- 1Department of Neurology, The First Affiliated Hospital of Nanjing Medical University, Nanjing, China
- 2Department of Radiology, The First Affiliated Hospital of Nanjing Medical University, Nanjing, China
- 3Department of Neurosurgery, The First Affiliated Hospital of Nanjing Medical University, Nanjing, China
Introduction: There is a high risk of stroke occurrence and recurrence in patients with intracranial atherosclerotic stenosis (ICAS) despite aggressive medical therapy. Evolocumab is a monoclonal antibody which can inhibit proprotein convertase subtilisin-kexin type 9 (PCSK9) and effectively reduce the level of low-density lipoprotein cholesterol. We hypothesize that evolocumab added to statin therapy (EAST) can stabilize intracranial plaques in patients with symptomatic ICAS.
Methods and analysis: This is a prospective, randomized, open-label, blinded end-point study, which will assess the efficacy and safety of evolocumab in patients with symptomatic ICAS. Eighty patients who suffer a stroke/transient ischemic attack (TIA) caused by ICAS recently will be randomly allocated in a 1:1 ratio to the evolocumab plus statin treatment group or the statin treatment group. High resolution vessel wall magnetic resonance imaging (HR-vwMRI) will be performed at recruitment and after 6 months and 12 months. The primary outcome is changes in plaque characteristics assessed by HR-vwMRI at 6th month and 12th month after treatment. Cognitive and neurological function will also be evaluated at recruitment and follow-up. This trial is being conducted at the first affiliated hospital of Nanjing medical university, China.
Ethics and dissemination: All participants will sign written informed consents. Peer-reviewed articles will be published to disseminate study outcomes.
Clinical trial registration: ClinicalTrials.gov, identifier: NCT05741086.
1 Introduction
Intracranial atherosclerosis stenosis (ICAS) is one of the important causes of ischemic stroke occurrence and recurrence worldwide and is especially common in Asian (1, 2). It accounts for 30–50% of strokes in Asian, but only 5–10% in the West (3, 4). Aggressive medical management (e.g., high intensity statin therapy to reduce low-density lipoprotein (LDL) cholesterol <1.8 mmol/L) is superior to the percutaneous transluminal angioplasty and stenting for symptomatic ICAS in the SAMMPRIS study. However, around one in five patients still experienced stroke recurrence or death during 3-year follow-up in the aggressive medical treatment group (5).
As a fully human monoclonal antibody and a member of proprotein convertase subtilisin-kexin type 9 (PCSK9) inhibitors, evolocumab has the potential to decrease LDL cholesterol levels by around 60%. The FOURIER study showed that evolocumab decreased cardiovascular events (i.e., cardiovascular death, myocardial infarction, and stroke), in patients with atherosclerotic cardiovascular disease during 2.2 years follow-up (6). It’s still largely unknown whether evolocumab could affect the evolution of ICAS and reduce ischemic events in patients with stroke.
We thus designed this prospective, randomized, open-label, blinded end-point study to evaluate the effects of evolocumab added to statin therapy (EAST) in patients with stroke/transient ischemic attack (TIA) caused by ICAS. Eighty patients will be enrolled and assigned into two arms with or without evolocumab. Changes in plaque characteristics on high resolution vessel wall magnetic resonance imaging (HR-vwMRI), neurological function and cognitive function will be evaluated and analyzed between groups over a period of 1 year follow-up.
2 Materials and methods
2.1 Study design
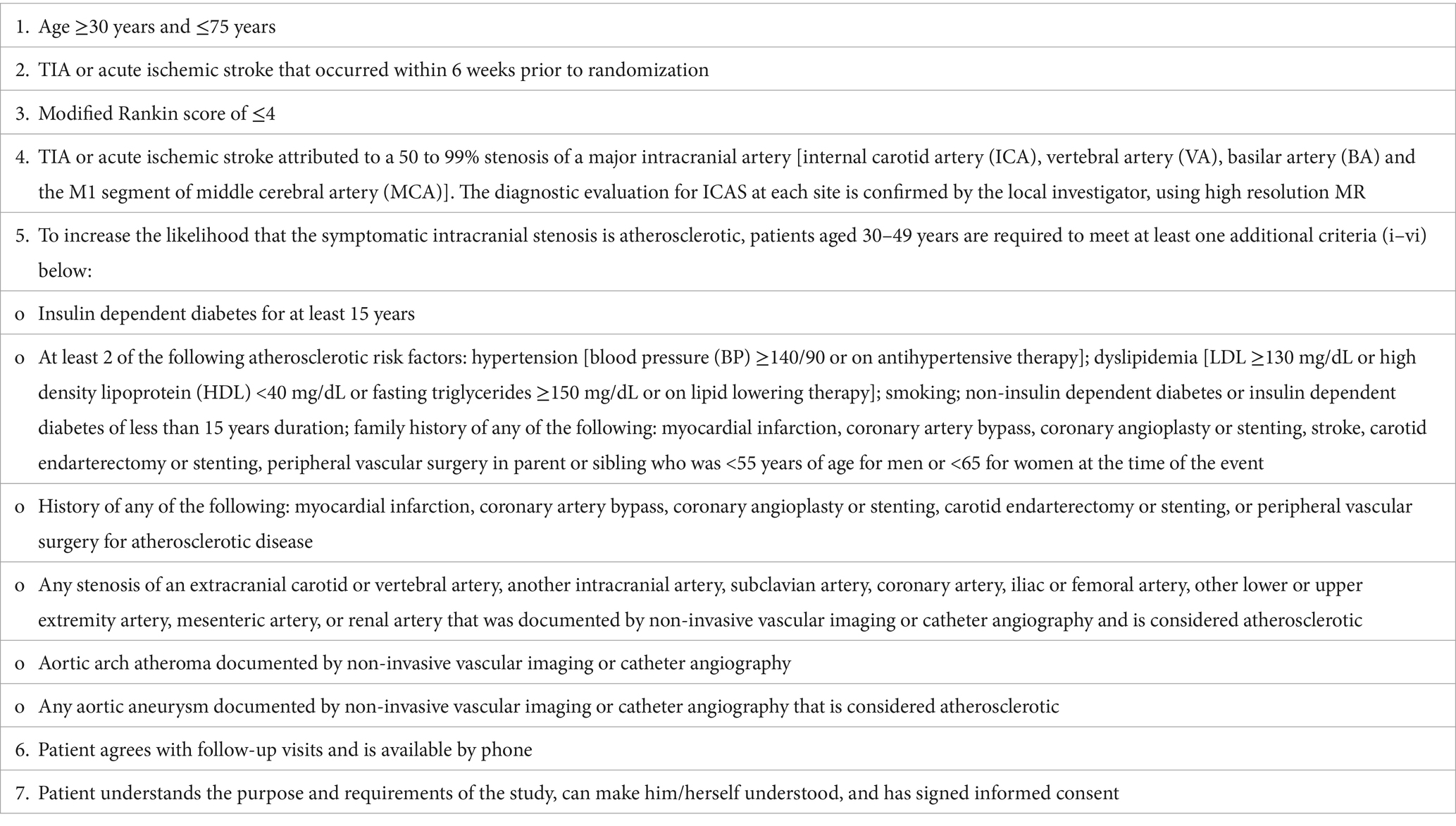
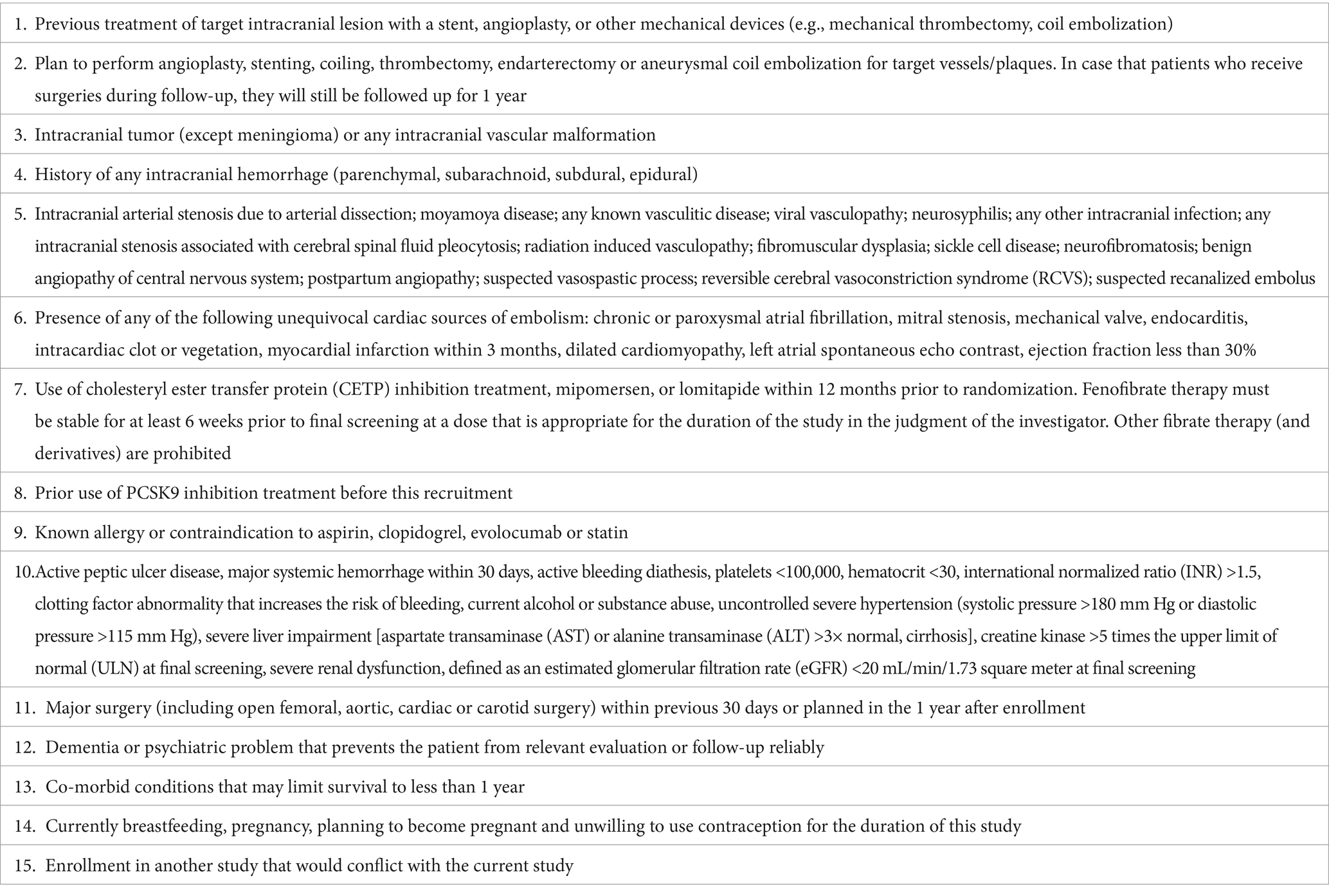
This is a prospective, randomized, open-label, and blinded endpoint assessment study. We will recruit 80 patients who have recently suffered a stroke/transient ischemic attack (TIA) due to ICAS. The inclusion and exclusion criteria are shown in Tables 1, 2, respectively. Patients will be allocated in a 1:1 ratio to the evolocumab plus statin treatment arm or the statin alone treatment arm. Evolocumab (150 mg) will be injected subcutaneously every 2 weeks for 12 months. Both arms will receive statins for 12 months, including atorvastatin 20–80 mg or rosuvastatin 10–20 mg. According to current guideline and the standards in SAMMPRIS study, optimal medical management with aggressive control of the risk factors will be provided to all patients, such as proper application of antiplatelet, antihypertensive, and antidiabetic drugs. HR-vwMRI will be performed at baseline and at 6th and 12th month after recruitment. Blood test will be conducted at baseline and at 1st, 3rd, 6th, and 12th month after recruitment. National Institute of Health Stroke Scale (NIHSS), modified Rankin Scale (mRS), Barthel index, Mini-Mental State Examination (MMSE), and Montreal Cognitive Assessment (MoCA) will be evaluated at baseline and at 3rd, 6th, and 12th month post-recruitment. This study was approved by the ethics board of the first affiliated hospital of Nanjing Medical University. Written informed consents will be obtained. The flow chart of this study is shown in Figure 1.
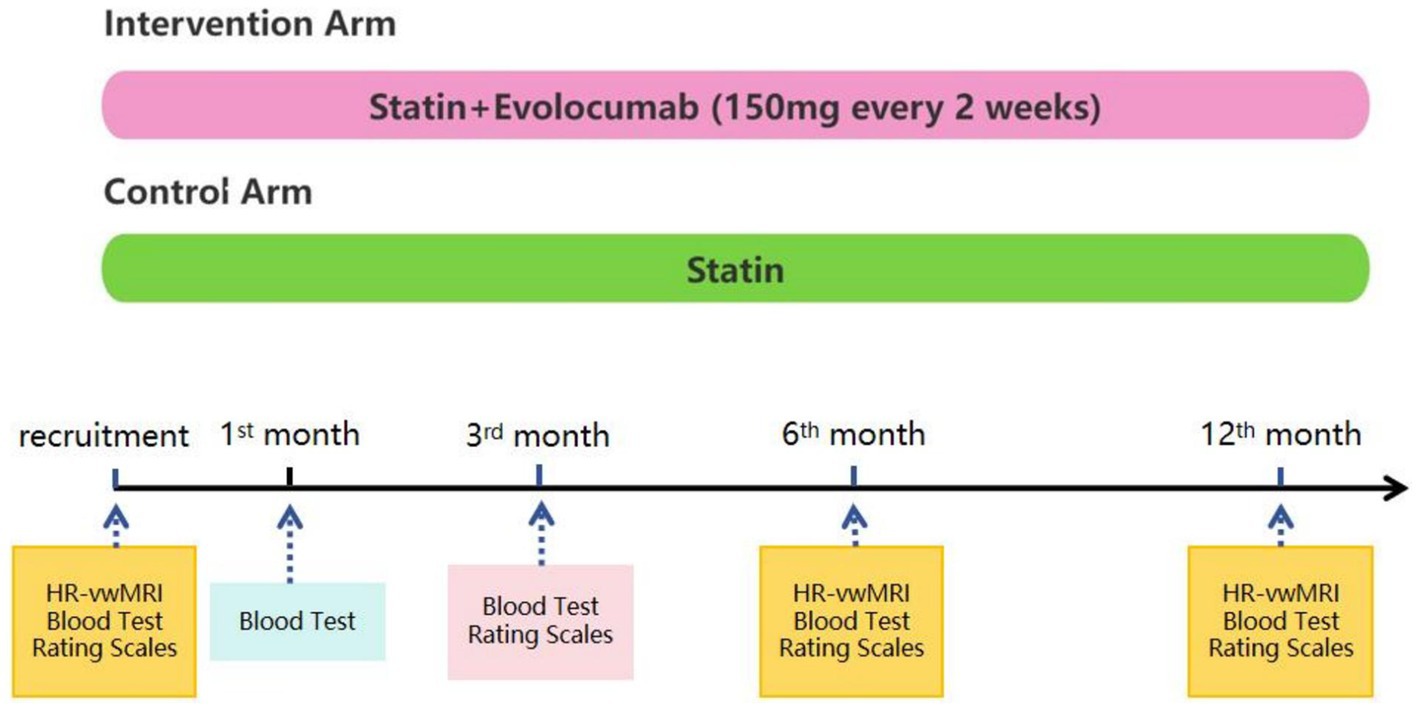
Figure 1. Schematic of the study design and treatment schedule. Statin, including rosuvastatin 10–20 mg or atorvastatin 20–80 mg, will be administered in both arms for 12 months. All patients will receive optimal medical management with aggressive control of the risk factors (e.g., appropriate use of antiplatelet, antihypertensive, and antidiabetic drugs). Patients in the intervention arm will receive an additional treatment of evolocumab 150 mg subcutaneously every 2 weeks for 12 months. All patients will undergo high resolution vessel wall magnetic resonance imaging (HR-vwMRI) at recruitment and after 6 and 12 months. Blood tests will be performed at baseline and at 1, 3, 6, and 12 months after recruitment. Rating scales, including NIHSS, Barthel index, modified Rankin Scale, MoCA, and MMSE, will be used to assess neurological functions and cognitive functions at baseline and at the 3rd, 6th, and 12th months after recruitment.
2.2 Clinical data
We will collect demographic information, vascular risk factors, and blood test results. Vascular risk factors include smoking, hypertension, diabetes, hyperlipidemia, coronary artery disease and previous ischemic stroke. Blood test data include low-density lipoprotein (LDL), high-density lipoprotein (HDL), total cholesterol, triglyceride, urea nitrogen, serum creatinine, aspartate transaminase (AST) or alanine transaminase (ALT), C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), interleukin 6 (IL6), procalcitonin, glycosylated hemoglobin, D-dimmer and fibrinogen (FIB), and blood routine examination.
2.3 HR-vwMRI protocol
All images were performed using a 3.0 T magnetic resonance (MR) (Magnetom Verio or Skyra, Siemens, Erlangen, Germany) equipped with a 32-channel or a 20-channel head-matrix coil. The detailed protocol has been described before (7). Briefly, this HR-vwMRI includes sequences as below: three-dimensional time-of-flight MR angiography (3D TOF-MRA), axial DWI (diffusion weighted imaging), 3D T1-weighted SPACE (sampling perfection with application optimized contrast using different angle evolutions), two-dimensional black blood (2D-BB) T2WI, dynamic susceptibility contrast-perfusion weighted imaging (DSC-PWI) conducted with a gradient echo planar sequence using of a bolus of 0.1 mmol/kg contrast agent (gadodiamide, GE Healthcare, Cork, Ireland). In addition, contrast-enhanced 3D T1-weighted SPACE will be acquired with an around 5-min delay time after contrast administration. It takes approximately 25 min to complete all sequences above.
2.4 Image analysis
Two neuroradiologists will independently analyze HR-MRI images on commercially available software (Carestream Vue PACS v12.1, Carestream Health), and they were blinded to all clinical information. A senior neuroradiologist will give a further review on images with uncertainty grading to reach a consensus.
The characteristics of the plaque, such as stenosis degree, enhancement, the presence of T1 hyperintensity, remodeling index, plaque burden, and the distribution of the plaque, will be assessed. The detailed imaging analysis on HR-vwMRI has been described before (7). Briefly: (1) degree of stenosis = (1 − Dplaque/Dreference) × 100%, where Dplaque means the diameter of the stenotic artery at the most stenotic site, and Dreference is the diameter of the normal artery proximal to the plaque; (2) grading of plaque enhancement: grade 0 means enhancement is similar to or less than that of normal-appearing intracranial arterial walls in the same subject; grade 1, enhancement is greater than that of grade 0 but less than that of the pituitary infundibulum; and grade 2, enhancement is similar to or greater than that of the infundibulum (8); (3) plaque enhancement ratio (ER): neuroradiologists will draw circular region of interest (ROI) within the plaque on pre-contrast and post-contrast T1-weighted SPACE images, respectively. The mean signal intensity (SI) of plaques will be acquired. ER = (SIpost − SIpre)/SIpre × 100% (9); (4) remodeling index (RI): the outer wall area (OWA) is manually contoured at the most stenotic site (OWAplaque) and the reference site (OWAreference) on T1-weighted SPACE. RI = OWAplaque/OWAreference × 100%. Arterial remodeling is classified as positive if RI >1.05, intermediate if 0.95 ≤ RI ≤ 1.05, and negative if RI <0.95 (10); (5) plaque burden (PB): lumen area (LA) is manually contoured at the most stenotic site (LAplaque) on T1-weighted SPACE. PB = (1 − LAplaque/OWAplauqe) × 100% (11, 12); (6) presence of T1 hyperintensity: the brightest spot of the plaque whose SI is >150% of that of the reference vessel wall on pre-contrast T1 image (13); (7) plaque distribution: a concentric plaque is defined if the wall involvement is at least 75%, and the minimum wall thickness is bigger than 50% of the maximum wall thickness.
For patients experiencing an acute ischemic stroke within the middle cerebral artery (MCA) territory, infarct patterns on DWI will be categorized into four types: internal borderzone, cortical borderzone, core MCA and perforator (14). For infarction lesion that crossed these territories, the territory which is predominantly involved is chosen. DSC-PWI is derived through the application of the singular value decomposition deconvolution technique, facilitated by the software NeuBrainCARE (v1.1.10). The arterial input function is determined automatically, taken from the MCA on the opposite side. Hypoperfusion volume is automatically calculated using time to maximum (Tmax) with time thresholds of >4 s and >6 s, respectively.
2.5 Neurological function and cognitive function
At baseline and then after 3, 6, and 12 months, we will evaluate NIHSS for neurological deficits, Barthel index for activities of daily living, mRS for the degree of disability in daily activities, MMSE, and MoCA for cognitive functions.
3 Results
3.1 Study outcomes
Primary endpoints:
1) Changes in plaque enhancement.
A reduction of ≥ 1 grading in plaque enhancement after 12 months’ treatment was regarded as relief of plaque enhancement. For example, a patient’s plaque enhancement reduces to grade 1 or even 0 after 12 months’ treatment, compared to grade 2 at the time of enrollment.
2) Changes in degree of stenosis.
3) Changes in plaque enhancement ratio.
4) Changes in plaque burden.
5) Changes in hypoperfusion volume.
6) T1 hyperintensity.
We define three types of T1 hyperintensity changes at 12 month after recruitment: type A, no changes before and after treatment; type B, disappearance of T1 heperintensity; type C, new T1 hyperintensity.
7) Changes in remodeling index (RI) of the plaque.
Secondary endpoints:
1) Recurrent stroke (ischemic or hemorrhagic) or death during follow-up.
2) Changes in LDL-cholesterol levels.
3) Changes in NIHSS/Barthel index/mRS score.
4) Changes in MoCA/MMSE score.
All changes above before and after treatment in each arm will be calculated and compared between arms.
3.2 Adverse events
We shall document any adverse events that happen throughout the trial ensuring that patients receive prompt medical attention at our facilities or at local hospitals. Interim analyses are conducted to facilitate decisions on either halting or proceeding with the trial based on the data that has been accumulated.
3.3 Statistical analysis
Appropriate statistical methods, such as Fisher’s exact test or Pearson’s chi-squared test, will be employed for the analysis of categorical data. Continuous variables will be compared by an independent samples Student’s t-test or Mann–Whitney U-test, as appropriate. We will present the cumulative incidence of stroke or mortality over a 12-month maximum follow-up period using Kaplan–Meier analysis, and we will determine the hazard ratios along with their 95% confidence intervals through the application of Cox proportional hazards modeling and the log-rank test to assess the efficacy of the treatment. Statistical significance is defined as a p value of < 0.05.
4 Discussion
This prospective, randomized, open-label, blinded end-point study seeks to investigate the impact of evolocumab plus statin on ICAS. We hypothesize that evolocumab added to statin therapy (EAST) will demonstrate greater efficacy in stabilizing intracranial plaques, as assessed by HR-vwMRI, when compared to the arm of statin alone without evolocumab. Multiple plaque features (enhancement, plaque burden, degree of stenosis, remodeling index, T1 hyperintensity) and hemodynamic markers (hypoperfusion volume defined by Tmax) will be compared between patients with and without evolocumab. Additionally, we will also document and compare neurological function, cognitive performance, blood parameters levels, and the incidence of recurrent stroke between arms.
Nowadays, the treatment of ICAS is mainly composed of antiplatelet therapy, lipid-lowering strategies, the modification of risk factors and interventional procedures (15). Statins are the preferred treatment option for lowering LDL-C (16). It has been observed that intensive statin therapy can stabilize symptomatic intracranial atherosclerotic plaques, as evidenced by a reduction in enhancement and stenosis on sequential HR-MRI assessments. In addition, greater reductions in LDL levels are associated with a more pronounced decrease in plaque enhancement (17). However, more than a third of these patients demonstrated either no improvement or a worsening of plaque enhancement and stenosis after high-dose statin therapy, suggesting a lack of or minimal responsiveness to statins (17). This is consistent with earlier findings regarding the progression of ICAS in patients treated with statins (3, 18). Meanwhile, there is an association between high-intensity statin therapy and increased side effects, including myalgia and liver dysfunction, which can result in inadequate treatment adherence. Endovascular treatments such as stenting may be a promising treatment method for ICAS. Nevertheless, the CASSISS study found that there was no significant difference between stenting combined with drug therapy and drug therapy alone in preventing stroke or death (19). As a member of PCSK9 inhibitor family, evolocumab significantly reduces LDL cholesterol levels, and associated with greater plaque regression and stabilization by optical coherence tomography measures of plaque composition (20), reducing the risk of cardiovascular events (6). Therefore, it is plausible that evolocumab added to statin therapy (EAST) could also stabilize intracranial plaques in stroke/TIA patients with ICAS.
In this study, symptomatic ICAS patients will be randomized into the arm of evolocumab plus statin or the arm of statin alone. The evolution of these plaques will be monitored through sequential HR-vwMRI scans at 6-months and 12-months after enrollment. Future multicenter studies with a large sample size are needed to further investigate the efficacy of this “EAST” strategy in symptomatic ICAS.
5 Strengths and limitations of this study
This is one of the pioneering studies to evaluate the impact of a PCSK9 inhibitor on plaque evolution in patients with symptomatic ICAS, extending beyond the scope of conventional medical therapy. This study, characterized by its prospective, randomized, open-label, and blinded end-point design, facilitates patient recruitment and keeps costs low. Considering the limited sample size and single-center recruitment, the findings of this study should be applied with caution, and there is a need for future large-scale, multi-center studies to validate the results.
Ethics statement
The study is registered on ClinicalTrials.gov (NCT05741086). The Ethics Committee of the First Affiliated Hospital of Nanjing Medical University approved this study (Approval No. 2023-SR-147). Written informed consents will be obtained from all participants. We shall publish the study outcomes in peer-reviewed journals and academic conferences. Research data can be obtained from the corresponding author on reasonable request.
Author contributions
JD: Conceptualization, Methodology, Project administration, Writing – original draft, Writing – review & editing, Investigation, Supervision. HZ: Investigation, Methodology, Writing – review & editing. XC: Investigation, Writing – review & editing. PN: Investigation, Writing – review & editing. JG: Supervision, Writing – review & editing. MW: Writing – review & editing. KZ: Writing – review & editing. ZW: Writing – review & editing. HL: Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer BH declared a shared parent affiliation with the authors to the handling editor at the time of review.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Wang, Y, Zhao, X, Liu, L, Soo, YOY, Pu, Y, Pan, Y, et al. Prevalence and outcomes of symptomatic intracranial large artery stenoses and occlusions in China: the Chinese intracranial atherosclerosis (CICAS) study. Stroke. (2014) 45:663–9. doi: 10.1161/STROKEAHA.113.003508
2. Holmstedt, CA, Turan, TN, and Chimowitz, MI. Atherosclerotic intracranial arterial stenosis: risk factors, diagnosis, and treatment. Lancet Neurol. (2013) 12:1106–14. doi: 10.1016/S1474-4422(13)70195-9
3. Tan, T-Y, Kuo, Y-L, Lin, W-C, and Chen, T-Y. Effect of lipid-lowering therapy on the progression of intracranial arterial stenosis. J Neurol. (2009) 256:187–93. doi: 10.1007/s00415-009-0960-9
4. Wong, KS, and Li, H. Long-term mortality and recurrent stroke risk among Chinese stroke patients with predominant intracranial atherosclerosis. Stroke. (2003) 34:2361–6. doi: 10.1161/01.STR.0000089017.90037.7A
5. Derdeyn, CP, Chimowitz, MI, Lynn, MJ, Fiorella, D, Turan, TN, Janis, LS, et al. Aggressive medical treatment with or without stenting in high-risk patients with intracranial artery stenosis (SAMMPRIS): the final results of a randomised trial. Lancet. (2014) 383:333–41. doi: 10.1016/S0140-6736(13)62038-3
6. Sabatine, MS, Giugliano, RP, Keech, AC, Honarpour, N, Wiviott, SD, Murphy, SA, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. (2017) 376:1713–22. doi: 10.1056/NEJMoa1615664
7. Chen, X, Dai, J, Nie, P, Gu, P, Wang, M, Zhang, K, et al. Design of the “EAST” strategy in patients with asymptomatic intracranial atherosclerotic stenosis. Clin Neurol Neurosurg. (2024) 245:108507. doi: 10.1016/j.clineuro.2024.108507
8. Qiao, Y, Zeiler, SR, Mirbagheri, S, Leigh, R, Urrutia, V, Wityk, R, et al. Intracranial plaque enhancement in patients with cerebrovascular events on high-spatial-resolution MR images. Radiology. (2014) 271:534–42. doi: 10.1148/radiol.13122812
9. Alexander, MD, de Havenon, A, Kim, S-E, Parker, DL, and McNally, JS. Assessment of quantitative methods for enhancement measurement on vessel wall magnetic resonance imaging evaluation of intracranial atherosclerosis. Neuroradiology. (2019) 61:643–50. doi: 10.1007/s00234-019-02167-3
10. Qiao, Y, Anwar, Z, Intrapiromkul, J, Liu, L, Zeiler, SR, Leigh, R, et al. Patterns and implications of intracranial arterial remodeling in stroke patients. Stroke. (2016) 47:434–40. doi: 10.1161/STROKEAHA.115.009955
11. Teng, Z, Peng, W, Zhan, Q, Zhang, X, Liu, Q, Chen, S, et al. An assessment on the incremental value of high-resolution magnetic resonance imaging to identify culprit plaques in atherosclerotic disease of the middle cerebral artery. Eur Radiol. (2016) 26:2206–14. doi: 10.1007/s00330-015-4008-5
12. Ran, Y, Wang, Y, Zhu, M, Wu, X, Malhotra, A, Lei, X, et al. Higher plaque burden of middle cerebral artery is associated with recurrent ischemic stroke: a quantitative magnetic resonance imaging study. Stroke. (2020) 51:659–62. doi: 10.1161/STROKEAHA.119.028405
13. Xu, W-H, Li, M-L, Gao, S, Ni, J, Yao, M, Zhou, L-X, et al. Middle cerebral artery intraplaque hemorrhage: prevalence and clinical relevance. Ann Neurol. (2012) 71:195–8. doi: 10.1002/ana.22626
14. Wabnitz, AM, Derdeyn, CP, Fiorella, DJ, Lynn, MJ, Cotsonis, GA, Liebeskind, DS, et al. Hemodynamic markers in the anterior circulation as predictors of recurrent stroke in patients with intracranial stenosis. Stroke. (2019) 50:143–7. doi: 10.1161/STROKEAHA.118.020840
15. Liu, D, Liu, J, Cai, Y, Wong, KSL, and Liu, L. Is the future of symptomatic intracranial atherosclerotic stenosis management promising? J Neurol Neurosurg Psychiatry. (2020) 91:122–4. doi: 10.1136/jnnp-2019-321564
16. Kim, H-J, Kim, E-K, Kwon, SU, Kim, JS, and Kang, D-W. Effect of statin on progression of symptomatic intracranial atherosclerosis. Can J Neurol Sci. (2012) 39:801–6. doi: 10.1017/s031716710001564x
17. Chung, J-W, Cha, J, Lee, MJ, Yu, I-W, Park, M-S, Seo, W-K, et al. Intensive statin treatment in acute ischaemic stroke patients with intracranial atherosclerosis: a high-resolution magnetic resonance imaging study (STAMINA-MRI study). J Neurol Neurosurg Psychiatry. (2020) 91:204–11. doi: 10.1136/jnnp-2019-320893
18. Leung, TW, Wang, L, Soo, YOY, Ip, VHL, Chan, AYY, Au, LWC, et al. Evolution of intracranial atherosclerotic disease under modern medical therapy. Ann Neurol. (2015) 77:478–86. doi: 10.1002/ana.24340
19. Gao, P, Wang, T, Wang, D, Liebeskind, DS, Shi, H, Li, T, et al. Effect of stenting plus medical therapy vs medical therapy alone on risk of stroke and death in patients with symptomatic intracranial stenosis: the CASSISS randomized clinical trial. JAMA. (2022) 328:534–42. doi: 10.1001/jama.2022.12000
Keywords: statin, intracranial atherosclerotic stenosis, treatment, HR-vwMRI, PCSK9 inhibitor
Citation: Dai J, Zhao H, Chen X, Nie P, Gong J, Wang M, Zhang K, Wang Z and Lu H (2025) Design of the “EAST” strategy in patients with symptomatic intracranial atherosclerotic stenosis. Front. Neurol. 16:1520356. doi: 10.3389/fneur.2025.1520356
Edited by:
Tianxiao Li, Henan Provincial People’s Hospital, ChinaReviewed by:
Qazi Zeeshan, University of Pittsburgh Medical Center, United StatesBaosheng Huang, Nanjing Medical University, China
Copyright © 2025 Dai, Zhao, Chen, Nie, Gong, Wang, Zhang, Wang and Lu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhaolu Wang, emhhb2x1d2FuZ0Buam11LmVkdS5jbg==; Hua Lu, bHVodWFAbmptdS5lZHUuY24=
 Jiaqi Dai
Jiaqi Dai Haobo Zhao1
Haobo Zhao1 Kezhong Zhang
Kezhong Zhang Zhaolu Wang
Zhaolu Wang Hua Lu
Hua Lu