- 1Guy's and St Thomas' NHS Foundation Trust, London, United Kingdom
- 2Kings College Hospital NHS Foundation Trust, London, United Kingdom
- 3Ear Institute, University College London, London, United Kingdom
Background: Postural tachycardia syndrome (PoTS) is a chronic condition characterized by an increased heart rate upon standing and is recognized to be associated with dizziness and migraines. This study aimed to investigate the multifaceted nature of dizziness in PoTS from a vestibular perspective, including the relationship with vestibular migraine.
Methods: A retrospective review of 80 patients with confirmed PoTS, attending a tertiary neuro-otology clinic, was conducted to evaluate standardized detailed assessments {validated symptom questionnaires, history/physical examination, neuro-otological diagnostics [including video head impulse test (VHIT), videonystagmography (VNG), and caloric testing], and management}. The PoTS cohort was also compared with an age- and sex-matched control group of dizzy patients.
Results: A total of 80 patients were included (mean age: 35.3 years ± 12.1; 93% women). Approximately 84% had migraine, and 30% had vestibular migraine. Clinical examination of static and dynamic balance was most frequently abnormal. VHIT was abnormal in 2%, and VNG canal paresis and directional preponderance were abnormal in 19 and 22%, respectively. In total, 49% of cases were offered migraine management advice, and 58% were offered vestibular rehabilitation. The PoTS group showed higher rates of vestibular symptoms, including vertigo, unsteadiness, and positional vertigo, and high Disability Rating Scale (DRS) scores.
Conclusion: Our findings reflect the challenges of distinguishing dizziness phenotypes in PoTS, particularly vestibular migraine. This study illustrates the importance of judicious clinical enquiry to investigate non-orthostatic dizziness mechanisms, such as vestibular migraine, for which evidence-based management exists, and recommends further research to elucidate the multifaceted nature of PoTS-associated dizziness.
Introduction
Postural tachycardia syndrome (PoTS, also known as postural orthostatic tachycardia syndrome [POTS]) is a chronic heterogeneous condition defined clinically by a minimum of two consecutive recordings demonstrating a sustained increase in heart rate of more than 30 beats per minute over 10 minutes of standing in the absence of orthostatic hypotension (1). The cardinal symptom of PoTS is dizziness, especially of orthostatic type. Haemodynamic orthostatic dizziness has been discussed in detail in a Barany Society Consensus document (2).
Widely recognized for its female preponderance, typically affecting those between 12 and 50 years of age, the prevalence of PoTS is not truly appreciated due to underdiagnosis (3, 4). Published estimates from the United States report a prevalence between 0.1 and 1% (4). Migraine, a common comorbidity in PoTS, is also well known as a cause of dizziness in neurology and neuro-otology clinics, primarily in the form of vestibular migraine (5). Association with Ehlers–Danlos Syndrome (EDS), hypermobility, fibromyalgia, depression, and anxiety further illustrate the complexity of the PoTS spectrum (6).
Currently, there is a paucity of evidence investigating the nature of dizziness in PoTS beyond orthostatic intolerance. This study, therefore, intended to examine dizziness in PoTS from a neuro-otological perspective to identify the prevalence of common dizziness presentations and conditions, including vestibular migraine (VM).
Methods
Design
A retrospective observational cohort study was conducted by sequentially selecting patients reviewed in 2020, with a diagnosis of confirmed PoTS in accordance with the Heart Rhythm Society Consensus Statement (7), at a single publicly funded neuro-otology tertiary clinic in London, United Kingdom. Institutional Ethics approval was obtained [IRAS ID: 257283; Research Ethics Committee (REC) reference number: 20/EM/0112], and the study protocol and execution were informed by discussion with volunteer patient representatives from PoTS UK.
Subjects
Electronic clinical case notes were reviewed to identify patients with a diagnosis of PoTS, confirmed by a neuro-cardiology specialist. These patients were then compared, where appropriate, to an age- and sex-matched control group of new patients attending the Balance Clinic during the same period, who did not have confirmed or suspected PoTS.
Outcome measures
Demographic data, the presence of defined vestibular symptoms (head motion-triggered dizziness, visually induced dizziness, spinning vertigo, positional vertigo, unsteadiness) (8), and examination findings were collated. Common PoTS-associated conditions were also recorded—potentially relevant to balance assessment and treatment. Specifically, migraine and vestibular migraine were diagnosed according to the IHS criteria (9) and the Barany Society criteria (10), respectively. Both definite and probable vestibular migraine were included. Other comorbidities (depression, anxiety, chronic pain, and hypermobility) were recorded as present if they were coded in the patient's health record.
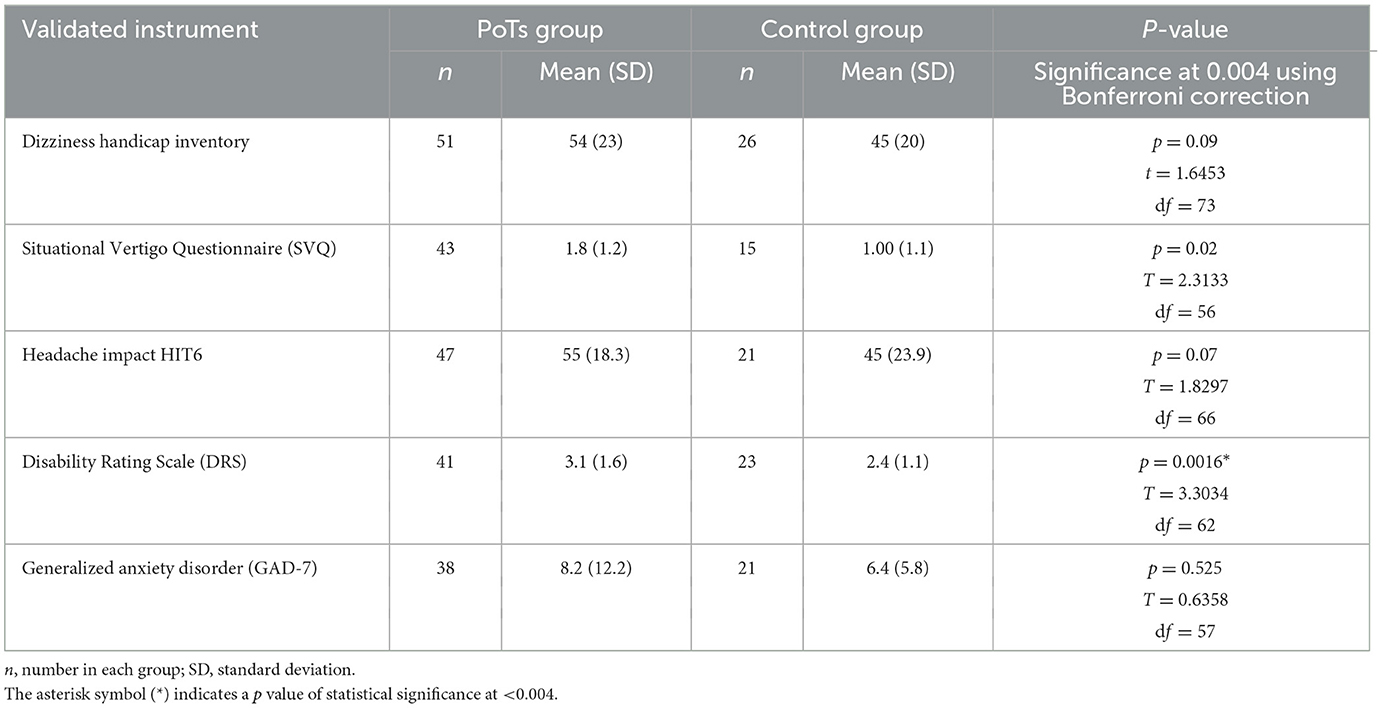
Validated symptom questionnaires were recorded, specifically the Headache Impact Test (HIT6) (11), Generalized Anxiety Disorder Questionnaire (GAD-7) (12), Situational Vertigo Questionnaire (SVQ) (13), Dizziness Handicap Inventory (DHI) (14), and Disability Rating Scale (DRS) (15).
Examination findings, including Dix-Hallpike positional testing and clinical static and dynamic balance, were recorded. Specifically, static balance was recorded using the modified clinical test of sensory integration in balance (16). Dynamic balance was assessed by visual inspection of gait and tandem gait over 10 steps, with any side step or gap of more than 2 cm counted as an error, and with the best effort on three trials considered. Vestibular diagnostic test findings obtained from the six canal video head impulse test (VHIT), videonystagmography (VNG), oculomotor, and bithermal water stimulus VNG caloric assessments were recorded. Specifically, VNG was conducted according to standard protocol (17); gaze testing was performed in central and lateral gaze (+/– 30°) to assess for nystagmus with and without fixation. For smooth pursuit testing, participants were required to follow a target moving in a sinusoidal fashion at 0.2, 0.3, and 0.4 Hz. Horizontal saccadic testing employed a projected target moving randomly over a 5°-30° arc. Optokinetic testing subjected participants to remain stationary while the visual surround revolved around their vertical axis at a speed of 40°/s, alternating direction every 5–10 s for ~30 s. VHIT was considered abnormal if gain was below 0.8 for lateral canals and below 0.7 for vertical canals, combined with consistent grouped catch-up saccades. The caloric test was performed and recorded according to national guidelines (18).
Treatment recommendations for migraine management and subsequent referral for vestibular rehabilitation were noted in the medical record. National guidelines for migraine from the British Association for the Study of Headache were followed (19). Patients were asked to provide a Global Impression of Change on medication (overall benefit—neutral—overall worse), and a decision to stay on medication or not was documented. In terms of physiotherapy, benefit was additionally determined by the proportion benefitting from the intervention provided and determined by a change (decrease) on the Dizziness Handicap Inventory of more than 8 points before and after therapy.
All clinical assessments were performed by Consultant Audiovestibular Physicians or Highly Specialist Physiotherapists, and formal vestibular testing was conducted by specialist Audiologists, working collaboratively in a team within a large specialist tertiary Multidisciplinary Balance Clinic.
Statistical analysis was done using Statistical Package for the Social Sciences (SPSS) version 27.0 IBM Corp, including t-tests and chi-squared tests, with multiple hypothesis correction applied (Bonferroni).
Results
A total of 80 eligible patients with PoTS were identified following the exclusion of non-consenting individuals. The cohort was predominantly female (74 of 80; 93%) with age ranging between 18 and 65 years and a mean age of 35.3 years (standard deviation, SD = 12.1). There were 52 control patients (48 women, 92%) with age ranging between 25 and 60 years and a mean age of 37 years (SD = 9.6). The groups showed no significant difference for sex (chi-squared test: 0.002; df = 1; p = 0.9675) and age (t-test, p = 0.3207, t = 0.9968, df = 129). The primary diagnosis in the control group was benign paroxysmal positional vertigo (n = 10), acute unilateral vestibular loss or its sequelae (n = 9), vestibular migraine (n = 10), persistent postural perceptual dizziness (n = 11), and other mixed diagnoses in the rest (n = 12).
The results related to symptoms, examination, comorbidity, and vestibular tests are listed in Table 1. Table 2 presents the questionnaires for the PoTS and control cohorts. Vestibular symptoms most commonly recorded in PoTS included unsteadiness (84%), and there were also relatively high rates of positional vertigo (68%) followed by spontaneous vertigo (48%). Comparison data for controls are given in Table 1. Controls had higher rates of head motion-triggered dizziness, with vertigo, whereas positional vertigo and unsteadiness were higher in the PoTS cohort.
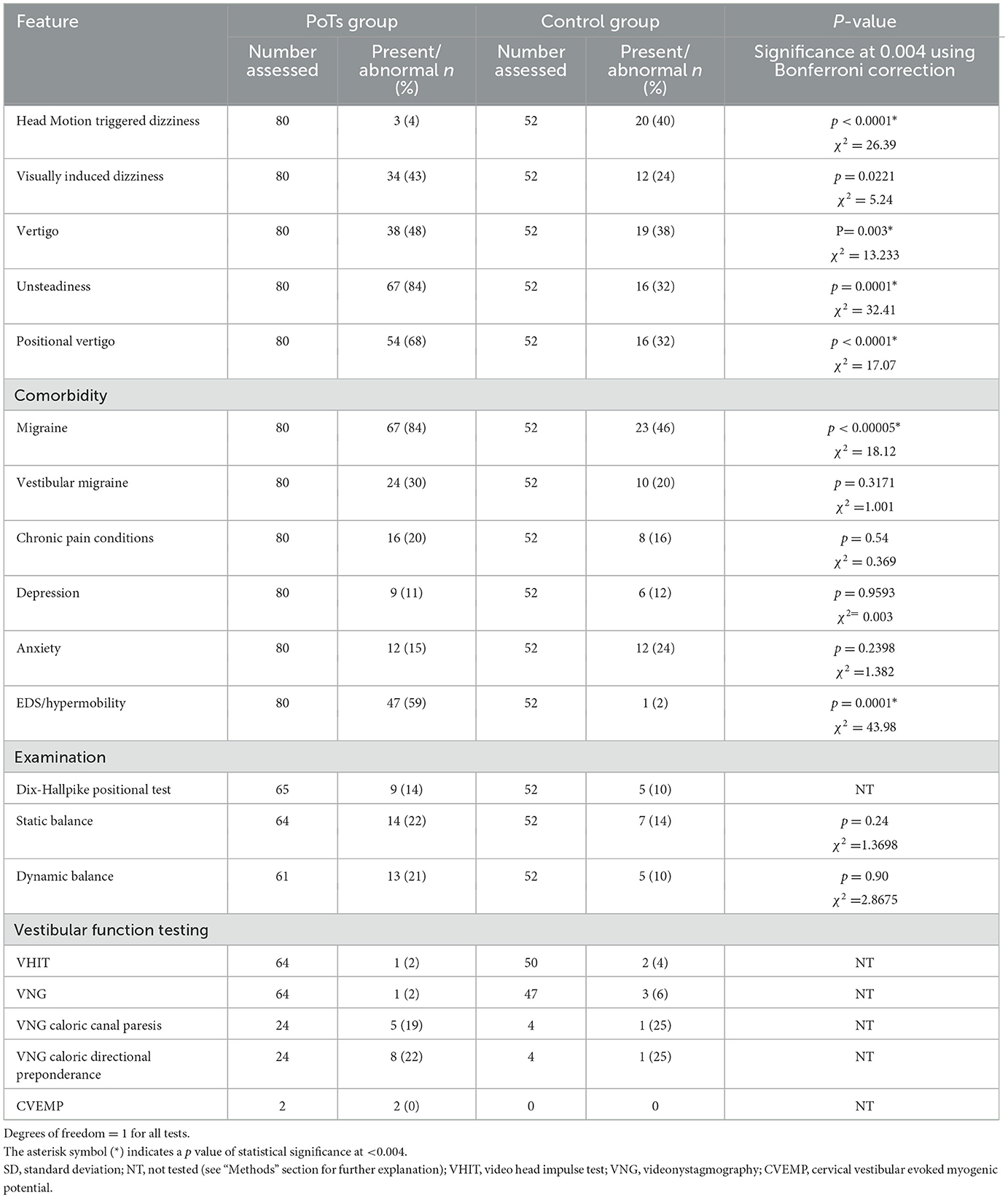
Table 1. Prevalence of vestibular symptoms (not observed during postural induced tachycardia), comorbidities, and proportion of abnormality in examination and vestibular function testing in both controls and PoTS patients.
The symptom questionnaires in the PoTS group demonstrated an SVQ mean score of 1.8 (SD = 1.2), Dizziness Handicap Inventory mean score of 54 (SD = 22.9), GAD7 mean score of 8.2 (SD = 12.2), HIT-6 mean score of 54.7 (SD = 18.3), and DRS mean score of 3.1 (SD = ±1.6) (Table 2). PoTS patients had higher scores than controls in all measures, which was statistically significant (p = 0.0016) for the Disability Rating Score. For the SVQ, the p-value was 0.02, but this was not considered statistically significant when multiple hypothesis correction was applied.
Due to restrictions placed by the COVID-19 pandemic during this data collection phase, face-to-face balance examination and vestibular function testing could not be performed for all patients. Of those assessed, 21% and 22% demonstrated abnormal dynamic or static balance, respectively, and 14% exhibited a positive Dix–Hallpike test. Comparison figures for the control group are provided in Table 1. However, statistical tests were not completed due to low absolute numbers of abnormal findings.
A total of 39 PoTS cases (49%) had migraine treatment and a care plan. In 8 of these 39 cases, this included using a beta blocker, which can be effective for both PoTS and migraine. Other medications offered included nortriptyline or amitriptyline (n = 11), topiramate (n = 3), candesartan (n = 2), and other specific advice in the rest (n = 15) which included use of other prescription medicines, management of menstrual migraine, guidance on acute attack management with medications, lifestyle modifications, and possible evidence-based nutritional supplements or other management strategies.
Of the 39 given migraine management advice, follow-up data (at least 12 weeks following intervention recommendation) were available for 18 patients; 9 patients reported a noticeable migraine improvement reporting both a favorable Global Impression of Change and continued their medication [including nortriptyline, candesartan, beta blockers, topiramate, frovatriptan, and gabapentin (NB: gabapentin was previously included in national guidelines for migraine management, though it has since been removed)]. The remaining nine patients required further intervention. A total of 46 PoTS patients (58%) were offered formal vestibular physiotherapy. Of the 30 patients with available follow-up data (at least 12 weeks following intervention recommendation), 19 patients (63%) demonstrated clinically significant benefit according to the definition, and the remaining 11 patients (37%) reported no benefit.
For comparison, in the control group, 46/50 (92%) underwent vestibular rehabilitation and 14/50 (28%) received additional migraine advice.
Discussion
Our study reports a real-world neurotological perspective in a large cohort of PoTS patients who have also received expert neuro-cardiac evaluation.
Compared to the general population, where 6.5% of men and 18.2% of women experience migraines, the PoTS population in previous studies demonstrates a greater prevalence of migraine of up to 40% (5, 20, 21). It is noted that studies investigating the prevalence of headaches in PoTS seldom identify each headache subtype to determine a representative migraine prevalence for comparison (4, 10, 12). Similarly, dizziness is rarely characterized in detail from a neuro-otological perspective in studies on PoTS.
A relatively high proportion of characteristically “vestibular” symptoms, including visually triggered dizziness, spontaneous vertigo, and positional vertigo, were reported in our cohort.
We also report a migraine prevalence of 84%, which is evidently greater than previously described. Although this disparity is partly attributed to cohort size and referral bias, since our clinic is known for expertise in managing migraine-related dizziness conditions, there was a significantly greater prevalence of migraine in our PoTs population compared to our comparator group.
Although our primary aim in this study was to describe Neuro-otological findings in a PoTS cohort, for a reference point, we compared our PoTS cohort to an age- and sex-matched control group of other dizzy patients reviewed in our clinic. It should be remembered that this control population is atypical for the clinic population due to the need for age and sex matching, which, in this case, necessitates a higher proportion of younger adult women. Compared to the controls, the PoTS cohort had a higher prevalence of vertigo, unsteadiness, and positional vertigo, while the control group had a higher prevalence of head motion-triggered dizziness. This higher prevalence is likely due to a control group with a greater number of younger women. The exclusion of older adults may have reduced the number of individuals with BPPV, who may experience more vertigo.
The diagnosis of Ehlers–Danlos syndrome is complex, and we did not distinguish these in the analysis. Hypermobility was confirmed in the PoTS clinic using the Beighton score, with a cutoff of 4. Visually triggered dizziness was similar in both groups. The rate of visually triggered dizziness in the PoTS group is noteworthy, with 43% describing visually triggered dizziness, similar to the control group. Notably, none of these symptoms are directly attributable to the cardiovascular manifestations of PoTS, suggesting the possibility that there are other mechanisms contributing to dizziness in this group. Since migraine is a recognized comorbidity in PoTs, it is likely that migraine (or vestibular migraine) is responsible for some of these symptoms.
Clinicians diagnosed VM according to recognized criteria in 30% of our PoTs patients, a higher rate than the comparison group (20%), but not statistically significantly different.
Like PoTS, vestibular migraine manifests symptomatically with both migraine and dizziness, demonstrates a strong female preponderance, and is underdiagnosed by healthcare professionals (22). When considering diagnostic criteria for VM (10), it is necessary for clinicians to exclude other causes of symptoms, and this may be challenging in the presence of a comorbidity that is also associated with both dizziness and migraine, such as PoTS. We consider if this may be a reason why the prevalence of migraine was very high (84%) in our cohort, but only 30% were diagnosed with vestibular migraine. Further research to develop instruments to assess dizziness and migraines, particularly vestibular migraine, in complex populations such as those with PoTS would be beneficial.
Approximately 59% of our PoTS cohort have a diagnosis of hypermobility, similar to the combined finding of 55% described by Miller et al. in the first cross-sectional prospective study evaluating the prevalence of hypermobile EDS and general joint hypermobility in PoTS (23). To the best of our knowledge, despite being well documented as an association, it is not known why PoTS and EDS/hypermobility syndrome overlap, nor why there is an association with migraine. Furthermore, we identified the prevalence of chronic pain (20%), anxiety (15%), and depression (11%), all of which may influence illness perception; particularly, anxiety may potentially cause or complicate dizziness.
Our PoTS cohort also had an abnormal Dix-Hallpike positioning test in 14%, including cases of benign paroxysmal positional vertigo, again confirming that the mechanism of dizziness in this cohort can be complex and multifaceted. The positive Dix–Hallpike test finding may have several explanations. Benign paroxysmal positional vertigo and migraine can both cause vertigo and nystagmus on positional tests. Since approximately a third of the PoTS population experience recurrent syncopal episodes (24), a predisposition to head trauma and thereby an association with benign paroxysmal positional vertigo may be theorized. This is a presentation the authors have observed; however, the proposed mechanism remains speculative. A similar rationale may apply to the abnormalities observed in caloric and VHIT testing, which could, in part, be due to a post-traumatic etiology.
Similarly, clinical static and dynamic balance tests were frequently abnormal. This may be secondary to the influence of other comorbidities, such as joint hypermobility, or directly related to the pathophysiology of PoTS, such as in cases associated with a lower limb small fiber neuropathy (25, 26). Further research is warranted to elucidate the mechanisms of impaired balance control in this population.
A considerable proportion were offered migraine management advice and continued the medication at follow-up. This is consistent with our clinical experience, where migraine management, for which several efficacious evidence-based treatments exist, may be easily neglected in the context of a patient population with significant comorbidity. Addressing migraine has the potential to improve quality of life. Similarly, vestibular physiotherapy is beneficial for managing symptoms in selected cases. This observation demonstrates the value of a multidisciplinary approach to assessing and managing dizziness in individuals with PoTS. While it may not be practical for all patients to access specialist neuro-otological or neurological assessments, research could be directed toward developing standardized assessment tools to identify which patients may benefit from specific interventions.
The primary limitations of this study are its retrospective design, observational nature in relation to clinical practice, and referral bias, the latter of which is typical of a specialist clinic cohort and may limit generalizing findings to the PoTS population more widely. Although our study was conducted during the COVID-19 pandemic, which has influenced data completeness, our findings still illustrate the heterogeneity of dizziness in PoTS; several atypical dizziness conditions, often considered atypical of PoTS, were additionally identified within this study. We were able to carry out clinical neuro-otological assessments in a cohort that had been well characterized from a neuro-cardiological perspective, providing a grounding for future work in this area.
Conclusion
Our findings reflect the challenges of distinguishing dizziness phenotypes, including vestibular disorders and vestibular migraine, within the PoTS population. Vestibular migraine appears to be common in patients with PoTS. We advocate judicious clinical assessment of dizziness in PoTS to identify non-orthostatic mechanisms that may cross traditional disciplines and necessitate contributions from neurologists, vestibular specialists, and cardiologists, among others. We provide a basis for further prospective research to elucidate the multifaceted nature of PoTS-associated dizziness and for the development of clinical tools to assess vestibular migraine and migraine in complex patient populations.
Data availability statement
The raw data supporting the conclusions of this article will be made available upon reasonable request and subject to institutional approvals. Requests to access the datasets should be directed to bG91aXNhLm11cmRpbkBnc3R0Lm5ocy51aw==.
Ethics statement
The studies involving humans were approved by the NHS Research Ethics Committee (IRAS ID: 257283 Ref 20/EM/0112). The studies were conducted in accordance with the local legislation and institutional requirements. The Ethics Committee/Institutional Review Board waived the requirement of written informed consent for participation from the participants or the participants' legal guardians/next of kin because the institutional ethics approval was for the use of pooled, anonymized, routinely collected clinical data, analyzed by the treating clinician, with safeguards for opt-out in the institution for any who did not wish for their data to be used. There is a full institutional review process for any projects completed under this method which was completed.
Author contributions
RS: Writing – original draft, Writing – review & editing. NG: Conceptualization, Investigation, Writing – original draft, Writing – review & editing. CA: Writing – original draft, Writing – review & editing. LM: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We would like to thank individuals with PoTS we have seen in our clinical practice, the representatives of PoTS UK who provided study proposal comments, and the Multidisciplinary Balance Team at Guy's Hospital.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Freeman R, Wieling W, Axelrod FB, Benditt DG, Benarroch E, Biaggioni I, et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin Autonom Res. (2011) 21:69–72. doi: 10.1007/s10286-011-0119-5
2. Kim HA, Bisdorff A, Bronstein AM, Lempert T, Rossi-Izquierdo M, Staab JP, et al. Hemodynamic orthostatic dizziness/vertigo: diagnostic criteria. J Vestibul Res Equilibr Orient. (2019) 29:45–56. doi: 10.3233/VES-190655
3. Bryarly M, Phillips LT, Fu Q, Vernino S, Levine BD. Postural orthostatic tachycardia syndrome: JACC focus seminar. J Am Coll Cardiol. (2019) 73:1207–28. doi: 10.1016/j.jacc.2018.11.059
4. Arnold AC, Ng J, Raj SR. Postural tachycardia syndrome—diagnosis, physiology, and prognosis. Autonom Neurosci Basic Clin. (2018) 215:3–11. doi: 10.1016/j.autneu.2018.02.005
5. Thieben MJ, Sandroni P, Sletten DM, Benrud-Larson LM, Fealey RD, Vernino S, et al. Postural orthostatic tachycardia syndrome: the Mayo clinic experience. Mayo Clin Proc. (2007) 82:308–13. doi: 10.1016/S0025-6196(11)61027-6
6. Benarroch EE. Postural tachycardia syndrome: a heterogeneous and multifactorial disorder. Mayo Clin Proc. (2012) 87:1214–25. doi: 10.1016/j.mayocp.2012.08.013
7. Sheldon RS, Grubb BP II, Olshansky B, Shen WK, Calkins H, Brignole M, et al. 2015 heart rhythm society expert consensus statement on the diagnosis and treatment of postural tachycardia syndrome, inappropriate sinus tachycardia, and vasovagal syncope. Heart Rhythm. (2015) 12:e41–63. doi: 10.1016/j.hrthm.2015.03.029
8. Bisdorff A, Von Brevern M, Lempert T, Newman-Toker DE. Classification of vestibular symptoms: Towards an international classification of vestibular disorders. J Vestib Res Equilibr Orient. (2009) 19:1–13. doi: 10.3233/VES-2009-0343
9. ICHD. Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. Cephalalgia. (2018) 38:1–211. doi: 10.1177/0333102417738202
10. Lempert T, Olesen J, Furman J, Waterston J, Seemungal B, Carey J, et al. Vestibular migraine: diagnostic criteria (Update). J Vestibul Res Equilibr Orient. (2021) 22:167–72. doi: 10.3233/VES-201644
11. Yang M, Rendas-Baum R, Varon SF, Kosinski M. Validation of the Headache Impact Test (HIT-6™) across episodic and chronic migraine. Cephalalgia. (2011) 31:357–67. doi: 10.1177/0333102410379890
12. Spitzer RL, Kroenke K, Williams JB, Löwe B, A. brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. (2006) 166:1092–7. doi: 10.1001/archinte.166.10.1092
13. Jacob RG, Lilienfeld SO, Furman JM, Durrant JD, et al. Panic disorder with vestibular dysfunction: Further clinical observations and description of space and motion phobic stimuli. J Anxiety Disord. (1989) 3:117–30. doi: 10.1016/0887-6185(89)90006-6
14. Jacobson GP, Newman CW. The development of the Dizziness Handicap Inventory. Arch Otolaryngol Head Neck Surg. (1990) 116:424–7. doi: 10.1001/archotol.1990.01870040046011
15. Shepard NT, Telian SA, Smith-Wheelock M. Habituation and balance retraining therapy. A retrospective review. Neurol Clin. (1990) 8:459–75. doi: 10.1016/S0733-8619(18)30367-0
16. Horn LB, Rice T, Stoskus JL, Lambert KH, Dannenbaum E, Scherer MR. Measurement Characteristics and Clinical Utility of the Clinical Test of Sensory Interaction on Balance (CTSIB) and Modified CTSIB in Individuals With Vestibular Dysfunction. Arch Phys Med Rehabil. (2015) 96:1747–8. doi: 10.1016/j.apmr.2015.04.003
17. British Society of Audiology. Vestibular Assessment—Eye Movement Recordings (2015). Seafield: British Society of Audiology. Available online at: http://www.thebas.org.uk
18. British Society of Audiology. Recommended Procedure: The Caloric Test (2010). Seafield: British Society of Audiology. Available online at: http://www.thebas.org.uk
19. The British Association for the Study of Headache. National Headache Management System for Adults (2019). London: The British Association for the Study of Headache.
20. Lipton RB, Stewart WF, Diamond S, Diamond ML, Reed M. Prevalence and burden of migraine in the United States: data from the American Migraine Study II. Headache. (2001) 41:646–57. doi: 10.1046/j.1526-4610.2001.041007646.x
21. D'Antona LaM M. “Headache in postural tachycardia syndrome”. In: Gall N KL, Lobo MD, editor, Postural Tachycardia Syndrome. Switzerland: Springer Nature (2021).
22. Lempert T, Neuhauser H. Epidemiology of vertigo, migraine and vestibular migraine. J Neurol. (2009) 256:333–8. doi: 10.1007/s00415-009-0149-2
23. Miller AJ, Stiles LE, Sheehan T, Bascom R, Levy HP, Francomano CA, et al. Prevalence of hypermobile Ehlers-Danlos syndrome in postural orthostatic tachycardia syndrome. Autonom Neurosci Basic Clin. (2020) 224:102637. doi: 10.1016/j.autneu.2020.102637
25. Gibbons CH, Bonyhay I, Benson A, Wang N, Freeman R. Structural and functional small fiber abnormalities in the neuropathic postural tachycardia syndrome. PLoS ONE. (2013) 8:e84716. doi: 10.1371/journal.pone.0084716
Keywords: postural tachycardia syndrome, migraine, vertigo, clinical observation, dizziness
Citation: Sekhon R, Gall N, Ainsworth C and Murdin L (2025) Dizziness in postural tachycardia syndrome and its link to vestibular migraine. Front. Neurol. 16:1583348. doi: 10.3389/fneur.2025.1583348
Received: 25 February 2025; Accepted: 29 April 2025;
Published: 30 May 2025.
Edited by:
Thomas Lempert, Schlosspark-Klinik, GermanyReviewed by:
Alfarghal Mohamad, National Guard Hospital, Saudi ArabiaFernando Gananca, Federal University of São Paulo, Brazil
Copyright © 2025 Sekhon, Gall, Ainsworth and Murdin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Louisa Murdin, bG91aXNhLm11cmRpbkBnc3R0Lm5ocy51aw==
 Ruby Sekhon1
Ruby Sekhon1 Carolyn Ainsworth
Carolyn Ainsworth Louisa Murdin
Louisa Murdin