- 1Department of Internal Medicine, Catholic University of Health and Allied Sciences, Mwanza, Tanzania
- 2Department of Internal Medicine, Bugando Medical Centre, Mwanza, Tanzania
- 3Department of Neurology, Harvard Medical School, Massachusetts General Hospital, Boston, MA, United States
- 4Department of Internal Medicine, Muhimbili University of Health and Allied Sciences, Dar es Salaam, Tanzania
- 5Department of Cardiology, Jakaya Kikwete Cardiac Institute, Dar es Salaam, Tanzania
- 6Department of Neurology, Texas Tech University Health Sciences Center, Paul L. Foster School of Medicine, El Paso, TX, United States
- 7Stroke Medicine Department, Counties Manukau Health, Auckland, New Zealand
Background: Stroke is the second leading cause of death worldwide, with the highest mortality rates in low- to middle-income countries, particularly in sub-Saharan Africa. We aimed to investigate the predictors of 30-day mortality among patients with stroke admitted at a tertiary teaching hospital in Northwestern Tanzania.
Methods: This cohort study recruited patients with the World Health Organization's clinical definition of stroke. Data were collected on baseline characteristics, the degree of neurological impairment at admission (measured using the National Institutes of Health Stroke Scale), imaging and electrocardiogram (ECG) findings, and post-stroke complications. The modified Rankin scale (mRS) was used to assess stroke outcomes. Kaplan–Meier analysis was used to describe survival, and the Cox proportional hazards model was used to examine predictors of mortality.
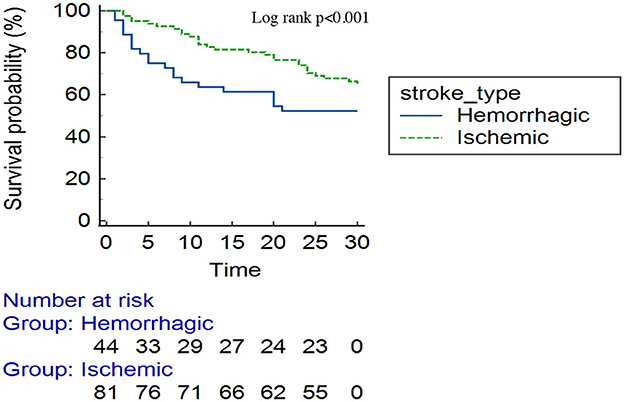
Results: A total of 135 patients were enrolled, with a mean age of 64.5 years. Hypertension was observed in 76%, and 20% were on regular anti-hypertensive medications. The overall 30-day mortality rate was 37%. Comparing patients with hemorrhagic and ischemic stroke, 25% had died by day 5 [25th percentile survival time (in days): 5 (95% CI: 2–14)] versus day 23 [25th percentile survival time (in days): 23 (95% CI: 11–30) (log-rank p < 0.001)], respectively. Aspiration pneumonia was the most common medical complication, occurring in 41.3% of patients. ECG abnormalities were observed in 54.6 and 46.9% of patients with hemorrhagic and ischemic stroke, respectively. The most common patterns were as follows: ST changes 29.6 vs. 30.9%, T-wave inversion 34.1 vs. 38.3%, and U-waves 18.2 vs. 1.2% in hemorrhagic and ischemic stroke, respectively. Independent predictors for case mortality were as follows: mRS score at presentation (4–5) [aHR 5.50 (95% CI: 2.02–15.04)], aspiration pneumonia [aHR 3.69 (95% CI: 1.71–13.69)], ECG abnormalities [aHR 2.28 (95% CI: 1.86–5.86)], and baseline stroke severity [aHR 1.09 (95% CI: 1.02–1.17)].
Conclusion: Stroke is associated with a high 30-day mortality rate in Northwestern Tanzania. Concerted efforts are warranted in managing patients with stroke, with particular attention to individuals with severe strokes, ECG abnormalities, and swallowing difficulties to reduce early morbidity and mortality.
Introduction
Stroke is the second leading cause of death and the third leading cause of death and disability-adjusted life years (DALYs) combined worldwide, according to the 2019 Global Burden of Disease report (1). In contrast to high-income countries (HIC), where there was a decrease in age-standardized stroke incidence, DALY, and mortality, low-middle-income countries (LMIC) had the highest age-standardized stroke-related mortality of more than 3.6 times (86%) with 87% DALYs (1). This decline in stroke morbidity and mortality in HIC reflects advancements in stroke management, leading to more favorable outcomes.
Countries in sub-Saharan Africa (SSA) have a high stroke burden and mortality (1): Stroke in Africa occurs at a younger age, which has significant socioeconomic implications (2–5). Previous hospital-based studies in Tanzania have reported varied 30-day case fatality rates, ranging from 30 to 60%, with a limited characterization of predictors of mortality (5, 6). In SSA, stroke severity, infections, elevated glucose levels, and fever are known predictors of 30-day mortality (5–7). There is an urgent need to find solutions to mitigate the rising number of strokes and the associated mortality in Tanzania. We aimed to investigate the predictors of 30-day mortality among patients with stroke admitted at a large tertiary teaching hospital in Tanzania.
Materials and methods
Study design and population
This cohort study was conducted at a tertiary teaching hospital, Bugando Medical Center (BMC), in Northwestern Tanzania. BMC offers specialized care to all in and outpatients from all over the country, particularly along the shores of Lake Victoria. Patients with stroke who met the World Health Organization (WHO) clinical definition were consecutively recruited between February 2022 and May 2022 after obtaining written informed consent from the patient, or next of kin (for those unable to consent due to stroke-related disabilities) (8).
Data collection
Data were collected and managed using an electronic data-capturing database developed and hosted by VervigR. Information captured included baseline data, including gender, age, residency, marital status, level of educational achievement, and at least three available mobile numbers from the patient and next of kin. We also recorded any previous history or prescriptions for hypertension and diabetes mellitus and inquired about smoking and alcohol consumption.
Clinical measurement
Physical examination included assessment of the Glasgow coma scale score, temperature, pulse rate, and rhythm. Blood pressure readings were taken using a standard digital sphygmomanometer (Omron 10, Healthcare); three readings were taken 5-min apart. Hypertension was defined as systolic blood pressure (SBP) of ≥140 mmHg and/or diastolic blood pressure (DBP) of ≥90 mmHg or previous/current use of anti-hypertensive medications (9). Waist and hip circumference were measured using a tape measure and recorded in centimeters. The waist-hip ratio was computed and interpreted according to the WHO guidelines (10).
Laboratory investigations
We aseptically collected 15 ml of venous blood for complete blood count (Sysmex 1000 machine) and random total cholesterol (BIO-SYSTEMS machine). Capillary fingertip blood samples were collected to check for random blood glucose (RBG) levels and HIV rapid testing using a glucometer GLUCOPLUSTM and SD Bioline, respectively. A fasting blood glucose (FBG) sample was collected the following morning for patients with RBG levels of ≥11.1 mmol/l. Diabetes mellitus diagnosis was defined as an RBG reading of ≥11.1 mmol/l or an FBG reading of ≥7 mmol/l. For patients who were HIV reactive to SD Bioline, confirmatory testing was performed using Unigold Biotech.
Medical complications
This included clinical seizures, infections: urinary tract infection and aspiration pneumonia (confirmed aspiration pneumonia or probable) (11), and the presence of bedsores (12, 13). Other complications included new onset fever lasting more than 24 h from an unknown source (13). Presumed venous thromboembolism was clinically diagnosed using Well's score (14). Miscellaneous complications were defined as any documented complication resulting in a specific medical or surgical intervention (e.g., gastrointestinal hemorrhage, constipation, episodes of cardiac failure, cardiac arrhythmias, and arthritis) (13).
Brain imaging
A non-contrasted brain computed tomography scan, acquired on a 128-slice CT Scanner (Siemens Somatom Perspective, Siemens Healthcare GmbH, Germany), was performed on all patients on admission, and images were interpreted by a neuro-radiologist. For ischemic stroke, the Alberta Stroke Program Early CT (ASPECTS) score was dichotomized to <7 and ≥7 and used for analysis (15). Hemorrhagic transformation was defined per European Cooperative Acute Stroke Study (ECASS II) (16). Midline shift was defined as any measurable shift of midline cerebral structures seen on axial view, specifically the septum pellucidum and/or the pineal gland (17). For those with hemorrhagic stroke, the location was documented, and the hematoma volume was measured using the modified ABC/2 method (18). We calculated the intracerebral hemorrhage (ICH) score with scores ranging from 0 to 5 (19).
Cardiovascular assessment
A 12-lead electrocardiography (ECG) (model ECG-3312B) was performed on all patients, and results were interpreted by a cardiologist for the presence of ST-segment depression or elevation, T-wave abnormalities, U-waves, and the presence of atrial fibrillation.
Stroke outcomes
Stroke severity was assessed using the National Institutes of Health Stroke Scale (NIHSS) on admission (20). Stroke outcomes were assessed using a modified Rankin scale (mRS) with scores ranging from 0 (no symptoms) to 6 (death) on admission up to 30 days (20). The date of death was recorded, and the time-to-event was computed using the date difference between the date of last contact (date of death or date of the last follow-up) and the date of symptom onset.
Study variables
The dependent variable was case mortality, and the independent variables included were as follows: demographic data, medical co-morbidities and complications, stroke severity, stroke subtype, and ECG changes.
Data analysis
Data analysis was done using STATA software version 15.0. Continuous variables were summarized and presented as means and standard deviation (SD) or medians and interquartile range [IQR]. Categorical variables were summarized as frequencies and proportions. Kaplan–Meier analysis was used to describe survival, where the 25th percentile survival time with respective 95% confidence intervals was estimated, and significant differences in survival times by stroke subtype were tested using the log-rank test. Crude and adjusted analyses were done using a Cox proportional hazards model. Hazard ratios (HRs), 95% confidence intervals (CIs), and corresponding p-values were obtained from the models adjusting for potential confounders. Factors for multivariable analyses were selected based on prior knowledge of the possible associations between stroke and mortality. These included age, sex, and previous history of stroke. Other variables with a p-value of <0.20 in the univariable model were included in the multivariable analysis, and a significance level was set as a p-value of <0.05.
Results
The proportion of strokes compared to the total medical admissions
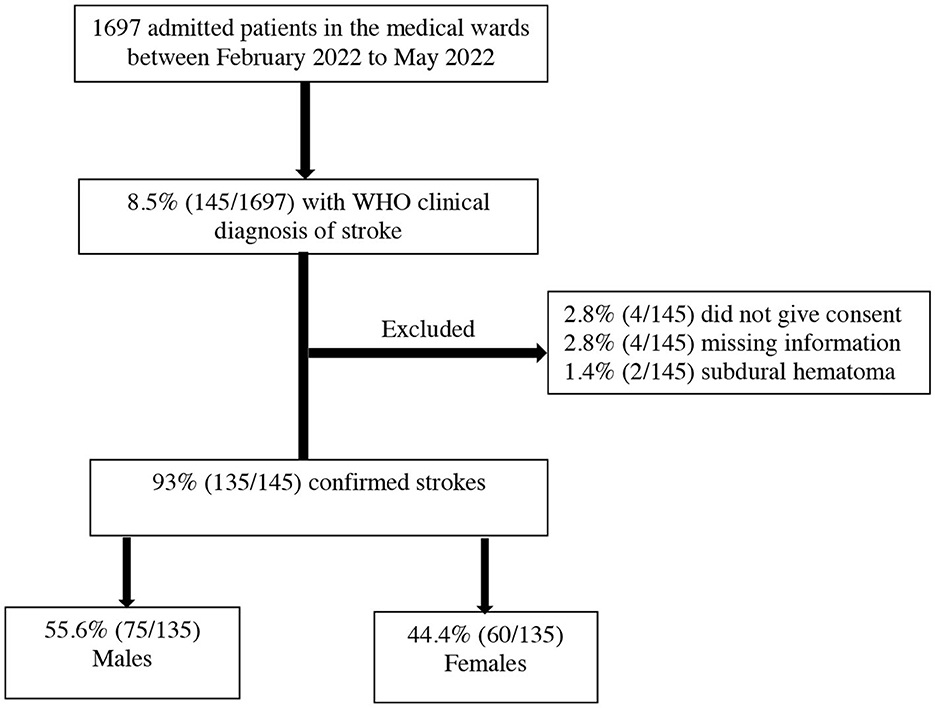
During the study period from February 2022 to May 2022, there were 1,697 medical admissions. Out of these admissions, 8.5% (145/1697) met the WHO's clinical definition of stroke. We excluded 6.9% (10/145) patients for the following reasons: 2.8% (4/145) did not give consent to participate in the study, 2.8% (4/145) had missing information, and 1.38% (2/145) had subdural hematoma as summarized in Figure 1. The final proportion of patients with stroke compared to the total medical admissions was 7.9% (135/1697) (95% CI 6.7–9.4%).
Demographic characteristics of the study patients
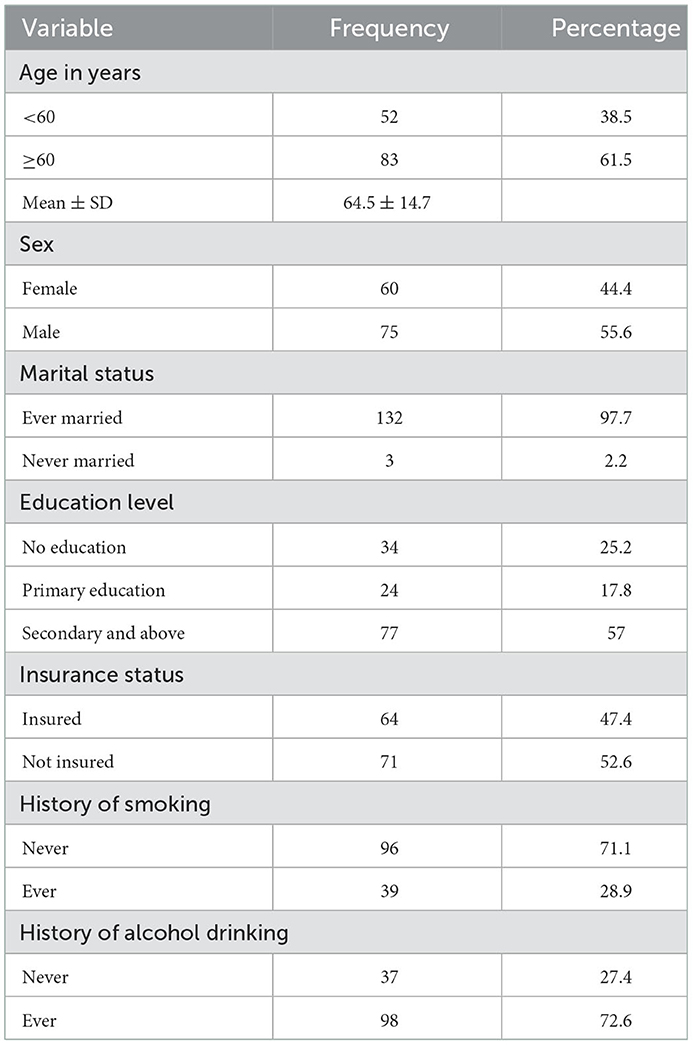
The mean age was 64.5 ± 14.7 years, and the majority were male patients, 55.6% (75/135). Notably, 57% (77/135) had secondary education or higher and 52.6% (71/135) were uninsured. The proportion of patients with a history of smoking or alcohol consumption was 28.9% (39/135) and 72.6% (98/135), respectively, as summarized in Table 1. The mean time from stroke symptom onset to hospital arrival was 2.67 ± 4.9 days.
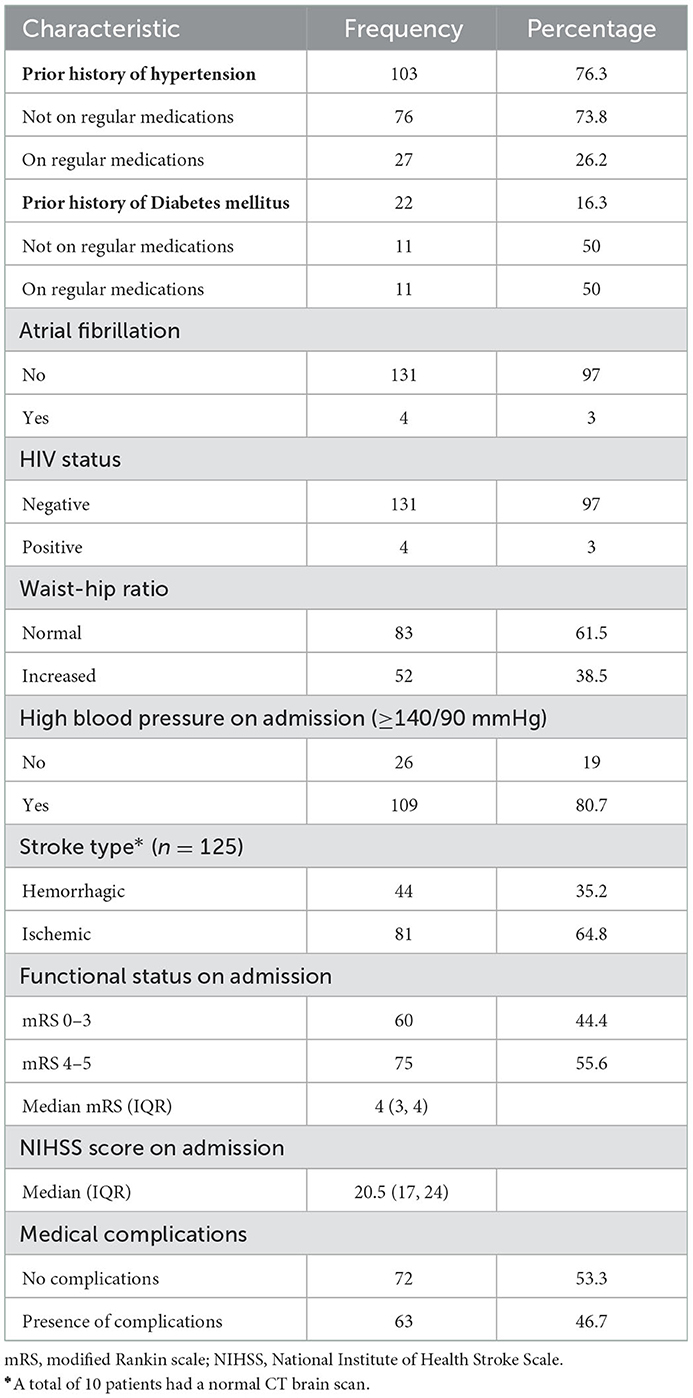
A prior history of hypertension and diabetes mellitus was present in 76.3% (103/135) and 16.3% (22/135) of subjects, respectively. One-quarter of hypertensive (27/103) and one-half of patients with diabetes (11/22) were taking relevant regular medication. The mean time from stroke onset to brain imaging was 3.43 ± 3.8 days, and ischemic stroke occurred in two-thirds (81/135) of the patients. The median mRS and NIHSS scores on admission were 4 (IQR 3, 4) and 20.5 (17, 21), respectively. At presentation, 80.7% (109/135) were hypertensive and 3% (4/135) were HIV positive, as summarized in Table 2.
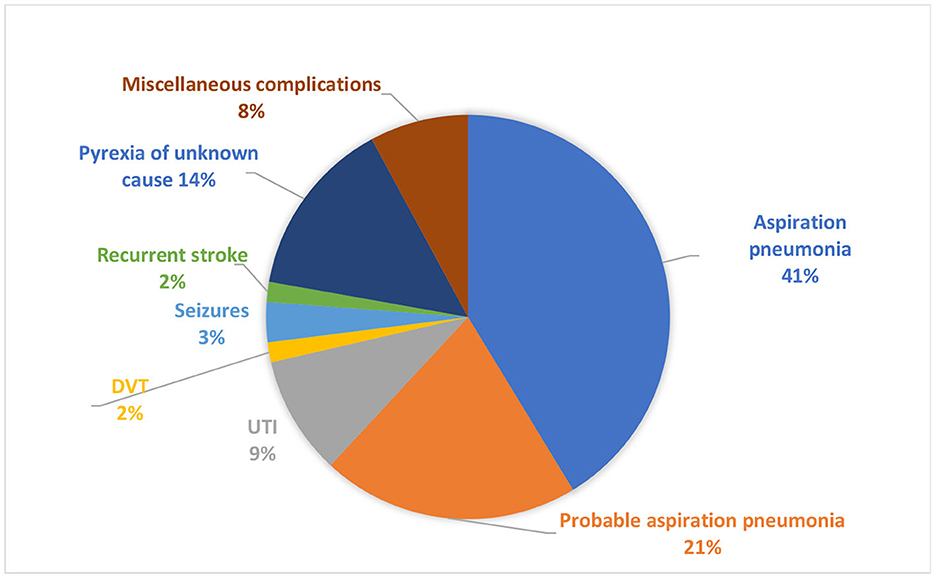
Post-stroke medical complications occurred in 46.7% (63/135) of patients. Confirmed aspiration pneumonia was the leading medical complication, seen in 41% (26/63), followed by probable aspiration pneumonia in 21% (13/63) and pyrexia of unknown cause in 14% (9/63) (Figure 2).
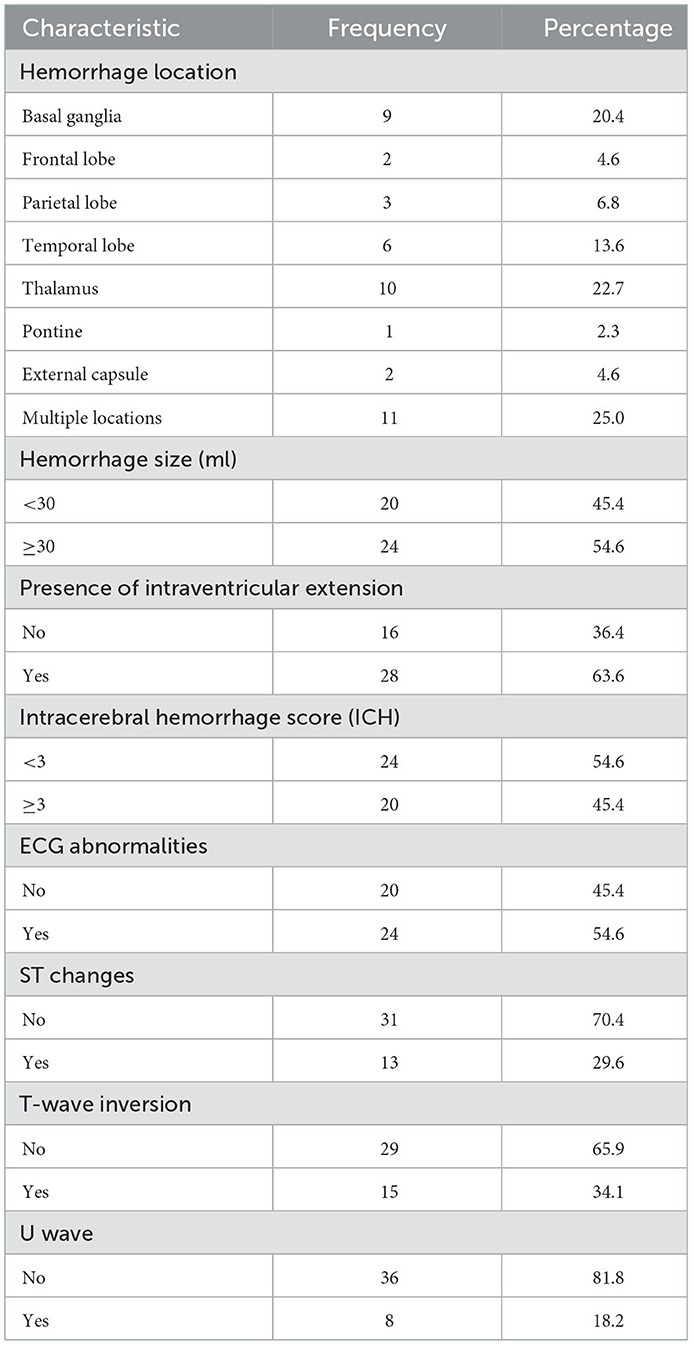
Neuroimaging and ECG abnormalities among patients with hemorrhagic stroke
Neuroimaging and ECG features of the study patients with hemorrhagic stroke are presented in Table 3. A quarter (11/44) had a hemorrhage in multiple locations (both the cortical and subcortical regions), followed by the thalamus in 22.7% (10/44). In more than half (24/44), the volume of hematoma exceeded 30 ml and 63.6% (28/44) had an intraventricular extension. ECG abnormalities were present in 54.6% (24/44); the most common patterns were T-wave inversion in 34.1% (15/44), followed by ST segment changes in 29.6% (13/44).
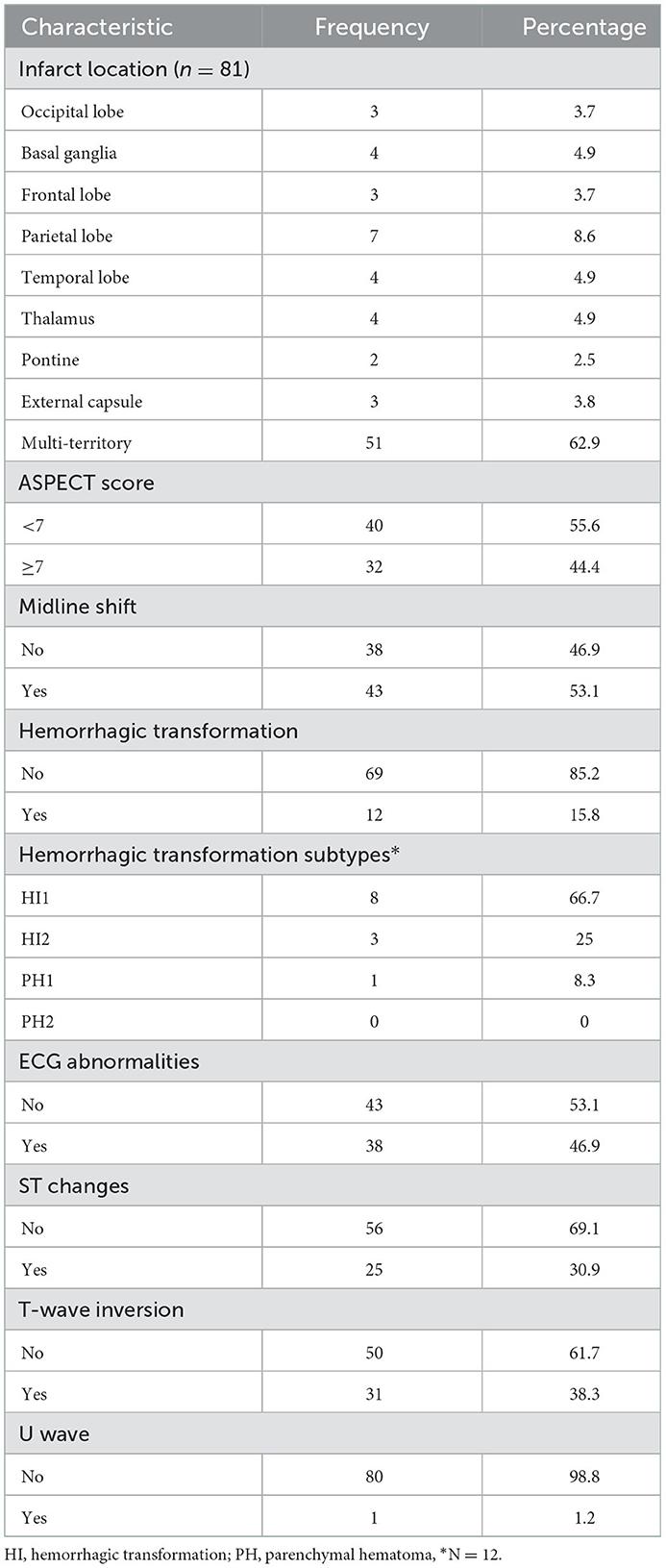
Neuroimaging and ECG abnormalities among patients with ischemic stroke
The majority were observed to have multi-territory infarction; 62.9% (51/81) and 53.1% (43/81) had midline shifts. ECG abnormalities were present in 46.9% (38/81); the most common patterns included T-wave inversion in 38.3% (31/81), followed by ST changes in 30.9% (25/81) (Table 4).
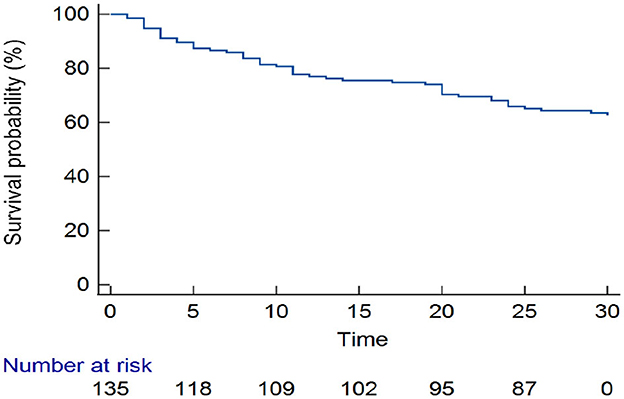
Survival experience and mortality by stroke subtype
Approximately one-third (50/135) of the patients with stroke had died by 30-day follow-up. Overall, one-quarter of the patients had died by day 17 [25th percentile survival time (in days): 17 (95% CI: 9–24)] (Figure 3). Comparing patients with hemorrhagic and ischemic stroke, 25% of the patients had died by day 5 [25th percentile survival time (in days): 5 (95% CI: 2–14)] compared with day 23 [25th percentile survival time (in days): 23 (95% CI: 11–30)], respectively (log-rank p < 0.001) (Figure 4).
Predictors of 30-day mortality
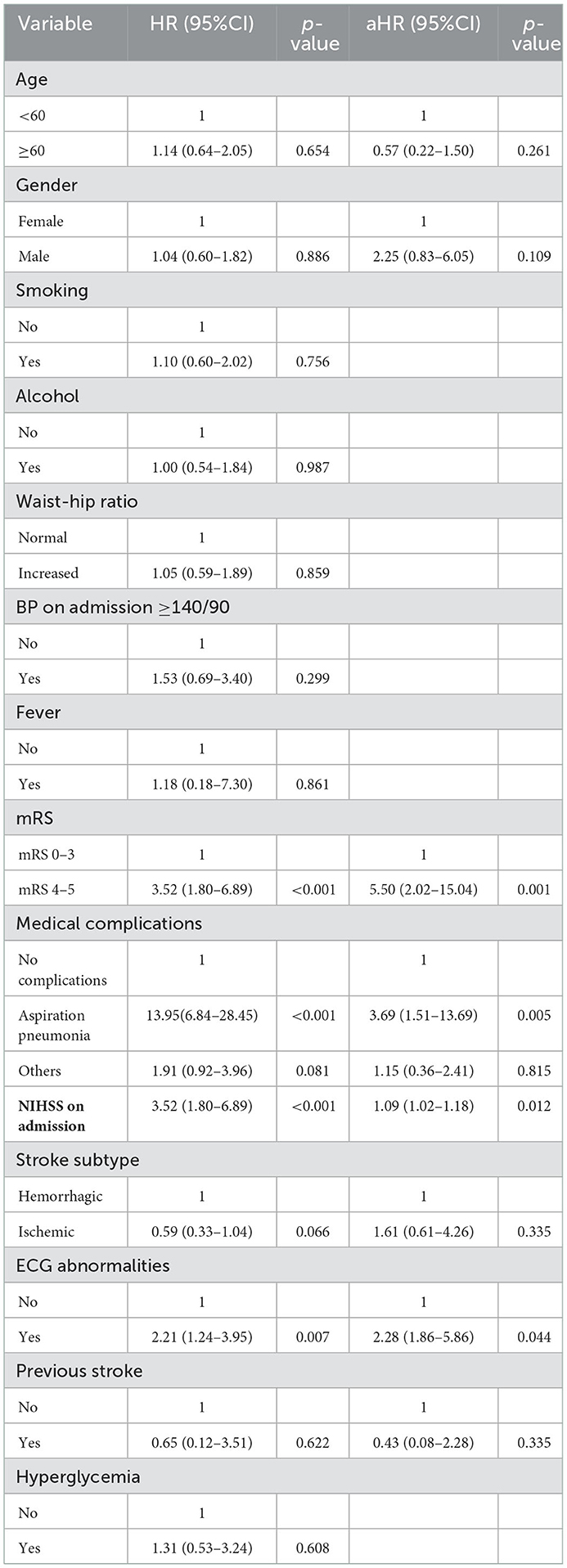
In the multivariable-adjusted analysis, independent predictors of 30-day mortality were NIHSS score on admission (30-day mortality increased by 9% for every unit increase in NIHSS score) [aHR 1.09 (95% CI: 1.02–1.17), p = 0.012], degree of disability on admission (mRS 4–5) [aHR 5.50 (95% CI: 2.02–15.04), p = 0.001], aspiration pneumonia [aHR 3.69 (95% CI: 1.51–13.6), p = 0.005], and ECG abnormalities [aHR 2.28 (95% CI: 1.86–5.86), p = 0.044] (Table 5).
Discussion
In this study, we found that the 30-day post-stroke mortality rate was 37%. This mortality is high compared to mortality rates observed in HIC; for instance, the rate is 12.7% in patients admitted to Canadian stroke services (22). Furthermore, our mortality rate is higher than other comparable studies conducted in LMIC, for example, Nigeria, where the 30-day mortality rate was 23.8% (23). The differences in mortality could be attributed to the nature of the study design: The Nigerian study was a retrospective study of first-ever strokes, whereas this study was prospective and included all strokes (first and recurrent). Our findings are quite alarming and highlight the barriers associated with the management of acute stroke in a resource-limited setting. It has been well demonstrated that the presence of specialized stroke units, specialists, the use of intravenous thrombolytic agents, and endovascular procedures are highly effective at reducing morbidity and mortality from stroke (24). However, these services and resources are neither readily available nor affordable in SSA. In the current study, half of the patients had no health insurance coverage, which is a major barrier to accessing affordable, good-quality healthcare services for patients with stroke in Tanzania. A recent systematic review on health financing for universal health coverage in SSA reported that 27 out of 48 countries are affected by direct out-of-pocket payments for healthcare services of >30%. Therefore, the cost is likely to be a significant barrier to accessing healthcare, thus contributing to the high burden of preventable deaths (21).
In the present study, patients with hemorrhagic stroke had higher mortality than those with ischemic stroke, with one-quarter of patients dying by day 5 vs. 23. This is similar to global data, where the hemorrhagic stroke is associated with poor outcomes compared to ischemic stroke (25). Hypertensive hemorrhagic stroke results from the rupture of the thinner-walled perforating arteries, which supply the sub-cortical regions of the brain (26). Untreated, ongoing hypertension increases the risk of further bleeding, hematoma expansion, and intraventricular extension. In the present study, more than half (56.4%) had a hematoma size of ≥30 ml, and intraventricular extension was observed in 63.6% of patients. Similarly, elevated blood pressure readings were observed in more than two-thirds of patients (80.7%). Uncontrolled hypertension appears to be a major etiology for stroke, particularly the hemorrhagic stroke subtype in the current study, as almost one-half of the bleeds were located in the sub-cortical regions (particularly the basal ganglia and the thalamus), signifying a hypertensive etiology. Hypertension is a leading risk factor for stroke in Tanzania, and its early detection, treatment, and control cannot be overemphasized (5). This is a call for increasing community awareness for screening and treating hypertension in SSA. Furthermore, our findings also highlight the need for multidisciplinary stroke management on dedicated stroke units, with input from neurosurgeons for consideration of hematoma evacuation, decompressive surgery, or placement of an external ventricular drain.
Our study found that severe stroke, severe neurological impairment, aspiration pneumonia, and ECG abnormalities were independent predictors for 30-day mortality. High NIHSS and mRS scores are known predictors for 30-day mortality (27, 28). Notably, patients with stroke in the current study presented late to the hospital from the time of stroke onset (mean time of 2.67 days). This delay could be a major contributor to high admission NIHSS scores, stemming from a lack of community awareness of early recognition of stroke symptoms and signs and challenges in the overall healthcare infrastructure in Tanzania. Similarly, the relatively high admission NIHSS may also indicate that patients with milder strokes are not routinely accessing healthcare. Patients with severe neurological deficits have a high risk of medical complications such as aspiration pneumonia. This is in line with other studies and is a leading cause of early mortality in SSA (11). This is a call to advocate for specialized stroke units in Tanzania to help manage patients with stroke to prevent or reduce stroke-related complications.
Overall, ECG abnormalities were observed in more than one-third (46%) of the patients. The most common patterns in both stroke subtypes were as follows: ST changes (46.9 vs. 29.6%) and T-wave inversion (38.3 vs. 34.1%) for ischemic and hemorrhagic stroke, respectively, in line with previous studies (29). ECG abnormalities are a result of bidirectional interaction between the brain and the heart. This is thought to be caused by the over-activation of sympathetic activity and the hypothalamic–pituitary–adrenal axis, as well as immune and inflammatory responses resulting in brain dysregulation following an acute stroke. Similarly, the release of catecholamines causes vasoconstriction of peripheral and coronary vessels, which leads to myocardial ischemia (29). Therefore, there is a need for continuous electrocardiographic monitoring among patients with stroke to reduce early mortality.
Our study is limited by the following: It was a single center with a small sample size. Therefore, the results may not be generalizable. Most patients did not have a baseline ECG, so the changes observed might be attributed to other medical conditions. There may be other unmeasured or unknown confounders responsible for the observed data, including interdisciplinary and nursing input and timing of antithrombotic or anti-hypertensive therapy. We did not collect data on the final causes of death, which limits our ability to draw further inferences. Continuous ECG (telemetry) was not performed due to limited resources.
Conclusion
In this present study, stroke is associated with a high 30-day mortality rate in Northwestern Tanzania. The hemorrhagic stroke subtype had the highest mortality, and independent predictors of death included higher NIHSS on admission with severe disabilities, aspiration pneumonia, and ECG abnormalities. Concerted efforts are warranted in the prevention and management of patients with stroke to reduce the associated morbidity and mortality.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the joint Catholic University of Health and Allied Sciences-Bugando Medical Center Institutional Review Board approval number CREC/528/2022. The study was carried out in accordance with the tenets of the Declaration of Helsinki. The patients/participants provided their written informed consent to participate in this study.
Author contributions
Conceptualization of the study: SSM, KM, FK, and GM. Data collection: GM, JS, LA, and JN. Interpretation of the results: GM, SSM, and PN. Data analysis: GM and SSM. Writing the initial manuscript: SSM. Critically reviewing and revising the manuscript: RA, FK, PN, BT, KK, RM, MM, FS, and KM. All authors read and approved the final manuscript.
Funding
We acknowledge support from the Catholic University of Health and Allied Sciences. The funder has no role in the design, analysis, final write-up of the manuscript, and decision to submit the paper for publication.
Acknowledgments
Our sincere gratitude goes to Dr. Henrik Juhl for his support in using the online data collection software.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
mRS, modified Rankin scale; NIHSS, National Institute of Health Stroke Scale; SSA, sub-Saharan Africa; WHO, World Health Organization.
References
1. Feigin VL, Stark BA, Johnson CO, Roth GA, Bisignano C, Abady GG, et al. Global, regional, and national burden of stroke and its risk factors, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. (2021) 20:795–820. doi: 10.1016/S1474-4422(21)00252-0
2. Urimubenshi G, Cadilhac DA, Kagwiza JN, Wu O, Langhorne P. Stroke care in Africa: a systematic review of the literature. Int J Stroke. (2018) 13:797–805. doi: 10.1177/1747493018772747
3. Adoukonou T, Kossi O, Fotso Mefo P, Agbétou M, Magne J, Gbaguidi G, et al. Stroke case fatality in sub-Saharan Africa: Systematic review and meta-analysis. Int J Stroke. (2021) 16:902–16. doi: 10.1177/1747493021990945
4. Owolabi M, Olowoyo P, Popoola F, Lackland D, Jenkins C, Arulogun O, et al. The epidemiology of stroke in Africa: a systematic review of existing methods and new approaches. J Clin Hypertens. (2018) 20:47–55. doi: 10.1111/jch.13152
5. Matuja S, Munseri P, Khanbhai K. The burden and outcomes of stroke in young adults at a tertiary hospital in Tanzania: a comparison with older adults. BMC Neurol. (2020) 20:206. doi: 10.1186/s12883-020-01793-2
6. Okeng'o K, Chillo P, Gray WK, Walker R, Matuja W. Early mortality and associated factors among patients with stroke admitted to a large teaching hospital in Tanzania. J Stroke Cerebrovasc Dis. (2017) 26:871–8. doi: 10.1016/j.jstrokecerebrovasdis.2016.10.037
7. Nakibuuka J, Sajatovic M, Nankabirwa J, Ssendikadiwa C, Furlan AJ, Katabira E, et al. Early mortality and functional outcome after acute stroke in Uganda: prospective study with 30 day follow-up. Springerplus. (2015) 4:450. doi: 10.1186/s40064-015-1252-8
8. WHO Noncommunicable Diseases and Mental Health. The WHO STEPwise Approach to Stroke Surveillance Report. (2005). Available online at: https://apps.who.int/iris/handle/10665/43420 (accessed November 26, 2022).
9. Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. (2003) 42:1206–52. doi: 10.1161/01.HYP.0000107251.49515.c2
10. World Health Organization. Waist Circumference and Waist-Hip Ratio: Report of a WHO Expert Consultation. World Health Organization (2011).
11. Fisher RS, Cross JH, French JA, Higurashi N, Hirsch E, Jansen FE, et al. Operational classification of seizure types by the International League Against Epilepsy: Position Paper of the ILAE commission for classification and terminology. Epilepsia. (2017) 58:522–30. doi: 10.1111/epi.13670
12. Kumar S, Selim MH, Caplan LR. Medical complications after stroke. Lancet Neurol. (2010) 9:105–18. doi: 10.1016/S1474-4422(09)70266-2
13. Prust ML, Nutakki A, Habanyama G, Chishimba L, Chomba M, Mataa M, et al. Aspiration Pneumonia in Adults Hospitalized With Stroke at a Large Academic Hospital in Zambia. Neurol Clin Pract. (2021) 11:e840–e847. doi: 10.1212/CPJ.0000000000001111
14. Modi S, Deisler R, Gozel K, Reicks P, Irwin E, Brunsvold M, et al. Wells criteria for DVT is a reliable clinical tool to assess the risk of deep venous thrombosis in trauma patients. World J Emerg Surg. (2016) 11:1–6. doi: 10.1186/s13017-016-0078-1
15. Esmael A, Elsherief M, Eltoukhy K. Predictive value of the alberta stroke program early CT score (ASPECTS) in the outcome of the acute ischemic stroke and its correlation with stroke subtypes, NIHSS, and cognitive impairment. Stroke Res Treat. (2021) 2021:1–10. doi: 10.1155/2021/5935170
16. G B. European Cooperative Acute Stroke Study (ECASS): (rt-PA-Thrombolysis in acute stroke) study design and progress report. Eur J Neurol. (1995) 1:213–9. doi: 10.1111/j.1468-1331.1995.tb00074.x
17. Ropper AH. Lateral displacement of the brain and level of consciousness in patients with an acute hemispheral mass. N Engl J Med. (2009) 314:953–8. doi: 10.1056/NEJM198604103141504
19. Hemphill JC, Bonovich DC, Besmertis L, Manley GT, Johnston SC. The ICH score: a simple, reliable grading scale for intracerebral hemorrhage. Stroke. (2001) 32:891–6. doi: 10.1161/01.STR.32.4.891
20. Farooq MU, Chaudhry AH, Amin K, Majid A. The WHO STEPwise approach to stroke surveillance. J Coll Phys Surg Pak. (2008) 18:665.
21. Ifeagwu SC, Yang JC, Parkes-Ratanshi R, Brayne C. Health financing for universal health coverage in Sub-Saharan Africa: a systematic review. Glob Health Res Policy. (2021) 6:1–9. doi: 10.1186/s41256-021-00190-7
22. Ganesh A, Lindsay P, Fang J, Kapral MK, Côté R, Joiner I, et al. Integrated systems of stroke care and reduction in 30-day mortality. Neurology. (2016) 86:898–904. doi: 10.1212/WNL.0000000000002443
23. Desalu OO, Wahab KW, Fawale B, Olarenwaju TO, Busari OA, Adekoya AO, et al. A review of stroke admissions at a tertiary hospital in rural Southwestern Nigeria. Ann Afr Med. (2011) 10:80–5. doi: 10.4103/1596-3519.82061
24. Wardlaw JM, Murray V, Berge E, del Zoppo GJ. Thrombolysis for acute ischaemic stroke. Cochrane Database Syst Rev. (2014) 29:CD000213. doi: 10.1002/14651858.CD000213.pub3
25. Krishnamurthi RV, Feigin VL, Forouzanfar MH, Mensah GA, Connor M, Bennett DA, et al. Global and regional burden of first-ever ischaemic and haemorrhagic stroke during 1990-2010: findings from the global burden of disease study 2010. Lancet Glob Health. (2013) 1:e259–81. doi: 10.1016/S2214-109X(13)70089-5
26. Unnithan AKA, Das JM, Mehta P. Hemorrhagic Stroke. StatPearls. (2022). Available online at: https://www.ncbi.nlm.nih.gov/books/NBK559173/ (accessed November 2, 2022).
27. Fonarow GC, Saver JL, Smith EE, Broderick JP, Kleindorfer DO, Sacco RL, et al. Relationship of national institutes of health stroke scale to 30-day mortality in medicare beneficiaries with acute ischemic stroke. J Am Heart Assoc. (2012) 1:42. doi: 10.1161/xJAHA.111.000034
28. Huybrechts KF, Caro JJ, Xenakis JJ, Vemmos KN. The prognostic value of the modified Rankin Scale score for long-term survival after first-ever stroke. Results from the Athens stroke registry. Cerebrovasc Dis. (2008) 26:381–7. doi: 10.1159/000151678
Keywords: stroke, predictors, morbidity, mortality, Tanzania
Citation: Matuja SS, Mlay G, Kalokola F, Ngoya P, Shindika J, Andrew L, Ngimbwa J, Ahmed RA, Tumaini B, Khanbhai K, Mutagaywa R, Manji M, Sheriff F and Mahawish K (2023) Predictors of 30-day mortality among patients with stroke admitted at a tertiary teaching hospital in Northwestern Tanzania: A prospective cohort study. Front. Neurol. 13:1100477. doi: 10.3389/fneur.2022.1100477
Received: 16 November 2022; Accepted: 29 December 2022;
Published: 18 January 2023.
Edited by:
Bin Qiu, Yale University, United StatesReviewed by:
Ahmed Y. Azzam, October 6 University, EgyptPaul Olowoyo, Federal Teaching Hospital Ido-Ekiti, Nigeria
Copyright © 2023 Matuja, Mlay, Kalokola, Ngoya, Shindika, Andrew, Ngimbwa, Ahmed, Tumaini, Khanbhai, Mutagaywa, Manji, Sheriff and Mahawish. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sarah Shali Matuja,  ZHIubWF0dWphanVuaW9yQGdtYWlsLmNvbQ==
ZHIubWF0dWphanVuaW9yQGdtYWlsLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Sarah Shali Matuja
Sarah Shali Matuja Gilbert Mlay2†
Gilbert Mlay2† Rashid Ali Ahmed
Rashid Ali Ahmed Khuzeima Khanbhai
Khuzeima Khanbhai Reuben Mutagaywa
Reuben Mutagaywa Mohamed Manji
Mohamed Manji Faheem Sheriff
Faheem Sheriff