- 1Doctor of Philosophy Program in Medical Sciences (International Program), Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand
- 2Chulalongkorn Centre of Excellence for Parkinson’s Disease and Related Disorders, Department of Medicine, Faculty of Medicine, Chulalongkorn University, and King Chulalongkorn Memorial Hospital, Thai Red Cross Society, Bangkok, Thailand
- 3Wat Pho Thai Traditional Medical School, Bangkok, Thailand
- 4Biostatistics Excellence Centre, Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand
- 5The Kirby Institute, University of New South Wales, Sydney, NSW, Australia
- 6Department of Acupuncture, Shonan Keiiku Hospital, Fujisawa, Japan
- 7Toriumi Acupuncture Clinic, Tokyo, Japan
- 8The Academy of Science, The Royal Society of Thailand, Bangkok, Thailand
Background: ON-freezing of gait (ON-FOG) in Parkinson’s disease (PD), often resistant to medication, is linked to sensory deficits and proprioceptive impairment, and results in falls and reduced life quality. While visual cues from a laser cane (LC), which rapidly accesses the motor cortex, are commonly used to compensate for proprioceptive impairment, increased visual reliance may be affected by disease progression. Emerging evidence suggests that modulation of peripheral sensory processing may alleviate ON-FOG, and therapeutic Thai acupressure (TTA) may be a solution. This study aims to evaluate the effect of TTA in alleviating ON-FOG and compare its effectiveness to LC in patients with PD.
Methods: This open-label, non-inferiority trial randomized 90 PD patients with ON-FOG equally into three arms: TTA for plantar nerve stimulation for 96 s, LC for visual cueing, and sham control (SC). Stride length was the primary non-inferiority endpoint [non-inferiority margin: lower limit of 95% confidence interval (CI) above −10 cm in mean change difference in pre- and immediately post-intervention in TTA versus LC (one-sided)]. Secondary outcomes included FOG episodes, double support time, velocity, cadence, step length, timed up and go (TUG) test, and visual analog scale (VAS) score.
Results: TTA showed non-inferiority to LC in stride length (mean = −0.7 cm; 95% CI: −6.55; 5.15) (one-sided). The improvements with TTA and LC versus SC were comparable between (mean = 13.11 cm; 95% CI: 7.26; 18.96) and (mean = 13.8 cm; 95% CI: 7.96; 19.65) (one-sided). Secondary outcomes favored TTA and LC over SC with improved FOG, velocity, step length, and VAS scores, while only TTA resulted in improved double support time, cadence, and TUG test results. No complications occurred.
Conclusion: The efficacy of TTA, which improves stride length, is non-inferior to that of LC and consequently alleviates FOG comparable to LC. TTA might enhance proprioceptive function and reduce visual dependence. Therefore, TTA, characterized by its non-invasive, simple, and safe techniques, is a potential non-pharmacological alternative for ON-FOG treatment and might enhance overall quality of life. However, further research into the mechanism, efficacy, and utilization of TTA is essential.
Clinical trial registration: https://www.thaiclinicaltrials.org/show/TCTR20200317001, identifier TCTR20200317001.
1 Introduction
In Parkinson’s disease (PD), freezing of gait (FOG) is a disabling motor symptom. This phenomenon is described as “a brief, episodic absence or marked reduction in forward progression of the feet despite the intention to walk,” resulting in falls and reduced quality of life (1). As PD progresses, the prevalence of FOG also increases. Although its milder forms can be recognized early, over half of patients with PD will ultimately experience FOG (2). FOG presents in two distinct forms: ON-FOG, which occurs when the medication takes full effect (ON-state), and OFF-FOG, which happens when the medication efficacy wanes. Notably, ON-FOG is particularly challenging owing to its resistance to many medications (3).
Although the underlying causes of ON-FOG remain under investigation, it is considered a dysfunction either in dopaminergic function or in extra-striatal regions, where projections of the cortex and cerebellum where motor and sensory signals interact (2, 4, 5). However, prevalent theories underscore sensory deficits in gait and balance control from peripheral side (6). A significant loss of Aβ cutaneous mechanoreceptors may link to peripheral neuronal degeneration to cause peripheral deafferentation to central nerve system on PD patients (7). A key element is impaired proprioceptive feedback (8). For instance, during the ON state, FOG increases in situations relying heavily on proprioception—such as navigating doorways in the dark (9). A combination of inadequate proprioceptive feedback and visuomotor disruptions might contribute to FOG (5, 9). Insufficient proprioceptive feedback can confuse the position sense attributed to space perception, thereby hindering motor planning and leading to shortened stride lengths and the onset of FOG (10). Consequently, stride length is often a FOG indicator (11, 12). To counter proprioceptive deficits, visual cues, notably from a laser cane (LC), have gained attention as effective gait-improvement tools (13). These cues, produced by laser light, either sharpen focus on a target or facilitate movement, aiding patients in modulating stride length to counter ON-FOG (13–15). Thailand has even adopted LC as a standard complementary and alternative medicine (CAM) treatment (14, 16). However, there are some concerns that increased reliance on visual cues, against a backdrop of PD-induced visual dysfunction, might exacerbate FOG in the long term (15, 17–19). The need for alternative treatments that address the core issue of proprioceptive impairment is underscored.
Emerging evidence supports the efficacy of peripheral sensory processing manipulations in mitigating ON-FOG (20). Tools such as metallic mechanical stimulators and silicone pads have garnered attention. Previous studies suggest their potential in not only reducing the severity of FOG but also in refining balance control and various gait metrics, such as stride length (21–23). The principle behind this is enhancing afferent input to the central pattern generators (CPGs) from the periphery, which in turn improves sensorimotor function.
Traditional Asian medical treatments may have an underlying scientific basis for their treatment of PD (24). Acupressure, a CAM sensory manipulation, holds potential in the treatment of ON-FOG. In Thailand, the deep therapeutic Thai acupressure (TTA) technique has recognized benefits in mitigating musculoskeletal disorders and pain (25–27). TTA involves applying thumb pressure on specific acupoints situated along meridian lines. The pressure varies in intensity, tailored to the pain threshold of each recipient, and is typically repeated multiple times for each point (26, 28, 29). Such pressure potentially stimulates proprioceptors including spindle cells and Golgi tendon organs, harmonizing muscle tension and neuromuscular excitability and boosting proprioceptive feedback (26, 28, 29). TTA provides benefits that include improved balance, foot sensation in diabetic neuropathy patients, and even increased muscle strength in ON-state patients with PD (28, 30). These improvements are primarily attributed to enhanced proprioception (28, 30). However, the efficacy of TTA for managing ON-FOG is still unclear. Similarly, although plantar stimulations appear promising, their potential in alleviating increased visual dependency is yet to be determined. Here, it has to be noted that patients with PD rely heavily on visual cues, because they provide the most powerful and rapid access to visuomotor function to compensate for the inadequate proprioceptive feedback and facilitate the initial movement (31). Therefore, we hypothesized that TTA might alleviate ON-FOG with a non-inferior magnitude compared with LC in terms of the immediate effect.
Taken together, we aimed to compare the efficacy of TTA with that of LC in treating ON-FOG in patients with PD, particularly regarding the immediate effect. Toward this goal, we adopted a non-inferiority trial design that involved three arms: TTA, LC, and a placebo-controlled sham arm.
2 Materials and methods
2.1 Study design and participants
This study was an interventional, randomized, open-label, three-armed parallel-group, controlled, non-inferiority trial. The trial enrolled patients diagnosed with PD using the United Kingdom Parkinson’s Disease Society Brain Bank criteria at the Chulalongkorn Centre of Excellence for Parkinson’s Disease and Related Disorders at King Chulalongkorn Memorial Hospital in Bangkok, Thailand (ChulaPD). Only patients whose condition was both clinically verified by movement disorder specialists and had medically intractable ON-FOG were eligible. The inclusion criteria were age ≥40 years, ability to walk a minimum of 10 m independently, and showing ON-FOG symptoms even after maintaining a consistent medication regimen for at least 3 months. The exclusion criteria were as follows: inability to walk unaided and exhibiting any of the following: utilization of deep brain stimulation, presence of other neurological disorders apart from PD, dementia as identified by a score exceeding 1 on the Unified Parkinson’s Disease Rating Scale (UPDRS)-Part I item 1, acute visual impairments, severe depression, diabetic-induced peripheral neuropathy, active foot skin conditions, systolic blood pressure above 140 mmHg, and diastolic pressure above 90 mmHg.
FOG was determined based on a minimum score of 2 on item 14 related to walking freeze in the UPDRS-Part II (32). To evaluate the medical intractability of FOG, patients were requested to execute 540° turns both left and right at their regular and highest speeds during their ON-state and while on their typical medication.
This study was approved by the Human Ethics Committee of the Faculty of Medicine, Chulalongkorn University (Institutional Review Board No. 211/62) and was conducted according to the tenets of the Declaration of Helsinki. The study is also registered with the Thai Clinical Trial Registry (TCTR20200317001). All participants provided informed consent before participation.
2.2 Experimental protocol
The participants were randomized in a 1:1:1 ratio into three arms: stimulation of plantar nerves via TTA as a novel active treatment, visual cues through LC walking as a standard active treatment, or light touch on the plantar surface as a placebo internal sham control (SC) (33). The allocation was conducted using sex-stratified permuted block randomization (Figure 1). Evaluations took place in the ON-state at 30–60 min after taking their usual dopaminergic medication. To establish baseline characteristics, movement disorder specialists assessed the Hoehn & Yahr stage, UPDRS-Part III, levodopa equivalence dosage, and disease duration (32, 34). The freezing of gait questionnaire (FOG-Q) was administered to determine daily life FOG episodes (35). The FOG-Q has 6 items with scores ranging from 0 to 24, where a higher score signifies increased severity of FOG symptoms.
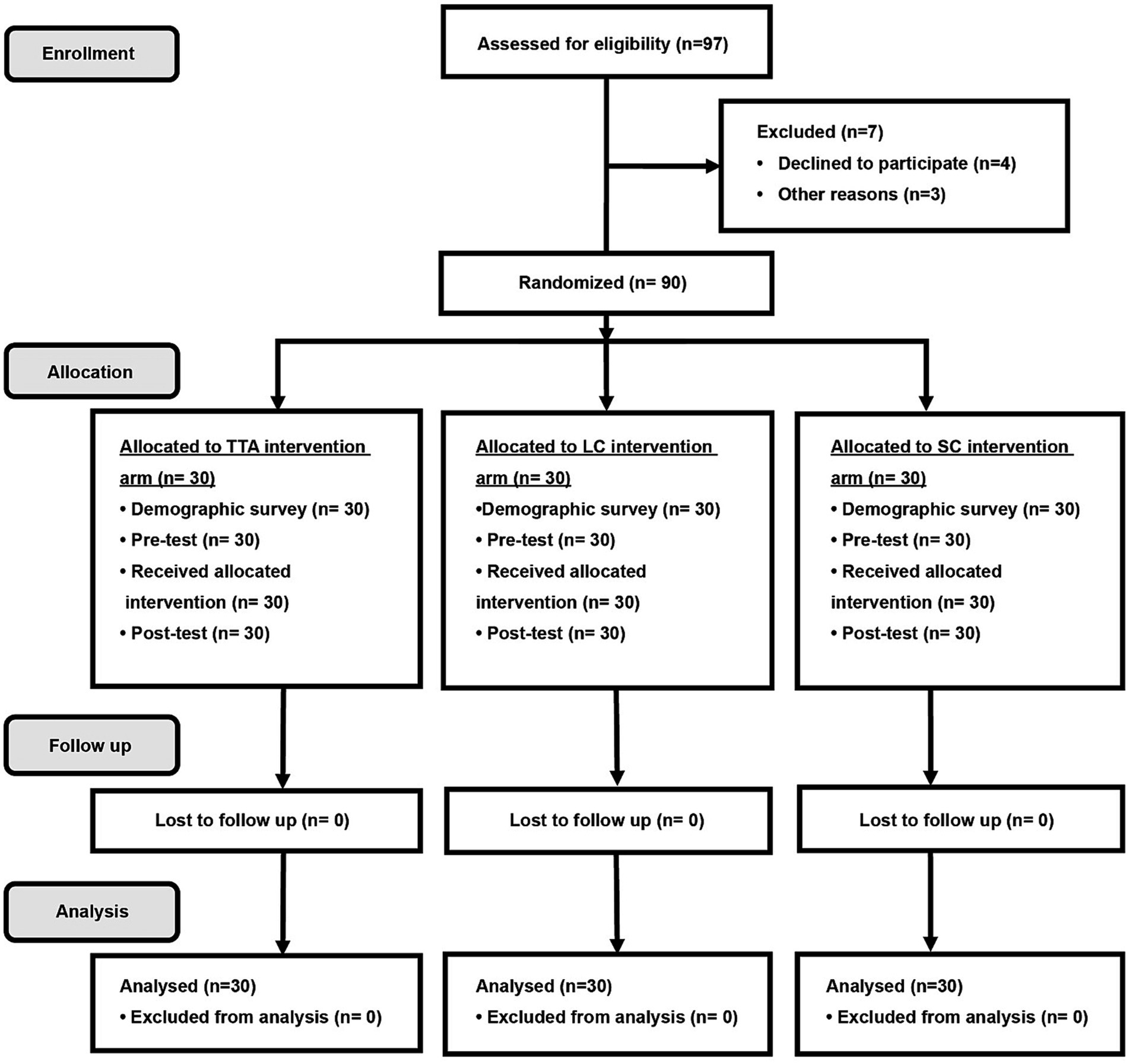
Figure 1. CONSORT randomization flow diagram. TTA, therapeutic Thai acupressure; LC, laser cane; SC, sham-control of light touch.
On the randomization day, the participants underwent a single session that comprised a pre-test, the randomized intervention, and an immediate post-test. Each test was facilitated using the GAITRite® software version 3.95 (CIR Systems, Inc., New Jersey, United States), and the participants walked a 10 m hallway at their own pace, starting and stopping 1 m away from the mat to negate recording phases of acceleration and deceleration (36). Mobility was gauged using the timed up and go (TUG) test, which entailed standing from a chair, walking 3 m, turning, walking back, and sitting (37). This test was performed twice with averages used for subsequent analyses. Notably, only the post-test in the LC arm utilized the LC. A 10 min interval was allowed between tests for participant comfort. Finally, subjective discomfort levels during walking were assessed using the visual analog scale (VAS) at both pre and post-test. The VAS scores ranged from 0 to 10, with 10 indicating the most severe discomfort (38) (Figure 2).
2.3 Intervention
The intervention procedure encompassed four distinct stages: initial rest and vital signs checks, preparation, the intervention itself, and concluding with rest and vital signs checks. The initial and concluding stages were identical across all study arms, involving a 10 min rest period where participants sat on a chair. Vital signs checks, predominantly focused on blood pressure measurements, were carried out during these stages. Following the completion of the intervention, participants could depart, provided their condition remained stable.
2.3.1 Therapeutic Thai acupressure treatment arm
This study defined TTA as deep acupressure applied to specific acupoints using the thumb (26, 28, 29). In this study, the pressure intensity was gradually increased within 3–5 s until the participants reported mild discomfort, and this pressure level was sustained for 6 s, after which it was gradually reduced over roughly 5 s (26, 28, 29, 39). This procedure was executed by YM, the principal investigator and a certified therapeutic Thai massage practitioner accredited by the Ministry of Education, Thailand, and with 18 years of professional experience.
In the preparation stage, participants’ feet were sanitized using alcohol. For the intervention, acupressure was applied to four predetermined acupoints, split between both feet: the head of the big toe and the base of the first metatarsal bone, located between the sesamoid bones (21, 22, 40). These acupoints were selected based on their significant vibratory and touch pressure sensitivity thresholds in patients with PD, as well as the emergence of the monosynaptic reflex in the tibialis anterior muscle (21, 22, 40). The chosen acupoints aligned with the standard therapeutic Thai acupoints on the plantar, which are recognized for their role in influencing motor function in the lower extremities (25, 26). Adhering to Thai traditional medicine principles, acupressure was initially applied to the left foot before shifting to the right (26). This procedure was repeated four times (21, 22, 40). In total, TTA took 96 s which consisted of 6 s at the discomfort threshold per acupoint, i.e., on 4 target acupoints in total with 2 acupoints per foot, and repeated 4 times (21, 22, 40) (Figure 2).
2.3.2 Laser cane treatment arm
An LC is a specialized walking aid that emits laser light to assist foot placement (14, 31). This study defined LC treatment as using a laser line to which participants attempted to step “on,” not “over” (31). In this study, the LC used was designed by the ChulaPD team. This LC has been widely distributed nationwide with support from the Thai Red Cross Society and the Ministry of Social Development and Human Security. Importantly, this LC is specifically tailored for patients with PD with FOG (14, 16).
During the preparation stage, the height of the LC was first calibrated to each participant, and then the participants were briefed on its usage. For the intervention, participants underwent a 5 min training session walking with the LC. They were given the flexibility to use the cane on either their dominant right or left side. The orientation of the laser was adjusted forward, and the participants were instructed to step comfortably on the trajectory of the laser (Figure 2).
2.3.3 Sham-control of light touch treatment arm
This study defined the SC treatment as involving light touch, without any pressure, applied to the acupoints on the plantar surface using the thumb and sustained for 6 s per acupoint (21, 22, 40). This method was executed by the same practitioner responsible for the TTA treatment. All procedural steps were the same as those in the TTA arm, except the third step wherein light touch was applied instead of TTA. In total, SC took 96 s which consisted of 6 s per each acupoint, i.e., on 4 target acupoints in total with 2 acupoints per foot, and repeated 4 times (21, 22, 40). This method ensured that no reflex withdrawal was triggered, eradicating potential confounding effects (21, 22, 40) (Figure 2).
2.4 Outcomes
The primary non-inferiority outcome was stride length. The secondary outcomes included the number of FOG episodes, velocity, double support time, step length, cadence (1, 11), TUG results, and VAS scores. In this study, a step within a FOG episode was defined by each individual’s objective spatial-temporal gait evaluated using GAITRite, which fulfilled two conditions: a double support time exceeding 1.65 standard deviations (SD) above the average and a velocity falling below 90% of the average (41). Improvement in these parameters is denoted by increased stride length, velocity, cadence, and step length and by reductions in FOG episodes, double support time, TUG results, and VAS scores.
2.5 Statistical analysis
The sample size was determined using the standard formula specific to non-inferiority trials (42). Based on an assumption that the SD change in stride length from the pre-test to the post-test in the LC arm was 15 cm (14), treatment was deemed non-inferior if the lower limit of the 95% confidence interval (CI) for the mean change in pre- and post-test stride length was above −10 cm in the comparison between the TTA arm and the LC arm. To achieve an 80% statistical power and determine non-inferiority at a one-sided 5% significance level, 28 participants were recruited for each group, subsequently expanded to 30 participants per group. The SC arm served as a reference point to compare against the two active intervention arms.
Baseline characteristics were organized by treatment arm. Continuous data were presented as the mean (SD) and compared by the one-way analysis of variance test, while categorical data were presented as n (%) and compared by the chi-square test. Any duplicate pre- and post-test results were consolidated into an average value. For the primary outcome, the main analysis computed the mean change difference from the baseline (pre-test) to post-test in the TTA arm compared to that in the LC arm, assessing non-inferiority based on the 95% CIs. For the secondary outcomes, the mean difference (95% CI) pre and post-test for each factor in the TTA versus LC arms was calculated using an independent t-test with a two-sided 5% significance level. Additional formal comparisons of the primary and the secondary outcomes aimed to determine the mean difference (95% CI) between each active intervention arm and the SC arm. The analysis method was the intention-to-treat. A p-value less than 0.05 was considered statistically significant. All statistical analyses were performed using SPSS (version 22.0; IBM Corp., Armonk, NY, United States) and Stata 16.1 (StataCorp., College Station, TX, United States).
3 Results
3.1 Participant characteristics
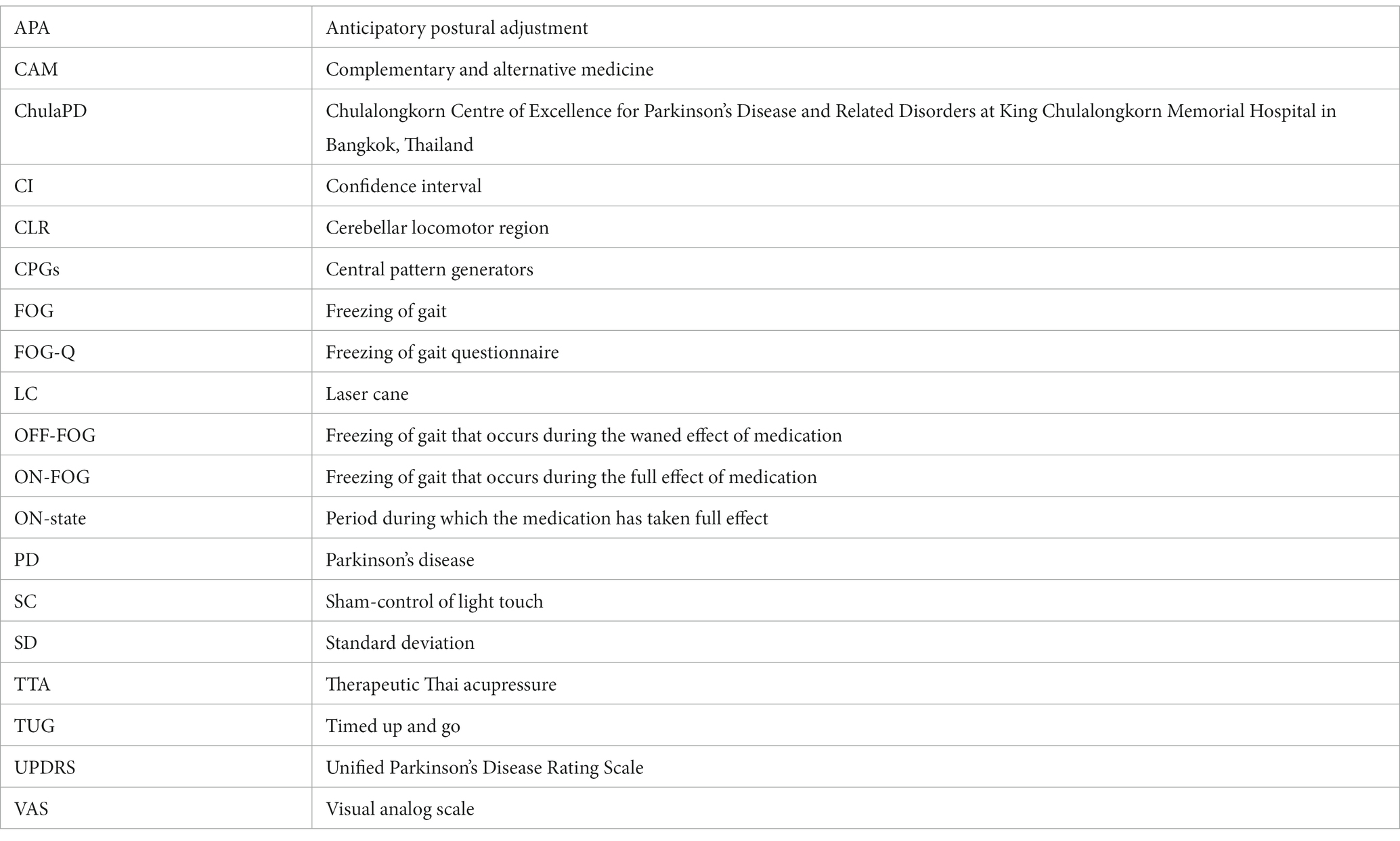
In total, 97 participants were enrolled from March 2020 to August 2021. Among them, 7 participants were either ineligible or declined participation due to time constraints. The remaining 90 patients were randomly allocated to the TTA, LC, and SC arms: 30 participants per arm. All participants complied with the protocol, as shown in Figure 1. All clinicodemographic characteristics were uniformly distributed across the study groups. Overall, 50% (45/90) of the participants were females, and the mean age was 67.5 (8.6) years. The mean UPDRS-III and FOG-Q scores were 19.2 (5.1) points and 9.4 (3.6) points, respectively. There were no significant differences among the 3 arms regarding demographic and disease-related characteristics. The participant characteristics are shown in Table 1. No adverse events occurred.
3.2 Primary non-inferiority outcome
The average stride length difference pre- and post-test between the TTA and LC arms was −0.7 cm (95% CI, −6.55 to 5.15) (p = 0.41, one-sided), satisfying the non-inferiority criteria. The 95% CI indicated a marginally better stride length improvement in the LC arm. In addition, both the TTA and LC arms showed similar improvements when compared to the SC arm, with an increase of 13.11 cm (95% CI, 7.26 to 18.96) (p < 0.001) for the TTA arm and 13.8 cm (95% CI, 7.96 to 19.65) (p < 0.001) for the LC arm. The details are presented in Table 2 and Figure 3A (33).
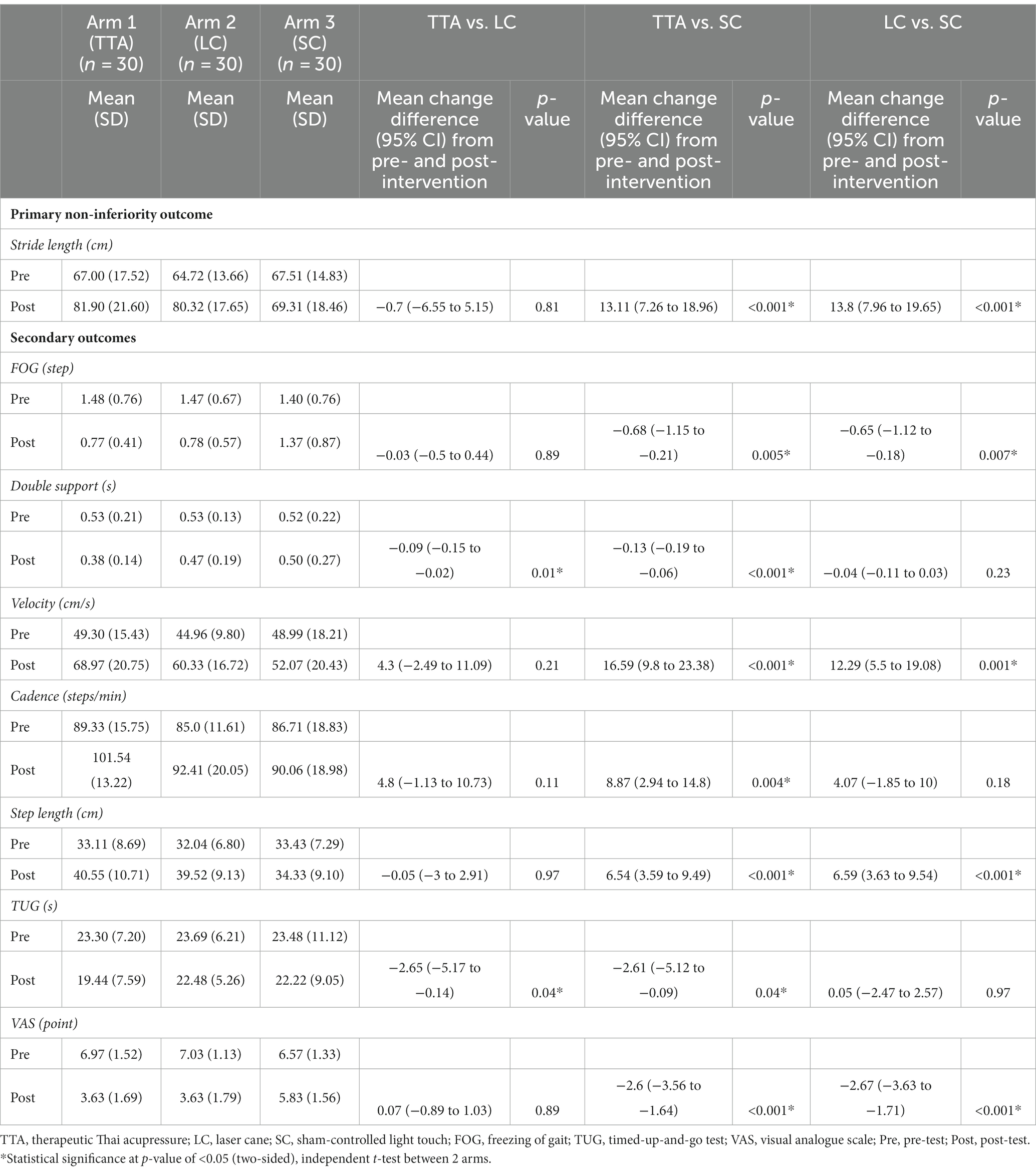
Table 2. The pre- and post-test mean and SD gait parameter estimates, and pairwise comparisons of the mean difference in change from baseline (95% CI) for TTA versus LC arms, and the TTA and LC arms versus the SC arm.
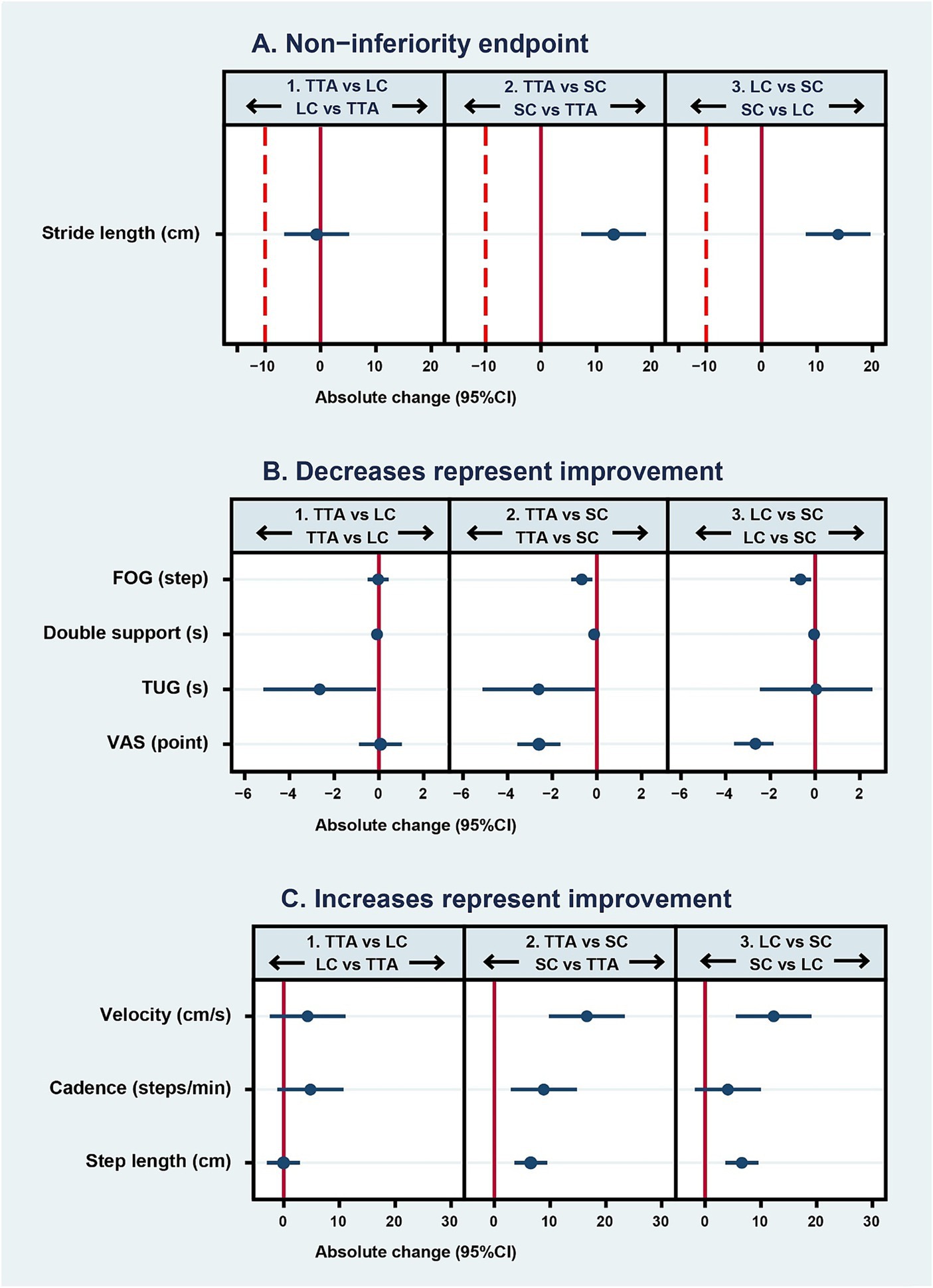
Figure 3. Mean differences (95% CI) in pre- to post-intervention parameter changes between randomized arms. In graph (A), the solid line represents the no-effect level, and the dashed line is the non-inferiority margin. In panel 1, estimates to the left of the dashed line favor the LC arm and those to the right favor the TTA arm. In panels 2 and 3, estimates to the right of the dashed line favor the TTA and LC arms, respectively. In graph (B) where decreased scores represent improvement, the solid line represents the no-effect level. In panel 1, estimates to the left of the solid line favor the TTA arm. In panels 2 and 3, estimates to the left of the solid line favor the active intervention arm (TTA and LC, respectively) over the sham comparator arm (SC). In graph (C) where increased scores represent improvement, the solid line represents the no-effect level. In panel 1, estimates to the right of the solid line favor the TTA arm over the LC arm. In panels 2 and 3, estimates to the right of the solid line favor the active intervention arm (TTA and LC, respectively) over the sham control (SC). Arrows represent the direction of effect that would be favorable for each arm. TTA, therapeutic Thai acupressure; LC, laser cane; SC, sham-control of light touch.
3.3 Secondary outcomes
In the study, researchers compared the effect of three distinct interventions: TTA, LC, and SC. The comparative effects among these arms regarding secondary outcomes were manifested differently.
3.3.1 TTA vs. LC arms
There was a marked improvement in two parameters in the TTA arm. The double support time was reduced by −0.09 s (95% CI: −0.15 to −0.02, p = 0.01), and the TUG test results decreased by −2.65 s (95% CI: −5.17 to −0.14; p = 0.04). Meanwhile, there was no significant difference between the two arms with respect to the FOG that changed by −0.03 steps (95% CI: −0.5 to 0.44; p = 0.89), velocity that changed by 4.3 cm/s (95% CI: −2.49 to 11.09; p = 0.21), cadence that increased by 4.8 steps/min (95% CI: −1.13 to 10.73; p = 0.11), step length that decreased minimally by −0.05 (95% CI: −3 to 2.91; p = 0.97), and the VAS score that increased by 0.07 points (95% CI: −0.89 to 1.03; p = 0.89). Interestingly, although there was no significant difference, the 95% CIs indicated the TTA arm had greater improvements in velocity and cadence. LC use might lead to lesser improvements in cadence, double support time, and TUG than TTA as the TUG test takes longer to complete when using a cane (Table 2 and Figures 3B,C) (43).
3.3.2 TTA, LC vs. SC arms
The improvements in the TTA arm were either comparable or superior to those in the LC arm across various parameters. Both the TTA and LC arms showed significant improvements over the SC arm in FOG, with a mean difference in pre- and post-test values of −0.68 steps (95% CI: −1.15 to −0.21; p = 0.005) for TTA and −0.65 steps (95% CI: −1.12 to −0.18; p = 0.007) for LC. Similar findings were obtained for velocity, where it was improved by 16.59 cm/s (95% CI: 9.8 to 23.38; p < 0.001) in the TTA arm and by 12.29 cm/s (95% CI: 5.5 to 19.08; p = 0.001) in the LC arm. Step length was also better in both intervention arms than in the SC arm, with the TTA arm achieving 6.54 cm (95% CI: 3.59 to 9.49; p < 0.001) and the LC arm achieving 6.59 cm (95% CI: 3.63 to 9.54; p < 0.001). Further, the VAS scores were higher in both the TTA arm by −2.6 points (95% CI: −3.56 to −1.64; p < 0.001) and the LC arm by −2.67 points (95% CI: −3.63 to −1.71; p < 0.001).
However, in the comparison between the TTA and SC arms, the TTA arm showed significantly better double support time by −0.13 s (95% CI: −0.19 to −0.06; p < 0.001), cadence by 8.87 steps/min (95% CI: 2.94 to 14.8; p = 0.004), and TUG by −2.61 s (95% CI: −5.12 to −0.09; p = 0.04). These parameters were not significantly different between the LC and SC arms (Table 2 and Figures 3B,C).
3.4 Duration of intervention efficacy
Although we did not formally evaluate the efficacy duration of the interventions, a post-intervention telephone follow-up interview provided subjective insights. The participants in the TTA and LC arms reported retained effects for around 3–6 h, whereas those in the SC arm reported persistent effects for approximately 1–3 h. Most participants in all arms reported experiencing good sleep, with the remainder sleeping as usual. No adverse events were reported.
4 Discussion
In this study, stride length, the primary non-inferiority endpoint indicating FOG, under TTA treatment, was non-inferior to that under LC treatment. The results showed that TTA improves spatial-temporal gait parameters, mobility and reduces ON-FOG to a similar magnitude or even better relative to LC versus SC. These findings are consistent with previous data and establish the potential of the proposed plantar nerve stimulation therapy and LC therapy for managing gait and FOG (14, 21–23).
It is notable that the difference in mean stride length from pre- to post-intervention between the TTA and LC arms barely reached −1 cm, with the lower limit falling above −7 cm, although some researchers might view the non-inferiority margin of −10 cm as overly generous. Additionally, most gait parameters and comfortable level during walk were comparable between the TTA and LC arms, highlighting that the efficacy of TTA is comparable to that of LC in patients with PD experiencing ON-FOG.
This study found that TTA was effective in mitigating ON-FOG. Although our research did not investigate the mechanisms by which TTA exerted its effects, we hypothesize that TTA could stimulate the degenerated mechanoreceptor response. The benefits can be attributed to the greatest thresholds of the vibratory and touch sensitivity areas brought about by cutaneous sensation. Moreover, tapping into deep sensation, TTA could improve proprioceptive deficits, which can be attributed to the activation of the Golgi tendon organs and spindle cells found in the tibialis anterior muscle. Further, by animating cutaneous and joint receptors, it could be hypothesized that TTA can potentially bridge the proprioceptive feedback from the peripheral sensory afferents to the CPGs in the central nervous system (21, 23, 44, 45). Notably, stimulating peripheral regions at the designated four acupoints is linked to increased resting-state functional connectivity (40). This enhancement is mainly observed between cerebral territories pivotal for visuomotor functionality and proprioception, evident in the sensorimotor cortex which is closely associated with anticipatory postural adjustment (APA) (40). These findings indicate that such upward activation might target the thalamus and potentially influence the cerebellar locomotor region (CLR), which is known to play a pivotal role in ON-FOG and is a cornerstone of the compensatory external pathway (3, 22, 40). The current study found that TTA has a certain effect; it augments proprioceptive functional connectivity, allowing for sustained anticipation of body movement and the positions of the body and limbs, even after the TTA is concluded. This proactive approach enriches motor movement planning (46). With improved proprioception, patients can easily combine visual and proprioceptive feedback, optimizing their visuomotor functionality. This improved proprioception, in turn, refines their ability to gauge spatio-perceptual distances accurately, thus, streamlining their gait movement planning and mobility (46, 47), possibly reducing the rigidity associated with FOG (48).
Visual stimulation techniques have been developed to manage diminished proprioception. A common method is laser light, aiding step verification, attention, and initiating a pronounced optic flow. This flow, driven by central and peripheral vision, enhances space perception and movement (15, 47, 49). Furthermore, there is an interesting interaction of visual stimulation with visuomotor cerebellocortical pathways. When activated, these pathways prevent the impaired functionality of the basal ganglia, enabling an alternate compensatory route (31, 49). TTA appears to affect similar overlapping pathways (40). Particularly, the significant correlation between heightened CLR activation and extended stride length indicates that TTA and LC can be beneficial methods for CLR activation (12). This improvement in stride length supports that external stimulation, whether from TTA or LC, might act as the key to unlocking neural pathways that command central motor functions at the non-inferiority level. This activation is pivotal for stimulating the tibialis anterior muscle and refining motor planning, which collectively enhance APA and stride length and mitigate ON-FOG (50). Therefore, TTA might be beneficial for improving proprioception and space perception, and accordingly alleviating ON-FOG. The improved proprioception might possibly alleviate the visual reliance, magnify multisensory capabilities and decelerate disease progression (15, 17–19). However, these benefits are contingent on individual patient conditions and their environments. Given this intricate interrelation, a comprehensive study into the concurrent effects of both TTA and LC is imperative.
Dopaminergic medications, while beneficial, have implications for both dopaminergic and non-dopaminergic neurotransmitter systems. Furthermore, dopaminergic medications might negatively impact proprioception (51–54). Consequently, these drugs can lead to irregular limb movements and ON-FOG manifestation (4). Such effects suggest that these drugs could contribute to FOG, muscle debilitation, balance issues, and postural instability, even in the early stages of PD (8, 10, 51, 55). Our study clarifies the capability of TTA to modify sensory deficits, especially in proprioception, among patients with ON-FOG. Additionally, TTA was observed to improve muscle strength, especially during the ON-state, in patients with PD (30). This improvement highlights the potential of TTA in counteracting ON-state symptoms. Additionally, data from our phone-based follow-up interviews indicate that subjective effects were markedly longer in both active intervention arms than in the SC arm, highlighting the efficacy of active treatments. Furthermore, most participants described comfort levels during and after treatment in both arms. Interestingly, a significant proportion of participants in all three arms reported sound sleep following the interventions. Lower sleep quality in patients with PD is related to decreased CLR and visuospatial functions and is associated with FOG and a decreased response to levodopa (56–58). Future studies could delve deeper into the sustained efficacy and psychological effects of TTA by objective measurements, other freezing symptoms such as hand movements and speech, and ON-state symptoms.
Our study operated on an open-label format, posing blinding challenges. To counter this limitation, we incorporated a sham control, mirroring the TTA arm but without pressure application, to minimize the impact of this limitation. Our approach to replicating the intervention involved a maneuver that applied light touch, which might stimulate the dorsal column and potentially boost sensory processing. However, the sensory enhancement derived from such light touch—without pressure—is subtle and different from that with TTA (40, 46). Furthermore, another potential study limitation is the demographic characteristics of our study cohort, which was exclusively enrolled from a specialized clinic within a singular tertiary referral hospital in Thailand. Expanding the research to encompass diverse populations would be the logical next step.
Taken together, TTA offers an upfront, noninvasive, and safe CAM approach to manage ON-FOG. A significant contribution of our study is the application of traditional therapeutic methods from Thai medicine, tailored to the unique physical and psychological needs of each patient at every treatment session (26, 29, 39). TTA offers patients with PD an opportunity to hone their physical self-awareness while having a therapeutic experience. In our clinic, ON-FOG management involves a multimodal approach that goes beyond dopaminergic medication and includes various non-pharmacological strategies to counter sensory deficits. This approach for ON-FOG management includes vitamin B12 and B6 treatments (59, 60), because nutritional status may be associated with FOG (61); a specialized Parkinson shoe (23); and, notably, the LC (14, 16). Overall, our data support that TTA is a promising modality that can be added to the multimodal treatment for FOG including homecare application.
5 Conclusion
TTA, as a noninvasive therapeutic approach, has comparable efficacy to LC for the treatment of ON-FOG in patients with PD. TTA improved stride length and alleviated ON-FOG by a similar magnitude to LC. This indicates that TTA might also reduce visual reliance during walking and increase life quality. Given its noninvasive nature with simple and safe techniques and potential benefits, TTA is considered a CAM option for PD treatment. However, the results should be interpreted in consideration of the study limitations. Further research is needed for a comprehensive insight into the long-term outcomes, efficacy mechanism, effective utilization strategies, and applicability in diverse patient cohorts.
Data availability statement
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Faculty of Medicine, Chulalongkorn University (IRB No. 211/62). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
YM: Conceptualization, Data curation, Formal analysis, Methodology, Writing – original draft. OP: Data curation, Formal analysis, Writing – review & editing. SK: Data curation, Formal analysis, Writing – review & editing. CA: Project administration, Resources, Writing – review & editing. HT: Visualization, Writing – review & editing. RB: Conceptualization, Funding acquisition, Methodology, Project administration, Resources, Supervision, Visualization, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was generously supported by the 90th Anniversary of Chulalongkorn University Fund under the Ratchadaphiseksomphot Endowment Fund, Batch No. 47(3/63). Additional fundings were provided by the 100th Anniversary Chulalongkorn University for Doctoral Scholarship, Thailand Science Research and Innovation for Senior Research Scholar (RTA6280016), and Chulalongkorn University Centre of Excellence Grant for Centre of Excellence for Parkinson’s Disease and Related Disorders (GCE 3300160003).
Acknowledgments
The authors would like to thank all patients who participated in this study and the fundings of Chulalongkorn University.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Nutt, JG, Bloem, BR, Giladi, N, Hallett, M, Horak, FB, and Nieuwboer, A. Freezing of gait: moving forward on a mysterious clinical phenomenon. Lancet Neurol. (2011) 10:734–44. doi: 10.1016/s1474-4422(11)70143-0
2. Forsaa, EB, Larsen, JP, Wentzel-Larsen, T, and Alves, G. A 12-year population-based study of freezing of gait in Parkinson’s disease. Parkinsonism Relat Disord. (2015) 21:254–8. doi: 10.1016/j.parkreldis.2014.12.020
3. Mei, S, Li, J, Middlebrooks, EH, Almeida, L, Hu, W, Zhang, Y, et al. New onset on-medication freezing of gait after STN-DBS in Parkinson’s disease. Front Neurol. (2019) 10:659. doi: 10.3389/fneur.2019.00659
4. Espay, AJ, Fasano, A, van Nuenen, BF, Payne, MM, Snijders, AH, and Bloem, BR. “On” state freezing of gait in Parkinson disease: a paradoxical levodopa-induced complication. Neurology. (2012) 78:454–7. doi: 10.1212/WNL.0b013e3182477ec0
5. Shine, JM, Naismith, SL, and Lewis, SJ. The pathophysiological mechanisms underlying freezing of gait in Parkinson’s disease. J Clin Neurosci. (2011) 18:1154–7. doi: 10.1016/j.jocn.2011.02.007
6. Ehgoetz Martens, KA, Pieruccini-Faria, F, and Almeida, QJ. Could sensory mechanisms be a core factor that underlies freezing of gait in Parkinson’s disease? PLoS One. (2013) 8:e62602. doi: 10.1371/journal.pone.0062602
7. Nolano, M, Provitera, V, Estraneo, A, Selim, MM, Caporaso, G, Stancanelli, A, et al. Sensory deficit in Parkinson’s disease: evidence of a cutaneous denervation. Brain. (2008) 131:1903–11. doi: 10.1093/brain/awn102
8. Tan, T, Almeida, QJ, and Rahimi, F. Proprioceptive deficits in Parkinson’s disease patients with freezing of gait. Neuroscience. (2011) 192:746–52. doi: 10.1016/j.neuroscience.2011.06.071
9. Cowie, D, Limousin, P, Peters, A, and Day, BL. Insights into the neural control of locomotion from walking through doorways in Parkinson’s disease. Neuropsychologia. (2010) 48:2750–7. doi: 10.1016/j.neuropsychologia.2010.05.022
10. Martens, KAE, Ellard, CG, and Almeida, QJ. Dopaminergic medication amplifies sensory integration deficits during static balance in Parkinson’s patients with freezing of gait. BAOJ Neurol. (2016) 2:021. doi: 10.24947/baojn/2/3/122
11. Heremans, E, Nieuwboer, A, and Vercruysse, S. Freezing of gait in Parkinson’s disease: where are we now? Curr Neurol Neurosci Rep. (2013) 13:350. doi: 10.1007/s11910-013-0350-7
12. Vieira-Yano, B, Martini, DN, Horak, FB, de Lima-Pardini, A, Almeida, F, Santana, VP, et al. The adapted resistance training with instability randomized controlled trial for gait automaticity. Mov Disord. (2021) 36:152–63. doi: 10.1002/mds.28298
13. Cosentino, C, Putzolu, M, Mezzarobba, S, Cecchella, M, Innocenti, T, Bonassi, G, et al. One cue does not fit all: a systematic review with meta-analysis of the effectiveness of cueing on freezing of gait in Parkinson’s disease. Neurosci Biobehav Rev. (2023) 150:105189. doi: 10.1016/j.neubiorev.2023.105189
14. Buated, W, Sriyudthsak, M, Sribunruangrit, N, and Bhidayasiri, R. A low-cost intervention for improving gait in Parkinson’s disease patients: a cane providing visual cues. Eur Geriatr Med. (2012) 3:126–30. doi: 10.1016/j.eurger.2012.01.006
15. Azulay, JP, Mesure, S, and Blin, O. Influence of visual cues on gait in Parkinson’s disease: contribution to attention or sensory dependence? J Neurol Sci. (2006) 248:192–5. doi: 10.1016/j.jns.2006.05.008
16. Thai Red Cross Society . Handover of royal laser pointer canes to royal Thai air force for distribution to patients with Parkinson’s and those with walking disabilities (Thai Red Cross Society News) : Thai Red Cross Society (2021). Avairable at: https://english.redcross.or.th/news/medical-and-health-care-services/5838/ (Accessed: 5 December 2022)).
17. Yakubovich, S, Israeli-Korn, S, Halperin, O, Yahalom, G, Hassin-Baer, S, and Zaidel, A. Visual self-motion cues are impaired yet overweighted during visual-vestibular integration in Parkinson’s disease. Brain Commun. (2020) 2:fcaa035. doi: 10.1093/braincomms/fcaa035
18. Halperin, O, Israeli-Korn, S, Yakubovich, S, Hassin-Baer, S, and Zaidel, A. Self-motion perception in Parkinson’s disease. Eur J Neurosci. (2021) 53:2376–87. doi: 10.1111/ejn.14716
19. Huh, YE, Hwang, S, Kim, K, Chung, WH, Youn, J, and Cho, JW. Postural sensory correlates of freezing of gait in Parkinson’s disease. Parkinsonism Relat Disord. (2016) 25:72–7. doi: 10.1016/j.parkreldis.2016.02.004
20. Mazzetta, I, Zampogna, A, Suppa, A, Gumiero, A, Pessione, M, and Irrera, F. Wearable sensors system for an improved analysis of freezing of gait in Parkinson’s disease using electromyography and inertial signals. Sensors. (2019) 19:948. doi: 10.3390/s19040948
21. Kleiner, AFR, Souza Pagnussat, A, Pinto, C, Redivo Marchese, R, Salazar, AP, and Galli, M. Automated mechanical peripheral stimulation effects on gait variability in individuals with Parkinson disease and freezing of gait: a double-blind, randomized controlled trial. Arch Phys Med Rehabil. (2018) 99:2420–9. doi: 10.1016/j.apmr.2018.05.009
22. Pinto, C, Pagnussat, AS, Rozin Kleiner, AF, Marchese, RR, Salazar, AP, Rieder, CRM, et al. Automated mechanical peripheral stimulation improves gait parameters in subjects with Parkinson disease and freezing of gait: a randomized clinical trial. Am J Phys Med Rehabil. (2018) 97:383–9. doi: 10.1097/phm.0000000000000890
23. Phuenpathom, W, Panyakaew, P, Vateekul, P, Surangsrirat, D, Hiransuthikul, A, and Bhidayasiri, R. Vibratory and plantar pressure stimulation: steps to improve freezing of gait in Parkinson’s disease. Parkinsonism Relat Disord. (2022) 105:43–51. doi: 10.1016/j.parkreldis.2022.10.024
24. Han, L, Xie, YH, Wu, R, Chen, C, Zhang, Y, and Wang, XP. Traditional Chinese medicine for modern treatment of Parkinson’s disease. Chin J Integr Med. (2017) 23:635–40. doi: 10.1007/s11655-016-2537-7
25. World Health Organization . Benchmark for training in Nuad Thai. Geneva: WHO Press (2010). 36 p.
26. Tangtrongchit, P. Knowledge of Thai massage (in Thai and in English). Bangkok: Wat Pho Thai Traditional Medical School and Chetawan Health School (2004). 100 p.
27. Laohapand, T, and Jaturatamrong, U. Thai traditional medicine in the Faculty of Medicine Siriraj Hospital. Bangkok: Supavanich Press (2014). 109 p.
28. Chatchawan, U, Eungpinichpong, W, Plandee, P, and Yamauchi, J. Effects of Thai foot massage on balance performance in diabetic patients with peripheral neuropathy: a randomized parallel-controlled trial. Med Sci Monit Basic Res. (2015) 21:68–75. doi: 10.12659/msmbr.894163
29. Chatchawan, U, Eungpinichpong, W, Sooktho, S, Tiamkao, S, and Yamauchi, J. Effects of Thai traditional massage on pressure pain threshold and headache intensity in patients with chronic tension-type and migraine headaches. J Altern Complement Med. (2014) 20:486–92. doi: 10.1089/acm.2013.0176
30. Miyahara, Y, Jitkritsadakul, O, Sringean, J, Aungkab, N, Khongprasert, S, and Bhidayasiri, R. Can therapeutic Thai massage improve upper limb muscle strength in Parkinson’s disease? An objective randomized-controlled trial. J Tradit Complement Med. (2018) 8:261–6. doi: 10.1016/j.jtcme.2018.01.004
31. Kompoliti, K, Goetz, CG, Leurgans, S, Morrissey, M, and Siegel, IM. “On” freezing in Parkinson’s disease: resistance to visual cue walking devices. Mov Disord. (2000) 15:309–12. doi: 10.1002/1531-8257(200003)15:2<309::aid-mds1016>3.0.co;2-p
32. Kremens, DE, and Gilbart, J. Use of the unified Parkinson’s disease rating scale activities of daily living subscale to assess response to Rasagiline in early Parkinson’s disease. US Neurol. (2011) 7:91. doi: 10.17925/USN.2011.07.02.91
33. Piaggio, G, Elbourne, DR, Pocock, SJ, Evans, SJ, and Altman, DG. Reporting of noninferiority and equivalence randomized trials: extension of the CONSORT 2010 statement. J Am Med Assoc. (2012) 308:2594–604. doi: 10.1001/jama.2012.87802
34. Goetz, CG, Poewe, W, Rascol, O, Sampaio, C, Stebbins, GT, Counsell, C, et al. Movement Disorder Society Task Force report on the Hoehn and Yahr staging scale: status and recommendations. Mov Disord. (2004) 19:1020–8. doi: 10.1002/mds.20213
35. Giladi, N, Tal, J, Azulay, T, Rascol, O, Brooks, DJ, Melamed, E, et al. Validation of the freezing of gait questionnaire in patients with Parkinson’s disease. Mov Disord. (2009) 24:655–61. doi: 10.1002/mds.21745
36. Panyakaew, P, and Bhidayasiri, R. The spectrum of preclinical gait disorders in early Parkinson’s disease: subclinical gait abnormalities and compensatory mechanisms revealed with dual tasking. J Neural Transm. (2013) 120:1665–72. doi: 10.1007/s00702-013-1051-8
37. Morris, S, Morris, ME, and Iansek, R. Reliability of measurements obtained with the timed “up & go” test in people with Parkinson disease. Phys Ther. (2001) 81:810–8. doi: 10.1093/ptj/81.2.810
38. Boonstra, AM, Schiphorst Preuper, HR, Reneman, MF, Posthumus, JB, and Stewart, RE. Reliability and validity of the visual analogue scale for disability in patients with chronic musculoskeletal pain. Int J Rehabil Res. (2008) 31:165–9. doi: 10.1097/MRR.0b013e3282fc0f93
39. Buttagat, V, Eungpinichpong, W, Chatchawan, U, and Arayawichanon, P. Therapeutic effects of traditional Thai massage on pain, muscle tension and anxiety in patients with scapulocostal syndrome: a randomized single-blinded pilot study. J Bodyw Mov Ther. (2012) 16:57–63. doi: 10.1016/j.jbmt.2011.04.005
40. Quattrocchi, CC, de Pandis, MF, Piervincenzi, C, Galli, M, Melgari, JM, Salomone, G, et al. Acute modulation of brain connectivity in Parkinson disease after automatic mechanical peripheral stimulation: a pilot study. PLoS One. (2015) 10:e0137977. doi: 10.1371/journal.pone.0137977
41. Cowie, D, Limousin, P, Peters, A, Hariz, M, and Day, BL. Doorway-provoked freezing of gait in Parkinson’s disease. Mov Disord. (2012) 27:492–9. doi: 10.1002/mds.23990
42. Julious, SA. Sample sizes for clinical trials with normal data. Stat Med. (2004) 23:1921–86. doi: 10.1002/sim.1783
43. Fudickar, S, Kiselev, J, Frenken, T, Wegel, S, Dimitrowska, S, Steinhagen-Thiessen, E, et al. Validation of the ambient tug chair with light barriers and force sensors in a clinical trial. Assist Technol. (2020) 32:1–8. doi: 10.1080/10400435.2018.1446195
44. Witzel, T, Napadow, V, Kettner, NW, Vangel, MG, Hamalainen, MS, and Dhond, RP. Differences in cortical response to acupressure and electroacupuncture stimuli. BMC Neurosci. (2011) 12:73. doi: 10.1186/1471-2202-12-73
45. Mehta, P, Dhapte, V, Kadam, S, and Dhapte, V. Contemporary acupressure therapy: adroit cure for painless recovery of therapeutic ailments. J Tradit Complement Med. (2016) 7:251–63. doi: 10.1016/j.jtcme.2016.06.004
46. Marasco, PD, and de Nooij, JC. Proprioception: a new era set in motion by emerging genetic and bionic strategies? Annu Rev Physiol. (2023) 85:1–24. doi: 10.1146/annurev-physiol-040122-081302
47. Beck, EN, Ehgoetz Martens, KA, and Almeida, QJ. Freezing of gait in Parkinson’s disease: an overload problem? PLoS One. (2015) 10:e0144986. doi: 10.1371/journal.pone.0144986
48. Taximaimaiti, R, and Wang, XP. Comparing the clinical and neuropsychological characteristics of Parkinson’s disease with and without freezing of gait. Front Neurosci. (2021) 15:660340. doi: 10.3389/fnins.2021.660340
49. Ferrarin, M, Rabuffetti, M, Tettamanti, M, Pignatti, R, Mauro, A, and Albani, G. Effect of optical flow versus attentional strategy on gait in Parkinson’s disease: a study with a portable optical stimulating device. J Neuroeng Rehabil. (2008) 5:3. doi: 10.1186/1743-0003-5-3
50. Marquez, JS, Hasan, SMS, Siddiquee, MR, Luca, CC, Mishra, VR, Mari, Z, et al. Neural correlates of freezing of gait in Parkinson’s disease: an electrophysiology mini-review. Front Neurol. (2020) 11:571086. doi: 10.3389/fneur.2020.571086
51. O’Suilleabhain, P, Bullard, J, and Dewey, RB. Proprioception in Parkinson’s disease is acutely depressed by dopaminergic medications. J Neurol Neurosurg Psychiatry. (2001) 71:607–10. doi: 10.1136/jnnp.71.5.607
52. Konczak, J, Corcos, DM, Horak, F, Poizner, H, Shapiro, M, Tuite, P, et al. Proprioception and motor control in Parkinson’s disease. J Mot Behav. (2009) 41:543–52. doi: 10.3200/35-09-002
53. Vinding, MC, Tsitsi, P, Piitulainen, H, Waldthaler, J, Jousmäki, V, Ingvar, M, et al. Attenuated Beta rebound to proprioceptive afferent feedback in Parkinson’s disease. Sci Rep. (2019) 9:2604. doi: 10.1038/s41598-019-39204-3
54. Haas, CT, Buhlmann, A, Turbanski, S, and Schmidtbleicher, D. Proprioceptive and sensorimotor performance in Parkinson’s disease. Res Sports Med. (2006) 14:273–87. doi: 10.1080/15438620600985902
55. Hwang, S, Agada, P, Grill, S, Kiemel, T, and Jeka, JJ. A central processing sensory deficit with Parkinson’s disease. Exp Brain Res. (2016) 234:2369–79. doi: 10.1007/s00221-016-4642-4
56. Liu, J, Shuai, G, Fang, W, Zhu, Y, Chen, H, Wang, Y, et al. Altered regional homogeneity and connectivity in cerebellum and visual-motor relevant cortex in Parkinson’s disease with rapid eye movement sleep behavior disorder. Sleep Med. (2021) 82:125–33. doi: 10.1016/j.sleep.2021.03.041
57. de Almeida, FO, Ugrinowitsch, C, Brito, LC, Milliato, A, Marquesini, R, Moreira-Neto, A, et al. Poor sleep quality is associated with cognitive, mobility, and anxiety disability that underlie freezing of gait in Parkinson’s disease. Gait Posture. (2021) 85:157–63. doi: 10.1016/j.gaitpost.2021.01.026
58. Nobleza, CMN, Siddiqui, M, Shah, PV, Balani, P, Lopez, AR, and Khan, S. The relationship of rapid eye movement sleep behavior disorder and freezing of gait in Parkinson’s disease. Cureus. (2020) 12:e12385. doi: 10.7759/cureus.12385
59. Phokaewvarangkul, O, Bhidayasiri, R, Garcia-Ruiz, P, Odin, P, Riederer, P, and Müller, T. Homocysteine, vitamin B metabolites, dopamine-substituting compounds, and symptomatology in Parkinson’s disease: clinical and therapeutic considerations. J Neural Transm. (2023) 130:1451–62. doi: 10.1007/s00702-023-02684-9
60. Qiu, F, Wu, Y, Cao, H, Liu, B, Du, M, Jiang, H, et al. Changes of peripheral nerve function and vitamin B12 level in people with Parkinson’s disease. Front Neurol. (2020) 11:549159. doi: 10.3389/fneur.2020.549159
61. Zhang, LL, Zhang, L, Dong, J, Zhao, Y, and Wang, XP. Factors contributing to malnutrition in Parkinson’s disease patients with freezing of gait. Front Neurol. (2022) 13:816315. doi: 10.3389/fneur.2022.816315
Glossary
Keywords: Parkinson’s disease, neurologic gait disorders, freezing of gait, quality of life, cues, acupressure, proprioception
Citation: Miyahara Y, Phokaewvarangkul O, Kerr S, Anan C, Toriumi H and Bhidayasiri R (2024) Comparing the efficacy of therapeutic Thai acupressure on plantar acupoints and laser cane therapy on freezing of gait in Parkinson’s disease: a randomized non-inferiority trial. Front. Neurol. 15:1327448. doi: 10.3389/fneur.2024.1327448
Edited by:
Mario Bernardo-Filho, Rio de Janeiro State University, BrazilReviewed by:
Shyang Chang, National Tsing Hua University, TaiwanXiao-Ping Wang, Shanghai Jiao Tong University School of Medicine, China
Copyright © 2024 Miyahara, Phokaewvarangkul, Kerr, Anan, Toriumi and Bhidayasiri. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Roongroj Bhidayasiri, cmJoQGNodWxhcGQub3Jn
 Yuka Miyahara
Yuka Miyahara Onanong Phokaewvarangkul
Onanong Phokaewvarangkul Stephen Kerr
Stephen Kerr Chanawat Anan
Chanawat Anan Haruki Toriumi
Haruki Toriumi Roongroj Bhidayasiri
Roongroj Bhidayasiri