- Neurovascular Center, Changhai Hospital, Naval Medical University, Shanghai, China
Objective: In the study, we explored the safety and effectiveness of staged stenting strategy for acutely wide-neck ruptured intracranial aneurysms.
Methods: Online databases, including PubMed, EMBASE, the Cochrane database, and Web of Science, were retrospectively and systematically searched. The main observation indicators were the procedure-related complication rate, complete occlusion rate, and favorable clinical outcome. Meta-analysis was performed using a random or fixed effect model based on heterogeneity.
Results: A total of 5 studies with 143 patients were included. The hemorrhagic complication rate of the initial coiling and staged stenting was 2.8% (4 of 143) and 0, respectively. The ischemic complication rate of the coiling and supplemental stenting was 3.5% (5 of 143) and 2.9% (4 of 139), respectively. There were no deaths due to procedure-related complications in two stages. The aneurysm complete occlusion rate was 25% (95% CI, 0.13–0.03; I2 = 4.4%; P = 0.168) after initial coiling, 54% (95% CI, 0.63–0.64; I2 = 0%; P = 0.872) after staged stenting, and 74% (95% CI, 0.66–0.81; I2 = 56.4%; P = 0.562) at follow-up, respectively. Favorable clinical outcome rate 74% (95% CI, 0.61–0.86; I2 = 50.5%; P = 0.133) after discharge of initial coiling treatment, and 86% (95% CI, 0.80–0.92; I2 = 0; P = 0.410) after discharge from stenting, and 97% (95% CI, 0.93–1.01; I2 = 43.8%; P = 0.130) at follow-up.
Conclusion: Staged stenting treatment of wide-neck RIA with coiling in the acute phase followed by delayed regular stent or flow-diverter stent had high aneurysm occlusion rate, favorable clinical outcome rate and low procedure-related complication rate. A more dedicated and well-designed controlled study is warranted for further evaluation of staged stenting treatment compared to SCA in wide-neck RIA.
Introduction
Endovascular embolization has become the standard treatment strategy for ruptured intracranial aneurysms (RIA) (1). The indications of endovascular embolization are increasingly well defined, however it remains controversial for the wide-neck RIA (2). For wide-neck RIA, stents are often necessary to provide permanent protection for the coil inside the aneurysm sac, which may prevent coil prolapse or migration during the procedure (3). Stent assisted coiling (SAC) also allows to obtain a better immediate occlusion, however, the application of stent and antiplatelet medication can lead to more unexpected ischemic and hemorrhagic complications in the acute phase of subarachnoid hemorrhage (SAH) (4, 5). The overall rate of perioperative complications of SAC in RIA was about 20.2% as we previously reported, which was significantly higher than coiling only (6).
To avoid the high complication risk of SAC due to antiplatelet medication use in acute phase of SAH for wide-neck RIA, staged stenting strategy was gradually being applied. In a recent study, acute coiling followed by staged stenting in the treatment of 20 RIA showed that the perioperative complication during acute coiling was 3.7% and the staged treatment was no complication occurred, which suggested that staged stenting could avoid the potential risk of stent placement in the acute phase of SAH (7). As reported, staged stenting strategy was divided into two stages (7–11). The patients underwent conventional coiling in the acute phase of SAH, the purpose of which was to embolize the target aneurysms to avoid early rebleeding without stenting. Then adequate antiplatelet medication and supplemental stenting treatment (regular stent or flow-diverter stent) were scheduled after 2 weeks at least when the patient's condition was stable. Considering the distinctive characteristics of wide-neck RIA, the argument of whether staged stenting strategy would be more appropriate for managing wide-neck RIA remains unclear. Therefore, we performed a meta-analysis to evaluate the safety and outcomes of staged stenting treatment for wide-neck RIA.
Materials and methods
Literature search strategy
This systematic review and single-arm meta-analysis was conducted in accordance with the PRISMA guidelines (12). A systematic search and critical review of the reported data were from January 2006 to June 2022. A thorough search of published English language literature was performed using PubMed, EMBASE, the Cochrane database, and Web of Science. The terms “ruptured”, “intracranial aneurysms”, “cerebral aneurysms”, “intracranial aneurysm”, “cerebral aneurysm”, “staged”, “subtotal”, “planned”, “partial”, “targeted”, “stent”, and “flow-diverter” were combined as either keywords or Medical Subject Headings terms to identify all eligible studies. The reference lists of included studies were searched manually. All identified articles were systematically evaluated using the inclusion and exclusion criteria.
Selection criteria
The inclusion criteria were: (1) studies reported patients with wide-neck RIA verified by CT scan and CTA /MRA/DSA, who underwent staged stenting (regular stent or flow-diverter stent) treatment; (2) studies included at least 10 patients; (3) studies reported the clinical or angiographic outcomes of aneurysms. The exclusion criteria were as follows: (1) unextractable or unclear data; (2) second staged treatment using alternative modalities; (3) duplicated reports; (4) fusiform, dissecting, mycotic aneurysms; (5) unpublished studies, reviews, meta-analyses, comments, letters, pilot studies, conference-only reports, technical notes, case reports, and abstract only and non-English language studies. The database search and study selection were performed by two junior physicians (Wei and Zhang) independently, with disagreements settled by the senior physicians (Zuo and Liu).
Data extraction and item definition
The following information was extracted for the included studies: author, country, publication year, number of patients, baseline patient information, time between coiling and stent, complications, and so forth. The investigators were contacted if additional data were necessary.
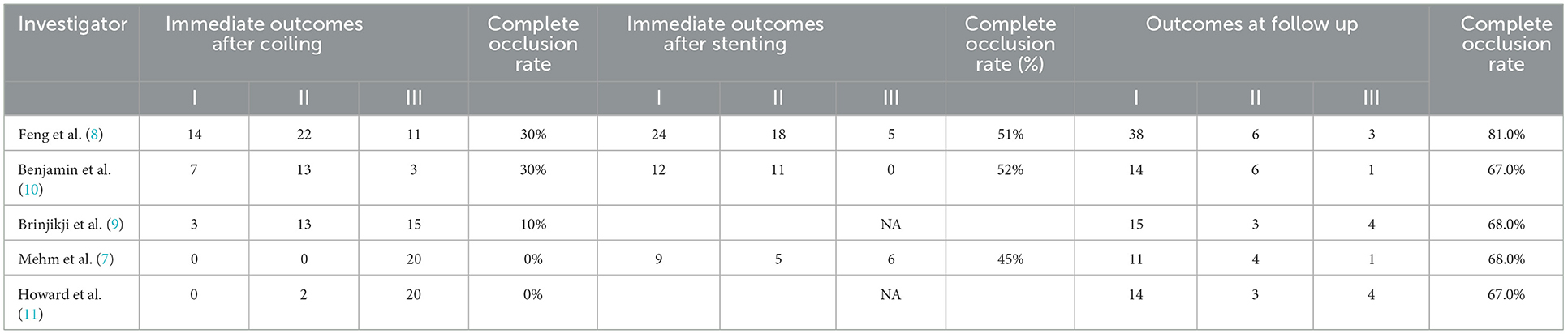
The main observation indicators include the following: (1) Perioperative procedure-related complications and mortality in both phases. Procedure-related complications included hemorrhagic and ischemic complications. Hemorrhagic complications were defined as intraoperative aneurysm rupture and early rebleeding in two stages. Ischemic complications included acute in-stent thrombus formation, thromboembolic event, parent or branch artery occlusion. Procedure-related mortality was defined as death caused by a procedure-related complication other than deterioration of a severe condition. (2) The intracranial aneurysms complete occlusion rate immediately after both procedures and at follow-up. The intracranial aneurysm occlusion was evaluated using Raymond-Roy grade scale: (I) complete occlusion, (II) neck remnant, and (III) incomplete occlusion (13). (3) Favorable clinical outcome after both procedures immediately and at follow-up. Favorable clinical outcomes were defined as a modified Rankin scale score of 0–2.
Quality assessment and statistical analysis
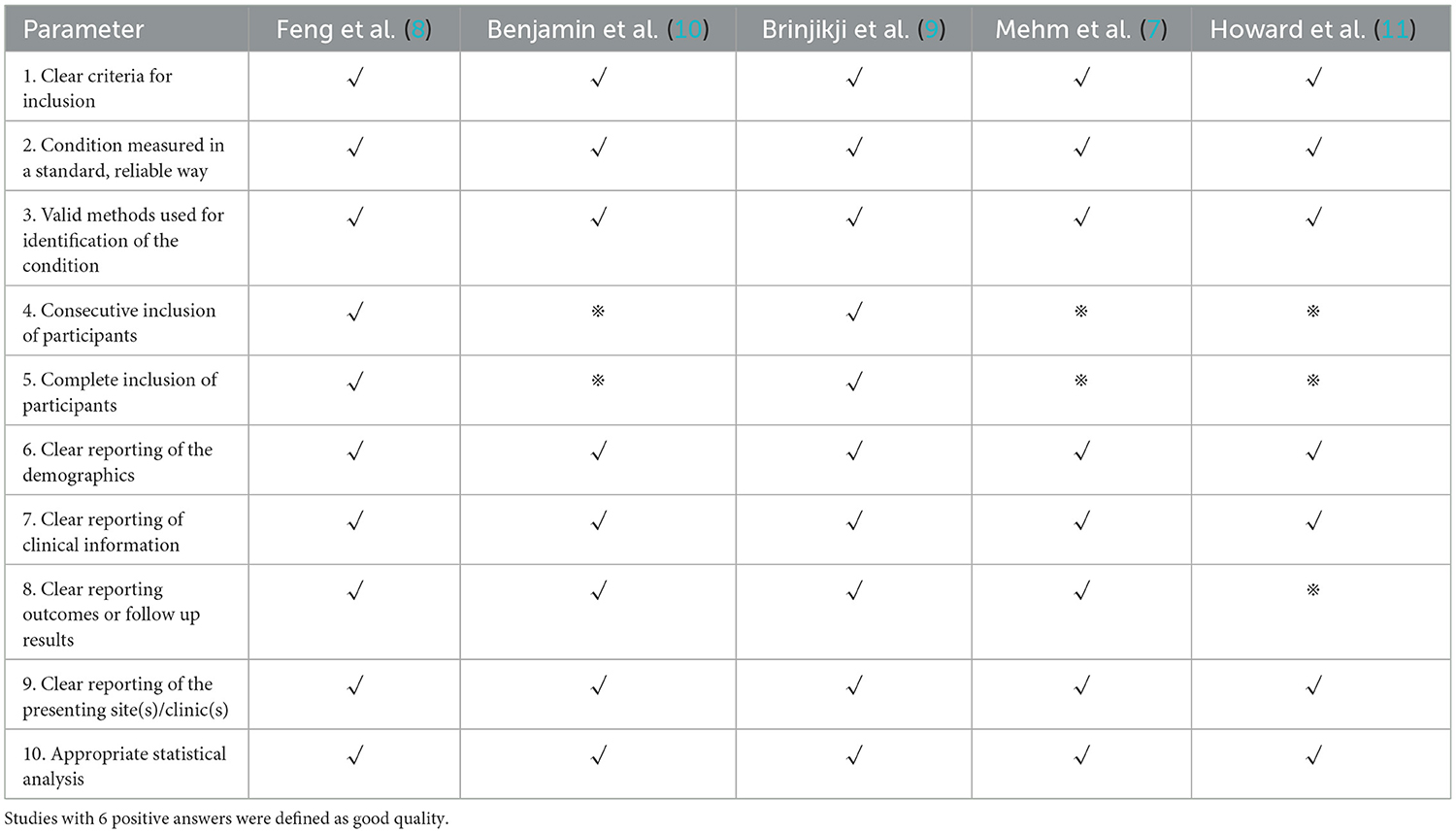
Assessment of study quality was performed using a Joanna Briggs institute scale for 6 studies (Table 1).
A meta-analysis was performed using Stata, version 16 (Stata Corp., College Station, Texas, USA). Continuous variables are presented as mean values or median and range. Dichotomous variables are presented as risk ratios with 95% confidence intervals (CI). Statistical heterogeneity was assessed using I2, a random effect model was used for analysis if the I2 was >50%, and a sensitivity analysis was further performed. For analysis with an I2 <50%, a fixed-effect model was used and a sensitivity analysis was not performed. Significance was set at P < 0.05.
Results
Literature search, study characteristics, and quality assessment
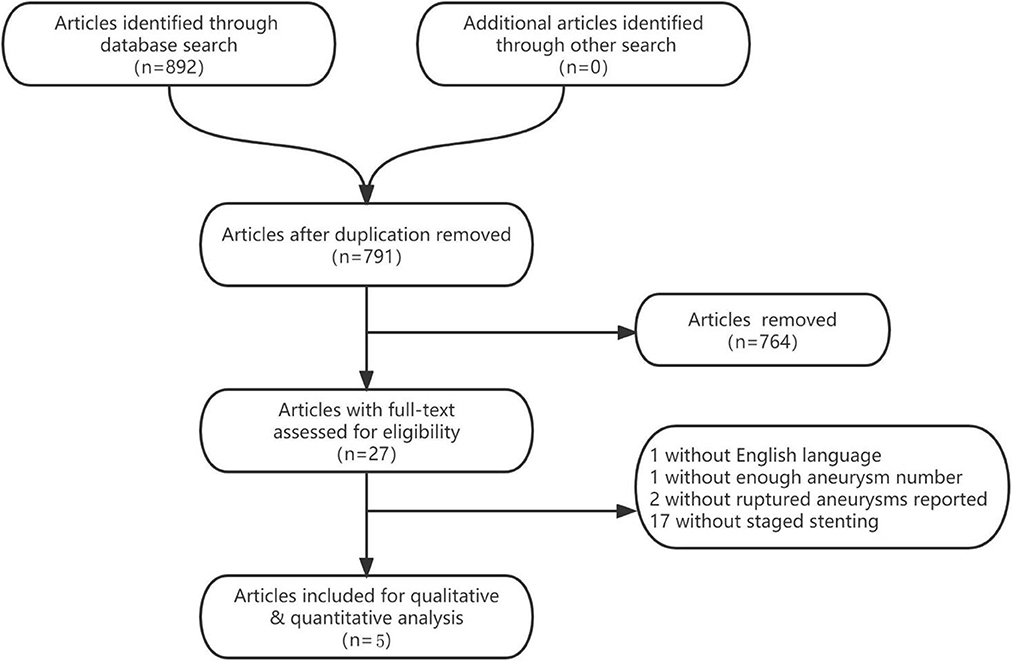
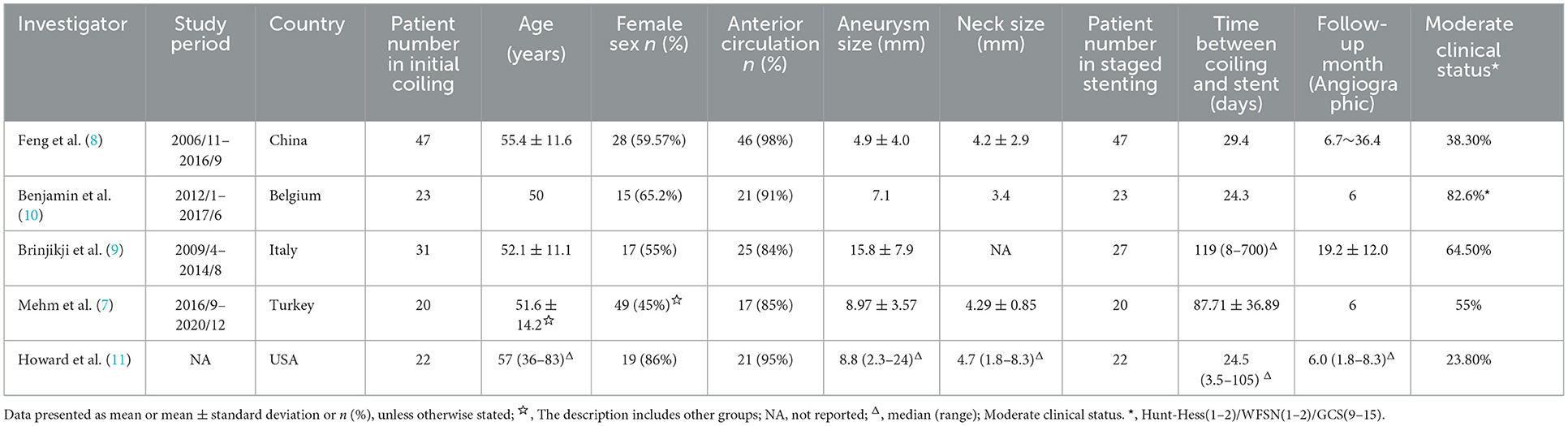
The literature search process was presented in a Preferred Reporting Items for flow chart showing the number of studies screened and excluded at each stage (Figure 1). The basic characteristics of the included eligible studies were summarized in Table 2. All included studies were retrospective studies using data from retrospective or prospective databases. The quality of the included studies using a Joanna Briggs institute scale (score range, 0~10), with 6 positive answers taken to define a good quality study (Table 1).
We identified 892 studies through the database search, and a total of 5 studies (7–11) were included in the present meta-analysis, with 143 ruptured intracranial aneurysms patients (Table 2).
Analysis of safety and outcomes
In all the 5 studies, the patients underwent conventional coiling in the acute phase of SAH without antiplatelet therapy. In 3 studies, the researchers described detailed antiplatelet strategy when patients underwent the supplemental stenting treatment. The regular dual antiplatelet drugs were administered for at least 3 days before stenting or a loading dose of dual antiplatelet drugs was used before the procedure.
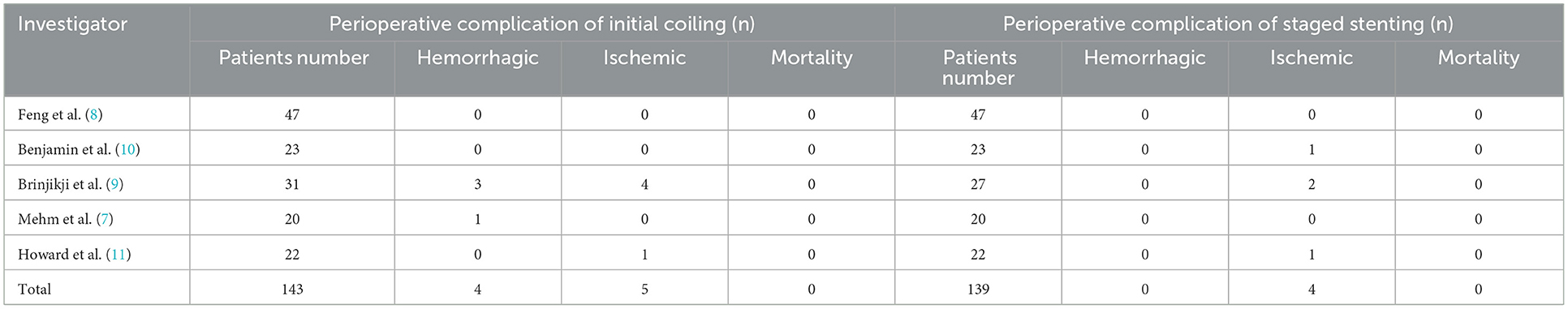
The hemorrhagic complications only occurred in 4 patients (4 of 143, 2.8%) in perioperative of coiling. Among them the intraoperative aneurysm bleeding rate was 2.1% (3/143), and the early rebleeding rate was 0.7% (1 of 143). The ischemic complications occurred in 5 patients (5 of 139, 3.5%) in perioperative of coiling and 4 patients (4 of 139, 2.8%) in perioperative of stenting. There were no deaths due to procedure-related complications in two stages (Table 3).
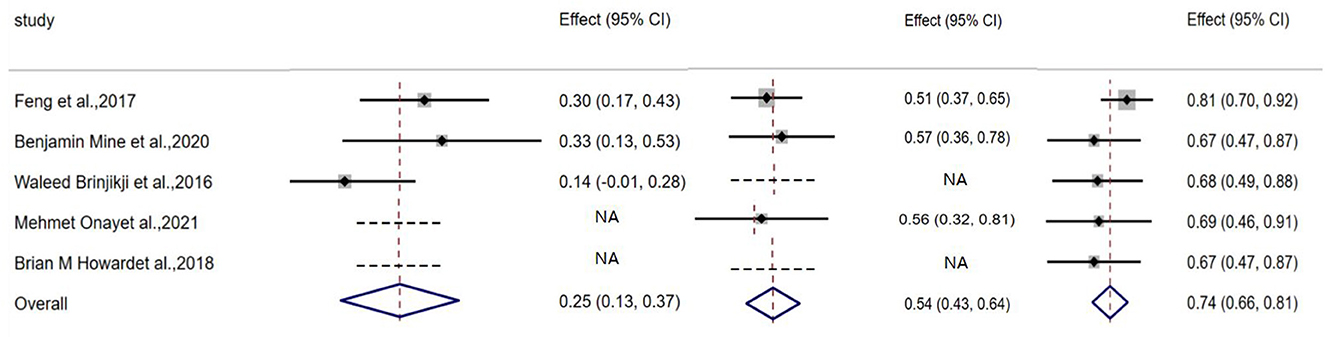
The aneurysm complete occlusion rate was 25% (95% CI, 0.13–0.03; I2 = 4.4%; P = 0.168) after initial coiling, 54% (95% CI, 0.63–0.64; I2 = 0%; P = 0.872) after staged stenting, and 74% (95% CI, 0.66–0.81; I2 = 56.4%; P = 0.562) at follow-up, respectively (Table 4, Figure 2).
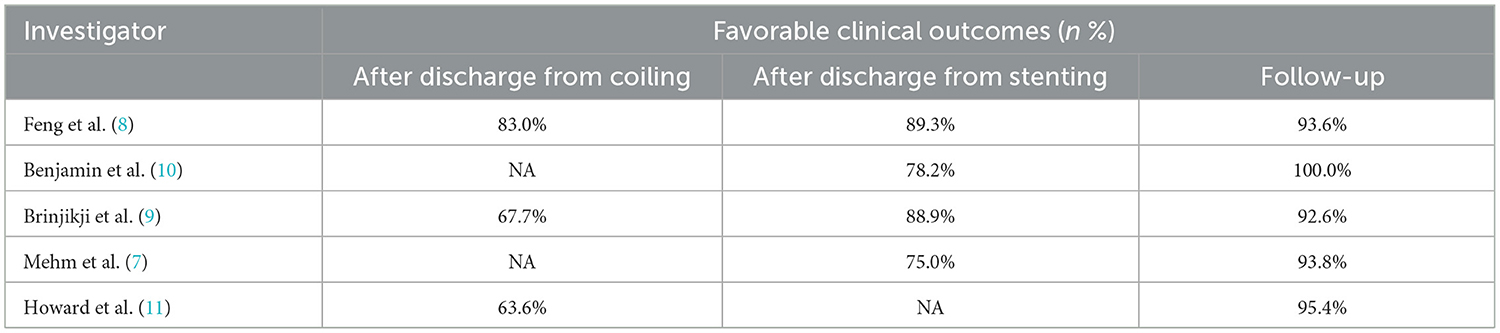
Favorable clinical outcome rate was 74% (95% CI, 0.61–0.86; I2 = 50.5%; P = 0.133) after discharge of initial coiling treatment, and 86% (95% CI, 0.80–0.92; I2 = 0; P = 0.410) after discharge from stenting, and 97% (95% CI, 0.93–1.01; I2 = 43.8%; P = 0.130) at follow-up, respectively (Table 5, Figure 3).
Sensitivity analysis and publication bias
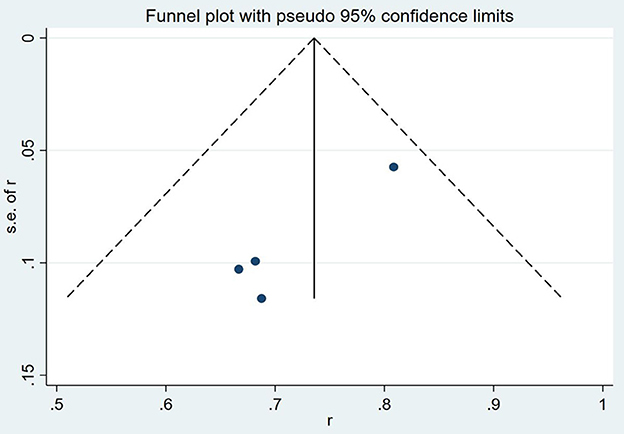
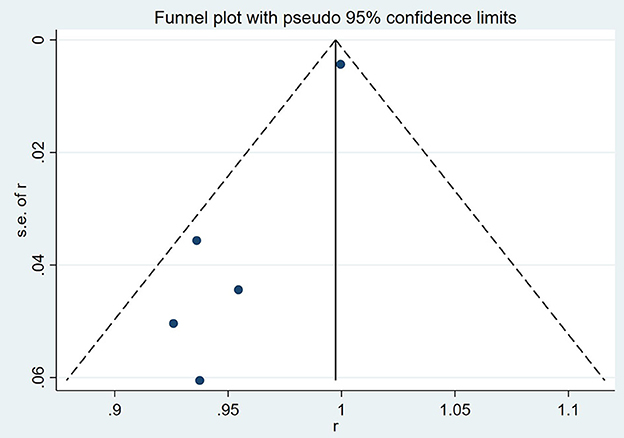
The results of a funnel plot analysis of favorable clinical outcome at discharge was shown in Figures 4, 5, which indicated obvious publication bias. Similar results were also obtained for the immediate occlusion rate (Figures 4, 5).
Discussion
Endovascular treatment has long been part of the standard strategies in the treatment of RIA to prevent aneurysm rebleeding after ISAT trial (14). With the development of skills and devices, the use of intracranial stents for endovascular treatment of intracranial aneurysms has dramatically widened its indications to wide-neck RIA (15, 16). However, SAC is not the first option for treating wide-neck RIA in the acute phase of SAH. Studies related to SAC of RIA have been performed in several centers, and the use of intracranial stents may be associated with a higher risk of ischemic and hemorrhagic complications if the antiplatelet therapy is not proper (4, 17). In our previous study on SAC, the ischemic complication rate was 3.1–8.1% and the hemorrhagic complication rate was 4.5–6.2% in different periods and with different stents (3, 18). Meanwhile, antiplatelet therapy may also increase the risk of hemorrhagic complications during invasive surgeries that may be necessary within the early time of severe SAH such as ventricular drainage, hematoma clearance, or decompressive craniectomy. The study of Kung et al. (19) showed that the surgery-related bleeding risk was approximately three times higher in patients who used dual antiplatelet drugs with RIA than in those without dual antiplatelet drugs use (95% CI 1.46–8.04, p = 0.0048).
To effectively reduce the high rate of hemorrhagic and ischemic complications, several studies have reported the staged treatment of wide-neck RIA with coiling in the acute phase followed by delayed stenting, which may be a safe and effective strategy compared to SAC for acutely ruptured aneurysms. It allowed patients to avoid dual antiplatelet therapy during the acute phase and allowed for transitioning of the patients to a more subacute phase when the patient and aneurysm were stabilized, and dual antiplatelet therapy was safer.
Waldau et al. (20) firstly described the staged stent strategy for wide-neck RIA in 2010, with 5 patients who had complex ruptured aneurysms receiving intentional partial coiling dome protection and staged stent placement, and none of them experienced aneurysm rerupture before the supplementary stent treatment. We previously reported a series of 47 RIA patients with staged stenting treatment, and the results showed that no perioperative procedure-related complications and related death in both phases (8). Moreover, Onay et al. (7) proposed a different and radical technique of only targeted aneurysm bleb embolization and staged stenting treatment. They used small coils to embolize the bleb only instead of embolizing the aneurysm sac, which may lead to an increase in the formation of thromboses at the bleb and ensure the stabilization of the bleb to avoid early rebleeding as they considered. In their study, although the early occlusion rate was 0, no patient suffered rebleeding before the second-stage stenting and the occlusion rate was 68% at follow-up. The safety of this new approach needs to be further validated with more studies.
Among the 143 included patients in this meta-analysis, only 4 (2.8%) patients had hemorrhagic events, and 5 (3.5%) patients had ischemic events, with no deaths due to procedure-related complications. The rate of complications seemed lower than our previous study about SAC (3, 18). Also in Mehmet's study, the staged stenting strategy had significantly lower complication rates compared to the SAC (p = 0.047).
In the previous meta-analysis of wide-neck RIA, the immediate occlusion rate of single coiling was 64.2% (6). It was indeed an interesting result that a low rebleeding rate was observed after the staged stenting strategy even with a relatively low rate of aneurysm immediate occlusion (25%). We shared the view of these researchers that non-dense embolization of wide-neck RIA may provide adequate protection against aneurysms rerupture in the acute phase. More evidence is still needed on whether non-dense embolization could prevent aneurysm rerupture.
At the same time, compared with our previous study about SAC of wide-neck RIA, the results of this meta-analysis showed similar rate of favorable clinical outcome (94 vs. 85.6%) and long-term angiographic complete occlusion (75 vs. 74%) at follow-up (3). Thus, staged stenting strategy may be a safe and effective way for wide-neck RIA treatment without antiplatelet management in the acute phase and with adequate antiplatelet preparation before the second-stage stent placement.
There were some limitations in the meta-analysis. First, in these 5 searched publications, the sample size was small. And only in 1 study the results of the staged stenting strategy were compared with SAC in acute phase. Therefore, we only performed a single-arm meta-analysis to summarize the preliminary results and experiences about the staged stenting strategy for wide-neck RIA. Second, the enrolled patients were carefully selected and treated with staged stenting strategy. These results may not truly represent the safety and effectiveness of staged stenting strategy. That was an unavoidable drawback of retrospective studies.
Third, the antiplatelet strategies differed across these studies, which may affect complications when supplementing stents. A more dedicated and well-designed controlled study is warranted for further evaluation of staged treatment with stent compared to SAC in acute phase of wide-neck RIA.
Conclusion
Staged stenting strategy of wide-neck RIA with coiling in the acute phase followed by delayed regular stent or flow-diverter stent had low procedure-related complication rates, favorable clinical outcome and high aneurysms occlusion rate at follow-up based on this single arm meta-analysis. We advocate for future prospective, randomized controlled trials of this promising therapy.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
YW, XZ, and RZhan made substantial contributions to the conception and design, acquisition of data, analysis, and drafting of the manuscript. GZ, CS, RC, DL, MH, CW, and KZ searched for relevant studies and selected the studies. ZF, DD, QL, QH, YX, PY, RZhao, QZ, and JL assisted in the evaluation of analysis and their interpretation. All authors read and approved the final manuscript.
Funding
The present study was supported by the Changhai Hospital of the Naval Medical University “234 Discipline Climbing Plan” (2020YXK060) and Project from Shanghai Science and Technology Commission (19DZ1930302).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Petridis AK, Kamp MA, Cornelius JF, Beez T, Beseoglu K, Turowski B, et al. Diagnosis and surgical treatment versus endovascular of subarachnoid aneurismatic hemorrhage. Neurol Argentina. (2020) 12:223–32.
2. Phan K, Huo YR, Jia F, Phan S, Rao PJ, Mobbs RJ, et al. Meta-analysis of stent-assisted coiling versus coiling-only for the treatment of intracranial aneurysms. J Clin Neurosci. (2016) 31:15–22. doi: 10.1016/j.jocn.2016.01.035
3. Yang P, Zhao K, Zhou Y, Zhao R, Zhang L, Zhao W, et al. Stent-assisted Coil Placement for the Treatment of 211 Acutely Ruptured Wide-necked Intracranial Aneurysms: A Single-Center 11-Year Experience. Radiology. (2015) 276:545–52. doi: 10.1148/radiol.2015140974
4. Ryu CW, Park S, Shin HS, Koh JS. Complications in Stent-Assisted Endovascular Therapy of Ruptured Intracranial Aneurysms and Relevance to Antiplatelet Administration: A Systematic Review. AJNR Am J Neuroradiol. (2015) 36:1682–8. doi: 10.3174/ajnr.A4365
5. Santos GA, Petersen N, Zamani AA, Du R, LaRose S, Monk A, et al. Pathophysiologic differences in cerebral autoregulation after subarachnoid hemorrhage. Neurology. (2016) 86:1950–6. doi: 10.1212/WNL.0000000000002696
6. Zhang X, Zuo Q, Tang H, Xue G, Yang P, Zhao R, et al. Stent assisted coiling versus non-stent assisted coiling for the management of ruptured intracranial aneurysms: a meta-analysis and systematic review. J Neurointerv Surg. (2019) 11:489–96. doi: 10.1136/neurintsurg-2018-014388
7. Onay M, Altay CM, Binboga AB. Targeted and Staged Treatment for Ruptured Wide-neck Intracranial Aneurysms: Bleb Coiling Strategy as a New Approach. Acad Radiol. (2022) 29:S132–s40. doi: 10.1016/j.acra.2021.05.020
8. Feng Z, Zuo Q, Yang P, Li Q, Zhao R, Hong B, et al. Staged stenting with or without additional coils after conventional initial coiling of acute ruptured wide-neck intracranial aneurysms. World Neurosurg. (2017) 108:506–12. doi: 10.1016/j.wneu.2017.09.040
9. Brinjikji W, Piano M, Fang S, Pero G, Kallmes DF, Quilici L, et al. Treatment of ruptured complex and large/giant ruptured cerebral aneurysms by acute coiling followed by staged flow diversion. J Neurosurg. (2016) 125:120–7. doi: 10.3171/2015.6.JNS151038
10. Benjamin M, Thomas B, Carlos VSJ, Noemie L, Boris L. Evaluation of clinical and anatomical outcome of staged stenting after acute coiling of ruptured intracranial aneurysms. Interv Neuroradiol. (2020) 26:260–7. doi: 10.1177/1591019919891602
11. Howard BM, Frerich JM, Madaelil TP, Dion JE, Tong FC, Cawley CM, et al. 'Plug and pipe' strategy for treatment of ruptured intracranial aneurysms. J Neurointerv Surg. (2019) 11:43–8. doi: 10.1136/neurintsurg-2018-014058
12. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. J Clin Epidemiol. (2021) 134:178–89. doi: 10.1016/j.jclinepi.2021.02.003
13. Roy D, Milot G, Raymond J. Endovascular treatment of unruptured aneurysms. Stroke. (2001) 32:1998–2004. doi: 10.1161/hs0901.095600
14. Molyneux A, Kerr R, Stratton I, Sandercock P, Clarke M, Shrimpton J, et al. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised trial. Lancet (London, England). (2002) 360:1267–74. doi: 10.1016/S0140-6736(02)11314-6
15. Hong Y, Wang YJ, Deng Z, Wu Q, Zhang JM. Stent-assisted coiling versus coiling in treatment of intracranial aneurysm: a systematic review and meta-analysis. PLoS ONE. (2014) 9:e82311. doi: 10.1371/journal.pone.0082311
16. Wanke I, Forsting M. Stents for intracranial wide-necked aneurysms: more than mechanical protection. Neuroradiology. (2008) 50:991–8. doi: 10.1007/s00234-008-0460-0
17. Santillan A, Greenberg E, Patsalides A, Salvaggio K, Riina HA, Gobin YP. Long-term clinical and angiographic results of Neuroform stent-assisted coil embolization in wide-necked intracranial aneurysms. Neurosurgery (2012) 70:1232–7. doi: 10.1227/NEU.0b013e3182422a68
18. Zuo Q, Yang P, Lv N, Huang Q, Zhou Y, Zhang X, et al. Safety of coiling with stent placement for the treatment of ruptured wide-necked intracranial aneurysms: a contemporary cohort study in a high-volume center after improvement of skills and strategy. J Neurosurg. (2018) 131:435–41. doi: 10.3171/2018.3.JNS172199
19. Kung DK, Policeni BA, Capuano AW, Rossen JD, Jabbour PM, Torner JC, et al. Risk of ventriculostomy-related hemorrhage in patients with acutely ruptured aneurysms treated using stent-assisted coiling. J Neurosurg. (2011) 114:1021–7. doi: 10.3171/2010.9.JNS10445
20. Waldau B, Reavey-Cantwell JF, Lawson MF, Jahshan S, Levy EI, Siddiqui AH, et al. Intentional partial coiling dome protection of complex ruptured cerebral aneurysms prevents acute rebleeding and produces favorable clinical outcomes. Acta Neurochir. (2012) 154:27–31. doi: 10.1007/s00701-011-1214-z
Keywords: wide-neck, ruptured intracranial aneurysms (RIA), staged stenting, complications, initial coiling
Citation: Wei Y, Zhang X, Zhang R, Zhang G, Shang C, Chen R, Li D, Huyan M, Wu C, Zong K, Feng Z, Dai D, Li Q, Huang Q, Xu Y, Yang P, Zhao R, Zuo Q and Liu J (2023) Staged stenting strategy of acutely wide-neck ruptured intracranial aneurysms: A meta-analysis and systematic review. Front. Neurol. 14:1070847. doi: 10.3389/fneur.2023.1070847
Received: 15 October 2022; Accepted: 17 January 2023;
Published: 03 February 2023.
Edited by:
Bu-Lang Gao, Hebei Medical University, ChinaReviewed by:
Ansaar Rai, West Virginia University, United StatesWei Ni, Huashan Hospital, Fudan University, China
Bing Zhao, Shanghai Jiao Tong University, China
Copyright © 2023 Wei, Zhang, Zhang, Zhang, Shang, Chen, Li, Huyan, Wu, Zong, Feng, Dai, Li, Huang, Xu, Yang, Zhao, Zuo and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qiao Zuo,  ZHJfenVvQDEyNi5jb20=; Jianmin Liu,
ZHJfenVvQDEyNi5jb20=; Jianmin Liu,  Y2hzdHJva2VAMTYzLmNvbQ==
Y2hzdHJva2VAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Yanpeng Wei
Yanpeng Wei Xiaoxi Zhang
Xiaoxi Zhang Renkun Zhang†
Renkun Zhang† Guanghao Zhang
Guanghao Zhang Chenghao Shang
Chenghao Shang Rundong Chen
Rundong Chen Kang Zong
Kang Zong Zhengzhe Feng
Zhengzhe Feng Qiang Li
Qiang Li Qinghai Huang
Qinghai Huang Rui Zhao
Rui Zhao Qiao Zuo
Qiao Zuo Jianmin Liu
Jianmin Liu