- 1School of Clinical Science, Auckland University of Technology, Auckland, New Zealand
- 2Eisdell Moore Centre for Hearing and Balance Research, University of Auckland, Auckland, New Zealand
- 3Department of Pharmacology and Toxicology, Faculty of Biomedical and Molecular Sciences, The Brain Health Research Centre, University of Otago, Dunedin, New Zealand
- 4Department of Physiology, University of Auckland, Auckland, New Zealand
- 5New Zealand Dizziness and Balance Centre, Auckland, New Zealand
An established aspect of noisy galvanic vestibular stimulation (nGVS) is tuning the nGVS signal to optimize stability on an individual basis. However, conventional tuning methods are strongly influenced by historical approaches and fail to integrate contemporary research findings. We outline a process used to integrate current physiological and neuroscientific insights into a robust method for personalizing nGVS signals to improve stability. We argue that an optimization protocol for a neuromodulatory nGVS signal designed to facilitate postural control needs to include: (1) A task that is relevant to the population, and which can be modified to give an appropriate level of challenge at an individual level; (2) Elements that can be reliably measured and are responsive to changes in postural control; (3) Well controlled and defined signal parameters; (4) Potential to be translated into the clinical setting. Questioning conventional methods enabled us to develop an alternative nGVS optimization assessment to enhance postural control in people with bilateral vestibulopathy. Refining this optimization assessment represents a crucial step in developing individualized nGVS interventions. The fundamental principles applied to develop our method can be adapted to other neuromodulatory stimuli across different impairments and populations.
Introduction
Postural control refers to our ability to maintain, achieve or restore a state of balance (1). Adequate postural control is a prerequisite for independent mobility, and if lacking can lead to reduced mobility, loss of confidence, imbalance, falls, injury, and social isolation (2, 3). There are large social and economic costs associated with poor balance (4). On this basis, interventions to restore postural control have been explored extensively (5, 6). Noisy galvanic vestibular stimulation (nGVS) is one treatment option that has been investigated. While nGVS has been found to improve postural control in research trials (7–27) and meta-analyses (28, 29), results have not been consistent. The dynamic and interdependent nature of postural control mechanisms, alongside the diverse signal parameters and varied methods of signal optimization, appear to contribute to the discrepancies noted in research (30).
nGVS is a stochastic noisy neuromodulatory stimulus, typically delivered as zero mean and Gaussian via electrodes placed bilaterally over the mastoid process (30). It has been used to facilitate postural control in people with impaired balance stemming from aging or neurological deficits (7, 25, 26, 31, 32). Optimizing the noisy galvanic vestibular stimulation (nGVS) signal to give optimum postural control has been identified as an important component of this neuromodulatory intervention, as the most effective parameters can vary between individuals (30, 31, 33–37). However, despite evidence for the efficacy of nGVS to improve stability, and for the importance of optimizing the signal (28, 30), there has been little research investigating the processes involved in optimizing nGVS to improve postural control.
Central to optimization is the premise that everyone's physiology is unique, thus nGVS parameters must be personalized to achieve the best response. The dominant theory underpinning nGVS is stochastic resonance; the theory that in non-linear sensory systems—i.e., systems characterized by a discrete threshold for sensory transmission—a noisy signal can enhance the detection of weak afferent inputs (38, 39). However, research findings are conflicting, with some studies supporting this theory (25, 31, 40, 41) while others report a response to nGVS that does not follow a stochastic pattern (31, 42, 43). A neuroplastic mechanism may operate alongside the stochastic process. This could help explain postural response patterns that do not follow a stochastic curve, cortical changes observed during and after nGVS, and the sustained effects following stimulation (32, 44, 45).
Conventional optimization methods are strongly influenced by historical approaches. As nGVS research advances, it is important to assess prior assumptions in light of emerging knowledge. In this paper we explore the influence of the task, task challenge, parameter choice, population, responsiveness and choice of outcome measures, on the optimization assessment. We propose an approach to help researchers identify factors that will bolster the optimization process when using nGVS to improve postural control. The overarching principles applied to develop our methodology may apply to other neuromodulatory stimuli that are used to improve postural control.
What has been tried to date?
To date, three primary methods of tuning the amplitude of the signal to improve postural control have been reported. These methods have varied in time to complete, equipment required and theoretical underpinnings.
Motion perception to a 1 Hz sinusoidal galvanic vestibular stimulation (GVS) waveform has been used to determine a threshold amplitude. A 1 Hz sinusoidal GVS waveform is delivered, and the amplitude at which an individual senses mediolateral motion or, is observed moving on a force plate, is taken as the sensory threshold. A percentage of this threshold amplitude (between 50% and 100%) is then applied to an nGVS waveform (17, 21, 24, 46–50). While a conceptually sound method to investigate the responsiveness of the vestibular system, there is no evidence for a commensurate relationship between the motion perception threshold to a sinusoidal signal and a postural response to a noisy signal (49). Although this method has been used historically, it has fallen out of favor in recent years. It also requires equipment that can provide both a sinusoidal and a noisy signal at a variety of amplitudes; this type of equipment is not readily available at present.
The cutaneous nGVS threshold has been used as a quick, simple method of optimization, appealing for research and clinical practice (15, 16, 27, 51). The cutaneous threshold is determined by finding the point at which nGVS elicits cutaneous sensation under the electrodes (15, 16, 27, 51). Stimulation is then delivered at around 80% of this cutaneous threshold. Hesitancy exists around this method as the relationship between cutaneous sensation and vestibular function is unclear (30). Sensation from the skin over the mastoid process, travels through the posterior branch of the auricular nerve, through the dorsal root ganglion to the C2/3 spinal root, then ascends to synapse in the medulla, before transmission to the sensory cortex. In contrast the vestibular system sends signals from the vestibular apparatus via the vestibulocochlear nerve, to the vestibular nuclei in the brainstem prior to making diffuse connections within the brain, including the cerebellum and hippocampus (52). In addition, the threshold for cutaneous sensation is influenced by a complex interplay of physiological, environmental and temporal factors. Stress levels, circadian rhythm and airborne allergens, are among some of the factors that can influence sensory perception, mitigating the reliability of this method (53–55).
The most direct method for optimizing nGVS stimulation is to present nGVS at different amplitudes to identify the point at which postural stability is maximally enhanced (7, 8, 20, 22, 23, 42, 49, 50, 56). Although time intensive, this method has been the most frequently employed (30), most closely aligns with the principle of stochastic resonance, and serves as the gold standard in comparative studies (8, 19, 49, 50). Wuehr et al. (26) applied various amplitudes to optimize nGVS in people with bilateral vestibulopathy (BVP). Their findings revealed that most participants exhibited stochastic response patterns. This supports the theoretical foundation for using postural control measurements across different amplitudes to identify the optimum stimulation amplitude. While three different optimization methods appear in the nGVS literature, directly testing postural stability across a range of amplitudes remains the most conceptually sound approach. Although this method requires more time, this disadvantage is offset by the greater certainty it provides in determining the optimal stimulation parameters.
What factors should we consider in task selection?
Postural stability requires appropriate motor output in response to multisensory integration of vestibular, visual, and proprioceptive afferent information. Historically, the majority of nGVS studies have focussed on optimizing the stimulation signal based on changes observed during standing (30). However, loss of standing postural control is seldom mentioned in the clinical BVP literature (2). The Bilateral Vestibulopathy Questionnaire does not include standing balance, and the Oscillopsia Functional Impact Scale has only one question referencing standing, out of a total of 43 (57, 58). In contrast, gait instability is widely recognized as a defining feature of BVP (59). In the Bilateral Vestibulopathy Questionnaire five of the 20 questions relate to gait (57) and in the Oscillopsia Functional Impact Scale 12 of 43 questions refer to gait (58). Recent preliminary research indicates that responses to nGVS may be task specific. Peto et al. (60) found that signals optimized in a standing position had no effect during gait in individuals with Parkinson's disease. Further research is required to determine whether this task specific response to nGVS is generalized to other populations. We propose that in studies to improve postural control, choosing an optimization task that is relevant to the individual and their deficit is preferable.
Typically, nGVS optimization in standing has utilized the velocity of the center of pressure during postural sway, sometimes combined with sway area and root mean square (RMS) displacement (30). In standing the body is commonly modeled as a single-segment inverted pendulum pivoting around the ankle (61). Quiet stance is characterized by body sway produced by gravity acting on the center of mass and by intrinsic forces. Equilibrium is maintained primarily by steady state control, where the musculoskeletal system makes small postural adjustments to maintain stability. Postural sway gives us insight into the sensory-motor integrity of the nervous system in a situation where the primary destabilizing forces are gravity and small internal forces (62). The argument for using postural sway as an optimization task is that limiting sway is advantageous to a point. Reducing mediolateral sway appears to be particularly beneficial to maintain balance (1, 63). While using standing sway as an optimization task has merit, it is reliant on the assumption that less sway is better. In reality, there is a “Goldilocks zone” for sway. Too much sway can indicate insufficient sensory information is available for the sensitive and fine control required to maintain quiet standing. Conversely, too little sway can indicate the individual is using excessive rigidity to maintain postural control and lacks the flexibility characteristic of a healthy biological system (64–66). Thus, a reduction in sway may not always indicate improved postural control. In addition, while imbalance and oscillopsia are the primary deficits reported in BVP (2), patients seldom report that these deficits limit their function in quiet standing (57, 58). Consequently, standing may not be a task that adequately represents situations people with BVP find challenging.
An alternative to standing is the use of gait as the optimization task. A strong argument in favor of this is that the primary deficit reported by people with BVP is imbalance during gait (2, 3, 67). This reinforces the role of the vestibular system in more dynamic activity involving greater head movement and postural challenge. Gait requires the coordination of numerous muscles and joints to progress forward, orientate body segments and adjust to environmental demands (68). Vestibular information regarding the acceleration and translation of the head is integrated with afferent inputs from the visual, and somatosensory systems (69). During gait the center of mass sits outside the base of support for 60–80% of the gait cycle, making the task inherently unstable (53, 70). We maintain that, for people with BVP, assessing spatiotemporal gait parameters is a more relevant and meaningful task on which to base neuromodulatory signal optimization.
What aspects of task challenge do we need to consider?
Task challenge appears to affect the ability of nGVS to influence postural control (71). When an optimization task is too easy, the vestibular system already has capacity to meet task demands. For example, nGVS has no effect on postural control when healthy individuals walk across a well-lit room (22). The healthy vestibular system has sufficient capacity to easily meet the demands of the task, and nGVS facilitation has no effect on motor output. In contrast, when people with BVP receive nGVS facilitation walking in a well-lit room, their gait stability improves (22). The gait task challenges the capacity of people with BVP; thus, facilitation of the vestibular afferent signal improves gait stability.
At the other extreme, a highly challenging task can exceed the capabilities of the postural control system to the point where despite an nGVS boost to the vestibular system, the system will still fail. For example, nGVS failed to improve postural control when people with BVP stood on foam with their eyes closed (21). An extremely challenging task such as this may be so far beyond the individual's capacity there is not a measurable response to an enhanced vestibular signal. Consequently, a task that is too easy or too hard can obscure the potential benefit of neuromodulation.
Individual capacity influences task challenge (71). Overground walking is sufficiently challenging to see the effects of nGVS in most people with BVP, making it a suitable task (8, 22). However, higher functioning participants may require a more challenging option (69, 72, 73). Thus, it is worthwhile considering how the task may be modified to provide an ideal level of challenge. A hallmark of BVP is imbalance when visual input is reduced, or somatosensory information becomes less reliable (59). Therefore, we can consider how these components can be manipulated during the optimization task. For example, walking with eyes open or closed will alter the visual condition, and the choice of surface may influence the somatosensory feedback. Similarly, modifying gait speed may offer an alternative approach to influence task challenge. Slow gait speeds are associated with increased gait variability in people with BVP (69, 72), as well as greater responsiveness to nGVS (15).
What aspect of gait should we measure, and how does this relate to the output we are modulating?
Historically, gait motor control theory has been dominated by the role of central pattern generators; neuronal circuits at a spinal level that can produce rhythmical motor patterns, such as those used in gait (74). While the central pattern generator has a crucial role to play, this focus has led to less emphasis on the influence of afferent sensory feedback, and cortical control which enables variability and flexibility in the gait cycle (75). This variability and flexibility, particularly the ability to make precise adjustments to foot placement and step timing are prerequisites for walking in the real world (76, 77).
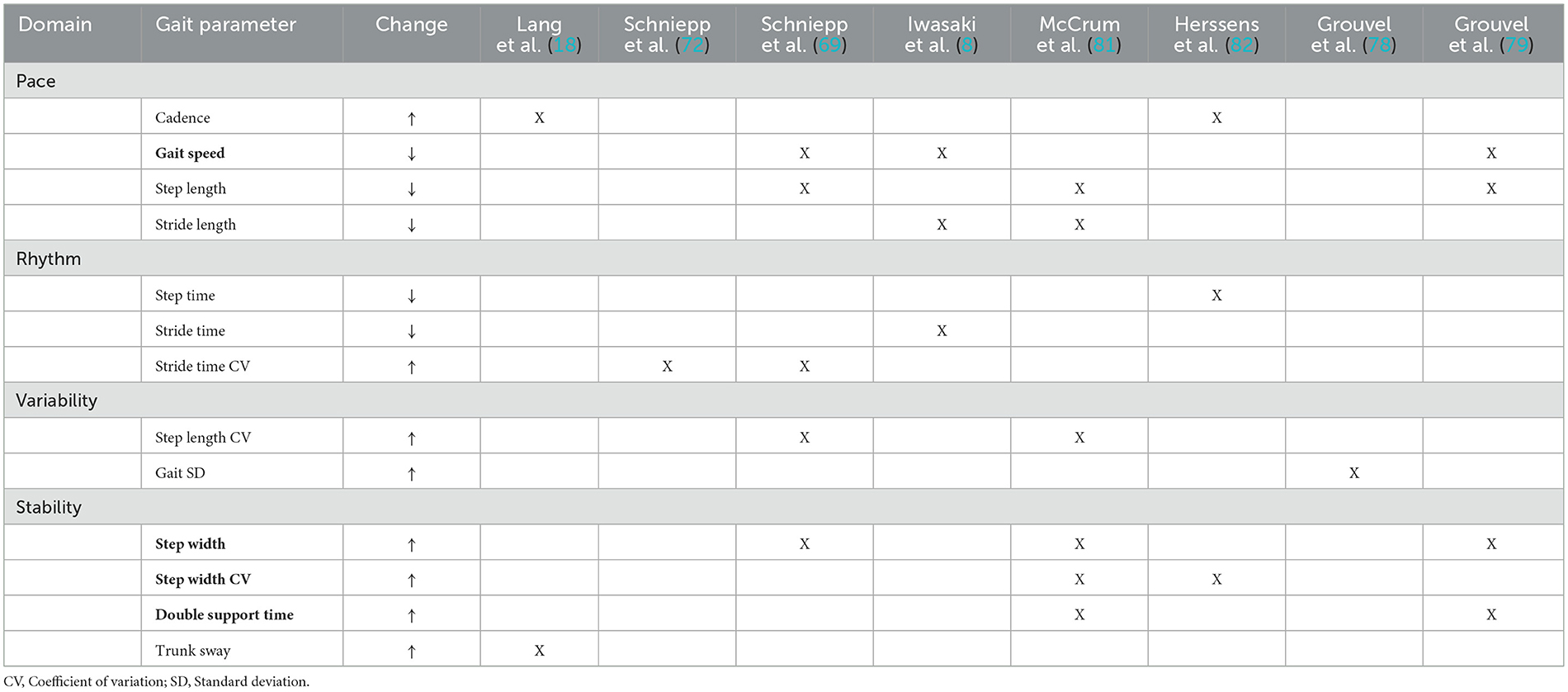
The spatiotemporal parameters of gait have been divided into domains that represent characteristics of gait: namely, pace, rhythm, variability, asymmetry and stability. People with BVP demonstrate marked changes in the domains of pace, variability and stability (69, 73, 75, 78–80). The gait patterns observed in individuals with BVP typically involve spatial and temporal adaptations that build more stability into the gait pattern. People with BVP tend to have a slower preferred gait speed, with shorter quicker steps- this reduces the duration of time where the center of mass lies outside of the base of support (8, 69, 80–82). Steps tend to be wider, with a larger proportion of the gait cycle spent in double support. This improves lateral stability and increases the opportunity to use motor strategies to control the center of mass (69, 79, 81, 82). Gait is also thought to become more variable in the absence of vestibular information. Studies have found people with BVP demonstrate more variability in foot placement, with higher standard deviations and coefficients of variation of stride time, step length and step width (69, 72, 78, 81). Changes to the spatiotemporal parameters of gait are hypothesized to result from the lack of vestibular afferent information affecting feedforward mechanisms of motor control and the fine tuning of foot placement (75, 76, 83).
There is little work investigating optimization of nGVS using gait. Mulavara et al. (49) used a cost function derived from 7 gait stability measures during perturbed walking at different nGVS signal amplitudes. This method was complex, requiring extensive equipment and calculations and has not been repeated in the literature. Iwasaki et al. (8) used the nGVS amplitude that resulted in the greatest increase in gait speed. This decision was based on the premise that the vestibular system plays a role in maintaining a consistent pace (75). In contrast to the complex approach adopted by Mulavara et al. (49), the Iwasaki et al. (8) approach may oversimplify the vestibular impact on gait. Although people with BVP typically have a slower preferred gait speed (72), they may periodically maintain or even increase walking velocity to reduce their reliance on afferent sensory information (73, 82).
To find a middle ground between existing approaches, we propose a theoretically informed methodology for parameter optimization based on assimilation of the gait and BVP literature. Lord et al. (84) divided gait into five key domains: pace, rhythm, variability, asymmetry, and stability (Table 1). Using the BVP literature, we identified key spatiotemporal features of gait affected when vestibular afferent information is lacking and categorized them by domain (Table 1) (75). We hypothesized that if these gait parameters were influenced by the absence of vestibular signals, then restoration of these afferent signals would change the parameters in the direction of improved stability. By interpreting the BVP literature through the lens of gait theory, we were able to combine theoretical understanding with practical application to develop a robust approach to optimization assessment (Table 2).
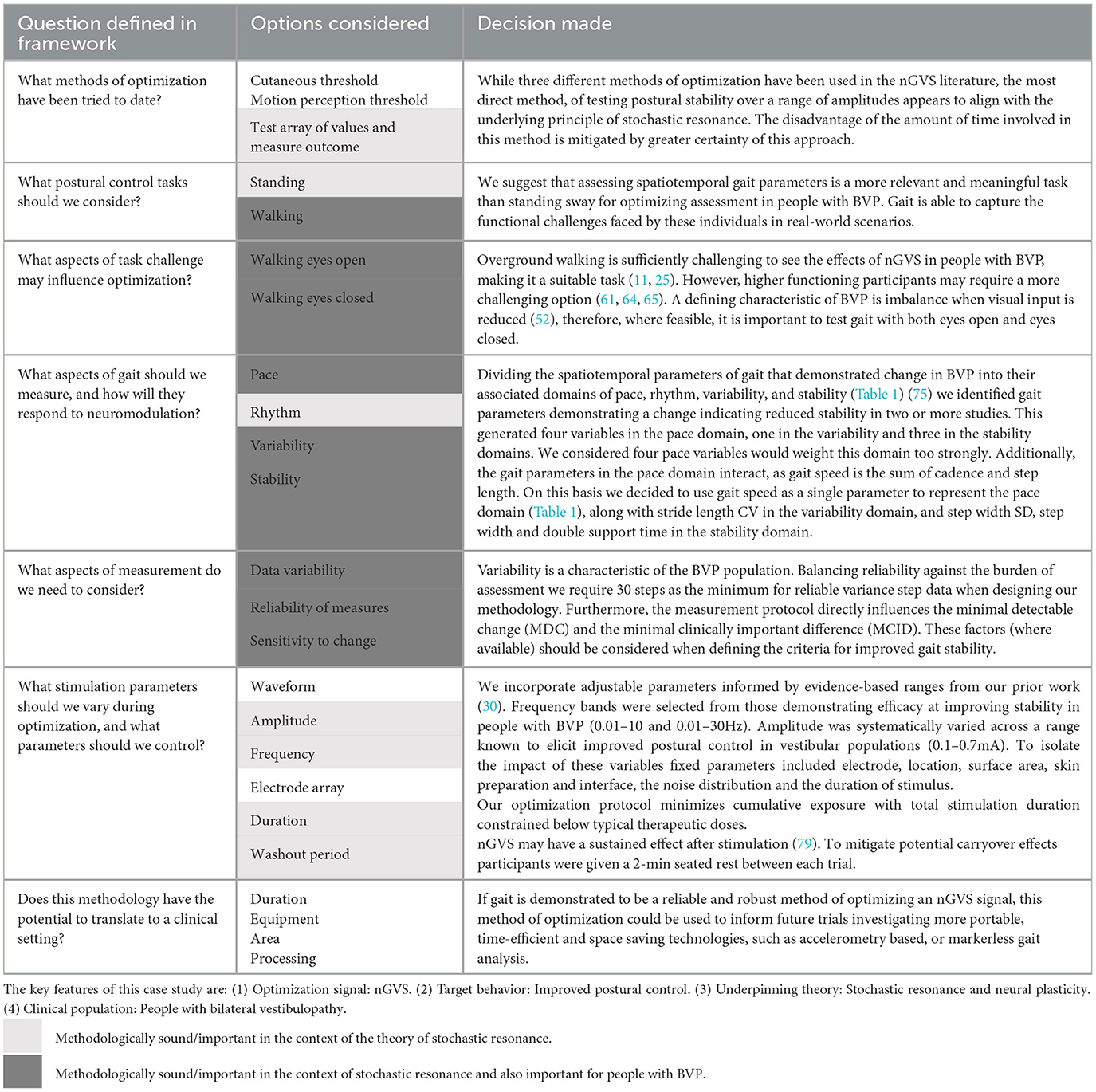
Table 2. Application of the theoretical optimization framework to an nGVS optimization protocol for people with bilateral vestibulopathy.
Measurement considerations during testing and analysis?
Neuromodulatory optimization studies evaluate within session changes, which are generally modest. Therefore, it is crucial to consider both reliability of the testing methods and how population characteristics might influence the data. In people with vestibular disorders within session test- retest reliability of preferred gait speed is excellent using both manual (ICC = 0.88) and instrumented (ICC = 0.94) recording methods (85, 86). However, the measurement of other gait domains can be challenging as we manage both internal and external sources of variability. Internal sources represent the normal variability of gait as well as variability that represents deficit due to pathological mechanisms (87). Internal variability provides important insights about the stability of gait and the neurological and biomechanical control of posture. It is generally accepted that people with BVP typically exhibit highly variable step time, step and stride length and step width (69, 72, 73, 78, 82).
External variability is the variability that occurs due to measurement error. This variability needs to be minimized to ensure that the variability that is seen in the data primarily represents the internal variability. For example, many studies investigating gait in people with BVP have not reported the number of steps analyzed, and those that do, report using 4–14 steps in their analysis, increasing the risk of external variability in the results (72, 82). It is critical that our methodology is robust and minimizes the risk of measurement error. For example, when measuring variance, research in older adults suggests that between 30 and 220 steps are required to achieve reliable step variance data (88, 89). Of the 5 studies investigating overground walking in people with BVP (8, 69, 72, 78, 79), only one study captured gait over a distance >12 m (69). Therefore, it is unlikely that the volume of steps in these data reached the number required to establish reliable variance measures.
A further cause of external variability can be instrumentation error. A high number of outliers have been reported in the spatiotemporal gait data of people with BVP (69, 72, 73, 78, 79). This can be challenging to manage as outliers relating to the use of foot placement to control the center of mass and maintain postural stability should be retained (internal variability). Conversely, outlier data relating to instrumentation error and not representative of spatiotemporal gait parameters must be removed to maintain data integrity.
What nGVS parameters should we use, and how may these affect the method?
Guidelines for the stimulation parameters that influence the signal delivered have been covered extensively in our previous paper (30). While nGVS holds promise as a means of improving gait and balance in people with vestibular disorders (28), its transition into clinical practice is hindered by the vast array of different parameters that have been used, and a lack of consensus on optimizing them effectively (30). Parameters such as the electrode surface area, electrode/skin interface, frequency band, amplitude and noise distribution have potential to influence the efficacy of the signal and must be prescribed thoughtfully and reported fastidiously so we can further our knowledge in this area (30). Prior research informs us of parameters and parameter ranges that are likely to be effective, and that require further investigation. Other parameter variables should remain consistent, so the effect of the experimental parameters can be realized.
Additionally, the dose of stimulation over the course of the protocol must remain within safe limits. That is, the total duration of stimulation participants received in the optimization session should remain less than they would receive in a treatment session.
Residual effects and the need for a washout period between stimuli also become important when we are looking for subtle dose-related changes (31). nGVS may have a sustained effect after stimulation (32). The duration of washout between optimization trials has been poorly reported in the nGVS literature with most papers failing to report on the washout period (7, 20, 22, 23, 31, 32, 49, 56, 90). Those that report this metric (8, 19, 22, 42, 48, 50, 91) have a break ranging between 20 s (19, 50) and 3 min (7, 19) between stimulation trials. It has not been ascertained whether these durations are sufficient to eliminate the effects of nGVS. Some studies have found no residual effects (33), while others have noted effects for up to 4 h (7, 14). There is also the potential for cumulative stimulation effects that have not been explored to date. The washout period for stimulation effects warrants further investigation as it has the potential to influence both optimization processes, and the duration of residual facilitatory effects post-nGVS treatment.
Does this methodology have the potential to translate to optimization in a clinical setting?
While to date, nGVS optimisation has been conducted in research settings, the future of this technology is its adoption in the clinical space. Laboratory- based optimization relies on laboratory equipment such as force plates, motion capture systems and treadmills, which are typically not available in clinical settings due to their high costs, space requirements, and the technical expertise and time required for data acquisition, processing and analysis (30, 92). However, the strength of laboratory-based optimization protocols lies in their ability to generate rich data sets, providing a robust foundation for the development of simpler methods to assess stability in future clinical applications. Emerging technologies, such as body worn sensors, accelerometery and markerless gait analysis systems, are capable of capturing metrics that reliably measure postural stability and show potential as efficient and robust tools for movement analysis within clinical settings (93–95). As nGVS technology approaches clinical feasibility, it is increasingly important to consider how the principles established and validated in the laboratory can be effectively translated into more portable, time efficient and space saving technologies.
Conclusion
As the importance of individualizing neuromodulation parameters becomes established, it is timely to critically examine current optimization practice, with a view to improve methodological rigor. By critically examining existing methods and incorporating our knowledge of neuroscience and motor control, we have developed a methodology that prioritizes task relevance, appropriate levels of challenge and population specific considerations. Our approach emphasizes the importance of selecting meaningful outcome measures, ensuring data quality and considering the potential for clinical translation from the conceptual stages. The seven key questions proposed in this paper (Table 2), provide a framework for researchers to design rigorous optimization protocols for neuromodulatory stimuli and a foundation for developing individualized interventions.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
RM: Funding acquisition, Conceptualization, Writing – review & editing, Methodology, Writing – original draft. PS: Funding acquisition, Writing – review & editing, Supervision. RT: Writing – review & editing, Supervision, Funding acquisition. DT: Supervision, Methodology, Writing – review & editing, Conceptualization, Funding acquisition.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Health Research Council of New Zealand (Grant Number HRC22/363).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that Gen AI was used in the creation of this manuscript. To edit some portions of the manuscript. The content edited using the Generative AI has been checked for factual accuracy and plagiarism.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Morelli N, Hoch M. A Proposed postural control theory synthesizing optimal feedback control theory, postural motor learning, and cerebellar supervision learning. Percept Mot Skills. (2020) 127:1118–33. doi: 10.1177/0031512520930868
2. Lucieer F, Duijn S, Van Rompaey V, Perez Fornos A, Guinand N, Guyot JP, et al. Full spectrum of reported symptoms of bilateral vestibulopathy needs further investigation- a systematic review. Front Neurol. (2018) 9:352. doi: 10.3389/fneur.2018.00352
3. Lucieer FMP, Van Hecke R, van Stiphout L, Duijn S, Perez-Fornos A, Guinand N, et al. Bilateral vestibulopathy: beyond imbalance and oscillopsia. J Neurol. (2020) 267:241–55. doi: 10.1007/s00415-020-10243-5
4. Agrawal Y, Carey JP, Della Santina CC, Schubert MC, Minor LB. Disorders of balance and vestibular function in US adults. Arch Intern Med. (2009) 169:938–44. doi: 10.1001/archinternmed.2009.66
5. James SL, Lucchesi LR, Bisignano C, Castle CD, Dingels ZV, Fox JT, et al. The global burden of falls: global, regional and national estimates of morbidity and mortality from the Global Burden of Disease Study 2017. Inj Prev. (2020) 26:i3–i11. doi: 10.1136/injuryprev-2019-043286
6. Sun DQ, Ward BK, Semenov YR, Carey JP, Della Santina CC. Bilateral vestibular deficiency: quality of life and economic implications. JAMA Otolaryngol Head Neck Surg. (2015) 140:527–534. doi: 10.1001/jamaoto.2014.490
7. Fujimoto C, Egami N, Kawahara T, Uemura Y, Yamamoto Y, Yamasoba T, et al. Noisy galvanic vestibular stimulation sustainably improves posture in bilateral vestibulopathy. Front Neurol. (2018) 9:900. doi: 10.3389/fneur.2018.00900
8. Iwasaki S, Fujimoto C, Egami N, Kinoshita M, Togo F, Yamamoto Y, et al. Noisy vestibular stimulation increases gait speed in normals and in bilateral vestibulopathy. Brain Stimul. (2018) 11:709–15. doi: 10.1016/j.brs.2018.03.005
9. Fujimoto C, Kinoshita M, Kamogashira T, Egami N, Kawahara T, Uemura Y, et al. Noisy galvanic vestibular stimulation has a greater ameliorating effect on posture in unstable subjects: a feasibility study. Sci Rep. (2019) 9:17189. doi: 10.1038/s41598-019-53834-7
10. Inukai Y, Masaki M, Otsuru N, Saito K, Miyaguchi S, Kojima S, et al. Effect of noisy galvanic vestibular stimulation in community-dwelling elderly people: a randomised controlled trial. J Neuroeng Rehabil. (2018) 15:63. doi: 10.1186/s12984-018-0407-6
11. Inukai Y, Miyaguchi S, Kobayashi N, Otsuru N, Onishi H. Noisy galvanic vestibular stimulation effect on center of pressure sway during one-legged standing. J Clin Neurosci. (2020) 82:173–8. doi: 10.1016/j.jocn.2020.10.050
12. Inukai Y, Miyaguchi S, Saito M, Otsuru N, Onishi H. Effects of different stimulation conditions on the stimulation effect of noisy galvanic vestibular stimulation. Front Hum Neurosci. (2020) 14:581405. doi: 10.3389/fnhum.2020.581405
13. Inukai Y, Otsuru N, Masaki M, Saito K, Miyaguchi S, Kojima S, et al. Effect of noisy galvanic vestibular stimulation on center of pressure sway of static standing posture. Brain Stimul. (2018) 11:85–93. doi: 10.1016/j.brs.2017.10.007
14. Inukai Y, Otsuru N, Saito K, Miyaguchi S, Kojima S, Yokota H, et al. The after-effect of noisy galvanic vestibular stimulation on postural control in young people: a randomized controlled trial. Neurosci Lett. (2020) 729:135009. doi: 10.1016/j.neulet.2020.135009
15. Wuehr M, Nusser E, Decker J, Krafczyk S, Straube A, Brandt T, et al. Noisy vestibular stimulation improves dynamic walking stability in bilateral vestibulopathy. Neurology. (2016) 86:2196–202. doi: 10.1212/WNL.0000000000002748
16. Wuehr M, Nusser E, Krafczyk S, Straube A, Brandt T, Jahn K, et al. Noise-enhanced vestibular input improves dynamic walking stability in healthy subjects. Brain Stimul. (2016) 9:109–16. doi: 10.1016/j.brs.2015.08.017
17. Temple DR, De Dios YE, Layne CS, Bloomberg JJ, Mulavara AP. Efficacy of stochastic vestibular stimulation to improve locomotor performance during adaptation to visuomotor and somatosensory distortion. Front Physiol. (2018) 9:301. doi: 10.3389/fphys.2018.00301
18. Nooristani M, Bigras C, Lafontaine L, Bacon BA, Maheu M, Champoux F. Vestibular function modulates the impact of nGVS on postural control in older adults. J Neurophysiol. (2021) 125:489–95. doi: 10.1152/jn.00512.2020
19. Iwasaki S, Yamamoto Y, Togo F, Kinoshita M, Yoshifuji Y, Fujimoto C, et al. Noisy vestibular stimulation improves body balance in bilateral vestibulopathy. Neurology. (2014) 82:969–75. doi: 10.1212/WNL.0000000000000215
20. Ko LW, Chikara RK, Chen PY, Jheng YC, Wang CC, Yang YC, et al. Noisy galvanic vestibular stimulation (stochastic resonance) changes electroencephalography activities and postural control in patients with bilateral vestibular hypofunction. Brain Sci. (2020) 10:740 doi: 10.3390/brainsci10100740
21. Sprenger A, Spliethoff P, Rother M, Machner B, Helmchen C. Effects of perceptible and imperceptible galvanic vestibular stimulation on the postural control of patients with bilateral vestibulopathy. J Neurol. (2020) 267:2383–97. doi: 10.1007/s00415-020-09852-x
22. Chen PY, Jheng YC, Wang CC, Huang SE, Yang TH, Hsu PC, et al. Effect of noisy galvanic vestibular stimulation on dynamic posture sway under visual deprivation in patients with bilateral vestibular hypofunction. Sci Rep. (2021) 11:4229. doi: 10.1038/s41598-021-83206-z
23. Eder J, Kellerer S, Amberger T, Keywan A, Dlugaiczyk J, Wuehr M, et al. Combining vestibular rehabilitation with noisy galvanic vestibular stimulation for treatment of bilateral vestibulopathy. J Neurol. (2022) 269:5731–7. doi: 10.1007/s00415-022-11033-x
24. Samoudi G, Jivegard M, Mulavara AP, Bergquist F. Effects of stochastic vestibular galvanic stimulation and LDOPA on balance and motor symptoms in patients with Parkinson's disease. Brain Stimul. (2015) 8:474–80. doi: 10.1016/j.brs.2014.11.019
25. Wuehr M, Schmidmeier F, Katzdobler S, Fietzek UM, Levin J, Zwergal A. Effects of low-intensity vestibular noise stimulation on postural instability in patients with Parkinson's disease. J Parkinsons Dis. (2022) 12:1611–8. doi: 10.3233/JPD-213127
26. Wuehr M, Peto D, Fietzek UM, Katzdobler S, Nubling G, Zaganjori M, et al. Low-intensity vestibular noise stimulation improves postural symptoms in progressive supranuclear palsy. J Neurol. (2024) 271:4577–86. doi: 10.1007/s00415-024-12419-9
27. Lotfi Y, Farahani A, Azimiyan M, Moossavi A, Bakhshi E. Comparison of efficacy of vestibular rehabilitation and noisy galvanic vestibular stimulation to improve dizziness and balance in patients with multiple sclerosis. J Vestib Res. (2021) 31:541–51. doi: 10.3233/VES-201609
28. McLaren R, Smith PF, Taylor RL, Ravindran S, Rashid U, Taylor D. Efficacy of nGVS to improve postural stability in people with bilateral vestibulopathy: a systematic review and meta-analysis. Front Neurosci. (2022) 16:101239. doi: 10.3389/fnins.2022.1010239
29. Mahmud M, Hadi Z, Prendergast M, Ciocca M, Saad AR, Pondeca Y, et al. The effect of galvanic vestibular stimulation on postural balance in Parkinson's disease: a systematic review and meta-analysis. J Neurol Sci. (2022) 442:120414. doi: 10.1016/j.jns.2022.120414
30. McLaren R, Smith PF, Taylor RL, Niazi IK, Taylor D. Scoping out noisy galvanic vestibular stimulation: a review of the parameters used to improve postural control. Front Neurosci. (2023) 17:1156796. doi: 10.3389/fnins.2023.1156796
31. Wuehr M, Eder J, Kellerer S, Amberger T, Jahn K. Mechanisms underlying treatment effects of vestibular noise stimulation on postural instability in patients with bilateral vestibulopathy. J Neurol. (2024) 271:1408–15. doi: 10.1007/s00415-023-12085-3
32. Fujimoto C, Yamamoto Y, Kamogashira T, Kinoshita M, Egami N, Uemura Y, et al. Noisy galvanic vestibular stimulation induces a sustained improvement in body balance in elderly adults. Sci Rep. (2016) 6:37575. doi: 10.1038/srep37575
33. Nooristani M, Maheu M, Houde MS, Bacon BA, Champoux F. Questioning the lasting effect of galvanic vestibular stimulation on postural control. PLoS ONE. (2019) 14:e0224619. doi: 10.1371/journal.pone.0224619
34. Nooristani M, Maheu M, Bacon BA, Champoux F. The importance of nGVS current density for postural control enhancement. Brain Stimul. (2019) 12:1592–4. doi: 10.1016/j.brs.2019.07.022
35. Herssens N, McCrum C. Stimulating balance: recent advances in vestibular stimulation for balance and gait. J Neurophysiol. (2019) 122:447–50. doi: 10.1152/jn.00851.2018
36. Haxby F, Akrami M, Zamani R. Finding a balance: a systematic review of the biomechanical effects of vestibular prostheses on stability in humans. J Funct Morphol Kinesiol. (2020) 5:23 doi: 10.3390/jfmk5020023
37. Lajoie K, Marigold DS, Valdes BA, Menon C. The potential of noisy galvanic vestibular stimulation for optimizing and assisting human performance. Neuropsychologia. (2021) 152:107751. doi: 10.1016/j.neuropsychologia.2021.107751
38. Moss F, Ward LM, Sannita WG. Stochastic resonance and sensory information processing: a tutorial and review of application. Clin Neurophysiol. (2004) 115:267–81. doi: 10.1016/j.clinph.2003.09.014
39. Dlugaiczyk J, Gensberger KD, Straka H. Galvanic vestibular stimulation: from basic concepts to clinical applications. J Neurophysiol. (2019) 121:2237–55. doi: 10.1152/jn.00035.2019
40. Schniepp R, Boerner JC, Decker J, Jahn K, Brandt T, Wuehr M. Noisy vestibular stimulation improves vestibulospinal function in patients with bilateral vestibulopathy. J Neurol. (2018) 265:57–62. doi: 10.1007/s00415-018-8814-y
41. Wuehr M, Boerner JC, Pradhan C, Decker J, Jahn K, Brandt T, et al. Stochastic resonance in the human vestibular system –Noise-induced facilitation of vestibulospinal reflexes. Brain Stimul. (2018) 11:261–3. doi: 10.1016/j.brs.2017.10.016
42. Asslander L, Giboin LS, Gruber M, Schniepp R, Wuehr M. No evidence for stochastic resonance effects on standing balance when applying noisy galvanic vestibular stimulation in young healthy adults. Sci Rep. (2021) 11:12327. doi: 10.1038/s41598-021-91808-w
43. Gavriilidou A, Mylonas V, Tsalavoutas I, Konstantakos V, Psillas G, Wuehr M, et al. Effects of individually calibrated white and pink noise vestibular stimulation on standing balance of young healthy adults. Exp Brain Res. (2025) 243:33. doi: 10.1007/s00221-024-06979-5
44. Wuehr M, Eilles E, Lindner M, Grosch M, Beck R, Ziegler S, et al. Repetitive low-intensity vestibular noise stimulation partly reverses behavioral and brain activity changes following bilateral vestibular loss in rats. Biomolecules. (2023) 13:1580. doi: 10.3390/biom13111580
45. Valdes BA, Lajoie K, Marigold DS, Menon C. Cortical effects of noisy galvanic vestibular stimulation using functional near-infrared spectroscopy. Sensors. (2021) 21:1476 doi: 10.3390/s21041476
46. Pavlik AE, Inglis JT, Lauk M, Oddsson L, Collins J. The effects of stochastic galvanic vestibular stimulation on human postural sway. Exp Brain Res. (1999) 124:273–280. doi: 10.1007/s002210050623
47. Woll J, Sprenger A, Helmchen C. Postural control during galvanic vestibular stimulation in patients with persistent perceptual-postural dizziness. J Neurol. (2019) 266:1236–49. doi: 10.1007/s00415-019-09255-7
48. Piccolo C, Bakkum A, Marigold DS. Subthreshold stochastic vestibular stimulation affects balance-challenged standing and walking. PLoS ONE. (2020) 15:e0231334. doi: 10.1371/journal.pone.0231334
49. Mulavara AP, Kofman IS, De Dios YE, Miller C, Peters BT, Goel R, et al. Using low levels of stochastic vestibular stimulation to improve locomotor stability. Front Syst Neurosci. (2015) 9:117. doi: 10.3389/fnsys.2015.00117
50. Goel R, Kofman I, Jeevarajan J, De Dios Y, Cohen HS, Bloomberg JJ, et al. Using low levels of stochastic vestibular stimulation to improve balance function. PLoS ONE. (2015) 10:e0136335. doi: 10.1371/journal.pone.0136335
51. Mitsutake T, Taniguchi T, Nakazono H, Yoshizuka H, Sakamoto M. Effects of noisy galvanic vestibular stimulation on the muscle activity and joint movements in different standing postures conditions. Front Hum Neurosci. (2022) 16:891669. doi: 10.3389/fnhum.2022.891669
53. Shumway-Cook A, Woollacott MH. Normal postural control. in Motor Control: Translating Research Into Clinical Practice (3rd Edn.). Philadelphia, PA: Lippincott Williams & Wilkins (2007). p. 176–181.
54. McGirr A, LeDue J, Chan AW, Boyd JD, Metzak PD, Murphy TH. Stress impacts sensory variability through cortical sensory activity motifs. Transl Psychiatry. (2020) 10:20. doi: 10.1038/s41398-020-0713-1
55. Vellei M, Pigliautile I, Pisello AL. Effect of time-of-day on human dynamic thermal perception. Sci Rep. (2023) 13:2367. doi: 10.1038/s41598-023-29615-8
56. Mulavara AP, Fiedler MJ, Kofman IS, Wood SJ, Serrador JM, Peters B, et al. Improving balance function using vestibular stochastic resonance: optimizing stimulus characteristics. Exp Brain Res. (2011) 210:303–12. doi: 10.1007/s00221-011-2633-z
57. van Stiphout L, Rolfes J, Waardenburg S, Kimman M, Guinand N, Perez Fornos A, et al. Construct validity and reliability of the Bilateral Vestibulopathy Questionnaire (BVQ). Front Neurol. (2023) 14:1221037. doi: 10.3389/fneur.2023.1221037
58. Anson ER, Gimmon Y, Kiemel T, Jeka JJ, Carey JP. A tool to quantify the functional impact of oscillopsia. Front Neurol. (2018) 9:142. doi: 10.3389/fneur.2018.00142
59. Strupp M, Kim JS, Murofushi T, Straumann D, Jen JC, Rosengren SM, et al. Bilateral vestibulopathy: diagnostic criteria consensus document of the classification committee of the Barany Society. J Vestib Res. (2017) 27:177–89. doi: 10.3233/VES-170619
60. Peto D, Schmidmeier F, Katzdobler S, Fietzek UM, Levin J, Wuehr M, et al. No evidence for effects of low-intensity vestibular noise stimulation on mild-to-moderate gait impairments in patients with Parkinson's disease. J Neurol. (2024) 271:5489–97. doi: 10.1007/s00415-024-12504-z
61. Winter DA. Human balance and posture control during standing and walking. Gait Posture. (1995) 3:193–214. doi: 10.1016/0966-6362(96)82849-9
62. Quijoux F, Nicolai A, Chairi I, Bargiotas I, Ricard D, Yelnik A, et al. A review of center of pressure (COP) variables to quantify standing balance in elderly people: Algorithms and open-access code. Physiol Rep. (2021) 9:e15067. doi: 10.14814/phy2.15067
63. Solomon AJ, Jacobs JV, Lomond KV, Henry SM. Detection of postural sway abnormalities by wireless inertial sensors in minimally disabled patients with multiple sclerosis: a case-control study. J Neuroeng Rehabil. (2015) 12:74. doi: 10.1186/s12984-015-0066-9
64. Anderson N, Button C. Development of static postural control: An overview and summary of entropy analysis. J Motor Learn Dev. (2017) 5:126–47. doi: 10.1123/jmld.2016-0011
65. Karmali F, Goodworth AD, Valko Y, Leeder T, Peterka RJ, Merfeld DM. The role of vestibular cues in postural sway. J Neurophysiol. (2021) 125:672–86. doi: 10.1152/jn.00168.2020
66. Bonnet CT, Kechabia YR, Magnani I, Polastri PF, Rodrigues ST. Benefits of postural sway to succeed in goal-directed visual tasks. Hum Mov Sci. (2024) 97:103277. doi: 10.1016/j.humov.2024.103277
67. Lucieer F, Vonk P, Guinand N, Stokroos R, Kingma H, van de Berg R. Bilateral vestibular hypofunction: insights in etiologies, clinical subtypes, and diagnostics. Front Neurol. (2016) 7:26. doi: 10.3389/fneur.2016.00026
68. Bayot M, Dujardin K, Tard C, Defebvre L, Bonnet CT, Allart E, et al. The interaction between cognition and motor control: a theoretical framework for dual-task interference effects on posture, gait initiation, gait and turning. Neurophysiol Clin. (2018) 48:361–75. doi: 10.1016/j.neucli.2018.10.003
69. Schniepp R, Schlick C, Schenkel F, Pradhan C, Jahn K, Brandt T, et al. Clinical and neurophysiological risk factors for falls in patients with bilateral vestibulopathy. J Neurol. (2017) 264:277–83. doi: 10.1007/s00415-016-8342-6
70. Horwood A, Chockalingam N. Clinical Biomechanics in Human Locomotion: Gait and Pathomechanical Principles. Amsterdam, Netherlands: Elsevier (2023).
71. Gomes E, Alder G, Bright FAS, Signal N. Understanding task “challenge” in stroke rehabilitation: an interdisciplinary concept analysis. Disabil Rehabil. (2024) 47:560–70. doi: 10.1080/09638288.2024.2356010
72. Schniepp R, Wuehr M, Neuhaeusser M, Kamenova M, Dimitriadis K, Klopstock T, et al. Locomotion speed determines gait variability in cerebellar ataxia and vestibular failure. Mov Disord. (2012) 27:125–31. doi: 10.1002/mds.23978
73. Schniepp R, Mohwald K, Wuehr M. Clinical and automated gait analysis in patients with vestibular, cerebellar, and functional gait disorders: perspectives and limitations. J Neurol. (2019) 266:118–22. doi: 10.1007/s00415-019-09378-x
74. Marder E, Bucher D. Central pattern generators and the control of rhythmic movements. Curr Biol. (2001) 11:R986–96. doi: 10.1016/S0960-9822(01)00581-4
75. Akay T, Murray AJ. Relative contribution of proprioceptive and vestibular sensory systems to locomotion: opportunities for discovery in the age of molecular science. Int J Mol Sci. (2021) 22:1467. doi: 10.3390/ijms22031467
76. Bruijn SM, van Dieen JH. Control of human gait stability through foot placement. J R Soc Interface. (2018) 15:20170816. doi: 10.1098/rsif.2017.0816
77. Reimann H, Fettrow T, Thompson ED, Jeka JJ. Neural control of balance during walking. Front Physiol. (2018) 9:1271. doi: 10.3389/fphys.2018.01271
78. Grouvel G, Boutabla A, Armand S, Corre J, Revol R, Cavuscens S, et al. Exploring gait kinematic variability in patients with severe vestibulopathy. Gait Posture. (2023) 106:S69–70. doi: 10.1016/j.gaitpost.2023.07.086
79. Grouvel G, Boutabla A, Armand S, Corre J, Revol R, Cavuscens S, et al. Impaired spatiotemporal gait parameters in patients with unilateral and bilateral vestibular deficits. Gait Posture. (2023) 106:S70–1. doi: 10.1016/j.gaitpost.2023.07.087
80. Lang J, Ishikawa K, Hatakeyama K, Wong WH, Yin M, Saito T, et al. 3D body segment oscillation and gait analysis for vestibular disorders. Auris Nasus Larynx. (2013) 40:18–24. doi: 10.1016/j.anl.2011.11.007
81. McCrum C, Lucieer F, van de Berg R, Willems P, Perez Fornos A, Guinand N, et al. The walking speed-dependency of gait variability in bilateral vestibulopathy and its association with clinical tests of vestibular function. Sci Rep. (2019) 9:18392. doi: 10.1038/s41598-019-54605-0
82. Herssens N, Saeys W, Vereeck L, Meijer K, van de Berg R, Van Rompaey V, et al. An exploratory investigation on spatiotemporal parameters, margins of stability, and their interaction in bilateral vestibulopathy. Sci Rep. (2021) 11:6427. doi: 10.1038/s41598-021-85870-7
83. Magnani RM, van Dieen JH, Bruijn SM. Effects of vestibular stimulation on gait stability when walking at different step widths. Exp Brain Res. (2022) 241:49–58. doi: 10.1101/2021.09.09.459650
84. Lord S, Galna B, Rochester L. Moving forward on gait measurement: toward a more refined approach. Mov Disord. (2013) 28:1534–43. doi: 10.1002/mds.25545
85. Hall C, Herdman SJ. Reliability of clinical measures used to assess patients with peripheral vestibular disorders. J Neurol Phys Ther. (2006) 30:74–81. doi: 10.1097/01.NPT.0000282571.55673.ed
86. Schmidheiny A, Swanenburg J, Straumann D, de Bruin ED, Knols RH. Discriminant validity and test re-test reproducibility of a gait assessment in patients with vestibular dysfunction. BMC Ear Nose Throat Disord. (2015) 15:6. doi: 10.1186/s12901-015-0019-8
87. Chau T, Young S, Redekop S. Managing variability in the summary and comparison of gait data. J Neuroeng Rehabil. (2005) 2:22. doi: 10.1186/1743-0003-2-22
88. Galna B, Lord S, Rochester L. Is gait variability reliable in older adults and Parkinson's disease? Towards an optimal testing protocol. Gait Posture. (2013) 37:580–5. doi: 10.1016/j.gaitpost.2012.09.025
89. Hollman JH, Childs KB, McNeil ML, Mueller AC, Quilter CM, Youdas JW. Number of strides required for reliable measurements of pace, rhythm and variability parameters of gait during normal and dual task walking in older individuals. Gait Posture. (2010) 32:23–8. doi: 10.1016/j.gaitpost.2010.02.017
90. Wuehr M, Eder J, Keywan A, Jahn K. Noisy galvanic vestibular stimulation improves vestibular perception in bilateral vestibulopathy. J Neurol. (2022) 270:938–43. doi: 10.2139/ssrn.4129070
91. Mitsutake T, Sakamoto M, Horikawa E. Comparing activated brain regions between noisy and conventional galvanic vestibular stimulation using functional magnetic resonance imaging. Neuroreport. (2021) 32:583–7. doi: 10.1097/WNR.0000000000001629
92. McGrath M, Wood J, Walsh J, Window P. The impact of Three-Dimensional Gait Analysis in adults with pathological gait on management recommendations. Gait Posture. (2023) 105:75–80. doi: 10.1016/j.gaitpost.2023.06.014
93. Scataglini S, Abts E, Van Bocxlaer C, Van den Bussche M, Meletani S, Truijen S. Accuracy, validity, and reliability of markerless camera-based 3D motion capture systems vs. marker-based 3D motion capture systems in gait analysis: a systematic review and meta-analysis. Sensors. (2024) 24:3686. doi: 10.3390/s24113686
94. Olsen S, Rashid U, Allerby C, Brown E, Leyser M, McDonnell G, et al. Smartphone-based gait and balance accelerometry is sensitive to age and correlates with clinical and kinematic data. Gait Posture. (2023) 100:57–64. doi: 10.1016/j.gaitpost.2022.11.014
Keywords: neuromodulation, galvanic vestibular stimulation, nGVS, vestibular, posture, balance, stochastic resonance, optimization
Citation: McLaren R, Smith PF, Taylor RL and Taylor D (2025) Optimizing noisy galvanic vestibular stimulation (nGVS) for postural control: methodological considerations when individualizing the signal for people with bilateral vestibulopathy. Front. Neurol. 16:1609123. doi: 10.3389/fneur.2025.1609123
Received: 10 April 2025; Accepted: 28 May 2025;
Published: 20 June 2025.
Edited by:
Thomas Platz, University of Greifswald, GermanyReviewed by:
Martin J. McKeown, University of British Columbia, CanadaEnrique Soto, Meritorious Autonomous University of Puebla, Mexico
Max Wuehr, Ludwig Maximilian University of Munich, Germany
Copyright © 2025 McLaren, Smith, Taylor and Taylor. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ruth McLaren, cnV0aC5tY2xhcmVuQGF1dC5hYy5ueg==
 Ruth McLaren
Ruth McLaren Paul F. Smith
Paul F. Smith Rachael L. Taylor
Rachael L. Taylor Denise Taylor
Denise Taylor