- 1Department of Neurology, National Key Clinical Department and Key Discipline of Neurology, The First Affiliated Hospital, Sun Yat-sen University, Guangzhou, China
- 2Guangzhou Women and Children's Medical Center, Guangzhou Medical University, Guangzhou, China
Moyamoya disease (MMD), most often diagnosed in children and adolescents, is a chronic cerebrovascular disease characterized by progressive stenosis at the terminal portion of the internal carotid artery and an abnormal vascular network at the base of the brain. Recently, many investigators show a great interest in MMD with pulmonary arterial hypertension (PAH). Ring finger protein 213 (RNF213) is a major susceptibility gene for MMD and also has strong correlations with PAH. Therefore, this review encapsulates current cases of MMD with PAH and discusses MMD with PAH in the aspects of epidemiology, pathology, possible pathogenesis, clinical manifestations, diagnosis, and treatment.
Introduction
Moyamoya disease (MMD) is a chronic and rare cerebrovascular disease characterized by an abnormal vascular network at the base of the brain and progressive stenosis or occlusion at the terminal part of the internal carotid artery and the initial part of the middle cerebral artery or the anterior cerebral artery. It is most common in East Asian populations, especially in Japanese, Korean, and Chinese. The MMD prevalence in Japan peaks at two ages: 5–10 and 25–49 years of age (1). The peak in the child group of MMD accounts for 16.2% of all incident cases, and the peak in the adult group accounts for 22.8% (2). The clinical presentations of MMD include transient ischemic attack (TIA), stroke, headache, epileptic seizures, and impaired mental function (3–6). Studies involving Asian populations indicate that adults with MMD have a much higher rate of hemorrhage stroke, whereas children with MMD have a higher rate of ischemic stroke or TIA (6). MMD has become one of the most important reasons for stroke in adolescents (7) and children (8), though it is an independent risk factor for recurrent stroke in children.
Recent studies found that MMD is not only related to intracranial vascular but is also related to some extracranial vascular, such as pulmonary arteries, renal arteries, and coronary arteries, among which the involvement of pulmonary arteries is common (9–11). Since 1990, Kapusta reported the first clinical case of MMD with pulmonary hypertension, more than 10 cases of MMD with pulmonary arterial hypertension (PAH) have been covered (12–20). PAH is a pathological condition in which pulmonary artery pressure is abnormally elevated with known and unknown etiology and can easily lead to right heart failure and even death as the disease progresses (21). Peripheral pulmonary artery stenosis (PPAS), with multiple stenosis and blockage of peripheral pulmonary arteries, can be seen in pediatric patients with congenital abnormalities or chromosomal syndromes (22–25). In addition, some cases of pulmonary hypertension for unknown reasons, called idiopathic pulmonary arterial hypertension (IPAH), are often associated with genetic factors (26).
The Ring Finger Protein 213(RNF213) on chromosome 17q25.3 has been identified as a susceptibility gene for MMD (27, 28). RNF213 encodes a 591-kDa protein that possesses AAA+ ATPase domains and E3 ubiquitin ligase domains, which are closely related to various activities, such as angiogenesis, autophagy, autoimmunity, and lipid metabolism (29–32). RNF213 p.R4810K has a strong relation with MMD in East Asian, which was detected in 95% of familial MMD cases (28) and 79% of sporadic cases (33). Other mutations are also associated with MMD in other areas in the world. On the other hand, a study has suggested a causal relationship between RNF213 and PAH (34). RNF213 p.R4810K was detected in 7.9% of patients with IPAH, resulting in a higher risk of lung transplantation and death and an earlier onset age (35). The incidence of PPAS in RNF213 wild type, heterozygous p.R4810K, and homozygous p.R4810K MMD/quasi-MMD was 0% (0/101), 0.5% (1/200), and 40% (2/5), respectively (36). The positive detection effect of RNF213 p.R4810k was no less than that of the recognized bone morphogenic protein receptor type 2 (BMPR2), which is one of the causative genes in PAH (35). However, the relationship between RNF213 and MMD with PAH is unknown. We summarized the current cases of MMD with PAH and discussed the epidemiology, pathology, possible pathogenesis, clinical manifestations, diagnosis, and treatment.
Literature Search
We performed a search of the literature in the PubMed and Web of Science databases to identify articles related to MMD and PAH on November 20th, 2021. The titles and abstracts of those articles were reviewed by two reviewers to confirm their quality and eligibility for further examination. The inclusion criteria were as follows: (1) MMD and PAH, PPAS, or IPAH were simultaneously mentioned in the title or abstract, and (2) case reports. The exclusion criteria were as follows: (1) without a definite diagnosis of MMD and PAH, PPAS, or IPAH; (2) non-English article; and (3) Moyamoya syndrome. MMD was diagnosed when there was no specific underlying disease, including genetic, hereditary disorders, hematological disorders, connective-tissue diseases, infectious or chronic inflammatory conditions, metabolic diseases, and vascular injury in this study.
Epidemiology and Clinical Manifestations
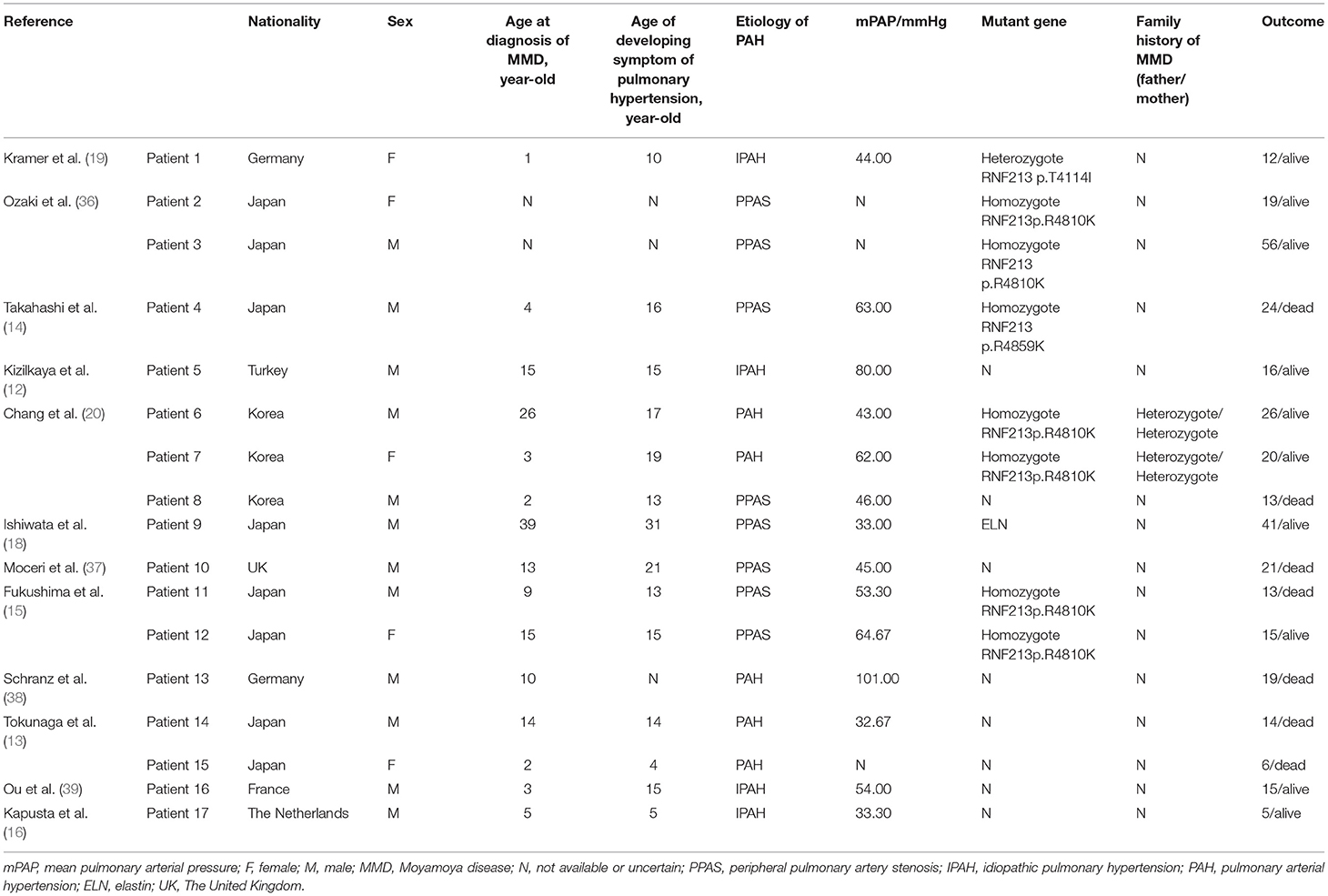
The prevalence of MMD with PAH is relatively high in East Asian countries (Table 1). Since the first clinical case of MMD with PAH was reported, we searched for patients with MMD accompanied by PAH and found 17 patients in 12 pieces of literature ranging from 1990 to 2021, excluding MMD syndrome and other patients with an unknown diagnosis. Among these cases, the minimum age at diagnosis of MMD was 1 year and the maximum age at diagnosis was 39 years. These cases have shown a younger age of MMD with PAH at diagnosis. Of the 14 patients with definite age of diagnosis, MMD often appears before the diagnosis of PAH (8/14), but sometimes appears simultaneously at the diagnosis of PAH (4/14). Additionally, the male-to-female ratio is 12:5, which is not consistent with the epidemiological phenomenon that MMD is predominant in women. The PPAS is most common in MMD with PAH, followed by IPAH. Seven patients died prematurely, with an average age of 15.71 years. Some patients died suddenly (14, 20), some patients died of postoperative complications like cerebral hernia or pneumonia (13), and some patients died for uncertain reasons.
Nine patients conducted genetic testing and up to 77.8% (7/9) of the patients carried homozygous RNF213 p.R4810K mutation [RNF213 p.R4859K and p.R4810K are identical, and p.R4810K is more widely used because it reflects the major transcript that lacks exon 4 (27, 31)]. One patient carried heterozygous RNF213 p.T4114I mutation, and one patient carried elastin (ELN) mutation. The parents of two patients with homozygous p.R4810K mutation carried the heterozygous mutation, whereas the other family history remained unknown. RNF213 p.R4810K is the most important variation in MMD, which is carried by about 80% of East Asians with MMD but is almost absent in European and American patients (27, 28, 40–43). This mutation is predominantly heterozygous in MMD, and homozygous mutations can be observed in 7–8% of patients (33, 44). In this study, the rate of homozygous p.R4810K mutation carrier in patients with MMD and PAH was significantly higher than that in patients with MMD alone. It is interesting that homozygous individuals with MMD and PAH were also found in Western populations. The mutations of RNF213 p.R4810K can affect both the severity and presentation of the disease. The penetrance of MMD in heterozygous is 0.33–0.67%, whereas the incidence of MMD in homozygous is as high as 78% (44). The p.R4810K homozygous variant of RNF213 predicted an early-onset, severe, and higher frequency of bilateral and posterior circulation form of MMD (33, 44, 45). Fukushima believed that the phenotype observed after RNF213 mutation was gene-dosage-dependent manner (15). Heterozygous carriers of p.R4810K mostly presented MMD or PAH alone, whereas homozygous carriers were more likely to develop systemic vascular diseases including intracranial artery and peripheral artery like pulmonary artery involvement (15).
A recent whole-exome sequencing identified RNF213 as the susceptibility gene for PAH (34). RNF213 p.R4810K mutation has a higher positive detection effect than BMPR2, resulting in a higher risk of lung transplantation and death, an earlier onset age, less responsiveness to vasodilators, and a poor prognosis (35). PPAS was the most common cause of pulmonary hypertension in the patients included in this review (8/17), IPAH followed (4/17). PPAS may be underdiagnosed; thus, some patients with IPAH may have PPAS (23). The carrier rate of p.R4810K homozygous in patients with PPAS was much higher than heterozygous and wild type (36).
Thus, these results have suggested the importance of RNF213 in MMD with PAH. In addition, several reports documented that RNF213 gene mutations not only increase the risk of MMD but also are associated with intracranial atherosclerosis (46, 47) and systemic vascular diseases, such as PPAS (15, 20), renal artery stenosis (48), and coronary artery disease (49, 50). Therefore, MMD, PAH, and renal artery stenosis may be the specific manifestations of pathophysiological changes caused by RNF213 mutation, which can be summarized as spectrums of RNF213 vasculopathy (51, 52). The association between RNF213 p.R4810K mutation and individual disease manifestation needs further study.
Pathology
The pathological changes of MMD with PAH are partly similar to MMD or PAH. The typical pathological changes induced by MMD in the stenotic segment are thickening of the intima, medial thinness, and irregular undulation of the internal elastic laminae (53), with no infiltration of inflammatory cells and fats in the vascular wall (54). However, the thickened intima was composed predominantly of smooth muscle cells with an admixture of some macrophages and T-cell scattering in the superficial layer of the intimal thickening (55). Marked perivascular inflammation was present in a high number of PAH lungs and correlated with intima plus media remodeling (56). There are two cases of IPAH (or hereditary) carrying heterozygous RNF213 p.R4810K (35). Pathological examination of the first patient indicated plexiform and concentric neointimal lesions in the pulmonary arteries. In the second case, pathologic examination demonstrated marked venous wall thickness and venous occlusion accompanied by dilation of capillary vessels and lymphedema of the interlobular septa, and arterial wall thickening. PPAS has been widely reported in clinical cases of MMD with PAH (14–16, 20). Takahashi et al. (14) recently reported a case of MMD with PPAS with homozygous RNF213 p.R4810K. Autopsy results showed the proximal pulmonary artery was dilated (the diameter of the pulmonary valve was 65 mm), and multiple stenoses in the branch pulmonary arteries with post-stenotic dilation. In this case, the membrane became thicker and the inner elastic lamina showed continuous and irregular waving, compared with patients with MMD. There are significant differences among the internal elastic layer between the patients with MMD and PAH and patients with PPAS, even though they share the same pathologic changes in intima and media. The irregular waving of the internal elastic (57) is the pathological feature that is not present in PPAS. As the family of the patient refused craniotomy, it is unknown whether the patient's cerebrovascular pathology showed similar pathological changes with the pulmonary artery. Thus, existing pathological results suggest that the mutation of the RNF213 p.R4810K has an important influence on the pathology of MMD with PAH, and further exploration with more samples is required.
Pathogenesis Underlying MMD With PAH
RNF213 and Angiogenesis
The abnormal angiogenesis has a strong association with the development of MMD (58, 59) and PAH (60, 61). Recent studies have suggested the importance of RNF213 in the pathogenesis of MMD with PAH (14, 35). Mysterin, encoded by RNF213 containing enzymatically active AAA+ ATPase and E3 ubiquitin ligase domains, has a complex structure and function, which can participate in several physiological activities in cells, especially plays an important role in angiogenesis. Ito et al. (62) found that angiogenesis in RNF213 knockout mice was enhanced after chronic hind-limb ischemia, suggesting that abnormal RNF213 may be involved in the development of the vascular network in chronic ischemia. The p.R4810K mutation is located in the E3 core at the C-terminal of the RNF213 and affects the function of vascular endothelial cells (63). Meanwhile, Kobayashi et al. (57) found that RNF213 p.R4810K had antiangiogenic activity through decreasing ATPase activity to stabilize oligomers. In vitro experiments, endothelial cells derived from pluripotent stem cells with RNF213 p.R4810K induced significantly lower angiogenesis activity than wild-type cells (64). Further studies showed that RNF213 p.R4810K induced downregulation of securin (64). However, it remains unable to have a comprehensive and systematic explanation for the abnormal angiogenesis of MMD with PAH. We need more investigations to provide an overall insight into their mechanism.
RNF213 and Caveolin-1
Caveolin-1 (Cav-1), the signature protein of endothelial cell caveolae, is closely related to the function of endothelial cells and smooth muscle cells (65, 66). Caveolins participate in many important cellular processes, including vesicular transport, cholesterol homeostasis, signal transduction, and tumor suppression (66). In patients with MMD and homozygous RNF213, p.R4810K mutation, Cav-1 positive expression of RNF213 was found in the thickened intima (14). Recently, researchers found that the expression of Cav-1 in plasma of patients with MMD was decreased, especially in patients carrying RNF213 p.R4810K (30). The decrease of Cav-1 was positively correlated with the narrowing of the distal external diameter of the internal carotid artery in adult patients with MMD (59). In vitro study, the downregulation of Cav-1 expression could induce apoptosis in endothelial cells and lead to lumen formation disorder, which may play a role in the reduction and remodeling of intracranial arterial external diameter (59). In patients with IPAH, Cav-1 protein was reduced in human pulmonary artery endothelial cells (67) but increased in human pulmonary smooth muscle cells (68). Serum Cav-1 level was also significantly decreased in IPAH (69). Cav-1 knockout mice have some prominent features including adverse lung phenotype with thickened alveolar septa, smaller alveolar spaces, and an interstitial hypercellularity, and it is further characterized by substantial PAH accompanied by a hypertrophied right ventricle (70–73). Moreover, Kobayashi, H et al. found that increased ventricular pressure, end-diastolic ventricular diameter ratio, pulmonary vascular muscularization, ablation of pulmonary vascular endothelial cells from the basal membrane, and decreased Cav-1 in mice with RNF213 p.R4810K gene mutation under hypoxia (74). Meanwhile, a study demonstrated that the artery stiffness increased in Cav-1deficient mice, especially the circumferential stiffness of the pulmonary arteries (75). The mutation of the RNF213 p.R4810K may affect endothelial cell function by interference with the Cav-1. In human pulmonary smooth muscle cells of PAH, Cav-1 may influence intracellular calcium concentration (68, 76) and cytoplasmic vesicle transport (77). In MMD with PAH, RNF213 p.R4810K may affect vascular smooth muscle cells via Cav-1. The relationship between RNF213 and Cav-1 will provide a new direction for us to explore the pathogenesis of MMD with PAH. In a word, the mutation of the RNF213 p.R4810K is an important factor leading to pathological abnormalities of PAH in MMD, but the specific mechanism of the effect on pulmonary vascular and cerebrovascular needs to be further studied.
Other Factors
In some cases of MMD with PAH, pulmonary angiography suggests peripheral pulmonary stenosis with beading and bending (15, 20, 39). Arterial beaded lesions are an important feature of fibromuscular dysplasia (78). Fibromuscular dysplasia is a rare non-atherosclerotic and non-inflammatory arterial disease that mainly involves small and medium arteries. There are two patients with homozygous RNF213 p.R4810K mutations accompanying MMD with bead-like pulmonary angiography (15, 20). Fibromuscular dysplasia (FMD) may be the basis of intracranial and extracranial vascular lesions in MMD. Recently, a study reported that RNF213 rare coding variants suggested a gene-based association with multifocal FMD (79). However, p.R4810k was not present in the exome array that generated genotypes in FMD cases (79). Therefore, the relationship between RNF213 other gene mutations and FMD needs further study. In addition, a study reported a clinical case of ELN mutation in MMD with PAH. Elastic fibers play an important role in maintaining the elasticity of tissues, such as arteries, lungs, and skin. Elastic fiber abnormality can cause a variety of cardiovascular and skin diseases, such as descending aortic stenosis, descending aortic stenosis, and skin laxity (80–82). This suggests that abnormal vascular wall components may lead to systemic vascular diseases, such as MMD, PAH, renal artery stenosis, and other symptoms.
Diagnosis and Treatment
When young patients, with a clear history of MMD, show dyspnea and/or syncope, we should be vigilant for PAH (14, 15, 19, 20, 36). In the same way, when the patient with PAH showed unprovoked and spontaneously recurring epileptic seizures, movement disorder, and retardation of psychomotor development, we should be suspicious about MMD (12, 19). The patients with MMD and PAH did not show any specific signs in physical examination (20). From a clinical standpoint, cerebrovascular or pulmonary vascular investigations may be warranted in patients with PAH or MMD, respectively. As MMD and PAH have their complete diagnostic criteria, respectively, it's not difficult to have definite diagnoses for both diseases. In patients with MMD with RNF213 p.R4810k, they can accompany PAH (35). Thus, regular echocardiographic screening for early signs of PAH in patients with MMD should be part of regular clinical workup (19). RNF213 gene mutations, especially p.R4810k are associated with various intracranial and extracranial vascular lesions, such as intracranial artery stenosis/occlusion disease (83, 84), intracranial atherosclerosis (85), PPAS (15, 20), renal artery stenosis (48), coronary artery disease (49, 50), superior mesenteric arteries stenosis (86), and so on (87). Therefore, if patients with MMD had a homozygous (or compound heterozygous) RNF213 mutation, systemic screening may be useful. In this way, early detection and treatment of MMD with PAH might help to improve the long-term outcome and quality of life.
As the treatment of MMD with PAH, there is still in the exploratory stage. Some scholars suggest patients with MMD and PAH should be treated as early as possible with dual/triple therapy (19). However, the therapeutic effect of drugs on MMD with PAH is not obvious (12, 20), and there are not enough case studies. At the same time, there is a controversy about the application of angioplasty in MMD with PAH (20, 37). Vasculopathy in MMD involves intimal hyperplasia of smooth muscle cells and possibly results in immediate elastic recoil or progressive restenosis, which has been noted during angioplasty of cerebral arteries in patients with MMD (88). As MMD with PAH shares the same pathologic changes in intima and media with MMD, the same thing may happen in the patients of MMD with PAH (15). Hence, we should be cautious when treating these patients with MMD and PAH with percutaneous angioplasty. A palliative Potts shunt may be a good choice for end-stage patients with MMD and PAH (38). Thus, more studies on MMD with PAH are needed to find an effective treatment for the disease.
Conclusion
In conclusion, we have reviewed that RNF213 plays an important role in MMD with PAH at epidemiology, pathology, possible pathogenesis, clinical manifestations, clinical manifestations, diagnosis, and treatment. Most patients with MMD complicated with PAH carried RNF213 p.R4810K and were accompanied by mutation-associated pathophysiological changes. Thus, the detection of RNF213 mutation, especially p.R4810K, could be used as a part of the etiology study for MMD with PAH. At the same time, RNF213 p.R4810K may be a predictor for systemic vascular examination. Although it is unable to have a comprehensive and systematic explanation for the occurrence and development of MMD with PAH, we can regard RNF213 as a target to better understand the possible pathogenesis of this disease. In particular, significant research will have to be undertaken to uncover the relationship between RNF213 and vascular disease, which is not limited to MMD.
Author Contributions
XS and SW designed this review. YL drafted the manuscript. YL and ZC searched and reviewed the database and all included articles and reviewed the articles based on inclusion and exclusion criteria. XS, SW, and YL provided comments and revised the manuscript. All authors have approved the final version of the manuscript.
Funding
This study was funded by the National Natural Science Foundation of China (82071292, 81601004, and 81102248), Kelin New Talent Program (R08016), the Natural Science Foundation of Guangdong Province (2016A030310139), the Fundamental Research Funds for the Central Universities (20ykpy66), the Guangdong Provincial Key Laboratory of Diagnosis and Treatment of Major Neurological Diseases (2020B1212060017), the Guangdong Provincial Clinical Research Center for Neurological Diseases (2020B1111170002), the Southern China International Cooperation Base for Early Intervention and Functional Rehabilitation of Neurological Diseases (2015B050501003 and 2020A0505020004), the Guangdong Provincial Engineering Center for Major Neurological Disease Treatment, and the Guangdong Provincial Translational Medicine Innovation Platform for Diagnosis and Treatment of Major Neurological Disease.
Conflict of Interest
The authors declare that the review was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank all lab members for constructive discussions.
References
1. Shang S, Zhou D, Ya J, Li S, Yang Q, Ding Y, et al. Progress in Moyamoya disease. Neurosurg Rev. (2020) 43:371–82. doi: 10.1007/s10143-018-0994-5
2. Ahn IM, Park DH, Hann HJ, Kim KH, Kim HJ, Ahn HS. Incidence, prevalence, and survival of Moyamoya disease in Korea: a nationwide, population-based study. Stroke. (2014) 45:1090–5. doi: 10.1161/STROKEAHA.113.004273
3. Fukui M. Guidelines for the diagnosis and treatment of spontaneous occlusion of the circle of Willis (‘Moyamoya’ disease) Research Committee on Spontaneous Occlusion of the Circle of Willis (Moyamoya Disease) of the Ministry of Health and Welfare, Japan. Clin Neurol Neurosurg. (1997) 99:S238–40. doi: 10.1016/S0303-8467(97)00082-6
4. Tho-Calvi SC, Thompson D, Saunders D, Agrawal S, Basu A, Chitre M, et al. Clinical features, course, and outcomes of a UK cohort of pediatric Moyamoya. Neurology. (2018) 90:e763–70. doi: 10.1212/WNL.0000000000005026
5. Zhang X, Xiao W, Zhang Q, Xia D, Gao P, Su J, et al. Progression in Moyamoya Disease: Clinical Feature, Neuroimaging Evaluation and Treatment. Curr Neuropharmacol. (2021).
6. Scott RM, Smith ER. Moyamoya disease and Moyamoya syndrome. N Engl J Med. (2009) 360:1226–37. doi: 10.1056/NEJMra0804622
7. Unda SR, Antoniazzi AM, Miller R, Klyde D, Javed K, Fluss R, et al. Moyamoya Disease and Syndrome: A National Inpatient Study of Ischemic Stroke Predictors. J Stroke Cerebrovasc Dis. (2021) 30:105965. doi: 10.1016/j.jstrokecerebrovasdis.2021.105965
8. Amlie-Lefond C, Bernard TJ, Sébire G, Friedman NR, Heyer GL, Lerner NB, et al. Predictors of cerebral arteriopathy in children with arterial ischemic stroke: results of the International Pediatric Stroke Study. Circulation. (2009) 119:1417–23. doi: 10.1161/CIRCULATIONAHA.108.806307
9. Lee JH, Youn TJ, Yoon YE, Park JJ, Hong SJ, Chun EJ, et al. Coronary artery stenosis in Moyamoya disease: tissue characterization by 256-slice multi-detector CT and virtual histology. Circulation. (2013) 127:2063–5. doi: 10.1161/CIRCULATIONAHA.112.136473
10. Baek JW, Jo KI, Park JJ, Jeon P, Kim KH. Prevalence and clinical implications of renal artery stenosis in pediatric Moyamoya disease. Eur J Paediatr Neurol. (2016) 20:20–4. doi: 10.1016/j.ejpn.2015.11.002
11. Choi Y, Kang BC, Kim KJ, Cheong HI, Hwang YS, Wang KC, et al. Renovascular hypertension in children with Moyamoya disease. J Pediatr. (1997) 131:258–63. doi: 10.1016/S0022-3476(97)70163-X
12. Kizilkaya MH, Uysal F, Gurbuz E, Taskapilioglu MO, Bostan OM. Atypical presentation of Moyamoya disease with pulmonary hypertension: a case report. Anatol J Cardiol. (2018) 19:350–1. doi: 10.14744/AnatolJCardiol.2018.65642
13. Tokunaga K, Hishikawa T, Sugiu K, Date I. Fatal outcomes of pediatric patients with Moyamoya disease associated with pulmonary arterial hypertension. Report of two cases Clin Neurol Neurosurg. (2013) 115:335–8. doi: 10.1016/j.clineuro.2012.05.002
14. Takahashi K, Nakamura J, Sakiyama S, Nakaya T, Sato T, Watanabe T, et al. A histopathological report of a 16-year-old male with peripheral pulmonary artery stenosis and Moyamoya disease with a homozygous RNF213 mutation. Respir Med Case Rep. (2020) 29:100977. doi: 10.1016/j.rmcr.2019.100977
15. Fukushima H, Takenouchi T, Kosaki K. Homozygosity for Moyamoya disease risk allele leads to Moyamoya disease with extracranial systemic and pulmonary vasculopathy. Am J Med Genet A. (2016) 170:2453–6. doi: 10.1002/ajmg.a.37829
16. Kapusta L, Daniels O, Renier WO. Moya-Moya syndrome and primary pulmonary hypertension in childhood. Neuropediatrics. (1990) 21:162–3. doi: 10.1055/s-2008-1071486
17. de Vries RR, Nikkels PG, van der Laag J, Broere G, Braun KP. Moyamoya and extracranial vascular involvement: fibromuscular dysplasia? A report of two children Neuropediatrics. (2003) 34:318–21. doi: 10.1055/s-2003-44664
18. Ishiwata T, Tanabe N, Shigeta A, Yokota H, Tsushima K, Terada J, et al. Moyamoya disease and artery tortuosity as rare phenotypes in a patient with an elastin mutation. Am J Med Genet A. (2016) 170:1924–7. doi: 10.1002/ajmg.a.37662
19. Kramer J, Beer M, Kaestner M, Bride P, Winter B, Apitz C. Moyamoya disease associated with pediatric pulmonary hypertension-a case report. Cardiovasc Diagn Ther. (2021) 11:1052–6. doi: 10.21037/cdt-20-249
20. Chang SA, Song JS, Park TK, Yang JH, Kwon WC, Kim SR, et al. Nonsyndromic Peripheral Pulmonary Artery Stenosis Is Associated With Homozygosity of RNF213 p.Arg4810Lys Regardless of Co-occurrence of Moyamoya Disease. Chest. (2018) 153:404–13. doi: 10.1016/j.chest.2017.09.023
21. Galiè N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J. (2016) 37:67–119. doi: 10.1093/eurheartj/ehv317
22. Al-Khaldi A, Mohammed Y, Tamimi O, Alharbi A. Early outcomes of total pulmonary arterial reconstruction in patients with arterial tortuosity syndrome. Ann Thorac Surg. (2011) 92:698–704. doi: 10.1016/j.athoracsur.2011.03.068
23. Tonelli AR, Ahmed M, Hamed F, Prieto LR. Peripheral pulmonary artery stenosis as a cause of pulmonary hypertension in adults. Pulm Circ. (2015) 5:204–10. doi: 10.1086/679727
24. Delaney TB, Nadas AS. Peripheral Pulmonic Stenosis. Am J Cardiol. (1964) 13:451–61. doi: 10.1016/0002-9149(64)90152-3
25. Beuren AJ, Schulze C, Eberle P, Harmjanz D, Apitz J. The Syndrome of Supravalvular Aortic Stenosis, Peripheral Pulmonary Stenosis, Mental Retardation and Similar Facial Appearance. Am J Cardiol. (1964) 13:471–83. doi: 10.1016/0002-9149(64)90154-7
26. Hemnes AR, Zhao M, West J, Newman JH, Rich S, Archer SL, et al. Critical genomic networks and vasoreactive variants in idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med. (2016) 194:464–75. doi: 10.1164/rccm.201508-1678OC
27. Liu W, Morito D, Takashima S, Mineharu Y, Kobayashi H, Hitomi T, et al. Identification of RNF213 as a susceptibility gene for Moyamoya disease and its possible role in vascular development. PLoS ONE. (2011) 6:e22542. doi: 10.1371/journal.pone.0022542
28. Kamada F, Aoki Y, Narisawa A, Abe Y, Komatsuzaki S, Kikuchi A, et al. A genome-wide association study identifies RNF213 as the first Moyamoya disease gene. J Hum Genet. (2011) 56:34–40. doi: 10.1038/jhg.2010.132
29. Sugihara M, Morito D, Ainuki S, Hirano Y, Ogino K, Kitamura A, et al. The AAA+ ATPase/ubiquitin ligase mysterin stabilizes cytoplasmic lipid droplets. J Cell Biol. (2019) 218:949–60. doi: 10.1083/jcb.201712120
30. Bang OY, Chung JW, Kim SJ, Oh MJ, Kim SY, Cho YH, et al. Caveolin-1, Ring finger protein 213, and endothelial function in Moyamoya disease. Int J Stroke. (2016) 11:999–1008. doi: 10.1177/1747493016662039
31. Ahel J, Lehner A, Vogel A, Schleiffer A, Meinhart A, Haselbach D, et al. Moyamoya disease factor RNF213 is a giant E3 ligase with a dynein-like core and a distinct ubiquitin-transfer mechanism. Elife. (2020) 9:e56185. doi: 10.7554/eLife.56185.sa2
32. Morito D, Nishikawa K, Hoseki J, Kitamura A, Kotani Y, Kiso K, et al. Moyamoya disease-associated protein mysterin/RNF213 is a novel AAA+ ATPase, which dynamically changes its oligomeric state. Sci Rep. (2014) 4:4442. doi: 10.1038/srep04442
33. Kim EH, Yum MS, Ra YS, Park JB, Ahn JS, Kim GH, et al. Importance of RNF213 polymorphism on clinical features and long-term outcome in Moyamoya disease. J Neurosurg. (2016) 124:1221–7. doi: 10.3171/2015.4.JNS142900
34. Suzuki H, Kataoka M, Hiraide T, Aimi Y, Yamada Y, Katsumata Y, et al. Genomic comparison with supercentenarians identifies RNF213 as a risk gene for pulmonary arterial hypertension. Circ Genom Precis Med. (2018) 11:e002317. doi: 10.1161/CIRCGEN.118.002317
35. Hiraide T, Kataoka M, Suzuki H, Aimi Y, Chiba T, Isobe S, et al. Poor outcomes in carriers of the RNF213 variant. (p.Arg4810Lys) with pulmonary arterial hypertension. J Heart Lung Transplant. (2020) 39:103–12. doi: 10.1016/j.healun.2019.08.022
36. Ozaki D, Endo H, Tashiro R, Sugimura K, Tatebe S, Yasuda S, et al. Association between RNF213 c.14576G>A Variant. (rs112735431) and Peripheral Pulmonary Artery Stenosis in Moyamoya Disease. Cerebrovasc Dis. (2021) 1–6. doi: 10.1159/000519717
37. Moceri P, Laïk J, Bouvaist H, Fraisse A, Ferrari E. Peripheral pulmonary artery stenoses in the setting of Moyamoya. Eur Heart J Cardiovasc Imaging. (2016) 17:575. doi: 10.1093/ehjci/jew006
38. Schranz D, Kerst G, Menges T, Akintürk H, van Alversleben I, Ostermayer S, et al. Transcatheter creation of a reverse Potts shunt in a patient with severe pulmonary arterial hypertension associated with Moyamoya syndrome. EuroIntervention. (2015) 11:121. doi: 10.4244/EIJV11I1A21
39. Ou P, Dupont P, Bonnet D. Fibromuscular dysplasia as the substrate for systemic and pulmonary hypertension in the setting of Moya-Moya disease. Cardiol Young. (2006) 16:495–7. doi: 10.1017/S104795110600045X
40. Moteki Y, Onda H, Kasuya H, Yoneyama T, Okada Y, Hirota K, et al. Systematic Validation of RNF213 Coding Variants in Japanese Patients With Moyamoya Disease. J Am Heart Assoc. (2015) 4:e001862. doi: 10.1161/JAHA.115.001862
41. Cao Y, Kobayashi H, Morimoto T, Kabata R, Harada KH, Koizumi A. Frequency of RNF213 p.R4810K a susceptibility variant for Moyamoya disease, and health characteristics of carriers in the Japanese population. Environ Health Prev Med. (2016) 21:387–90. doi: 10.1007/s12199-016-0549-8
42. Jang MA, Shin S, Yoon JH Ki CS. Frequency of the Moyamoya-related RNF213 p.Arg4810Lys variant in 1,516 Korean individuals. BMC Med Genet. (2015) 16:109. doi: 10.1186/s12881-015-0252-4
43. Ma J, Liu Y, Ma L, Huang S, Li H, You C. RNF213 polymorphism and Moyamoya disease: A systematic review and meta-analysis. Neurol India. (2013) 61:35–9. doi: 10.4103/0028-3886.107927
44. Miyatake S, Miyake N, Touho H, Nishimura-Tadaki A, Kondo Y, Okada I, et al. Homozygous c.14576G>A variant of RNF213 predicts early-onset and severe form of Moyamoya disease. Neurology. (2012) 78:803–10. doi: 10.1212/WNL.0b013e318249f71f
45. Wang Y, Zhang Z, Wei L, Zhang Q, Zou Z, Yang L, et al. Predictive role of heterozygous p.R4810K of RNF213 in the phenotype of Chinese Moyamoya disease. Neurology. (2020) 94:e678–e86. doi: 10.1212/WNL.0000000000008901
46. Bang OY, Chung JW, Cha J, Lee MJ, Yeon JY Ki CS, et al. A Polymorphism in RNF213 Is a Susceptibility Gene for Intracranial Atherosclerosis. PLoS ONE. (2016) 11:e0156607. doi: 10.1371/journal.pone.0156607
47. Okazaki S, Morimoto T, Kamatani Y, Kamimura T, Kobayashi H, Harada K, et al. Moyamoya Disease Susceptibility Variant RNF213 p.R4810K Increases the Risk of Ischemic Stroke Attributable to Large-Artery Atherosclerosis. Circulation. (2019) 139:295–8. doi: 10.1161/CIRCULATIONAHA.118.038439
48. Pinard A, Fiander MDJ, Cecchi AC, Rideout AL, Azouz M, Fraser SM, et al. Association of De Novo RNF213 Variants With Childhood Onset Moyamoya Disease and Diffuse Occlusive Vasculopathy. Neurology. (2021) 96:e1783–e91. doi: 10.1212/WNL.0000000000011653
49. Morimoto T, Mineharu Y, Ono K, Nakatochi M, Ichihara S, Kabata R, et al. Significant association of RNF213 p.R4810K a Moyamoya susceptibility variant, with coronary artery disease. PLoS ONE. (2017) 12:e0175649. doi: 10.1371/journal.pone.0175649
50. Koyama S, Ito K, Terao C, Akiyama M, Horikoshi M, Momozawa Y, et al. Population-specific and trans-ancestry genome-wide analyses identify distinct and shared genetic risk loci for coronary artery disease. Nat Genet. (2020) 52:1169–77. doi: 10.1038/s41588-020-0705-3
51. Bang OY, Chung JW, Kim DH, Won HH, Yeon JY Ki CS, et al. Moyamoya Disease and Spectrums of RNF213 Vasculopathy. Transl Stroke Res. (2020) 11:580–9. doi: 10.1007/s12975-019-00743-6
52. Mineharu Y, Takagi Y, Miyamoto S. Significance of RNF213 in clinical management in Japan. Moyamoya disease explored through RNF213. Curr Top Environ Health Prevent Med. (2017) 137–50. doi: 10.1007/978-981-10-2711-6_11
53. Fujimura M, Bang OY, Kim JS. Moyamoya Disease. Front Neurol Neurosci. (2016) 40:204–20. doi: 10.1159/000448314
54. Sun SJ, Zhang JJ Li ZW, Xiong ZW, Wu XL, Wang S, et al. Histopathological features of middle cerebral artery and superficial temporal artery from patients with Moyamoya disease and enlightenments on clinical treatment. J Huazhong Univ Sci Technolog Med Sci. (2016) 36:871–5. doi: 10.1007/s11596-016-1677-5
55. Masuda J, Ogata J, Yutani C. Smooth muscle cell proliferation and localization of macrophages and T cells in the occlusive intracranial major arteries in Moyamoya disease. Stroke. (1993) 24:1960–7. doi: 10.1161/01.STR.24.12.1960
56. Stacher E, Graham BB, Hunt JM, Gandjeva A, Groshong SD, McLaughlin VV, et al. Modern age pathology of pulmonary arterial hypertension. Am J Respir Crit Care Med. (2012) 186:261–72. doi: 10.1164/rccm.201201-0164OC
57. Kobayashi H, Matsuda Y, Hitomi T, Okuda H, Shioi H, Matsuda T, et al. Biochemical and Functional Characterization of RNF213. (Mysterin) R4810K, a Susceptibility Mutation of Moyamoya Disease, in Angiogenesis In Vitro and In Vivo. J Am Heart Assoc. (2015) 4:e002146. doi: 10.1161/JAHA.115.002146
58. Hu J, Luo J, Chen Q. The Susceptibility Pathogenesis of Moyamoya Disease. World Neurosurg. (2017) 101:731–41. doi: 10.1016/j.wneu.2017.01.083
59. Chung JW, Kim DH, Oh MJ, Cho YH, Kim EH, Moon GJ, et al. Cav-1. (Caveolin-1) and arterial remodeling in adult Moyamoya disease. Stroke. (2018) 49:2597–604. doi: 10.1161/STROKEAHA.118.021888
60. Hennigs JK, Cao A, Li CG, Shi M, Mienert J, Miyagawa K, et al. PPARγ-p53-mediated vasculoregenerative program to reverse pulmonary hypertension. Circ Res. (2021) 128:401–18. doi: 10.1161/CIRCRESAHA.119.316339
61. Oliveira SDS, Chen J, Castellon M, Mao M, Raj JU, Comhair S, et al. Injury-induced shedding of extracellular vesicles depletes endothelial cells of Cav-1 (Caveolin-1) and Enables TGF-β (Transforming Growth Factor-β)-dependent pulmonary arterial hypertension. Arterioscler Thromb Vasc Biol. (2019) 39:1191–202. doi: 10.1161/ATVBAHA.118.312038
62. Ito A, Fujimura M, Niizuma K, Kanoke A, Sakata H, Morita-Fujimura Y, et al. Enhanced post-ischemic angiogenesis in mice lacking RNF213; a susceptibility gene for Moyamoya disease. Brain Res. (2015) 1594:310–20. doi: 10.1016/j.brainres.2014.11.014
63. Mineharu Y, Miyamoto S. RNF213 and GUCY1A3 in Moyamoya disease: key regulators of metabolism, inflammation, and vascular stability. Front Neurol. (2021) 12:687088. doi: 10.3389/fneur.2021.687088
64. Hitomi T, Habu T, Kobayashi H, Okuda H, Harada KH, Osafune K, et al. Downregulation of Securin by the variant RNF213 R4810K. (rs112735431. G>A) reduces angiogenic activity of induced pluripotent stem cell-derived vascular endothelial cells from Moyamoya patients. Biochem Biophys Res Commun. (2013) 438:13–9. doi: 10.1016/j.bbrc.2013.07.004
65. Chang SH, Feng D, Nagy JA, Sciuto TE, Dvorak AM, Dvorak HF. Vascular permeability and pathological angiogenesis in caveolin-1-null mice. Am J Pathol. (2009) 175:1768–76. doi: 10.2353/ajpath.2009.090171
66. Williams TM, Lisanti MP. The caveolin proteins. Genome Biol. (2004) 5:214. doi: 10.1186/gb-2004-5-3-214
67. Bakhshi FR, Mao M, Shajahan AN, Piegeler T, Chen Z, Chernaya O, et al. Nitrosation-dependent caveolin 1 phosphorylation, ubiquitination, and degradation and its association with idiopathic pulmonary arterial hypertension. Pulm Circ. (2013) 3:816–30. doi: 10.1086/674753
68. Patel HH, Zhang S, Murray F, Suda RY, Head BP, Yokoyama U, et al. Increased smooth muscle cell expression of caveolin-1 and caveolae contribute to the pathophysiology of idiopathic pulmonary arterial hypertension. FASEB J. (2007) 21:2970–9. doi: 10.1096/fj.07-8424com
69. Wang KY, Lee MF, Ho HC, Liang KW, Liu CC, Tsai WJ, et al. Serum Caveolin-1 as a novel biomarker in idiopathic pulmonary artery hypertension. Biomed Res Int. (2015) 2015:173970. doi: 10.1155/2015/173970
70. Wunderlich C, Schmeisser A, Heerwagen C, Ebner B, Schober K, Braun-Dullaeus RC, et al. Chronic NOS inhibition prevents adverse lung remodeling and pulmonary arterial hypertension in caveolin-1 knockout mice. Pulm Pharmacol Ther. (2008) 21:507–15. doi: 10.1016/j.pupt.2007.11.005
71. Razani B, Engelman JA, Wang XB, Schubert W, Zhang XL, Marks CB, et al. Caveolin-1 null mice are viable but show evidence of hyperproliferative and vascular abnormalities. J Biol Chem. (2001) 276:38121–38. doi: 10.1074/jbc.M105408200
72. Drab M, Verkade P, Elger M, Kasper M, Lohn M, Lauterbach B, et al. Loss of caveolae, vascular dysfunction, and pulmonary defects in caveolin-1 gene-disrupted mice. Science. (2001) 293:2449–52. doi: 10.1126/science.1062688
73. Zhao YY, Liu Y, Stan RV, Fan L, Gu Y, Dalton N, et al. Defects in caveolin-1 cause dilated cardiomyopathy and pulmonary hypertension in knockout mice. Proc Natl Acad Sci USA. (2002) 99:11375–80. doi: 10.1073/pnas.172360799
74. Kobayashi H, Kabata R, Kinoshita H, Morimoto T, Ono K, Takeda M, et al. Rare variants in RNF213, a susceptibility gene for Moyamoya disease, are found in patients with pulmonary hypertension and aggravate hypoxia-induced pulmonary hypertension in mice. Pulm Circ. (2018) 8:2045894018778155. doi: 10.1177/2045894018778155
75. Moreno J, Escobedo D, Calhoun C, Le Saux CJ, Han HC. Arterial Wall Stiffening in Caveolin-1 Deficiency-Induced Pulmonary Artery Hypertension in Mice. Exp Mech. (2021) 6:217–28. doi: 10.1007/s11340-020-00666-6
76. Mu YP, Lin DC, Yan FR, Jiao HX, Gui LX, Lin MJ. Alterations in Caveolin-1 Expression and Receptor-Operated Ca2+ Entry in the Aortas of Rats with Pulmonary Hypertension. Cell Physiol Biochem. (2016) 39:438–52. doi: 10.1159/000445637
77. Mukhopadhyay S, Shah M, Xu F, Patel K, Tuder RM, Sehgal PB. Cytoplasmic provenance of STAT3 and PY-STAT3 in the endolysosomal compartments in pulmonary arterial endothelial and smooth muscle cells: implications in pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol. (2008) 294:L449–68. doi: 10.1152/ajplung.00377.2007
78. Khoury MH, Gornik HL. Fibromuscular dysplasia (FMD). Vasc Med. (2017) 22:248–52. doi: 10.1177/1358863X17700716
79. Kiando SR, Barlassina C, Cusi D, Galan P, Lathrop M, Plouin PF, et al. Exome sequencing in seven families and gene-based association studies indicate genetic heterogeneity and suggest possible candidates for fibromuscular dysplasia. J Hypertens. (2015) 33:1802–10; discussion 10. doi: 10.1097/HJH.0000000000000625
80. Corbett E, Glaisyer H, Chan C, Madden B, Khaghani A, Yacoub M. Congenital cutis laxa with a dominant inheritance and early onset emphysema. Thorax. (1994) 49:836–7. doi: 10.1136/thx.49.8.836
81. Carta L, Wagenseil JE, Knutsen RH, Mariko B, Faury G, Davis EC, et al. Discrete contributions of elastic fiber components to arterial development and mechanical compliance. Arterioscler Thromb Vasc Biol. (2009) 29:2083–9. doi: 10.1161/ATVBAHA.109.193227
82. Baldwin AK, Simpson A, Steer R, Cain SA, Kielty CM. Elastic fibres in health and disease. Expert Rev Mol Med. (2013) 15:e8. doi: 10.1017/erm.2013.9
83. Wang Y, Mambiya M, Li Q, Yang L, Jia H, Han Y, et al. RNF213 p.R4810K Polymorphism and the Risk of Moyamoya Disease. Intracranial major artery stenosis/occlusion and quasi-Moyamoya disease: a meta-analysis. J Stroke Cerebrovasc Dis. (2018) 27:2259–70. doi: 10.1016/j.jstrokecerebrovasdis.2018.04.013
84. Miyawaki S, Imai H, Shimizu M, Yagi S, Ono H, Mukasa A, et al. Genetic variant RNF213 c.14576G>A in various phenotypes of intracranial major artery stenosis/occlusion. Stroke. (2013) 44:2894–7. doi: 10.1161/STROKEAHA.113.002477
85. Hongo H, Miyawaki S, Imai H, Shinya Y, Ono H, Mori H, et al. Smaller outer diameter of atherosclerotic middle cerebral artery associated with RNF213 c.14576G>A Variant. (rs112735431). Surg Neurol Int. (2017) 8:104. doi: 10.4103/sni.sni_59_17
86. Nomura S, Aihara Y, Akagawa H, Chiba K, Yamaguchi K, Kawashima A, et al. Can Moyamoya disease susceptibility gene affect extracranial systemic artery stenosis? J Stroke Cerebrovasc Dis. (2020) 29:104532. doi: 10.1016/j.jstrokecerebrovasdis.2019.104532
87. Jee TK, Yeon JY, Kim SM, Bang OY, Kim JS, Hong SC. Prospective screening of extracranial systemic arteriopathy in young adults with Moyamoya disease. J Am Heart Assoc. (2020) 9:e016670. doi: 10.1161/JAHA.120.016670
Keywords: Moyamoya disease, pulmonary arterial hypertension, RNF213, idiopathic pulmonary arterial hypertension, peripheral pulmonary artery stenosis
Citation: Luo Y, Cao Z, Wu S and Sun X (2022) Ring Finger Protein 213 in Moyamoya Disease With Pulmonary Arterial Hypertension: A Mini-Review. Front. Neurol. 13:843927. doi: 10.3389/fneur.2022.843927
Received: 27 December 2021; Accepted: 09 February 2022;
Published: 24 March 2022.
Edited by:
Yasushi Takagi, Tokushima University, JapanCopyright © 2022 Luo, Cao, Wu and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shaoqing Wu, d3Nxb25lQDE2My5jb20=; Xunsha Sun, c3VueHNoM0BtYWlsLnN5c3UuZWR1LmNu
 Yuting Luo
Yuting Luo Zhixin Cao1
Zhixin Cao1 Xunsha Sun
Xunsha Sun