- 1Department of Interventional Neuroradiology, Beijing Neurosurgical Institute and Beijing Tiantan Hospital, Capital Medical University, Beijing, China
- 2Beijing Engineering Research Center, Beijing, China
Background and Purpose: The Neuroform EZ stent system (Boston Scientific Corporation, Fremont, CA, United States) is a fourth-generation intracranial aneurysm stent designed specifically for the cerebrovasculature to support aneurysm treatment. In this study, we analyzed our consecutive series of patients with aneurysm treated with the Neuroform EZ stent, with special attention to the occurrence of in-stent stenosis (ISS).
Methods: A retrospective review of our center's electronic database was conducted to identify all patients with intracranial aneurysms who underwent aneurysm treatment with the Neuroform EZ stent between January 2016 and October 2018. Patients with at least one digital subtraction angiography (DSA) follow-up in our hospital were enrolled in this study. In-stent stenosis (ISS) was graded as mild (<2–5%), moderate (25–50%), or severe (>50%).
Results: The study included 114 patients (78 women, 68.4%; median age 57.2 ± 9 years) with a total of 116 aneurysms. Of the 116 lesions, 20 were identified with ISS (17.2%) at a mean follow-up of 6.9 ± 1.7 months, and ISS was mild in 30% (6/20), moderate in 50% (10/20), and severe in 20% (4/20). No patients were symptomatic or required further intervention. Patients who developed ISS were younger than those without ISS (52.6 ± 7.8 vs. 57.9 ± 9; p = 0.016). The proportion of aneurysms located at the artery bifurcation was significantly higher in patients with stenosis than located at the sidewall artery (37.9 vs. 10.3%; p = 0.002). In the multivariable analysis, the patients' age (OR = 0.94; 95% CI 0.88–0.998; p = 0.02) and bifurcated aneurysm location (OR = 4.59; 95% CI 1.54–13.67; p = 0.006) were independent predictors of ISS.
Conclusions: In this retrospective study, the incidence of ISS after Neuroform EZ stent placement was 17.2%, and all the ISS cases were asymptomatic. Patients with younger age and bifurcated aneurysm location are more likely to develop ISS. Although Neuroform EZ stent is particularly suitable for bifurcated aneurysms, the ISS for this location should be focused upon.
Introduction
The Neuroform Microdelivery Stent System (Stryker Neurovascular, Fremont, CA, United States) was the first device approved by the Food and Drug Administration (FDA) in 2002 to treat intracranial aneurysms. Subsequent product iterations, especially the Neuroform EZ stent system (NF-EZ), were approved in 2010. The NF-EZ stent is an excellent choice for most intracranial aneurysms, specifically those with tight bends in the parent vessel or aneurysms located at the artery bifurcation (1).
Although much attention has been paid to the cure rate, ischemic complications, and hemorrhage of aneurysms, delayed complications of in-stent stenosis (ISS) have not been extensively studied. The reported incidence of ISS after stent-assisted coiling (SAC) depends on the type of stent, and ranges from 2.3 to 17.9% (2–7). However, there is lack of data on ISS after NF-EZ stenting for an aneurysm. In this study, we analyzed our consecutive series of patients with aneurysm treated with NF-EZ SAC, with special attention to the occurrence of ISS.
Methods
Study Population
We retrospectively reviewed patients with intracranial aneurysms who received SAC therapy with the NF-EZ stent at the neurological intervention center of our hospital between January 2016 and October 2018. Patients were included in this study if they underwent at least one cerebral digital subtraction angiography (DSA) follow-up examination in our hospital. Patient demographics, aneurysm characteristics, and clinical and angiographic outcomes were reviewed. This study was approved by the institutional review board of our hospital. The consent of each patient was obtained before study enrollment.
Endovascular Procedure
Patients who were considered for treatment were receiving antiplatelet pharmacotherapy (100 mg aspirin and 75 mg clopidogrel daily) for at least 5 days before the procedure, including on the day of the procedure. Patients with preoperative subarachnoid hemorrhage were provided with an antiplatelet medication following the procedure. All the procedures were performed under general anesthesia via a femoral approach. When the stent was released completely, visualization of the 4 platinum marker bands at both ends of the stent affirmed the proximal and distal positions of the stent in the parent vessel. Coil embolization was mainly performed with the introduction of a microcatheter through the cell of the stent. Following the procedure, dual antiplatelet therapy was maintained for 6 months, and aspirin was continued indefinitely thereafter.
Assessment of ISS
ISS is defined as any loss in the diameter of the parent artery around the stent implantation segment in angiographic follow-up images. The degree of stenosis was evaluated based on the European Carotid Surgery Trial standard (8). The percentage of stenosis was calculated as 1–(narrowest vessel diameter within the stenosis/maximum vessel diameter of the artery) ×100%. ISS was graded as mild (<25%), moderate (25–50%), or severe (>50%). Additionally, ISS was classified as proximal, middle, or distal based on location, and as focal or diffuse based on whether the stenosis extended more than 10 mm. The assessment of ISS was performed by a neuroradiologist with 3 years of experience through DSA follow-up images, and the assessments were reviewed by a senior neuroradiologist.
Statistical Analysis
Data are presented as frequency for categorical variables and as the mean with range for continuous variables. Chi-square test or Fisher exact test was conducted to analyze categorical variables, and independent samples t-test was conducted to analyze continuous variables. Binary logistic regression analysis was conducted to identify significant independent predictors of severe ISS. Variables that were found to be significant at the level of 0.1 in univariate analysis or based on clinical relevance were subjected to binary logistic regression analysis. The results are presented in the form of odds ratio (OR) and corresponding 95% confidence interval (CI). A value of p < 0.05 was considered to indicate statistical significance. A receiver operating characteristic (ROC) curve was used to analyze the performance of a logistic regression classification model. We performed statistical analysis and plotted the figures using SPSS and GraphPad software.
Results
Of the 301 patients that received SAC treatment using NF-EZ stent in our center between January 2016 and October 2018, 114 (78 women, 68.4%; median age 57.2 ± 9 years) with a total of 116 aneurysms had at least one DSA follow-up examination and were eventually enrolled in this study. Demographics, aneurysm characteristics, and angiographic outcomes of the patients are presented in Table 1.
The mean max length of the aneurysms and mean aneurysm neck size were 6.4 ± 3.8 and 4.6 ± 2.3 mm, respectively. Ruptured aneurysms accounted for 8.6% (10/116) of the cases. Five aneurysms had been previously treated, four by coiling and one by clipping. Most of the aneurysms were located in the internal carotid artery (57.8%, 67/116), 6.9% (8/116) in vertebral arteries, 12.9% (15/116) in the basilar and other posterior cerebral arteries, and 22.4% (26/116) in the distal circle of Willis (including middle cerebral artery, anterior cerebral artery, anterior communicating artery, and posterior communicating artery). Twenty-five (29/116) percent of the aneurysms were located at the artery bifurcation and 80.2% (93/116) in the anterior circulation. Bifurcation aneurysms were located at the middle cerebral artery bifurcation (44.8%, 13/29), basilar artery tip (37.9%, 11/29), and anterior communicating artery (17.2%, 5/29).
All the patients received SAC therapy for aneurysm. Multiple stenting was performed using the Y-configuration for three cases (2.6%), in which the aneurysms were all located at the tip of the basilar artery. Immediate complete and nearly complete aneurysm occlusions were achieved in 78.4% of the cases (grade 1, 62.1%; grade 2, 16.4%). Follow-up angiography (mean follow-up duration 7.5 ± 2.4 months) showed complete and nearly complete aneurysm occlusions in 87.9% of the cases (grade 1, 77.6%; grade 2, 10.3%). Retreatment by coiling was performed in two cases of recurrent aneurysm. Treatment-related complications were observed in 5/116 cases (4.3%) in the periprocedural period. There were two cases of aneurysm rupture during the treatment procedure, one case of parenchymal hemorrhage and one case of SAH; the rest of the infarctions occurred after the treatment procedure (<24 h). Transient deficits were observed in 4/5 cases (mRS <2) and permanent deficits in one case (mRS = 4).
There were 17.2% (20/116) cases identified with ISS at the first angiographic follow-up (mean follow-up duration 6.9 ± 1.7 months). The radiological and clinical characteristics of 20 ISS cases are shown in Table 2 and examples of ISS are show in Figure 1. ISS was mild in 30% of the cases (6/20), moderate in 50% of the cases (10/20), and severe in 20% of the cases (4/20). Stenosis was diffuse in 55% of the cases (11/20) and focal in 45% of the cases (9/20). Stenosis was located at the proximal end of the stent in 45% of the cases (11/20), in the middle in 15% of the cases (3/20), and at the distal end in 30% of the cases (6/20). Sidewall and bifurcation aneurysms accounted for 45 and 55% of the ISS cases, respectively. Three ISS cases (15%) had a mean angiography follow-up time of 18.3 ± 5.5 months, and showed complete resolution in one of the cases (Figure 2) and stable condition in the other two. None of the patients with ISS were symptomatic or required further intervention.
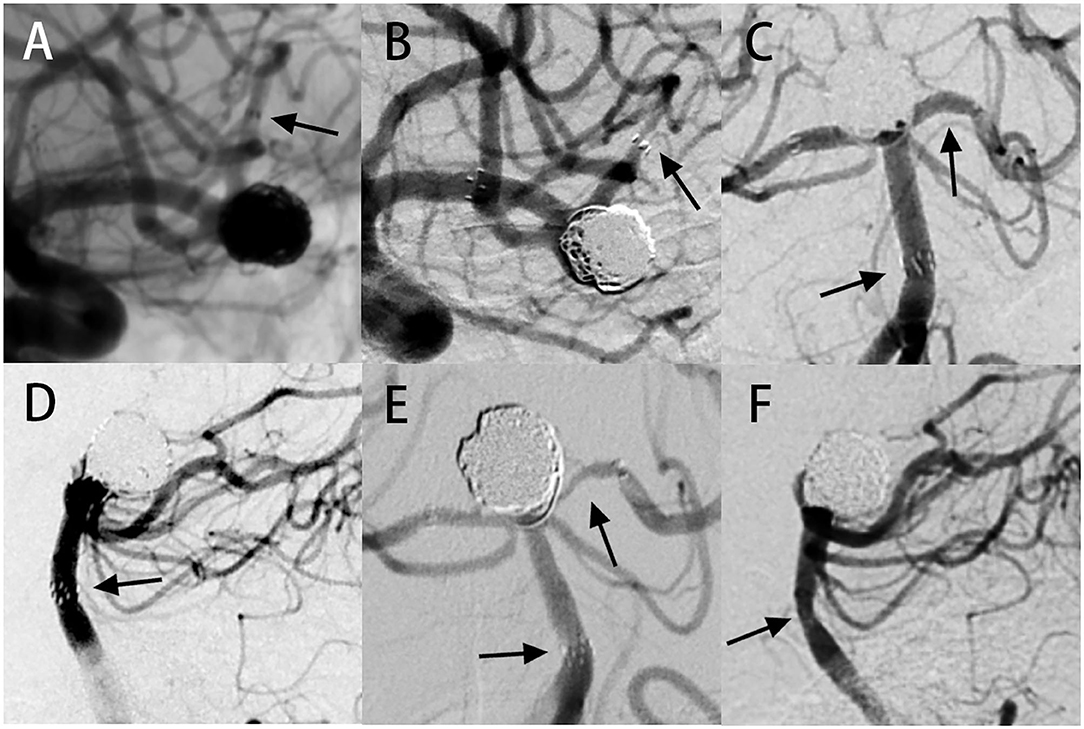
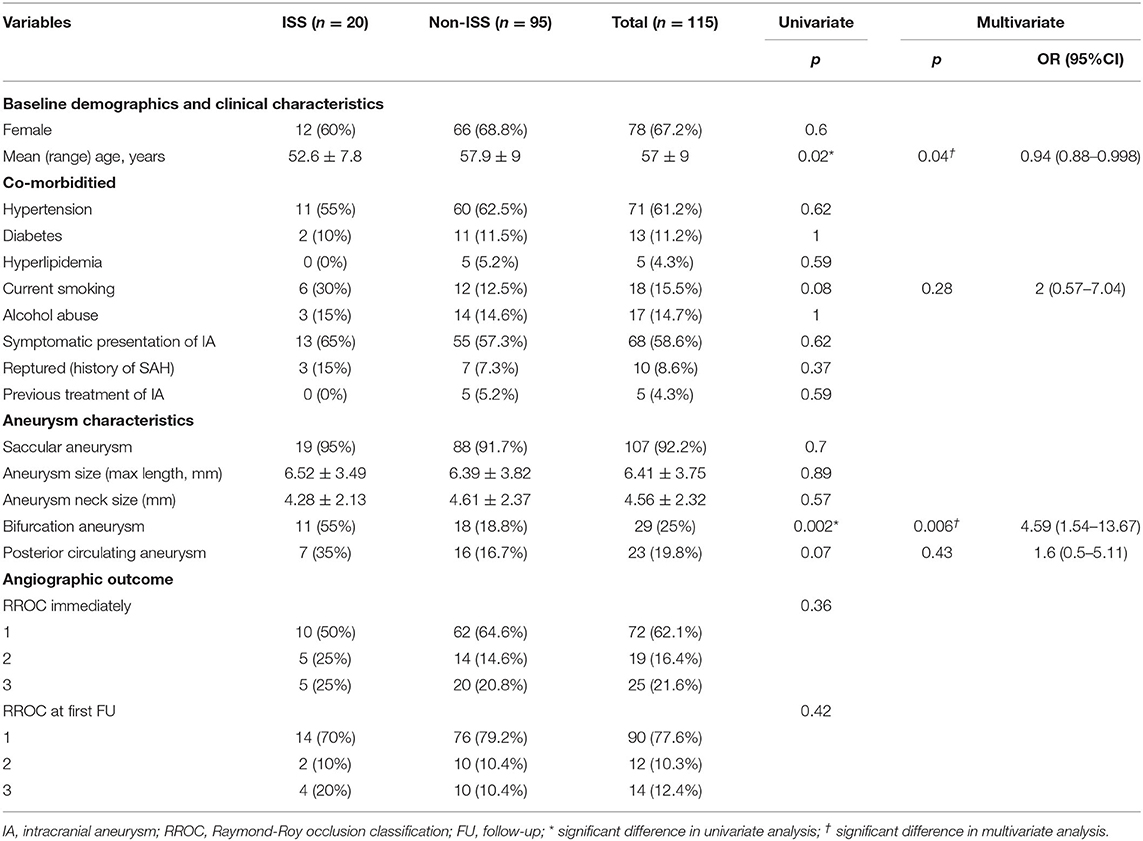
Figure 1. In-stent stenosis case presentation. Case 1: (A) digital subtraction angiography (DSA) images showing an aneurysm located at the bifurcation of the left middle cerebral artery. Stent-assisted coiling (SAC) therapy was performed with the NF-EZ stent. (B) Angiography follow-up at 13 months showing severe ISS at the distal end of the stent. Case 2: (C,D) DSA images showing an aneurysm at the tip of the basilar artery. Two NF-EZ stents were used for the “Y” type SAC operation. (E,F) Six-month follow-up angiography showing multiple ISS, including (E) severe ISS in the left posterior cerebral artery at the distal of the stent and (F) moderate ISS in the basilar artery at the proximal end of the stent. The arrow shows the location of ISS.
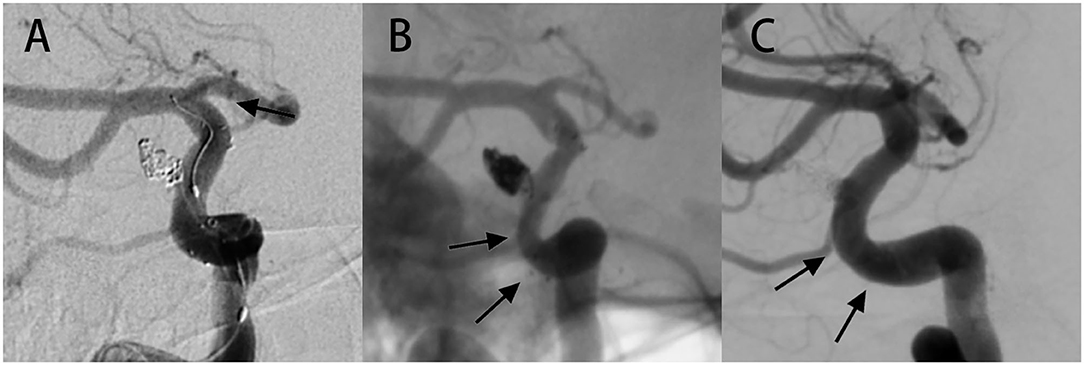
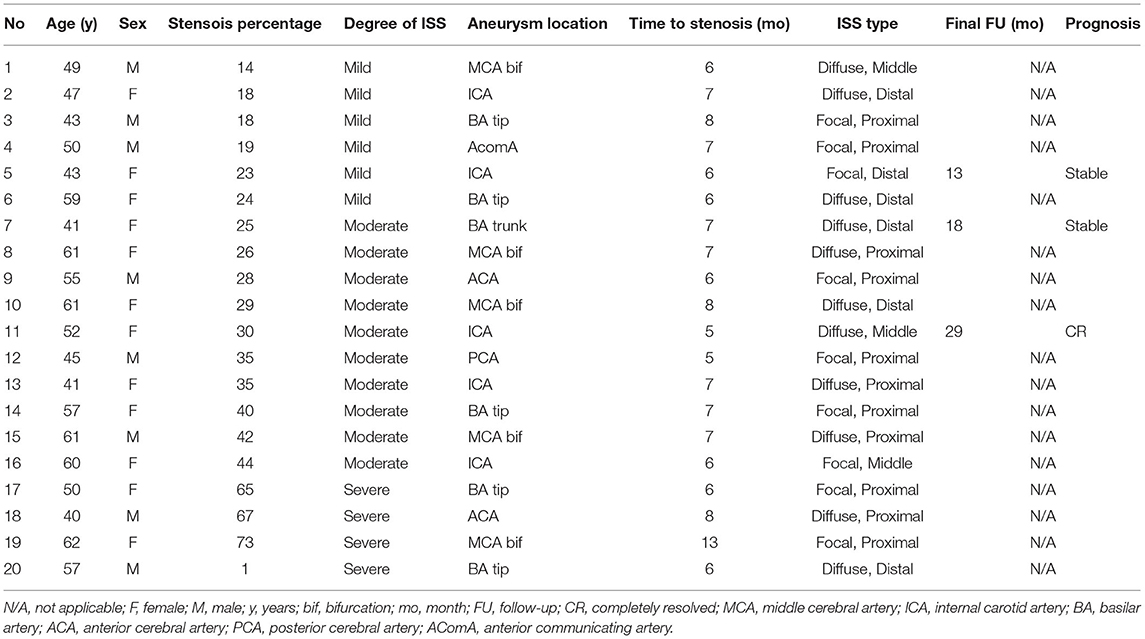
Figure 2. Case of ISS showing complete resolution during long-term angiographic follow-up. (A) A 52-year-old female had an aneurysm in the right internal carotid artery (ICA) a paraophthalmic aneurysm, and was treated with SAC therapy using the NF-EZ stent. On the immediate postoperative image, the marker at both ends of the stent showed that the stent was in the proper position. (B) Follow-up image at 5 months revealing extensive mild ISS. The patient was asymptomatic and continued to undergo dual antiplatelet therapy. (C) Angiography at 29 months follow-up revealing complete resolution of ISS. The arrow shows the location of ISS.
The univariate and multivariate logistic analyses, in association with ISS, are presented in Table 1. Patients who developed ISS were younger than those without ISS (52.6 ± 7.8 vs. 57.9 ± 9; p = 0.016). The proportion of aneurysms located at the artery bifurcation was significantly higher in patients with stenosis than those without stenosis (37.9 vs. 10.3%; p = 0.002). The proportion of patients with current smoking status (33.3 vs. 14.3%; p = 0.08) and posterior circulation aneurysm (30.4 vs. 14%; p = 0.072) was higher but not significantly in the ISS group than in the non-ISS group. In the univariate analysis, significant variables at the threshold of 10% were subjected to binary logistic regression analysis. In the multivariate analysis, the patients' age (OR = 0.94; 95% CI 0.88–0.998; p = 0.02) and bifurcated aneurysm location (OR = 4.59; 95% CI 1.54–13.67; p = 0.006) exhibited strong independent associations with ISS. Specifically, the younger patients and those with aneurysms at the artery bifurcation were more likely to develop ISS. ROC curve was used to analyze the performance of the logistic regression classification model, and the area under the curve (AUC) was 0.8 (Figure 3).
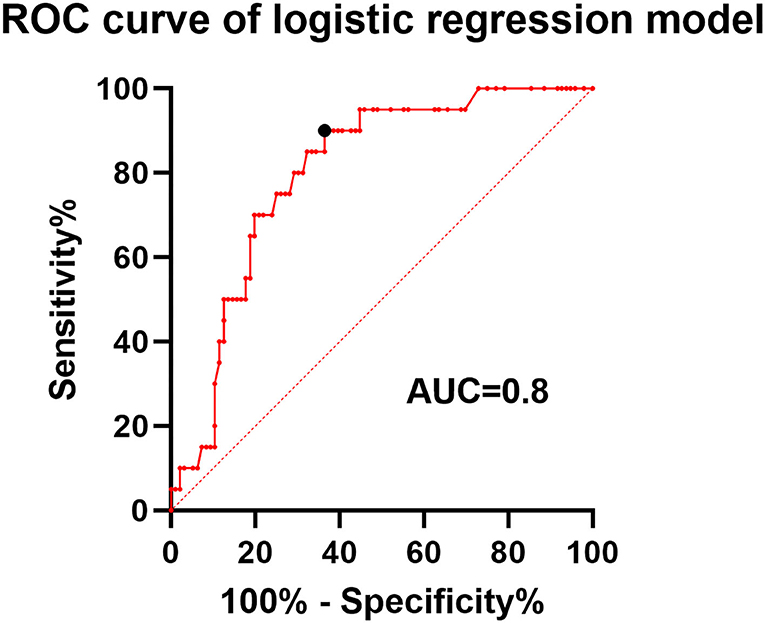
Figure 3. Receiver operating characteristic (ROC) curve of the logistic regression classification model. The area under the curve (AUC) was 0.8 with a sensitivity and specificity of 0.9 and 0.64 at the point of the cutoff value.
Discussion
The occurrence of ISS after NF-EZ stenting for the treatment of aneurysms is not well-studied. We found that 17.2% of the patients in our study had a radiographically identifiable ISS, but no patient showed symptoms or required further intervention. The multivariate analysis identified younger age and bifurcated aneurysm location as independent predictors of ISS.
The reported incidence of ISS and symptomatic stenosis after SAC is 2.3–17.9 and 0–1.3%, respectively (2–7, 9). ISS incidence after Neuroform stent implantation is relatively low, mostly between 2.4 and 7.5% (2, 3, 10). In our study, the incidence of ISS after NF-EZ stenting was 17.2% (20/116), which was relatively higher than that previously reported. The difference in ISS incidence may be because of the difference in ISS criterion. Some suggest <25% narrowing as intimal hyperplasia (11, 12), while some suggest that only >50% narrowing is significant (3–5, 10, 13). Our high incidence rate (17.9%) would be reasonable considering that our criterion of ISS is below 25%. However, stenosis <25% should not be overlooked, as it is known that ISS is a dynamic phenomenon, and the observed progression of stenosis may indicate the need for timely intervention. In the report of Kim et al. (9), a patient with stenosis of <25% progressed to occlusion during long-term follow-up.
ISS is a well-known issue of endovascular stents, especially in the treatment of coronary artery and atheromatous diseases. Abnormal vascular remodeling and neointimal hyperplasia are considered to be the underlying causes of ISS (14). Disruption of endothelial function by vascular endothelial injury leads to proliferation of local smooth muscle cells, formation of neointimal tissue, and eventually ISS (15). Overlying the stent with the endothelium may lead to prolonged endothelialization, because the middle of the stent will be reached from the edge of the stent. Persistent neointimal hyperplasia in the stented segment may significantly compromise the parent vessel (3, 7, 16, 17). The longer time required for reendothelialization further stimulates more proliferation of smooth muscle cells at the edge of the stent, since the process continues as long as the endothelial layer is incomplete (18, 19).
The degree of neointimal hyperplasia and ISS after SAC may be related to the severity of endothelial injury during stenting and further manipulations that may affect the stability of the stent during the initial process (10). There is a striking difference in the incidence and natural history of ISS between balloon-expanded stents used for atheromatous disease and NF-EZ stent (20). The deployment of a balloon-expanded stent or balloon angioplasty for atheromatous disease will inevitably lead to disruption and denudation of the endothelium over the treated vascular segment and result in a relatively high rate of postoperative restenosis (32%) (20). Deployment of the NF-EZ stent causes much less injury than either angioplasty alone or deployment of a conventional balloon-mounted stent, because the self-expanding NF-EZ stent has a lower radial force (16) and does not require balloon angioplasty.
The Neuroform EZ stent system is a fourth-generation intracranial aneurysm stent with an integrated navigation guidewire, and is designed specifically for the cerebrovasculature to support aneurysm treatment. It has an open-cell design to allow for high navigability. The Neuroform EZ stent is an excellent choice for most intracranial aneurysms, especially those with tight bends in the parent vessel or those located at the artery bifurcation (1). The operator may obtain access with their choice of microwire and microcatheter and avoid the difficulty of en bloc advancement through curved anatomical structures. However, our results showed that patients with bifurcated aneurysms were more likely to develop ISS. Our finding that bifurcated aneurysm location is a predictor of ISS is not based on previous literature and may be due to complex procedures for bifurcation aneurysms. Compared with sidewall aneurysms, the treatment of bifurcated aneurysms requires delicate operation and high-skilled technology to protect the branch vessels, which inevitably increases operation time (21) and the risk of potential endothelial injury.
Younger age was also identified as an independent predictor of ISS. This finding is consistent with the study by Chalouhi et al. (22) on ISS after aneurysm stenting and the study by Turk et al. (23) on in-stent restenosis after stenting for atherosclerotic disease. They found that the rate of postoperative stenosis or restenosis was significantly higher in younger patients than in elderly patients possibly because the response of neointimal hyperplasia induced by stenting is more aggressive in younger patients. This hypothesis is supported by the study by Du et al. (24) who observed a significant reduction in neointimal growth after coronary stenting in elderly patients than in younger patients.
The prognosis of ISS is a benign process after stent implantation for aneurysm, since most ISS cases eventually become stable or improve (3, 9, 10, 25, 26). In this study, three ISS cases had long-term angiographic follow-up data, in which the stenosis was completely relieved in one of cases and stable in the other two. All the patients with ISS in this series remained asymptomatic during the follow-up period. Nevertheless, the occurrence of symptomatic stenosis in previously asymptomatic patients with ISS represents a considerable issue. Therefore, close observation and careful imaging follow-up are recommended for patients with ISS.
In most studies, ISS was identified during radiological follow-up by both magnetic resolution angiography (MRA) and catheter-selected angiography (3, 10, 27, 28). Although Prabhakaran et al. have suggested that MRA could be a promising screening method for detecting ISS, we chose DSA as the only follow-up imaging method in our research, since it is still the gold standard for cerebral disease. In addition, we only performed angiography for image evaluation, so image differences affected our assessment results less.
There are some limitations to this study. This is a single-center retrospective study that may increase the risk of selection bias. Some of the patients had an angiographic follow-up in local hospitals, leading to some loss in follow-up. Further exploration of large-scale cohorts with long-term follow-up data is needed.
Conclusion
In this retrospective study, the incidence of ISS and its predictors were determined. We found that the incidence of ISS after NF-EZ stent placement was 17.9%, and that all the ISS cases were asymptomatic. Patients with younger age and bifurcated aneurysm location are more prone to developing ISS. Although the NF-EZ stent is particularly suitable for bifurcated aneurysms, our findings suggest that more attention should be paid to ISS after treatment.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Institutional Review Board at Tiantan Hospital. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
YL, YJ, and XL designed the study. WY and PL contributed to data collection. WY and JF performed the data analysis and drafted the manuscript. HJ and HG performed the revision of the current literature. All authors contributed to the manuscript and approved the final version.
Funding
This study has received funding by the National Natural Science Foundation of China (82171289 and 81901197), the National Key Research and Development Program of China (2017YFB1304400).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Mangubat EZ, Johnson AK, Keigher KM, Lopes DK. Initial experience with neuroform EZ in the treatment of wide-neck cerebral aneurysms. Neurointervention. (2012) 7:34–9. doi: 10.5469/neuroint.2012.7.1.34
2. Biondi A, Janardhan V, Katz JM, Salvaggio K, Riina HA, Gobin YP. Neuroform stent-assisted coil embolization of wide-neck intracranial aneurysms: strategies in stent deployment and midterm follow-up. Neurosurgery. (2007) 61:460–8; discussion: 468–9. doi: 10.1227/01.NEU.0000290890.62201.A9
3. Fiorella D, Albuquerque FC, Woo H, Rasmussen PA, Masaryk TJ, McDougall CG. Neuroform in-stent stenosis: incidence, natural history, and treatment strategies. Neurosurgery. (2006) 59:34–42; discussion: 34–42. doi: 10.1227/01.NEU.0000219853.56553.71
4. Kanaan H, Jankowitz B, Aleu A, Kostov D, Lin R, Lee K, et al. In-stent thrombosis and stenosis after neck-remodeling device-assisted coil embolization of intracranial aneurysms. Neurosurgery. (2010) 67:1523–32; discussion: 1532–3. doi: 10.1227/NEU.0b013e3181f8d194
5. Mocco J, Fargen KM, Albuquerque FC, Bendok BR, Boulos AS, Carpenter JS, et al. Delayed thrombosis or stenosis following enterprise-assisted stent-coiling: is it safe? Midterm results of the interstate collaboration of enterprise stent coiling. Neurosurgery. (2011) 69:908–13; discussion: 913–4. doi: 10.1227/NEU.0b013e318228490c
6. Takemoto K, Tateshima S, Rastogi S, Gonzalez N, Jahan R, Duckwiler G, et al. Disappearance of a small intracranial aneurysm as a result of vessel straightening and in-stent stenosis following use of an Enterprise vascular reconstruction device. J Neurointerven Surg. (2014) 6:e4. doi: 10.1136/neurintsurg-2012-010583.rep
7. Yoon KW, Kim YJ. In-stent stenosis of stent assisted endovascular treatment on intracranial complex aneurysms. J Korean Neurosurg Soc. (2010) 48:485–9. doi: 10.3340/jkns.2010.48.6.485
8. Randomised trial of endarterectomy for recently symptomatic carotid stenosis: final results of the MRC European Carotid Surgery Trial (ECST). Lancet. (1998) 351:1379–87. doi: 10.1016/S0140-6736(97)09292-1
9. Kim SJ, Kim YJ, Ko JH. Long term outcome of in-stent stenosis after stent assisted coil embolization for cerebral aneurysm. J Korean Neurosurg Soc. (2019) 62:536–44. doi: 10.3340/jkns.2019.0087
10. Kulcsar Z, Goricke SL, Gizewski ER, Schlamann M, Sure U, Sandalcioglu IE, et al. Neuroform stent-assisted treatment of intracranial aneurysms: long-term follow-up study of aneurysm recurrence and in-stent stenosis rates. Neuroradiology. (2013) 55:459–65. doi: 10.1007/s00234-013-1143-z
11. John S, Bain MD, Hui FK, Hussain MS, Masaryk TJ, Rasmussen PA, et al. Long-term follow-up of in-stent stenosis after pipeline flow diversion treatment of intracranial aneurysms. Neurosurgery. (2016) 78:862–7. doi: 10.1227/NEU.0000000000001146
12. Lylyk P, Miranda C, Ceratto R, Ferrario A, Scrivano E, Luna HR, et al. Curative endovascular reconstruction of cerebral aneurysms with the pipeline embolization device: the Buenos Aires experience. Neurosurgery. (2009) 64:632–42; discussion: 642–3. doi: 10.1227/01.NEU.0000339109.98070.65
13. Lubicz B, Van der Elst O, Collignon L, Mine B, Alghamdi F. Silk flow-diverter stent for the treatment of intracranial aneurysms: a series of 58 patients with emphasis on long-term results. AJNR. Am J Neuroradiol. (2015) 36:542–6. doi: 10.3174/ajnr.A4143
14. Buccheri D, Piraino D, Andolina G, Cortese B. Understanding and managing in-stent restenosis: a review of clinical data, from pathogenesis to treatment. J Thorac Dis. (2016) 8:E1150–62. doi: 10.21037/jtd.2016.10.93
15. Kipshidze N, Dangas G, Tsapenko M, Moses J, Leon MB, Kutryk M, et al. Role of the endothelium in modulating neointimal formation: vasculoprotective approaches to attenuate restenosis after percutaneous coronary interventions. J Am Coll Cardiol. (2004) 44:733–9. doi: 10.1016/S0735-1097(04)01083-6
16. Lee JI, Ko JK, Choi BK, Choi CH. In-stent stenosis of stent-assisted coil embolization of the supraclinoid internal carotid artery aneurysm. J Korean Neurosurg Soc. (2012) 51:370–3. doi: 10.3340/jkns.2012.51.6.370
17. Kearney M, Pieczek A, Haley L, Losordo DW, Andres V, Schainfeld R, et al. Histopathology of in-stent restenosis in patients with peripheral artery disease. Circulation. (1997) 95:1998–2002. doi: 10.1161/01.CIR.95.8.1998
18. Sick PB, Brosteanu O, Niebauer J, Hehrlein C, Schuler G. Neointima formation after stent implantation in an experimental model of restenosis: polytetrafluoroethylene-covered versus uncovered stainless steel stents. Heart Dis. (2002) 4:18–25. doi: 10.1097/00132580-200201000-00004
19. Rogers C, Edelman ER. Endovascular stent design dictates experimental restenosis and thrombosis. Circulation. (1995) 91:2995–3001. doi: 10.1161/01.CIR.91.12.2995
20. Investigators SS. Stenting of Symptomatic Atherosclerotic Lesions in the Vertebral or Intracranial Arteries (SSYLVIA): study results. Stroke. (2004) 35:1388–92. doi: 10.1161/01.STR.0000128708.86762.d6
21. de Gast AN, Soepboer A, Sluzewski M, van Rooij WJ, Beute GN. How long does it take to coil an intracranial aneurysm? Neuroradiology. (2008) 50:53–6. doi: 10.1007/s00234-007-0301-6
22. Chalouhi N, Drueding R, Starke RM, Jabbour P, Dumont AS, Gonzalez LF, et al. In-stent stenosis after stent-assisted coiling: incidence, predictors and clinical outcomes of 435 cases. Neurosurgery. (2013) 72:390–6. doi: 10.1227/NEU.0b013e31828046a6
23. Turk AS, Levy EI, Albuquerque FC, Pride GL Jr, Woo H, Welch BG, et al. Influence of patient age and stenosis location on wingspan in-stent restenosis. Am J Neuroradiol. (2008) 29:23–7. doi: 10.3174/ajnr.A0869
24. Du R, Zhang RY, Zhang Q, Shi YH, Hu J, Yang ZK, et al. Assessment of the relation between IVUS measurements and clinical outcome in elderly patients after sirolimus-eluting stent implantation for de novo coronary lesions. Int J Cardiovasc Imaging. (2012) 28:1653–62. doi: 10.1007/s10554-011-0007-z
25. Aguilar Perez M, Bhogal P, Henkes E, Ganslandt O, Bazner H, Henkes H. In-stent stenosis after p64 flow diverter treatment. Clin Neuroradiol. (2018) 28:563–8. doi: 10.1007/s00062-017-0591-y
26. Ravindran K, Salem MM, Enriquez-Marulanda A, Alturki AY, Moore JM, Thomas AJ, et al. Quantitative assessment of in-stent stenosis after pipeline embolization device treatment of intracranial aneurysms: a single-institution series and systematic review. World Neurosurg. (2018) 120:e1031–40. doi: 10.1016/j.wneu.2018.08.225
27. Maldonado IL, Machi P, Costalat V, Mura T, Bonafe A. Neuroform stent-assisted coiling of unruptured intracranial aneurysms: short- and midterm results from a single-center experience with 68 patients. Am J Neuroradiol. (2011) 32:131–6. doi: 10.3174/ajnr.A2245
Keywords: neuroform-EZ stent, stent-assisted coiling, in-stent stenosis, intracranial aneurysm, bifurcated aneurysm
Citation: You W, Feng J, Ge H, Jin H, Liu P, Li Y, Jiang Y and Liu X (2022) Bifurcated Aneurysm Location Predicts In-Stent Stenosis After Neuroform-EZ Stent-Assisted Coiling for Intracranial Aneurysm. Front. Neurol. 13:873014. doi: 10.3389/fneur.2022.873014
Received: 10 February 2022; Accepted: 20 April 2022;
Published: 13 May 2022.
Edited by:
Osama O. Zaidat, Northeast Ohio Medical University, United StatesReviewed by:
Basil Erwin Grüter, Aarau Cantonal Hospital, SwitzerlandCarmen Parra-Farinas, University of Toronto, Canada
Copyright © 2022 You, Feng, Ge, Jin, Liu, Li, Jiang and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuhua Jiang, anh5MjAwMzIxQGdtYWlsLmNvbQ==; Xinke Liu, eGlua2UubGl1QGhvdG1haWwuY29t
†These authors have contributed equally to this work and share first authorship
 Wei You
Wei You Junqiang Feng1†
Junqiang Feng1† Huijian Ge
Huijian Ge Hengwei Jin
Hengwei Jin Peng Liu
Peng Liu Youxiang Li
Youxiang Li Xinke Liu
Xinke Liu