- 1The Affiliated Hospital of Yan'an University, Yan'an, Shaanxi, China
- 2Department of Neurology, Yan'an People's Hospital, Yan'an, China
Background: Capsular warning syndrome (CWS), a rare subtype of transient ischemic attack, is associated with a high risk of progression to acute cerebral infarction. However, predictive factors for infarction and determinants of functional outcome remain inadequately defined.
Methods: In this retrospective study, we analyzed 89 CWS patients admitted between January 2021 and December 2024. Clinical, laboratory, imaging, and treatment data were collected. Patients were stratified into infarcted and non-infarcted groups based on DWI findings. Infarcted patients were followed for 90 days, with outcomes assessed using the modified Rankin Scale (mRS). LASSO regression was used for variable selection, followed by multivariable logistic regression to identify independent predictors of infarction and favorable 90-day outcomes (mRS 0–2).
Results: Of 89 patients, 57 (64%) experienced infarctions. Independent predictors included elevated platelet count (OR = 1.02, 95% CI: 1.01–1.04, p = 0.002), higher NIHSS score (OR = 1.96, 95% CI: 1.25–3.55, p = 0.011), higher ABCD2 score (OR = 1.86, 95% CI: 1.04–3.60, p = 0.047), intracranial atherosclerosis (OR = 10.5, 95% CI: 1.54–99.0, p = 0.024), and male sex (OR = 5.57, 95% CI: 1.32–27.9, p = 0.024). Among 57 infarcted patients, tirofiban infusion was the only independent predictor of favorable outcome (OR = 0.01, CI: 0.00–0.07, p < 0.001).
Conclusion: In CWS, infarction risk is independently associated with platelet count, clinical severity, vascular pathology, and sex. Tirofiban may improve short-term outcomes in infarcted patients. Prospective multicenter studies are needed to validate these findings.
Introduction
Capsular warning syndrome (CWS) is an uncommon but distinctive clinical manifestation of acute ischemic cerebrovascular disease, currently recognized as a specific subtype of transient ischemic attack (TIA) (1). First characterized by Donnan in 1993, CWS is clinically defined by at least three stereotyped, transient, and completely reversible episodes of unilateral motor or sensory impairment occurring within a 24-h period. These episodes must involve at least two regions among the face, arm, or leg, without associated cortical dysfunctions such as aphasia, apraxia, agnosia, or cognitive impairment (2). CWS is most commonly associated with lacunar infarctions, which result from the occlusion of small penetrating arteries. Furthermore, TIA have been observed in approximately 10% of patients prior to the onset of lacunar infarction, suggesting a prodromal phase in some cases (3, 4). Recent advancements in neuroimaging have clarified that lesions underlying CWS are not restricted solely to the internal capsule, as initially postulated, but may extend to other deep cerebral structures, including the pons, thalamus, centrum semiovale, and corpus callosum (5, 6). Additionally, the temporal definition of CWS has evolved, with recent studies proposing expanded diagnostic windows of 48 h, 72 h, or even up to 7 days, leading to broader and more inclusive diagnostic criteria (7–9).
Despite clinical parallels with conventional TIA, CWS is associated with a significantly increased risk of progression to ischemic stroke. The 90-day stroke risk among general TIA patients is approximately 1.4% (10), whereas the reported rate of infarction among CWS patients ranges from 71.2 to 80.55% (11, 12). However, comprehensive investigations into independent risk factors specifically predicting infarction in CWS remain limited. Current literature predominantly addresses conventional vascular risk factors such as hypertension, atherosclerosis, and hyperhomocysteinemia (13). Currently, clinical management of CWS often mirrors that of TIA or acute ischemic stroke, involving antiplatelet therapy and intravenous thrombolysis (14). Among antiplatelet options, tirofiban is a fast-acting, reversible glycoprotein IIb/IIIa antagonist that inhibits the final step of platelet aggregation. Its safety and potential benefits in acute ischemic stroke have been supported by several studies (15, 16). Considering the potential contribution of platelet hyperreactivity and small-vessel pathology in CWS, tirofiban represents a biologically rational candidate for mitigating infarct development. Nevertheless, the efficacy and safety of these strategies in CWS remain uncertain due to the lack of high-quality, CWS-specific evidence. Heterogeneous designs and limited sample sizes further contribute to inconsistent conclusions and the absence of standardized treatment guidelines.
This study aims to review the clinical and imaging characteristics of CWS, identify predictors of infarction and poor prognosis, and evaluate the impact of different therapeutic strategies on clinical outcomes. By elucidating risk profiles and treatment effects in this unique population, we hope to inform earlier recognition and tailored interventions for patients at high risk of progression.
Materials and methods
Study design and population
This retrospective observational study included consecutive patients admitted to the Department of Neurology at Yan’an University Affiliated Cardiovascular and Cerebrovascular Hospital between January 2021 and December 2024 for acute ischemic stroke. All patients met the clinical criteria for CWS and were managed according to standard institutional protocols. The study was approved by the hospital’s institutional ethics committee (Approval No. L2020184), and all patient data were anonymized for confidentiality.
Inclusion criteria
1. Age ≥18 years.
2. Clinical diagnosis of CWS, defined as ≥3 stereotyped, transient, and fully reversible motor or sensory episodes within 24 h, involving at least two of the face, arm, or leg, without cortical signs (e.g., aphasia, neglect).
3. Time from symptom onset to hospital admission ≤24 h.
Exclusion criteria
1. Altered level of consciousness at admission.
2. Neuroimaging or medical history indicating alternative cerebrovascular or intracranial diseases (e.g., intracerebral hemorrhage, chronic subdural hematoma, neoplasm, subarachnoid hemorrhage, arteriovenous malformation, or aneurysm).
3. Stroke mimics (e.g., postictal paresis, functional neurological disorder, migraine aura, neuromyelitis optica spectrum disorder).
4. Severe systemic illness precluding standard stroke care (e.g., end-stage renal disease, decompensated heart failure).
5. Use of anticoagulation with high bleeding risk, known coagulopathy, or platelet count <100 × 109/L.
Definitions of comorbidities
1. Hypertension: Documented history or use of antihypertensives, or admission blood pressure ≥130/90 mmHg.
2. Diabetes Mellitus: Documented history or use of antidiabetic drugs, or fasting glucose ≥7.0 mmol/L, or HbA1c ≥ 6.5%.
3. Dyslipidemia: Documented diagnosis or lipid-lowering therapy, or LDL-C ≥ 3.4 mmol/L, total cholesterol ≥5.2 mmol/L, or triglycerides ≥1.7 mmol/L.
4. Hyperhomocysteinemia: Known diagnosis or fasting plasma homocysteine >15 μmol/L.
5. Atherosclerosis: Based on prior history or neurovascular imaging showing vessel wall thickening, plaque, or stenosis.
6. Prior stroke/TIA, demyelinating lesions, smoking, and alcohol use were determined from medical records and patient interviews.
Data collection
1. Demographics: age, sex.
2. Clinical presentation: frequency and duration of attacks.
3. Medical history and comorbidities (as defined above).
4. Laboratory data: CBC, coagulation, glucose, lipid panel, homocysteine.
5. Imaging: DWI for infarction status and lesion localization; CTA/MRA for atherosclerosis. (Emergency repeat DWI imaging was conducted during hospitalization upon neurological deterioration lasting over 1 hour or an NIHSS increase of ≥2 points, guiding subsequent therapeutic decisions).
6. Stroke scales: ABCD2 and NIHSS at presentation; modified Rankin Scale (mRS) at 90 days.
7. Treatment: rt-PA, intravenous tirofiban, and oral antiplatelet therapy.
Treatment protocol
All patients received standard oral antiplatelet therapy upon admission. Tirofiban therapy was initiated once patients exhibited persistent, unrelieved CWS symptoms—defined as a neurological deficit lasting > 60 min or recurrent attacks without full recovery within 1 h—despite standard dual oral antiplatelet therapy, irrespective of DWI findings. The standardized tirofiban protocol was:
1. Loading: 0.4 μg·kg−1·min−1 for 30 min.
2. Maintenance: 0.1 μg·kg−1·min−1 for 24 h (halved if eGFR <30 mL·min−1).
3. Antiplatelet overlap: Aspirin (100 mg) + clopidogrel (75 mg) started 4 h before infusion discontinuation.
Study design and outcomes
1. Phase 1: All patients clinically diagnosed with CWS were included and grouped based on DWI results (positive vs. negative). The primary outcome was DWI-confirmed infarction.
2. Phase 2: Patients with confirmed infarction (DWI-positive) were followed for 90 days. The primary outcome was functional independence (mRS 0–2).
Statistical analysis
Comparisons were made using chi-square or Fisher’s exact test for categorical variables, and t-test or Mann–Whitney U test for continuous variables, as appropriate. LASSO regression (using the 1-SE rule) was used for variable selection, followed by multivariable logistic regression. Odds ratios (ORs) with 95% confidence intervals (CIs) were reported. A p-value <0.05 was considered statistically significant. To evaluate the predictive performance of the final multivariate logistic regression model, we performed receiver operating characteristic (ROC) curve analysis. Analyses were performed using R software (version 4.2.2).
Results
Baseline characteristics
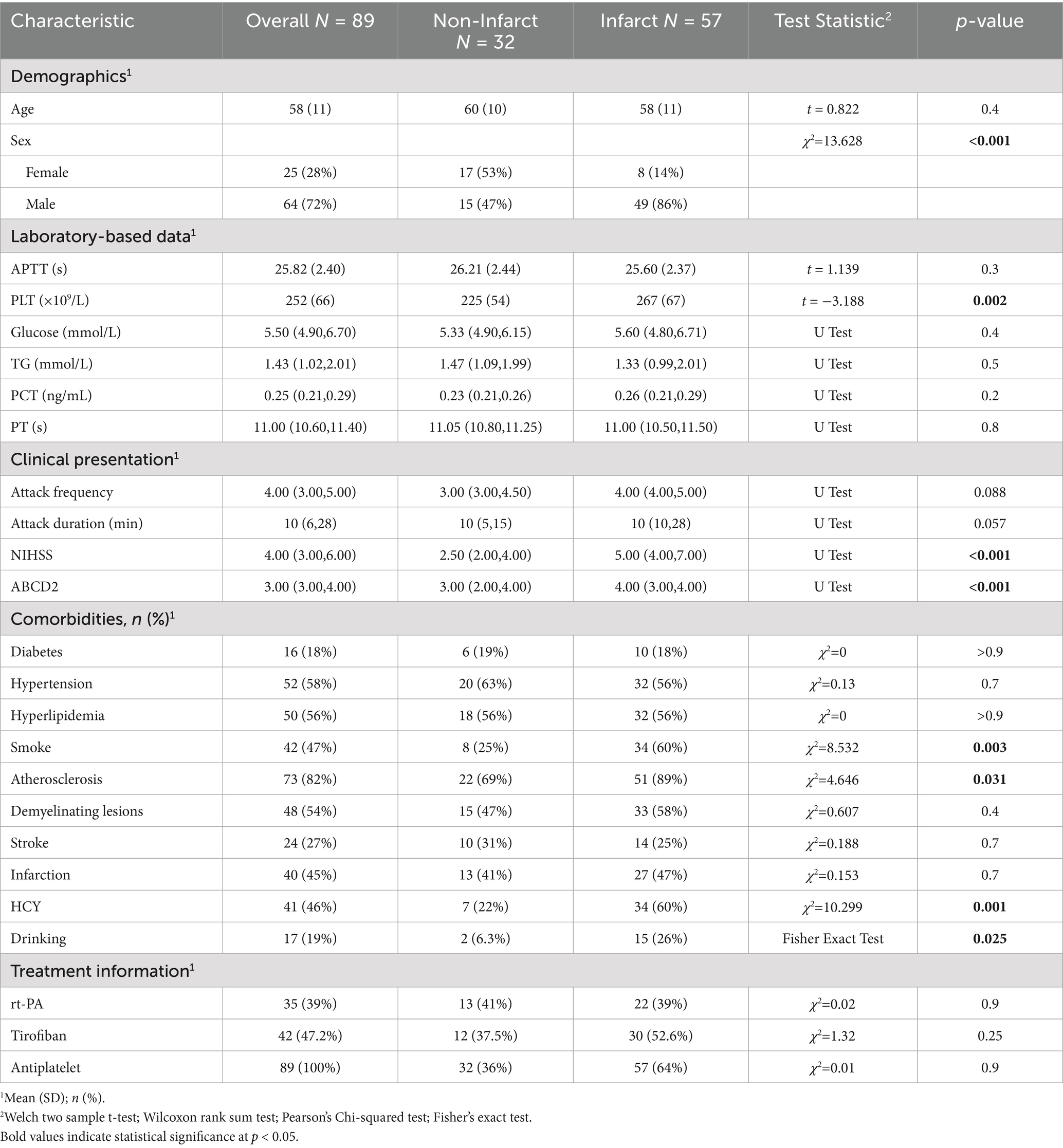
A total of 89 patients diagnosed with CWS were included. Of these, 64 (72%) were male and 25 (28%) females, with a mean age of 58 ± 11 years. The most common comorbidities included hypertension (58%), dyslipidemia (56%), prior stroke (27%), and diabetes (18%). Additionally, 47% had a history of smoking and 19% reported alcohol use.
All patients presented with recurrent, transient neurological deficits. The median number of episodes per patient was 4 (range: 3–20). Symptom patterns were classified as purely motor in 49 patients (55.1%), combined motor and sensory in 31 (34.8%), and purely sensory in 9 (10.1%) (Figure 1A). Median ABCD2 and NIHSS scores at presentation were 3 and 4, respectively. The median duration of each episode was 10 min.
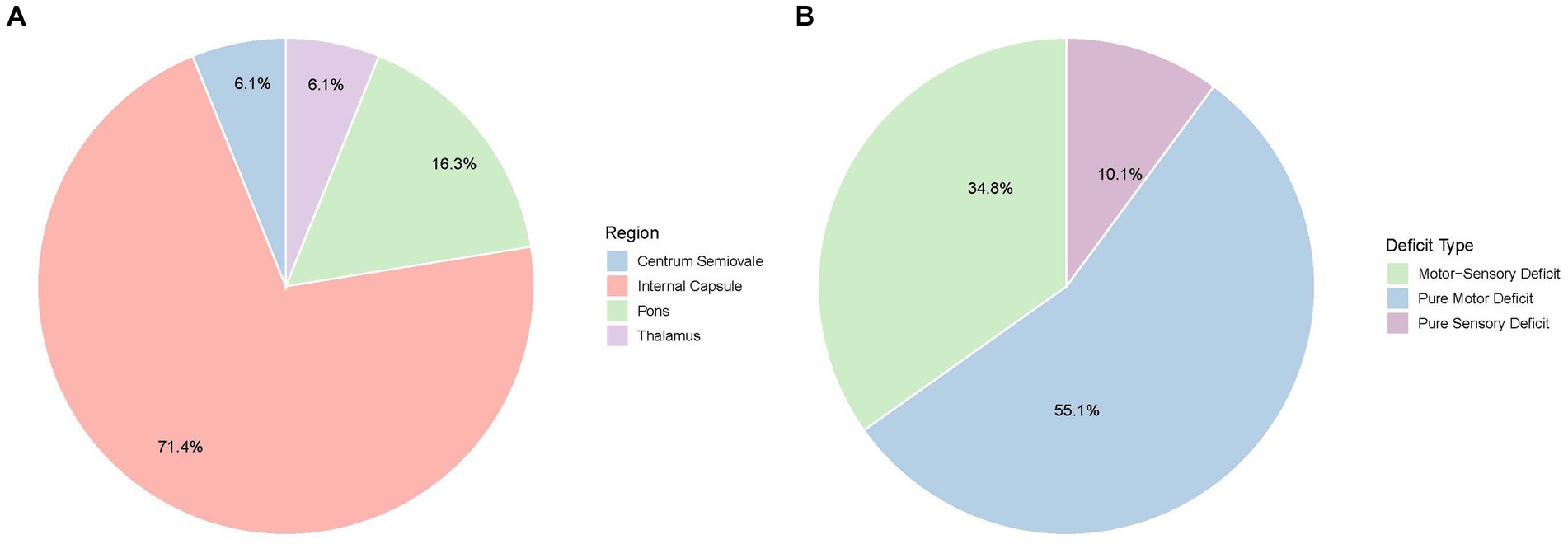
Figure 1. Clinical and imaging characteristics of capsular warning syndrome. (A) Distribution of present symptoms among patients with CWS. (B) Anatomic locations of acute infarcts detected by diffusion-weighted imaging (DWI).
Imaging findings
DWI revealed acute infarctions in 57 patients (64%). The most commonly affected region was the internal capsule (42 patients, 73.7%), particularly the posterior limb. Additional infarcts were observed in the pons (15.8%), thalamus (5.3%), and centrum semiovale (5.3%) (Figure 1B). Intracranial atherosclerosis was identified in 73 patients (82%) via vascular imaging, though only 5 (5.6%) had significant large artery stenosis or occlusion.
Risk factors for infarction
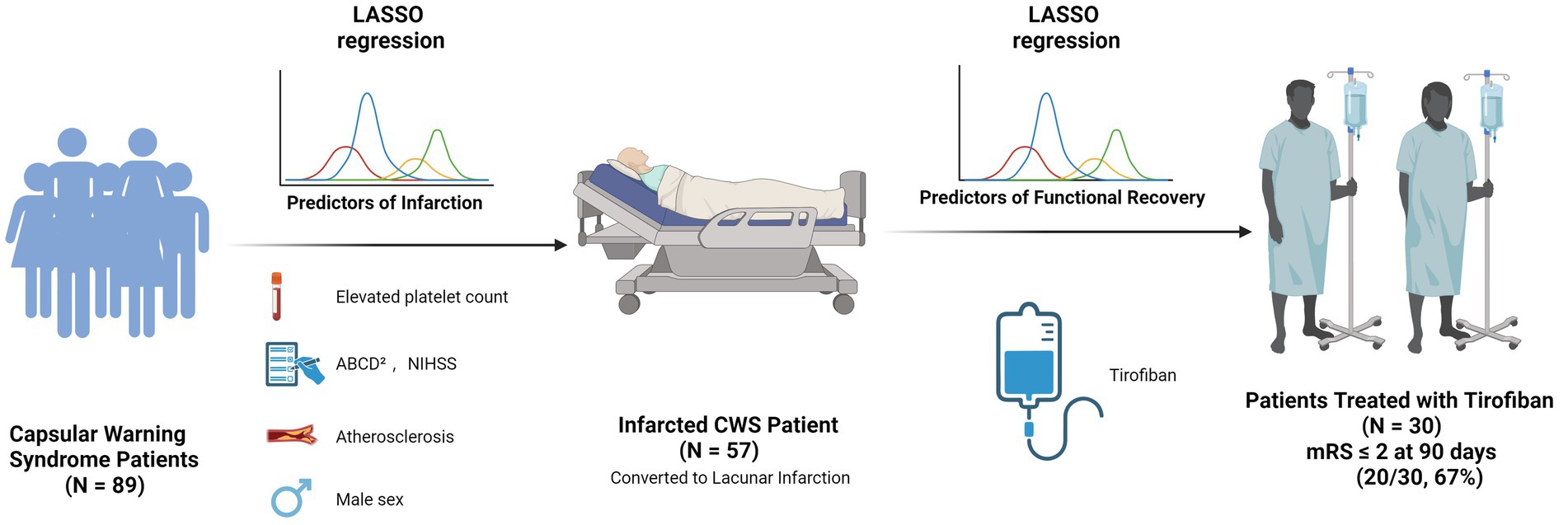
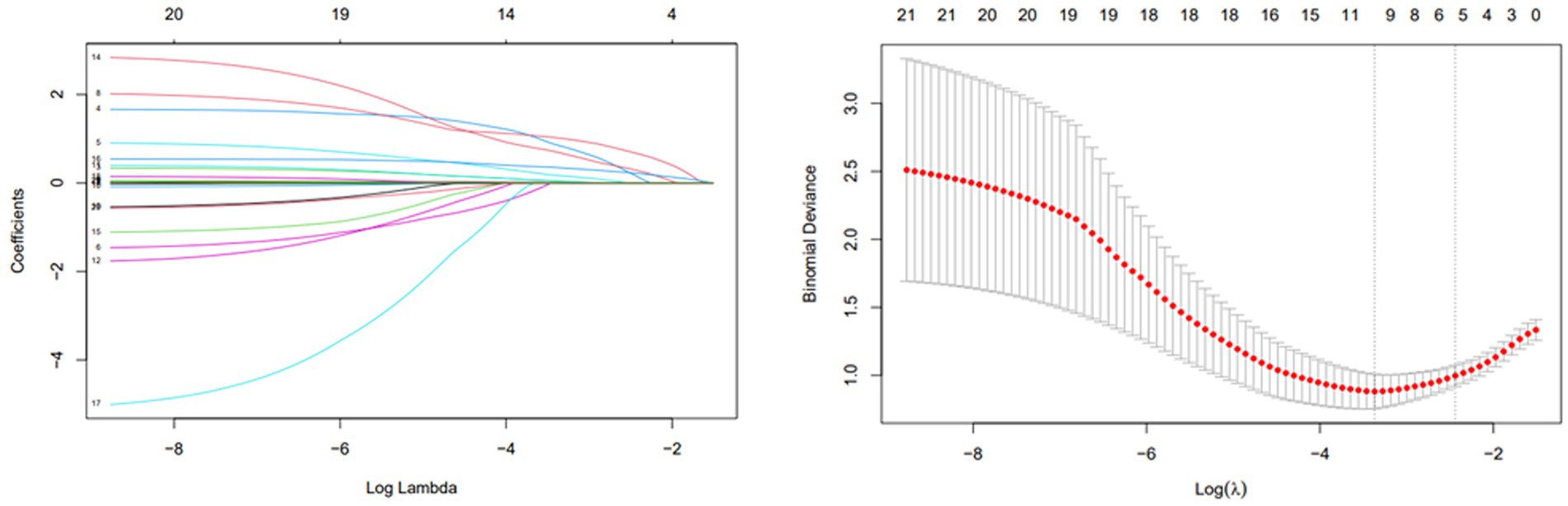
Patients were categorized into infarcted (n = 57) and non-infarcted (n = 32) groups based on DWI findings. Clinical and laboratory comparisons were made between the two groups (Table 1). LASSO regression (Figure 2) followed by multivariable logistic regression revealed that elevated platelet count (OR = 1.02, 95% CI: 1.01–1.04, p = 0.002), higher NIHSS score (OR = 1.96, 95% CI: 1.25–3.55, p = 0.011), higher ABCD2 score (OR = 1.86, 95% CI: 1.04–3.60, p = 0.047),presence of intracranial atherosclerosis (OR = 10.5, 95% CI: 1.54–99.0, p = 0.024), and male sex (OR = 5.57, 95% CI: 1.32–27.9, p = 0.024) were independent predictors of infarction (Table 2).
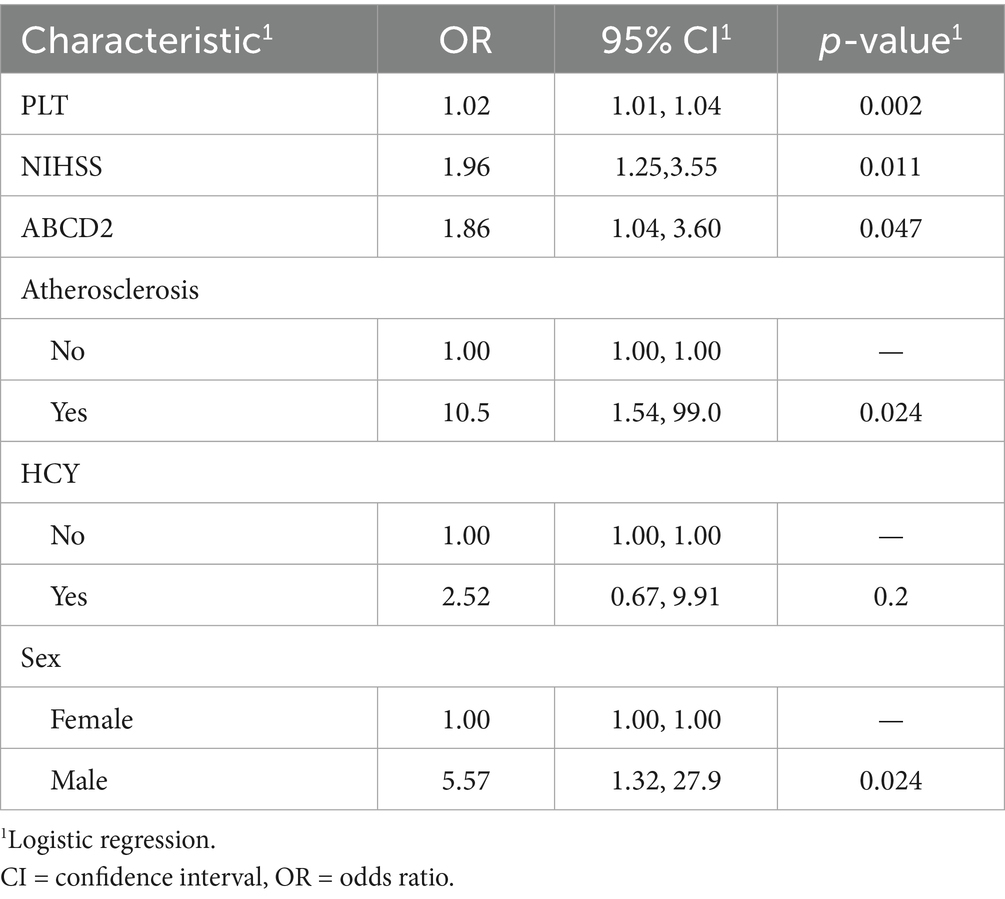
Table 2. Multivariate logistic regression of LASSO-selected risk factors for acute infarction in CWS.
To further evaluate the diagnostic performance of the multivariate model, receiver operating characteristic (ROC) analysis was conducted. The model yielded an area under the curve (AUC) of 0.906, indicating excellent discrimination. At the optimal cutoff value of 0.581 (determined by the Youden index), the model demonstrated a sensitivity of 87.7%, specificity of 78.1%, positive predictive value (PPV) of 87.7%, negative predictive value (NPV) of 78.1%, and overall diagnostic accuracy of 84.3% (Figure 3).
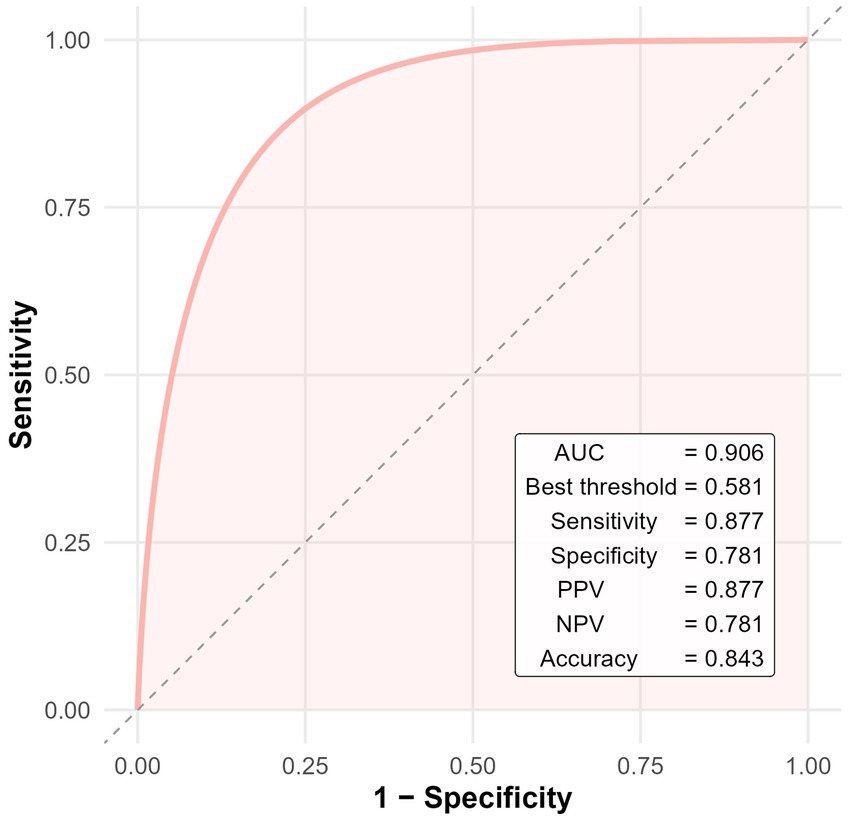
Figure 3. ROC curve of the multivariate logistic regression model for predicting cerebral infarction in patients with CWS.
Predictors of 90-day outcome among infarcted patients
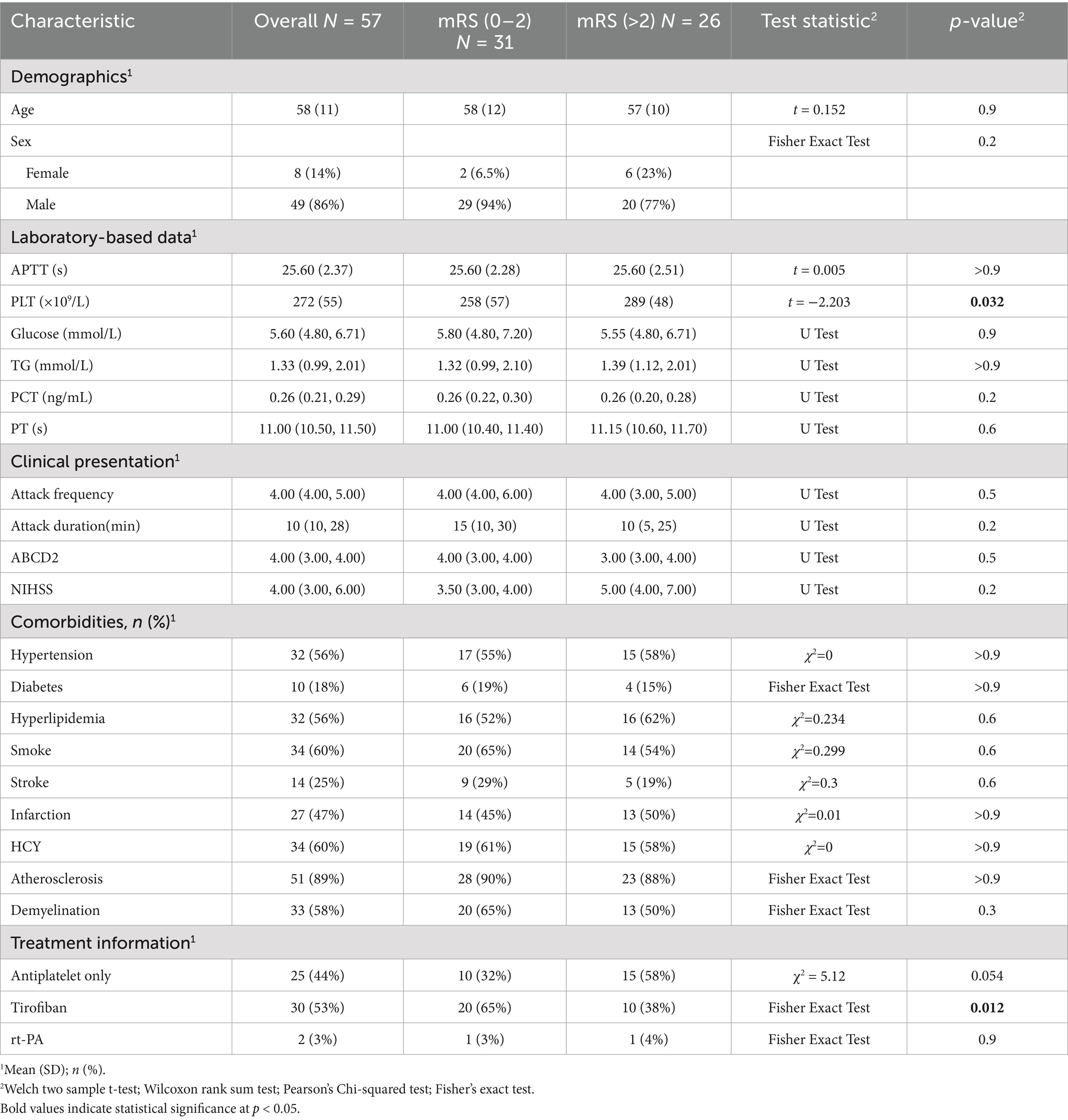
Of the 57 patients with DWI-confirmed infarction, 30 (53%) met escalation criteria and received an intravenous tirofiban infusion, whereas the remaining 25 (44%) were treated with dual oral antiplatelet therapy alone. At the 90-day evaluation, 31patients (54.3%) achieved functional independence (mRS 0–2), while 26 patients (45.7%) had an unfavorable outcome (mRS > 2). Favorable outcome occurred in 20 of 30 patients who received tirofiban (65%) and in 10 of 25 patients managed with antiplatelet therapy alone (40%). Baseline clinical and treatment characteristics stratified by outcome are summarized in Table 3. LASSO variable selection (Figure 4) followed by multivariable logistic regression identified tirofiban use as the only independent predictor of functional independence at 90 days OR = 0.01, CI: 0.00–0.07, p < 0.001 (Table 4).
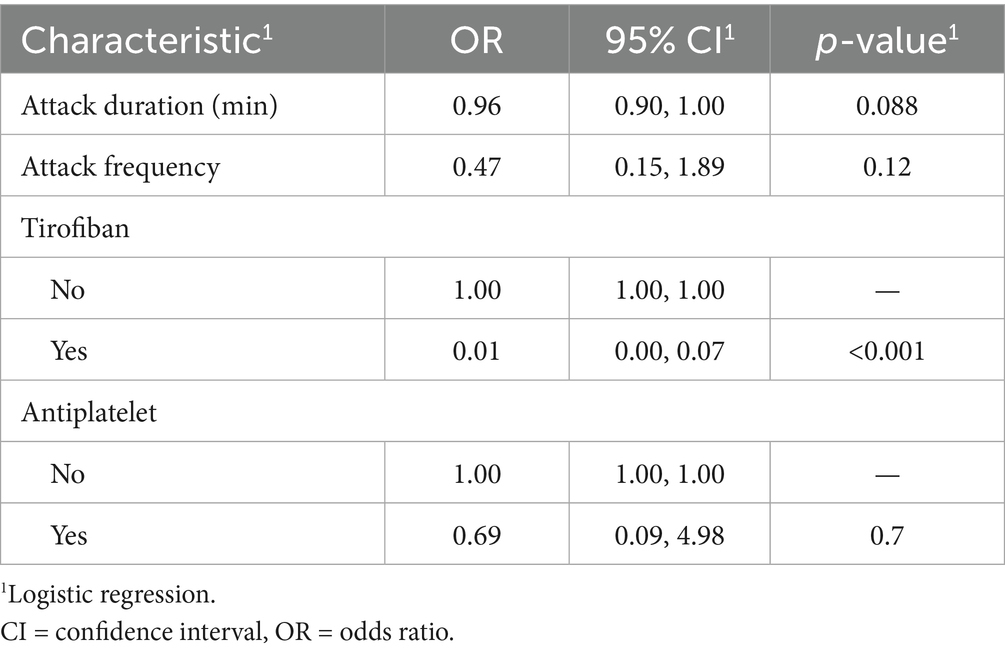
Table 4. Multivariate logistic regression of LASSO-selected predictors for favorable 90-day outcome in infarcted CWS patients.
Discussion
Our study set out to clarify two unresolved questions in CWS: (i) which bedside or laboratory variables truly forecast progression to infarction, and (ii) whether intensive, mechanism-directed antiplatelet therapy can improve functional recovery once infarction occurs. By analyzing 89 consecutive patients we show, first, that a composite profile of platelet hyperreactivity (elevated platelet count), early clinical severity (higher NIHSS and ABCD2 scores), intracranial atherosclerosis, and male sex independently signals imminent infarction, and second, that early, weight-adjusted tirofiban infusion is associated with a three- to four-fold increase in 90-day independence relative to best-medical therapy alone.
Even modest increases within the high-normal platelet range intensified the probability of permanent infarction (17). Platelets with greater surface density not only raise the encounter rate between GP IIb/IIIa receptors and fibrinogen but also shed pro-coagulant macrovesicles that accelerate thrombin generation (18, 19). Male sex retained significance after multivariable adjustment; testosterone is known to up-regulate platelet thromboxane A₂ receptor density and augment aggregation (20) Intracranial atherosclerosis predicted infarction despite minimal large-artery stenosis in most patients, underscoring the “branch-occlusive” model in which ostial plaques obstruct perforating arteries or create platelet-rich micro emboli (21). Chronic hypertension, smoking, dyslipidemia and hyperhomocysteinemia—present in >70% of our cohort—promote lipohyalinosis, endothelial nitric-oxide depletion and basement-membrane thickening, further narrowing the perforator lumen and predisposing to flow arrest (22, 23).
Tirofiban is a short-acting, reversible glycoprotein IIb/IIIa antagonist that blocks the final common pathway of platelet aggregation without the immunogenicity of abciximab. Experimental models confirm that perforator-type infarcts are exquisitely platelet-dependent and respond poorly to P2Y12 inhibition alone (24). The SaTIS trial established safety in moderate ischemic stroke (25), and successive randomized data have documented functional benefits in selected populations—large-artery atherosclerosis (26), non-occlusive strokes, and, most recently, in combination with intravenous thrombolysis (27). Our cohort complements these trials by focusing on small-vessel-dominant pathology, a context in which platelet-rich thrombi and endothelial plugging are central. The magnitude of benefit we observed is concordant with the pooled effect of four randomized controlled trials in non-repercussed stroke (28). Nevertheless, it contrasts with a neutral signal in a prior open-label CWS series (29), possibly because our protocol mandated (i) early initiation after first sustained deficit and (ii) overlap with dual oral antiplatelets to prevent rebound aggregation. These findings are consistent with recent evidence from a multicenter cohort study, which demonstrated that tirofiban administration improved outcomes in patients with large artery atherosclerosis who achieved complete reperfusion and were presented with high NIHSS scores (30). This further supports the potential utility of tirofiban in patients with CWS, especially those with underlying atherosclerotic pathology and severe initial neurological deficits.
Our conclusions must be interpreted in light of several constraints. First, the retrospective design invites selection bias, particularly in treatment allocation—patients receiving tirofiban had more severe intrahospital fluctuations, which could lead to confounding by indication despite multivariate adjustment. Second, sample size is modest, limiting power for subgroup analyses (e.g., sex-specific efficacy) and inflating confidence intervals around some estimates. Third, lack of randomization and blinding leaves room for unmeasured confounders, including clinician preference and temporal improvements in ancillary care. Fourth, precise symptom onset-to-admission intervals were not consistently documented. However, all included patients were admitted within 24 h from symptom onset. Finally, generalizability is restricted: our hospital is a tertiary referral center in north-west China with a high background prevalence of intracranial atherosclerosis; centers with different risk profiles or practice patterns may not reproduce identical effect sizes.
Taken together, our data support a two-step management algorithm: bedside triage using the platelet-NIHSS-ABCD2 composite to identify high-risk patients, followed by early tirofiban infusion in those who convert to infarction despite standard therapy. Prospective, multicenter randomized trials with stratification by platelet function and imaging-defined perforator disease are urgently required. Incorporation of blood biomarkers of endothelial dysfunction and high-resolution vessel-wall MRI could further unravel the microvascular mechanisms precipitating CWS and refine candidate selection for intensive antiplatelet therapy.
Conclusion
In this single-center cohort, elevated platelet count, high initial clinical scores, intracranial atherosclerosis, and male sex independently predicted infarction in CWS, while low-dose tirofiban was the only modifiable factor associated with superior 90-day functional outcomes. These findings strengthen the biological rationale for early, mechanism-specific platelet inhibition in high-risk CWS and lay the groundwork for definitive randomized evaluation.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Ethics Committee of the Affiliated Hospital of Yan'an University (Approval number: L2020184). The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants' legal guardians/next of kin due to the retrospective nature of the study.
Author contributions
R-xG: Writing – review & editing, Writing – original draft, Methodology, Data curation, Formal analysis. TW: Writing – original draft, Validation, Supervision, Writing – review & editing, Conceptualization, Investigation, Software.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sales, C, and Calma, AD. Stroke warning syndrome. Clin Neurol Neurosurg. (2022) 213:107120. doi: 10.1016/j.clineuro.2022.107120
2. Donnan, GA, O'Malley, HM, Quang, L, Hurley, S, and Bladin, PF. The capsular warning syndrome: pathogenesis and clinical features. Neurology. (1993) 43:957–62. doi: 10.1212/wnl.43.5.957
3. Arboix, A, Massons, J, García-Eroles, L, Targa, C, Comes, E, Parra, O, et al. Nineteen-year trends in risk factors, clinical characteristics and prognosis in lacunar infarcts. Neuroepidemiology. (2010) 35:231–6. doi: 10.1159/000319460
4. Onuska, KD, and Hill, SA. Effect of rheumatoid factor on cardiac troponin I measurement using two commercial measurement systems. Clin Chem. (2000) 46:307–8. doi: 10.1093/clinchem/46.2.307
5. Muengtaweepongsa, S, Singh, NN, and Cruz-Flores, S. Pontine warning syndrome: case series and review of literature. J Stroke Cerebrovasc Dis. (2010) 19:353–6. doi: 10.1016/j.jstrokecerebrovasdis.2009.06.008
6. Nandhagopal, R, Al-Asmi, A, Johnston, WJ, Jacob, PC, and Arunodaya, GR. Callosal warning syndrome. J Neurol Sci. (2012) 314:178–80. doi: 10.1016/j.jns.2011.10.007
7. Paul, NL, Simoni, M, Chandratheva, A, and Rothwell, PM. Population-based study of capsular warning syndrome and prognosis after early recurrent TIA. Neurology. (2012) 79:1356–62. doi: 10.1212/WNL.0b013e31826c1af8
8. Foschi, M, Pavolucci, L, Rondelli, F, Barone, V, Rinaldi, R, Spinardi, L, et al. Capsular warning syndrome: features, risk profile, and prognosis in a large prospective TIA cohort. Cerebrovasc Dis. (2023) 52:218–25. doi: 10.1159/000525954
9. Sen, A, Birns, J, and Bhalla, A. Stroke warning syndromes. Br J Hosp Med (Lond). (2020) 81:1–5. doi: 10.12968/hmed.2019.0222
10. Garg, A, Limaye, K, Shaban, A, Leira, EC, and Adams, HP. Risk of ischemic stroke after an inpatient hospitalization for transient ischemic attack in the United States. Neuroepidemiology. (2021) 55:40–6. doi: 10.1159/000511829
11. Camps-Renom, P, Delgado-Mederos, R, Martinez-Domeno, A, Prats-Sanchez, L, Cortes-Vicente, E, et al. Clinical characteristics and outcome of the capsular warning syndrome: a multicenter study. Int J Stroke. (2015) 10:571–5. doi: 10.1111/ijs.12432
12. Payus, AO, Ibrahim, A, Sheaker, VC, and Yahya, WNNW. 'Crescendo transient ischemic attack'-an uncommon presentation of a very common disease: a case report on capsular warning syndrome. Oxf Med Case Rep. (2022) 2022:omab129. doi: 10.1093/omcr/omab129
13. He, L, Xu, R, Wang, J, Zhang, L, Zhang, L, Zhou, F, et al. Capsular warning syndrome: clinical analysis and treatment. BMC Neurol. (2019) 19:285. doi: 10.1186/s12883-019-1522-0
14. Martínez, HR, Figueroa-Sanchez, JA, Arreola-Aldape, CA, Moran Guerrero, JA, Trujillo-Bracho, AL, Cantú López, A, et al. Capsular warning syndrome and its clinical awareness and therapeutic approach: two case reports and a systematic review of the literature. Front Neurol. (2023) 14:1177660. doi: 10.3389/fneur.2023.1177660
15. Baek, BH, Yoon, W, Lee, YY, Kim, SK, Kim, JT, and Park, MS. Intravenous tirofiban infusion after angioplasty and stenting in intracranial atherosclerotic stenosis-related stroke. Stroke. (2021) 52:1601–8. doi: 10.1161/STROKEAHA.120.033551
16. Zi, W, Song, J, Kong, W, Huang, J, Guo, C, He, W, et al. Tirofiban for stroke without large or medium-sized vessel occlusion. N Engl J Med. (2023) 388:2025–36. doi: 10.1056/NEJMoa2214299
17. Sadeghi, F, Kovács, S, Zsóri, KS, Csiki, Z, Bereczky, Z, Shemirani, AH, et al. Platelet count and mean volume in acute stroke: a systematic review and meta-analysis. Platelets. (2020) 31:731–9. doi: 10.1080/09537104.2019.1680826
18. Zhang, T, Lemoi, BA, and Sentman, CL. Chimeric NK-receptor-bearing T cells mediate antitumor immunotherapy. Blood. (2005) 106:1544–51. doi: 10.1182/blood-2004-11-4365
19. Fitsanakis, VA, Thompson, KN, Deery, SE, Milatovic, D, Shihabi, ZK, Erikson, KM, et al. A chronic iron-deficient/high-manganese diet in rodents results in increased brain oxidative stress and behavioral deficits in the Morris water maze. Neurotox Res. (2009) 15:167–78. doi: 10.1007/s12640-009-9017-1
20. Ajayi, AA, Mathur, R, and Halushka, PV. Testosterone increases human platelet thromboxane A2 receptor density and aggregation responses. Circulation. (1995) 91:2742–7. doi: 10.1161/01.cir.91.11.2742
21. Wardlaw, JM, Chabriat, H, de Leeuw, FE, Debette, S, Dichgans, M, Doubal, F, et al. European stroke organisation (ESO) guideline on cerebral small vessel disease, part 2, lacunar ischaemic stroke. Eur Stroke J. (2024) 9:5–68. doi: 10.1177/23969873231219416
22. Markus, HS, and de Leeuw, FE. Cerebral small vessel disease: recent advances and future directions. Int J Stroke. (2023) 18:4–14. doi: 10.1177/17474930221144911
23. Hassan, A, Hunt, BJ, O’Sullivan, M, Bell, R, D’Souza, R, Jeffery, S, et al. Homocysteine is a risk factor for cerebral small vessel disease, acting via endothelial dysfunction. Brain. (2004) 127:212–9. doi: 10.1093/brain/awh023
24. Yang, M, Huo, X, Miao, Z, and Wang, Y. Platelet glycoprotein IIb/IIIa receptor inhibitor Tirofiban in acute ischemic stroke. Drugs. (2019) 79:515–29. doi: 10.1007/s40265-019-01078-0
25. Siebler, M, Hennerici, MG, Schneider, D, von Reutern, GM, Seitz, RJ, Röther, J, et al. Safety of Tirofiban in acute ischemic stroke: the SaTIS trial. Stroke. (2011) 42:2388–92. doi: 10.1161/STROKEAHA.110.599662
26. R.B.T. InvestigatorsQiu, Z, Li, F, Sang, H, Luo, W, Shuai, J, et al. Effect of intravenous tirofiban vs placebo before endovascular thrombectomy on functional outcomes in large vessel occlusion stroke: the RESCUE BT randomized clinical trial. JAMA. (2022) 328:543–53. doi: 10.1001/jama.2022.12584
27. Tao, C, Liu, T, Cui, T, Liu, J, Li, Z, Ren, Y, et al. Early Tirofiban infusion after intravenous thrombolysis for stroke. N Engl J Med. (2025). doi: 10.1056/NEJMoa2503678
28. Alqurashi, S, Alqahtani, MS, Albeladi, SM, Almahdawi, S, Danish, H, Alshaikh, H, et al. Intravenous tirofiban in acute ischemic stroke patients not receiving reperfusion treatments: a systematic review and meta-analysis of randomized controlled trials. Front Neurol. (2025) 16:1552658. doi: 10.3389/fneur.2025.1552658
29. Li, W, Wu, Y, Li, XS, Liu, CC, Huang, SH, Liang, CR, et al. Intravenous tirofiban therapy for patients with capsular warning syndrome. Stroke Vasc Neurol. (2019) 4:22–7. doi: 10.1136/svn-2018-000163
Keywords: capsular warning syndrome, transient ischemic attack (TIA), LASSO regression, infarction, tirofiban
Citation: Guo R-x and Wang T (2025) Predictors of infarction and outcomes in capsular warning syndrome: a retrospective observational study. Front. Neurol. 16:1617623. doi: 10.3389/fneur.2025.1617623
Edited by:
Aleksandras Vilionskis, Vilnius University, LithuaniaReviewed by:
Adria Arboix, Sacred Heart University Hospital, SpainThomas Lindner, Stavanger University Hospital, Norway
Copyright © 2025 Guo and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tao Wang, d2FuZ3Rhb0B5YXUuZWR1LmNu
 Rui-xi Guo1
Rui-xi Guo1 Tao Wang
Tao Wang