- 1Department of Pediatric Neurosurgery, APHP, Hôpital Necker Enfants Malades, Paris, France
- 2Université de Paris Cité, Paris, France
- 3Department of Pediatric Neurology, Hôpital Necker Enfants Malades, Paris, France
- 4Department of Radiology, GHU Sainte-Anne, Paris, France
- 5Department of Pediatric Oncology, Institut Curie, Paris, France
- 6Department of Pediatric Oncology, Institut Gustave Roussy, Villejuif, France
- 7Department of Pediatric Radiology, APHP, Hôpital Necker Enfants Malades, Paris, France
- 8Université Paris Cité, UMR 1163, Institut Imagine, Paris, France
Background: The co-occurrence of moyamoya vasculopathy and extra-optic pathway tumors is rare in neurofibromatosis type 1 (NF1), with only four cases described in the literature. Brain surgery in these patients may be challenging because of the risk of brain infarction after skin and dural incision. Given its percutaneous and minimally invasive nature, laser interstitial thermal therapy (LITT) is an ideal option for the treatment of brain tumors in these patients. Here, we report on two patients with NF1 and moyamoya syndrome (MMS) treated for a brain glioma with LITT, after cerebral revascularization.
Cases: The first patient, with familial NF1, underwent bilateral indirect revascularization with multiple burr holes (MBH) for symptomatic MMS. Two years later, she was diagnosed with a left temporal tumor, with evidence of radiologic progression over 10 months. The second patient, also with familial NF1, developed unilateral MMS when he was 6 years old and was treated with MBH. At the age of 15 years, MRI showed a right cingular lesion, growing on serial MRIs. Both patients underwent LITT with no perioperative complications; they are progression free at 10 and 12 months, respectively, and the tumors have decreased in volume.
Discussion: While the association of extra-optic neoplasm and moyamoya angiopathy is seldom reported in NF1, tumor treatment is challenging in terms of both avoiding stroke and achieving oncological control. Here, we show in 2 cases, that LITT could be a safe and effective option in these rare conditions.
Introduction
MR-guided laser interstitial thermal therapy (MRgLITT) is one of the most recent advances introduced in neurosurgery. Since its approval by the FDA in 2007, it has been successfully used in the fields of neuro-oncology (1), epilepsy (2), and recently functional neurosurgery (3). The most attractive advantage of this technique is the possibility of performing laser ablation of a given cerebral volume through a stereotactic percutaneous trajectory under real-time monitoring of brain temperature. For this reason, LITT has established itself as one of the preferred techniques for deep-seated lesions, in order to avoid brain retraction injuries during open surgery. Furthermore, its percutaneous and minimally invasive nature makes it a suitable option for superficial cortical lesions in patients with contraindications to traditional craniotomy (4). In pediatric neuro-oncology, LITT has been shown to be effective for the treatment of brain tumors in a recent multicenter study on 86 children, which reported a progression-free survival rate of 92% at 72 months (5).
Herein, we report the use of LITT in two cases of pediatric patients with neurofibromatosis type 1 (NF1) who presented with the very rare association of moyamoya syndrome (MMS) and extra-optic pathway gliomas [only 4 cases of which are described in the literature (6–9)]. The rationale behind the use of LITT was to minimize the risk of perioperative stroke as a result of the interruption of spontaneous collaterals and external–internal carotid artery anastomoses, which may occur during open brain surgery at the skin or dural incision.
Case description
Case 1
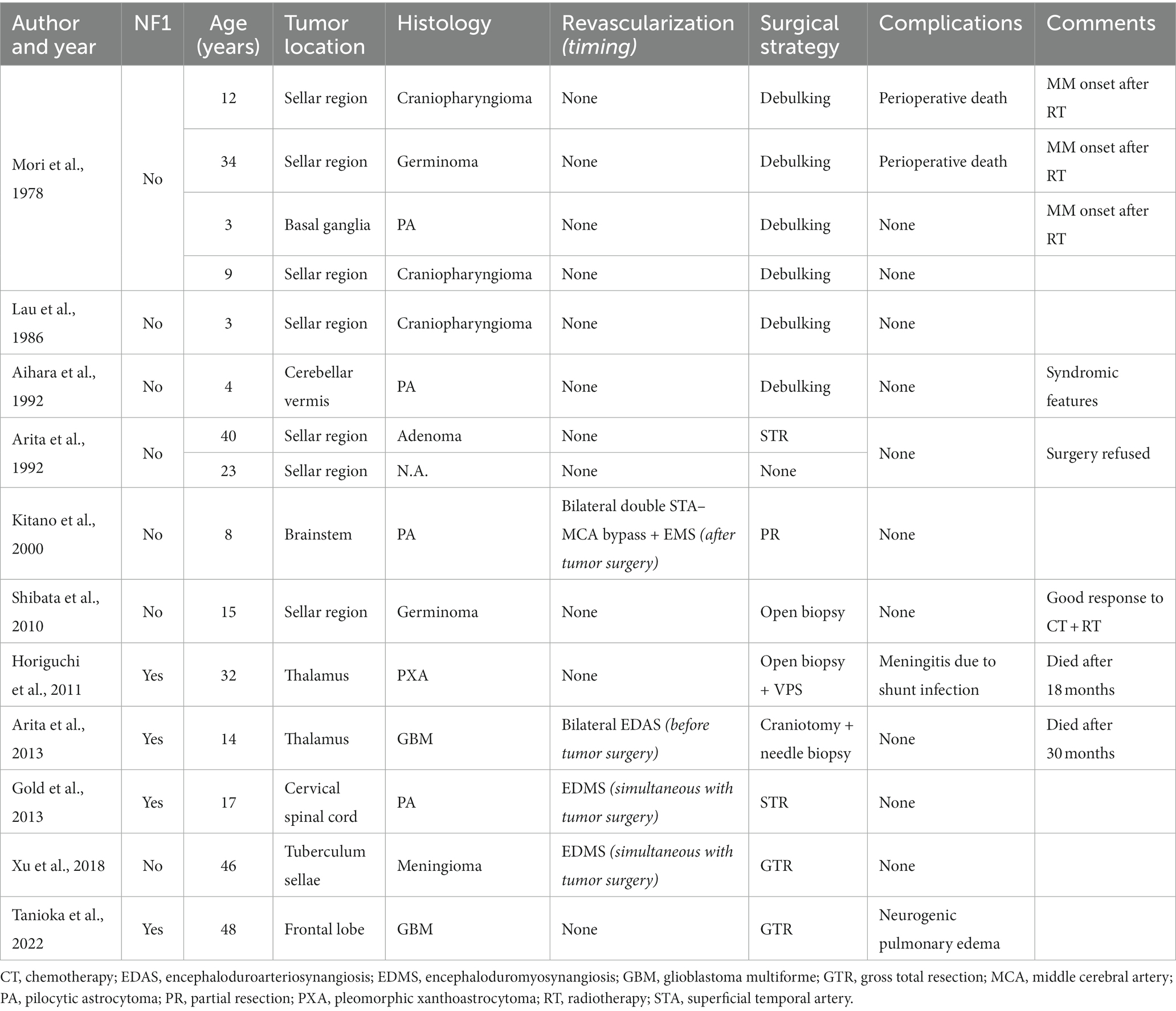
The first case had been diagnosed with familial NF1 associated to MMS and OPG. Following two strokes and recurrent transient ischemic attacks (TIAs), at the age of 9 years she underwent bilateral indirect revascularization through multiple burr hole (MBH) surgery (Figure 1A), conducted in children as described by our team (10, 11). In the postoperative course, no further stroke occurred, and 1-year postoperative digital subtraction angiography (DSA) showed Matsushima grade A revascularization. Two years later, follow-up MRI showed a left posterior middle temporal gyrus tumor suggestive of low-grade glioma, nodular with a small cystic component, homogeneously enhancing, that progressively increased in volume over the next 10 months (Figure 1B). After multidisciplinary discussion, we established surgical indication for tumor removal, given the tumor progression and the potential increased perioperative risks in case of further evolution. DSA showed collaterals from the middle meningeal and posterior auricular arteries supplying the left hemisphere (Figure 1C), and notably the supramarginal gyrus. These branches were above the theoretical location of the craniotomy for tumor resection. To avoid perioperative brain infarction and subsequent aphasia with open surgery, we performed an MR-guided (3 T General Electrics), stereotactic, robot-assisted (ROSA One Brain, Zimmer Biomet) laser ablation surgery (Visualase, Medtronic, United States) with a temporal trajectory, at the same time as a stereotactic biopsy (Figure 1D). Pathologic exam revealed a pilocytic astrocytoma. There were no perioperative complications, nor was there tumor recurrence after 10 months of follow-up (Figure 1E).
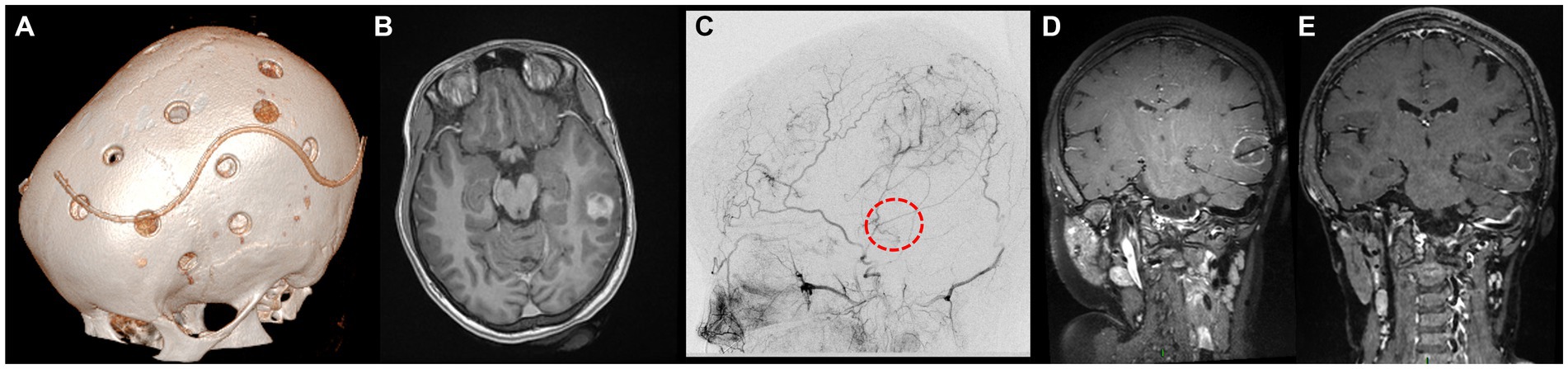
Figure 1. Panel showcasing the most relevant events in the medical history of L.M. (A) Day 1 postoperative cranial CT scan showing the position of the burr holes at the left side after revascularization surgery (9 years old). (B) Axial enhanced T1-weighted cerebral MRI at the age of 11, showing the appearance of a left middle temporal gyrus lesion, nodular with a small posterior cyst, surrounded by brain edema. (C) DSA of the branches of the external carotid artery showing the revascularization of the left hemisphere by the middle meningeal artery and posterior auricular artery. The red circle represents the putative location of the tumor. (D) Coronal enhanced T1-weighted cerebral MRI showing the trajectory of the LITT fiber at the end of the procedure. (E) Coronal enhanced T1-weighted cerebral MRI 10 months after the surgery, documenting the reduction in tumor size.
Case 2
The second case was that of a 6-year-old boy with familial NF1, who was diagnosed with unilateral severe MMD following focal seizures. He underwent left MBH surgery (Figure 2A) through a left coronal skin incision exceeding the midline, with no postoperative stroke and satisfactory revascularization of the left hemisphere on serial postoperative perfusion imaging (Figure 2B) with ASL MRI (12). Nine years later, follow-up MRI showed a right posterior cingular tumor suggestive of a low-grade glioma, nodular, homogeneously enhancing, and growing after 6 months (Figure 2C). As in the previously described case, the indication for tumor removal was discussed due to tumor progression and major perioperative risks in case of further volumetric increase. Open surgery was not deemed a safe option, as a biparietal skin incision and interhemispheric approach would have carried the risk of transosseous and transdural anastomosis damage over the right parietal lobe.
Figure 2. Panel illustrating the different steps of the treatment of E.B.S. (A) day-30 postoperative cranial CT scan showing the burr holes at the right side after revascularization surgery (6 years-old); (B) ASL-MRI of the brain showing cortical hyperperfusion at the level of frontoparietal burr holes and on the medial part of the hemispheres; (C) Axial enhanced T1-weighted cerebral MRI at the age of 15 documenting the appearance of a posterior cingular lesion, nodular and homogeneously enhancing; (D) Axial enhanced T1-weighted cerebral MRI showing the trajectory of the LITT fiber at the end of the procedure; (E) Axial enhanced T1-weighted cerebral MRI 12 months after the surgery displaying the reduction in tumor size.
Therefore, we carried out MR-guided, stereotactic, robot-assisted laser ablation surgery (Visualase, Medtronic, United States) with a right intraparietal trajectory (Figure 2D). We did not perform a needle biopsy at the same time because of radiological features typical of low-grade glioma on MRI in the context of molecular diagnosis of NF1. No perioperative complications occurred, and the tumor size showed a significant reduction of 65% at 12-month follow-up (Figure 2E).
Discussion
These two case reports demonstrate that LITT represents a good option for the ablation of supratentorial brain tumors in the context of NF1 after revascularization surgery with multiple burr holes. To our knowledge, this treatment strategy has not been described previously.
The association of moyamoya angiopathy and brain tumors outside the optic pathways is rare: only 4 NF1 patients (6–9) and 10 other cases (7 adults, 3 children) with concomitant CNS tumor and MMD have been described in the literature (13–19). As shown in Table 1, revascularization was carried out in only four of these patients (6, 9, 14, 19), mainly after brain tumor surgery. In the other reports, the vasculopathy occurred after tumor treatment (surgery or radiotherapy) or was not symptomatic at the time of tumor diagnosis. The 14-year-old NF1 patient reported by Arita et al. (6) is the sole patient who has undergone bilateral encephaloduroarteriosynangiosis (EDAS) 19 months before surgical biopsy of a thalamic malignant tumor. In this case, the authors used a small craniotomy with a neuronavigation-guided needle biopsy to avoid damage to dural collaterals.
Treating a brain tumor in the context of NF1 and moyamoya is complicated and bears a high risk of stroke, because on the one hand, radiotherapy exacerbates the vasculopathy (20, 21), and on the other hand, microsurgery may require interrupting spontaneous transdural collaterals; medical treatments with chemotherapy or MEK inhibitors may be an active alternative, but the long duration of treatment, the burden of side effects, and the risk of tumor recurrence after treatment discontinuation lead us to prefer a radical local treatment whenever possible.
At our center, indirect revascularization is performed via the multiple burr hole technique, because bilateral revascularization can be carried out in a single procedure, and because burr holes are realized all over the cranial vault, offering whole hemispheric revascularization, and ultimately excellent (>90%) stroke control (10, 12). However, brain collaterals will inevitably occur in the vicinity of any surgical access if a craniotomy is further needed.
LITT can therefore represent the first choice procedure in these cases, given that: (i) it is performed in stereotactic conditions through a 3.2 mm burr hole, with an entry point that can be chosen according to preoperative MRI and DSA to avoid any vessel; (ii) a stereotactic needle biopsy can be performed during the same procedure, providing further treatment possibilities according to the molecular profile if required; (iii) it is performed under real-time MR imaging, allowing simulation of the ablation volume and tailoring of the therapy; (iv) it can be repeated along the same or another trajectory in case of relapse. Provided that the lesion conformation is favorable (a round or elliptic shape), LITT may achieve satisfactory ablation volumes (22) and assure sustained control of the tumor (4, 5): this is particularly attractive in NF1 patients to reduce the need for radiation and prolonged medical treatment, recognizing that the use of radiotherapy is not recommended (20, 21, 23). The choice of whether or not to perform a stereotactic needle biopsy before the introduction of the laser fiber is still under discussion, as it has been pointed out that this could cause MRI artifacts at the biopsy site, resulting in sub-optimal MR thermometry and potentially decreased LITT efficacy (24, 25). A recent retrospective registry-based comparative study involving 678 patients with malignant brain tumors undergoing LITT alone and 373 cases treated through needle biopsy before LITT did not indicate differences in the safety profile (26).
Finally, tumoral size may limit LITT indications, both because the whole tumor may not be ablated, and because the risk of complications is correlated to the tumor size and has been found to reach 26.7% in a multicentric series, with a 5.8% risk of neurological deficits and 2.3% risk of mortality (5). This might justify prolonged MRI follow-up of NF1 patients with previously revascularized MMS, with the aim of detecting tumors when they are morphologically and volumetrically suitable for laser ablation.
Conclusion
Association of moyamoya angiopathy and brain neoplasms is rare but may occur, notably in neurofibromatosis type 1. Avoiding stroke while preserving EC–IC anastomosis is a challenge that LITT can overcome, provided that the tumor location and volume are suitable. This increases the potential indications for this technique, proposed here as an alternative to irradiation (not recommended in the context of NF1 and moyamoya) or prolonged medical treatment.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The institutional review board (Assistance Publique Hôpitaux de Paris) authorized the waiver of written informed consent for this study because of its non-interventional retrospective design, and patients were informed they could oppose the use of their health-related data for research purposes. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent was obtained from the minor(s)’ legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article.
Author contributions
LG: Conceptualization, Formal analysis, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. KB: Writing – review & editing. SB: Writing – review & editing. MK: Writing – review & editing. ON: Writing – review & editing. MB: Writing – review & editing. FB: Writing – review & editing. SA: Writing – review & editing. VD-R: Writing – review & editing. NB: Writing – review & editing. TB: Funding acquisition, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
TB is a consultant for Medtronic.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Rahmathulla, G, Recinos, PF, Kamian, K, Mohammadi, AM, Ahluwalia, MS, and Barnett, GH. MRI-guided Laser interstitial thermal therapy in neuro-oncology: a review of its current clinical applications. Oncology. (2014) 87:67–82. doi: 10.1159/000362817
2. Youngerman, BE, Save, AV, and McKhann, GM. Magnetic resonance imaging-guided Laser interstitial thermal therapy for epilepsy: systematic review of technique, indications, and outcomes. Neurosurgery. (2020) 86:E366–82. doi: 10.1093/neuros/nyz556
3. Aubignat, M, Tir, M, Ouendo, M, Constans, JM, and Lefranc, M. Stereotactic robot-assisted MRI-guided laser interstitial thermal therapy thalamotomy for medically intractable Parkinson’s disease tremor: technical note and preliminary effects on 2 cases. Acta Neurochir. (2023) 165:1453–60. doi: 10.1007/s00701-023-05614-6
4. Kamath, AA, Friedman, DD, Hacker, CD, Smyth, MD, Limbrick, DD Jr, Kim, AH, et al. MRI-guided interstitial Laser ablation for intracranial lesions: a large single-institution experience of 133 cases. Stereotact Funct Neurosurg. (2017) 95:417–28. doi: 10.1159/000485387
5. Arocho-Quinones, EV, Lew, SM, Handler, MH, Tovar-Spinoza, Z, Smyth, M, Bollo, R, et al. Magnetic resonance–guided stereotactic laser ablation therapy for the treatment of pediatric brain tumors: a multiinstitutional retrospective study. J Neurosurg Pediatr. (2020) 26:13–21. doi: 10.3171/2020.1.PEDS19496
6. Arita, H, Narita, Y, Ohno, M, Miyakita, Y, Okita, Y, Ide, T, et al. Management of glioblastoma in an NF1 patient with moyamoya syndrome: a case report. Childs Nerv Syst. (2013) 29:341–5. doi: 10.1007/s00381-012-1948-9
7. Horiguchi, S, Mitsuya, K, Watanabe, R, Yagishita, S, and Nakasu, Y. Pleomorphic Xanthoastrocytoma and Moyamoya disease in a patient with Neurofibromatosis type 1-case report. Neurol Med Chir. (2011) 51:310–4. doi: 10.2176/nmc.51.310
8. Tanioka, S, Fujiwara, M, Yago, T, Tanaka, K, Ishida, F, and Suzuki, H. Glioblastoma with concomitant moyamoya vasculopathy in neurofibromatosis type 1: illustrative case. J Neurosurg. (2022) 3:CASE21708. doi: 10.3171/CASE21708
9. Gold, JJ, Dory, CE, Levy, ML, and Crawford, JR. Simultaneous Moyamoya disease and cervical spinal cord low-grade astrocytoma in a child with neurofibromatosis type 1. Case Rep. (2013) 2013:bcr2013009812. doi: 10.1136/bcr-2013-009812
10. Blauwblomme, T, Mathon, B, Naggara, O, Kossorotoff, M, Bourgeois, M, Puget, S, et al. Long-term outcome after multiple Burr hole surgery in children with Moyamoya Angiopathy: a single-center experience in 108 hemispheres. Neurosurgery. (2017) 80:950–6. doi: 10.1093/neuros/nyw161
11. Sainte-Rose, C, Oliveira, R, Puget, S, Beni-Adani, L, Boddaert, N, Thorne, J, et al. Multiple bur hole surgery for the treatment of moyamoya disease in children. J Neurosurg Pediatr. (2006) 105:437–43. doi: 10.3171/ped.2006.105.6.437
12. Blauwblomme, T, Lemaitre, H, Naggara, O, Calmon, R, Kossorotoff, M, Bourgeois, M, et al. Cerebral blood flow improvement after indirect revascularization for Pediatric Moyamoya disease: a statistical analysis of arterial spin-labeling MRI. AJNR Am J Neuroradiol. (2016) 37:706–12. doi: 10.3174/ajnr.A4592
13. Aihara, N, Nagai, H, Mase, M, Kanai, H, Wakabayashi, S, and Mabe, H. A typical moyamoya disease associated with brain tumor. Surg Neurol. (1992) 37:46–50. doi: 10.1016/0090-3019(92)90065-U
14. Kitano, S, Sakamoto, H, Fujitani, K, and Kobayashi, Y. Moyamoya disease associated with a brain stem glioma. Childs Nerv Syst. (2000) 16:251–5. doi: 10.1007/s003810050508
15. Lau, YLDN, and Milligan, DWAA. Atypical presentation of craniopharyngioma associated with Moyamoya disease. J R Soc Med. (1986) 79:236–7. doi: 10.1177/014107688607900414
16. Mori, K, Takeuchi, F, Ishikawa, M, Handa, H, Toyama, M, and Yamaki, T. Occlusive arteriopathy and brain tumor. J Neurosurg. (1978) 49:22–35. doi: 10.3171/jns.1978.49.1.0022
17. Shibata, Y, Matsuda, M, Suzuki, K, and Matsumura, A. Cystic neurohypophysial germinoma associated with moyamoya disease. Neurol Sci. (2010) 31:189–92. doi: 10.1007/s10072-009-0172-1
18. Arita, K, Uozumi, T, Oki, S, Kuwabara, S, Ohba, S, Muttaqin, Z, et al. MoyaMoya disease associated with pituitary adenoma report of two cases. Neurol Med Chir. (1992) 32:753–7. doi: 10.2176/nmc.32.753
19. Xu, F, Tang, H, Xiong, J, and Liu, X. Moyamoya disease associated with tuberculum Sellae meningioma and cavernous sinus hemangioma. World Neurosurg. (2018) 109:89–95. doi: 10.1016/j.wneu.2017.09.116
20. Grill, J, Couanet, D, Cappelli, C, Habrand, JL, Rodriguez, D, Sainte-Rose, C, et al. Radiation-induced cerebral vasculopathy in children with neurofibromatosis and optic pathway glioma. Ann Neurol. (1999) 45:393–6. doi: 10.1002/1531-8249(199903)45:3<393::AID-ANA17>3.0.CO;2-B
21. Cappelli, C, Grill, J, Raquin, M, Pierre-Kahn, A, Lellouch-Tubiana, A, Terrier-Lacombe, MJ, et al. Long term follow up of 69 patients treated for optic pathway tumours before the chemotherapy era. Arch Dis Child. (1998) 79:334–8. doi: 10.1136/adc.79.4.334
22. Munier, SM, Hargreaves, EL, Patel, NV, and Danish, SF. Ablation dynamics of subsequent thermal doses delivered to previously heat-damaged tissue during magnetic resonance–guided laser-induced thermal therapy. J Neurosurg. (2019) 131:1958–65. doi: 10.3171/2018.7.JNS18886
23. Carr, CM, Benson, JC, DeLone, DR, Diehn, FE, Kim, DK, Merrell, KW, et al. Intracranial long-term complications of radiation therapy: an image-based review. Neuroradiology. (2021) 63:471–82. doi: 10.1007/s00234-020-02621-7
24. Bakr, SM, Kantak, PA, MJD, J, Budnick, HC, and Raskin, J. Thermal damage estimate artifact following antecedent biopsy: a case report. Cureus. (2022) 14:e31913. doi: 10.7759/cureus.31913
25. Noh, T, Juvekar, P, Huang, R, Lee, G, Ogasawara, CT, and Golby, AJ. Biopsy artifact in Laser interstitial thermal therapy: a technical note. Front Oncol. (2021) 11:746416. doi: 10.3389/fonc.2021.746416
26. Sharma, M, Do, TH, Palzer, EF, Huling, JD, and Chen, CC. Comparable safety profile between neuro-oncology procedures involving stereotactic needle biopsy (SNB) followed by laser interstitial thermal therapy (LITT) and LITT alone procedures. J Neuro-Oncol. (2023) 162:147–56. doi: 10.1007/s11060-023-04275-w
Keywords: laser interstitial thermal therapy (LITT), pediatrics, oncology, moyamoya, revascularization, case report
Citation: Guida L, Beccaria K, Benichi S, Kossorotof M, Naggara O, Bourgeois M, Bourdeaut F, Abbou S, Dangouloff-Ros V, Boddaert N and Blauwblomme T (2023) Laser interstitial thermal therapy is effective and safe for the treatment of brain tumors in NF1 patients after cerebral revascularization for moyamoya angiopathy: a report on two cases. Front. Neurol. 14:1291207. doi: 10.3389/fneur.2023.1291207
Edited by:
Jesse Skoch, Cincinnati Children's Hospital Medical Center, United StatesReviewed by:
Flavio Giordano, University of Florence, ItalyAlessandro Consales, Giannina Gaslini Institute (IRCCS), Italy
Copyright © 2023 Guida, Beccaria, Benichi, Kossorotof, Naggara, Bourgeois, Bourdeaut, Abbou, Dangouloff-Ros, Boddaert and Blauwblomme. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Thomas Blauwblomme, dGhvbWFzLmJsYXV3YmxvbW1lQGFwaHAuZnI=
 Lelio Guida
Lelio Guida Kevin Beccaria1,2
Kevin Beccaria1,2 Thomas Blauwblomme
Thomas Blauwblomme