- 1Department of Neurology, Beijing Luhe Hospital, Capital Medical University, Beijing, China
- 2China-America Institute of Neuroscience, Department of Neurology, Beijing Luhe Hospital, Capital Medical University, Beijing, China
Dyskinesia-hyperpyrexia syndrome, a rare medical emergency in Parkinson's disease, is first described in 2010. It is characterized by severe continuous dyskinesia associated with rhabdomyolysis, hyperthermia and subsequent alteration of the mental state. Gradual reduction of dopaminergic dose or DBS is recommended treatment. The prognosis is usually good, but sometimes fatal. But so far, this potentially fatal complication is not widely recognized by clinicians. In emergency, if clinicians fail to make prompt diagnosis and treatment, patients' conditions may get worse, and their lives may be threatened in serious cases.
Introduction
Acute hyperpyrexia syndrome related to Parkinson's disease usually occurs in advanced PD patients, especially in those who have received long-term treatment with levodopa. Acute hyperpyrexia syndrome includes Parkinson hyperpyrexia syndrome (PHS), dyskinesia-hyperpyrexia syndrome (DHS) and serotonin syndrome (SS) (1, 2). These acute hyperthermia syndromes are easily confused by clinicians. If emergency physicians cannot recognize these three hyperthermia syndromes timely and give treatment optimally, it may lead to poor prognosis, even threaten lives. Here, we reported a PD patient who presented with severe dyskinesia-hyperpyrexia syndrome, meanwhile performed a comprehensive literature.
Case description
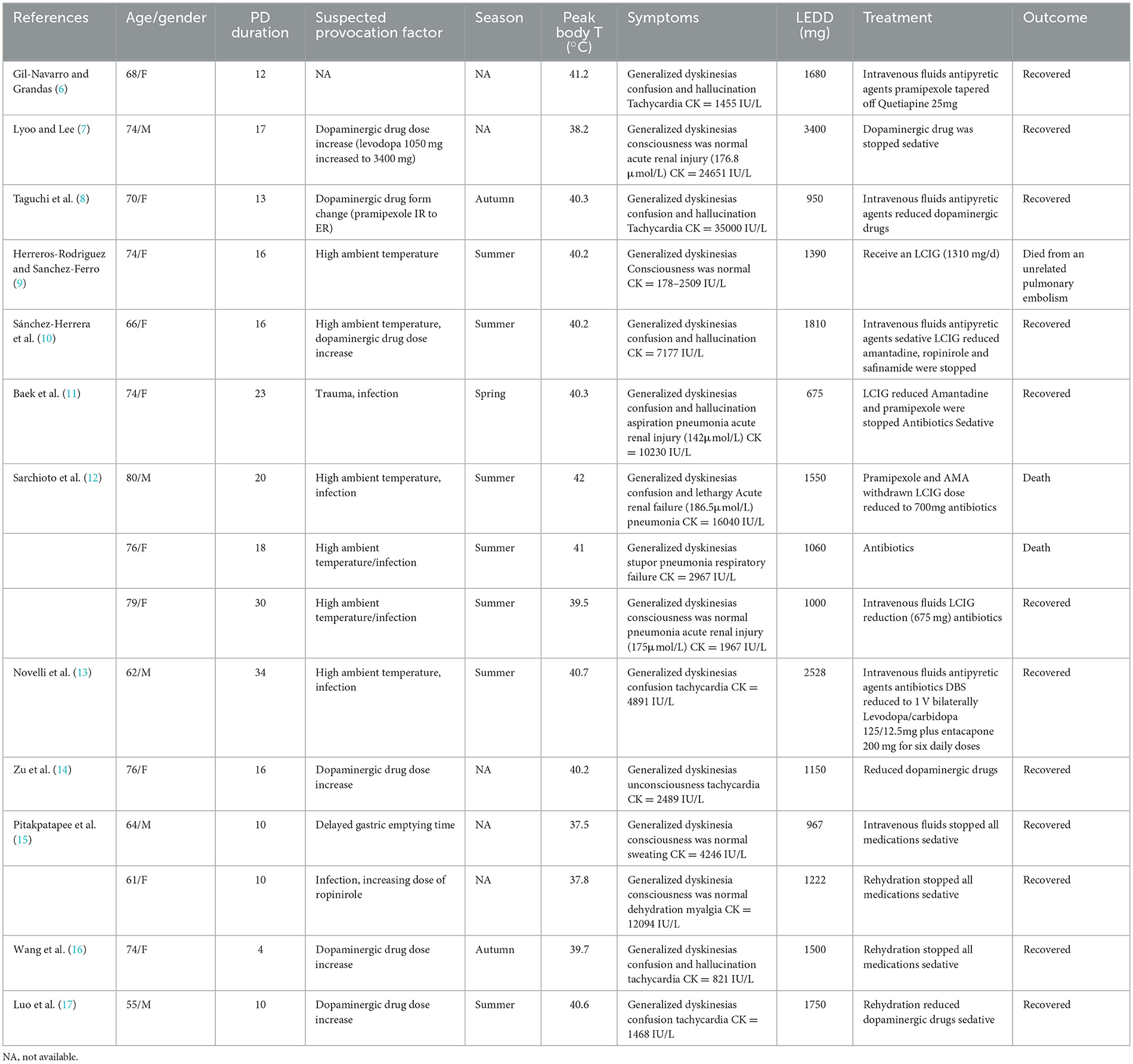
Our patient was a 78-year-old female with a history of PD for 10 years. She had been taking levodopa/benserazide 600/150 mg/day, pramipexole 0.75 mg/day and selegiline 10 mg/day. She denied a history of current statins or serotoninergic drugs use. The season was summer during which she had a slight weight loss. A week before admission she had head trauma secondary to a fall, followed by appetite loss accompanied with a mild dyskinesia. The patient's family considered the symptoms to be tremors and added another compound tablet of levodopa/benserazide (200/50 mg), along with daily oral medication, including levodopa/benserazide 600/150 mg, pramipexole 0.75 mg and selegiline 10 mg, the LEDD was 975 mg. After that the abnormal movements worsened significantly.
On admission, she was in coma because of hypoglycemia, even though she had no history of diabetes and never received hypoglycemic treatment. In the emergency room, her body temperature was 39.6°C, and the heart rate was 118 beats/min. When hypoglycemia is rapidly corrected, the patient's consciousness recovered and severe generalized involuntary dyskinesia of limbs occurred, more intense in the upper limbs. Laboratory findings were mild leukocytosis (white blood cell count 10.07 × 109/L). Elevated BUN (13.6 mmol/L) with serum creatinine (87umol/L) indicated a mild decrease in renal function. Lac (8.4 mmol/L) and serum CK level (1579 U/L) were highly elevated. A chest CT scan showed ill-defined ground-glass opacity in right lower lobes. No space-occupying inflammatory lesion was observed. Head CT basically ruled out intracranial hemorrhage, head injury and strategic infarction (Figure 1). Unfortunately, No head MRI was performed due to the patient's involuntary movements. Since low PCT (0.1 ng/ml) and bacterial culture of sputum were negative, pneumonia was not considered as a fever source, and antibiotics were not prescribed.

Figure 1. Head CT basically ruled out intracranial hemorrhage, head injury and strategic infarction.
Based on the clinical features and laboratory findings, a diagnosis of dyskinesia hyperpyrexia syndrome was made. We discontinued pramipexole and reduced the levodopa/benserazide dose to 300/75 mg/day. The dose of antiparkinsonian drugs was reduced progressively but the dyskinesia never disappeared. After the patient's drug was completely reduced on the 10th day of hospitalization, the abnormal movement completely disappeared but the patient developed fever again. So we added selegiline 5 mg/day, and after that the patient did not show any abnormal movement, with the temperature returned to normal.
Discussion
Acute hyperpyrexia syndrome related to Parkinson's disease is rare but life-threatening complication of PD. Approximately 30–40% of PD patients who have been treated with levodopa for more than 5 years may develop levodopa-induced dyskinesia (3, 4). When a PD patient experiences acute hyperpyrexia, the PHS, DHS and SS need to be considered. Wang's study elucidated the similarities and differences between PHS, DHS, and SS (5). The similarity of these three syndromes can exhibit hyperpyrexia, neuromuscular symptoms, autonomic symptoms and consciousness disturbance. Concerning potential triggers, it should be noted that high ambient temperature, dehydration, infection, and trauma can be common triggers for PHS and DHS, while excessive serotonin drugs are the only cause of SS. In terms of disease course, the course of PHS and DHS usually lasts for 1–2 weeks, while the course of SS usually lasts for < 24 h. The neuromuscular symptoms of DHS are often manifested as dyskinesia, while PHS is often characterized by rigidity and oligokinesia. Compare to PHS, SS may have symptoms such as increased tendon reflexes and clonus in addition to rigidity. PHS and SS usually manifest as tachycardia, sweating, and unstable blood pressure as autonomic nervous symptoms, which are rare in DHS. The consciousness disorder of PHS usually manifests as a decrease in consciousness level from drowsiness to coma, while DHS typically manifests as blurred consciousness and hallucinations. SS usually exhibit anxiety and irritability, while severe patients may experience symptoms such as delirium and coma. The most effective treatment for the three syndromes is to remove potential triggers. PHS needs to gradually increase the dosage of dopaminergic drugs or restart DBS. Conversely, DHS needs to gradually reduce the dosage of dopaminergic drugs or reduce the stimulation of DBS. In addition, SS needs to discontinue the serotonergic drugs. Dyskinesia associated with hyperpyrexia was first described in a 68-year-old advanced PD patient by Gil-Navarro and Grandas (6). And DHS is even rarer compared with PHS or SS. To date, a total of 15 cases of DHS have been reported in 12 publications (Table 1). Sometimes, the dyskinesia may lead to rhabdomyolysis, acute renal failure and respiratory distress, which may become severe and life-threatening.
In our review, we found that DHS was more likely to appear in advanced PD patients accompanied with motor symptom fluctuation, particularly with high-dose dopaminergic therapy. The dyskinesia in DHS patients is usually systemic and persistent and may precede fever. Therefore, some patients may only present with dyskinesia without elevated body temperature in the early stages. The body temperature may fluctuate from 37.5°C to 42°C, and most of which is above 40°C. Elevations of CK are generally considered as secondary to severe dyskinesia, ranging from hundreds to 35000 IU/L, but not all DHS patients CK elevation. There were no characteristic changes through our patient's head CT scan. We speculate that other imaging examinations may have more implications. Previously study rarely described characteristic neuroimaging features of DHS. A recent research provides new insights into DHS and expands on its neuroimaging features. Luo's study showed that DHS can cause reversible encephalopathy, which was detected through head MRI (17). Thus, DHS still has many characteristics to be discovered. In previous study, there were 9 out of 14 patients manifested as confusion and hallucinations, which might be caused by dopaminergic hyperactivity in the mesocorticolimbic system. Three patients manifested with consciousness level reduction (stupor or lethargy). Furthermore, we observed that female patients are prone to develop this complication (ten female and four male). These gender differences might be induced by female hormonal patterns which could increase the individual dyskinetic sensitivity to levodopa (18). Besides, compared with male, female patients with lighter body weight are more likely to have higher plasma concentrations of levodopa with the same treatment protocol (19). Therefore, clinicians should pay more attention to individualized drug therapy.
The pathogenesis is still unclear. Previous literature review suggested a variety of suspected triggering factors, including adjustment in dopaminergic therapy, trauma, infection, hot weather, dehydration, gastrointestinal diseases and changes in deep brain stimulation (5). Dopamine dysregulation syndrome (DDS), an uncommon complication of medical treatment for PD, is characterized by addictive behavior and excessive use of dopaminergic medication. Levodopa is more likely to be associated with DDS compared to other drugs; in addition, there were higher rates of dyskinesias and motor fluctuations. Warren's study suggested that individuals at risk may have less efficient inhibitory dopaminergic systems. Thus, we speculate that DDS is also one of the reasons for DHS (20). The current hypothesis was considered as that DHS are prone to occur in high temperature environments. Dopamine is an important neurotransmitter in the hypothalamus, which can promote body heat dissipation and play an important role in regulating body temperature. In patients with advanced PD, degeneration of dopaminergic neurons in the substantia nigra leads to dopaminergic deficiency (10). Therefore, it is speculated that in summer, it is easier to trigger dyskinesia in advanced PD patients treated with high-dose dopaminergic drugs when under high ambient temperature and long daylight duration which may increase the dopaminergic activity (16). Furthermore, hyperpyrexia was also related to increased thermogenesis caused by excessive dyskinesia.
The combination of reduced dopaminergic medications with standard medical care is usually effective and the prognosis is generally favorable. Prompt identification and optimal treatment may improve patient outcomes. Reducing dopaminergic drug dosage as soon as possible is the most effective way to treat DHS. Sedation is effective for patients with refractory dyskinesia (15). Supportive treatments such as intravenous rehydration, cooling, anti-infection, and electrolyte balance are also critical for DHS. Among the 13 patients reported so far, only two patients died due to DHS (12). Complications including rhabdomyolysis, acute renal failure, and respiratory failure suggest a poor prognosis for DHS.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
Ethics statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent from the patients/participants or patients/participants' legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
XD: Writing – original draft, Writing – review & editing. XW: Writing – review & editing. XG: Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Simonet C, Tolosa E, Camara A, Valldeoriola F. Emergencies and critical issues in Parkinson's disease. Pract Neurol. (2020) 20:15–25.
2. Ghosh R, Liddle BJ. Emergency presentations of Parkinson's disease: early recognition and treatment are crucial for optimum outcome. Postgrad Med J. (2011) 87:125–31. doi: 10.1136/pgmj.2010.104976
3. Prange S, Danaila T, Laurencin C, Caire C, Metereau E, Merle H, et al. Age and time course of long-term motor and nonmotor complications in Parkinson's disease. Neurology. (2019) 92:e148–e60. doi: 10.1212/WNL.0000000000006737
4. Cossu G, Colosimo C. Hyperkinetic movement disorder emergencies. Curr Neurol Neurosci Rep. (2017) 17:6. doi: 10.1007/s11910-017-0712-7
5. Wang M, Wang W, Gao Z, Yin X, Chen T, Jiang Z, et al. Dyskinesia-hyperpyrexia syndrome in Parkinson's disease: a systematic review. Clin Auton Res. (2021) 31:529–42. doi: 10.1007/s10286-021-00801-w
6. Gil-Navarro S, Grandas F. Dyskinesia-hyperpyrexia syndrome: another Parkinson's disease emergency. Movem Disorders Off J Movem Disorder Soc. (2010) 25:2691–2. doi: 10.1002/mds.23255
7. Lyoo CH, Lee MS. Rhabdomyolysis induced by severe levodopa induced dyskinesia in a patient with Parkinson's disease. J Neurol. (2011) 258:1893–4. doi: 10.1007/s00415-011-6041-x
8. Taguchi S, Niwa J-i, Ibi T, Doyu M. [Dyskinesia-hyperpyrexia syndrome in a patient with Parkinson's disease: a case report]. Clin Neurol. (2015) 55:182–4. doi: 10.5692/clinicalneurol.55.182
9. Herreros-Rodriguez J, Sanchez-Ferro A. Summertime dyskinesia-hyperpyrexia syndrome: the “Dual Heat” hypothesis. Clin Neuropharmacol. (2016) 39:210–1. doi: 10.1097/WNF.0000000000000155
10. Sánchez-Herrera FA, García-Barragán N, Estévez-Fraga C, Martínez-Castrillo JC, Moreno JLL-S. Dyskinesia-hyperpyrexia syndrome under continuous dopaminergic stimulation. Parkinsonism Relat Disord. (2017) 36:103–4. doi: 10.1016/j.parkreldis.2016.12.018
11. Baek MS, Lee HW, Lyoo CH. A patient with recurrent dyskinesia and hyperpyrexia syndrome. J Move Disord. (2017) 10:154–7. doi: 10.14802/jmd.17022
12. Sarchioto M, Ricchi V, Melis M, Deriu M, Arca R, Melis M, et al. Dyskinesia-hyperpyrexia syndrome in Parkinson's disease: A heat shock-related emergency? Movem Disorders Clin Prac. (2018) 5:534–7. doi: 10.1002/mdc3.12663
13. Novelli A, Di Vico IA, Terenzi F, Sorbi S, Ramat S. Dyskinesia-hyperpyrexia syndrome in Parkinson's disease with deep brain stimulation and high-dose levodopa/carbidopa and entacapone. Parkinson Relat Disord. (2019) 64:352–3. doi: 10.1016/j.parkreldis.2019.05.018
14. Zu J, Raza HK, Chansysouphanthong T, Xu C, Zhang W, Cui G. Dyskinesia and hyperpyrexia syndrome: a case report and review of the literature. Revue Neurol. (2021) 177:710–3. doi: 10.1016/j.neurol.2020.10.002
15. Pitakpatapee Y, Srikajon J, Sangpeamsook T, Srivanitchapoom P. Rhabdomyolysis Associated with severe levodopa-induced dyskinesia in Parkinson's disease: a report of two cases and literature review. Tremor Hyperkinetic Movem. (2021) 11:39. doi: 10.5334/tohm.641
16. Wang JY, Huang JF, Zhu SG, Huang SS, Liu RP, Hu BL, et al. Parkinsonism-hyperpyrexia syndrome and dyskinesia-hyperpyrexia syndrome in Parkinson's Disease: two cases and literature review. J Parkinson's Dis. (2022) 12:1727–35. doi: 10.3233/JPD-223362
17. Luo B, Zhang H, Qin L. Case report: reversible encephalopathy caused by dyskinesia-hyperpyrexia syndrome. Front Neurol. (2023) 14:1234974. doi: 10.3389/fneur.2023.1234974
18. Blanchet PJ, Fang J, Hyland K, Arnold LA, Mouradian MM, Chase TN. Short-term effects of high-dose 17beta-estradiol in postmenopausal PD patients: a crossover study. Neurology. (1999) 53:91–5. doi: 10.1212/WNL.53.1.91
19. Zappia M, Crescibene L, Arabia G, Nicoletti G, Bagalà A, Bastone L, et al. Body weight influences pharmacokinetics of levodopa in Parkinson's disease. Clin Neuropharmacol. (2002) 25:79–82. doi: 10.1097/00002826-200203000-00004
Keywords: Parkinson's disease, acute hyperpyrexia syndrome, dyskinesia, hyperpyrexia, treatment
Citation: Du X, Wang X and Geng X (2024) Dyskinesia-hyperpyrexia syndrome in Parkinson's disease triggered by overdose of levodopa — a case report and literature review. Front. Neurol. 14:1323717. doi: 10.3389/fneur.2023.1323717
Received: 18 October 2023; Accepted: 21 November 2023;
Published: 05 January 2024.
Edited by:
Antonio Emanuele Elia, IRCCS Carlo Besta Neurological Institute Foundation, ItalyReviewed by:
Antonio Suppa, Sapienza University of Rome, ItalyJacky Ganguly, Institute of Neurosciences Kolkata (I-NK), India
Mariachiara Malaguti, Azienda Provinciale per i Servizi Sanitari (APSS), Italy
Copyright © 2024 Du, Wang and Geng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaokun Geng, eGdlbmdAY2NtdS5lZHUuY24=
 Xiangnan Du
Xiangnan Du Xuemei Wang1
Xuemei Wang1 Xiaokun Geng
Xiaokun Geng