- 1Department of Medicine and Surgery, Unit of Headache and Neurosonology, Università Campus Bio-Medico di Roma, Roma, Italy
- 2Fondazione Policlinico Universitario Campus Bio-Medico, Roma, Italy
- 3Neurological Clinic, Marche Polytechnic University, Ancona, Italy
- 4Neurology and Stroke Unit, ASST Grande Ospedale Metropolitano Niguarda, Milan, Italy
- 5Neurology Unit, ASST Bergamo Est, Seriate, Italy
- 6Department of Medicine and Surgery, Unit of Neurology, Neurophysiology, Neurobiology, and Psychiatry, Università Campus Bio-Medico di Roma, Roma, Italy
Introduction: The mechanisms subtending the increased stroke risk in migraine with aura (MA) are not fully understood. Our study aims to evaluate if the clinical profile in stroke patients with MA differentiates from those without MA.
Methods: We retrieved the prospective registered electronic clinical dossiers of adult patients younger than 60 years with acute ischemic stroke admitted in four hospitals between January 2016 and June 2022. Patients were classified by the history of MA (MA+ and MA–).
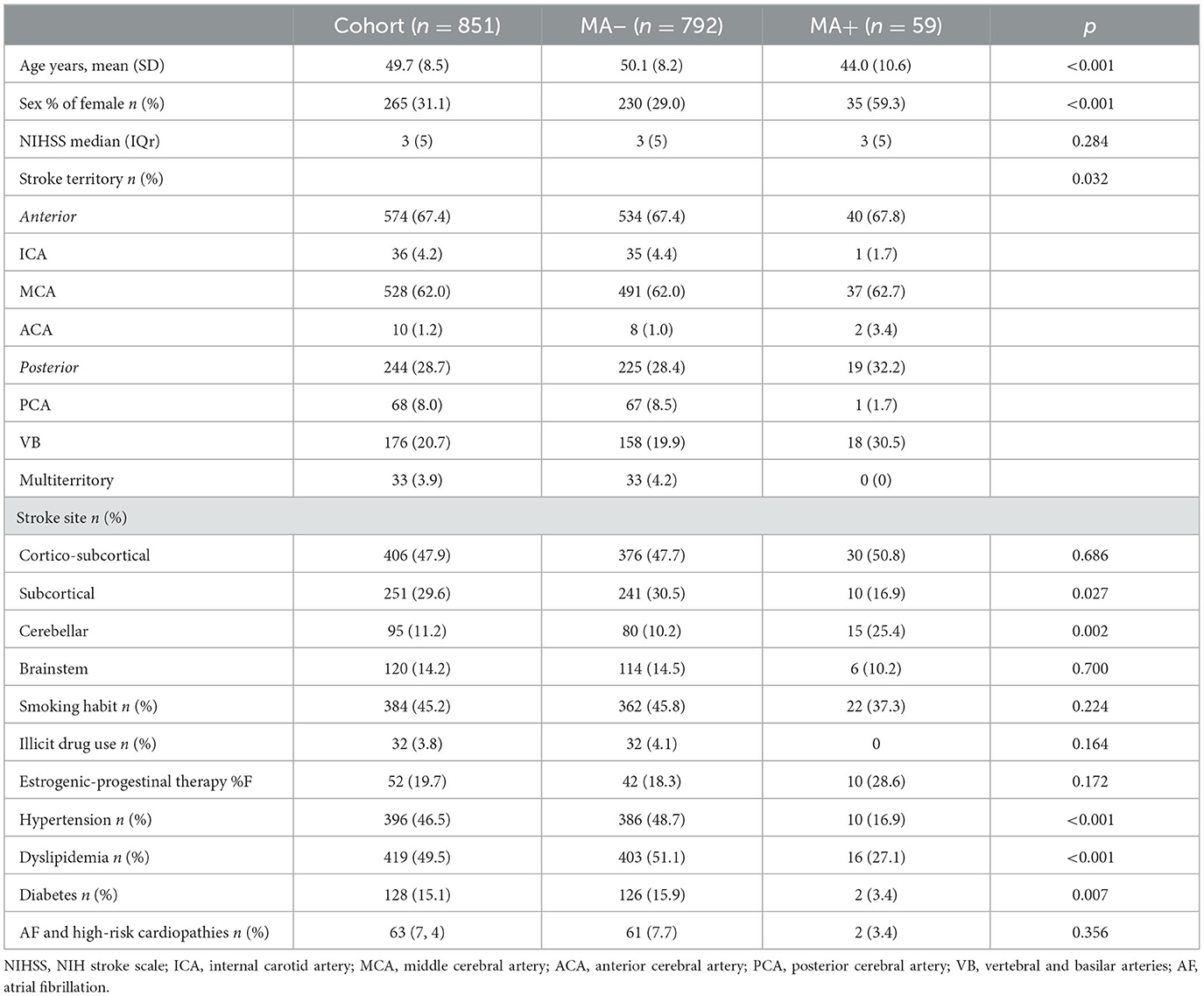
Results: We identified 851 stroke patients (59 MA+, 6.9%). Compared to MA−, MA+ patients were characterized by younger age (44.0 ± 10.6 vs 50.1 ± 8.2 years), female sex (59.3% vs 29.0%), and affected by cryptogenic (OR 2.594 95% CI 1.483–4.537), and cerebellar stroke (OR 3.218 95% CI 1.657–6.250; p ≤ 0.001 for all comparisons). After adjusting for age and sex, MA+ patients presented less frequently hypertension (OR 0.349 95% CI 0.167–0.470; p=0.005) and dyslipidemia (OR 0.523 95% CI 0.280–0.974; p = 0.041). After adjusting also for risk factors, the MA+ group had less frequently symptomatic large vessel stenosis (OR 0.126 95% CI 0.017–0,924; p = 0.042) and clinical atherosclerosis (OR 0.103 95% CI 0.014–0.761; p = 0.026), while intima–media thickness did not differ (p = 0.395).
Discussion: Cryptogenic and cerebellar stroke and fewer vascular risk factors and clinical atherosclerosis seem to characterize stroke patients with MA.
Introduction
Migraine is one of the most prevalent and disabling neurological conditions worldwide (1). Recurrent headaches accompanied by typical symptoms such as nausea, noise, and light sensitivity allow migraine diagnosis to be made solely on clinical criteria (2). Approximately 30% of patients with migraine can experience transient neurological (i.e., visual, sensory, or aphasic) symptoms that characterize typical aura. While migraine can easily be diagnosed, its pathogenetic mechanisms are a complex entanglement that is not fully unraveled (3). These multifaceted mechanisms are also shared with a plethora of other clinical conditions which are comorbid with migraine (4). Among these disorders, acute vascular accidents are probably the most dreaded event in the lifetime of patients with migraine (5). Stroke risk is more consistent in patients with migraine with aura (MA), where, while the absolute risk of ischemic stroke is low, the relative risk is doubled when compared to the general population (6). Ischemic stroke in patients with MA can rarely occur in concomitance with an attack with a prolonged aura (i.e., migrainous infarction) (2), while most vascular events hit patients interictally.
Different pathogenetic mechanisms have been called into question to explain both conditions: a predisposing genetic background, thromboembolism, endothelial and hemodynamic dysfunction, and the inefficient use of energetic supplies (7, 8).
The increased prevalence and larger patent foramen ovale (PFO) (9) in patients with MA compared with controls led to the hypothesis that PFO might play a role in MA pathogenesis and may be a common cardio-embolism source in MA patients with stroke. Indeed, patients with MA most often suffer from cryptogenic stroke, independent of PFO presence (10, 11).
In contrast, the clinical impact of atrial fibrillation has still to be clearly established (12, 13). An impairment in cerebral hemodynamics, either constitutional or as the result of recurrent attacks or triptan intake, has often been claimed as another potential determinant of stroke in patients with migraine (14). Nevertheless, as an apparent paradox, patients with MA tend to have more reactive inter-ictal cerebral hemodynamics, at least in the anterior circulation (15, 16) and, with some reports, also in the posterior circulation (14). Along the same line, the lack of traditional vascular risk factors (17) and large artery atherosclerosis characterize migraine patients with stroke (18, 19).
The carotid wall [i.e., intima–media thickness (IMT)] is influenced by endothelial health. Local environment changes induce the endothelium to secrete several substances, including endothelin-1 (ET-1), von Willebrand Factor (vWF), plasminogen activator inhibitor-1, and platelet activation. These can result in local inflammation and thrombosis, a phenomenon defined as endothelial activation (7). Platelet activation induces augmented aggregation and interaction with leucocytes, which have been observed in migraine (20). Patients with migraine also present a pro-inflammatory and pro-coagulative milieu (21, 22) that justifies the exponential effect that smoking and estrogens exert on stroke risk in MA (23).
Cohort studies addressing carotid IMT consistently reported higher values in patients with migraine with or without aura than controls (24–26).
This study investigates subclinical and clinical large vessel atherosclerosis in stroke patients with MA compared with other young and middle-aged stroke patients without MA.
Methods
Participants and study design
This multicenter cohort study was conducted in four hospitals in Northern and Central Italy: ASST Grande Ospedale Metropolitano Niguarda in Milan, ASST Bergamo Est in Seriate (Bergamo), Marche Polytechnic University Hospital in Ancona, and Foundation Campus Bio-Medico University Hospital in Rome.
Our primary endpoints were to investigate whether stroke patients with MA (MA+) present a different burden of subclinical and clinical atherosclerosis than those without MA (MA–).
We retrospectively analyzed the prospectively collected electronic clinical dossiers of consecutive patients discharged from our hospitals with the diagnosis of acute ischemic stroke from January 2016 to June 2022.
Inclusion criteria:
a. clinical diagnosis of acute ischemic stroke confirmed by radiological findings;
b. age 18 years or older and younger than 60 years.
Exclusion criteria: Clinical manifestations lasting longer than 24 h but with negative radiologic findings were excluded to avoid stroke mimics confounders.
Clinical assessment
All patients received diagnostic and therapeutic care according to the Italian Stroke Association guidelines (https://isa-aii.com/). Stroke was defined as focal neurological symptoms associated with radiological findings (either MR or CT) of acute cerebral ischemia. Migraine with aura and migrainous infarction was diagnosed according to the International Headache Society classification (2).
Our hospitals were provided with an electronic clinical dossier where patient medical history is recorded according to a semi-structured clinical chart model. All patients were interviewed (with the help of family members in case of aphasia or impaired alertness) according to a semi-structured clinical chart model. In MA+ patients, characteristics of aura were also collected: (a) type (pure visual compared to non-visual or multimodal), (b) frequency (i.e., number of episodes) in the last year, (c) the usual duration of the aura phenomenon, and (d) the onset age of MA.
Vascular risk factors
Hypertension was defined as a history of high blood pressure, systolic blood pressure of ≥140 mmHg, diastolic blood pressure of ≥90 mmHg, or the use of antihypertensive therapies before stroke onset. Dyslipidemia was defined as a history of dyslipidemia, fasting serum total cholesterol level of ≥6.22 mmol/L (2.4 g/L), a triglyceride level of ≥2.26 mmol/L (2 g/L), or the use of statins or fibrates before stroke onset. Diabetes mellitus was defined as a previous diagnosis of type 1 or type 2 diabetes, a history of diabetes mellitus, a fasting serum glucose level of ≥7.0 mmol/L (1.26 g/L), or using an oral antihyperglycemic or insulin before stroke onset. Smoking was defined as active tobacco smoking. The active use of recreational drugs and estrogenic-progestin therapy was also collected. Seventy-two-hour electrocardiographic monitoring or 24 h Holter electrocardiography in case of negative findings was performed to detect atrial fibrillation or other arrhythmias. In selected patients, prolonged non-invasive or invasive EKG monitoring was carried out. We defined the presence of autoimmunity as a positive finding of autoantibodies against extractable nuclear antigens (ENAs) and/or antinuclear antibodies (ANAs) analyzed during the hospital stay.
Large vessel atherosclerosis and carotid IMT
Carotid and vertebral artery atherosclerosis were investigated with ultrasound duplex as described elsewhere (27). Briefly, the neck vessel arteries were assessed by continuous-wave Doppler and color flow B-mode Doppler ultrasound using high-resolution 7.5 MHz transducers (Philips iU22, Bothell, WA, USA). The best images were digitized and stored for central reading and interpretation. The degree of carotid stenosis was established by means of combined criteria considering blood flow velocities as well as morphological characteristics (28). According to the Mannheim consensus, a carotid plaque was defined as a focal structure protruding into the arterial lumen of at least 0.5 mm or 50% of the surrounding IMT value or showing a thickness of >1.5 mm measured from the media–adventitia interface to the intima–lumen interface (29). For each segment, a plaque scoring system was set as follows: 0, no plaque; 1, small plaque, i.e., stenosis <30%; 2, mild stenosis, i.e., (range 30–49%); 3, moderate stenosis (range 50–69%); and 4, severe stenosis or occlusion (≥70%). “High-risk” plaque was defined as grades 3 and 4 and/or with the morphological characteristics of instability (i.e., echolucency and ulcer) (30). We named “symptomatic large vessel stenosis” the presence of carotid “high-risk” plaques or vertebral or intracranial stenosis in the territory of the ischemic stroke while “clinical atherosclerosis” the presence of carotid stenosis of at least a moderate degree, and/or any other vertebral or intracranial stenosis in any vascular territory.
Neck vessel stenosis >50% or occlusion was confirmed with magnetic resonance imaging (MRI) and/or computed tomography (CT) angiography.
Carotid IMT was measured as described elsewhere according to the Mannheim consensus (27, 29) in Ancona, Bergamo, and Roma hospitals. Interrater reproducibility has been previously assessed in the Ancona and Rome ultrasound labs (31). We considered right and left IMT and the mean value of both sides (mean IMT).
Intracranial stenoses were investigated with MRI and/or CT angiography and transcranial ultrasound duplex as complimentary evaluation.
Patent foramen ovale
Transthoracic echocardiography (TTE) was performed in all patients. In patients without a definite cause of stroke, upon clinical indication, transcranial Doppler and/or transesophageal echocardiography (TEE) (at rest and during provocative maneuvers using IV injection of agitated saline) were performed to detect PFO and septal interatrial aneurysm (SIA) and other potential cardiac sources of embolism. The presence of PFO with SIA and large PFO (>30 bubbles at rest) was classified as high-risk PFO (32). Further investigations (e.g., screening for acquired or genetic thrombophilia and homocysteine blood levels) were performed upon clinical indication. The stroke severity at admission was assessed by the National Institutes of Health Stroke Scale (NIHSS) (http://nihstrokescale.org). Stroke etiology was defined in all patients according to the TOAST classification (33).
The study received approval from the local Ethical Committee (Prot: 17/20 OSS ComEt CBM), which waived participants' need for written consent because of the retrospective study design and the anonymous data set. Anonymized data will be shared by request from any qualified investigator.
Statistical analysis
No sample size calculation was performed as this convenience sample was based on the available data. Statistical analyses were performed with SPSS version 28.0 (SPSS Inc., Chicago, IL, USA). The interval variables between groups were compared with independent t-test (expressed as means with standard deviations [SD]) or Mann–Whitney U-tests (medians with interquartile range [IQR]) according to data distribution assessed with contingency tables (chi-square and two-tailed Fisher exact tests), and unadjusted odds ratios (ORs) with their 95% confidence intervals (CIs) were run to compare frequencies between groups. All tests were two-tailed. Statistical significance was set with a p-value of < 0.05.
Thereafter, we ran forced entry binary logistic regression to adjust risk factors for age and sex and investigated if MA was correlated with IMT and large vessel atherosclerosis. Subjects with missing information on extracranial or intracranial atherosclerosis were excluded. Variables were considered for the analyses if available for at least two-thirds of the patients with the exception of IMT. No imputation was done for missing baseline data. Stepwise linear or binary regression analysis was carried out to adjust correlations for sex, age, and vascular risk factors.
Results
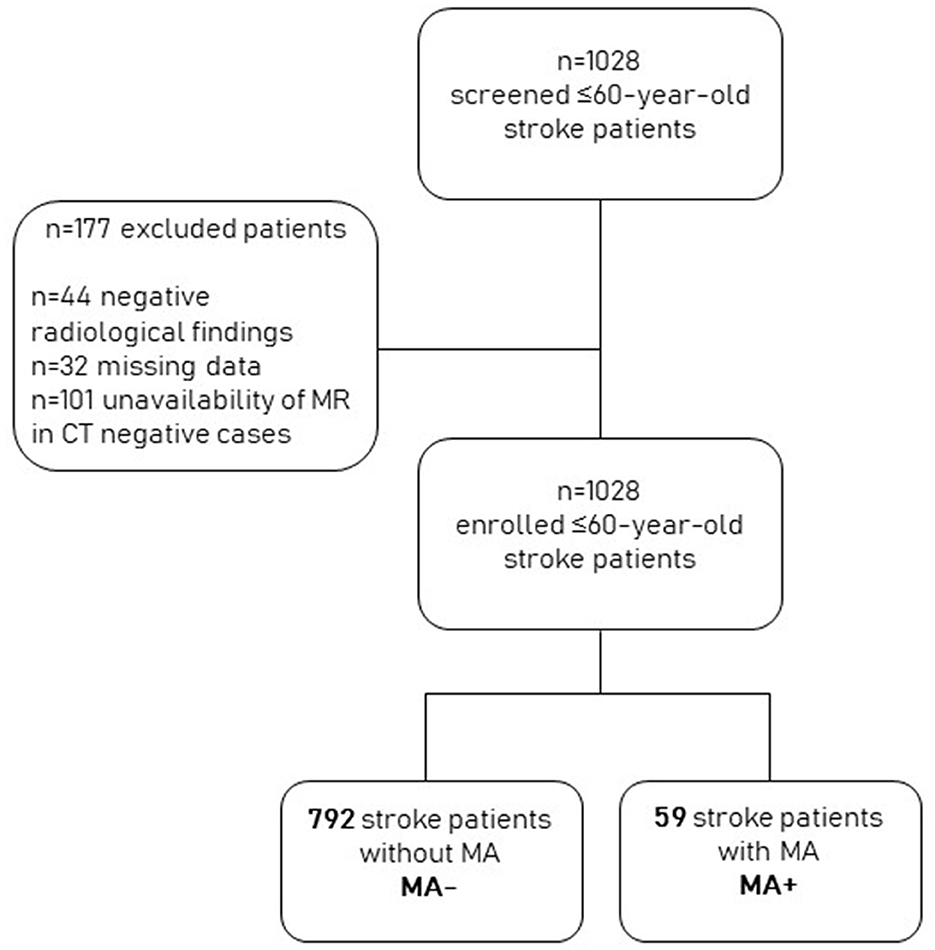
Of the 1,028 screened patients, 101 were excluded because they had a negative brain CT scan and had not undergone MRI during their hospital stay, 44 because they had negative radiological findings, and 32 because they had missing data. We finally included 851 patients with stroke. Of these, 59 (6.9%) also had an MA diagnosis (MA+). The remaining 792 were classified as MA– (Figure 1).
Table 1 summarizes demographics, clinical variables, and stroke features in MA+ compared to MA– participants. Overall, MA+ participants were younger and characterized by a lower burden of vascular risk factors (diabetes [OR 0.185, 95% C,I 0.045–0.768], hypertension [OR 0.215, 95%CI, 0.107–0.430], and dyslipidemia [OR 0.355, 95%CI, 0.197–0.642]), were mainly female [OR 3.225, 95% CI,1.958–5.310], presented cerebellar stroke more often [OR 3.017, 95% CI, 1.607–5.665], and subcortical infarct less frequently [OR 0.281, 95% CI, 0.163–0.482] compared to MA– participants. After adjusting for age and sex, hypertension (OR 0.349, 95%CI, 0.167–0.470; p = 0.005) and dyslipidemia (OR 0.523, 95% CI, 0.280–0.974; p = 0.041) but not diabetes (p = 0.129) were confirmed as less prevalent in the MA+ group; cerebellar stroke (OR 3.218, 95%CI, 1.657–6.250; p ≤ 0.001) showed still distinctive of MA+ patients, while subcortical ischemic lesions were not (p = 0.117).
Stroke patients with MA presented visual aura more commonly (55, 88.1%) with a mean duration of 19.8 min (SD 11.6) and a frequency of 11.4 attacks per year (SD 11.7, min 2, max 120). They reported an MA onset age of 26.8 years (SD 12.8), with a mean time interval of 17.3 years (SD 12.9) from the MA onset to stroke occurrence.
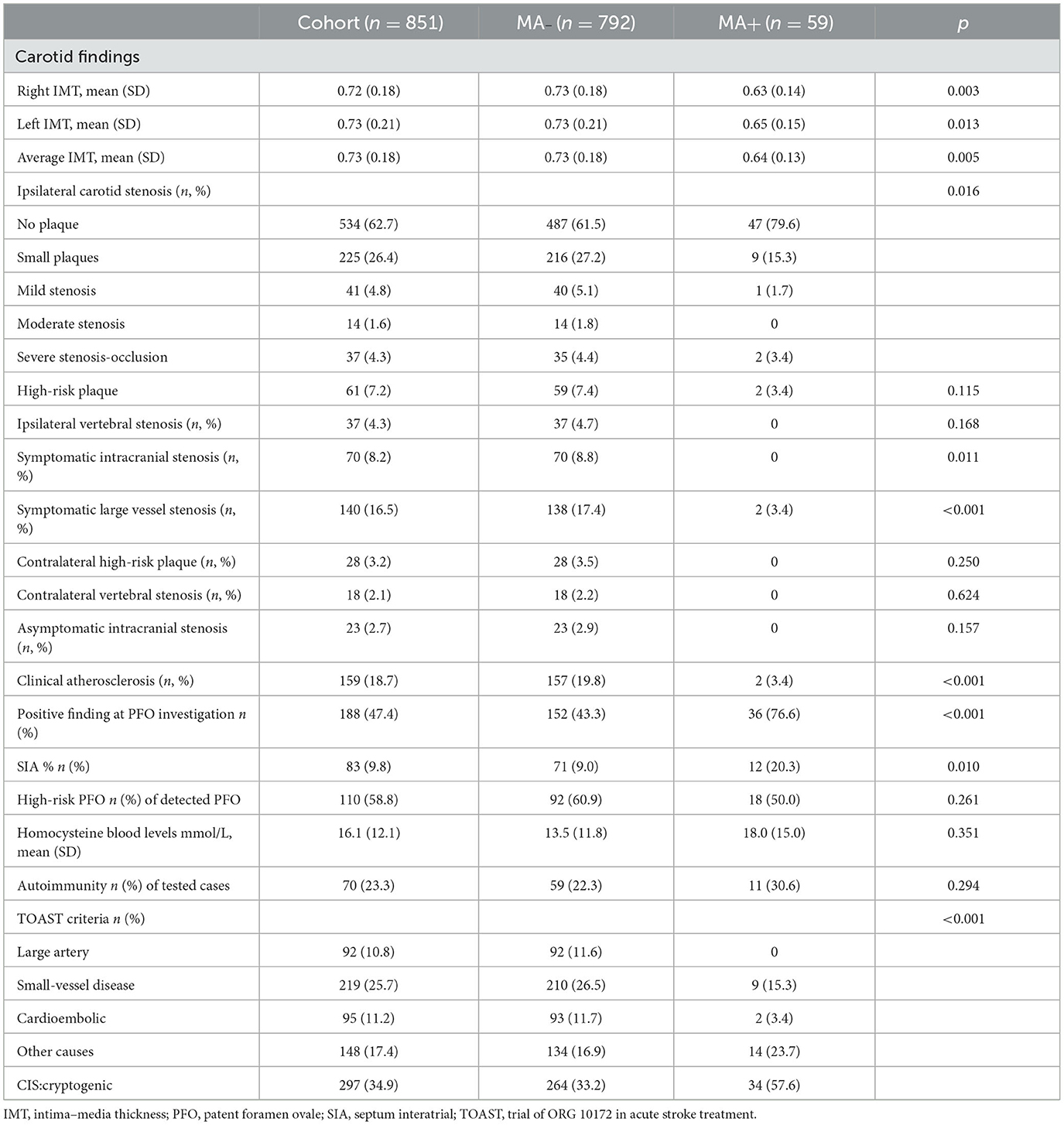
Table 2 shows the results of diagnostic workup and stroke classification in MA+ and MA- participants. Carotid IMT was evaluated in 347 subjects (32 MA+, 54.2%, and 315 MA–, 39.8%; p = 0.084), and serum homocysteine was analyzed in 548 patients (50 MA+, 84.7% and 498 MA–, 62.9%; P < 0.001).
The clinical investigations to detect PFO were performed in 47 (79.7%) MA+ patients and in 350 (44.2%) MA– patients (p < 0.001). Similarly, autoimmunity was investigated more often in MA+ (36, 61%) than in MA– (264, 33.3%) patients (p < 0.001).
After adjusting for age and sex, IMT was not different in MA+ compared to MA– participants (p = 0.692), being largely correlated with age (p ≤ 0.001). Carotid IMT was also correlated with serum homocysteine after correction for age and sex (p = 0.041).
MA+patients were more often affected by cryptogenic stroke (OR 3.144, 95% CI, 1.825–5.416, p < 0.001) also when adjusted for age and sex (OR 2.594, 95% CI, 1.483–4.537; p < 0.001).
Table 3 displays the association between MA and high-risk PFO, carotid IMT, symptomatic large vessel stenosis, and clinical atherosclerosis after adjusting for age, sex, diabetes, hypertension, dyslipidemia, and smoking habit. Cerebellar strokes did not correlate with the presence of high-risk PFO (19.1 vs. 18.8%; p = 1.000).
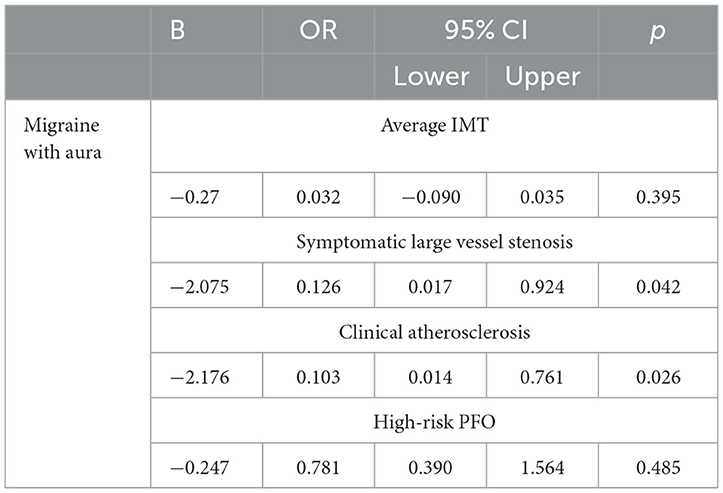
Table 3. Association of MA (independent variable) with high-risk PFO, carotid IMT, symptomatic large vessel stenosis, and clinical atherosclerosis (dependent variables) after adjusting for age and sex, diabetes, hypertension, dyslipidemia, and smoking habit.
Discussion
Migraine with aura is one of the most common stroke mimics that clinicians deal with in emergency settings (34). Indeed, migraine, especially with aura, increases stroke risk, and given its high prevalence, it should be given more consideration in vascular prevention programs. In this light, it is of pivotal importance to fully understand the pathological mechanisms subtending stroke in patients with migraine. On the one hand, it would allow for the implementation of tailored effective stroke prevention strategies; on the other hand, it would increase the knowledge of migraine physiopathology.
The findings of this study outline a peculiar profile of patients with stroke with MA. In our cohort, patients with MA were younger, more often female, and had fewer vascular risk factors (i.e., diabetes and dyslipidemia) even when adjusted for sex and age. We also observed a negative association between symptomatic large vessel stenosis, any relevant atherosclerosis, and MA diagnosis. This result suggests that the lack of large vessel atherogenesis is a distinctive feature of patients with MA with stroke and confirms earlier reports drawing similar conclusions in cohorts with different age ranges (18, 19, 35, 36). Our study also addressed subclinical atherosclerosis. Carotid IMT is a marker of endothelial activation leading to atherosclerosis and is associated with stroke risk, plaque progression, and neurodegenerative disorders (27, 30, 37). Carotid IMT is also largely dependent on inflammation and oxidative processes (38). Cohort studies have shown that patients with migraine present intima–media thickening (24–26), and here we confirm that, in contrast to large vessel atherosclerosis, patients with MA present age and sex-adjusted IMT similar to other patients with stroke. This observation highlights the potential pathogenic role of endothelial activation in predisposing patients with migraine to stroke.
The endothelium is an active endocrine and paracrine organ playing a central role in multiple functions. Its release of paracrine substances mediates vascular permeability, vascular tone, local inflammation, and thrombosis. The endothelium homeostasis is kept by balanced actions of substances with opposing functions. Some of these (e.g., nitric oxide, ET-1, vWF, platelet-activating factor, and homocysteine) have been consistently implied in migraine pathogenesis, especially in MA (7, 22). If the endothelium is damaged by mechanisms related to vascular risk factors or genetic or exogenous factors, this balance is lost, resulting in the so-called endothelial activation. To note, in our cohort serum, homocysteine was correlated with IMT after age and sex adjustment. The detrimental relationship between endothelial activation and migraine is probably bidirectional. On the one side, the unbalanced release of endothelial substances and microcirculation thrombosis can trigger cortical spreading depression (39); on the other side, frequent migraine attacks seem to interfere with endothelial functioning (26). In any case, the cerebral cortex of patients with MA seems to be particularly vulnerable to ischemic insults, displaying larger infarct volume (40) and a reduced ratio between hypoperfused and infarcted ischemic areas (41). This sensitivity to ischemic insults can result from different factors, among which the most relevant seems to be an impairment of vascular tone regulation secondary to endothelial dysfunction as an inefficient use of energy supplies (42). The younger age and the lower impact of traditional risk factors in patients with MA in our and other cohorts (19, 34) support these hypotheses.
Our study also observed that MA patients more frequently presented cerebellar stroke than MA– participants. The camera study (43) reported for the first time a higher prevalence of silent ischemic infarct in the posterior circulation (i.e., cerebellum) of patients with migraine, suggesting a combination of (possibly migraine attack-related) hypoperfusion and embolism but not atherosclerosis or small-vessel disease as the possible determinants. More recently, the AGES-Reykjavik study confirmed the higher prevalence of cerebellar infarcts in MA and higher incidence in patients with any migraine (44), as also observed in the acute settings (45).
We report a higher frequency of clinically relevant cerebellar strokes in patients with MA that were not correlated with the presence of high-risk PFO. It should be noted that, while PFOs of any degree were more frequently detected in patients with MA [~50–55% (46)], they were only high-risk PFOs in half of the cases. The prevalence of PFO is also very common in the general population, but it is responsible for ischemic stroke only in a small percentage of cases (32). Moreover, no patients with MA presented the steno-occlusive disease of vertebral and basilar arteries. The cerebellar involvement in migraine is supported by neurophysiological, neuroimaging, and genetic studies (47). Patients with MA can also display impairment in cerebellar circulation autoregulation (48). In this line, cerebellar hypoperfusion and crossed cerebellar diaschisis are common in patients with migraine with aura. However, this phenomenon is usually of benign origin as it is not associated with cerebellar infarctions, even in patients with prolonged symptom-related perfusion abnormalities persisting for up to 24 h (49). Nevertheless, it is possible that under specific conditions, these perfusion alterations produce permanent damage. It should be noted that Purkinje cells are selectively prone to hypoxic insults (50). The brain of patients with MA, which is highly energy-demanding but unable to use energetic supply effectively, can easily subdue the ischemic damage in vulnerable neurons, especially in the presence of altered autoregulation and/or endothelial activation.
The main limitation of our study is the retrospective design. Our structured electronic dossiers, however, enabled us to collect prospectively in detail the studied variables. Another limitation is the lack of identification and characterization of migraine without aura in all stroke subjects. However, when not accompanied by transient focal symptoms, headache is rarely investigated or adequately reported in real-life clinical settings, producing a detection bias. Therefore, we preferred not to consider this aspect as it may have been largely underestimated in the whole cohort.
In summary, our findings suggest that young and middle-aged patients with stroke with MA present a peculiar clinical profile characterized by a less prominent role of traditional vascular risk factors and clinical atherosclerosis and by ischemic damage in vulnerable brain areas, probably mediated by endothelial and local hemodynamic impairment and inefficient energetic use (Figure 2).
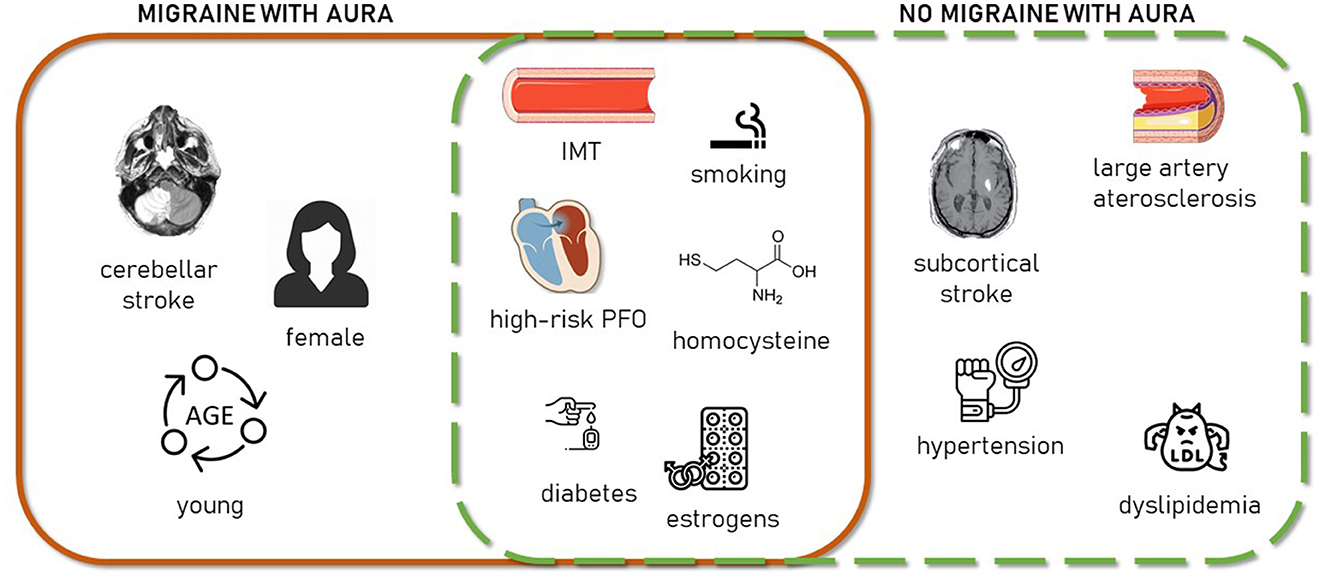
Figure 2. Schematic representation of clinical stroke-related aspects characterizing MA+ patients (continuous line square, left), MA– patients (dashed line square, right), or both (square intersection).
Our study warrants more research in the field of vascular aspects of migraine as the prevention of common vascular risks is insufficient to address migraine-related stroke risk. This perspective appears even more relevant since the introduction of the new migraine preventive therapies targeting the calcitonin gene-related peptide, one of the most potent vasodilatory peptides in cerebral circulation. Although clinical studies do not support any detrimental impact on cerebral and systemic hemodynamics (51), cautious vigilance is necessary.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Campus Bio-Medico University. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
CA conceptualized the study and analyzed and interpreted the findings. GV, AR, and PM collected the data and interpreted the findings. NB and MM collected the data. VL, FF, EA, MS, and FV interpreted the findings and revised the manuscript for intellectual content. All authors contributed to the article and approved the submitted version.
Acknowledgments
We would like to thank Marcel Bach Pages for revising the content of this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer RO declared a past co-authorship/collaboration with the authors CA, NB, and FV.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Steiner TJ, Stovner LJ, Jensen R, Uluduz D, Katsarava Z. Migraine remains second among the world's causes of disability, and first among young women: findings from GBD2019. J Headache Pain. (2020) 21:137. doi: 10.1186/s10194-020-01208-0
2. Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition. Cephalalgia. (2018) 38:1–211. doi: 10.1177/0333102417738202
4. Altamura C, Corbelli I, de Tommaso M, di Lorenzo C, di Lorenzo G, di Renzo A, et al. Pathophysiological bases of comorbidity in migraine. Front Hum Neurosci. (2021) 15:640574. doi: 10.3389/fnhum.2021.640574
5. Ng CYH, Tan BYQ, Teo YN, Teo YH, Syn NLX, Leow AST, et al. Myocardial infarction, stroke and cardiovascular mortality among migraine patients: a systematic review and meta-analysis. J Neurol. (2022) 269:2346–58. doi: 10.1007/s00415-021-10930-x
6. Øie LR, Øie LR, Kurth T, Gulati S, Gulati S, Dodick DW. Migraine and risk of stroke. J Neurol Neurosurg Psychiatry. (2020) 91:593–604. doi: 10.1136/jnnp-2018-318254
7. Paolucci M, Altamura C, Vernieri F. The role of endothelial dysfunction in the pathophysiology and cerebrovascular effects of migraine: a narrative review. J Clin Neurol. (2021) 17:164–75. doi: 10.3988/jcn.2021.17.2.164
8. Sacco S, Harriott AM, Ayata C, Ornello R, Bagur R, Jimenez-Ruiz A, et al. Microembolism and other links between migraine and stroke: clinical and pathophysiologic update. Neurology. (2022) 20:1699. doi: 10.1212/WNL.0000000000201699
9. Schwerzmann M, Nedeltchev K, Lagger F, Mattle HP, Windecker S, Meier B, et al. Prevalence and size of directly detected patent foramen ovale in migraine with aura. Neurology. (2005) 65:1415–8. doi: 10.1212/01.wnl.0000179800.73706.20
10. Martinez-Majander N, Artto V, Ylikotila P, Sarnowski B, Waje-Andreassen U, Yesilot N, et al. Association between migraine and cryptogenic ischemic stroke in young adults. Ann Neurol. (2021) 89:242–53. doi: 10.1002/ana.25937
11. Altamura C, Cascio Rizzo A, Viticchi G, Maggio P, Costa CM, Brunelli N, et al. Shorter visual aura characterizes young and middle-aged stroke patients with migraine with aura. J Neurol. (2022) 269:897–906. doi: 10.1007/s00415-021-10671-x
12. Sen S, Michelle Androulakis X, Duda V, Alonso A, Chen LY, Soliman EZ, et al. Migraine with visual aura is a risk factor for incident atrial fibrillation: a cohort study. Neurology. (2018) 91:E2202–10. doi: 10.1212/WNL.0000000000006650
13. De Giuli V, Grassi M, Locatelli M, Gamba M, Morotti A, Bonacina S, et al. Cardiac sources of cerebral embolism in people with migraine. Eur J Neurol Ene. (2020) 20:14556. doi: 10.1111/ene.14556
14. Dzator JS, Howe PR, Wong RH. Profiling cerebrovascular function in migraine: a systematic review and meta-analysis. J Cerebr Blood Flow Metabol. (2021) 41:919–44. doi: 10.1177/0271678X20964344
15. Vernieri F, Tibuzzi F, Pasqualetti P, Altamura C, Palazzo P, Rossini PM, et al. Increased cerebral vasomotor reactivity in migraine with aura: an autoregulation disorder? a transcranial Doppler and near-infrared spectroscopy study. Cephalalgia. (2008) 28:689–95. doi: 10.1111/j.1468-2982.2008.01579.x
16. Altamura C, Paolucci M, Brunelli N, Cascio Rizzo A, Cecchi G, Assenza F, et al. Right-to-left shunts and hormonal therapy influence cerebral vasomotor reactivity in patients with migraine with aura. PLoS One. (2019) 14:e0220637. doi: 10.1371/journal.pone.0220637
17. Sacco S, Pistoia F, Degan D, Carolei A. Conventional vascular risk factors: Their role in the association between migraine and cardiovascular diseases. Cephalalgia. (2015) 35:146–64. doi: 10.1177/0333102414559551
18. van Os HJA, Mulder IA, Broersen A, Algra A, van der Schaaf IC, Kappelle LJ, et al. Migraine and cerebrovascular atherosclerosis in patients with ischemic stroke. Stroke. (2017) 48:1973–5. doi: 10.1161/STROKEAHA.116.016133
19. Gollion C, Guidolin B, Lerebours F, Rousseau V, Barbieux-Guillot M, Larrue V. Migraine and large artery atherosclerosis in young adults with ischemic stroke. Headache. (2022) 62:191–7. doi: 10.1111/head.14265
20. Borgdorff P, Tangelder GJ. Migraine: Possible role of shear-induced platelet aggregation with serotonin release. Headache. (2012) 52:1298–318. doi: 10.1111/j.1526-4610.2012.02162.x
21. Tietjen GE, Collins SA. Hypercoagulability and migraine. Headache J Head Face Pain. (2018) 58:173–83. doi: 10.1111/head.13044
22. Tietjen GE, Maly EF. Migraine and ischemic stroke in women: a narrative review. Headache. (2020) 60:843–63. doi: 10.1111/head.13796
23. Sacco S, Merki-Feld GS, Ægidius KL, Bitzer J, Canonico M, Kurth T, et al. Hormonal contraceptives and risk of ischemic stroke in women with migraine: a consensus statement from the European Headache Federation (EHF) and the European Society of Contraception and Reproductive Health (ESC). J Headache Pain. (2017) 18:108. doi: 10.1186/s10194-017-0815-1
24. Magalhães JE, Barros IML, de Pedrosa RP, Sampaio Rocha-Filho PA. Migraine and markers of carotid atherosclerosis in middle-aged women: a cross-sectional study. Headache. (2019) 59, 77–85. doi: 10.1111/head.13460
25. Wang Q, Liu Z-Y, Zhou J. Ultrasonic assessment of carotid intima-media thickness in migraine: a meta-analysis. J Int Med Res. (2019) 47:2848–55. doi: 10.1177/0300060519851354
26. Yilmaz Avci A, Akkucuk MH, Torun E, Arikan S, Can U, Tekindal MA. Migraine and subclinical atherosclerosis: endothelial dysfunction biomarkers and carotid intima-media thickness: a case-control study. Neurologic Sci. (2019) 40:703–11. doi: 10.1007/s10072-019-3710-5
27. Brunelli N, Altamura C, Costa CM, Altavilla R, Palazzo P, Maggio P, et al. Carotid artery plaque progression: proposal of a new predictive score and role of carotid intima-media thickness. Int J Environ Res Public Health. (2022) 19:758. doi: 10.3390/ijerph19020758
28. de Bray JM, Glatt B. Quantification of atheromatous stenosis in the extracranial internal carotid artery. Cerebrovascul Dis. (1995) 5:414–26. doi: 10.1159/000107895
29. Touboul P-J, Hennerici MG, Meairs S, Adams H, Amarenco P, Bornstein N, et al. Mannheim carotid intima-media thickness and plaque consensus (2004–2006–2011). Cerebrovascul Dis. (2012) 34:290–6. doi: 10.1159/000343145
30. Silvestrini M, Altamura C, Cerqua R, Pasqualetti P, Viticchi G, Provinciali L, et al. Ultrasonographic markers of vascular risk in patients with asymptomatic carotid stenosis. J Cerebr Blood Flow Metabol. (2013) 33:619–24. doi: 10.1038/jcbfm.2013.5
31. Buratti L, Balestrini S, Avitabile E, Altamura C, Vernieri F, Viticchi G, et al. Sex-associated differences in the modulation of vascular risk in patients with asymptomatic carotid stenosis. J Cerebr Blood Flow Metabol. (2015) 35:684–8. doi: 10.1038/jcbfm.2014.248
32. Elgendy AY, Saver JL, Amin Z, Boudoulas KD, Carroll JD, Elgendy IY, et al. Proposal for updated nomenclature and classification of potential causative mechanism in patent foramen ovale–associated stroke. JAMA Neurol. (2020) 77:878–86. doi: 10.1001/jamaneurol.2020.0458
33. Adams HP, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke: definitions for use in a multicenter clinical trial TOAST Trial of Org 10172 in Acute Stroke Treatment. Stroke. (1993) 24:35–41. doi: 10.1161/01.STR.24.1.35
34. Terrin A, Toldo G, Ermani M, Mainardi F, Maggioni F. When migraine mimics stroke: a systematic review. Cephalalgia. (2018) 38:2068–78. doi: 10.1177/0333102418767999
35. Pezzini A, Grassi M, Lodigiani C, Patella R, Gandolfo C, Casoni F, et al. Predictors of migraine subtypes in young adults with ischemic stroke: The Italian project on stroke in young adults. Stroke. (2011) 42:17–21. doi: 10.1161/STROKEAHA.110.592246
36. Androulakis XM, Kodumuri N, Giamberardino LD, Rosamond WD, Gottesman RF, Yim E, et al. Ischemic stroke subtypes and migraine with visual aura in the ARIC study. Neurology. (2016) 87:2527–32. doi: 10.1212/WNL.0000000000003428
37. Altamura C, Squitti R, Pasqualetti P, Tibuzzi F, Silvestrini M, Ventriglia MC, et al. What is the relationship among atherosclerosis markers, apolipoprotein E polymorphism and dementia? Eur J Neurol. (2007) 14:679–82. doi: 10.1111/j.1468-1331.2007.01714.x
38. Corrado E, Rizzo M, Coppola G, Fattouch K, Novo G, Marturana I, et al. An update on the role of markers of inflammation in atherosclerosis. J Atheroscler Thromb. (2010) 17:1–11. doi: 10.5551/jat.2600
39. Lemale CL, Lückl J, Horst V, Reiffurth C, Major S, Hecht N, et al. Migraine Aura, transient ischemic attacks, stroke, and dying of the brain share the same key pathophysiological process in neurons driven by gibbs–donnan forces, namely spreading depolarization. Front Cell Neurosci. (2022) 16:837650. doi: 10.3389/fncel.2022.837650
40. de Giuli V, Besana M, Grassi M, Zedde M, Zini A, Lodigiani C, et al. History of migraine and volume of brain infarcts: The italian project on stroke at young age (IPSYS). J Stroke. (2019) 21:324–31. doi: 10.5853/jos.2019.00332
41. Pezzini A, Busto G, Zedde M, Gamba M, Zini A, Poli L, et al. Vulnerability to infarction during cerebral ischemia in migraine sufferers. Stroke. (2018) 49:573–8. doi: 10.1161/STROKEAHA.118.020554
42. Gross EC, Lisicki M, Fischer D, Sándor PS, Schoenen J. The metabolic face of migraine — from pathophysiology to treatment. Nat Rev Neurol. (2019) 15:627–43. doi: 10.1038/s41582-019-0255-4
43. Kruit MC, Launer LJ, Ferrari MD, van Buchem MA. Infarcts in the posterior circulation territory in migraine: the population-based MRI CAMERA study. Brain. (2005) 128:2068–77. doi: 10.1093/brain/awh542
44. Sigurdsson S, Aspelund T, Kjartansson O, Gudmundsson E, Jonsson P, et al. Cerebrovascular risk-factors of prevalent and incident brain infarcts in the general population: the AGES-Reykjavik Study. Stroke. (2022) 53:1199–206. doi: 10.1161/STROKEAHA.121.034130
45. Øygarden H, Kvistad CE, Bjørk M, Thomassen L, Waje-Andreassen U, Naess H. Diffusion-weighted lesions in acute ischaemic stroke patients with migraine. Acta Neurol Scand. (2014) 12:41–46. doi: 10.1111/ane.12236
46. Altamura C, Paolucci M, Costa CM, Brunelli N, Cascio Rizzo A, Cecchi G, et al. Right-to-left shunt and the clinical features of migraine with aura: earlier but not more. Cerebrovascul Dis. (2019) 47:268–74. doi: 10.1159/000501544
47. Kros L, Angueyra Aristizábal CA, Khodakhah K. Cerebellar involvement in migraine. Cephalalgia. (2018) 38:1782–91. doi: 10.1177/0333102417752120
48. Reinhard M, Schork J, Allignol A, Weiller C, Kaube H. Cerebellar and cerebral autoregulation in migraine. Stroke. (2012) 43:987–93. doi: 10.1161/STROKEAHA.111.644674
49. Kellner-Weldon F, El-Koussy M, Jung S, Jossen M, Klinger-Gratz PP, Wiest R. Cerebellar hypoperfusion in migraine attack: incidence and significance. AJNR Am J Neuroradiol. (2018) 39:435–40. doi: 10.3174/ajnr.A5508
50. Koeppen AH. The neuropathology of the adult cerebellum. Handb Clin Neurol. (2018) 154:129. doi: 10.1016/B978-0-444-63956-1.00008-4
Keywords: migraine aura, stroke, intima-media thickness, atherosclerosis, cerebral blood circulation
Citation: Altamura C, Viticchi G, Rizzo AC, Maggio P, Brunelli N, Marcosano M, Lazzaro VD, Fiacco F, Agostoni EC, Silvestrini M and Vernieri F (2023) Stroke territory and atherosclerosis in ischemic stroke patients with a history of migraine with aura. Front. Neurol. 14:1142424. doi: 10.3389/fneur.2023.1142424
Received: 11 January 2023; Accepted: 02 February 2023;
Published: 27 February 2023.
Edited by:
Anna Ambrosini, Mediterranean Neurological Institute Neuromed (IRCCS), ItalyReviewed by:
Raffaele Ornello, University of L'Aquila, ItalyIstván Fekete, University of Debrecen, Hungary
Copyright © 2023 Altamura, Viticchi, Rizzo, Maggio, Brunelli, Marcosano, Lazzaro, Fiacco, Agostoni, Silvestrini and Vernieri. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Claudia Altamura,  Yy5hbHRhbXVyYUBwb2xpY2xpbmljb2NhbXB1cy5pdA==
Yy5hbHRhbXVyYUBwb2xpY2xpbmljb2NhbXB1cy5pdA==
 Claudia Altamura
Claudia Altamura Giovanna Viticchi3
Giovanna Viticchi3 Angelo Cascio Rizzo
Angelo Cascio Rizzo Paola Maggio
Paola Maggio Vincenzo Di Lazzaro
Vincenzo Di Lazzaro Mauro Silvestrini
Mauro Silvestrini