- 1Department of Transplant Surgery, The Third Xiangya Hospital of Central South University, Changsha, China
- 2Engineering and Technology Research Center for Transplantation Medicine of National Health Commission, The Third Xiangya Hospital of Central South University, Changsha, China
- 3Class 2, Grade 2019, 8-Year Clinical Medicine Program, Xiangya School of Medicine, Central South University, Changsha, China
Objective: To analyze the incidence, timing, risk factors and prognosis of delirium after liver transplantation (LT).
Methods: The clinical data of 321 patients undergoing LT in the Third Xiangya Hospital of Central South University from January 2018 to December 2022 were collected to investigate the incidence, onset, and risk factors for post-LT delirium and the impact of delirium on LT recipients’ prognosis by statistical analysis.
Results: The incidence of post-LT delirium was 19.3% (62/321), and the median interval between LT and onset of delirium was 20.1 h. Univariate analysis showed that pre-LT variables (Model for End Stage Liver Disease (MELD) score, hospital stay, hepatic encephalopathy, infection, white blood cell (WBC) count, lymphocyte count, abnormal potassium, lactulose use), intraoperative variables (red blood cell transfusion, remimazolam use, dexmedetomidine use) and post-LT variables (hypernatraemia, acute rejection, reoperation, basiliximab use, tacrolimus concentration) were associated with post-LT delirium. Multivariate logistic regression analysis revealed that MELD score at LT ≥22 [OR = 3.400, 95% CI:1.468–7.876, p = 0.004], pre-LT hepatic encephalopathy [OR = 3.224, 95% CI:1.664–6.244, p = 0.001], infection within 2 months prior to LT [OR = 2.238, 95% CI:1.151–4.351, p = 0.018], acute rejection [OR = 2.974, 95% CI:1.322–6.690, p = 0.008], and reoperation [OR = 11.919, 95% CI:2.938–48.350, p = 0.001] were independent risk factors for post-LT delirium. Post-LT delirium was reduced in LT recipients exposing to intraoperative remimazolam [OR = 0.287, 95% CI: 0.113–0.733, p = 0.009] or ≥ 25 μg of intraoperative dexmedetomidine [OR = 0.441, 95% CI 0.225–0.867, p = 0.018]. As for clinical outcomes, patients with delirium had a higher percentage of staying at the (ICU) ≥7 d after LT than those without delirium [OR = 2.559, 95% CI 1.418–4.617, p = 0.002].
Conclusion: The incidence of delirium was high and the onset of delirium was early after LT. Risk factors for post-LT delirium included high MELD score at LT, pre-LT hepatic encephalopathy and infections, acute rejection and reoperation. Intraoperative use of remimazolam or dexmedetomidine reduced post-LT delirium. Delirium had a negative impact on the length of ICU stay.
Introduction
Delirium is a cognitive disorder that occurs in a short time and involves changes in attention, consciousness, orientation, memory, and perception (1). There are high incidence of postoperative delirium in liver transplant (LT) recipients, ranging from 7.5 to 47% (2–13). Delirium in liver transplant recipients is often associated with metabolic disorders, infection, organ failure, hepatic or uremic encephalopathy, and admission to an intensive care unit (ICU), as well as the neurotoxic side effects from the use of immunosuppressive drugs such as calcineurin inhibitors or high-dose steroids (14–16). Delirium mainly occurs within 1 month after LT, with a median onset time of 2.0–5.5 d postoperatively (2, 4, 6).
Previous studies have shown that various factors can lead to delirium after LT. Preoperative factors include advanced age, high international normalized ratio, hyperbilirubinemia, renal replacement therapy, hepatic encephalopathy, alcohol abuse, alcoholic liver cirrhosis, high Model for End Stage Liver Disease (MELD) score, Child-Turcotte-Pugh classification, acute physiology and chronic health evaluation (APACHE) II score, depression, recent use of antidepressants, and hospitalization. Intraoperative factors include red blood cell (RBC) transfusion, significant blood loss, use of fentanyl, and post-reperfusion syndrome. Postoperative factors include hyperammonemia, high APACHE II score, use of vasopressor drugs or midazolam, mechanical ventilation, reintubation, and prolonged ICU stay and hospitalization (2–6, 8, 9, 11, 13, 17–19).
Some researches have also shown that there are adverse effects on the prognosis for delirium after LT including prolonged postoperative ICU stay, total hospital stay, and mechanical ventilation, increased frequency of blood purification therapy, and mortality, and even long-term cognitive impairment (2, 5, 6, 8, 9, 12, 13). Our present study found that the risk factors for delirium after LT were associated with pre-, intra- and, post-operative variables and delirium was associated with longer ICU stay. Identifying risk factors for delirium after LT could help in early prevention and improve patient outcomes.
Methods and materials
General information
A retrospective cohort study was conducted to collect demographic, clinical, and laboratory data from 321 LT recipients of grafts from donation after citizens’ death from January 2018 to December 2022 in The Third Xiangya Hospital of Central South University. All liver grafts, but one which was from circulatory death, were from brain death. There were 264 males and 57 females, with a mean age of 47.1 ± 10.0 years. There were 246 cases with hepatitis virus-related cirrhosis/necrosis/tumor, 20 with alcoholic liver disease, 18 with mixed cirrhosis, 11 with autoimmune hepatitis, 8 with primary biliary cirrhosis, 5 with Budd-Chiari syndrome, 4 with cryptogenic cirrhosis, 3 each with hepatolenticular degeneration and transplanted liver failure, 2 with drug-induced liver injury, and 1 with polycystic liver. Our study was approved by the ethics committee of The Third Xiangya Hospital of Central South University.
Inclusion and exclusion criteria
We included the adult recipients who underwent LT in our center during the study period, and we excluded the recipients who were under 18 years old, developed infections within 2 weeks before LT, were unconscious and could not be evaluated for delirium, or died during the perioperative period for anesthesia accidents or surgical complications. A total of 321 patients of LT met the inclusion criteria, and 7 patients were excluded from our study, including 1 patient who died of massive bleeding during the operation, 4 patients who were persistent coma after LT, and 2 patients younger than 18 years old.
Definitions and assessment of delirium
Infection was evaluated according to the criteria of the Centers for Disease Control and Prevention/National Healthcare Safety Network (CDC/NHSN) (20). The cutoff value for the normal serum sodium level in our hospital was defined as 137 and 147 mmol/L. The normal range for serum potassium level in our hospital was 3.5–5.3 mmol/L. Reoperation referred to retransplantation or post-LT laparotomy. Acute rejection is referred to as T cell-mediated rejection or antibody mediated rejection confirmed by pathology. On-duty nurses examined patients for delirium through the ICU Confusion Assessment Method (CAM-ICU) every 8 h after LT and continued for 7 d, or until either discharge from the hospital or death (19, 21). Definitions of post-LT delirium met the diagnostic criteria of delirium in the Diagnostic and Statistical Manual for Mental Disorders, fifth edition (DSM-5) (22). Delirium was defined as an acute episode of neuropsychiatric condition with a fluctuating process of confusion or an altered mental status. Delirium has four characteristics in the CAM-ICU: (1) an acute change in mental status; (2) fluctuations in attention; (3) disordered thinking; and (4) fluctuations in consciousness.
Treatment strategy
All patients were performed modified piggyback LT under general anesthesia with endotracheal intubation. The initial induction of general anesthesia were propofol (1–1.5 mg/kg), cisatracurium (0.15–0.2 mg/kg), and/or sufentanil (0.07–0.08 μg/kg) and the maintenance were sevoflurane (1%), propofol (2–3 mg/kg/h), remifentanil (5–8 μg/kg/h), and/or cisatracurium (0.05–0.1 mg/kg/h). Dexmedetomidine was given at a dose of 0.5–1 μg/kg when the liver graft was implanted. The bispectral index was maintained at 45–60, and the monitoring count of four successive muscle relaxant stimulations was <3.
Routine cholecystectomy was performed for liver grafts, and a T-tube was placed for biliary drainage in 4 (1.2%) patients. Third-generation cephalosporins or carbapenem antibiotics were used for prophylaxis against Gram-negative bacteria infection during surgery and postoperatively. Prophylaxis against Gram-positive bacteria and fungal infections was given as needed, with a treatment duration of 5–7 d. Immune induction therapy was given to 208 (64.8%) patients with basiliximab, and tacrolimus or ciclosporin A, plus glucocorticoids, was used as the initial immunosuppressive maintenance treatment. The tacrolimus trough concentration was maintained at 8–10 ng/mL within 1 month after LT, then 6–8 ng/mL for months 2–6, and 5–6 ng/mL after 6 months. Methylprednisolone was used as an initial glucocorticoid treatment, and oral prednisone tablets were given on the seventh day. Mycophenolate mofetil or enteric-coated mycophenolate sodium and anti-thymocyte globulin were used if needed. All patients were closely monitored for 3–7 d in the ICU after LT.
The content and acquisition methods of the data
All of the patients were followed up for 6 months after LT. The data were obtained through electronic medical records, outpatient or telephone follow-up, etc. We included general preoperative, intraoperative, and postoperative conditions of the patients, all demographic, laboratory, and clinical data related to delirium, and postoperative outcomes including survival, ICU length of stay, and length of hospitalization after LT.
Statistics
We analyzed the data with SPSS 26.0 statistical software package (IBM SPSS Statistics, IBM Corporation, Armonk, NY, USA). Continuous variables were expressed as mean ± standard deviation or median (interquartile range), and categorical data were shown as percentages. Univariate analysis was performed using the Mann–Whitney U test, the t test, Pearson’s chi-square test or Fisher’s exact test, as appropriate. No missing data were required to handle. There is no collinearity between these 16 variables associated with post-LT delirium with all variance inflation factor < 2. Variables with statistically significant differences in univariate analysis were introduced into the final multivariate model, and performed using binary logistic regression based on forward stepwise logistic regression. The association was expressed as an odds ratio (OR) value and 95% confidence interval (CI) to describe the independent factors related to delirium. A two-tailed p value of <0.05 was considered statistically significant.
Results
General characteristics and prognosis of LT recipients
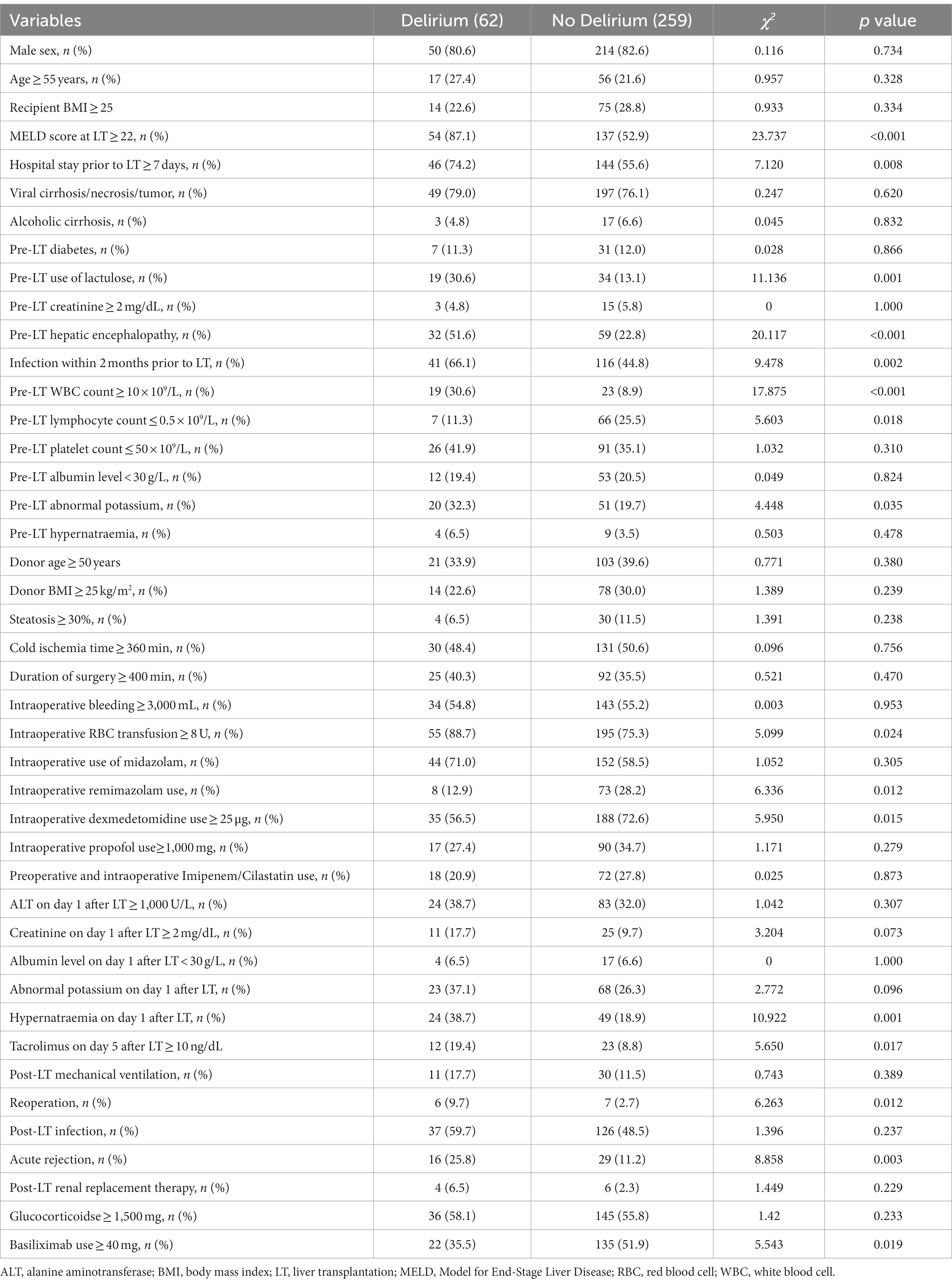
A total of 321 patients were eventually included in this retrospective study. There were 264 (82.2%) males with a mean age of 47.1 ± 10.0 years. A total of 62 (19.3%) patients developed delirium with a median onset time of 20.1 h after LT. Of these patients, 36 (58.1%) developed delirium within 24 h after LT. The median MELD score at LT was 24. A total of 157 (48.9%) patients had infections within 2 months before LT including 128 (39.9%) patients with pulmonary infections and 19 (5.9%) patients with multiple sites infections, and all these 19 patients had pulmonary infections. The primary liver diseases were mainly hepatitis viral-related cirrhosis/necrosis/tumor (246, 76.7%), and alcoholic cirrhosis (20, 6.2%). There were 91 (28.3%) patients with hepatic encephalopathy before LT. It was 0.8 mg/dL, 3.9 mmol/L, 138.2 mmol/L, 34.2 g/L, 5.3 × 109/L, 0.8 × 109/L, and 67.0 × 109/L for the median pre-LT creatinine, potassium, sodium, albumin, WBC count, lymphocyte count, and platelet count, respectively.
Eighty-one (25.2%) and 238 (74.1) patients received remimazolam with the maximal dosage 114 mg and dexmedetomidine with the maximal dosage 200 μg during surgery, respectively. It was 0 (0–5.0) mg, 2 (0–2.0) mg, 30.0 (0–50.0) μg and 850.0 (550.0–1085.0) mg for the median (interquartile range) dosage of remimazolam, midazolam, dexmedetomidine and propofol use, respectively. And it was 375.0 min for the median duration of LT, while the median blood loss was 3000.0 mL and a median RBC transfusion was 12.0 units.
Before opening the portal vein of the liver graft, all patients received 500 mg methylprednisolone and 208 (64.8%) patients received basiliximab for immune induction therapy. After LT, 17 patients (5.3%) were treated with anti-thymocyte immunoglobulin therapy, 318 (99.1%) with tacrolimus. The median ALT, creatinine, albumin, potassium, and sodium on day 1 after LT were 697.0 U/L, 1.1 mg/dL, 37.2 g/L, 3.7 mmol/L, and 145.0 mmol/L, respectively. After LT, 41 patients required mechanical ventilation, 45 developed acute rejection and 13 underwent reoperation. The median length of ICU stay was 6.0 d after LT. A total of 17 patients died within 1 month, 20 within 2 months, and 23 within 6 months after LT (Table 1).
Analysis of the risk factors for delirium after LT
The results of the univariate analysis showed that some factors were associated with post-LT delirium, including MELD score at LT ≥22 (p < 0.001), pre-LT hospitalization ≥7 d (p = 0.008), pre-LT use of lactulose (p = 0.001), pre-LT hepatic encephalopathy (p < 0.001), pre-LT infection cases within 2 months (p = 0.002), pre-LT WBC count ≥10 × 109/L (p < 0.001), pre-LT lymphocyte count ≤0.5 × 109/L (p = 0.018), pre-LT abnormal potassium (p = 0.035), intraoperative RBC transfusion ≥8 U (p = 0.024), intraoperative remimazolam use (p = 0.012), intraoperative dexmedetomidine use ≥25 μg (p = 0.015), hypernatraemia on day 1 after LT (p = 0.001), tacrolimus on day 5 after LT ≥ 10 ng/dL (p = 0.017), reoperation (p = 0.012), acute rejection (p = 0.003), and basiliximab use≥40 mg (p = 0.019) (Table 2).
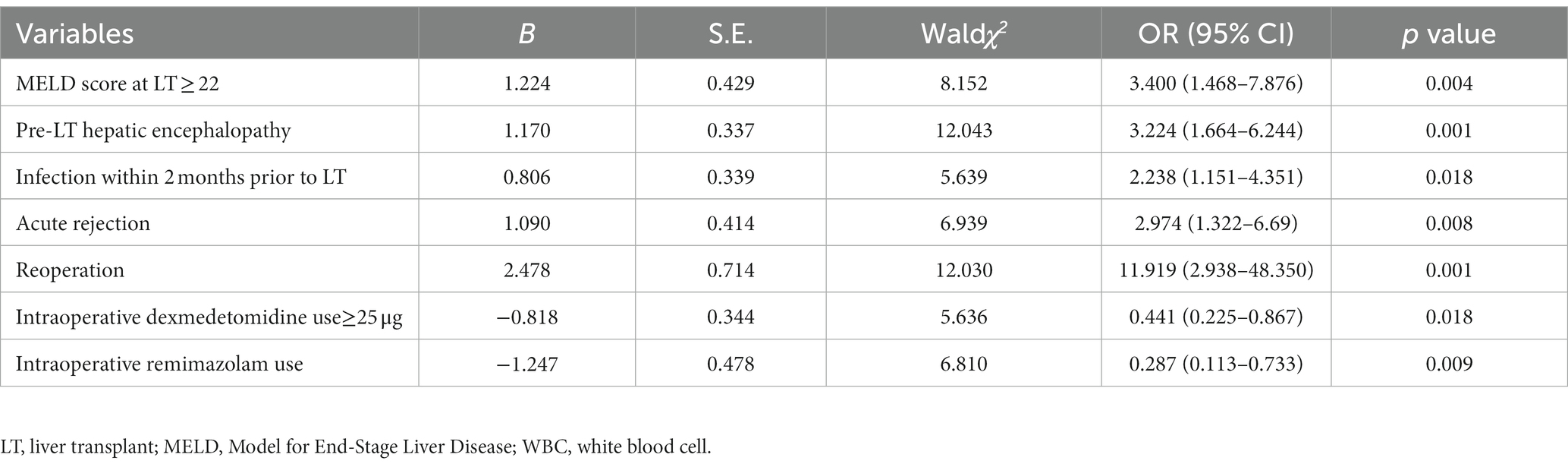
We further performed multivariate analysis and the results showed that MELD score at LT ≥22 [OR = 3.400, 95% CI:1.468–7.876, p = 0.004], pre-LT hepatic encephalopathy [OR = 3.224, 95% CI:1.664–6.244, p = 0.001], infection within 2 months prior to LT [OR = 2.238, 95% CI:1.151–4.351, p = 0.018], acute rejection [OR = 2.974, 95% CI:1.322–6.690, p = 0.008], and reoperation [OR = 11.919, 95% CI:2.938–48.350, p = 0.001] were independent risk factors for post-LT delirium. Post-LT delirium was reduced in LT recipients exposing to intraoperative remimazolam [OR = 0.287, 95% CI: 0.113–0.733, p = 0.009] or ≥ 25 μg of intraoperative dexmedetomidine [OR = 0.441, 95% CI 0.225–0.867, p = 0.018] (Table 3). The goodness of fit test showed a good result with a p value greater than 0.05 using Hosmer-Lemeshow test.
Prognosis of patients with delirium after LT
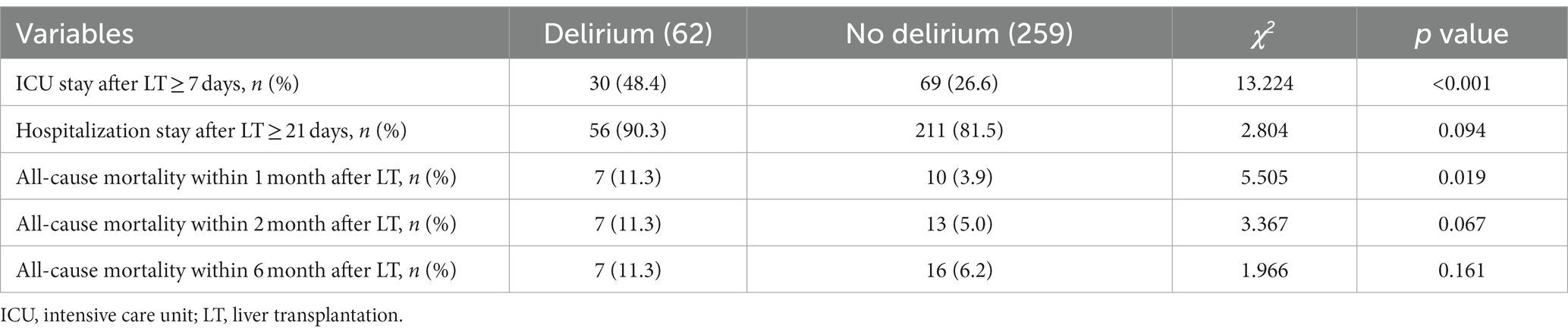
Our result revealed that more patients with delirium stayed at ICU ≥7 d after LT than those without delirium (p < 0.001). Delirium seems to increase mortality within 1 month (p = 0.019) after LT, but have no effects on mortality within 2 months (p = 0.067) and 6 months (p = 0.161) after LT compared to non-delirium (Table 4).
In order to decide if delirium was an independent risk factor for early mortality, we performed an analysis of those factors including delirium that might be associated with mortality and verified that recipient age ≥ 55 years [OR = 3.422, 95% CI:1.187–9.980, p = 0.023], post-LT infection [OR = 13.546, 95% CI:1.744–105.206, p = 0.013] and post-LT renal replacement therapy [OR = 9.477, 95% CI:2.035–44.131, p = 0.004], not delirium, were independently associated with mortality within 1 month after LT (Table 5). There is no collinearity between these 6 variables associated with all-cause mortality within 1 month after LT with all variance inflation factor < 2.

Table 5. Univariate and multivariate logistic regression analysis of risk factors for all-cause mortality within 1 month after LT.
However, when we performed an analysis of the factors related to prolonged ICU stay and found that delirium was independently associated with ICU stay after LT ≥ 7 days, among other variables such as creatinine on day 1 after LT ≥ 1.5, post-LT infection, and post-LT mechanical ventilation (Table 6).
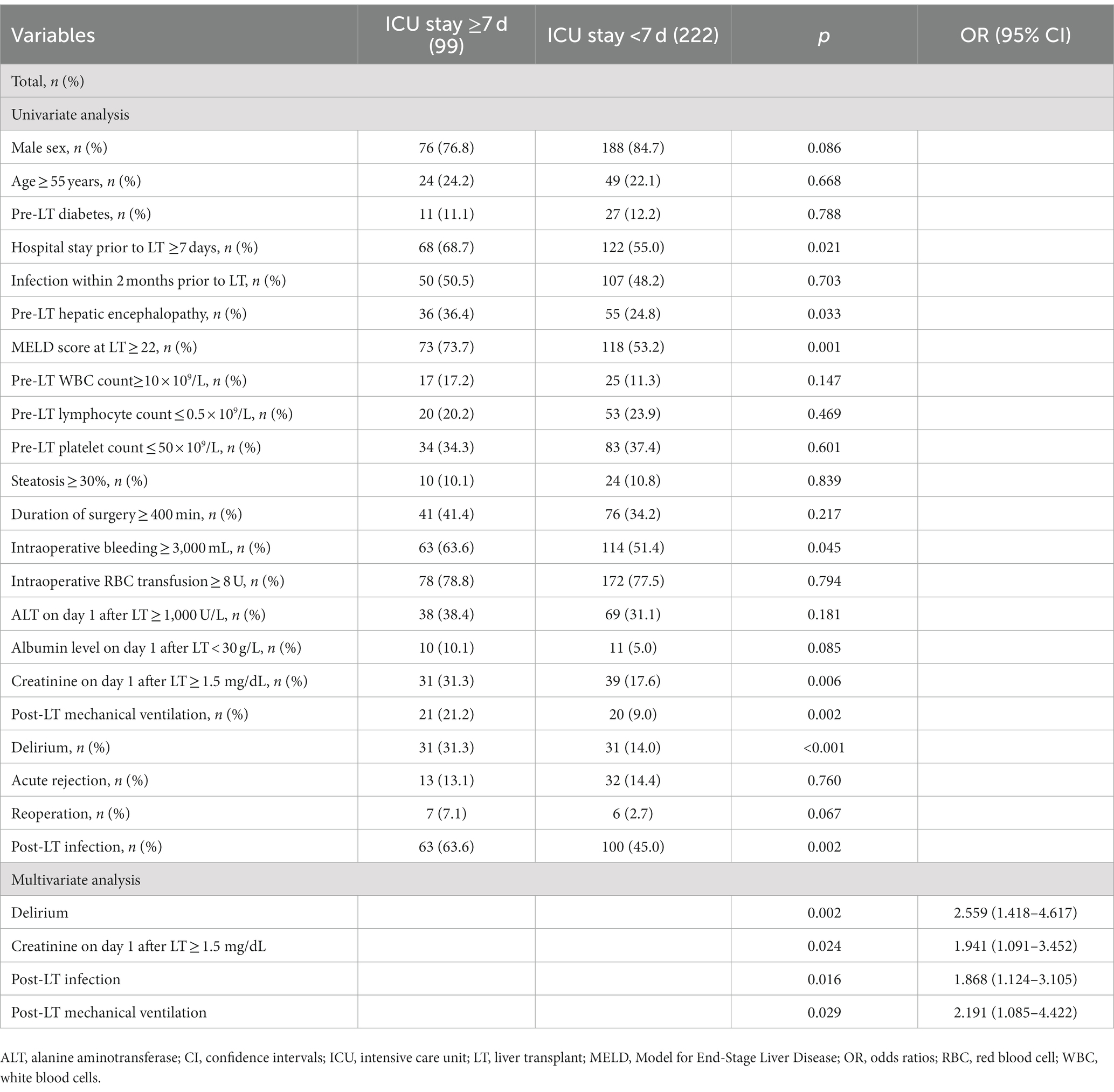
Table 6. Univariate and multivariate logistic regression analysis of risk factors for ICU stay after LT ≥ 7 days.
Discussion
The incidence of delirium after LT is high, but there are great differences among different studies, which is related to the variations in the diagnosis methods of delirium and the proportions of patients with alcoholic liver disease and hepatic encephalopathy (4). In our study, the incidence of delirium was 19.3 and 58.1% of them occurred within 24 h after LT, which was close to the incidence (17%) and the time of onset (88% on the day or the first day after surgery) reported by Lee et al. (4) and was extremely closely to the post-LT incidence (19.4%) reported by Lu et al. (19).
MELD score, which represents the severity of liver disease, affects the priority allocation of the liver and predicts the mortality of patients preparing for LT (23). The results of our study showed that a high MELD score at LT led to an increased incidence of delirium after LT, which was consistent with other studies (4, 8, 11, 13).
Our study also found that pre-LT infection within 2 months, not post-LT infection, was associated with the onset of delirium. Wang SH et al. and Bhattacharya et al. showed that infection after LT increased the occurrence of post-LT delirium (2, 13). However, whether infection is a cause or a consequence of delirium remains elusive, though infection is well-proven risk factors for delirium, especially pneumonia and urinary tract infection (13).
In our study, the results showed that pre-LT hepatic encephalopathy was associated with the occurrence of post-LT operative delirium, which was consistent with the results of several studies (3, 9, 11, 12). Patients with liver disease who underwent surgery and were admitted to the ICU were prone to delirium. The reason might be that liver diseases can affect the brain, in which activation of microglia cells plays a key role in leading to nervous system inflammation. In addition, the occurrence of delirium is also related to changes in astrocyte morphology, brain metabolism, brain perfusion, and blood–brain barrier permeability which were caused by hepatic encephalopathy (14, 24–26).
Some studies showed that it was prone to causing delirium for benzodiazepines such as midazolam, lorazepam, and propofol, while there was no correlation between midazolam, propofol and delirium in our study (8). Our study did not find the relationship between midazolam and delirium. The reason was that the amount (median dosage 2 mg) of midazolam used in all LT recipients was minimal, which could be insufficient to cause delirium. We found that dexmedetomidine reduced the occurrence of delirium. Dexmedetomidine, compared with benzodiazepines, was associated with less onset and shorter duration of delirium (27). Some research showed that dexmedetomidine might play a neuroprotective role by reducing the release of inflammatory mediators and neuroendocrine hormones, and better maintaining intracranial homeostasis (28).
Interestingly, we also found intraoperative use of remimazolam, one kind of benzodiazepines, reduced the occurrence of delirium. The finding was in line with a study reporting that in children undergoing tonsillectomy and adenoidectomy, the use of remimazolam led to a significantly lower delirium, compared with 0.9% saline (29). Another stduy revealed that remimazolam reduced the incidence of delirium after transcatheter aortic valve implantation under general anesthesia (30). Deng et al. claimed that both remimazolam and dexmedetomidine were equally effective at acutely mitigating postoperative delirium in older patients after orthopedic surgery with remimazolam having a longer time to delirium resolution than dexmedetomidine (31). The effect of remimazolam on delirium remains to be well elucidated but is of obvious interest. Specifically, we look forward to future studies addressing its effect on post-LT delirium.
Our findings also revealed that acute rejection and reoperation were two risk factors for delirium. Acute rejection means more immunosuppressant use and more infections occur, which can make delirium more likely to occur. Reoperation means more blood transfusions and more sedatives, which can also lead to delirium. At present, there are no reports of increased delirium caused by acute rejection and reoperation after LT. Whether they cause delirium needs to be confirmed by more studies, and the mechanism needs to be further explored in the future.
Lescot et al. and Zhou et al. reported that the amount of RBC infusion correlated with delirium after LT (6, 18). In our study, univariate analysis found that patients with intraoperative RBC infusion ≥8 U were more prone to develop postoperative delirium, but this statistical difference was not maintained in subsequent multivariate analysis. These results indicated that RBC infusion had less effect on delirium than other factors such as hepatic encephalopathy and high MELD score.
Electrolyte imbalance (sodium or potassium) was one of the most common risk factors for postoperative delirium (7). Hackworth et al. also claimed that pre-LT abnormal serum sodium was associated with an increased risk of post-LT delirium (32). Our present study found hypernatraemia on day 1 after LT was associated with post-LT delirium in univariate analysis but did remain as a significant risk factor for delirium in multivariate analysis.
In a previous publication, donor factors, such as graft macrovesicular steatosis, emerged as possibly associated with the likelihood of post-LT delirium (33). We inluded recipient age and body mass index, and steatosis of liver graft into the analysis and did not find their relationship with delirium.
The relationship between delirium and mortality is controversial. Several studies showed that delirium did not affect in-hospital or 1 year mortality with proper treatment (4, 34, 35). Nevertheless, Lescot et al. and Wang et al. reported that delirium after LT was related to in-hospital and 1 year mortality (2, 6). Beckmann et al. also found that patients with post-LT delirium had a shorter mean survival time than those without (12). In Oliver’s study, LT recipients with delirium were more likely to have higher 6 month mortality than patients who did not develop delirium (9). In univariate analysis, we found that patients with delirium had an increased mortality rate within 1 month after LT but had no effects on mortality within 2 and 6 months after LT compared with patients without delirium. However, when performed an analysis of these factors including delirium that might be associated with mortality, we verified that advanced recipient age, post-LT infection and renal replacement therapy, not delirium, were independently associated with mortality within 1 month after LT. There is no collinearity between those 6 variables associated with all-cause mortality within 1 month after LT with all variance inflation factor < 2. So delirium was not an independent risk factor for all-cause mortality within 1 month after LT.
Our study also investigated the effect of delirium on ICU and hospital stay after LT and found that delirium, among other variables such as creatinine on day 1 after LT ≥ 1.5, post-LT infection, and post-LT mechanical ventilation, was associated a longer time of postoperative ICU stay, consistent with previous reports (4, 6, 9, 13, 34, 36). We did not demonstrate a significant difference in postoperative length of hospital stay between patients with and without delirium, in line with the study from Chen et al. (36).
The pathophysiological mechanisms of delirium are far from fully understood (37). Considering the negative effect of delirium on outcomes and no single intervention or medication for treatment, early identification of correctable risk factors for delirium after LT could help treat it with multiple strategies.
Enhanced Recovery After Surgery (ERAS) is a multimodal and perioperative management pathway to minimize the severity of the surgical stress and to decrease postoperative complications (38). The use of ERAS program, which in part aim to minimize opioid exposure, have been related to reduced postoperative delirium among cardiac surgery ICU patients (39). ERAS has not yet been widely accepted in LT recipients, although studies verified that ERAS is safe and effective in accelerating discharge, shortening the duration of LT operation and the anhepatic period of the surgery, reducing intraoperative blood loss and blood transfusion rate, and shortening the ICU and hospital stays without increasing the incidence of complications, readmission and mortality, even reducing the incidence of total complications or severity of complications (40–45). Recommendations from the 2022 International Liver Transplantation Society consensus conference addressed the effect of short term outcomes on survival of both LT recipients and liver grafts, a clear trend toward lower survival in the presence of short-term complications which can be reduced potentially through elements of ERAS (46). Until now, there has been no studies on the effect of ERAS on the occurrence of post-LT delirium. Therefore, prospective trials are required to confirm the item in future.
In our study, we identified 5 risk factors and 2 protective factors for delirium after LT. These factors were classified into two categories. One was related to the severity and complexity of the disease including high MELD score at LT, pre-LT hepatic encephalopathy, infection within 2 months prior to LT, acute rejection and reoperation, and the other was related to the use of sedative drugs, including the intraoperative use of remimazolam and dexmedetomidine. Further research is still required to confirm the extent of the impact of these factors on delirium and to clarify their specific mechanisms.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the Ethics Committee of The Third Xiangya Hospital of Central South University (NO. 23607). The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because it is a retrospective cohort study.
Author contributions
QW conceived and designed the study. YM and CL analyzed and interpreted the data. QW, YM, and CL drafted the manuscript and had full access to all of the data in the study. All authors acquired the data, reviewed the manuscript and vouch for the accuracy and completeness of the data and for the adherence of the study to the protocol, and contributed to the article and approved the submitted version.
Funding
This work was support by the Key Project of Hunan Provincial Health Commission, China (grant numbers 202217012851).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-5. 5th ed. Washington, DC: American Psychiatric Association (2014).
2. Wang, SH, Wang, JY, Lin, PY, Lin, KH, Ko, CJ, Hsieh, CE, et al. Predisposing risk factors for delirium in living donor liver transplantation patients in intensive care units. PLoS One. (2014) 9:e96676. doi: 10.1371/journal.pone.0096676
3. Binda, F, Galazzi, A, Brambilla, A, Adamini, I, and Laquintana, D. Risk factors for delirium in intensive care unit in liver transplant patients. Assist Inferm Ric. (2017) 36:90–7. doi: 10.1702/2721.27754
4. Lee, H, Oh, SY, Yu, JH, Kim, J, Yoon, S, and Ryu, HG. Risk factors of postoperative Delirium in the intensive care unit after liver transplantation. World J Surg. (2018) 42:2992–9. doi: 10.1007/s00268-018-4563-4
5. Lee, H, Yang, SM, Chung, J, Oh, HW, Yi, NJ, Suh, KS, et al. Effect of perioperative low-dose Dexmedetomidine on postoperative Delirium after living-donor liver transplantation: a randomized controlled trial. Transplant Proc. (2020) 52:239–45. doi: 10.1016/j.transproceed.2019.11.015
6. Lescot, T, Karvellas, CJ, Chaudhury, P, Tchervenkov, J, Paraskevas, S, Barkun, J, et al. Postoperative delirium in the intensive care unit predicts worse outcomes in liver transplant recipients. Can J Gastroenterol. (2013) 27:207–12. doi: 10.1155/2013/289185
7. Ri, HS, Choi, YJ, Park, JY, Jin, SJ, Lee, YS, Son, JM, et al. Elevation of preoperative Ammonia level is not associated with the incidence of postoperative Delirium in patients with liver transplantation: a propensity score matching analysis. Transplant Proc. (2020) 52:219–26. doi: 10.1016/j.transproceed.2019.11.012
8. Zhou, S, Deng, F, Zhang, J, and Chen, G. Incidence and risk factors for postoperative delirium after liver transplantation: a systematic review and meta-analysis. Eur Rev Med Pharmacol Sci. (2021) 25:3246–53. doi: 10.26355/eurrev_202104_25733
9. Oliver, N, Bohorquez, H, Anders, S, Freeman, A, Fine, K, Ahmed, E, et al. Post-liver transplant delirium increases mortality and length of stay. Ochsner J. (2017) 17:25–30.
10. Mottaghi, S, Nikoupour, H, Firoozifar, M, Jalali, SS, Jamshidzadeh, A, Vazin, A, et al. The effect of taurine supplementation on delirium post liver transplantation: a randomized controlled trial. Clin Nutr. (2022) 41:2211–8. doi: 10.1016/j.clnu.2022.07.042
11. Yoon, JS, Kim, YR, Choi, JW, Ko, JS, Gwak, MS, and Kim, GS. Risk factors of postoperative delirium following liver transplantation. Korean J Anesthesiol. (2009) 57:584–9. doi: 10.4097/kjae.2009.57.5.584
12. Beckmann, S, Schubert, M, Burkhalter, H, Dutkowski, P, and de Geest, S. Postoperative Delirium after liver transplantation is associated with increased length of stay and lower survival in a prospective cohort. Prog Transplant. (2017) 27:23–30. doi: 10.1177/1526924816679838
13. Bhattacharya, B, Maung, A, Barre, K, Maerz, L, Rodriguez-Davalos, MI, Schilsky, M, et al. Postoperative delirium is associated with increased intensive care unit and hospital length of stays after liver transplantation. J Surg Res. (2017) 207:223–8. doi: 10.1016/j.jss.2016.08.084
14. Laubenberger, J, Haussinger, D, Bayer, S, Gufler, H, Hennig, J, and Langer, M. Proton magnetic resonance spectroscopy of the brain in symptomatic and asymptomatic patients with liver cirrhosis. Gastroenterology. (1997) 112:1610–6. doi: 10.1016/s0016-5085(97)70043-x
15. Dhar, R, Young, GB, and Marotta, P. Perioperative neurological complications after liver transplantation are best predicted by pre-transplant hepatic encephalopathy. Neurocrit Care. (2008) 8:253–8. doi: 10.1007/s12028-007-9020-4
16. Beresford, TP. Neuropsychiatric complications of liver and other solid organ transplantation. Liver Transpl. (2001) 7:S36–45. doi: 10.1053/jlts.2001.29095
17. Kork, F, Rimek, A, Andert, A, Becker, NJ, Heidenhain, C, Neumann, UP, et al. Visual quality assessment of the liver graft by the transplanting surgeon predicts postreperfusion syndrome after liver transplantation: a retrospective cohort study. BMC Anesthesiol. (2018) 18:29. doi: 10.1186/s12871-018-0493-9
18. Zhou, J, Xu, X, Liang, Y, Zhang, X, Tu, H, and Chu, H. Risk factors of postoperative delirium after liver transplantation: a systematic review and meta-analysis. Minerva Anestesiol. (2021) 87:684–94. doi: 10.23736/S0375-9393.21.15163-6
19. Lu, RY, Zhu, HK, Liu, XY, Zhuang, L, Wang, ZY, Lei, YL, et al. A non-linear relationship between preoperative Total bilirubin level and postoperative Delirium incidence after liver transplantation. J Pers Med. (2022) 12:141. doi: 10.3390/jpm12020141
20. Horan, TC, Andrus, M, and Dudeck, MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. (2008) 36:309–32. doi: 10.1016/j.ajic.2008.03.002
21. Ely, EW, Margolin, R, Francis, J, May, L, Truman, B, Dittus, R, et al. Evaluation of delirium in critically ill patients: validation of the confusion assessment method for the intensive care unit (CAM-ICU). Crit Care Med. (2001) 29:1370–9. doi: 10.1097/00003246-200107000-00012
22. Evered, L, Silbert, B, Knopman, DS, Scott, DA, DeKosky, ST, Rasmussen, LS, et al. Nomenclature consensus working group. Recommendations for the nomenclature of cognitive change associated with anaesthesia and surgery-2018. Anesthesiology. (2018) 129:872–9. doi: 10.1097/ALN.0000000000002334
23. Wiesner, R, Edwards, E, Freeman, R, Harper, A, Kim, R, Kamath, P, et al. Model for end-stage liver disease (MELD) and allocation of donor livers. Gastroenterology. (2003) 124:91–6. doi: 10.1053/gast.2003.50016
24. van Gool, WA, van de Beek, D, and Eikelenboom, P. Systemic infection and delirium: when cytokines and acetylcholine collide. Lancet. (2010) 375:773–5. doi: 10.1016/s0140-6736(09)61158-2
25. Häussinger, D, Kircheis, G, Fischer, R, Schliess, F, and Dahl,. Hepatic encephalopathy in chronic liver disease: a clinical manifestation of astrocyte swelling and low-grade cerebral edema? J Hepatol. (2000) 32:1035–8. doi: 10.1016/s0168-8278(00)80110-5
26. Catafau, AM, Kulisevsky, J, Bernà, L, Pujol, J, Martin, JC, Otermin, P, et al. Relationship between cerebral perfusion in frontal-limbic-basal ganglia circuits and neuropsychologic impairment in patients with subclinical hepatic encephalopathy. J Nucl Med. (2000) 41:405–10.
27. Riker, RR, Shehabi, Y, Bokesch, PM, Ceraso, D, Wisemandle, W, Koura, F, et al. Dexmedetomidine vs midazolam for sedation of critically ill patients: a randomized trial. JAMA. (2009) 301:489–99. doi: 10.1001/jama.2009.56
28. Perez-Zoghbi, JF, Zhu, W, Grafe, MR, and Brambrink, AM. Dexmedetomidine-mediated neuroprotection against sevoflurane-induced neurotoxicity extends to several brain regions in neonatal rats. Br J Anaesth. (2017) 119:506–16. doi: 10.1093/bja/aex222
29. Yang, X, Lin, C, Chen, S, Huang, Y, Cheng, Q, and Yao, Y. Remimazolam for the prevention of emergence Delirium in children following tonsillectomy and adenoidectomy under sevoflurane anesthesia: a randomized controlled study. Drug Des Devel Ther. (2022) 16:3413–20. doi: 10.2147/DDDT.S381611
30. Kaneko, S, Morimoto, T, Ichinomiya, T, Murata, H, Yoshitomi, O, and Hara, T. Effect of remimazolam on the incidence of delirium after transcatheter aortic valve implantation under general anesthesia: a retrospective exploratory study. J Anesth. (2023) 37:210–8. doi: 10.1007/s00540-022-03148-2
31. Deng, Y, Qin, Z, Wu, Q, Liu, L, Yang, X, Ju, X, et al. Liu L Efficacy and safety of remimazolam besylate versus dexmedetomidine for sedation in non-intubated older patients with agitated delirium after orthopedic surgery: a randomized controlled trial. Drug Des Devel Ther. (2022) 16:2439–51. doi: 10.2147/DDDT.S373772
32. Hackworth, WA, Heuman, DM, Sanyal, AJ, Fisher, RA, Sterling, RK, Luketic, VA, et al. Effect of hyponatraemia on outcomes following orthotopic liver transplantation. Liver Int. (2009) 29:1071–7. doi: 10.1111/j.1478-3231.2009.01982.x
33. Patrono, D, Rigo, F, Bormida, S, Berchialla, P, Giordanengo, L, Skurzak, S, et al. Romagnoli R Graft factors as determinants of postoperative delirium after liver transplantation. Updat Surg. (2020) 72:1053–63. doi: 10.1007/s13304-020-00887-3
34. Chang, WJ, Hsieh, CE, Hung, YJ, Hsu, YL, Lin, KH, and Chen, YL. Length of alcohol abstinence predicts posttransplant delirium in living donor liver transplant recipients with alcoholic cirrhosis. Exp Clin Transplant. (2022) 20:750–6. doi: 10.6002/ect.2022.0199
35. Tavabie, OD, Colwill, M, Adamson, R, McPhail, MJW, Bernal, W, Jassem, W, et al. A 'real-world' analysis of risk factors for post liver transplant delirium and the effect on length of stay. Eur J Gastroenterol Hepatol. (2020) 32:1373–80. doi: 10.1097/MEG.0000000000001661
36. Chen, J, Wang, H, He, Z, and Li, T. Analysis of risk factors for postoperative Delirium after liver transplantation. Neuropsychiatr Dis Treat. (2020) 16:1645–52. doi: 10.2147/NDT.S254920
38. Greco, M, Capretti, G, Beretta, L, Gemma, M, Pecorelli, N, and Braga, M. Enhanced recovery program in colorectal surgery: a meta-analysis of randomized controlled trials. World J Surg. (2014) 38:1531–41. doi: 10.1007/s00268-013-2416-8
39. McPherson, JA, Wagner, CE, Boehm, LM, Hall, JD, Johnson, DC, Miller, LR, et al. Pandhvaripande PP Delirium in the cardiovascular ICU: exploring modifiable risk factors. Crit Care Med. (2013) 41:405–13. doi: 10.1097/CCM.0b013e31826ab49b
40. Xu, Q, Zhu, M, Li, Z, Zhu, J, Xiao, F, Liu, F, et al. Enhanced recovery after surgery protocols in patients undergoing liver transplantation: a retrospective comparative cohort study. Int J Surg. (2020) 78:108–12. doi: 10.1016/j.ijsu.2020.03.081
41. King, AB, Kensinger, CD, Shi, Y, Shotwell, MS, Karp, SJ, Pandharipande, PP, et al. Intensive care unit enhanced recovery pathway for patients undergoing orthotopic liver transplants recipients: a prospective, observational study. Anesth Analg. (2018) 126:1495–503. doi: 10.1213/ANE.0000000000002851
42. Rao, JH, Zhang, F, Lu, H, Dai, XZ, Zhang, CY, Qian, XF, et al. Effects of multimodal fast-track surgery on liver transplantation outcomes. Hepatobiliary Pancreat Dis Int. (2017) 16:364–9. doi: 10.1016/S1499-3872(17)60020-1
43. Brustia, R, Monsel, A, Conti, F, Savier, E, Rousseau, G, Perdigao, F, et al. Scatton O Enhanced recovery in liver transplantation: a feasibility study. World J Surg. (2019) 43:230–41. doi: 10.1007/s00268-018-4747-y
44. Hillingsø, JG, Rostved, AA, Dengsø, KE, Sørensen, CL, Frederiksen, HJ, Krohn, PS, et al. Enhanced recovery after surgery is feasible and safe in liver transplantation: a cohort study. HPB. (2022) 24:2022–8. doi: 10.1016/j.hpb.2022.07.010
45. Zhang, Y, Xia, YM, Lin, DX, Zhuo, XB, and Yang, XF. Meta-analysis of effect of using enhanced recovery after surgery in perioperative period of liver transplantation. Chin J Gen Surg. (2021) 30:79–90.
46. Pollok, JM, Tinguely, P, Berenguer, M, Niemann, CU, Raptis, DA, Spiro, M, et al. Enhanced recovery for liver transplantation: recommendations from the 2022 international liver transplantation society consensus conference. Lancet Gastroenterol Hepatol. (2023) 8:81–94. doi: 10.1016/S2468-1253(22)00268-0
Keywords: liver transplantation, delirium, risk factors, prognosis, ICU stay
Citation: Ma Y, Li C, Peng W and Wan Q (2023) The influence of delirium on mortality and length of ICU stay and analysis of risk factors for delirium after liver transplantation. Front. Neurol. 14:1229990. doi: 10.3389/fneur.2023.1229990
Edited by:
Shang Yu Wang, Linkou Chang Gung Memorial Hospital, TaiwanReviewed by:
Oliver Tavabie, King’s College London, United KingdomDamiano Patrono, Azienda Ospedaliero Universitaria Città della Salute e della Scienza di Torino, Italy
Copyright © 2023 Ma, Li, Peng and Wan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qiquan Wan, MzU0ODY4NTU0MkAxNjMuY29t
†These authors have contributed equally to this work
 Ying Ma
Ying Ma Cuiying Li1,2†
Cuiying Li1,2† Qiquan Wan
Qiquan Wan