- 1Department of Neurology, Shanghai Ninth People's Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
- 2Department of Neurology, Huangpu Branch, Shanghai Ninth People's Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
Objective: A low serum 25-hydroxyvitamin D (25(OH)D) level is relevant to both the occurrence and recurrence of benign paroxysmal positional vertigo (BPPV). However, whether it also contributes to residual dizziness (RD) after successful repositioning maneuvers is unknown. Therefore, this study aimed to explore the correlation between the serum 25(OH)D level and short-term RD severity in patients with BPPV after successful repositioning maneuvers.
Methods: In total, 251 patients with BPPV after successful repositioning were enrolled and prospectively followed up for 1 week (W1). Serum 25(OH)D values were detected by chemiluminescence immunoassay at enrollment (W0). In addition, we explored the relationship between 25(OH)D values at baseline and RD severity at W1 in different subgroups stratified by sex and onset age (early-onset, ≤50 years; late-onset, >50 years).
Results: The serum 25(OH)D level of female patients was significantly lower than that of male patients (15.9 ± 6.8 vs. 19.8 ± 6.6 ng/ml, p < 0.001). Its level also decreased in early-onset patients compared to late-onset ones (15.3 ± 5.9 vs. 18.0 ± 7.3 ng/ml, p = 0.003). In addition, early-onset female patients had lower 25(OH)D values than late-onset female patients (14.0 ± 5.5 vs. 17.1 ± 7.2 ng/ml, p = 0.004). However, this difference was not observed between early- and late-onset male patients. Among early-onset female patients, the 25(OH)D values of the moderate-to-severe RD group were lower than those of the minor or no RD group (10.9 ± 3.3 vs. 14.7 ± 5.7 vs. 15.0 ± 5.9 ng/ml, p = 0.046). Multivariate analysis found that decreased 25(OH)D values were related to the occurrence of moderate-to-severe RD in early-onset female patients (OR = 0.801; p = 0.022). This effect did not exist in late-onset female or male patients with BPPV.
Conclusions: Age and sex differences in serum 25(OH)D levels exist in patients with BPPV. A decreased 25(OH)D level in early-onset female patients may increase the odds of moderate-to-severe RD 1 week after successful repositioning maneuvers.
Introduction
Benign paroxysmal positional vertigo (BPPV), the most common form of peripheral vestibular disorder, is caused by the movement of otoconia in the semicircular canal detached from the utricle. Although most of the patients suffering from BPPV can be treated with correct repositioning maneuvers (1–3), some patients will still suffer from residual symptoms, including undetermined dizziness or imbalance even after typical vertigo and nystagmus disappear (4, 5). With a reported prevalence ranging from 30% to 77%, these residual symptoms have been defined as residual dizziness (RD). Residual dizziness can last from several days to a few months, adversely affecting the physical and mental health of patients (4, 5). There are currently no established diagnostic criteria for RD. Several risk factors have been reported to be associated with RD, including the long duration of BPPV before treatment (6–8), multiple repositioning maneuvers (9), elderly onset age (9, 10), physical and psychological comorbidities (9, 10), and an abnormality of vestibular evoked myogenic potential (11).
In terms of biomarker research in blood, many studies have indicated that low serum 25-hydroxyvitamin D (25(OH)D) values are related to both the occurrence and recurrence of BPPV (12, 13). Talaat et al. (12, 13) found that low values of 25(OH)D were associated with the development of BPPV, while very low 25(OH)D values were related to the recurrence of BPPV. Ding et al. (13) indicated that 25(OH)D deficiency was correlated with BPPV occurrence and recurrence, and it was also negatively associated with the severity of BPPV. Even though accumulating evidence has revealed that the low 25(OH)D level in serum increases the odds of BPPV occurrence and recurrence, whether it also contributes to RD still needs further exploration. Currently, only one study explored its effect on RD in 123 patients with posterior semicircular canal BPPV and found that low vitamin D levels were also associated with RD after successful treatment of BPPV (14). Since there were sex- and age-related differences in the vitamin D level in BPPV (15), whether these factors may influence the effect of vitamin D level on RD merits investigation.
Consequently, the present prospective short-term follow-up study was conducted among patients with BPPV after successful repositioning maneuvers, so as to explore the correlation between baseline serum 25(OH)D levels stratified by sex and onset age and RD severity 1 week later to facilitate the precise evaluation and management of RD.
Materials and methods
Subjects
Subjects in this study were recruited from the Vertigo Clinic of the Neurology Department in Shanghai Ninth People's Hospital Affiliated to Shanghai Jiao Tong University School of Medicine. In this study, idiopathic BPPV was diagnosed following the criteria of the American Academy of Otolaryngology-Head and Neck Surgery Guideline (16) and Barany Society (17). Each patient underwent corresponding repositioning maneuvers promptly after the diagnosis was made at the first visit. To be specific, patients diagnosed with posterior semicircular canal BPPV were repositioned by Epley's maneuver, while those diagnosed with horizontal semicircular canal BPPV were repositioned by Barbecue's or Gufoni's maneuver. The patient inclusion criteria were as follows: (i) patients aged 18–80 years, (ii) those with unilateral posterior semicircular canal BPPV or horizontal semicircular canal BPPV, (iii) those with the disappearance of positional vertigo and nystagmus after repositioning maneuvers, indicating the successful repositioning treatment at the initial visit (W0), and (iv) those who completed face-to-face follow-up 1 week (W1) after the initial visit since this time point was regarded as the short-term evaluation time recommended by the Chinese and American BPPV diagnosis and treatment guidelines (16, 18). Moreover, provocative positional testing was conducted at W1. The patient exclusion criteria were as follows: (i) those whose positional vertigo and nystagmus reappeared in provocative positional testing at W1, indicating the recurrence of BPPV, (ii) those with a previous history of deafness, vestibular neuritis, Meniere's disease, vestibular migraine, cerebrovascular disease, severe brain trauma, epilepsy, schizophrenia, and other serious physical and psychological diseases, and (iii)those who took calcium or vitamin D tablets in the past 1 month (11). From September 2017 to August 2021, altogether 260 patients with BPPV were screened, and among them, nine were excluded for disease recurrence within 1 week, leaving 251 enrolled for final analysis (Figure 1).
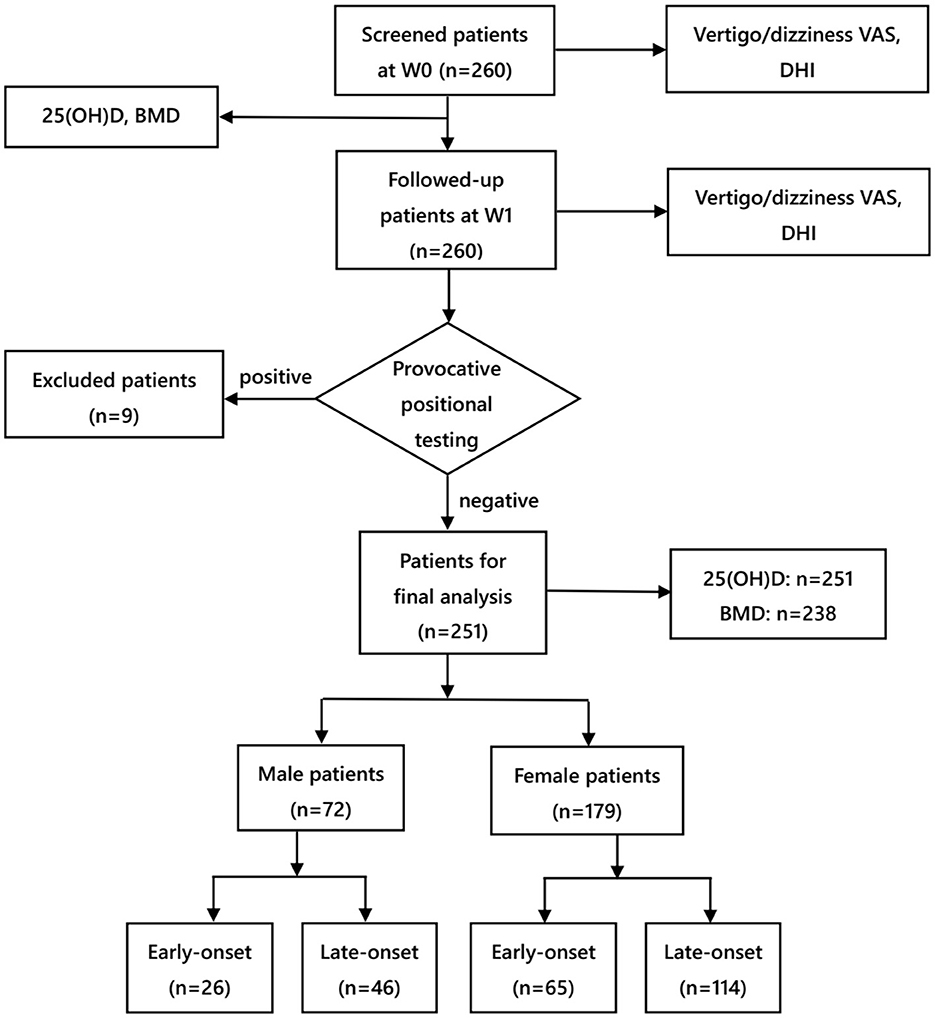
Figure 1. Flow chart of the study. VAS, visual analog scale; DHI, dizziness handicap inventory; BMD, bone mineral density; W0, at enrollment; W1, 1 week after enrollment.
Clinical profiles
Clinical parameters of all patients were collected at W0, including age, sex, body mass index (BMI), vascular comorbidities (such as hypertension, diabetes, coronary heart disease, and hyperlipidemia), history of headache, affected canals, duration of vertigo before treatment, and frequency of repositioning maneuvers. Most of our enrolled patients with BPPV were women, so the effect of estrogen on the pathogenesis of BPPV (15, 19) was also considered. According to the average menopausal age of Chinese women (20), patients aged ≤50 years were defined as the early-onset subgroup, whereas those aged >50 years were defined as the late-onset subgroup.
In this study, vertigo visual analog scale (VAS) and dizziness VAS were utilized to evaluate the severity of RD (21). The scores ranged from 0 (no symptoms) to 10 (very severe symptoms), representing the different symptom severity. Residual dizziness is a relatively subjective manifestation, and there are few relevant studies concerning the quantitative evaluation of RD. According to the principles in pain research and our previous study on BPPV (11, 22), RD was defined by the following criteria: (i) patients still suffered from dizziness or imbalanced symptoms without positional vertigo or nystagmus, as determined by provocative positional testing at W1 and (ii) the dizziness VAS score was more than 1 point at W1. Furthermore, patients with a dizziness VAS score of 1–3 were classified into a minor RD group (Group B), while those with a dizziness VAS score of >3 were in the moderate-to-severe RD group (Group C). Moreover, patients with a dizziness VAS score of 0 were classified into the no RD group (Group A). Furthermore, the Dizziness Handicap Inventory (DHI) questionnaire was used to assess the quality-of-life status of the patients. It covers three subdomains, namely, physical (DHI-P), functional (DHI-F), and emotional (DHI-E), with the total score ranging from 0 to 100 points (11, 23). A higher score indicates a greater impact on a patient's quality of life (24). DHI total and subdomain scores, together with vertigo/dizziness VAS, were evaluated and recorded at both W0 and W1 (Figure 1).
Venous blood was collected from each enrolled patient after repositioning maneuvers at W0. In our previous study, the serum 25(OH)D levels were determined by a chemiluminescence immunoassay method (Diasorin Inc, USA)(25). For the evaluation of bone mineral density, T scores of the lumbar spine and femur were measured by dual X-ray absorptiometry (GE Lunar iDXA). Osteoporosis was determined when the T score was ≤ −2.5 in either location (26).
Statistical analysis
Statistical analysis was performed by the SPSS 26.0 software (version 26.0 for Windows). Diagrams were plotted with GraphPad Prism 5.0 for Windows. Mean and standard deviation were used to represent numerical variables with a normal distribution, whereas the median and interquartile range (IQR, 25th−75th percentile) were employed to describe numerical variables with skewed distributions. The F-test and t-test were adopted to compare variables with normal distribution, whereas the Kruskal–Wallis test was employed to compare variables with skewed distributions. Meanwhile, the chi-square test or Fisher's exact test was applied to categorical variables. The independent factors associated with RD were investigated by binary logistic regressions. A P-value of <0.05 was considered statistically significant.
Results
Demographic data and RD incidence
The ages of our enrolled patients were between 21 and 80 (average, 55.2 ± 14.3) years. There were 91 patients aged under 50 years in the early-onset subgroup, and 160 aged over 50 years in the late-onset subgroup. Moreover, there were 179 (65 early- and 114 late-onset) female patients and 72 (26 early- and 46 late-onset) male patients in total. At W1, 131 patients developed RD, resulting in an RD prevalence of 52.2%, including 29.1% (n = 73) of minor RD while 23.1% (n = 58) of moderate-to-severe RD.
Serum 25(OH)D values correlated with age and sex
The serum 25(OH)D level in female patients significantly decreased compared to those in male patients (15.9 ± 6.8 vs. 19.8 ± 6.6 ng/ml, p < 0.001) (Figure 2A). In addition, the 25(OH)D level decreased in early-onset patients relative to late-onset patients (15.3 ± 5.9 vs. 18.0 ± 7.3 ng/ml, p = 0.003) (Figure 2B). Furthermore, early-onset female patients had a lower 25(OH)D level than late-onset female patients (14.0 ± 5.5 vs. 17.1 ± 7.2 ng/ml, p = 0.004) (Figure 2C). Since the 25(OH)D level was not significantly different between early- and late-onset male patients (18.5 ± 5.5 vs. 20.4 ± 7.1ng/ml, p = 0.248) (Figure 2C), the early- and late-onset male patients were integrated into one entity. The serum 25(OH)D level in early-onset female patients decreased compared with late-onset female and male patients (14.0 ± 5.5 vs. 17.1 ± 7.2 vs. 19.7 ± 6.6 ng/ml, p < 0.001) (Table 5).
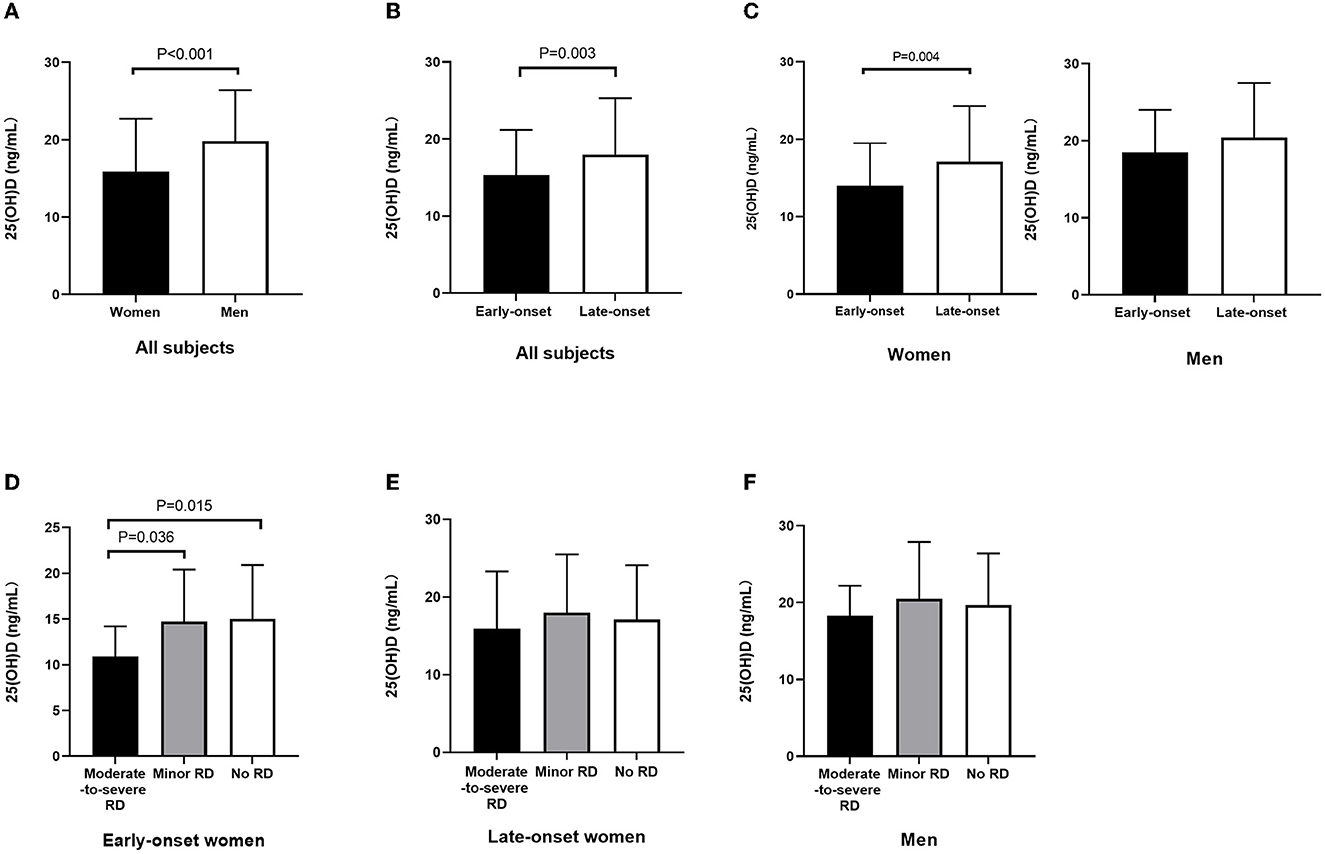
Figure 2. Serum 25(OH)D level among subgroups stratified by sex and onset age. (A) Decreased 25(OH)D level in female patients relative to male patients (p < 0.001). (B) Decreased 25(OH)D level in early-onset relative to late-onset cases (p = 0.003). (C) Decreased 25(OH)D level in early-onset female patients relative to late-onset female patients (p = 0.004); 25(OH)D level was not significantly different between early- and late-onset male patients. (D) Among early-onset female patients, 25(OH)D level in patients with moderate-to-severe RD decreased relative to those in the minor RD group (p = 0.036) and no RD group (p = 0.015). (E) Among late-onset female patients, the 25(OH)D level did not exhibit any significant difference among the three groups stratified by RD severity. (F) Among male patients, the 25(OH)D level did not exhibit any significant difference among the three groups stratified by RD severity. 25(OH)D, 25-hydroxyvitamin D; RD, residual dizziness.
Serum 25(OH)D values correlated with RD severity in the early-onset female subgroup
Among the early-onset female patients, the serum 25(OH)D level in the moderate-to-severe RD group decreased relative to the minor RD group (10.9 ± 3.3 vs. 14.7 ± 5.7 ng/ml, p = 0.036) and no RD group (10.9 ± 3.3 vs. 15.0 ± 5.9 ng/ml, p = 0.015). Furthermore, the serum 25(OH)D level decreased significantly in the moderate-to-severe RD group, and there was a significant difference among the three groups (p = 0.046). (Figure 2D, Table 1). As for late-onset female and male patients, the 25(OH)D level did not exhibit any significant difference among the three groups stratified by RD severity (Figures 2E, F, Tables 2, 3). Logistic regression analysis indicated that the low serum 25(OH)D level was closely related to the occurrence of moderate-to-severe RD in the early-onset female subgroup (OR = 0.801, 95% CI: 0.662–0.969; p = 0.022) (Table 4).
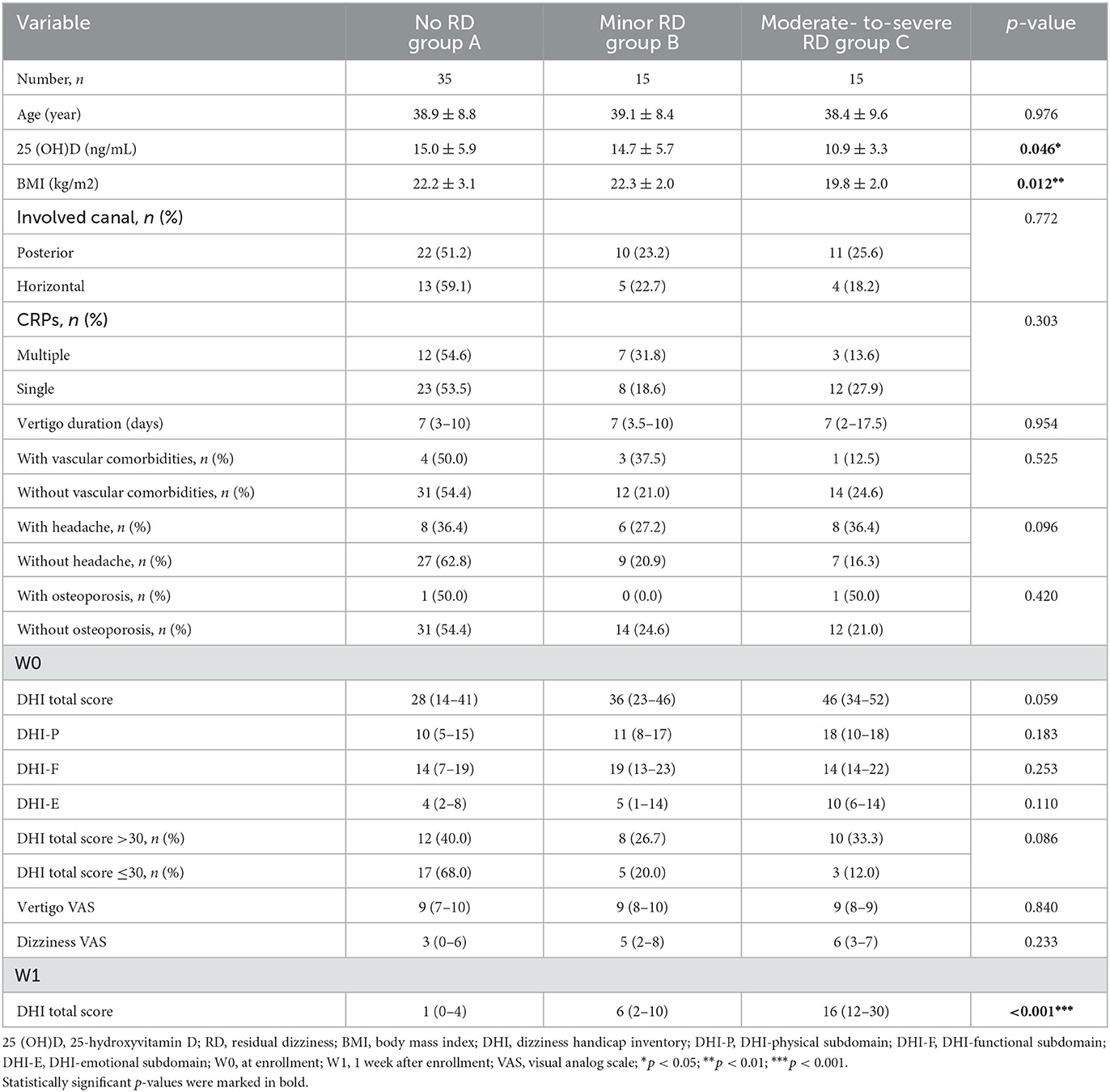
Table 1. Serum 25(OH)D level and clinical characteristics in early-onset female patients stratified by RD severity.
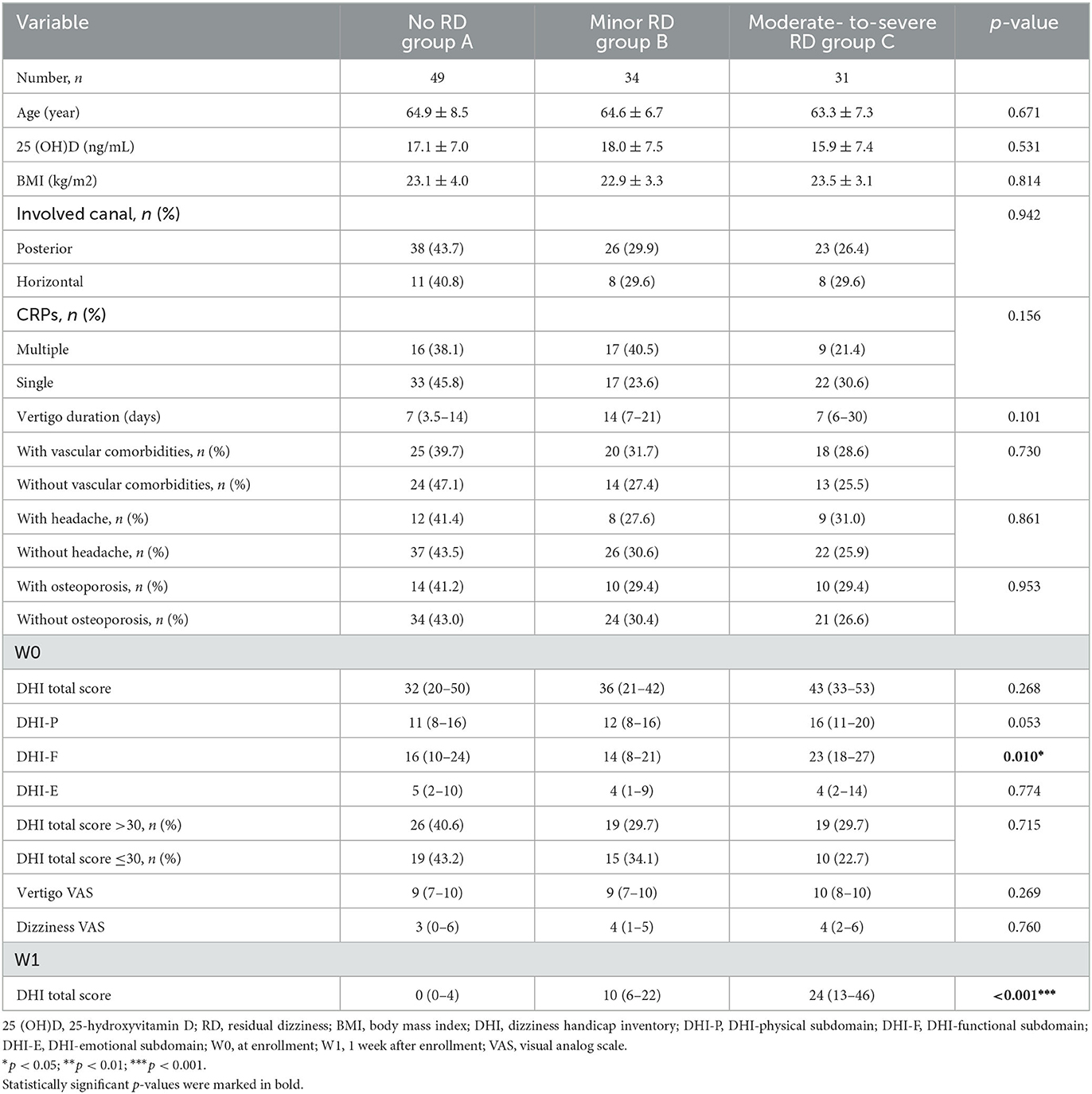
Table 2. Serum 25 (OH)D level and clinical characteristics in late-onset female patients stratified by RD severity.
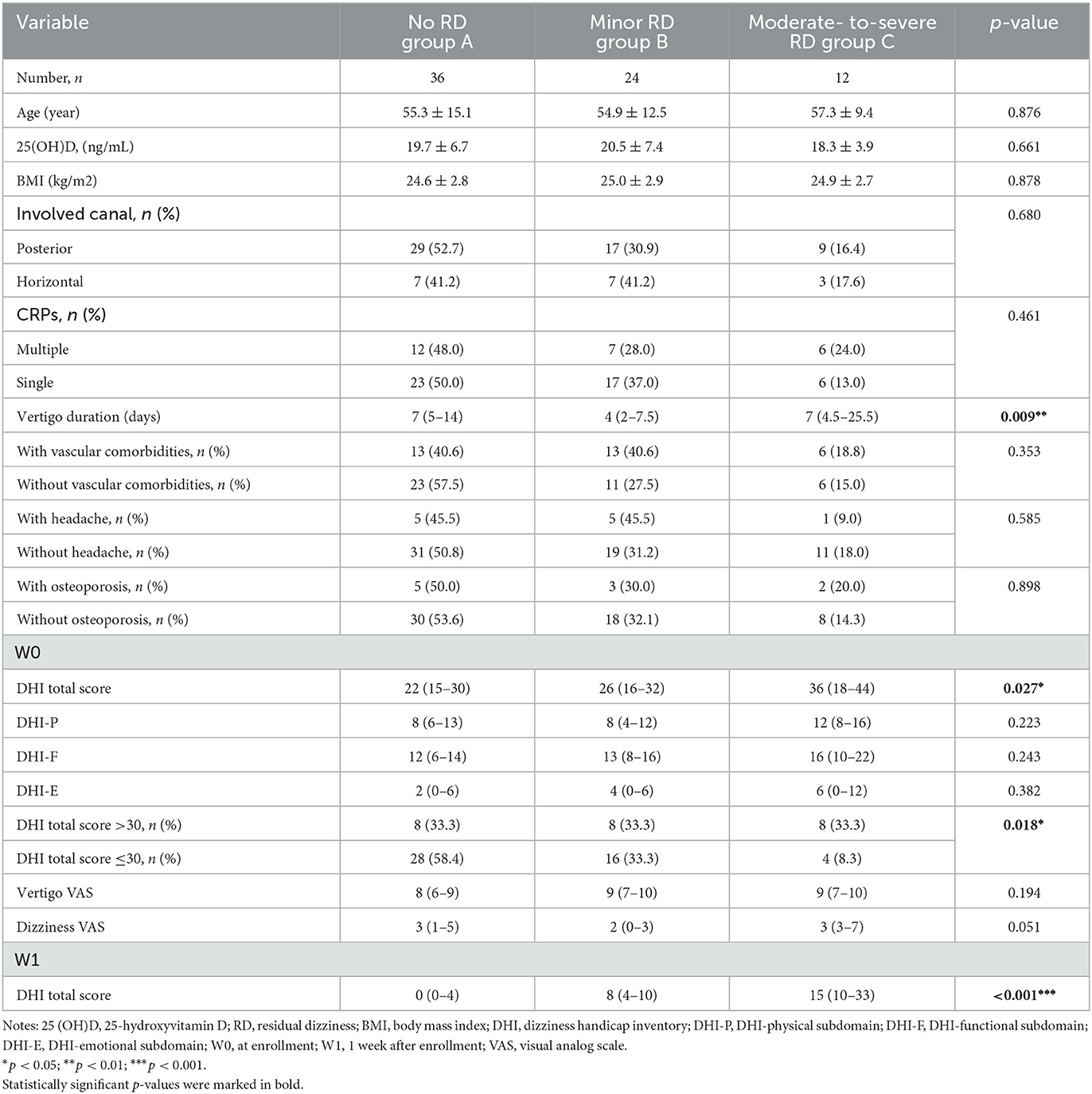
Table 3. Serum 25 (OH)D level and clinical characteristics in male patients stratified by RD severity.
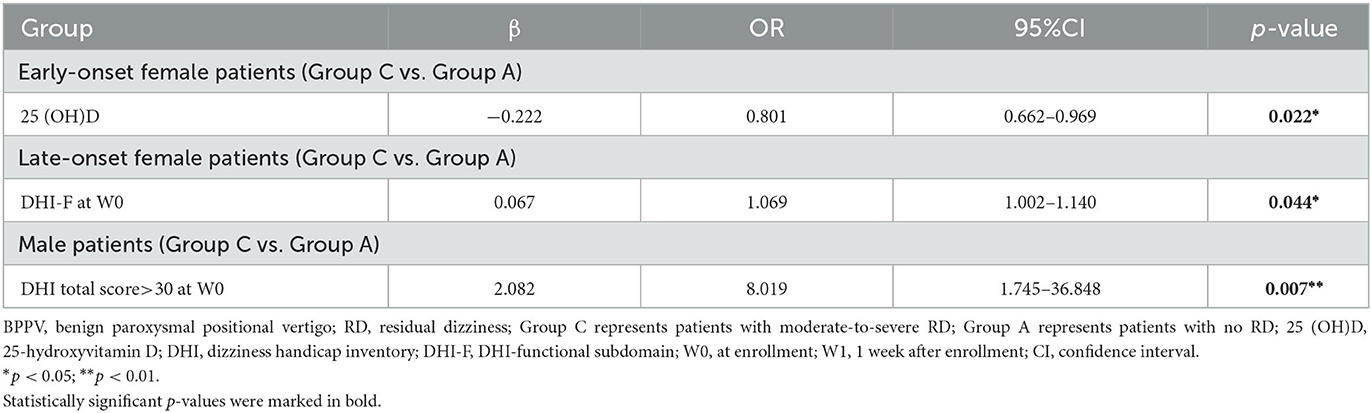
Table 4. Logistic regression analyses for moderate-to-severe RD in different subgroups of patients with BPPV.
Clinical characteristics associated with RD severity in late-onset female and male subgroups
Among the late-onset female patients, DHI-F scores were closely related to the RD severity (p = 0.010) (Table 2). Based on logistic regression analysis, the higher DHI-F scores at W0 increased the risk of moderate-to-severe RD in this subgroup (OR = 1.069, 95% CI: 1.002–1.140; p = 0.044) (Table 4). With regard to the male subgroup, DHI total scores (p = 0.027) and duration before treatment (p = 0.009) were associated with RD severity (Table 3). According to logistic regression analysis, DHI total scores of >30 at W0 were related to the occurrence of moderate-to-severe RD in this subgroup (OR = 8.019, 95% CI: 1.745–36.848; p = 0.007) (Table 4).
Other clinical findings
Compared with the other two subgroups, the late-onset female subgroup exhibited the highest proportion of osteoporosis (p < 0.001), vascular comorbidities (p < 0.001), longest vertigo duration (p = 0.007), highest DHI total scores (p = 0.001) and three subdomain scores (p < 0.01) at W0. By contrast, the early-onset female subgroup had the highest proportion of headaches (p = 0.041) (Table 5).
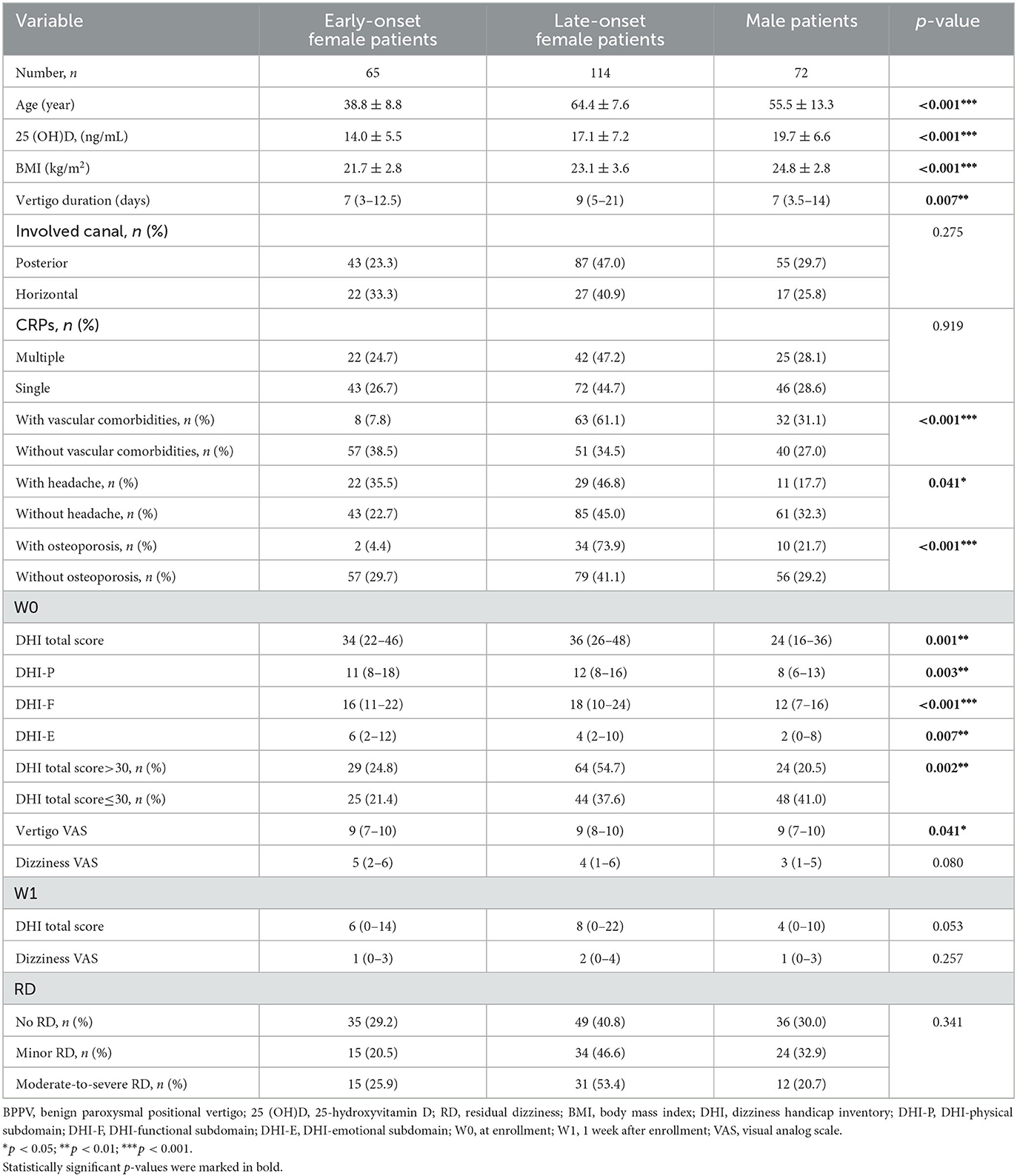
Table 5. Serum 25 (OH)D level and clinical characteristics among different subgroups of patients with BPPV.
Discussion
Residual dizziness is a common complaint by patients with BPPV, even after successful repositioning. This short-term follow-up study was conducted on 251 patients, aiming to analyze the correlation between baseline serum 25(OH)D levels and RD severity 1 week after successful repositioning maneuvers. Our results revealed that there were differences in serum 25(OH)D levels among patients with regard to sex and onset age, and early-onset female patients had the lowest 25(OH)D level relative to late-onset female and male patients. Moreover, in the subgroup of early-onset female patients, the decreased 25(OH)D level increased the risk of moderate-to-severe RD 1 week after successful repositioning maneuvers.
Interestingly, it was found that compared with late-onset patients with BPPV, the 25(OH)D level decreased in early-onset patients, with early-onset female patients having the lowest 25(OH)D level, which was consistent with other studies on vitamin D levels in Asian populations (15, 27, 28). Serum 25(OH)D levels may be affected by several factors, such as age, sex, seasonality, hormonal factors, geographic location, nutrition, lifestyle habits, and preexisting metabolic disorders. As demonstrated in a systematic review (27), in the Asia/Pacific region, 25(OH)D levels in children and adolescents are significantly lower than those in adults and elderly people. Han et al. (28) observed a significant association between 25(OH)D and increasing age, and the older individuals had higher serum 25(OH)D concentrations than the younger ones. Furthermore, Song et al. (15) also found that vitamin D levels varied with sex and age. Serum 25(OH)D level in female patients was significantly lower than those in male patients, and their level gradually increased with age in both BPPV and control groups. Solar radiation remains the most important source of vitamin D in human beings (29, 30), while serum 25(OH)D is the main circulating form of vitamin D, which is commonly used to assess the vitamin D status in a patient (29–31). The main cause of vitamin D deficiency in early-onset female patients in our study remains unknown, which may be attributed to inadequate exposure to sunlight due to the lack of outdoor activity, the use of sunscreen, and increased physiological demands, such as pregnancy or lactation (29, 31).
Previous studies have suggested the following possible mechanism of RD: (i) the persistence of otoconial debris in the semicircular canal, insufficient to provoke cupula deflection, leads to slight vertigo without positional nystagmus; (ii) a utricular dysfunction accompanying BPPV or an undiagnosed coexisting vestibular disorder; (iii) an incomplete or delayed central adaptation after repositioning maneuvers; (iv) sympathoneural dysregulation when the symptoms of RD are similar to those of autonomic dysfunction; and (v) emotional factors, anxiety developed after acute vertigo (4, 6–8). There is little relevant literature on whether 25(OH)D deficiency is associated with RD. Wu et al. (14) recently reported that low 25(OH)D levels are associated with the development of RD in patients with PC-BPPV. In our study, we quantified and stratified the severity of RD by VAS. A previous investigation indicated that VAS exhibits higher sensitivity and specificity in RD assessment (32). For RD, we have detailed severity evaluation and concrete evaluation time. In addition to 25(OH)D, more potential clinical factors associated with RD, such as vascular comorbidities, osteoporosis, and affected canals, were analyzed. The effect of vitamin D on RD was further stratified by sex and onset age in our study. A novel finding was that the serum 25(OH)D level was related to the severity of RD in early-onset female patients, and the low 25(OH)D level increased the risk of moderate-to-severe RD in this subgroup, but this effect was not found in late-onset female and male patients.
One of the main pathophysiological mechanisms of RD may be related to the existence of residual small otoconial debris in the semicircular canal after repositioning maneuvers, which is insufficient to provoke cupula deflection, causing mild dizziness or imbalance (4–7). Physiologically, 1, 25-dihydroxyvitamin D, the biologically active form of 25(OH)D, is beneficial for the upregulation of epithelial Ca2+ channel transporters, such as ECaC1, calbindin-D9k, and calbindin-D28k, in the semicircular canal, which can maintain the low endolymph Ca2+ concentration in the inner ear. Notably, a low endolymph Ca2+ concentration is significant for preventing the production of abnormal otoconia and maintaining the capacity to dissolve exfoliated otoconia. Therefore, a low vitamin D level can prompt the formation of otoconia or disturb the resorption of detached otoconia in endolymph (15, 33, 34). In addition, the physiological function of vitamin D depends on vitamin D receptors (VDR) in the inner ear epithelial cells. Vitamin D/VDR plays a very important role in vestibular function, and VDR-deficient mice show a decreased equilibrium function, suggesting that vitamin D deficiency may lead to vestibular dysfunction (13, 35). Therefore, it is inferred that vitamin D deficiency may cause the residues of small otoconial debris and additional dysfunction of otolith organs, contributing to the development of moderate-to-severe RD.
In late-onset female and male patients whose serum 25(OH)D levels were comparatively higher, vitamin D had an insignificant impact on RD severity. Late-onset female and male patients had higher proportions of vascular comorbidities and osteoporosis. As reported in a multicenter observational study, the presence of comorbidities such as hypertension, diabetes, and osteoporosis might worsen the status of the labyrinth, leading to more common otolith detachment and increasing the risk of BPPV recurrence (36). However, there is no clear conclusion about their correlation with RD, and the correlation between RD severity and comorbidities was not found in this study.
Instead, in this study, the higher DHI-F scores and DHI total scores at the baseline were related to the occurrence of moderate-to-severe RD 1 week later in late-onset female and male subgroups, respectively. Previous studies have revealed that the total DHI scores, especially DHI-F and DHI-E scores at the baseline, are significantly higher in the RD group than in the non-RD group. This indicates that patients with BPPV who have their life and emotional state more greatly affected before treatment are more likely to develop RD (37, 38). Fu et al. (39) found that patients with a DHI score of >30 had a nearly 2-fold higher risk of RD compared to those with a DHI score of ≤30. Previous investigations have found a strong correlation between DHI scores and comorbid anxiety and depression (40). Compared to early-onset female and male patients, late-onset female patients exhibited higher DHI scores at the baseline, indicating that this subgroup tended to have more serious mental health impairments. Cetin et al. (41) observed a correlation between RD and high anxiety levels in elderly patients with BPPV. Moreover, late-onset female patients had the longest vertigo duration. Furthermore, Faralli et al. (8) found that patients with BPPV who had longer durations before treatment tended to have higher DHI scores and a higher proportion of RD. The BPPV-induced peripheral vestibular asymmetry can lead to new central adaptations. The longer duration of otoconia debris in the endolymph will induce a better-established adaptation. Despite successful treatment, the brain cannot rapidly readapt to the pattern after repositioning maneuvers, resulting in more severe RD (8). It was inferred in this study that patients with higher DHI scores potentially had delayed central readaptation, which might contribute to persistent RD. An rs-fMRI study from our team found pontine functional alterations in patients with BPPV, which might reflect the central adaptation of RD (42). Further neuroimaging studies are warranted to explore the neural basis of RD in BPPV patients.
Our study has important clinical implications. Residual dizziness in BPPV may have varying etiology in patients of different sex and onset age, and a personalized treatment strategy is needed. To be specific, for early-onset female patients with low serum 25(OH)D level, the otolith organ disorder might be the most probable cause of RD, thus, the supplementation of vitamin D is necessary, and more outdoor activities and sun exposure are encouraged. For late-onset female patients, delayed central adaptation and comorbid anxiety and depression might be the most likely cause of RD, so timely diagnosis and treatment are particularly important for elderly patients with BPPV, and high attention should be paid to their potential psychological problems. Nonetheless, certain limitations should be noted in this study. First, the sample size of our study was relatively limited. Second, vitamin D data in healthy controls were not available, and several immune-related indicators, such as serum thyroid antibodies and rheumatoid factor, also may influence vitamin D expression. Further studies should be undertaken to correct these confounding factors. Third, the follow-up period was relatively short. Therefore, further follow-up investigations are required to reveal the role of vitamin D deficiency in the long-term RD of BPPV.
Conclusion
There were differences in serum 25(OH)D levels among patients with BPPV with regard to age and sex, and early-onset female patients had lower 25(OH)D levels than late-onset female and male patients. Decreased 25(OH)D level in early-onset female patients may increase the odds of short-term moderate-to-severe RD after successful repositioning maneuvers. In future, further follow-up study is warranted to disclose the effect of vitamin D deficiency on long-term RD of BPPV.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Shanghai Ninth People's Hospital Affiliated to Shanghai Jiao Tong University School of Medicine (ethical approval number: 2018-128-T106). The patients/participants provided their written informed consent to participate in this study.
Author contributions
JW contributed to the provision of study materials, data collection, analysis, and interpretation. C-YJ and Y-XB contributed to the collection and assembly of data, data analysis, and interpretation. QX contributed to the collection and assembly of data. X-HS, HP, and LS contributed to the provision of study materials. J-RL made important contributions to administrative support, data analysis, and interpretation. WC made important contributions to the conception, design, provision of study materials, data analysis, and interpretation. All authors contributed to the writing and approved the final version of the manuscript.
Funding
WC received grants from the Clinical Research Program of Shanghai Ninth People's Hospital Affiliated to Shanghai Jiao Tong University School of Medicine (JYLJ202003) and Project of Biobank from Shanghai Ninth People's Hospital Affiliated to Shanghai Jiao Tong University School of Medicine (YBKB202120). J-RL received grants from 200 talent project from the Shanghai Municipal Education Commission-Gaofeng Clinical Medicine Grant Support (20161422) and the Natural Science Foundation Project from the Shanghai Municipal Science and Technology Commission (22ZR1436900).
Acknowledgments
The authors would like to thank all the subjects for participating in the study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Kim JS. Clinical practice. Benign paroxysmal positional vertigo. N Engl J Med. (2014) 52:1138–47. doi: 10.1056/NEJMcp1309481
2. Balatsouras D, Koukoutsis G, Fassolis A, Moukos A, Aspris A. Benign paroxysmal positional vertigo in the elderly: current insights. Clin Interv Aging. (2018) 13:2251–66. doi: 10.2147/CIA.S144134
3. Kim H-J, Park J, Kim JS. Update on benign paroxysmal positional vertigo. J Neurol. (2020) 268:1995–2000. doi: 10.1007/s00415-020-10314-7
4. Giommetti G, Lapenna R, Panichi R, Dehgani Mobaraki P, Longari F, Ricci G, et al. Residual dizziness after successful repositioning maneuver for idiopathic benign paroxysmal positional vertigo: a review. Audiol Res. (2017) 7:178. doi: 10.4081/audiores.2017.178
5. Wu P, Cao W, Hu Y, Li H. Effects of vestibular rehabilitation, with or without betahistine, on managing residual dizziness after successful repositioning manoeuvres in patients with benign paroxysmal positional vertigo: a protocol for a randomised controlled trial. BMJ Open. (2019) 9:6711. doi: 10.1136/bmjopen-2018-026711
6. Seok JI LH, Yoo JH, Lee DK. Residual dizziness after successful repositioning treatment in patients with benign paroxysmal positional vertigo. J Clin Neurol. (2008) 3:107–10. doi: 10.3988/jcn.2008.4.3.107
7. Teggi R, Quaglieri S, Gatti O, Benazzo M, Bussi M. Residual dizziness after successful repositioning maneuvers for idiopathic benign paroxysmal positional vertigo. Orl. (2013) 75:74–81. doi: 10.1159/000350255
8. Faralli M, Lapenna R, Giommetti G, Pellegrino C, Ricci G. Residual dizziness after the first BPPV episode: role of otolithic function and of a delayed diagnosis. European Archives of Oto-Rhino-Laryngology. (2016) 273:3157–65. doi: 10.1007/s00405-016-3947-z
9. Vaduva C, Esteban-Sanchez J, Sanz-Fernandez R, Martin-Sanz E. Prevalence and management of post-BPPV residual symptoms. Eur Arch Otorhinolaryngol. (2018) 275:1429–37. doi: 10.1007/s00405-018-4980-x
10. Teggi R, Giordano L, Bondi S, Fabiano B, Bussi M. Residual dizziness after successful repositioning maneuvers for idiopathic benign paroxysmal positional vertigo in the elderly. Eur Arch Oto-Rhino-Laryngol. (2010) 268:507–11. doi: 10.1007/s00405-010-1422-9
11. Jiang CY, Wu J, Shu L, Sun XH, Pan H, Xu Q, et al. Clinical and cVEMP evaluation predict short-term residual dizziness after successful repositioning in benign paroxysmal positional vertigo. Front Med. (2022) 9:881307. doi: 10.3389/fmed.2022.881307
12. Talaat HS, Kabel AM, Khaliel LH, Abuhadied G, El-Naga HA, Talaat AS. Reduction of recurrence rate of benign paroxysmal positional vertigo by treatment of severe vitamin D deficiency. Auris Nasus Larynx. (2016) 43:237–41. doi: 10.1016/j.anl.2015.08.009
13. Ding J, Liu L, Kong WK, Chen XB, Liu X. Serum levels of 25-hydroxy vitamin D correlate with idiopathic benign paroxysmal positional vertigo. Biosci Rep. (2019) 39:BSR20190142. doi: 10.1042/BSR20190142
14. Wu Y, Han K, Han W, Fan Z, Zhou M, Lu X, et al. Low 25-Hydroxyvitamin D levels are associated with residual dizziness after successful treatment of benign paroxysmal positional vertigo. Front Neurol. (2022) 13:915239. doi: 10.3389/fneur.2022.915239
15. Song P, Zhao X, Xu Y, Zhao Z, Wang L, Liu Y, et al. Correlation between benign paroxysmal positional vertigo and 25-hydroxyvitamin D. Front Neurol. (2020) 11:576. doi: 10.3389/fneur.2020.00576
16. Bhattacharyya N, Gubbels SP, Schwartz SR, Edlow JA, El-Kashlan H, Fife T, et al. Clinical practice guideline: benign paroxysmal positional vertigo (Update). Otolaryngol Head Neck Surg. (2017) 156(3_suppl):S1-S47. doi: 10.1177/0194599816689667
17. von Brevern M, Bertholon P, Brandt T, Fife T, Imai T, Nuti D, et al. Benign paroxysmal positional vertigo: diagnostic criteria. J Vestib Res. (2015) 25:105–17. doi: 10.3233/VES-150553
18. Association Editorial Board of Chinese Journal of Otorhinolaryngology Head and Neck Surgery; Society of Otorhinolaryngology Head and Neck Surgery Chinese Medical Association. Guideline of diagnosis and treatment of benign paroxysmal positional vertigo. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. (2017) 52:173–7. doi: 10.3760/cma.j.issn.1673-0860.2017.03.003
19. Ling X, Zhao DH, Shen B, Si LH Li KZ, Hong Y, et al. Clinical characteristics of patients with benign paroxysmal positional vertigo diagnosed based on the diagnostic criteria of the barany society. Front Neurol. (2020) 11:602. doi: 10.3389/fneur.2020.00602
20. Zhang YM YZ, Li WX, Shi C, Yu YF. The relationship between the recurrence of benign paroxysmal positional vertigo and the level of bone mineral as well as estrogen in postmenopausal women. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. (2017) 52:881–4. doi: 10.3760/cma.j.issn.1673-0860.2017.12.001
21. Toupet M, Ferrary E, Grayeli AB. Visual analog scale to assess vertigo and dizziness after repositioning maneuvers for benign paroxysmal positional vertigo. J Vestib Res. (2011) 21:235–41. doi: 10.3233/VES-2011-0420
22. Reinpold WM, Nehls J, Eggert A. Nerve management and chronic pain after open inguinal hernia repair: a prospective two phase study. Ann Surg. (2011) 254:163–8. doi: 10.1097/SLA.0b013e31821d4a2d
23. Chen W, Shu L, Wang Q, Pan H, Wu J, Fang J, et al. Validation of 5-item and 2-item questionnaires in Chinese version of Dizziness Handicap Inventory for screening objective benign paroxysmal positional vertigo. Neurol Sci. (2016) 37:1241–6. doi: 10.1007/s10072-016-2573-2
24. Lee NH, Kwon HJ, Ban JH. Analysis of residual symptoms after treatment in benign paroxysmal positional vertigo using questionnaire. Otolaryngol Head Neck Surg. (2009) 141:232–6. doi: 10.1016/j.otohns.2009.04.006
25. Shu L, Wu J, Jiang CY, Sun XH, Pan H, Fang J, et al. Seasonal variation of idiopathic benign paroxysmal positional vertigo correlates with serum 25-hydroxyvitamin D levels: a six-year registry study in Shanghai, China. Sci Rep. (2019) 9:16230. doi: 10.1038/s41598-019-52803-4
26. Jeong SH CS, Kim JY, Koo JW, Kim HJ, Kim JS. Osteopenia and osteoporosis in idiopathic benign positional vertigo. Neurology. (2009) 5:1069–76. doi: 10.1212/01.wnl.0000345016.33983.e0
27. Hilger J, Friedel A, Herr R, Rausch T, Roos F, Wahl DA, et al. A systematic review of vitamin D status in populations worldwide. Br J Nutr. (2014) 111:23–45. doi: 10.1017/S0007114513001840
28. Han B, Wang X, Wang N, Li Q, Chen Y, Zhu C, et al. Investigation of vitamin D status and its correlation with insulin resistance in a Chinese population. Public Health Nutr. (2017) 20:1602–8. doi: 10.1017/S1368980017000490
29. Holick MF. The vitamin D deficiency pandemic: approaches for diagnosis, treatment and prevention. Rev Endocr Metab Disord. (2017) 18:153–65. doi: 10.1007/s11154-017-9424-1
30. Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. (2011) 96:1911–30. doi: 10.1210/jc.2011-0385
32. Fu W, He F, Bai Y, Wang Y, Wei D, Shi Y, et al. Assessment of residual dizziness after successful canalith repositioning maneuvre in benign paroxysmal positional vertigo patients: a questionnaire-based study. Eur Arch Otorhinolaryngol. (2023) 280:137–41. doi: 10.1007/s00405-022-07474-9
33. Yamauchi D, Raveendran NN, Pondugula SR, Kampalli SB, Sanneman JD, Harbidge DG, et al. Vitamin D upregulates expression of ECaC1 mRNA in semicircular canal. Biochem Biophys Res Commun. (2005) 331:1353–7. doi: 10.1016/j.bbrc.2005.04.053
34. Yamauchi D, Nakaya K, Raveendran NN, Harbidge DG, Singh R, Wangemann P, et al. Expression of epithelial calcium transport system in rat cochlea and vestibular labyrinth. BMC Physiol. (2010) 10:1. doi: 10.1186/1472-6793-10-1
35. Jeong SH, Lee SU, Kim JS. Prevention of recurrent benign paroxysmal positional vertigo with vitamin D supplementation: a meta-analysis. J Neurol. (2022) 269:619–26. doi: 10.1007/s00415-020-09952-8
36. De Stefano A, Dispenza F, Suarez H, Perez-Fernandez N, Manrique-Huarte R, Ban JH, et al. A multicenter observational study on the role of comorbidities in the recurrent episodes of benign paroxysmal positional vertigo. Auris Nasus Larynx. (2014) 41:31–6. doi: 10.1016/j.anl.2013.07.007
37. Ke Y, Ma X, Jing Y, Diao T, Yu L. Risk factors for residual dizziness in patients with benign paroxysmal positional vertigo after successful repositioning: a systematic review and meta-analysis. European Archives of Oto-Rhino-Laryngology. (2022) 279:3237–56. doi: 10.1007/s00405-022-07288-9
38. Martellucci S, Pagliuca G, de Vincentiis M, Greco A, De Virgilio A, Nobili Benedetti FM, et al. Features of residual dizziness after canalith repositioning procedures for benign paroxysmal positional vertigo. Otolaryngol Head Neck Surg. (2016) 154:693–701. doi: 10.1177/0194599815627624
39. Fu W, He F, Bai Y, An X, Shi Y, Han J, et al. Risk factors of residual dizziness after successful treatment for benign paroxysmal positional vertigo in middle-aged and older adults. Front Neurol. (2022) 13:850088. doi: 10.3389/fneur.2022.850088
40. Formeister EJ, Krauter R, Kirk L, Zhu TR, Rizk HG, Sharon JD. Understanding the dizziness handicap inventory (DHI): a cross sectional analysis of symptom factors that contribute to DHI variance. Otol Neurotol. (2020) 41:86–93. doi: 10.1097/MAO.0000000000002438
41. Cetin YS, Cagac A, Duzenli U, Bozan N, Elasan S. Residual Dizziness in elderly patients after benign paroxysmal positional vertigo. ORL J Otorhinolaryngol Relat Spec. (2022) 84:122–9. doi: 10.1159/000516961
Keywords: benign paroxysmal positional vertigo, residual dizziness, 25-hydroxyvitamin D, dizziness handicap inventory, peripheral vestibular disorder
Citation: Wu J, Jiang C-Y, Bai Y-X, Xu Q, Sun X-H, Pan H, Shu L, Liu J-R and Chen W (2023) Effect of the serum 25-hydroxyvitamin D level on risk for short-term residual dizziness after successful repositioning in benign paroxysmal positional vertigo stratified by sex and onset age. Front. Neurol. 14:1144958. doi: 10.3389/fneur.2023.1144958
Received: 15 January 2023; Accepted: 08 March 2023;
Published: 31 March 2023.
Edited by:
Weitian Tong, Georgia Southern University, United StatesReviewed by:
Xu Yang, Aerospace Clinical Medical College of Peking University, ChinaFangwei Zhou, Affiliated Hospital of Guizhou Medical University, China
Copyright © 2023 Wu, Jiang, Bai, Xu, Sun, Pan, Shu, Liu and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jian-Ren Liu, bGl1anIwMjFAdmlwLjE2My5jb20=; Wei Chen, ZGF2aWRfY2hlbjgxMDZAaG90bWFpbC5jb20=
†These authors have contributed equally to this work
 Jing Wu
Jing Wu Chun-Yan Jiang
Chun-Yan Jiang Ying-Xia Bai2†
Ying-Xia Bai2† Qian Xu
Qian Xu Liang Shu
Liang Shu Jian-Ren Liu
Jian-Ren Liu Wei Chen
Wei Chen