- 1Department of Neurology, The Second Affiliated Hospital of Chongqing Medical University, Chongqing, China
- 2Department of Neurology, West China Hospital, Sichuan University, Chengdu, Sichuan, China
Objective: This study aimed to investigate the prevalence and clinical characteristics of subjective constipation in Chinese patients with multiple system atrophy (MSA), as well as the timing of constipation onset relative to the occurrence of motor symptoms.
Methods: A total of 200 patients who were consecutively admitted to two large Chinese hospitals from February 2016 to June 2021 and subsequently diagnosed with probable MSA were enrolled in this cross-sectional study. Demographic and constipation-related clinical data were collected, and motor and non-motor symptoms were assessed using various scales and questionnaires. Subjective constipation was defined using ROME III criteria.
Results: The frequency of constipation was 53.5, 59.7, and 39.3% in MSA, MSA with predominately parkinsonism (MSA-P), and MSA with predominately cerebellar ataxia (MSA-C), respectively. MSA-P subtype and high total UMSARS scores were associated with constipation in MSA. Similarly, the high total UMSARS scores were associated with constipation in MSA-P and MSA-C patients. Among the 107 patients with constipation, 59.8% began experiencing it before the onset of motor symptoms, and the interval between constipation and occurrence of motor symptoms was significantly longer in these patients than in those who experienced constipation after onset of motor symptoms.
Conclusion: Constipation is a highly prevalent non-motor symptom in MSA and more often occurs before the onset of motor symptoms. The results of this study may help guide future research into MSA pathogenesis in its earliest stages.
Introduction
Multiple system atrophy (MSA) is a sporadic, rapidly progressive neurodegenerative disorder characterized by autonomic dysfunction, including orthostatic hypotension, bladder dysfunction, gastrointestinal dysfunction, and sexual dysfunction, along with parkinsonism or cerebellar ataxia (1). Constipation has also been observed in MSA patients, and it appears to emerge earlier than in patients with Parkinson’s disease (PD) (2). Constipation is considered an early pre-motor manifestation of PD, occurring in 8–70% of patients (3). The prevalence of constipation and its timing relative to the onset of motor symptoms in MSA are unclear, reflecting the low incidence of MSA.
Here we investigated these questions in Chinese patients with MSA. Our findings may help clarify how MSA arises and progresses in its earliest stages.
Methods
Participants
A total of 200 patients who were consecutively admitted to West China Hospital of Sichuan University and The Second Affiliated Hospital of Chongqing Medical University from February 2016 to June 2021 and subsequently diagnosed with probable MSA (4) were enrolled in this cross-sectional study. Patients with other neurodegenerative disorders, prior gastro-intestinal surgery, or any other identifiable cause of constipation were excluded.
All enrolled patients were interviewed in person, and their socio-demographic and clinical data were collected. The study was approved by the Ethics Committee of West China Hospital of Sichuan University, and all patients provided written informed consent.
Clinical assessments
Motor and non-motor symptoms were evaluated in all patients using the Unified MSA Rating Scale (UMSARS) (5), Non-motor Symptoms Scale (NMSS), Mini-mental State Examination (MMSE) (6), Hamilton Anxiety Rating Scale (HAMA), Hamilton Depression Rating Scale (HAMD), and Pittsburgh Sleep Quality Index (PSQI) (7).
Constipation, one of the most frequent non-motor symptoms of gastrointestinal dysfunction in the autonomic system, can be defined based on subjectively perceived symptoms or on objectively measured intestinal transit time. In the present study, Constipation was defined based on the ROME III criteria as the presence of two or more of the following symptoms (8): straining to defecate, lumpy/hard stool, sensation of incomplete evacuation, sensation of anorectal obstruction, manual maneuvering, and fewer than three bowel movements per week. These symptoms had to be present during at least 25% of defecations during at least 3 months after symptom onset, which had to be at least 6 months before the present study. Patients with constipation were asked to report the time of constipation onset relative to the onset of motor symptoms.
Statistical analysis
Continuous variables were reported as mean and standard deviation, and categorical variables as counts and percentages. Student’s t test was used to compare continuous variables between patients with and without constipation in the MSA group, MSA-P subgroup, and MSA-C subgroup. The chi-squared or Fisher’s exact tests was used to compare the categorical variables between different groups. Multivariate binary logistic regression models were used to determine the factors related to constipation. Variables associated with constipation at p values <0.05 or possibly associated with constipation based on clinical judgment in the univariate analysis were included in the multivariate binary logistic regression model. Results were expressed as odds ratios (ORs) and 95% confidence intervals (95% CIs). All statistical analyses were conducted using SPSS 19 (IBM, Chicago, IL, United States), and differences associated with p < 0.05 were considered significant.
Results
Demographic characteristics of MSA, MSA-P, and MSA-C patients with and without constipation are shown in Table 1. A total of 200 patients (77 female, 38.5%) with probable, idiopathic MSA were enrolled in the study, of whom 107 (53.5%) had constipation. The mean age of all patients was 63.88 ± 10.58 years, and they had MSA for a mean of 2.28 ± 1.78 years. The prevalence of constipation was 59.7% among the 139 patients with the parkinsonian subtype of MSA (MSA-P) and 39.3% among the 61 patients with the cerebellar subtype (MSA-C).
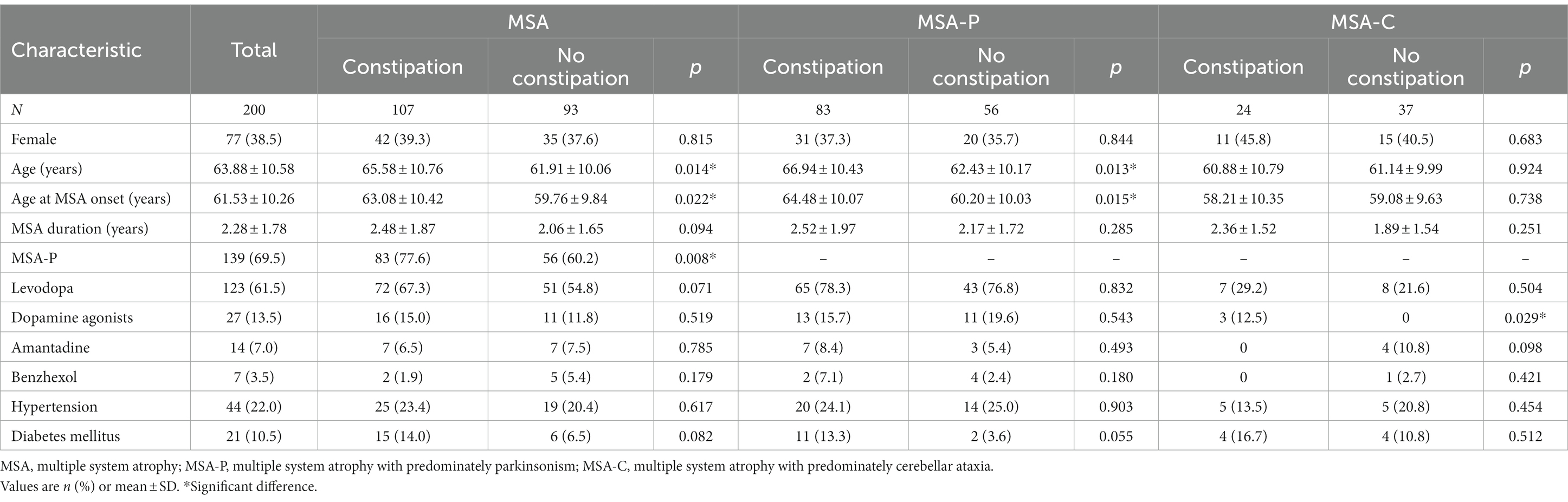
Table 1. Demographic characteristics of MSA, MSA-P, and MSA-C patients with and without constipation.
In the MSA group, patients with or without constipation did not differ significantly in sex distribution, concurrent diseases, medication, disease duration, or scores on the HAMD and MMSE. However, patients with constipation showed higher UMSARS scores total and separately on Parts I, II, and IV, indicating more advanced disease and greater disability. Patients with constipation were also significantly older, showed higher incidence of MSA-P subtype, and scored higher on the NMSS and HAMA (Table 2).
In the MSA-P subgroup, patients with constipation showed higher UMSARS scores total and separately on Parts I, II, and IV than those without constipation. They also scored higher on the NMSS and HAMA. In the MSA-C subgroup, patients with constipation showed higher UMSARS scores total and separately on Parts I, II, and IV. They also reported a higher frequency of dopamine agonists use and scored higher on the NMSS (Table 2).
To further investigate the factors associated with constipation, multivariate binary logistic regression was performed. In the MSA group, the MSA-P subtype (OR 2.077, 95% CI 1.065–4.049, p = 0.032) and total UMSARS score (OR 1.048, 95% CI 1.016–1.080, p = 0.003) were associated with constipation. In the MSA-P subgroup, the total UMSARS score (OR 1.038, 95% CI 1.005–1.073, p = 0.025) was associated with constipation. Similarly, the total UMSARS score (OR 1.063, 95% CI 1.011–1.119, p = 0.018) was associated with constipation in patients with MSA-C (Table 3).
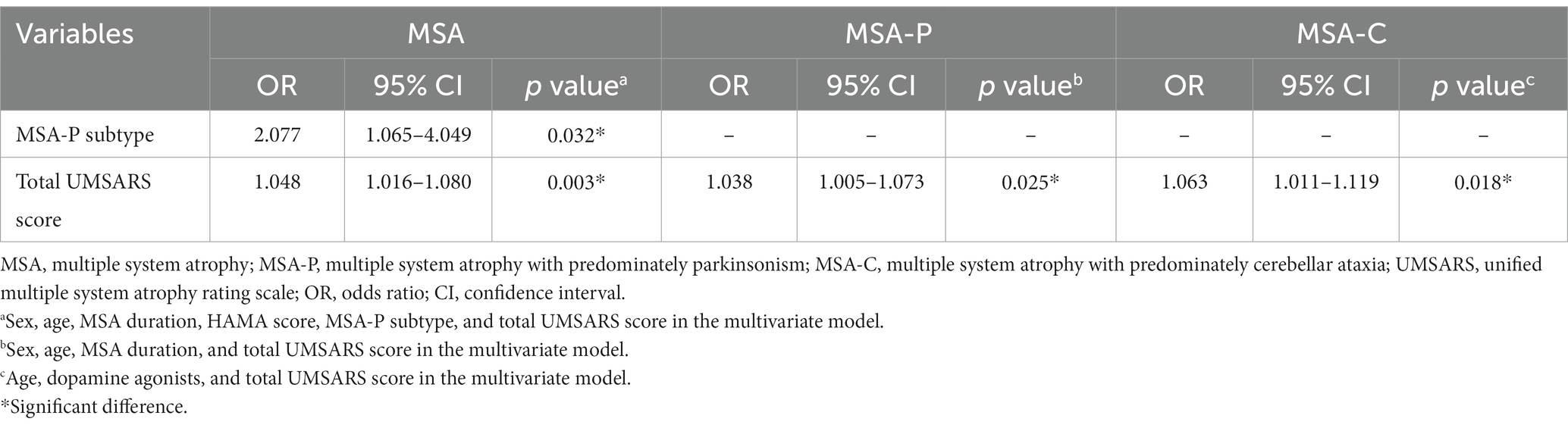
Table 3. Factors associated with constipation in MSA, MSA-P, and MSA-C patients in multivariate regression model.
Among the 107 MSA patients with constipation, 64 (59.8%) reported that it began before the onset of motor symptoms. The groups in whom constipation preceded or followed motor symptom onset did not differ significantly in age, sex distribution, medication, age at motor symptom onset, age at constipation onset, MSA subtype distribution, or scores on the UMSARS (total or separately on Parts I, II or IV), HAMA, HAMD, MMSE, NMSS, or PSQI. However, the interval between constipation onset and motor symptom onset was significantly longer among patients who experienced constipation before motor symptoms than among patients who first experienced motor symptoms (Table 4).
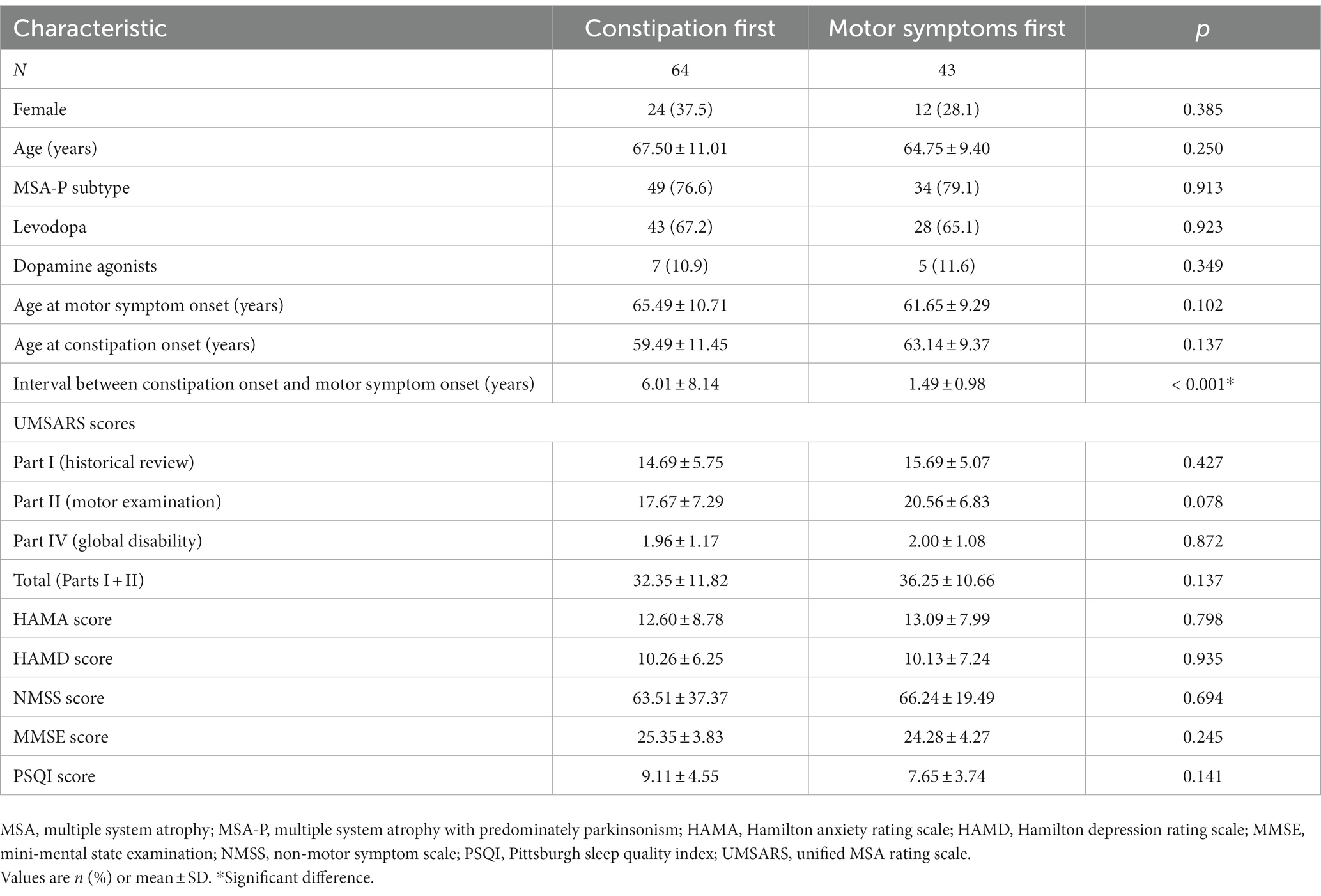
Table 4. Comparison of MSA patients in whom constipation preceded or followed onset of motor symptoms.
Discussion
In recent years, pre-motor symptoms in MSA, such as constipation, have received increasing attention (9, 10). However, the low incidence of MSA has made it difficult to research the prevalence of constipation among patients, or the timing of constipation relative to the onset of motor symptoms. Here we provide evidence in a Chinese cohort that constipation can affect a substantial proportion of MSA patients, and that it more often precedes, rather than follows, the onset of motor symptoms. We further identified MSA-P subtype and total UMSARS score as independent risk factors of constipation in MSA.
A wide range of subjective criteria have been reported for diagnosing constipation in PD (3), but only the ROME criteria are accepted by gastroenterologists worldwide (8), in part because these criteria take into account colonic and anorectal symptoms. Constipation in MSA probably results from slow colonic transit, decreased phasic rectal contraction, and weak abdominal strain (11). Based on the ROME III criteria, we found that 53.5% of our patients suffered subjective constipation, similar to the 54.7% reported for Chinese patients based solely on item 21 of the NMSS (12), but much lower than the 82% reported for South Korean patients based on item 12 of the UMSARS (13). This discrepancy can be attributed to different diagnostic criteria for constipation and to demographic differences among the samples. Consistent with both of those studies (12, 13), constipation in our sample was more frequent among MSA-P patients than MSA-C patients, which may reflect that the MSA-P subtype affects the basal ganglia to a greater extent, or peripheral causes like more α-synuclein deposition in autonomic plexus of gut in MSA-P patients. Future availability of an α-synuclein imaging tracer would allow more direct testing of the interesting hypothesis.
We found that constipation in MSA was associated with older age, reflecting the association between constipation and older age in the general population (14), which has been attributed to degeneration of epithelial, muscle, and neural cells of the colon and pelvic floor. In contrast, we did not detect an association between constipation and sex, in contrast to the association between female sex and chronic constipation in the general population (15). Similarly, we did not detect an association between constipation and use of medications, in contrast to the association between constipation and use of dopamine agonists or other dopaminergic medication in individuals with PD (16, 17). Regrettably, the dose of drugs taken for MSA patients at the time of the visit were not recorded. So, we cannot calculate the levodopa daily equivalent dose. These differences between our study and the literature likely reflect the pathology of MSA.
Our results link constipation in MSA to worse motor performance and non-motor performance, specifically anxiety and sleep quality. Whether constipation is a cause or effect of worse disease progression should be explored in future work. In this regard, it may be interesting that the interval between constipation onset and motor symptom onset was significantly longer among patients who experienced constipation before motor symptoms than among patients who first experienced motor symptoms. It would be important to clarify risk factors specifically for constipation that precedes motor symptoms in MSA, which we could not examine here because of the small sample.
Our study suggests that constipation can precede motor symptoms of MSA by as many as 6 years. One possible explanation for what triggers prodromal constipation in MSA is α-synuclein deposition in the enteric autonomic nervous system (18), especially given that PD seems to involve such deposition in the enteric system at an early stage, after which the deposition spreads to the brain (gut-first subtype) (19, 20). Considering the similar pathological features of MSA and PD, it seems that MSA can also be divided into “brain first” vs. “gut first” like that in PD (21). This study is an important and welcome addition to the clinical subtyping literature in MSA.
Our findings should be interpreted with caution in light of several limitations. There were no age and sex-matched healthy controls to compare the onset of constipation. We did not investigate the effects of diet, water intake, or exercise on the occurrence of constipation, and we relied on patients’ recall to analyze the timing of constipation and motor symptom onset. We relied on patients’ self-report of symptoms in order to diagnose constipation, which may underestimate the true incidence of constipation: among PD patients, diagnosing constipation based on the objective measure of prolonged colorectal transit time leads to higher incidence than diagnosis based on subjective symptoms (3, 22). Future studies should verify and extend our findings with larger samples, preferably using a longitudinal design and combining subjective and objective criteria to diagnose constipation.
Conclusion
Our study suggests that patients with MSA are prone to constipation, which more often precedes motor symptoms. MSA-P subtype and total UMSARS score may be independent risk factors for constipation in MSA. Our results may help guide future research to clarify MSA pathogenesis in its earliest stages.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Committee of West China Hospital of Sichuan University. The patients/participants provided their written informed consent to participate in this study.
Author contributions
YC collected the data, conducted the statistical analysis, interpreted the data, and writing the manuscript. HH and PZ collected the data. YX and YC revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was sponsored by Natural Science Foundation of Chongqing, China (Grant No. cstc2020jcyj-bshX0055).
Acknowledgments
The authors thank the patients and their families for their participation in the project.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Fanciulli, A, and Wenning, GK. Multiple-system atrophy. N Engl J Med. (2015) 372:249–63. doi: 10.1056/NEJMra1311488
2. Stocchi, F, Badiali, D, Vacca, L, D’Alba, L, Bracci, F, Ruggieri, S, et al. Anorectal function in multiple system atrophy and Parkinson’s disease. Mov Disord. (2000) 15:71–6. doi: 10.1002/1531-8257(200001)15:1<71::aid-mds1012>3.0.co;2-w
3. Knudsen, K, Krogh, K, Ostergaard, K, and Borghammer, P. Constipation in parkinson’s disease: subjective symptoms, objective markers, and new perspectives. Mov Disord. (2017b) 32:94–105. doi: 10.1002/mds.26866
4. Gilman, S, Wenning, GK, Low, PA, Brooks, DJ, Mathias, CJ, Trojanowski, JQ, et al. Second consensus statement on the diagnosis of multiple system atrophy. Neurology. (2008) 71:670–6. doi: 10.1212/01.wnl.0000324625.00404.15
5. Wenning, GK, Tison, F, Seppi, K, Sampaio, C, Diem, A, Yekhlef, F, et al. Development and validation of the unified multiple system atrophy rating scale (UMSARS). Mov Disord. (2004) 19:1391–402. doi: 10.1002/mds.20255
6. Folstein, MF, Folstein, SE, and McHugh, PR. Mini-mental state. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. (1975) 12:189–98. doi: 10.1016/0022-3956(75)90026-6
7. Buysse, DJ, Reynolds, CF 3rd, Monk, TH, Berman, SR, and Kupfer, DJ. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. (1989) 28:193–213. doi: 10.1016/0165-1781(89)90047-4
8. Rome, F. Guidelines--Rome III diagnostic criteria for functional gastrointestinal disorders. J Gastrointestin Liver Dis. (2006) 15:307–12.
9. Chelban, V, Catereniuc, D, Aftene, D, Gasnas, A, Vichayanrat, E, Iodice, V, et al. An update on MSA: premotor and non-motor features open a window of opportunities for early diagnosis and intervention. J Neurol. (2020) 267:2754–70. doi: 10.1007/s00415-020-09881-6
10. Jecmenica-Lukic, M, Poewe, W, Tolosa, E, and Wenning, GK. Premotor signs and symptoms of multiple system atrophy. Lancet Neurol. (2012) 11:361–8. doi: 10.1016/S1474-4422(12)70022-4
11. Sakakibara, R, Odaka, T, Uchiyama, T, Liu, R, Asahina, M, Yamaguchi, K, et al. Colonic transit time, sphincter EMG, and rectoanal videomanometry in multiple system atrophy. Mov Disord. (2004) 19:924–9. doi: 10.1002/mds.20165
12. Zhang, L, Cao, B, Ou, R, Wei, QQ, Zhao, B, Yang, J, et al. Non-motor symptoms and the quality of life in multiple system atrophy with different subtypes. Parkinsonism Relat Disord. (2017) 35:63–8. doi: 10.1016/j.parkreldis.2016.12.007
13. Jung, YJ, Kim, HJ, Yoo, D, Choi, JH, Im, JH, Yang, HJ, et al. Various motor and non-motor symptoms in early multiple system atrophy. Neurodegener Dis. (2019) 19:238–43. doi: 10.1159/000507292
14. Vriesman, MH, Koppen, IJN, Camilleri, M, Di Lorenzo, C, and Benninga, MA. Management of functional constipation in children and adults. Nat Rev Gastroenterol Hepatol. (2020) 17:21–39. doi: 10.1038/s41575-019-0222-y
15. Suares, NC, and Ford, AC. Prevalence of, and risk factors for, chronic idiopathic constipation in the community: systematic review and meta-analysis. Am J Gastroenterol. (2011) 106:1582–91; quiz 1581, 1592. doi: 10.1038/ajg.2011.164
16. Borovac, JA. Side effects of a dopamine agonist therapy for Parkinson’s disease: a mini-review of clinical pharmacology. Yale J Biol Med. (2016) 89:37–47.
17. Pagano, G, Tan, EE, Haider, JM, Bautista, A, and Tagliati, M. Constipation is reduced by beta-blockers and increased by dopaminergic medications in Parkinson’s disease. Parkinsonism Relat Disord. (2015) 21:120–5. doi: 10.1016/j.parkreldis.2014.11.015
18. Pouclet, H, Lebouvier, T, Coron, E, Rouaud, T, Flamant, M, Toulgoat, F, et al. Analysis of colonic alpha-synuclein pathology in multiple system atrophy. Parkinsonism Relat Disord. (2012) 18:893–5. doi: 10.1016/j.parkreldis.2012.04.020
19. Braak, H, Del Tredici, K, Rub, U, de Vos, RA, Jansen Steur, EN, and Braak, E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. (2003) 24:197–211. doi: 10.1016/s0197-4580(02)00065-9
20. Horsager, J, Andersen, KB, Knudsen, K, Skjaerbaek, C, Fedorova, TD, Okkels, N, et al. Brain-first versus body-first Parkinson’s disease: a multimodal imaging case-control study. Brain. (2020) 143:3077–88. doi: 10.1093/brain/awaa238
21. Borghammer, P, and Van Den Berge, N. Brain-first versus gut-first Parkinson’s disease: a hypothesis. J Parkinsons Dis. (2019) 9:S281–95. doi: 10.3233/JPD-191721
Keywords: multiple system atrophy, constipation, prevalence, non-motor symptom, ROME III criteria
Citation: Chen Y, Huang H, Zhang P, Xu Y and Chen Y (2023) Constipation in multiple system atrophy: a pilot study in Chinese patients. Front. Neurol. 14:1202279. doi: 10.3389/fneur.2023.1202279
Edited by:
Carlo Colosimo, Azienda Ospedaliera Santa Maria Terni, ItalyReviewed by:
Ryuji Sakakibara, Toho University Medical Center, Sakura Hospital, JapanJacky Ganguly, Institute of Neurosciences, Kolkata (I-NK), India
Copyright © 2023 Chen, Huang, Zhang, Xu and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yanming Xu, bmV1cm94eW05OTlAMTYzLmNvbQ==; Yangmei Chen, Y2hlbnltMTk5N0BjcW11LmVkdS5jbg==
 Yalan Chen
Yalan Chen Hongyan Huang2
Hongyan Huang2 Peng Zhang
Peng Zhang Yanming Xu
Yanming Xu Yangmei Chen
Yangmei Chen