- 1Department of Neurological Surgery, Washington University School of Medicine, St. Louis, MO, United States
- 2Department of Neurological Surgery, Medical College of Wisconsin, Milwaukee, WI, United States
- 3Department of Neurosurgery, Tehran University of Medical Sciences, Tehran, Iran
- 4Department of Neurological Surgery, Penn State University, Hershey, PA, United States
- 5Department of Neurological Surgery, University of Pittsburgh, Pittsburgh, PA, United States
Introduction: Approximately 1.2% of people in the United States have epilepsy. Accurate identification of seizure origin is critical for clinical management. Yan et al. published a systematic review up to 2018 comparing SDE and SEEG (two invasive monitoring modalities) on clinical effectiveness and safety. However, meta-analysis was not performed, and multiple centers have published key SDE and SEEG data since 2018.
Methods: We performed an updated literature search from Yan et al., through June 2023, of studies on patients who underwent SEEG or SDE for seizure focus localization. Inclusion criteria were: (1) randomized control trial, prospective or retrospective cohort study, or case series >5 patients, (2) at least one patient who underwent seizure focus resection, (3) outcomes of either seizure freedom or complications. Meta-5analytic methods were utilized for data analysis.
Results: An initial search resulted in 4,647 records; 81 studies were included, reflecting 3,482 SEEG and 2,816 SDE patients. Compared to SEEG, SDE exhibited similar operative time (164 vs. 185 min, p = 0.50), inpatient monitoring time (8.7 vs. 8.9 days, p = 0.81), and length of hospital stay (11.8 vs. 9.7 days, p = 0.17). Seizure foci were identified in 95.4% of SEEG patients and 91.9% of SDE patients (p = 0.25). A higher proportion of SDE patients underwent resective surgery (85.6 vs. 74.0%, p < 0.01). Overall, 8.0% of SEEG patients and 10.6% of SDE patients experienced adverse events (p = 0.22). Incidence of infection was higher for SDE (1.8%) than for SEEG (0.3%, p < 0.01). Overall, 62.7% of SEEG patients and 63.4% of SDE patients achieved seizure freedom (p = 0.87). Among studies which directly compared SEEG to SDE, there were no differences in seizure freedom attainment or overall morbidity.
Conclusion: SEEG and SDE are safe and effective modalities to localize seizure foci. SDE was associated with higher rates of subsequent resection, but infection rate was higher for SDE than SEEG.
1 Introduction
Approximately 1.2% of people in the United States have epilepsy, which reflects a population of 3.4 million nationwide (1). Approximately 30% of patients with epilepsy will have seizures resistant to antiseizure medications (ASMs) (2). In focal epilepsy, surgical resection is associated with decreased seizure frequency (3–6), increased rate of seizure freedom (6), improved quality of life (7), and better behavioral outcomes compared to ASM therapy alone. Accurately identifying the epileptogenic zone (EZ) for surgical resection is crucial. Scalp electroencephalography (EEG), magnetic resonance imaging (MRI), and semiology analysis are a few common non-invasive methods to identify the EZ. For up to 50% of resective epilepsy surgeries, invasive monitoring with direct brain recordings is necessary to identify the EZ. Invasive monitoring in epilepsy includes both stereoelectroencephalography (SEEG) and subdural electrodes (SDE). While SEEG utilizes thin, depth electrodes to sample superficial and deep cortex, SDE requires a craniotomy and records from the cortical surface of the brain. For the last decade, there has been extensive investigation into the comparative effectiveness of SEEG and SDE regarding (1) clinical effectiveness and (2) safety.
In 2019, Yan et al. published a systematic review comparing SEEG and SDE, reporting that SEEG was associated with higher seizure freedom rates after resection, lower mortality rates, and lower morbidity rates compared to SDE (8). However, this systematic review was limited because at the time, few centers outside of Europe had published their results. Of note, in 2021, Jehi et al. performed a registry analysis of 10 surgical epilepsy centers, reflecting 2,012 patients, to compare rates of surgical resection, seizure freedom, and complications among patients who underwent invasive intracranial monitoring with SDE versus SEEG (7). They found that while surgical resection rate was higher for those who underwent SDE, the complication rate and seizure freedom rates were both lower compared to SEEG (9). Changes in utilization of these invasive monitoring methods may affect clinical outcomes, given increased collective institutional experience. Therefore, the general objectives of this study were to (1) update the systematic review performed by Yan et al. in 2019, (2) to rigorously analyze SEEG and SDE seizure freedom outcomes and complication rates using meta-analytic methods, and (3) incorporate individual analyses into the meta-analysis that directly compared SDE and SEEG. We hypothesized that, akin to what Jehi et al. demonstrated in 2021, SEEG would be associated with a higher seizure freedom rate and a lower complication rate than SDE.
2 Methods
This study was registered on the PROSPERO database and was performed following Preferred Reporting Items of Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
2.1 Information sources and search strategy
We performed an updated literature search relative to Yan et al.’s November 2018 literature search of the Embase, Cochrane, and Ovid MEDLINE databases in December 2021 with the assistance of a librarian (M.A.). Appropriate keywords and subject headings for each database were used for the two concepts, epilepsy (epilepsy, seizure, etc.) and SEEG (stereoelectroencephalography, brain mapping, etc.). Results were limited to primary research articles about humans >2 years of age, written in English, and published since 1946. Conference materials were removed from Embase results. The literature search was subsequently updated in June 2023 to identify any additional articles for inclusion.
The reference lists of retrieved articles were screened by abstract for content and relevance to the research question to identify relevant articles for further review. Only studies written in English were included. Two investigators (N.M. and H.M.R) independently conducted the literature search, screen of abstracts, and selection of included trials.
2.2 Study selection
The goal of the selection process was to review and add to the prior systematic literature search performed by Yan et al. in 2018. Thus, our study selection criteria were identical. We included studies of patients with ASM-resistant epilepsy who underwent SEEG or SDE for seizure foci localization. Studies were included if they: (1) were a randomized control trial, prospective cohort study, retrospective cohort study, or a case series >5 patients, (2) had at least 1 patient who underwent seizure foci resection, (3) had outcomes of either seizure freedom or complications. As opposed to Yan et al.’s methodology, studies that reported on both SDE and SEEG outcomes were included in this analysis and were separately analyzed.
Studies with 5 or fewer patients were excluded from analysis. Other exclusion criteria were: (1) no primary data reported, (2) surgical resection was not a treatment that any patient in the study underwent (i.e., all underwent laser ablation, vagus nerve stimulation, or corpus callosotomy, etc.), (3) all patients underwent resective surgery irrespective of SEEG or SDE analysis, (4) overt selection bias (e.g., all patients had temporal lesion, increasing likelihood of resective surgery), and (5) the paper was not in English. Thermocoagulation and laser interstitial thermal therapy (LITT) were excluded, as these alternative treatment techniques have different seizure freedom rates compared to open resection, prohibiting a direct comparison between SEEG and SDE. There were no exclusions based on age. When duplicate studies were found (i.e., studies from the same epilepsy program over the same or overlapping time frames of data reporting), only the study with the least missing data or the most current study was included for quantitative assessment.
2.3 Data collection and critical appraisal
Each article was reviewed by two investigators independently (N.M. and H.M.R). A data collection form was utilized to create a record for each study and all data were extracted from article text, tables, and figures. Data extracted from articles included author name; year of publication; baseline characteristics, such as age and sex; epilepsy information, such as duration of epilepsy, etiology of temporal origin, tumor, focal cortical dysplasia type 1 (FCD1), FCD2, or lesional; and seizure outcomes, such as percentage seizure freedom following resective surgery, percentage seizure freedom independent of surgery, Engel class, and International League Against Epilepsy (ILAE) grading. Morbidity and mortality data were collected, with specific numbers related to all hemorrhages, intracranial hemorrhage, subdural or epidural hemorrhage, overall infection, meningitis, abscesses, or superficial hemorrhage, cerebrospinal fluid (CSF) leak, lead fractures, transient neurologic deficit, permanent neurologic deficit, and medical complications. Variables were collected as overall counts of participants, means, medians, and ranges where appropriate and available.
2.4 Study risk of bias assessment
The quality of the included studies was evaluated using the Methodological Index for Non-Randomized Studies (MINORS), designed for observational studies. Each study had a risk score for bias ranging from 0 to 16, with ≥13 being low-risk, 12–9 being moderate-risk, and ≤8 being high-risk. Risk of bias was assessed independently by two authors (N.M. and H.M.R).
2.5 Statistical analysis
All analyses were conducted in R statistical analysis software (version 4.1.2, R Foundation for Statistical Computing) using the “meta” package. Event outcomes were pooled using the “metaprop” function, which allows for calculation of 95% confidence intervals with the score statistic and the exact binomial method (10). Continuous outcomes were analyzed using the “metamean” function, which allows for comparison of difference of means. Due to inherent heterogeneity associated with surgical treatments and outcomes, a random effect model was used for reporting outputs. To explore differences in pooled outcomes between monitoring approaches, we conducted subgroup analyses with a Chi-squared tests for subgroup differences as implemented in the “meta” package in R. Meta-regression was conducted to assess the impact of a specific covariate on observed effect. A p-value less than 0.05 was considered significant.
3 Results
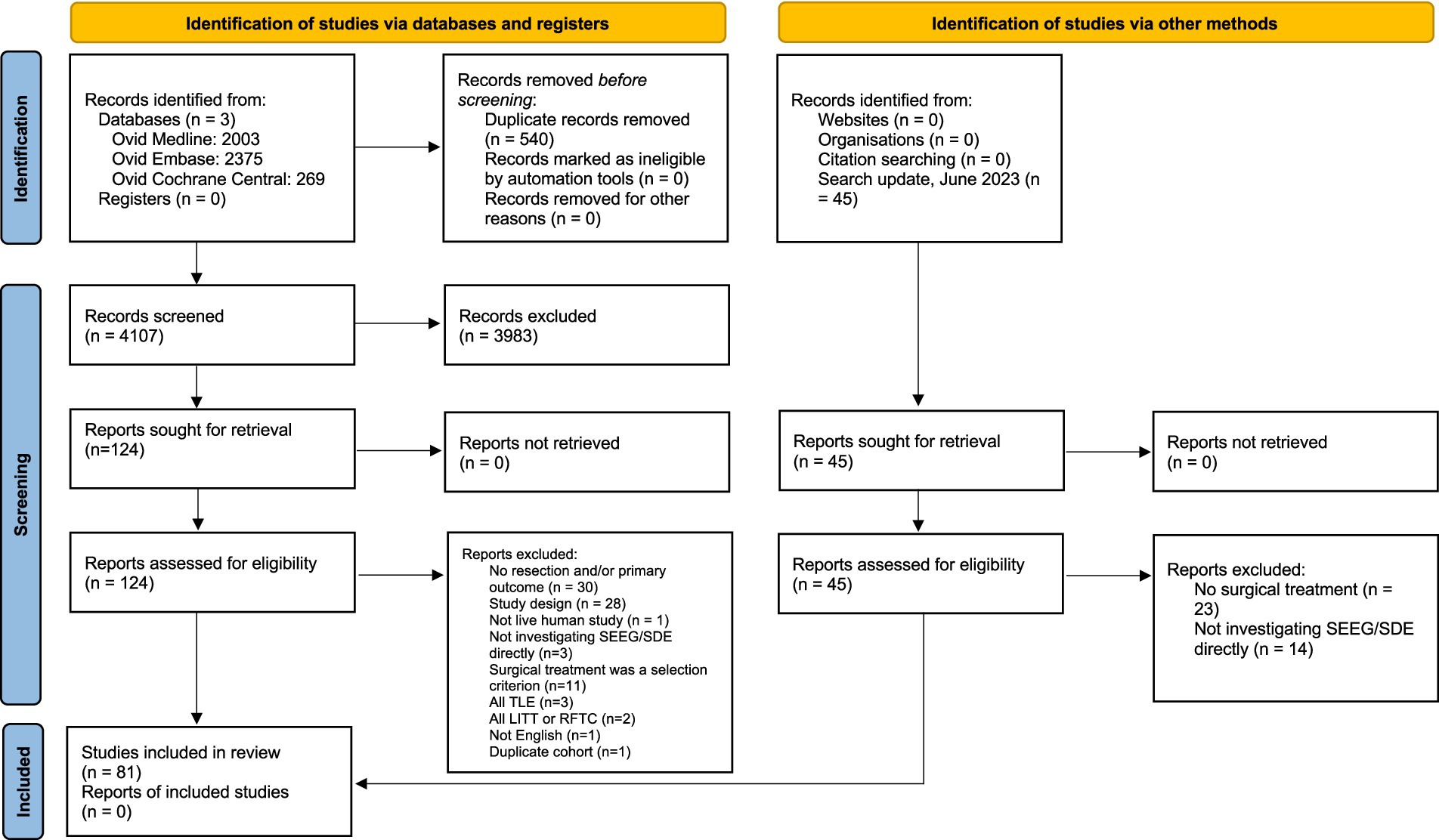
Our search string yielded a total of 4,647 records: 2,003 records from Ovid MEDLINE, 2,375 records from Embase, and 269 records from Cochrane Central. After duplicate removal (n = 540), 4,107 records were screened for title and abstract content. After title and abstract screening by our pre-defined inclusion and exclusion criteria, 124 articles were reviewed in detail via full text. Adding the 9 articles identified through the June 2023 search update, a total of 81 studies were included in this meta-analysis. The flow diagram of the screening process is depicted in Figure 1.
The 81 included studies were published between 1990 and 2023. Most were retrospective observational studies (Table 1). The studies were conducted in several countries including the United States, Sweden, and Korea. These included a total of 6,298 patients with drug-resistant epilepsy, 3,482 who were assessed using SEEG and 2,816 patients who underwent SDE. Among these, 24 studies with 1,350 patients (753 SEEG and 597 SDE) reflected only pediatric patients, 11 studies with 290 patients (225 SEEG and 65 SDE) reflected only adult patients, and 41 studies with 3,917 patients reflected both pediatric and adult patients (2,259 SEEG and 1,658 SDE). The remaining 5 studies with 741 patients (245 SEEG and 496 SDE) did not include sufficient detail to determine whether study samples comprised adult and/or pediatric patients.
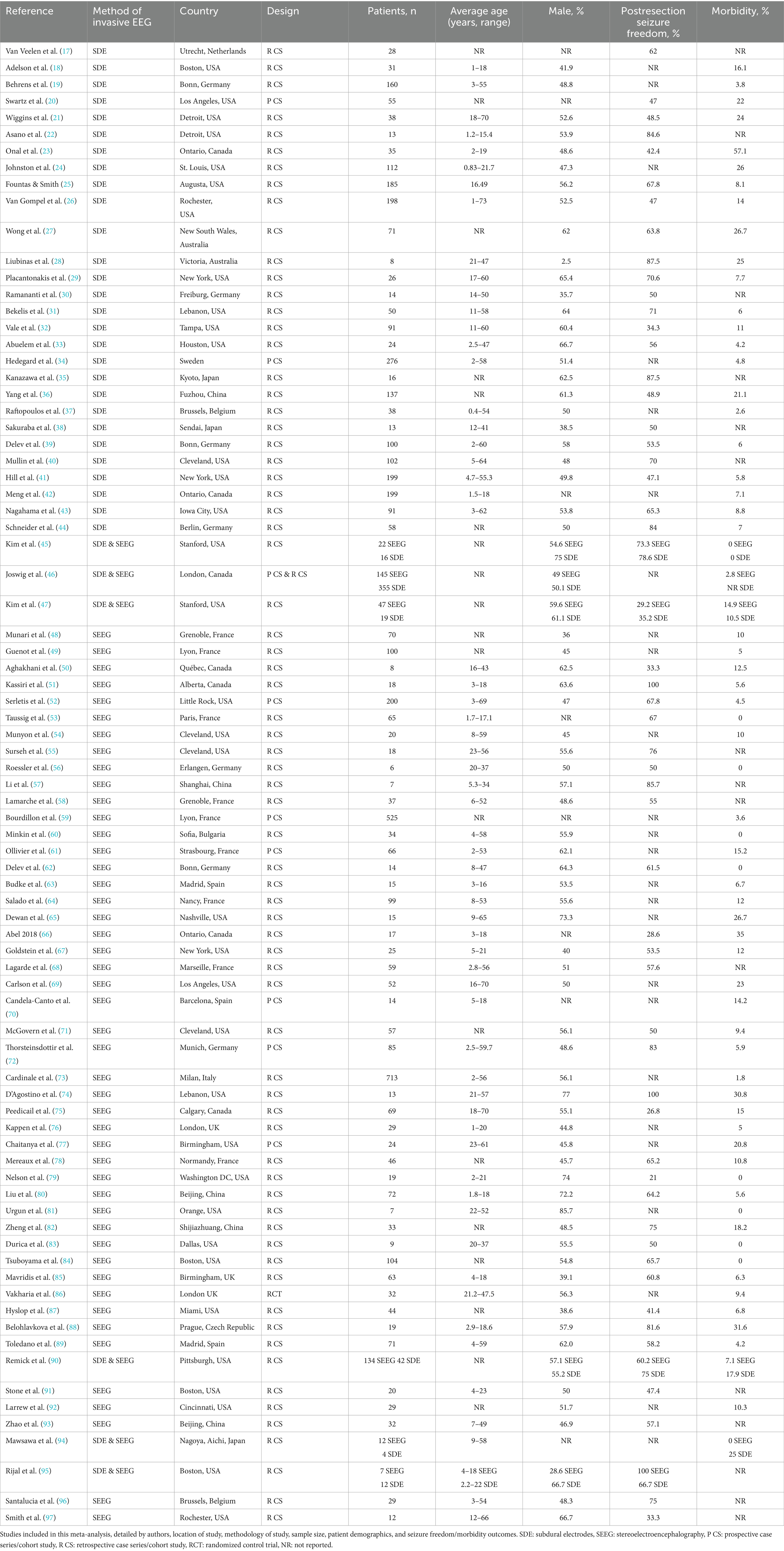
Table 1. Studies included in this meta-analysis, detailed by authors, location of study, methodology of study, sample size, patient demographics, and seizure freedom/morbidity outcomes.
3.1 Baseline cohort characteristics
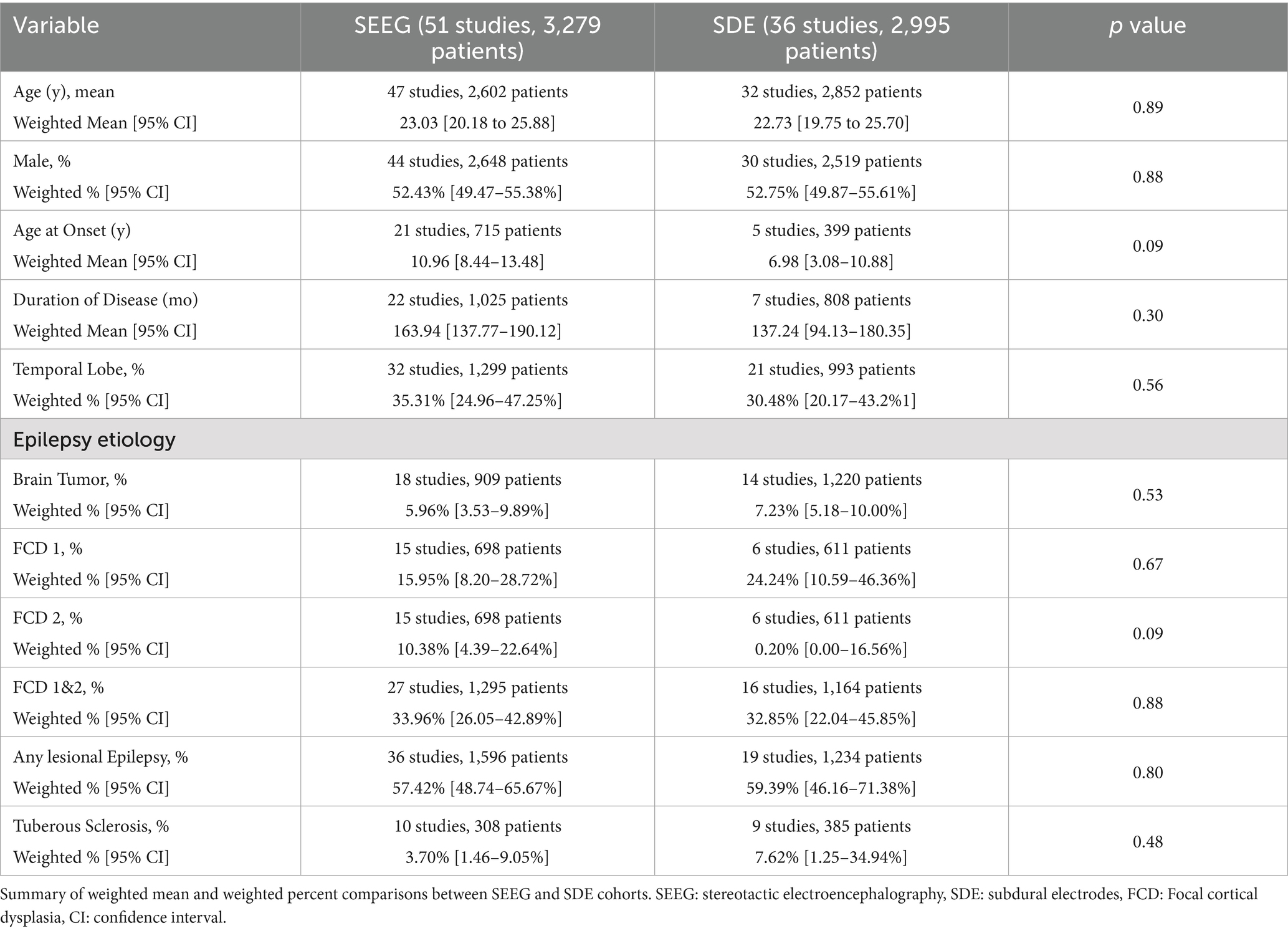
Table 2 demonstrates baseline cohort characteristics for SEEG and SDE patients in this sample. The mean age of patients who underwent SEEG ranged from 8.2 to 40 years with a pooled mean of 23.0 years, while the mean age in the SDE group ranged from 6.7 to 37.7 years with a pooled mean of 22.7 years. Within this cohort, 52.4% of SEEG patients and 52.7% of SDE patients were male. The mean age at seizure onset for SEEG patients was 11.0 years, while it was 7.0 years for SDE patients. The mean duration of epilepsy symptoms was 163.9 months among SEEG patients and 137.2 months among SDE patients. Etiologies of epilepsy were distributed similarly among the two cohorts, with 35% (SEEG) and 30% (SDE) of epilepsy being temporal lobe epilepsy and 57% (SEEG) and 59% (SDE) being lesion-related epilepsy (i.e., tumor, tuber, focal cortical dysplasia, etc.). No baseline characteristics differed significantly between the two invasive monitoring techniques.
3.2 Intracranial monitoring
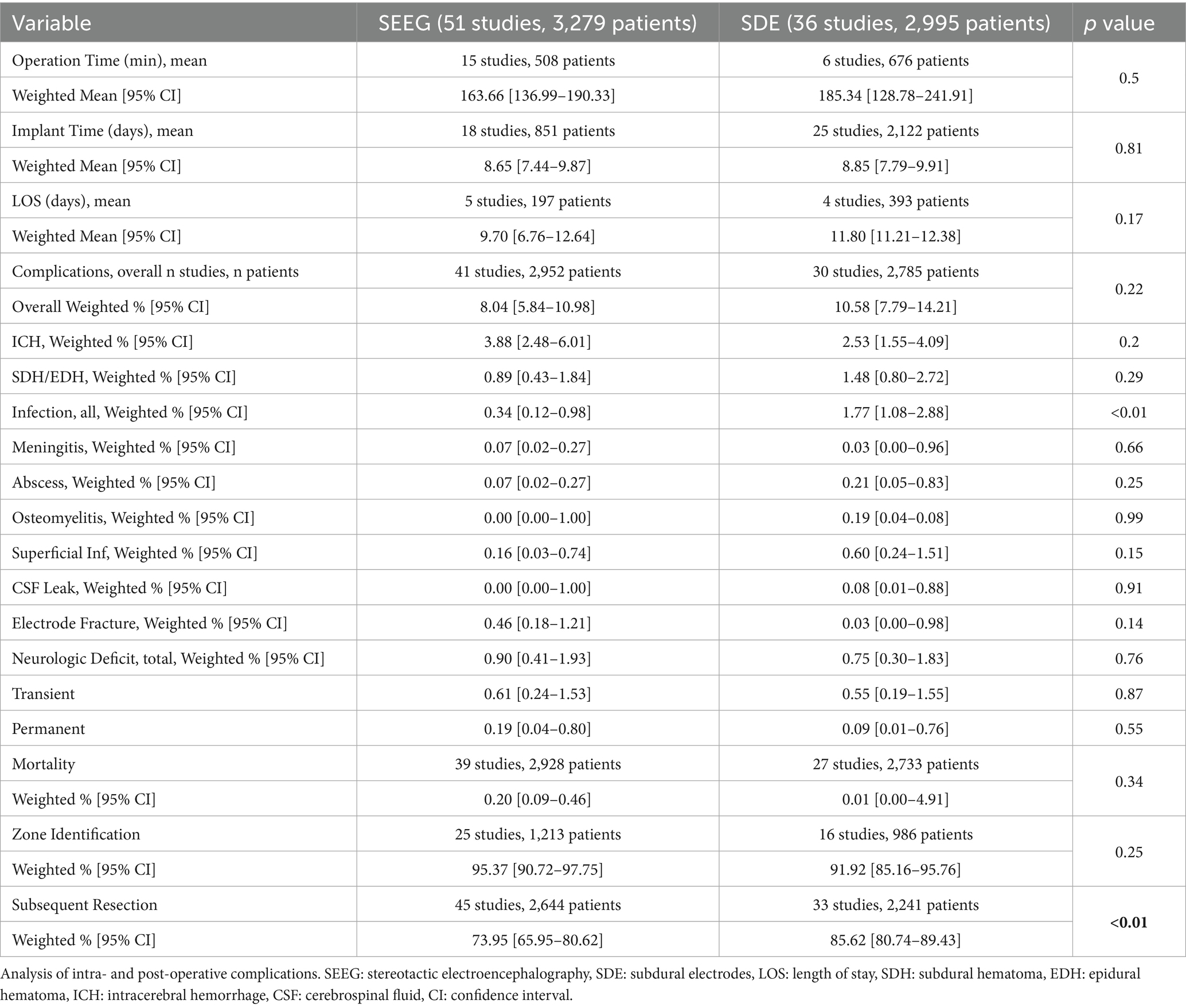
Table 3 demonstrates intraoperative variables associated with electrode placement. The mean operative time for electrode placement was 164 and 185 min for the SEEG and SDE groups, respectively (p = 0.50). Similarly, inpatient monitoring time was comparable between the SEEG and SDE groups (8.7 vs. 8.9 days, p = 0.81). The mean length of hospital stay after electrode placement, while slightly longer in the SDE group, did not significantly differ between the techniques (11.8 vs. 9.7 days, p = 0.17). Notably, the “length of hospital stay” for SDE patients included both invasive monitoring duration and surgical resection at the time of electrode removal if it occurred.
Table 3 additionally summarizes the morbidity and mortality of SEEG versus SDE. Overall, 8.0% of patients who underwent SEEG and 10.6% of patients who underwent SDE experienced adverse events (p = 0.22), with intracranial hemorrhage being the most common adverse event. The pooled mortality of SEEG and SDE were 0.2 and 0.01%, respectively (p = 0.34). Invasive monitoring resulted in seizure foci identification in 95.4% of SEEG patients and 91.9% of SDE patients (p = 0.25). A higher percentage of SDE patients underwent subsequent resective surgery compared to SEEG (85.6 vs. 74.0%, p < 0.01).
3.3 Post-monitoring course
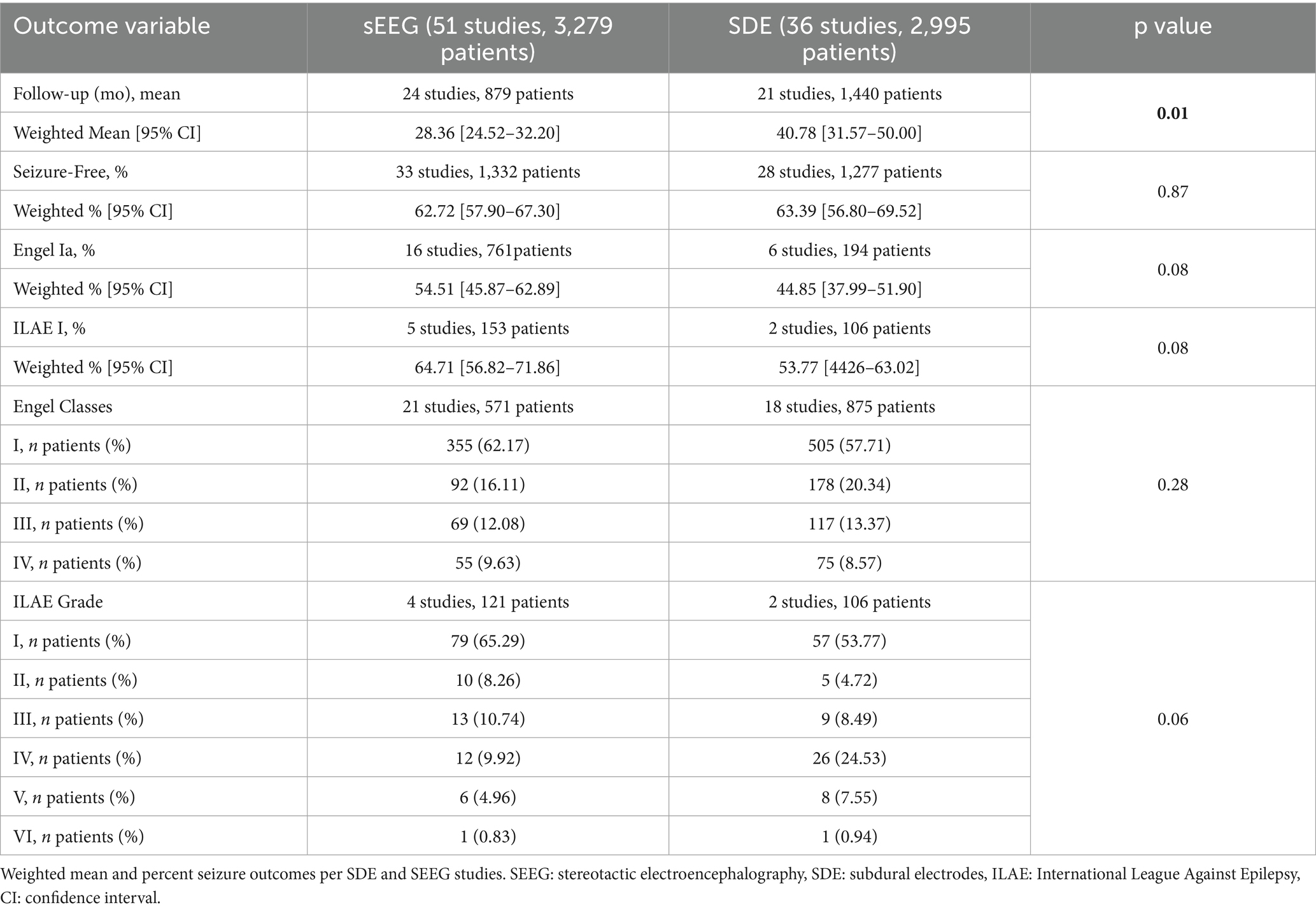
Details of follow duration and seizure outcomes are summarized in Table 4. The mean duration of postoperative follow-up was significantly higher in the SDE group (40.8 months) compared to the SEEG group (28.4 months, p = 0.01). Most studies which reported seizure freedom outcomes utilized the Engel classification (n = 16 SEEG studies reflecting 761 patients and n = 6 SDE studies reflecting 164 patients), while a minority utilized the ILAE classification (n = 5 SEEG studies reflecting 153 patients and n = 2 SDE studies reflecting 106 patients). Of note, some studies which reported Engel classes did not specifically sub-classify the Engel 1 class. Overall, there was no significant difference in seizure freedom outcomes (either ILAE 1 or Engel 1a, both corresponding to complete and sustained seizure freedom), with 62.7% of SEEG patients and 63.4% of SDE patients achieving seizure freedom (p = 0.87). Among studies reporting Engel classification, Engel 1a was achieved in 62.7% of SEEG surgeries and 44.9% of SDE surgeries (p = 0.08). Among studies reporting ILAE classification, ILAE 1 was achieved in 64.7% of SEEG patients and 53.8% of SDE patients (p = 0.08). The distribution of outcomes (either Engel classes or ILAE grades) was similar between the two approaches (p = 0.28 for Engel classes and p = 0.06 for ILAE grades).
Figure 2 demonstrates the rate of seizure freedom as a function of follow-up duration (2a,b) and study publication year (2c,d). This meta-regression demonstrated that the seizure freedom rate was not significantly associated with follow up duration for either cohort (Figures 2a,b, SEEG p = 0.15, SDE p = 0.10). There was also no significant association between year of study publication and seizure freedom rate for either cohort (Figures 2c,d, SEEG p = 0.61, SDE p = 0.67).
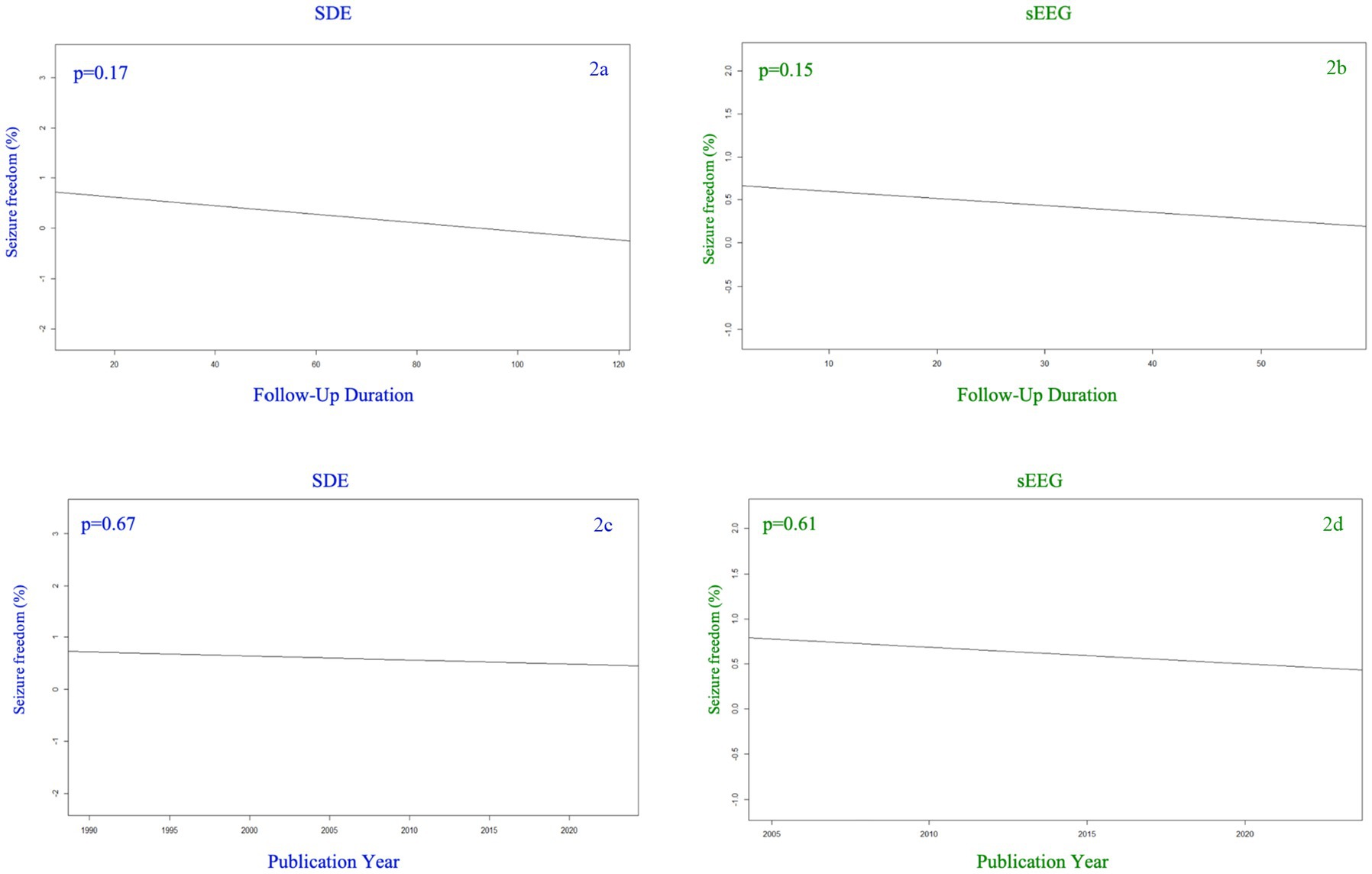
Figure 2. Seizure freedom percentage among SDE (left side) versus sEEG (right side) studies based on the year of publication (top row) and the duration of follow up (months) for the studies reported (bottom row).
Figure 3 demonstrates morbidity as a function of follow-up duration (3a,b) and study publication year (3c,d). While the SEEG group’s overall morbidity rate was affected by neither study publication year nor follow-up duration, the SDE group demonstrated a lower morbidity rate as follow-up duration increased (p = 0.03). There was no significant association between publication year and morbidity rate among the SDE group.
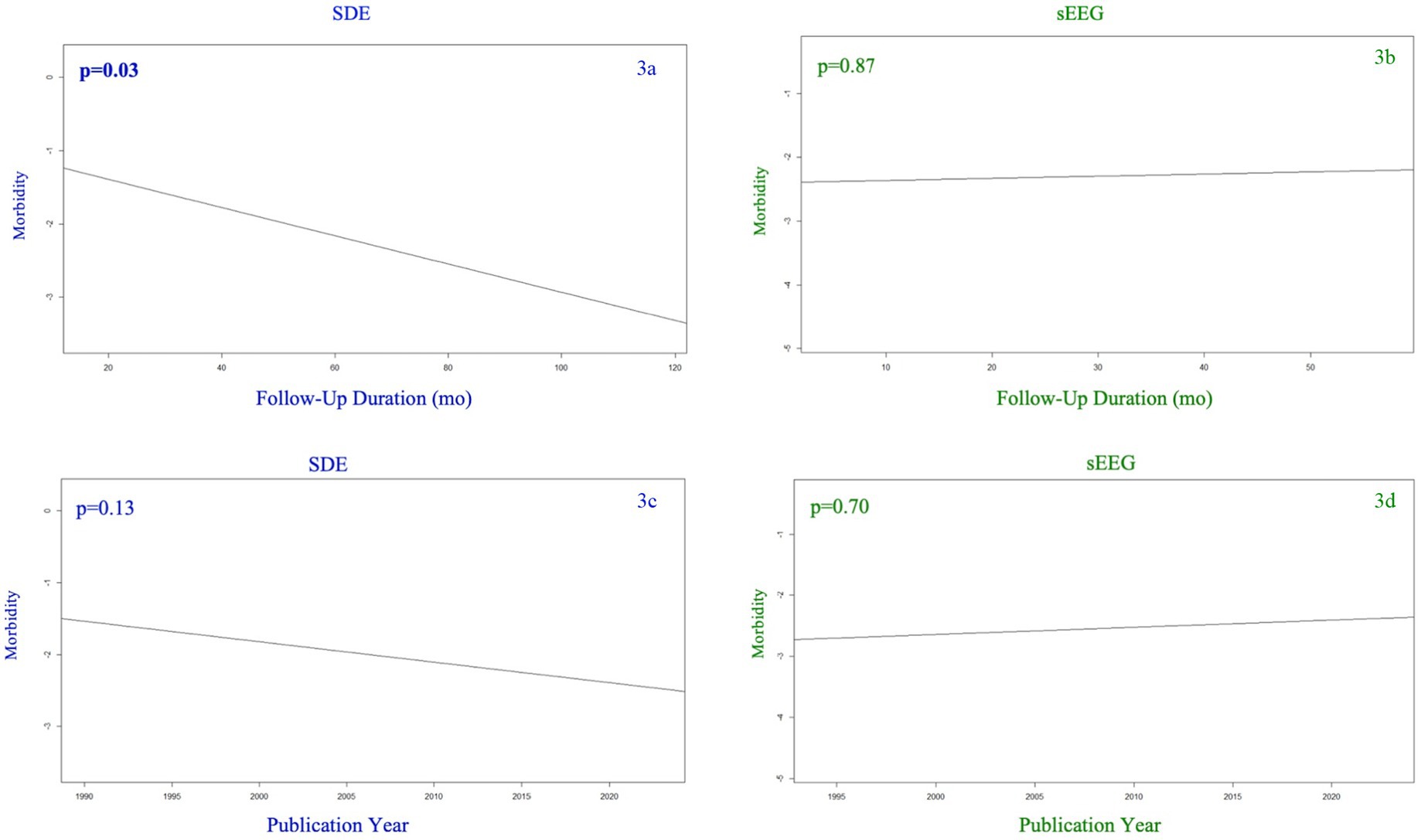
Figure 3. Morbidity (any complication) among SDE (left side) versus sEEG (right side) studies based on the year of publication (top row) and the duration of follow up (months) for the studies reported (bottom row).
3.4 Double arm studies
There were 6 studies in this analysis which reported data on both SEEG and SDE, and 3 reported seizure freedom outcomes using the Engel classification system. Figures 4a–c demonstrate forest plots of seizure freedom between SEEG and SDE (4a), outcome of Engel 1a. between SEEG and SDE (4b), and overall morbidity rates from the invasive monitoring procedure (4c). Overall, there were no differences between SEEG and SDE (among these studies in which the procedures were directly compared) with regards to seizure freedom attainment or overall morbidity.
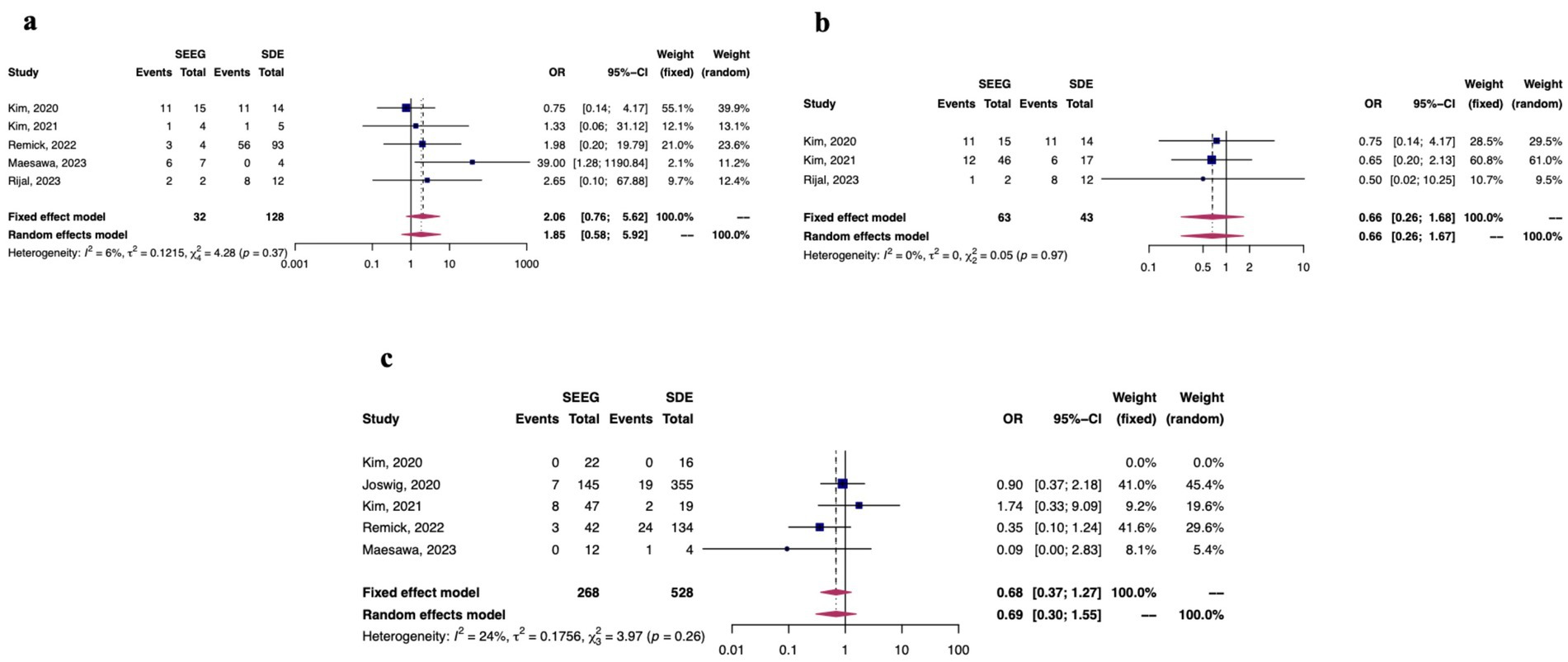
Figure 4. Among studies which directly compared SDE to sEEG (n = 6), fixed and random effects model results for seizure freedom (a), Engel 1a outcomes (b), and morbidity outcomes (c).
3.5 Risk of bias
The majority of studies included in this analysis (58.0%) demonstrated moderate risk of bias per the MINORS scale, and 25.9% of included studies demonstrated high risk of bias. The primary source of bias was rooted in retrospective study design. Loss to follow up was another source of bias.
4 Discussion
In this study, we present the first meta-analysis to directly compare SEEG with SDE, specifically including studies that compared SEEG to SDE directly. Our analysis utilized data from 81 articles, representing 6,274 patients with drug-resistant epilepsy who underwent either SEEG or SDE for seizure foci localization. We found SDE to be associated with significantly increased rate of subsequent surgical resection, but not associated with seizure freedom or morbidity. Further, we found that SDE was associated with an increased infection rate compared to SEEG.
SDE and SEEG are both important, albeit different, surgical techniques to more accurately localize seizure foci. Both techniques involve temporary implantation of electrodes and subsequent inpatient EEG monitoring while electrodes remain in place. In North America, SDE was previously the standard method for invasive EEG recording. SDE typically requires a large craniotomy and only allows for placement of electrodes on the surface of the cortex. In this way, a large portion of the cortical surface can be covered due to the size of the electrode grids and strips as well as the large craniotomy required for implantation. A relative advantage of SDE is this broad cortical surface coverage, which allows for easier implementation of functional mapping compared to SEEG (97). However, not all seizures originate from the cortical surface. For patients with seizure foci deeper than can be detected by subdural electrodes, SDE may not adequately capture the EZ with SDE unless depth electrodes are also implanted. In the past decade, SEEG techniques have become rapidly adopted across North America. SEEG is a less invasive technique which involves implanting depth electrodes through a percutaneous stereotactic approach. SEEG offers the unique advantages of avoiding a large craniotomy, implantation in both hemispheres when necessary, and access to deep cortical structures. Furthermore, with the increased availability of surgical robots such as ROSA, SEEG electrode implantation is more accurate, efficient, and safe. The startup of an SEEG program can be somewhat burdensome to institutions, however, as equipment is costly and requires specialized training (98).
Our analysis revealed no differences in surgical electrode placement time (185 min for SDE versus 163 min for SEEG), monitoring time (8.9 days for SDE vs. 8.6 days for SEEG), or length of hospital stay (11.8 days for SDE vs. 9.7 days for SEEG) between procedures. Recent evidence had demonstrated that with the advancements in neurosurgical robotics, SEEG cases were approximately one-third the duration of SDE cases (11). However, in our analysis, we did not specifically ascertain which SEEG procedures were performed with robots versus without, nor did we obtain the size of the craniotomies performed for SDE procedures. Thus, there is heterogeneity in the data, which may explain the lack of difference between procedures. Additionally, as mentioned earlier, experience has potentially optimized both surgeons’ efficiency with their preferred procedure and subsequent postoperative patient care.
SEEG successfully localized 95% of seizure foci, while SDE localized 92% of seizure foci. Although this was not statistically significant, it is a clinically important finding that a similar proportion of patients can achieve seizure foci localization with a less invasive procedure. Interestingly, a higher proportion of SDE patients underwent surgical resection of seizure than SEEG patients. This may be secondary to the fact that SDE involves a craniotomy for electrode implantation and requires reopening of the craniotomy for explantation. Therefore, if seizure foci are identified, they are easily accessible at the time of SDE explantation. For SEEG patients, seizure foci resection does not necessarily need to involve open craniotomy. Patients may opt to continue with minimally invasive approaches for treatment if the location is amenable, including LITT or radiofrequency ablation, and these patients are not reflected in our analysis. Further, localization by SEEG may afford the opportunity to resect seizure foci through a smaller incision than used to implant SDE electrodes.
We also found no difference in subsequent resection or seizure freedom rates between SEEG and SDE. This contrasts with Yan et al.’s previous finding (8), though it substantiates early evidence that the two procedures may be similar in effectiveness. The reason for this finding is likely multifaceted. As institutions have gained more experience with SEEG or SDE over the last several years, it is feasible that centers have opted primarily for one over the other. Therefore, each institution has developed expertise in their preferred method of invasive EEG. It is feasible that with this increased experience, surgeons have improved in the following aspects: selecting appropriate patients for each procedure, identifying cortical areas of interest based on non-invasive diagnostic studies, performing surgical implantation safely and precisely, interpreting data with more nuance, and/or improved management of patients postoperatively. In short, perhaps institutions are becoming better experts in their chosen invasive EEG method.
We were curious as to whether a study’s publication year or reported follow-up duration were associated with the finding that seizure freedom was similar between SEEG and SDE. As our baseline analysis demonstrated, studies in the SDE cohort reported longer follow up duration than studies in the SEEG cohort. This is likely secondary to the fact that SEEG is a more newly adopted technique, which is substantiated by the finding that studies published in 2018 or later were significantly biased toward reporting on SEEG (p < 0.001; Chi-squared test). Despite this, we found no significant association between either year of publication or follow-up duration and seizure freedom in either of our cohorts. We conducted a similar analysis for morbidity and found the SDE group demonstrated a lower morbidity rate as follow-up duration increased (p = 0.03). This is an interesting finding though it is without a clear explanation.
Some centers have rapidly adopted SEEG and have shown excellent results with the technique. Indeed, a recent large-scale, longitudinal, single-center analysis by Tandon et al. demonstrated that the adoption of SEEG was associated with lower complication rates, less narcotic utilization, and improved seizure localization compared to SDE (9). Lower resection rates following SEEG compared to SDE have been demonstrated previously (11). In being more selective with offering surgical resection, only patients with the greatest likelihood of having a positive outcome undergo the procedure. This minimizes the proportion of patients who undergo surgical resection and continue to have seizures. However, our finding may be interpreted from another perspective. It is possible that while SEEG was successful at localizing 95% of seizure foci, these foci were not able to be feasibly resected, which may have negatively influenced the seizure freedom rate. SEEG has been utilized for complex, difficult-to-localize seizure foci (12), which may influence outcomes following this procedure. Additionally, SEEG may be a preferred option in nonlateralized epilepsy as it avoids the requirement for bilateral craniotomies (97).
Regarding morbidity and mortality, 8.0% of patients who underwent SEEG and 10.6% of those who underwent SDE experienced any postoperative morbidity. The most common complication was intracranial hemorrhage (3.9% among SEEG patients and 2.5% among SDE patients). SDE was associated with a significantly higher infection rate than SEEG (1.8% versus 0.3%, respectively). This is consistent with existing literature. Given that SDEs are associated with larger incisions and larger exposed surface area, infection rates have been demonstrated to be higher for SDE procedures when compared to SEEG (11, 12). The incidence of intracranial hemorrhage between the two procedures is mixed. Even though SDE involves a larger incision and craniotomy, several small burr holes and depth electrodes are typically placed for SEEG, each of which can also be associated with small subdural and epidural hemorrhages as well as tract hemorrhages or small intraparenchymal hemorrhages along the electrode trajectory. Most studies did not report on the size or clinical significance of these intracranial hemorrhages (i.e., if they were able to be followed with serial imaging or required reoperation), so this nuance is missing in our meta-analysis. There were similar rates of CSF leak, electrode fracture, and neurologic deficits (both temporary and permanent) between groups. Finally, we found there was no difference in the mortality rate between SDE and SEEG (0.01 vs. 0.2%, respectively).
4.1 Limitations
This study has several limitations which are important to discuss. Firstly, it is important to note that there is likely strong selection bias as to which patients undergo SDE versus SEEG. Because of the requirement of craniotomy for SDE, often at institutions where SDE is common, only patients who are expected to eventually need a craniotomy for surgical resection based on pre-invasive monitoring data undergo SDE monitoring. In other words, while SEEG is more broadly utilized in epilepsy patients, SDE may be implanted in patients with a higher pre-test probability for resection. Furthermore, studies included in this analysis, while rigorous, were heterogenous. There are several confounders for which we are not able to account and may affect the primary outcomes of this analysis. Not all studies included potentially useful demographic data. For example, not all studies mentioned whether patients had clear lesions on MRI, if epilepsy was associated with prior traumatic brain injury, or if epilepsy was thought to be congenital. Still, we rigorously excluded studies with clear biases that could influence the decision to pursue surgical resection. For example, we excluded studies which reported exclusively temporal lobe epilepsy patients. An important avenue for subsequent research is to segregate etiologies of epilepsy and assess if SEEG or SDE are more likely to allow a certain population to attain seizure freedom. Similar to the Yan et al. study, ours is also limited by the heterogeneity of technique. Subdural electrodes may involve strips, grids, or a combination of both and can be placed via burr holes or craniotomies, while SEEG can be placed with a robot or manually. Further, both techniques can involve implantation with unilateral or bilateral coverage. These variables can significantly affect the operative time as well as the complication rates of these two procedures. Even in the perioperative period, there is heterogeneity in medical management. For example, some institutions give antibiotics for the duration of invasive monitoring, while other dose antibiotics only at the time of implantation, and this may possibly affect infection incidence. Not all manuscripts had the same rigor in reporting methodology, so the collection of data and synthesis of conclusions is limited. Finally, we acknowledge the years in which this meta-analysis includes, ending in 2023, which may exclude new studies that have been published within the last year.
5 Conclusion
In this meta-analysis comparing SEEG to SDE, utilizing data from 81 articles, representing 6,298 patients, we demonstrate that SEEG and SDE have similar proportions of patients who subsequently achieve seizure freedom. While SDE patients are more likely to undergo open resective surgery after successful seizure foci localization, SDE was also associated with a higher infection rate than SEEG. The mortality rate of both invasive monitoring procedures is low. Ultimately, SEEG is becoming more globally utilized for accurately capturing seizure foci, and in this analysis, allowed for successful localization in 95% of patients. These patients may go on to have open resective surgery but alternatively have the option of minimally invasive techniques for treatment of seizure foci, like LITT or radiofrequency ablation. Given similar outcomes, the selection of invasive monitoring techniques for a patient should thus be dictated by factors such as institutional availability of resources and expertise, clinical hypotheses of EZ localization, and may consider a combination of these methods for optimal results.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
NM: Data curation, Writing – review & editing, Validation, Methodology, Formal analysis, Writing – original draft, Investigation, Conceptualization, Visualization, Resources. HR: Writing – original draft, Writing – review & editing, Investigation, Methodology, Visualization, Validation, Data curation. SM: Validation, Formal analysis, Writing – review & editing, Data curation, Visualization, Software, Resources. AM: Writing – original draft, Resources, Project administration, Supervision, Conceptualization, Validation, Investigation. TA: Project administration, Validation, Methodology, Visualization, Conceptualization, Resources, Formal analysis, Data curation, Investigation, Supervision, Writing – review & editing, Funding acquisition, Writing – original draft. EH: Project administration, Validation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. National Center for Chronic Disease Prevention and Health Promotion DoPH. (2023). Epilepsy data and statistics. Updated 03/29/2023. Available online at: https://www.cdc.gov/epilepsy/data/index.html (Accessed April 19, 2024).
2. Sultana, B, Panzini, MA, Veilleux Carpentier, A, Comtois, J, Rioux, B, Gore, G, et al. Incidence and prevalence of drug-resistant epilepsy: a systematic review and Meta-analysis. Neurology. (2021) 96:805–17. doi: 10.1212/WNL.0000000000011839
3. Asadi-Pooya, AA, Brigo, F, Lattanzi, S, and Blumcke, I. Adult epilepsy. Lancet. (2023) 402:412–24. doi: 10.1016/S0140-6736(23)01048-6
4. Willard, A, Antonic-Baker, A, Chen, Z, O’Brien, TJ, Kwan, P, and Perucca, P. Seizure outcome after surgery for MRI-diagnosed focal cortical dysplasia: a systematic review and meta-analysis. Neurology. (2022) 98:e236–48. doi: 10.1212/WNL.0000000000013066
5. Rugg-Gunn, F, Miserocchi, A, and McEvoy, A. Epilepsy surgery. Pract Neurol. (2020) 20:4–14. doi: 10.1136/practneurol-2019-002192
6. Hsieh, JK, Pucci, FG, Sundar, SJ, Kondylis, E, Sharma, A, Sheikh, SR, et al. Beyond seizure freedom: dissecting long-term seizure control after surgical resection for drug-resistant epilepsy. Epilepsia. (2023) 64:103–13. doi: 10.1111/epi.17445
7. Dwivedi, R, Ramanujam, B, Chandra, PS, Sapra, S, Gulati, S, Kalaivani, M, et al. Surgery for drug-resistant epilepsy in children. N Engl J Med. (2017) 377:1639–47. doi: 10.1056/NEJMoa1615335
8. Yan, H, Katz, JS, Anderson, M, Mansouri, A, Remick, M, Ibrahim, GM, et al. Method of invasive monitoring in epilepsy surgery and seizure freedom and morbidity: a systematic review. Epilepsia. (2019) 60:1960–72. doi: 10.1111/epi.16315
9. Jehi, L, Morita-Sherman, M, Love, TE, Bartolomei, F, Bingaman, W, Braun, K, et al. Comparative effectiveness of stereotactic electroencephalography versus subdural grids in epilepsy surgery. Ann Neurol. (2021) 90:927–39. doi: 10.1002/ana.26238
10. Nyaga, VN, Arbyn, M, and Aerts, M. Metaprop: a Stata command to perform meta-analysis of binomial data. Arch Public Health. (2014) 72:39. doi: 10.1186/2049-3258-72-39
11. Tandon, N, Tong, BA, Friedman, ER, Johnson, JA, Von Allmen, G, Thomas, MS, et al. Analysis of morbidity and outcomes associated with use of subdural grids vs stereoelectroencephalography in patients with intractable epilepsy. JAMA Neurol. (2019) 76:672–81. doi: 10.1001/jamaneurol.2019.0098
12. Vadera, S, Mullin, J, Bulacio, J, Najm, I, Bingaman, W, and Gonzalez-Martinez, J. Stereoelectroencephalography following subdural grid placement for difficult to localize epilepsy. Neurosurgery. (2013) 72:723–9. doi: 10.1227/NEU.0b013e318285b4ae
13. Cardinale, F, Casaceli, G, Raneri, F, Miller, J, and Lo Russo, G. Implantation of stereoelectroencephalography electrodes: a systematic review. J Clin Neurophysiol. (2016) 33:490–502. doi: 10.1097/WNP.0000000000000249
14. Cardinale, F, Cossu, M, Castana, L, Casaceli, G, Schiariti, MP, Miserocchi, A, et al. Stereoelectroencephalography: surgical methodology, safety, and stereotactic application accuracy in 500 procedures. Neurosurgery. (2013) 72:353–66. doi: 10.1227/NEU.0b013e31827d1161
15. Lee, WS, Lee, JK, Lee, SA, Kang, JK, and Ko, TS. Complications and results of subdural grid electrode implantation in epilepsy surgery. Surg Neurol. (2000) 54:346–51. doi: 10.1016/S0090-3019(00)00324-4
16. Mullin, JP, Shriver, M, Alomar, S, Najm, I, Bulacio, J, Chauvel, P, et al. Is SEEG safe? A systematic review and meta-analysis of stereo-electroencephalography-related complications. Epilepsia. (2016) 57:386–401. doi: 10.1111/epi.13298
17. van Veelen, CW, Debets Rm,, van Huffelen, AC, van Emde Boas, W, Binnie, CD, Storm van Leeuwen, W, et al. Combined use of subdural and intracerebral electrodes in preoperative evaluation of epilepsy. Neurosurgery. (1990) 26:93–101. doi: 10.1227/00006123-199001000-00013
18. Adelson, PD, Black, PM, Madsen, JR, Adelson, D, Kramer, U, Rockoff, MA, et al. Use of subdural grids and strip electrodes to identify a seizure focus in children. Pediatr Neurosurg. (1995) 22:174–80. doi: 10.1159/000120898
19. Behrens, E, Zentner, J, van Roost, D, Hufnagel, A, Elger, CE, and Schramm, J. Subdural and depth electrodes in the presurgical evaluation of epilepsy. Acta Neurochir. (1994) 128:84–7. doi: 10.1007/BF01400656
20. Swartz, BE, Rich, JR, Dwan, PS, DeSalles, A, Kaufman, MH, Walsh, GO, et al. The safety and efficacy of chronically implanted subdural electrodes: a prospective study. Surg Neurol. (1996) 46:87–93. doi: 10.1016/0090-3019(96)00083-3
21. Wiggins, GC, Elisevich, K, and Smith, BJ. Morbidity and infection in combined subdural grid and strip electrode investigation for intractable epilepsy. Epilepsy Res. (1999) 37:73–80. doi: 10.1016/S0920-1211(99)00037-6
22. Asano, E, Muzik, O, Shah, A, Juhász, C, Chugani, DC, Sood, S, et al. Quantitative interictal subdural EEG analyses in children with neocortical epilepsy. Epilepsia. (2003) 44:425–34. doi: 10.1046/j.1528-1157.2003.38902.x
23. Onal, C, Otsubo, H, Araki, T, Chitoku, S, Ochi, A, Weiss, S, et al. Complications of invasive subdural grid monitoring in children with epilepsy. J Neurosurg. (2003) 98:1017–26. doi: 10.3171/jns.2003.98.5.1017
24. Johnston, JM Jr, Mangano, FT, Ojemann, JG, Park, TS, Trevathan, E, and Smyth, MD. Complications of invasive subdural electrode monitoring at St. Louis children’s hospital, 1994-2005. J Neurosurg. (2006) 105:343–7. doi: 10.3171/ped.2006.105.5.343
25. Fountas, KN, and Smith, JR. Subdural electrode-associated complications: a 20-year experience. Stereotact Funct Neurosurg. (2007) 85:264–72. doi: 10.1159/000107358
26. Van Gompel, JJ, Worrell, GA, Bell, ML, Patrick, TA, Cascino, GD, Raffel, C, et al. Intracranial electroencephalography with subdural grid electrodes: techniques, complications, and outcomes. Neurosurgery. (2008) 63:498–505. doi: 10.1227/01.NEU.0000324996.37228.F8
27. Wong, CH, Birkett, J, Byth, K, Dexter, M, Somerville, E, Gill, D, et al. Risk factors for complications during intracranial electrode recording in presurgical evaluation of drug resistant partial epilepsy. Acta Neurochir. (2009) 151:37–50. doi: 10.1007/s00701-008-0171-7
28. Liubinas, SV, Cassidy, D, Roten, A, Kaye, AH, and O’Brien, TJ. Tailored cortical resection following image guided subdural grid implantation for medically refractory epilepsy. J Clin Neurosci. (2009) 16:1398–408. doi: 10.1016/j.jocn.2009.03.012
29. Placantonakis, DG, Shariff, S, Lafaille, F, Labar, D, Harden, C, Hosain, S, et al. Bilateral intracranial electrodes for lateralizing intractable epilepsy: efficacy, risk, and outcome. Neurosurgery. (2010) 66:274–83. doi: 10.1227/01.NEU.0000363184.43723.94
30. Ramantani, G, Koessler, L, Colnat-Coulbois, S, Vignal, JP, Isnard, J, Catenoix, H, et al. Intracranial evaluation of the epileptogenic zone in regional infrasylvian polymicrogyria. Epilepsia. (2013) 54:296–304. doi: 10.1111/j.1528-1167.2012.03667.x
31. Bekelis, K, Radwan, TA, Desai, A, Moses, ZB, Thadani, VM, Jobst, BC, et al. Subdural interhemispheric grid electrodes for intracranial epilepsy monitoring: feasibility, safety, and utility: clinical article. J Neurosurg. (2012) 117:1182–8. doi: 10.3171/2012.8.JNS12258
32. Vale, FL, Pollock, G, Dionisio, J, Benbadis, SR, and Tatum, WO. Outcome and complications of chronically implanted subdural electrodes for the treatment of medically resistant epilepsy. Clin Neurol Neurosurg. (2013) 115:985–90. doi: 10.1016/j.clineuro.2012.10.007
33. Abuelem, T, Friedman, DE, Agadi, S, Wilfong, AA, and Yoshor, D. Interhemispheric subdural electrodes: technique, utility, and safety. Neurosurgery. (2013) 73:ons253–60. doi: 10.1227/01.neu.0000430287.08552.83
34. Hedegard, E, Bjellvi, J, Edelvik, A, Rydenhag, B, Flink, R, and Malmgren, K. Complications to invasive epilepsy surgery workup with subdural and depth electrodes: a prospective population-based observational study. J Neurol Neurosurg Psychiatry. (2014) 85:716–20. doi: 10.1136/jnnp-2013-306465
35. Kanazawa, K, Matsumoto, R, Imamura, H, Matsuhashi, M, Kikuchi, T, Kunieda, T, et al. Intracranially recorded ictal direct current shifts may precede high frequency oscillations in human epilepsy. Clin Neurophysiol. (2015) 126:47–59. doi: 10.1016/j.clinph.2014.05.028
36. Yang, PF, Zhang, HJ, Pei, JS, Tian, J, Lin, Q, Mei, Z, et al. Intracranial electroencephalography with subdural and/or depth electrodes in children with epilepsy: techniques, complications, and outcomes. Epilepsy Res. (2014) 108:1662–70. doi: 10.1016/j.eplepsyres.2014.08.011
37. Raftopoulos, C, Vaz, G, Tassigny, D, and Van Rijckevorsel, K. Invasive EEG in refractory epilepsy: insertion of subdural grids through linear craniectomy reduces complications and remains effective. Neurochirurgie. (2015) 61:16–21. doi: 10.1016/j.neuchi.2014.09.005
38. Sakuraba, R, Iwasaki, M, Okumura, E, Jin, K, Kakisaka, Y, Kato, K, et al. High frequency oscillations are less frequent but more specific to epileptogenicity during rapid eye movement sleep. Clin Neurophysiol. (2016) 127:179–86. doi: 10.1016/j.clinph.2015.05.019
39. Delev, D, Send, K, Malter, M, Ormond, DR, Parpaley, Y, von Lehe, M, et al. Role of subdural interhemispheric electrodes in Presurgical evaluation of epilepsy patients. World Neurosurg. (2015) 84:1719–25.e1. doi: 10.1016/j.wneu.2015.07.034
40. Mullin, JP, Sexton, D, Al-Omar, S, Bingaman, W, and Gonzalez-Martinez, J. Outcomes of subdural grid electrode monitoring in the Stereoelectroencephalography era. World Neurosurg. (2016) 89:255–8. doi: 10.1016/j.wneu.2016.02.034
41. Hill, TC, Rubin, BA, Tyagi, V, Theobald, J, Silverberg, A, Miceli, M, et al. The value of diagnostic bilateral intracranial electroencephalography in treatment-resistant focal epilepsy. World Neurosurg. (2017) 103:1–10. doi: 10.1016/j.wneu.2017.01.093
42. Meng, Y, Voisin, MR, Suppiah, S, Merali, Z, Moghaddamjou, A, Alotaibi, NM, et al. Risk factors for surgical site infection after intracranial electroencephalography monitoring for epilepsy in the pediatric population. J Neurosurg Pediatr. (2018) 22:31–6. doi: 10.3171/2018.1.PEDS17476
43. Nagahama, Y, Schmitt, AJ, Nakagawa, D, Vesole, AS, Kamm, J, Kovach, CK, et al. Intracranial EEG for seizure focus localization: evolving techniques, outcomes, complications, and utility of combining surface and depth electrodes. J Neurosurg. (2019) 130:1180–92. doi: 10.3171/2018.1.JNS171808
44. Schneider, UC, Oltmanns, F, Vajkoczy, P, Holtkamp, M, and Dehnicke, C. Craniotomy size for subdural grid electrode placement in invasive epilepsy diagnostics. Stereotact Funct Neurosurg. (2019) 97:160–8. doi: 10.1159/000501235
45. Kim, LH, Parker, JJ, Ho, AL, Pendharkar, AV, Sussman, ES, Halpern, CH, et al. Postoperative outcomes following pediatric intracranial electrode monitoring: a case for stereoelectroencephalography (SEEG). Epilepsy Behav. (2020) 104:106905. doi: 10.1016/j.yebeh.2020.106905
46. Joswig, H, Lau, JC, Abdallat, M, Parrent, AG, MacDougall, KW, McLachlan, RS, et al. Stereoelectroencephalography versus subdural strip electrode implantations: feasibility, complications, and outcomes in 500 intracranial monitoring cases for drug-resistant epilepsy. Neurosurgery. (2020) 87:E23–30. doi: 10.1093/neuros/nyaa112
47. Kim, LH, Parker, JJ, Ho, AL, Feng, AY, Kumar, KK, Chen, KS, et al. Contemporaneous evaluation of patient experience, surgical strategy, and seizure outcomes in patients undergoing stereoelectroencephalography or subdural electrode monitoring. Epilepsia. (2021) 62:74–84. doi: 10.1111/epi.16762
48. Munari, C, Hoffmann, D, Francione, S, Kahane, P, Tassi, L, Lo Russo, G, et al. Stereo-electroencephalography methodology: advantages and limits. Acta Neurol Scand Suppl. (1994) 152:56–67.
49. Guenot, M, Isnard, J, Ryvlin, P, Fischer, C, Ostrowsky, K, Mauguiere, F, et al. Neurophysiological monitoring for epilepsy surgery: the Talairach SEEG method. StereoElectroEncephaloGraphy. Indications, results, complications and therapeutic applications in a series of 100 consecutive cases. Stereotact Funct Neurosurg. (2001) 77:29–32. doi: 10.1159/000064595
50. Aghakhani, Y, Kinay, D, Gotman, J, Soualmi, L, Andermann, F, Olivier, A, et al. The role of periventricular nodular heterotopia in epileptogenesis. Brain. (2005) 128:641–51. doi: 10.1093/brain/awh388
51. Kassiri, J, Pugh, J, Carline, S, Jurasek, L, Snyder, T, Wheatley, M, et al. Depth electrodes in pediatric epilepsy surgery. Can J Neurol Sci. (2013) 40:48–55. doi: 10.1017/S0317167100012944
52. Serletis, D, Bulacio, J, Bingaman, W, Najm, I, and Gonzalez-Martinez, J. The stereotactic approach for mapping epileptic networks: a prospective study of 200 patients. J Neurosurg. (2014) 121:1239–46. doi: 10.3171/2014.7.JNS132306
53. Taussig, D, Chipaux, M, Lebas, A, Fohlen, M, Bulteau, C, Ternier, J, et al. Stereo-electroencephalography (SEEG) in 65 children: an effective and safe diagnostic method for pre-surgical diagnosis, independent of age. Epileptic Disord. (2014) 16:280–95. doi: 10.1684/epd.2014.0679
54. Munyon, C, Sweet, J, Luders, H, Lhatoo, S, and Miller, J. The 3-dimensional grid: a novel approach to stereoelectroencephalography. Neurosurgery. (2015) 11:127–34. doi: 10.1227/NEU.0000000000000649
55. Suresh, S, Sweet, J, Fastenau, PS, Luders, H, Landazuri, P, and Miller, J. Temporal lobe epilepsy in patients with nonlesional MRI and normal memory: an SEEG study. J Neurosurg. (2015) 123:1368–74. doi: 10.3171/2015.1.JNS141811
56. Roessler, K, Sommer, B, Merkel, A, Rampp, S, Gollwitzer, S, Hamer, HM, et al. A frameless stereotactic implantation technique for depth electrodes in refractory epilepsy using intraoperative magnetic resonance imaging. World Neurosurg. (2016) 94:206–10. doi: 10.1016/j.wneu.2016.06.114
57. Li, YH, Ye, XL, Liu, QQ, Mao, JW, Liang, PJ, Xu, JW, et al. Localization of epileptogenic zone based on graph analysis of stereo-EEG. Epilepsy Res. (2016) 128:149–57. doi: 10.1016/j.eplepsyres.2016.10.021
58. Lamarche, F, Job, AS, Deman, P, Bhattacharjee, M, Hoffmann, D, Gallazzini-Crépin, C, et al. Correlation of FDG-PET hypometabolism and SEEG epileptogenicity mapping in patients with drug-resistant focal epilepsy. Epilepsia. (2016) 57:2045–55. doi: 10.1111/epi.13592
59. Bourdillon, P, Ryvlin, P, Isnard, J, Montavont, A, Catenoix, H, Mauguière, F, et al. Stereotactic electroencephalography is a safe procedure, including for insular implantations. World Neurosurg. (2017) 99:353–61. doi: 10.1016/j.wneu.2016.12.025
60. Minkin, K, Gabrovski, K, Penkov, M, Todorov, Y, Tanova, R, Milenova, Y, et al. Stereoelectroencephalography using magnetic resonance angiography for avascular trajectory planning: technical report. Neurosurgery. (2017) 81:688–95. doi: 10.1093/neuros/nyx166
61. Ollivier, I, Behr, C, Cebula, H, Timofeev, A, Benmekhbi, M, Valenti, MP, et al. Efficacy and safety in frameless robot-assisted stereo-electroencephalography (SEEG) for drug-resistant epilepsy. Neurochirurgie. (2017) 63:286–90. doi: 10.1016/j.neuchi.2017.03.002
62. Delev, D, Quesada, CM, Grote, A, Boström, JP, Elger, C, Vatter, H, et al. A multimodal concept for invasive diagnostics and surgery based on neuronavigated voxel-based morphometric MRI postprocessing data in previously nonlesional epilepsy. J Neurosurg. (2018) 128:1178–86. doi: 10.3171/2016.12.JNS161676
63. Budke, M, Avecillas-Chasin, JM, and Villarejo, F. Implantation of depth electrodes in children using VarioGuide(R) frameless navigation system: technical note. Oper Neurosurg (Hagerstown). (2018) 15:302–9. doi: 10.1093/ons/opx192
64. Salado, AL, Koessler, L, De Mijolla, G, Schmitt, E, Vignal, JP, Civit, T, et al. sEEG is a safe procedure for a comprehensive anatomic exploration of the insula: a retrospective study of 108 procedures representing 254 Transopercular insular electrodes. Oper Neurosurg (Hagerstown). (2018) 14:1–8. doi: 10.1093/ons/opx106
65. Dewan, MC, Shults, R, Hale, AT, Sukul, V, Englot, DJ, Konrad, P, et al. Stereotactic EEG via multiple single-path omnidirectional trajectories within a single platform: institutional experience with a novel technique. J Neurosurg. (2018) 129:1173–81. doi: 10.3171/2017.6.JNS17881
66. Abel, TJ, Varela Osorio, R, Amorim-Leite, R, Mathieu, F, Kahane, P, Minotti, L, et al. Frameless robot-assisted stereoelectroencephalography in children: technical aspects and comparison with Talairach frame technique. J Neurosurg Pediatr. (2018) 22:37–46. doi: 10.3171/2018.1.PEDS17435
67. Goldstein, HE, Youngerman, BE, Shao, B, Akman, CI, Mandel, AM, McBrian, DK, et al. Safety and efficacy of stereoelectroencephalography in pediatric focal epilepsy: a single-center experience. J Neurosurg Pediatr. (2018) 22:444–52. doi: 10.3171/2018.5.PEDS1856
68. Lagarde, S, Roehri, N, Lambert, I, Trebuchon, A, McGonigal, A, Carron, R, et al. Interictal stereotactic-EEG functional connectivity in refractory focal epilepsies. Brain. (2018) 141:2966–80. doi: 10.1093/brain/awy214
69. Carlson, AA, Rutishauser, U, and Mamelak, AN. Safety and utility of hybrid depth electrodes for seizure localization and single-unit neuronal recording. Stereotact Funct Neurosurg. (2018) 96:311–9. doi: 10.1159/000493548
70. Candela-Cantó, S, Aparicio, J, López, JM, Baños-Carrasco, P, Ramírez-Camacho, A, Climent, A, et al. Frameless robot-assisted stereoelectroencephalography for refractory epilepsy in pediatric patients: accuracy, usefulness, and technical issues. Acta Neurochir. (2018) 160:2489–500. doi: 10.1007/s00701-018-3720-8
71. McGovern, RA, Knight, EP, Gupta, A, Moosa, ANV, Wyllie, E, Bingaman, WE, et al. Robot-assisted stereoelectroencephalography in children. J Neurosurg Pediatr. (2019) 23:288–96. doi: 10.3171/2018.7.PEDS18305
72. Thorsteinsdottir, J, Vollmar, C, Tonn, JC, Kreth, FW, Noachtar, S, and Peraud, A. Outcome after individualized stereoelectroencephalography (sEEG) implantation and navigated resection in patients with lesional and non-lesional focal epilepsy. J Neurol. (2019) 266:910–20. doi: 10.1007/s00415-019-09213-3
73. Cardinale, F, Rizzi, M, Vignati, E, Cossu, M, Castana, L, d’Orio, P, et al. Stereoelectroencephalography: retrospective analysis of 742 procedures in a single Centre. Brain. (2019) 142:2688–704. doi: 10.1093/brain/awz196
74. D’Agostino, E, Kanter, J, Song, Y, and Aronson, JP. Stereoencephalography electrode placement accuracy and utility using a frameless insertion platform without a rigid cannula. Oper Neurosurg. (2020) 18:409–16. doi: 10.1093/ons/opz200
75. Peedicail, JS, Almohawes, A, Hader, W, Starreveld, Y, Singh, S, Josephson, CB, et al. Outcomes of stereoelectroencephalography exploration at an epilepsy surgery center. Acta Neurol Scand. (2020) 141:463–72. doi: 10.1111/ane.13229
76. Kappen, P, Eltze, C, Tisdall, M, Cross, JH, Thornton, R, and Moeller, F. Stereo-EEG exploration in the insula/operculum in paediatric patients with refractory epilepsy. Seizure. (2020) 78:63–70. doi: 10.1016/j.seizure.2020.02.011
77. Chaitanya, G, Romeo, AK, Ilyas, A, Irannejad, A, Toth, E, Elsayed, G, et al. Robot-assisted stereoelectroencephalography exploration of the limbic thalamus in human focal epilepsy: implantation technique and complications in the first 24 patients. Neurosurg Focus. (2020) 48:E2. doi: 10.3171/2020.1.FOCUS19887
78. Mereaux, JL, Gilard, V, Le Goff, F, Chastan, N, Magne, N, Gerardin, E, et al. Practice of stereoelectroencephalography (sEEG) in drug-resistant epilepsy: retrospective series with surgery and thermocoagulation outcomes. Neurochirurgie. (2020) 66:139–43. doi: 10.1016/j.neuchi.2019.12.014
79. Nelson, JH, Brackett, SL, Oluigbo, CO, and Reddy, SK. Robotic stereotactic assistance (ROSA) for pediatric epilepsy: a single-center experience of 23 consecutive cases. Children (Basel). (2020) 7:94. doi: 10.3390/children7080094
80. Liu, Y, Chen, G, Chen, J, Zhou, J, Su, L, Zhao, T, et al. Individualized stereoelectroencephalography evaluation and navigated resection in medically refractory pediatric epilepsy. Epilepsy Behav. (2020) 112:107398. doi: 10.1016/j.yebeh.2020.107398
81. Urgun, K, Paff, M, Chan, A, Hsu, F, and Vadera, S. Surgical robot-enhanced implantation of intracranial depth electrodes for single neuron recording studies in patients with medically refractory epilepsy: a technical note. World Neurosurg. (2021) 145:210–9. doi: 10.1016/j.wneu.2020.09.100
82. Zheng, J, Liu, YL, Zhang, D, Cui, XH, Sang, LX, Xie, T, et al. Robot-assisted versus stereotactic frame-based stereoelectroencephalography in medically refractory epilepsy. Neurophysiol Clin. (2021) 51:111–9. doi: 10.1016/j.neucli.2020.11.001
83. Durica, SR, Caruso, JP, Podkorytova, I, Ding, K, Hays, R, Lega, B, et al. Stereo-EEG evaluation and surgical treatment in patients with drug-resistant focal epilepsy associated with nodular heterotopia. J Clin Neurophysiol. (2023) 40:17–26. doi: 10.1097/WNP.0000000000000850
84. Tsuboyama, M, Harini, C, Liu, S, Zhang, B, and Bolton, J. Subclinical seizures detected on intracranial EEG: patient characteristics and impact on surgical outcome in a single pediatric epilepsy surgery center. Epilepsy Behav. (2021) 121:108040. doi: 10.1016/j.yebeh.2021.108040
85. Mavridis, IN, Lo, WB, Wimalachandra, WSB, Philip, S, Agrawal, S, Scott, C, et al. Pediatric stereo-electroencephalography: effects of robot assistance and other variables on seizure outcome and complications. J Neurosurg Pediatr. (2021) 28:404–15. doi: 10.3171/2021.2.PEDS20810
86. Vakharia, VN, Rodionov, R, Miserocchi, A, McEvoy, AW, O’Keeffe, A, Granados, A, et al. Comparison of robotic and manual implantation of intracerebral electrodes: a single-Centre, single-blinded, randomised controlled trial. Sci Rep. (2021) 11:17127. doi: 10.1038/s41598-021-96662-4
87. Hyslop, A, Wang, S, Bryant, JP, Bhatia, S, Sandoval-Garcia, C, Karkare, K, et al. Stereo-electroencephalography (SEEG) in pediatric epilepsy: utility in children with and without prior epilepsy surgery failure. Epilepsy Res. (2021) 177:106765. doi: 10.1016/j.eplepsyres.2021.106765
88. Belohlavkova, A, Jahodova, A, Kudr, M, Benova, B, Ebel, M, Liby, P, et al. May intraoperative detection of stereotactically inserted intracerebral electrodes increase precision of resective epilepsy surgery? Eur J Paediatr Neurol. (2021) 35:49–55. doi: 10.1016/j.ejpn.2021.09.012
89. Toledano, R, Martínez-Alvarez, R, Jiménez-Huete, A, García-Morales, I, Aledo-Serrano, Á, Cabrera, W, et al. Stereoelectroencephalography in the preoperative assessment of patients with refractory focal epilepsy: experience at an epilepsy Centre. Neurologia (Engl Ed). (2022) 37:334–45. doi: 10.1016/j.nrl.2019.05.002
90. Remick, M, Akwayena, E, Harford, E, Chilukuri, A, White, GE, and Abel, TJ. Subdural electrodes versus stereoelectroencephalography for pediatric epileptogenic zone localization: a retrospective cohort study. Neurosurg Focus. (2022) 53:E4. doi: 10.3171/2022.7.FOCUS2269
91. Stone, SSD, Park, EH, Bolton, J, Harini, C, Libenson, MH, Rotenberg, A, et al. Interictal connectivity revealed by granger analysis of Stereoelectroencephalography: association with ictal onset zone, resection, and outcome. Neurosurgery. (2022) 91:583–9. doi: 10.1227/neu.0000000000002079
92. Larrew, T, Skoch, J, Ihnen, SKZ, Arya, R, Holland, KD, Tenney, JR, et al. Comparison of outcomes after stereoelectroencephalography and subdural grid monitoring in pediatric tuberous sclerosis complex. Neurosurg Focus. (2022) 53:E5. doi: 10.3171/2022.7.FOCUS22335
93. Zhao, B, McGonigal, A, Hu, W, Zhang, C, Wang, X, Mo, J, et al. Interictal HFO and FDG-PET correlation predicts surgical outcome following SEEG. Epilepsia. (2023) 64:667–77. doi: 10.1111/epi.17485
94. Maesawa, S, Ishizaki, T, Mutoh, M, Ito, Y, Torii, J, Tanei, T, et al. Clinical impacts of stereotactic electroencephalography on epilepsy surgery and associated issues in the current situation in Japan. Neurol Med Chir (Tokyo). (2023) 63:179–90. doi: 10.2176/jns-nmc.2022-0271
95. Rijal, S, Corona, L, Perry, MS, Tamilia, E, Madsen, JR, Stone, SSD, et al. Functional connectivity discriminates epileptogenic states and predicts surgical outcome in children with drug resistant epilepsy. Sci Rep. (2023) 13:9622. doi: 10.1038/s41598-023-36551-0
96. Santalucia, R, Carapancea, E, Vespa, S, Germany Morrison, E, Ghasemi Baroumand, A, Vrielynck, P, et al. Clinical added value of interictal automated electrical source imaging in the presurgical evaluation of MRI-negative epilepsy: a real-life experience in 29 consecutive patients. Epilepsy Behav. (2023) 143:109229. doi: 10.1016/j.yebeh.2023.109229
97. Smith, KM, Starnes, DK, Brinkmann, BH, So, E, Cox, BC, Marsh, WR, et al. Stereo-EEG localization of midline onset seizures on scalp EEG. Epilepsy Res. (2023) 193:107162
Keywords: SEEG, SDE, seizure freedom, complications, invasive monitoring
Citation: Muthiah N, Reecher HM, Maroufi SF, Mansouri A, Harford E and Abel TJ (2025) Seizure outcomes and complications associated with stereoelectroencephalography versus subdural electrodes for invasive monitoring in epilepsy surgery: a meta-analysis. Front. Neurol. 16:1619288. doi: 10.3389/fneur.2025.1619288
Edited by:
Noor Kamal Al-Qazzaz, University of Baghdad, IraqReviewed by:
Attila Racz, niversity Hospital Bonn, GermanyKapil Gururangan, Northwestern University, United States
Copyright © 2025 Muthiah, Reecher, Maroufi, Mansouri, Harford and Abel. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Taylor J. Abel, YWJlbHRqQHVwbWMuZWR1
 Nallammai Muthiah1
Nallammai Muthiah1 Hope M. Reecher
Hope M. Reecher Seyed Farzad Maroufi
Seyed Farzad Maroufi Alireza Mansouri
Alireza Mansouri Taylor J. Abel
Taylor J. Abel