotulinumtoxinA decreases interictal CGRP plasma levels in patients with chronic migraine. Pain. (2015) 156:820–4. doi: 10.1097/j.pain.0000000000000119
87. Meylakh N, Henderson LA. Exploring alterations in sensory pathways in migraine. J Headache Pain. (2022) 23:5. doi: 10.1186/s10194-021-01371-y
88. Filippi V, Steiger R, Beliveau V, Frank F, Kaltseis K, Gizewski ER, et al. Investigating the migraine cycle over 21 consecutive days using proton magnetic resonance spectroscopy and resting-state fMRI: a pilot study. Brain Sci. (2022) 12:646. doi: 10.3390/brainsci12050646
89. Young WB. De-stigmatizing migraine - with words. Headache. (2018) 58:319–21. doi: 10.1111/head.13209
90. Shapiro RE, Araujo AB, Nicholson RA, Reed ML, Buse DC, Ashina S, et al. Stigmatizing attitudes about migraine by people without migraine: results of the OVERCOME study. Headache. (2019) 59:14. doi: 10.1111/head.13549
91. Rutberg S, Öhrling K. Migraine–more than a headache: women's experiences of living with migraine. Disabil Rehabil. (2012) 34:329–36. doi: 10.3109/09638288.2011.607211
92. Karsan N, Goadsby PJ. Migraine is more than just headache: is the link to chronic fatigue and mood disorders simply due to shared biological systems? Front Hum Neurosci. (2021) 15:646692. doi: 10.3389/fnhum.2021.646692
93. Amoozegar F. Depression comorbidity in migraine. Int Rev Psychiatry. (2017) 29:504–15. doi: 10.1080/09540261.2017.1326882
94. Rist PM, Schürks M, Buring JE, Kurth T. Migraine, headache, and the risk of depression: prospective cohort study. Cephalalgia. (2013) 33:1017–25. doi: 10.1177/0333102413483930
95. Ligthart L, Hottenga J-J, Lewis CM, Farmer AE, Craig IW, Breen G, et al. Genetic risk score analysis indicates migraine with and without comorbid depression are genetically different disorders. Hum Genet. (2014) 133:173–86. doi: 10.1007/s00439-013-1370-8
96. Jahangir S, Adjepong D, Al-Shami HA, Malik BH. Is there an association between migraine and major depressive disorder? a narrative review. Cureus. (2020) 12:e8551. doi: 10.7759/cureus.8551
97. Stankewitz A, Keidel L, Rehm M, Irving S, Kaczmarz S, Preibisch C, et al. Migraine attacks as a result of hypothalamic loss of control. Neuroimage Clin. (2021) 32:102784. doi: 10.1016/j.nicl.2021.102784
98. van Staveren I. Migraine and stress-an exploratory cross-country study of external stress factors. BMC Res Notes. (2021) 14:174. doi: 10.1186/s13104-021-05587-8
Keywords: interictal burden, MIBS-4, migraine, quality of life, disability, co-morbidities, headache, pain
Citation: Vincent M, Viktrup L, Nicholson RA, Ossipov MH and Vargas BB (2022) The not so hidden impact of interictal burden in migraine: A narrative review. Front. Neurol. 13:1032103. doi: 10.3389/fneur.2022.1032103
Received: 30 August 2022; Accepted: 20 October 2022;
Published: 03 November 2022.
Edited by:
Milena De Felice, The University of Sheffield, United KingdomReviewed by:
Paola Torelli, University of Parma, ItalyAmanda Tinsley, Georgetown University, United States
Copyright © 2022 Vincent, Viktrup, Nicholson, Ossipov and Vargas. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maurice Vincent, dmluY2VudF9tYXVyaWNlQGxpbGx5LmNvbQ==
 Maurice Vincent
Maurice Vincent Lars Viktrup1
Lars Viktrup1 Michael H. Ossipov
Michael H. Ossipov