- 1Department of Life Sciences, Faculty of Natural Sciences, Imperial College London, London, United Kingdom
- 2Society of Meta-Research and Biomedical Innovation, London, United Kingdom
- 3Department of Nutrition and Dietetics, Musgrove Park Hospital, Taunton & Somerset NHS Foundation Trust, Taunton, United Kingdom
- 4Department of General Surgery, University College London Hospitals, NHS Foundation Trust, London, United Kingdom
- 5Department of Metabolism, Digestion and Reproduction, Faculty of Medicine, Imperial College London, London, United Kingdom
- 6Department of Obstetrics and Gynaecology, Chelsea and Westminster Hospital NHS Foundation Trust, London, United Kingdom
Symptoms, such as fever, dry cough, dyspnoea, and respiratory distress, are commonly described in patients infected with Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). Recently, a growing number of cases pertained to persistent hiccups have been reported by SARS-CoV-2 infected patients. The aim of this systematic review was to screen the current literature and provide a summary of the reported cases of SARS-CoV-2 infected patients presenting with persistent hiccups. According to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, PubMed, Scopus, and Web of Science databases were searched from inception until October 2021. Case reports or case series that provided a separate clinical description for patients with presenting complaints of persistent hiccups before or after COVID-19 diagnosis were retrieved. The critical appraisal checklist for case reports provided by the Joanna Briggs Institute (JBI) was employed to evaluate the overall quality of the eligible studies. We identified 13 eligible studies that included 16 hospitalized COVID-19 patients who complained of persistent hiccups. The mean duration of hiccups was 4.6 days reported in 88% (14/16) patients. Hypertension was the most common comorbidity present in 50% (8/16) of patients followed by diabetes mellitus (4/16). Moreover, 44% (7/16) of patients received only one medication for managing the hiccups with metoclopramide (5/16) followed by chlorpromazine and baclofen (4/16) used as primary treatment. Equally, 44% of patients (7/16) received dexamethasone followed by azithromycin (5/16), ivermectin (4/16), and ceftriaxone (4/16) for managing the infection from SARS-CoV-2. The majority of patients (14/16) improved after initiation of treatment. Persistent hiccups are possibly a rare symptom that clinicians may expect to encounter in patients infected with SARS-CoV-2. Although there is not ample proof to propose causation, increased awareness about the diversity of presentations of SARS-CoV-2 infection could be crucial in the early recognition of the disease.
Introduction
In December 2019, an atypical case of viral pneumonia caused by a novel coronavirus called Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) was discovered in Wuhan, Hubei Province of China (1, 2). Since then, the disease (COVID-19) has spread worldwide with currently more than 250,000,000 confirmed cases reported.
Patients infected with SARS-CoV-2 have a characteristic clinical presentation that includes fever, dry cough, progressive dyspnoea, and respiratory distress (3). The disease caused by this virus may also confer non-respiratory manifestations, including gastrointestinal symptoms, such as vomiting, diarrhea, and abdominal pain (4, 5). Beyond the most commonly presenting symptoms, a diverse range of complaints, including myalgia, dizziness, and headache, have been reported by patients (6, 7). More recently, there is a growing number of complaints pertained to persistent hiccups, which have been documented in patients with COVID-19 (8).
Hiccups, also known as singultus or hiccoughs, are involuntary reflex movements of diaphragmatic contractions that lead to the sudden closure of the glottis and the abrupt termination of inspiration (9, 10). Most commonly, hiccups are recognized as acute and are often of short duration, lasting less than 48 h (9–11). However, persistent hiccups or those longer than 48 h are often associated with cardiac or gastrointestinal disorders, which potentially underlie a manifestation of a major pathology (9–11).
Persistent hiccups can be burdensome to patients with a great impact on quality of life and often difficult to manage (12, 13). If left untreated, these can lead to sleep disturbances, physical exhaustion, and even depression (12, 13). To date, persistent hiccups as an atypical symptom of SARS-CoV-2 infection have not been considered. The aim of our systematic review was to comprehensively screen all currently available literature and provide a detailed summary of all the reported cases of SARS-CoV-2 infected patients presenting with persistent hiccups.
Materials and Methods
This review was composed on the basis of the “Preferred Reporting Items for Systematic Reviews and Meta-Analyses” (PRISMA) guidelines (14).
Literature Search
Two independent reviewers (PG and KKT) screened the PubMed, Scopus, and Web of Science databases from inception until October 2021, using the following terms: “(COVID 19) OR (SARS-COV2) OR (Coronavirus) OR (2019-nCoV) AND (hiccups) OR (hiccoughs) OR (singultus) OR (synchronous diaphragmatic flutter)”. A manual search of references cited in eligible publications, was also performed for undetected studies. No restrictions pertained to study design and geographic origin were applied to our search and all discrepancies were resolved by a third investigator (GG).
Eligibility Criteria
We included case reports or case series that provided a separate clinical description for patients with presenting complaints of persistent hiccups before or after the COVID-19 diagnosis. Non-English articles, review articles, abstracts and non-peer-reviewed sources were classified as ineligible for inclusion. Studies with in vitro and animal models were also excluded.
Data Extraction and Handling
We extracted individual patient data from eligible studies and collected the following information: gender, age, reported comorbidities and cardinal COVID-19 symptoms, onset of hiccups before or after COVID-19 diagnosis, duration of hiccups and outcome, use of laboratory tests, and treatment regime. Two authors (PG and KKT) conducted the data extraction independently and any disagreements were discussed and resolved by a third investigator (KSK).
Quality Assessment
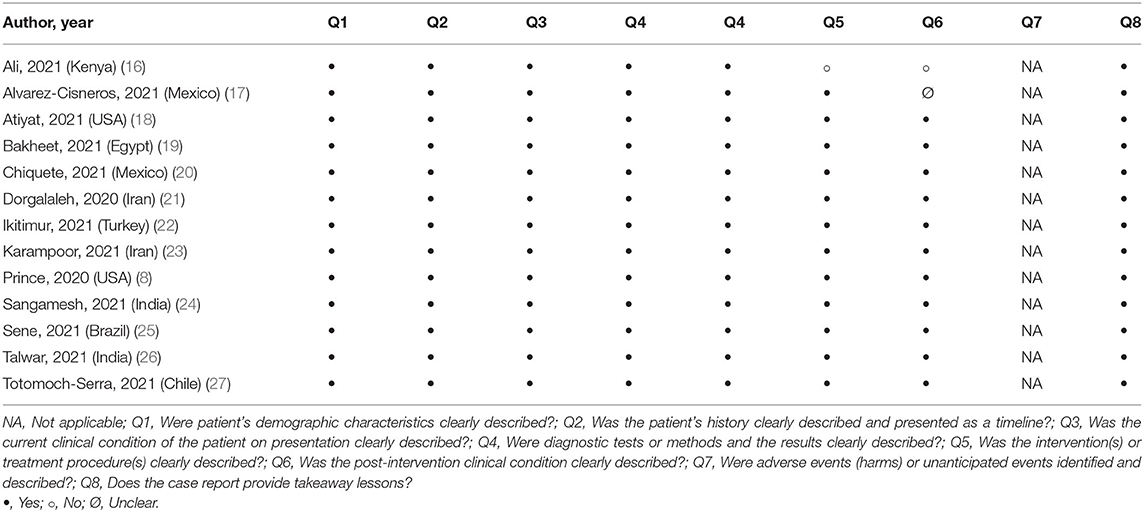
The critical appraisal checklist for case reports provided by the Joanna Briggs Institute (JBI) was employed to evaluate the overall quality of the included studies (15). The assessment was ensued based on the reporting of 8 different elements namely, patient demographics, medical history, health status, physical examination and diagnosis, concomitant therapies, post-intervention health status and drug administration reaction interface. The studies were scored either based on “Yes”, “No”, “Unclear or Not/Applicable” depending on the availability of information for every element.
Results
Characteristics of Studies
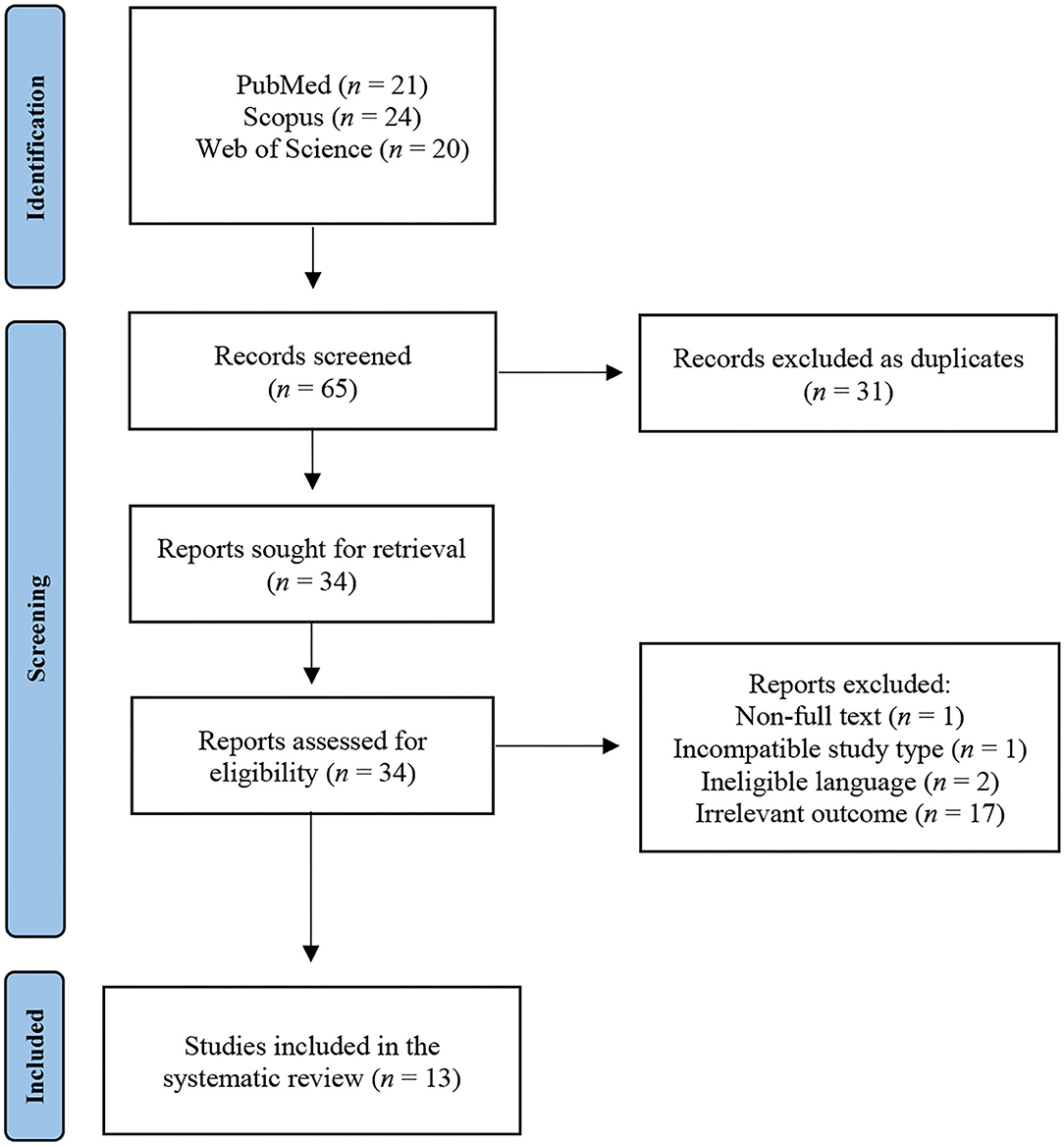
Our initial search of the literature resulted in 65 studies. After removal of 31 duplicates, 34 unique studies were retrieved. Upon screening of title and abstract, 17 studies containing irrelevant outcomes, two written in non-English language, one with incompatible study design, and one with no full-text availability, were excluded. Overall, 13 studies were regarded as eligible for inclusion in the systematic review (Figure 1). Eleven of the studies were case reports and two were case series. Six of the studies originated from the Americas, five from Asia, and two from Africa.
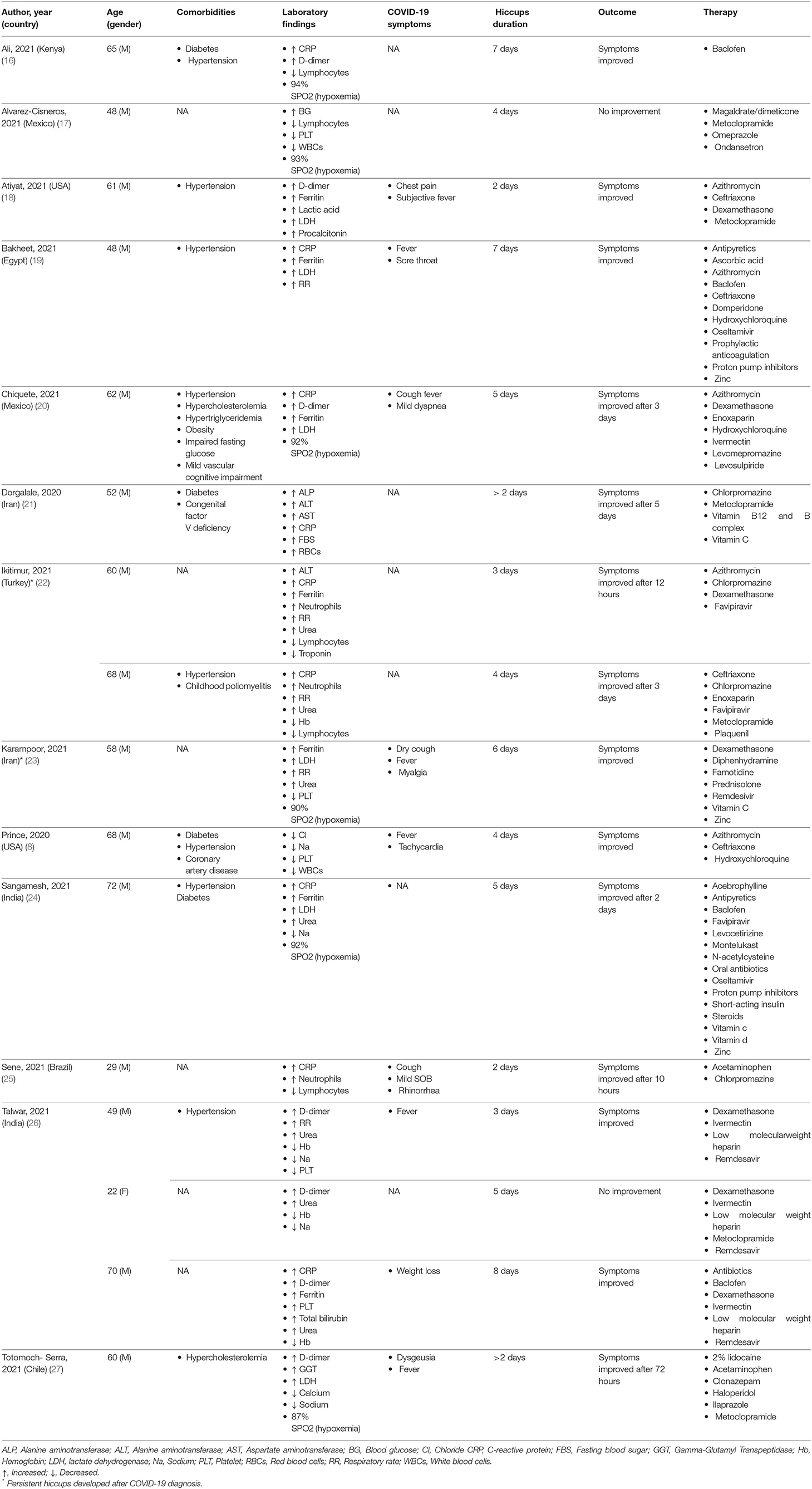
The majority of the included patients (14/16) had hiccups prior to COVID-19 diagnosis, with only two developing symptoms afterward.
We retrieved a total of 16 cases of COVID-19 patients with presenting complaints of persistent hiccups. The majority of the included patients (14/16) had hiccups before COVID-19 diagnosis, while in two symptoms developed thereafter. All patients were male apart from a single female, with a mean age of 56 years. Hypertension was the most common comorbidity present in 50% (8/16) of patients followed by diabetes mellitus in 25% (4/16) of patients. Laboratory measurements were available for all patients with the most common characteristic being elevated C-reactive protein, present in 56% (9/16) of patients. A drop in oxygen saturation below 95% was experienced in six patients and four patients were found with attenuated serum sodium levels below 135 mmol/L. Findings of interstitial pneumonia compatible with COVID-19 from chest X-ray or computed tomography scans were present in all the patients. Symptoms of fever along with hiccups were reported in six patients, while 44% (7/16) were free of typical COVID-19 symptoms. The mean duration of hiccups was 4.6 days reported in 88% (14/16) patients. In two studies hiccups lasting more than 2 days were reported, but without any mention of the exact duration before seeking medical advice. For managing the hiccups, 44% of patients (7/16) received metoclopramide (5/16) as medication followed by chlorpromazine and baclofen (4/16), given as primary treatment. Furthermore, 44% of patients (7/16) received dexamethasone followed by azithromycin (5/16), ivermectin (4/16) and ceftriaxone (4/16) for managing the infection from SARS-CoV-2. A larger proportion of patients (14/16) improved after initiation of treatment, while only two showed no signs of improvement (Table 1).
Quality of the Studies
Quality assessment of the eligible studies revealed that on average all of the recommended elements were fulfilled and thus, these were considered as low risk of bias. Only two studies did not attain a perfect score. The information most commonly marked as “Not/Applicable” across the studies was drug administration reaction interface (Table 2).
Discussion
Our systematic review examined the co-occurrence of persistent hiccups with SARS-CoV-2 infection. We included 13 reports of 16 patients in which presenting complaints of hiccups were reported by hospitalized COVID-19 patients. Our findings revealed that metoclopramide, chlorpromazine, and baclofen were used as the primary treatments for managing the hiccups, while dexamethasone, azithromycin, ivermectin, and ceftriaxone, for the infection from SARS-CoV-2. After the initiation of treatment, the majority of patients showed improvement of symptoms and were later recovered in stable condition.
A hiccup is a benign self-limiting reflex most commonly affecting males (9, 10, 28). It is characterized by repetitive sudden involuntary spasmodic contractions of the diaphragm and intercostal muscles (9, 10). These contractions usually are accompanied by a short inhalation, which is interrupted by closure of the glottis (9, 10). The hiccup response appears to involve a reflex arc that arises from the interaction of three neuroanatomical facets (29–31). An “afferent limb” composed of the phrenic and vagus nerves as well as sympathetic nerve fibers from the thoracic chain T6–T12, which mediates the outflow of visceral and somatic sensory inputs. An “efferent limb” consisted chiefly of the phrenic nerve, which conveys motor commands to the diaphragm and accessory respiratory muscles of the intercostal space. A central “processing unit” involving nonspecific anatomic structures between the upper spinal cord at the mid-cervical vertebrae (at levels) C3-C5 and the brainstem, which integrates the interaction between the afferent and efferent arms.
Persistent hiccups can arise from the direct structural or functional injury of the hiccup reflex arc itself, which leads to persistent triggering but may result from any underlying disease affecting the reflex nerves involved (32). In patients with COVID-19, the manifestation of persistent hiccups appears to be a complex phenomenon, with several theories proposed. The most compelling theory describes the ability of COVID-19 related-pneumonia to cause an injury on peripheral nerves, especially the vagus or phrenic nerves, which support the diaphragm musculature and lead to its irritation (33–36). Considering that the chest X-ray or computed tomography scan of the including patient cohort demonstrated findings of interstitial pneumonia compatible with COVID-19, an inflammatory-based pneumonic irritation of the vagus or phrenic nerve distributions and their pericardial, gastric, and esophageal branches, may explain the manifestation of persistent hiccups following SARS-CoV-2 infection.
Casual of persistent hiccups may also be the administration of pharmacological agents that may exert their action via the stimulation of the gastrointestinal or central nervous system (CNS) (30, 37–42). Although drug-induction is not widely considered as the triggering factor for recurrent episodic hiccups, drugs, such as corticosteroids and antibiotics, are often cited as a possible source of causation (39, 43–45). Particularly, with the absence of curative treatment pertained to antiviral or immunomodulatory interventions, a surge in corticosteroid therapy, such as dexamethasone, for controlling the severity of SARS-CoV-2 infection, has been increasingly popular (46–48). As reported, persistent hiccups secondary to corticosteroid administration, which often are supplementary to primary treatment and especially upon high doses, becomes worrisome (49). Equally, a substantial pre- and post-emptive use of antibiotics especially those available without a prescription, has been documented in SARS-CoV-2 infected patients, which has now developed into a deluge driven by both patients and physicians (50–53). Although information relevant to the diagnosis of co-infection from a bacterial source was not reported nor investigated in the included patients of which a plethora received azithromycin and ceftriaxone, antibiotic induced-persistent hiccups in the setting of COVID-19 treatment warrants further attention. Overall, there is not ample proof of drug-induced persistent hiccups to propose causation, however, the incidence may be adequate enough to raise this phenomenon and its potential association with SARS-CoV-2 infection, as a concern to practitioners.
Additional extrapulmonary factors to the manifestation of persistent hiccups have also been proposed and these include encephalitis lethargica and somatoform disorder. To date, there is increasing evidence of diverse neurological cases of encephalopathy following SARS-CoV-2 infection (54–57). Evidence from the influenza pandemic points out that the association between persistent hiccups and SARS-CoV-2 infection may be etiologically related to encephalitis lethargica (58–60). Considering that SARS-CoV-2 can be neuroinvasive with an olfactory transmucosal route to the CNS, this may hint the manifestation of persistent hiccups in COVID-19 patients (60, 61). Although examination of the cerebrospinal fluid (CSF) was not ensued in the included patients, differential diagnosis of viral encephalitis is largely contingent on CSF virus detection (55, 57). While SARS-CoV-2 dissemination in the CNS has been described as transient and/or limited with low CSF titres (55, 57), reports confirm negative CSF PCR positivity with delayed neurological involvement (62). Taken together, whether persistent hiccups in SARS-CoV-2 infected patients may reflect an encephalitis lethargica-like phenotype or simply immune-mediated para-infectious encephalitis, remains unexplored.
Patients with a history of SARS-CoV-2 infection also present a significantly higher risk of comorbid somatic symptom disorders, and thus, symptoms of psychological distress (63, 64). Reports of persistent hiccups of psychogenic origin have been documented and classified under somatic autonomic dysfunction according to the International Classification of Diseases (ICD) 10th revision and somatic symptom disorder based on the Diagnostic and Statistical Manual (DSM) of mental disorders (DSM-V) Text Revision (TR) (65–67). Nevertheless, whether acute SARS-CoV-2 infection (as part of our cohort) could trigger the development of somatoform symptoms in predisposed subjects, cannot be commented with complete accuracy as psychogenic symptoms often accompany convalescence in long COVID-19 patients.
Beyond COVID-19 settings, hiccups may be a rare manifestation of electrolyte abnormalities or deficiencies, such as hyponatremia (68–72). Although a less compelling hypothesis, hyponatremia-induced persistent hiccups have been previously documented and may occur from cellular swelling and cerebral oedema in response to the osmolar shift (73). Despite that none of the included patients presented with severely attenuated serum sodium levels, hyponatremia has been speculated to result in the higher center inhibition of sympathetic outflow in the hiccup reflex arc, a theory worth further exploration (73). Persistent hiccups may also be a manifestation of abdominal myoclonus in clinical settings (74–76). In the context of COVID-19, presenting cases of different transient abdominal complaints, including abdominal myoclonus, have been described (77–79). Despite that no reports of paroxysmal episodes of severe abdominal pain prevailed amongst the included patients (74), masking of abdominal myoclonus by the manifestation of persistent hiccups cannot be excluded in SARS-CoV-2 infected patients. Lastly, gastrogenic and cardiogenic causes have been proposed, involving disturbances in principal organs, spanning from abdominal distension and gastric irritation to angina pectoris, and acute myocardial infraction (73, 80–82). However, considering that most of the included patients were free of any prevailing comorbidities with their medical history and health status precluding the possibility of prior episodes of severe disease, these organic causes appear far from the cause of persistent hiccups following SARS-CoV-2 infection.
Strengths and Limitations
To our best knowledge, our study is the first to review the co-occurrence of persistent hiccups and SARS-CoV-2 infection. Our findings offer a comprehensive summary of the current literature and feature published data from included studies with quality assessment of increased scrutiny. However, our study is prone to limitations. A wider drawback involves the low-quality nature of case reports and series within our systematic review, which hampers the validity and interpretation of conclusions that can be attained. Especially, the underlying risk of bias in these studies and their selection remains unavoidable, as these become particularly vulnerable to the risk of misinterpretation or extrapolation. Thus, their reported findings although appealing, may not reflect the truth without underlying valid description.
Conclusion
Patients infected with SARS-CoV-2 have a clinical presentation that is commonly described by symptoms, such as fever, dry cough, dyspnoea, and respiratory distress. Beyond respiratory symptoms, a growing number of complaints pertained to persistent hiccups have been recently reported by hospitalized COVID-19 patients. While the above presentation is still underreported, persistent hiccups can be burdensome to patients and could have a great impact on quality of life especially to those infected with SARS-CoV-2. Although there is not ample proof to propose causation, persistent hiccups as an atypical manifestation of SARS-CoV-2 infection cannot yet be excluded. Familiarity with unusual signs of SARS-CoV-2 infection possibly, such as persistent hiccups, is vital in raising awareness to clinicians about the diversity of presentations and the early recognition of the disease.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Author Contributions
PG conceived and designed the study. PG and KKT acquired and collated the data. KSK supervised the study. All authors drafted the manuscript and critically revised the important intellectual content.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank the Imperial Open Access Fund for funding the article processing charges for this manuscript. PG thanks the Bodossaki Foundation and KK thanks the General Michael Arnaoutis Foundation for supporting their research activities.
References
1. Lu H, Stratton CW, Tang YW. Outbreak of pneumonia of unknown etiology in Wuhan, China: The mystery and the miracle. J Med Virol. (2020) 92:401. doi: 10.1002/jmv.25678
2. Yang X, Yu Y, Xu J, Shu H, Liu H, Wu Y, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. (2020) 8:475–81. doi: 10.1016/S2213-2600(20)30079-5
3. Tian S, Hu N, Lou J, Chen K, Kang X, Xiang Z, et al. Characteristics of COVID-19 infection in Beijing. J Infect. (2020) 80:401–6. doi: 10.1016/j.jinf.2020.02.018
4. Kalra RS, Tomar D, Meena AS, Kandimalla R. SARS-CoV-2, ACE2, and hydroxychloroquine: cardiovascular complications, therapeutics, and clinical readouts in the current settings. Pathogens. (2020) 9:546. doi: 10.3390/pathogens9070546
5. Katsikas Triantafyllidis K, Giannos P, Mian IT, Kyrtsonis G, Kechagias KS. Varicella zoster virus reactivation following COVID-19 vaccination: a systematic review of case reports. Vaccines. (2021) 9:1013. doi: 10.3390/vaccines9091013
6. Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. (2020) 395:507–13. doi: 10.1016/S0140-6736(20)30211-7
7. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. (2020) 395:497–506. doi: 10.1016/S0140-6736(20)30183-5
8. Prince G, Sergel M. Persistent hiccups as an atypical presenting complaint of COVID-19. Am J Emerg Med. (2020) 38:1546. e1545–1546. e1546. doi: 10.1016/j.ajem.2020.04.045
9. Lewis JH. Hiccups: causes and cures. J Clin Gastroenterol. (1985) 7:539–52. doi: 10.1097/00004836-198512000-00021
11. Howard RS. Persistent hiccups. BMJ: British Medical Journal. (1992) 305:1237. doi: 10.1136/bmj.305.6864.1237
12. Samuels L. Hiccup: a ten year review of anatomy, etiology, and treatment. Can Med Assoc J. (1952) 67:315.
14. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
15. Munn Z, Barker TH, Moola S, Tufanaru C, Stern C, Mcarthur A, et al. Methodological quality of case series studies: an introduction to the JBI critical appraisal tool. JBI Evidence Synthesis. (2020) 18:2127–33. doi: 10.11124/JBISRIR-D-19-00099
16. Ali SK, Muturi D, Sharma K. Be wary of hiccups: an unusual case of COVID-19. Cureus. (2021) 13:e12974. doi: 10.7759/cureus.12974
17. Alvarez-Cisneros T, Lara-Reyes A, Sansón-Tinoco S. Hiccups and psychosis: two atypical presentations of COVID-19. Int J Emerg Med. (2021) 14:1–5. doi: 10.1186/s12245-021-00333-0
18. Atiyat R, Veeraballi S, Al-Atiyat N, Chan KH, Slim J. A rare case report of persistent hiccups as an atypical presentation of COVID-19. Cureus. (2021) 13:e13625. doi: 10.7759/cureus.13625
19. Bakheet N, Fouad R, Kassem AM, Hussin W, El-Shazly M. Persistent hiccup: a rare presentation of COVID-19. Respir Investig. (2021) 59:263–5. doi: 10.1016/j.resinv.2020.11.003
20. Chiquete E, Toapanta-Yanchapaxi L, Aceves-Buendía JJ, Ruiz-Ruiz E, Rodríguez-Perea E, Durán-Coyote S, et al. Levosulpiride relieved persistent hiccups in a patient With COVID-19 and vascular cognitive impairment. Clin Neuropharmacol. (2021) 44:186–8. doi: 10.1097/WNF.0000000000000459
21. Dorgalaleh A, Dabbagh A, Tabibian S, Bahraini M, Rafieemehr H. Persistent hiccups in a patient with mild congenital factor V deficiency and COVID-19: clinical and laboratory finding of a rare bleeding disorder. Int J Lab Hematol. (2021) 43:e87–8. doi: 10.1111/ijlh.13385
22. Ikitimur H, Uysal BB, Ikitimur B, Umihanic S, Smajic J, Jahic R, et al. Case report: two cases of persistent hiccups complicating COVID-19. Am J Trop Med. (2021) 104:1713–5. doi: 10.4269/ajtmh.21-0190
23. Karampoor S, Afrashteh F, Laali A. Persistent hiccups after treatment of COVID-19 with dexamethasone: A case report. Respir Med Case Rep. (2021) 34:101515. doi: 10.1016/j.rmcr.2021.101515
24. Sangamesh S, Gosavi S, Shastry S, Johny SM. Hiccups and hyponatremia: unusual co-presentation in COVID-19. J Family Med Prim Care. (2021) 10:1040–3. doi: 10.4103/jfmpc.jfmpc_1582_20
25. Sene DR, Watashi DM, Bilitardo IO, Moreno CEC, Moreno MF. COVID-19 presenting as persistent hiccups: a case report. Revista do Instituto de Medicina Tropical de São Paulo. (2021) 63:e62. doi: 10.1590/S1678-9946202163062
26. Talwar D, Kumar S, Madaan S, Khanna S, Annadatha A. Intractable singultus: Atypical presentation of COVID 19. Med Sci. (2021) 25:1183–7.
27. Totomoch-Serra A, Ibarra-Miramon CB, Manterola C. Persistent hiccups as main COVID-19 symptom. Am J Med Sci. (2021) 361:799–800. doi: 10.1016/j.amjms.2021.01.001
28. Lee G-W, Kim RB, Go SI, Cho HS, Lee SJ, Hui D, et al. Gender differences in hiccup patients: analysis of published case reports and case-control studies. J Pain Symptom Manage. (2016) 51:278–83. doi: 10.1016/j.jpainsymman.2015.09.013
29. Launois S, Bizec J, Whitelaw W, Cabane J, Derenne JP. Hiccup in adults: an overview. Eur Respir J. (1993) 6:563–75.
30. Steger M, Schneemann M, Fox M. Systemic review: the pathogenesis and pharmacological treatment of hiccups. Aliment Pharm Ther. (2015) 42:1037–50. doi: 10.1111/apt.13374
31. Nausheen F, Mohsin H, Lakhan SE. Neurotransmitters in hiccups. Springerplus. (2016) 5:1–7. doi: 10.1186/s40064-016-3034-3
32. Usta Y. Persistent hiccups: an unusual presentation and treatment. J Pain Symptom Manage. (2012) 43:e7–8. doi: 10.1016/j.jpainsymman.2011.10.010
33. Burdette SD, Marinella MA. Pneumonia presenting as singultus. South Med J. (2004) 97:915–915. doi: 10.1097/01.SMJ.0000125174.62424.C6
34. Karakonstantis S, Pitsigavdaki S, Korela D, Galani D. Lower lobe pneumonia presenting as singultus (hiccups). Caspian J Intern Med. (2018) 9:403. doi: 10.1155/2018/9231989
35. Li YC, Bai WZ, Hashikawa T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J Med Virol. (2020) 92:552–5. doi: 10.1002/jmv.25728
36. Abdeldayem EH, Abdelrahman AS, Mansour MG. Recognition of phrenic paralysis as atypical presentation during CT chest examination of COVID-19 infection and its correlation with CT severity scoring: a local experience during pandemic era. Egypt J Radiol Nucl Med. (2021) 52:1–7. doi: 10.1186/s43055-021-00527-9
37. Macdonald RL, Rogers CJ, Twyman R. Barbiturate regulation of kinetic properties of the GABAA receptor channel of mouse spinal neurones in culture. J Physiol. (1989) 417:483–500. doi: 10.1113/jphysiol.1989.sp017814
38. Jones MV, Harrison NL, Pritchett DB, Hales TG. Modulation of the GABAA receptor by propofol is independent of the gamma subunit. J Pharmacol Exp Therapeut. (1995) 274:962–8.
39. Thompson DF, Landry JP. Drug-induced hiccups. Ann Pharmacother. (1997) 31:367–9. doi: 10.1177/106002809703100318
40. Liaw C-C, Wang C-H, Chang H-K, Wang H-M, Huang J-S, Lin Y-C, et al. Cisplatin-related hiccups: male predominance, induction by dexamethasone, and protection against nausea and vomiting. J Pain Symptom Manage. (2005) 30:359–66. doi: 10.1016/j.jpainsymman.2005.08.008
41. Hayashi M, Sugimura H, Suga Y, Kawahara M, Aimiya K, Miyamoto K. Study on risk factors for hiccups induced by cisplatin-based chemotherapy. J Pharm Health Care Sci. (2009) 35:89–95. doi: 10.5649/jjphcs.35.89
42. Ray P, Haq MZU, Nizamie SH. Aripiprazole-induced hiccups: a case report. Gen Hosp Psychiatry. (2009) 31:382–4. doi: 10.1016/j.genhosppsych.2008.09.014
43. Marinella M. A. (2009). Diagnosis and management of hiccups in the patient with advanced cancer. The journal of supportive oncology 7, 122-127, 130.
44. Peacock ME. (2013). Transient hiccups associated with oral dexamethasone. Case Rep Dent. (2013). doi: 10.1155/2013/426178
45. Rizzo C, Vitale C, Montagnini M. Management of intractable hiccups: an illustrative case and review. Am J Hosp Palliat Care ®. (2014) 31:220–4. doi: 10.1177/1049909113476916
46. Ahmed MH, Hassan A. Dexamethasone for the treatment of coronavirus disease (COVID-19): a review. SN Comprehensive Clinical Medicine. (2020) 1–10. doi: 10.1007/s42399-020-00610-8
47. Group TRC. Dexamethasone in hospitalized patients with Covid-19—preliminary report. N Engl J Med. (2020) 385:693–704. doi: 10.1056/NEJMoa2021436
48. Singh TU, Parida S, Lingaraju MC, Kesavan M, Kumar D, Singh RK. Drug repurposing approach to fight COVID-19. Pharmacological Reports. (2020) 1–30. doi: 10.1007/s43440-020-00155-6
49. Cersosimo RJ, Brophy MT. Hiccups with high dose dexamethasone administration: a case report. Cancer. (1998) 82:412–4. doi: 10.1002/(sici)1097-0142(19980115)82:2<415::aid-cncr23>3.0.co;2-0
50. Antinori S, Galimberti L, Milazzo L, Ridolfo AL. Bacterial and fungal infections among patients with SARS-CoV-2 pneumonia. Hospitals. (2020) 28:29–36.
51. Rawson TM, Moore LS, Zhu N, Ranganathan N, Skolimowska K, Gilchrist M, et al. Bacterial and fungal coinfection in individuals with coronavirus: a rapid review to support COVID-19 antimicrobial prescribing. Clin Infect Dis. (2020) 71:2459–68. doi: 10.1093/cid/ciaa530
52. Pelfrene E, Botgros R, Cavaleri M. Antimicrobial multidrug resistance in the era of COVID-19: a forgotten plight? Antimicrob Resist Infect Control. (2021) 10:1–6. doi: 10.1186/s13756-021-00893-z
53. Vaughn VM, Gandhi TN, Petty LA, Patel PK, Prescott HC, Malani AN, et al. Empiric antibacterial therapy and community-onset bacterial coinfection in patients hospitalized with coronavirus disease 2019 (COVID-19): a multi-hospital cohort study. Clin Infect Dis. (2021) 72:e533–41. doi: 10.1093/cid/ciaa1239
54. Farhadian S, Glick LR, Vogels CB, Thomas J, Chiarella J, Casanovas-Massana A, et al. Acute encephalopathy with elevated CSF inflammatory markers as the initial presentation of COVID-19. BMC Neurol. (2020) 20:1–5. doi: 10.1186/s12883-020-01812-2
55. Haider A, Siddiqa A, Ali N, Dhallu M. COVID-19 and the brain: acute encephalitis as a clinical manifestation. Cureus. (2020) 12. doi: 10.7759/cureus.10784
56. Pilotto A, Odolini S, Masciocchi S, Comelli A, Volonghi I, Gazzina S, et al. Steroid-responsive encephalitis in coronavirus disease 2019. Ann Neurol. (2020) 88:423–7. doi: 10.1002/ana.25783
57. Ye M, Ren Y, Lv T. Encephalitis as a clinical manifestation of COVID-19. Brain Behav Immun. (2020) 88:945. doi: 10.1016/j.bbi.2020.04.017
58. Hoffman LA, Vilensky JA. Encephalitis lethargica: 100 years after the epidemic. Brain. (2017) 140:2246–51. doi: 10.1093/brain/awx177
59. Badrfam R, Zandifar A. From encephalitis lethargica to COVID-19: Is there another epidemic ahead? Clin Neurol Neurosurg. (2020) 196:106065. doi: 10.1016/j.clineuro.2020.106065
60. Giordano A, Schwarz G, Cacciaguerra L, Esposito F, Filippi M. COVID-19: can we learn from encephalitis lethargica? Lancet Neurol. (2020) 19:570. doi: 10.1016/S1474-4422(20)30189-7
61. Meinhardt J, Radke J, Dittmayer C, Franz J, Thomas C, Mothes R, et al. Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19. Nat Neurosci. (2021) 24:168–75. doi: 10.1038/s41593-020-00758-5
62. Zamani R, Pouremamali R, Rezaei N. Central neuroinflammation in Covid-19: a systematic review of 182 cases with encephalitis, acute disseminated encephalomyelitis, and necrotizing encephalopathies. Rev Neurosci. (2021). doi: 10.1515/revneuro-2021-0082
63. Bhatia M, Agrawal P, Khastbir U, Rai S, Bhatia A, Bohra N, et al. A study of emergency psychiatric referrals in a government hospital. Indian J Psychiatry. (1988) 30:363.
64. Horn M, Fovet T, Vaiva G, D'hondt F, Amad A. Somatic symptom disorders and long COVID: A critical but overlooked topic. General Hospital Psychiatry. (2021). doi: 10.1016/j.genhosppsych.2021.06.007
65. WHO. The ICD-10 classification of mental and behavioural disorders: clinical descriptions and diagnostic guidelines. Genève: World Health Organization. (1992).
66. Association AP. Dsm-Iv-Tr. Diagnostic and statistical manual of mental disorders. Washington, DC: American Psychiatric Association (2000).
67. Mehra A, Subodh B, Sarkar S. Psychogenic hiccup in children and adolescents: a case series. Journal of family medicine and primary care. (2014) 3:161. doi: 10.4103/2249-4863.137666
68. Ramirez FC, Graham DY. Hiccups, compulsive water drinking, and hyponatremia. Ann Intern Med. (1993) 118:649. doi: 10.7326/0003-4819-118-8-199304150-00020
69. Lazarevic V, Hägg E, Wahlin A. Hiccups and severe hyponatremia associated with high-dose cyclophosphamide in conditioning regimen for allogeneic stem cell transplantation. Am J Hematol. (2007) 82:88–88. doi: 10.1002/ajh.20706
70. Goyal A, Mehmood S, Mishra S, Bhatnagar S. Persistent hiccups in cancer patient: a presentation of syndrome of inappropriate antidiuretic hormone induced hyponatremia. Indian J Palliat Care. (2013) 19:110. doi: 10.4103/0973-1075.116712
71. Gardecki J, Espinosa J, Lucerna A, Bernhardt J. Singultus: avoiding a hiccup in care. Am J Emerg Med. (2017) 35:938. e931–938. e933. doi: 10.1016/j.ajem.2016.12.056
72. Lim KY, Nawawi KNM. Hyponatremia-associated troublesome hiccups: a report of rare manifestation: a review. J Clin Diagn. (2020) 14. doi: 10.7860/JCDR/2020/43203.13430
73. George J, Thomas K, Jeyaseelan L, Peter J, An AC. Hyponatraernia and hiccups. Natl Med J India. (1996) 9.
74. Dutta SR, Hazarika I, Chakravarty BP. Abdominal epilepsy, an uncommon cause of recurrent abdominal pain: a brief report. Gut. (2007) 56:439–41. doi: 10.1136/gut.2006.094250
75. Harshe DG, Harshe SD, Harshe GR, Harshe GG. Abdominal epilepsy in an adult: a diagnosis often missed. J Clin Diagn. (2016) 10:VD01. doi: 10.7860/JCDR/2016/19873.8600
76. Assad S, Dobariya V, Zahid M, Malik SA. Abdominal epilepsy masked with hiccups in a patient with intracranial malignant glioma. Cureus. (2019) 11. doi: 10.7759/cureus.6338
77. Anand P, Zakaria A, Benameur K, Ong C, Putman M, O'shea S, et al. Myoclonus in patients with coronavirus disease 2019: a multicenter case series. Crit Care Med. (2020) 48:1664–9. doi: 10.1097/CCM.0000000000004570
78. Borroni B, Gazzina S, Dono F, Mazzoleni V, Liberini P, Carrarini C, et al. Diaphragmatic myoclonus due to SARS-CoV-2 infection. Neurological Sciences. (2020) 41:3471–4. doi: 10.1007/s10072-020-04766-y
79. Rábano-Suárez P, Bermejo-Guerrero L, Méndez-Guerrero A, Parra-Serrano J, Toledo-Alfocea D, Sánchez-Tejerina D, et al. Generalized myoclonus in COVID-19. Neurology. (2020) 95:e767–72. doi: 10.1212/WNL.0000000000009829
80. Walker P, Watanabe S, Bruera E. Baclofen, a treatment for chronic hiccup. J Pain Symptom Manage. (1998) 16:125–32. doi: 10.1016/S0885-3924(98)00039-6
81. Worku Hassen G, Singh MM, Kalantari H, Yemane-Merriwether S, Ferrante S, Shaw R. Persistent hiccups as a rare presenting symptom of pulmonary embolism. West J Emerg Med. (2012) 13:479. doi: 10.5811/westjem.2012.4.6894
Keywords: coronavirus, COVID-19, hiccups, singultus, hiccoughs, systematic review, SARS-CoV-2
Citation: Giannos P, Katsikas Triantafyllidis K, Geropoulos G and Kechagias KS (2022) Persistent Hiccups as an Atypical Presentation of SARS-CoV-2 Infection: A Systematic Review of Case Reports. Front. Neurol. 13:819624. doi: 10.3389/fneur.2022.819624
Received: 22 November 2021; Accepted: 15 February 2022;
Published: 04 April 2022.
Edited by:
Pankaj Seth, National Brain Research Centre (NBRC), IndiaReviewed by:
Fedele Dono, University of Studies G. d'Annunzio Chieti and Pescara, ItalyDomenico Antonio Restivo, Garibaldi Hospital, Italy
Copyright © 2022 Giannos, Katsikas Triantafyllidis, Geropoulos and Kechagias. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Panagiotis Giannos, cGFuYWdpb3Rpcy5naWFubm9zMTlAaW1wZXJpYWwuYWMudWs=; Konstantinos S. Kechagias, a29uc3RhbnRpbm9zLmtlY2hhZ2lhczE4QGltcGVyaWFsLmFjLnVr
 Panagiotis Giannos
Panagiotis Giannos Konstantinos Katsikas Triantafyllidis
Konstantinos Katsikas Triantafyllidis Georgios Geropoulos
Georgios Geropoulos Konstantinos S. Kechagias
Konstantinos S. Kechagias