- 1Shanxi Bethune Hospital, Shanxi Academy of Medical Sciences, Tongji Shanxi Hospital, Third Hospital of Shanxi Medical University, Taiyuan, China
- 2Shanxi Provincial People's Hospital, Taiyuan, China
Background: Hospital-acquired pneumonia (HBP) is a common and serious infections disease that affects the patients with traumatic brain injury (TBI). Severe pneumonia can lead to high mortality and morbidity in TBI patients. Therefore, it is important to investigate the risk factors and develop a prediction model for HBP following TBI.
Methods: The clinical data of 285 patients with TBI, admitted to Shanxi Bethune Hospital and Shanxi Provincial People’s Hospital, were collected. Patients were divided into two groups based on the presence or absence of pneumonia. Risk factors for HBP were identified, a predictive model was constructed, and its performance was validated.
Results: Significant differences were observed between the pneumonia and non-pneumonia groups regarding several factors, including age, history of diabetes, smoking history, white blood cell count, platelet count, albumin levels, Glasgow Coma Scale (GCS) score upon admission, thoracic trauma, craniocerebral surgery, and the need for tracheal intubation post-admission (p < 0.05). Among these, age, smoking history, thoracic trauma, white blood cell count, albumin levels, and admission GCS score were identified as independent risk factors for HBP following TBI. The predictive model based on these six factors demonstrated high accuracy.
Conclusion: Age, smoking history, thoracic trauma, white blood cell count, albumin levels, and admission GCS score are independent risk factors for HBP after TBI. The predictive model developed based on these factors shows strong predictive accuracy and clinical utility.
Introduction
Traumatic brain injury (TBI) refers to brain damage caused by trauma, car accidents, sports injuries, etc., and is a major global health problem (1). Over the past decade, the number of deaths resulting from TBI continues to rise significantly (2–4). Severe TBIs, such as brain contusions and diffuse axonal injuries, greatly impair brain function, often leading to late-onset bleeding, altered consciousness, and physical disabilities. These conditions are frequently accompanied by complications such as dysphagia, vomiting, impaired cough reflex, and an inability to clear oral secretions, all of which significantly increase the risk of aspiration and subsequent pneumonia (5). Recent studies have demonstrated that patients with TBI exhibit increased blood glucose variability, likely due to the traumatic stress response. This elevated blood glucose level is closely associated with a higher incidence of lung infections, contributing to increase in-hospital mortality and being positively correlated with poor clinical outcomes (6). In addition, studies have shown that the incidence of pneumonia in TBI patients can exceed 60%, making it an independent risk factor for poor prognosis (7). Severe pneumonia has even become one of the leading causes of death in TBI patients, not only affecting patient outcomes but also imposing a substantial financial burden on families (8, 9). Effective management of TBI requires not only treating the injury itself but also addressing complications, particularly pneumonia. Early identification of patients at high risk for pneumonia is therefore crucial in the care of TBI patients.
To comprehensively evaluate the risk factors for pneumonia following TBI and develop an early prediction model, we collected clinical data from 285 TBI patients treated at Shanxi Bethune Hospital and Shanxi Provincial People’s Hospital between January 2020 and May 2022. The aim of this study was to improve secondary prevention and management of hospital-acquired pneumonia (HBP) in patients with TBI.
Methods
Study design and population
A retrospective study was conducted on TBI patients admitted to Shanxi Bethune Hospital and Shanxi Provincial People’s Hospital between January 2020 and May 2022. The inclusion criteria were: (1) a confirmed history of trauma at admission; (2) brain imaging on admission showing traumatic changes such as brain contusion, laceration, skull fracture, or intracranial hematoma; (3) patients aged over 18 years. The exclusion criteria were: (1) unstable vital signs upon admission; (2) presence of serious underlying diseases that preclude the patient from tolerating trauma or surgery; (3) TBI caused by other primary neurological conditions, such as hypertensive cerebral hemorrhage; (4) pneumonia diagnosed at admission (including aspiration pneumonia, bacterial pneumonia, viral pneumonia, COVID-19, etc.); (5) refusal by the patient or their family to cooperate with treatment; (6) presence of other factors that may affect the study’s results.
A total of 285 TBI patients were included in this study, following the approval of more than three years of experience by neurosurgeons and the review by one neurosurgical director. The study protocol was approved by the Ethics Committee of Shanxi Bethune Hospital (YXLL-2022-124).
Data collection
Data collection for all enrolled patients was conducted by neurosurgery specialists and specialist nurse. General information gathered included gender, age, height, weight, smoking history, drinking history, and medical history. Clinical data comprised the Glasgow Coma Scale (GCS) score at admission, presence of chest trauma, tracheal intubation status, imaging examination results, routine blood tests, coagulation function, liver and kidney function, blood electrolytes, blood glucose levels, and serum albumin levels. All laboratory test results were obtained within 24 h of patient admission.
Diagnosis and case grouping of pneumonia
The diagnosis of pneumonia was made according to the Chinese guidelines for the diagnosis and treatment of HBP in adults and ventilator-associated pneumonia (2018 edition) (10). Pneumonia was diagnosed based on chest X-ray or CT findings showing new or progressive infiltrative shadows, consolidation, or ground-glass opacities, combined with two or more of the following three clinical symptoms: (1) fever with a temperature > 38°C; (2) purulent airway secretions; (3) peripheral blood leukocyte count > 10 × 109/L or < 4 × 109/L. Additionally, patients suspected of having ventilator-associated pneumonia (except for those who required ventilator support during surgery and anesthesia) and those diagnosed with pneumonia at admission (such as COVID-19 or other viral pneumonia) were excluded from the study.
It is important to note that this study focuses on early-onset pneumonia in TBI patients, and all risk factors were collected within the first 24 h of patient admission. Therefore, only patients diagnosed with pneumonia within 5 days of admission were included in the study. Pneumonia occurring after 5 days was considered to have missed the opportunity for early intervention, and these patients were excluded from the analysis. Pneumonia diagnoses were confirmed by two neurosurgery specialists following these criteria. In cases where diagnostic difficulties or disagreements arose, consultations were conducted with respiratory or critical care physicians with senior titles to resolve the issues and reach a final diagnosis. Ultimately, of the 285 TBI patients included in the study, 138 were diagnosed with pneumonia, while 147 were confirmed to be pneumonia-free.
Predictor variables
Refer to Table 1 for details on the indicators observed in the study. All measurements were taken on the first day after admission. Chest trauma primarily included rib fractures, hemopneumothorax, pulmonary contusion, and laceration. Chronic lung diseases encompassed conditions such as chronic bronchitis, interstitial pneumonia, and chronic obstructive pulmonary disease (COPD). Tracheal intubation and craniocerebral surgeries performed within the first 24 h of admission were also recorded.
Statistical analysis
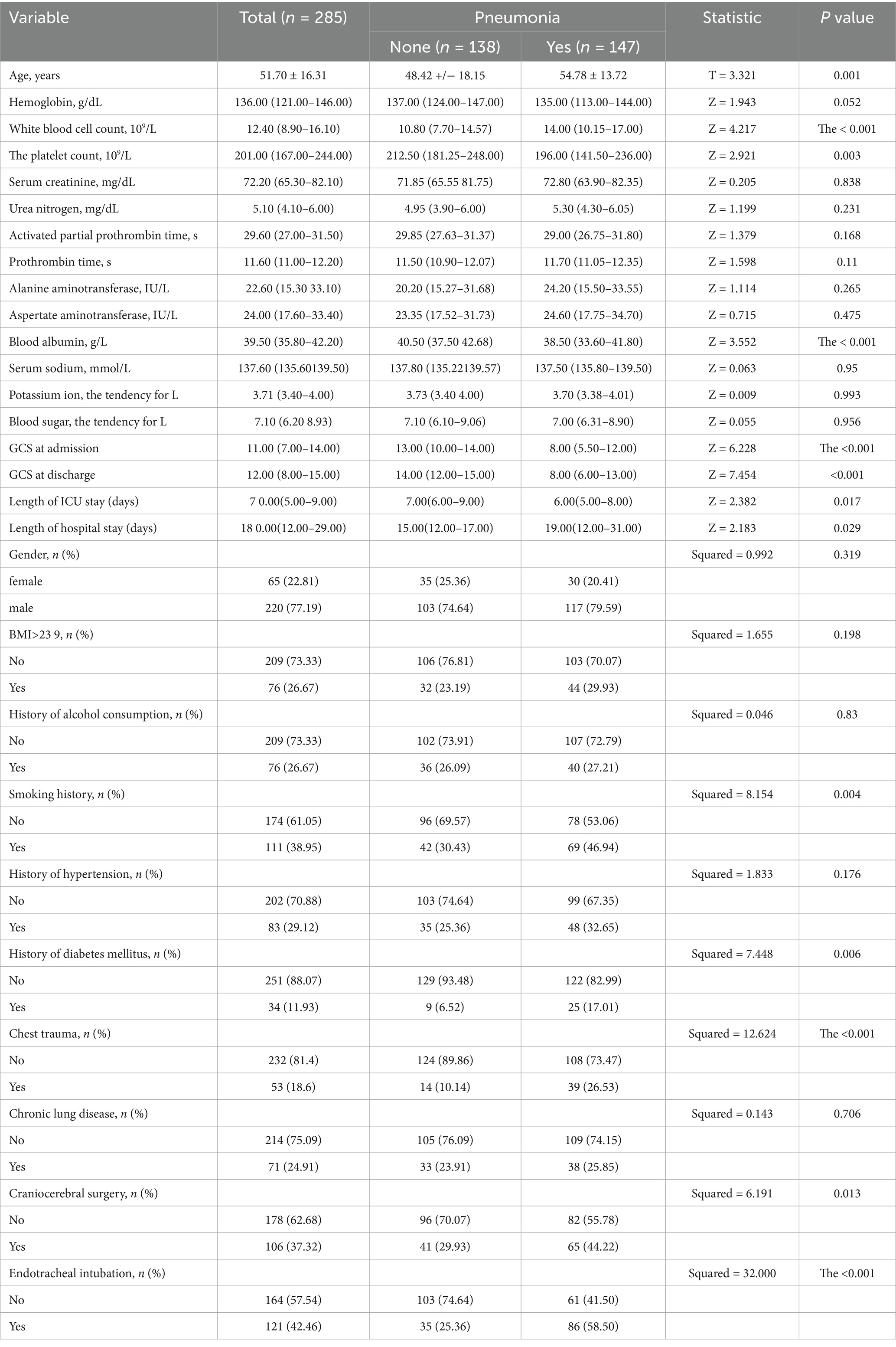
Data processing and analysis were performed using SPSS 26.0 statistical software. Categorical data were expressed as relative frequencies (%) or rates (%), and the chi-square test was used for comparisons. Normally distributed continuous data were expressed as mean ± standard deviation (x ± s), with group comparisons performed using the t-test. Non-normally distributed data were presented as median and interquartile range [M (P25, P75)], and group differences were analyzed using the Mann–Whitney U test.
Logistic regression and prediction model construction
Logistic regression was conducted to analyze the risk factors for pneumonia in TBI patients, with pneumonia status (presence or absence) as the dependent variable and the statistically significant factors as independent variables. The p value < 0.05 was considered statistically significant. Multicollinearity between variables was assessed using the variance inflation factor (VIF), with VIF values > 10 indicating the presence of multicollinearity. To assess the predictive value of independent and combined risk factors for pneumonia, receiver operating characteristic (ROC) curves were drawn. A nomogram was subsequently constructed using R software to visually represent the prediction model.
Results
Characteristics of the study population
Significant differences were observed between the two groups in terms of age, white blood cell count, platelet count, serum albumin levels, GCS score, smoking history, diabetes history, chest trauma, craniocerebral surgery, and endotracheal intubation. No significant differences were found for the remaining variables. Additionally, we observed that the median GCS of TBI patients who did not develop pneumonia increased from 13 at admission to 14 at discharge, whereas the median GCS of patients who developed pneumonia remained at 8 both at admission and discharge. Furthermore, TBI patients who did not develop pneumonia had shorter ICU stays and overall hospital stays compared to those who developed pneumonia (p < 0.05). These findings demonstrate the adverse impact of pneumonia on the prognosis of TBI patients. Detailed baseline data are provided in Table 1.
Risk factors analysis results
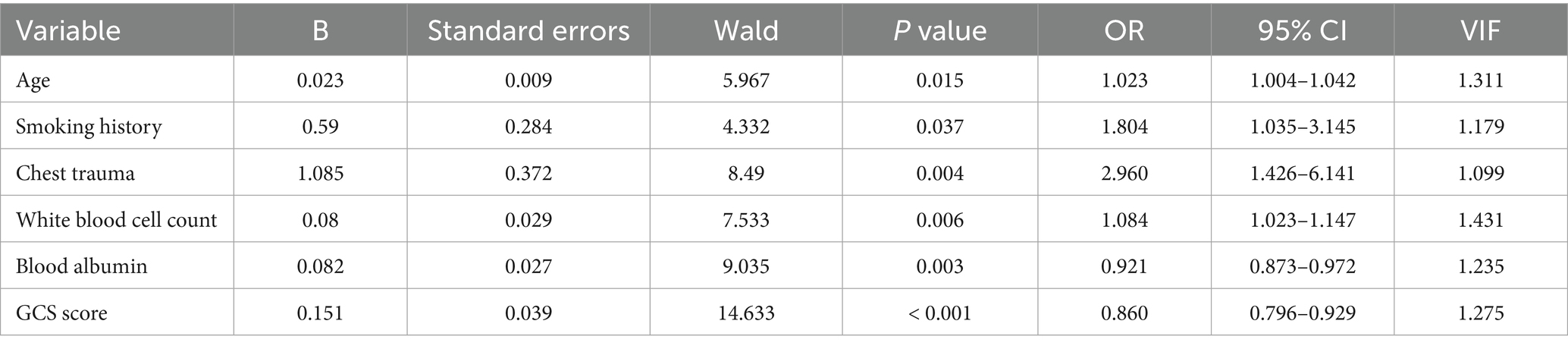
Multivariable logistic regression analysis was conducted to explore the risk factors for pneumonia, with pneumonia as the dependent variable and the factors showing statistically significant differences as independent variables. Due to the large number of independent variables, a two-way stepwise regression approach was employed. Ultimately, six factors of age, smoking history, chest trauma, white blood cell count, serum albumin levels, and GCS score were identified as independent risk factors for the development of pneumonia in TBI patients (Table 2). There is no multicollinearity between the variables (VIF < 10).
Prediction model construction
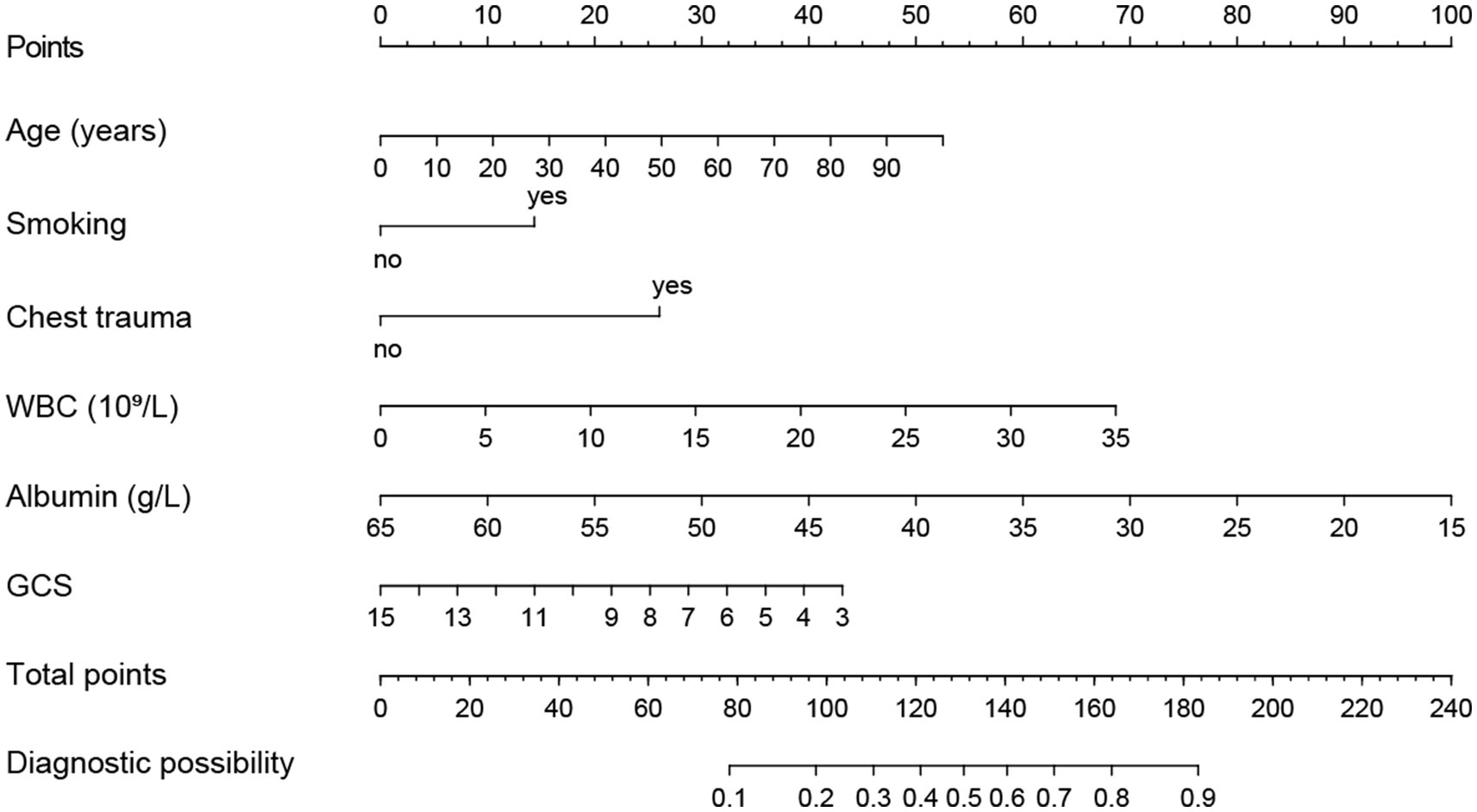
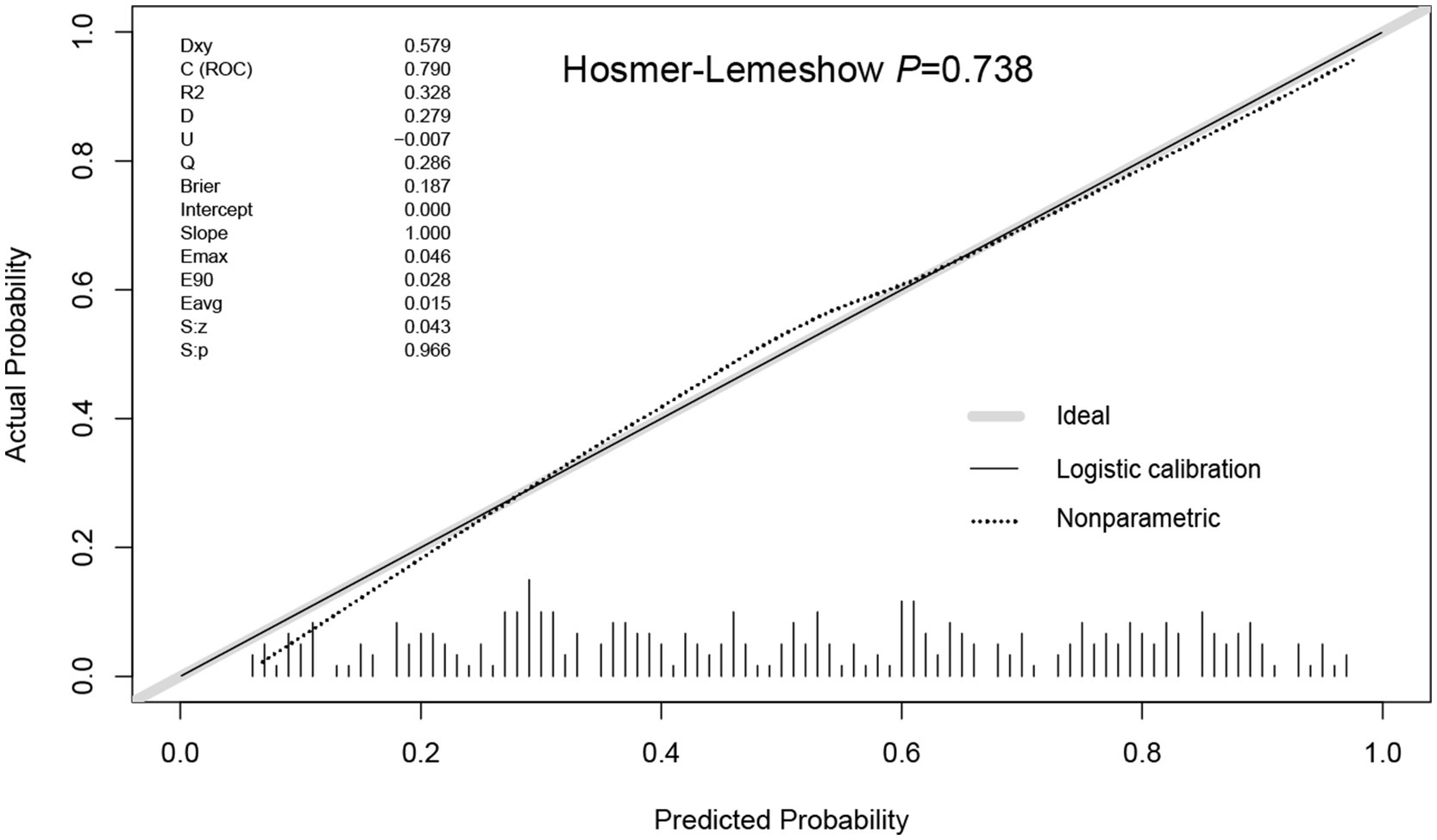
Using R software, we analyzed factors including age, smoking history, chest trauma, white blood cell count, blood albumin levels, and GCS score to develop a nomogram as a predictive model for pneumonia associated with TBI, as illustrated in Figure 1. The performance of the nomogram was tested using ROC curve, calibration curve, and DCA. The AUC of the nomogram was 0.790, which is higher than that of individual factors (AUC = 0.602, 0.583, 0.582, 0.622, 0.713, 0.645, 0.790; Figure 2), indicating a high discriminatory ability. The calibration curve closely followed the standard curve, and the p-value from the Hosmer-Lemeshow test was 0.738 (Figure 3), which indicates good calibration of the nomogram. The DCA showed that using the nomogram resulted in better clinical decision-making efficiency compared to using individual factors. Furthermore, the nomogram demonstrated good clinical decision-making efficiency across different risk thresholds (Figure 4).
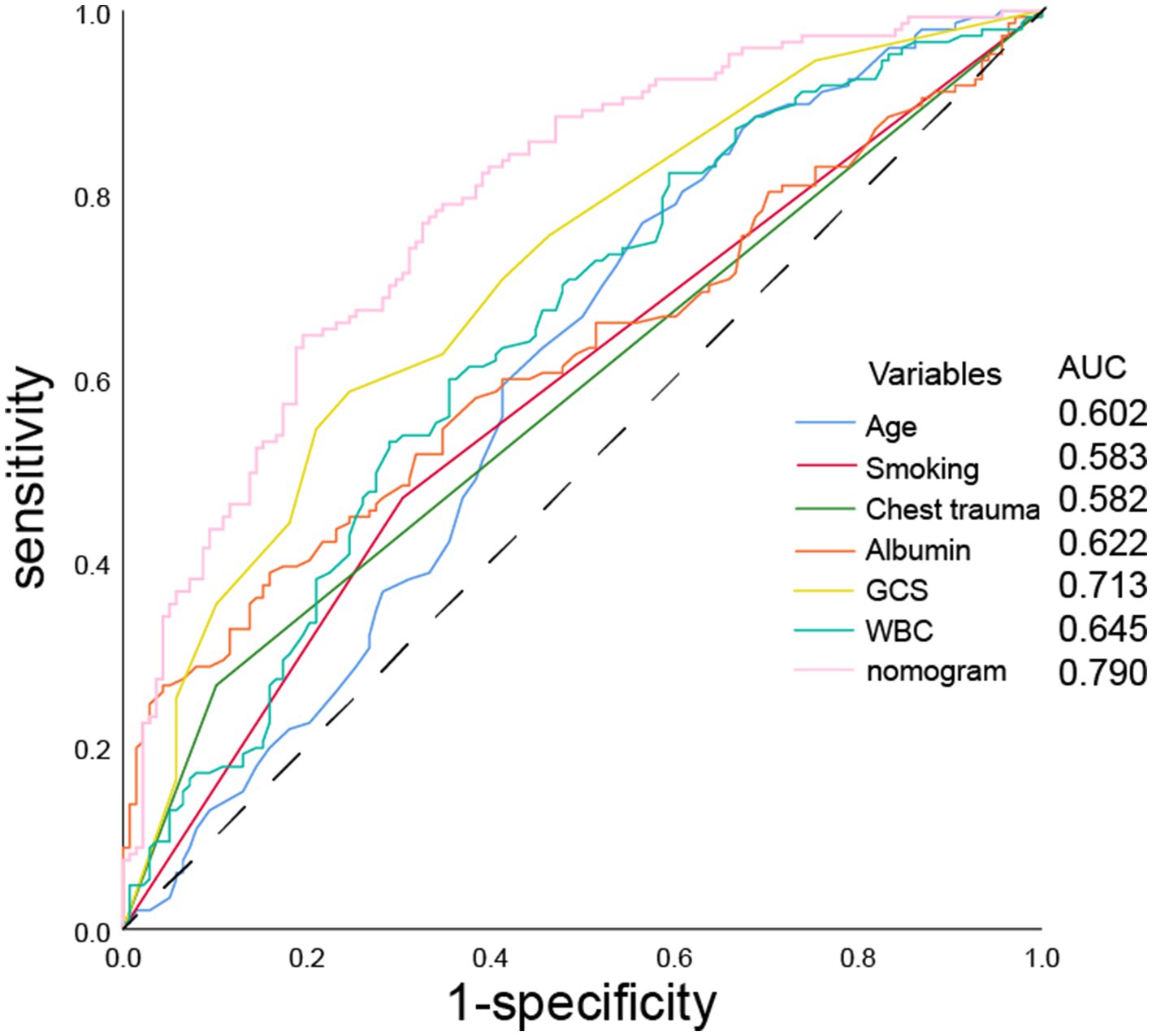
Figure 2. ROC curves of age, smoking history, chest trauma, white blood cell count, serum albumin, GCS score, and nomogram for predicting the incidence of pneumonia.
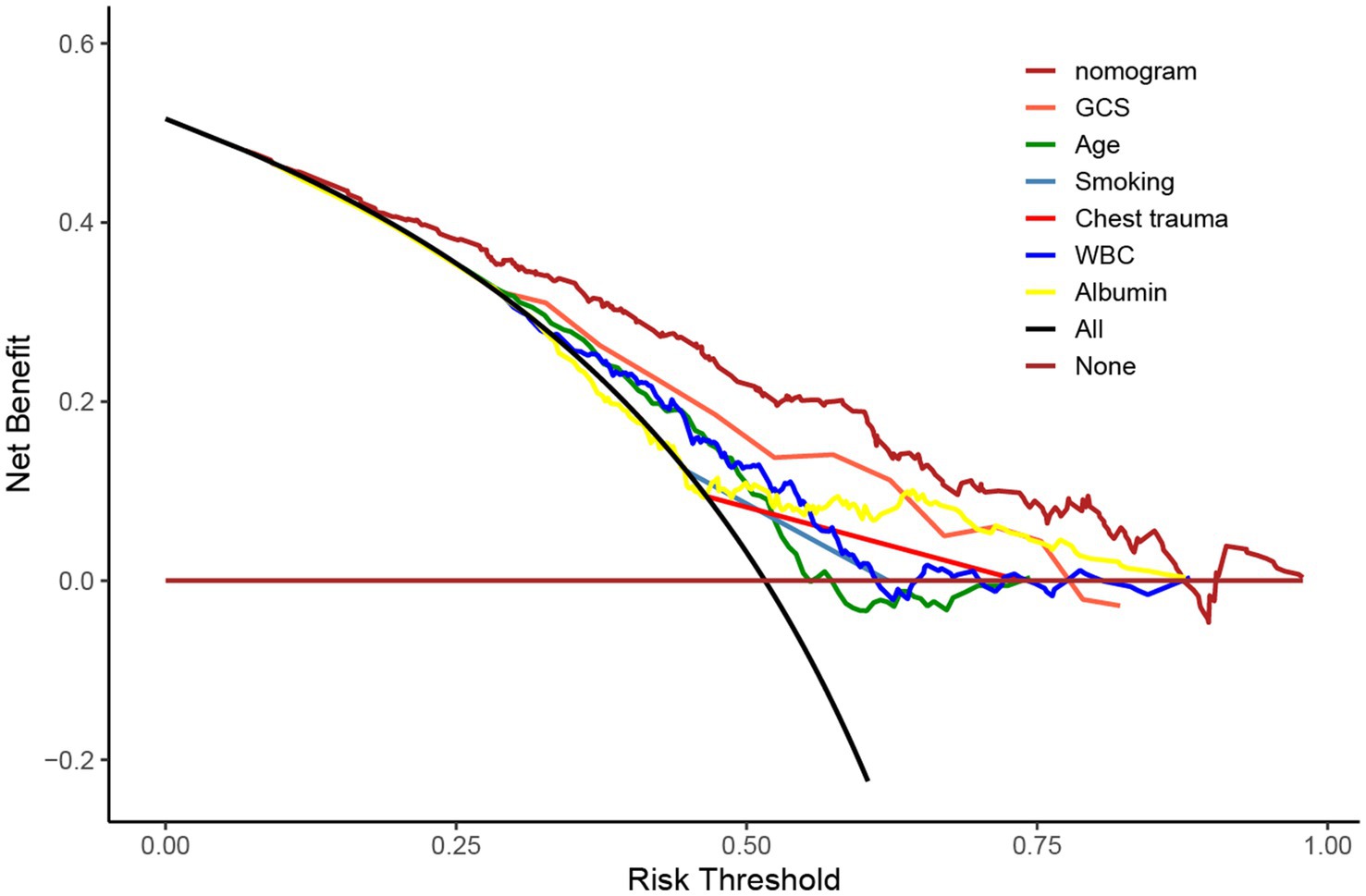
Figure 4. DCA curves of age, smoking history, chest trauma, white blood cell count, serum albumin, GCS score, and nomogram for predicting incidence of pneumonia.
Discussion
According to the U.S. National Institute on Disability and Rehabilitation, despite being the accepted standard for rehabilitating patients with moderately TBI, the mortality rate remains 2.2 times higher than that of the general population, with a reduced life expectancy of 6.6 years. Furthermore, TBI patients are 44 times more likely to die from pneumonia compared to the general population (11). Pneumonia is identified as an independent predictor of poor prognosis in severe TBI patients five years post-discharge (8). Severe pneumonia, particularly acute respiratory distress syndrome (ARDS), can lead to mortality in TBI patients (12). The risk of pneumonia in TBI patients is heightened due to neurological impairments, which can include altered consciousness, difficulty swallowing, compromised vomiting reflex, and impaired cough reflex, as well as ventilator-associated pneumonia.
Recent studies have revealed a complex interplay between the immune and nervous systems regarding TBI and pneumonia, suggesting a significant relationship between these two conditions (3, 13, 14). Following TBI, abnormalities such as dysregulated adrenal hormone secretion, activation of damage-associated molecular patterns (DAMPs), and cytokine release contribute to acute lung injury, pulmonary edema, and even ARDS, thereby exacerbating the peripheral inflammatory response. Additionally, inflammatory mediators may breach the damaged blood–brain barrier and the brain’s lymphatic-like system, influencing immune cells within brain tissue and further increasing blood–brain barrier permeability. This cascade results in an aggravated central inflammatory response, leading to brain edema (3). Simultaneously, TBI can diminish the functionality of immune cells, such as neutrophils and natural killer (NK) cells, through various mechanisms. This results in decreased production of pro-inflammatory cytokines like interleukin (IL)-1β and tumor necrosis factor (TNF)-α, while increasing the production of the anti-inflammatory cytokine IL-10, ultimately leading to immunosuppression and the exacerbation of pneumonia (13, 14).
The treatment of pneumonia typically involves the use of sensitive antibiotics, along with encouraging patients to cough and mobilize to facilitate inflammation absorption and recovery. However, in the context of TBI, neurological damage from trauma often leads to prolonged disturbances in consciousness, impairing the recovery of autonomic functions. This significantly extends the recovery period for pneumonia and increases the risk of antibiotic resistance among pathogenic bacteria, complicating treatment efforts. Therefore, emphasis should be placed on preventing pneumonia secondary to TBI. Early initiation of anti-infection treatments and high-quality nursing care for at-risk pneumonia groups can effectively reduce pneumonia-related mortality. Commonly used serum inflammatory markers for predicting HBP include procalcitonin (PCT), C-reactive protein (CRP), and interleukin-6 (IL-6). However, relying on a single blood marker may be overly simplistic. Consequently, in our study, we included a comprehensive array of indicators for predicting pneumonia occurrence, encompassing general demographic data, medical history, physical examination findings, imaging results, and blood inflammatory markers. This approach enabled us to construct a robust predictive model. The pneumonia prediction model, which incorporates factors such as age, smoking history, chest trauma, white blood cell count, serum albumin levels, and GCS, demonstrates high predictive accuracy. Each of these factors provides a comprehensive assessment of the actual lung injury in TBI patients and offers valuable insights into the severity of pneumonia.
Compared to previous studies (7), the factors included in our analysis were expanded to include serum albumin, which is consistent with clinical observations. In clinical practice, particularly in ICU settings, albumin is commonly used to assess a patient’s baseline nutritional status. Hypoalbuminemia is frequently observed in populations such as the elderly and malnourished individuals, who have a reduced ability to withstand traumatic insults and are therefore more susceptible to infections. Additionally, some factors that are considered high-risk for pneumonia in other studies, such as chronic pulmonary disease, were not included in our analysis. This may be attributed to the relatively small sample size and potential bias in the collection of patient histories, which is also a limitation of this study as a retrospective analysis. Furthermore, the lack of external validation data is another limitation of this study.
In conclusion, pneumonia is one of the most prevalent and serious complications in patients with TBI. Early diagnosis and targeted prevention and treatment strategies for high-risk groups are essential. The establishment of a pneumonia disease prediction model offers a reliable method for assessing the risk of HBP in TBI patients, facilitating effective secondary prevention and treatment measures.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by Ethics Committee of Shanxi Bethune Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because all patients’ names are replaced by IDs, so ethical approval is not applicable.
Author contributions
X-CW: Conceptualization, Data curation, Methodology, Project administration, Visualization, Writing – original draft, Writing – review & editing. Y-ZZ: Conceptualization, Data curation, Formal analysis, Methodology, Project administration, Writing – original draft. MG: Formal analysis, Methodology, Project administration, Writing – original draft, Writing – review & editing. H-BT: Formal analysis, Methodology, Writing – review & editing. Y-HW: Formal analysis, Methodology, Writing – review & editing. X-QW: Data curation, Visualization, Writing – review & editing. H-MJ: Data curation, Visualization, Writing – review & editing, BR: Conceptualization, Formal analysis, Methodology, Project administration, Writing – original draft, Writing – review & editing. HW: Conceptualization, Formal analysis, Methodology, Project administration, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study is supported by the Shanxi Provincial Science and Technology Department planning project (no. 202203021221241).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Lammy, S. Management of traumatic brain injury in China versus Europe. Lancet Neurol. (2020) 19:886. doi: 10.1016/s1474-4422(20)30345-8
2. Majdan, M, Plancikova, D, Brazinova, A, Rusnak, M, Nieboer, D, Feigin, V, et al. Epidemiology of traumatic brain injuries in Europe: a cross-sectional analysis. Lancet Public Health. (2016) 1:e76–83. doi: 10.1016/s2468-2667(16)30017-2
3. Hu, PJ, Pittet, JF, Kerby, JD, Bosarge, PL, and Wagener, BM. Acute brain trauma, lung injury, and pneumonia: more than just altered mental status and decreased airway protection. Am J Physiol Lung Cell Mol Physiol. (2017) 313:L1–l15. doi: 10.1152/ajplung.00485.2016
4. Robba, C, Rebora, P, Banzato, E, Wiegers, EJA, Stocchetti, N, Menon, DK, et al. Incidence, risk factors, and effects on outcome of ventilator-associated pneumonia in patients with traumatic brain injury: analysis of a large, multicenter, prospective, observational longitudinal study. Chest. (2020) 158:2292–303. doi: 10.1016/j.chest.2020.06.064
5. Wu, H, Geng, X, Liu, C, Ballah, AK, Li, F, Han, T, et al. Effect of folic acid treatment for patients with traumatic brain injury (TBI)-related hospital acquired pneumonia (HAP): a retrospective cohort study. J Clin Med. (2022) 11:7403. doi: 10.3390/jcm11247403
6. Cai, W, Li, Y, Guo, K, Wu, X, Chen, C, and Lin, X. Association of glycemic variability with death and severe consciousness disturbance among critically ill patients with cerebrovascular disease: analysis of the MIMIC-IV database. Cardiovasc Diabetol. (2023) 22:315. doi: 10.1186/s12933-023-02048-3
7. Xin, G, Hao, W, Chenan, L, Linrui, Q, Augustine, KB, Wenqiang, C, et al. Construction and validation of a predictive model of pneumonia for ICU patients with traumatic brain injury (TBI). Neurosurg Rev. (2023):46. doi: 10.1007/s10143-023-02208-9
8. Kesinger, MR, Kumar, RG, Wagner, AK, Puyana, JC, Peitzman, AP, Billiar, TR, et al. Hospital-acquired pneumonia is an independent predictor of poor global outcome in severe traumatic brain injury up to 5 years after discharge. J Trauma Acute Care Surg. (2015) 78:396–402. doi: 10.1097/ta.0000000000000526
9. Kumar, RG, Kesinger, MR, Juengst, SB, Brooks, MM, Fabio, A, Dams-O'Connor, K, et al. Effects of hospital-acquired pneumonia on long-term recovery and hospital resource utilization following moderate to severe traumatic brain injury. J Trauma Acute Care Surg. (2020) 88:491–500. doi: 10.1097/ta.0000000000002562
10. Shi, Y, Huang, Y, Zhang, TT, Cao, B, Wang, H, Zhuo, C, et al. Chinese guidelines for the diagnosis and treatment of hospital-acquired pneumonia and ventilator-associated pneumonia in adults (2018 edition). J Thorac Dis. (2019) 11:2581–616. doi: 10.21037/jtd.2019.06.09
11. Greenwald, BD, Hammond, FM, Harrison-Felix, C, Nakase-Richardson, R, Howe, LL, and Kreider, S. Mortality following traumatic brain injury among individuals unable to follow commands at the time of rehabilitation admission: a National Institute on Disability and Rehabilitation Research traumatic brain injury model systems study. J Neurotrauma. (2015) 32:1883–92. doi: 10.1089/neu.2014.3454
12. Komisarow, JM, Chen, F, Vavilala, MS, Laskowitz, D, James, ML, and Krishnamoorthy, V. Epidemiology and outcomes of acute respiratory distress syndrome following isolated severe traumatic brain injury. J Intensive Care Med. (2022) 37:68–74. doi: 10.1177/0885066620972001
13. Elkind, MSV, Boehme, AK, Smith, CJ, Meisel, A, and Buckwalter, MS. Infection as a stroke risk factor and determinant of outcome after stroke. Stroke. (2020) 51:3156–68. doi: 10.1161/strokeaha.120.030429
Keywords: traumatic brain injury, pneumonia, prediction model, risk factors analysis, hospital-acquired pneumonia (HAP)
Citation: Wei X-C, Zhang Y-Z, Guo M, Tong H-B, Wang Y-H, Wang X-Q, Ji H-M, Ren B and Wu H (2025) Risk factors analysis and prediction model construction of hospital-acquired pneumonia after traumatic brain injury. Front. Neurol. 16:1518599. doi: 10.3389/fneur.2025.1518599
Edited by:
Wiliam Panenka, University of British Columbia, CanadaReviewed by:
Elham Rostami, Uppsala University Hospital, SwedenEva Pettemeridou, University of Limassol, Cyprus
Copyright © 2025 Wei, Zhang, Guo, Tong, Wang, Wang, Ji, Ren and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bin Ren, cmVuYmlubGNAMTI2LmNvbQ==; Hao Wu, c2RkcHNvdW5kQDE2My5jb20=
†These authors have contributed equally to this work
 Xiao-Cong Wei
Xiao-Cong Wei Yi-Zi Zhang1†
Yi-Zi Zhang1† Yong-Hong Wang
Yong-Hong Wang Hao Wu
Hao Wu