- 1Institute of Basic Theory of Chinese Medicine, China Academy of Chinese Medical Sciences, Beijing, China
- 2College of Traditional Chinese Medicine, Jiangxi University of Traditional Chinese Medicine, Nanchang, China
Insomnia represents a significant global public health issue, affecting approximately 10% of adults with chronic symptoms and up to 20% with intermittent episodes. Closely associated with chronic diseases such as depression, anxiety, and cardiovascular conditions, insomnia markedly impairs patients’quality of life and imposes substantial economic burdens. Current treatments include pharmacological and non-pharmacological approaches. Pharmacotherapy primarily involves benzodiazepines, effective in short-term symptom relief but associated with long-term risks such as dependency, tolerance, and cognitive impairment. Recently introduced dual orexin receptor antagonists offer improved safety profiles but lack sufficient clinical evidence and remain costly. Melatonin and its receptor agonists have contentious efficacy, while antihistamines are discouraged for chronic use due to adverse effects. Herbal therapies have limited high-quality evidence to support routine clinical use. Among non-pharmacological treatments, cognitive behavioral therapy for insomnia (CBT-I) is recognized for clear efficacy, yet patient adherence and availability of trained specialists remain problematic. Exercise interventions, bright light therapy, and music therapy show preliminary positive effects; however, inconsistencies in intervention parameters and methodological quality necessitate further research. Recent advances highlight the potential of acupuncture and neuromodulation technologies, including transcranial magnetic stimulation (TMS), transcranial direct current stimulation (tDCS), and vagus nerve stimulation (VNS). Acupuncture effectively improves sleep through modulation of autonomic nervous function, endocrine regulation, and remodeling of sleep-related neural circuits, demonstrating sustained benefits and high safety. Neuromodulation techniques offer rapid onset and precise targeting. Preliminary evidence indicates that combining acupuncture and neuromodulation techniques could synergistically enhance treatment efficacy, efficiency, and personalization. However, existing studies remain limited. Large-scale, multicenter randomized controlled trials are needed to clarify efficacy, safety, and to optimize clinical protocols for these integrative approaches. We conducted a structured narrative review with a systematic search (2010–2025), including 93 studies for qualitative synthesis; evidence certainty was summarized using four qualitative categories (High/Moderate/Low/Very low).
1 Introduction
Insomnia is a significant global health concern affecting approximately 10% of adults chronically, with up to 20% experiencing occasional insomnia symptoms (1). Recent studies have demonstrated that insomnia not only adversely impacts patients’ daily lives but is also closely associated with various chronic conditions, including depression, anxiety, and cardiovascular diseases (2–4). Moreover, insomnia imposes substantial economic burdens on healthcare systems; in the United States alone, the direct and indirect costs associated with insomnia exceed 100 billion USD annually (5). Current clinical guidelines recommend pharmacological interventions and cognitive behavioral therapy for insomnia (CBT-I) as the primary treatments. However, both therapeutic approaches have notable limitations, such as the potential for dependency and high relapse rates associated with pharmacological treatments, and low patient adherence and difficulty maintaining efficacy for CBT-I (6).
Conventional pharmacological therapies, although clearly effective in rapidly alleviating insomnia symptoms, commonly lead to dependence, drug tolerance, and noticeable side effects, including headaches and memory impairment, when used long-term (7, 8). CBT-I, an important non-pharmacological therapy with demonstrated clinical efficacy (9), is heavily dependent on patient engagement and requires ongoing follow-up and lengthy treatment cycles (6). Although recognized as a first-line intervention in various clinical guidelines, CBT-I has not been widely implemented as a mainstream insomnia therapy in many regions due to economic constraints and poor patient adherence (10, 11). With the increased use of electronic devices such as smartphones (12) and rising life stress (2), the incidence of insomnia is currently trending upward. Therefore, exploring therapeutic methods that effectively avoid or mitigate these limitations is crucial for improving clinical treatment outcomes and alleviating patient suffering.
In recent years, with the deepening research on insomnia mechanisms and therapeutic interventions, acupuncture and modern neuromodulation techniques have demonstrated certain beneficial effects in improving insomnia (13–15). Some exploratory studies have attempted to combine acupuncture with neuromodulation techniques, aiming to provide multidimensional therapeutic outcomes and compensate for the limitations of individual therapies (16, 17). However, it is important to acknowledge that research into these integrative approaches remains limited and is still in the preliminary exploration phase.
Based on the above background, this article systematically reviews and evaluates comprehensive treatment methods for insomnia, with a particular emphasis on the potential clinical value of combining acupuncture with modern neuromodulation techniques such as transcranial magnetic stimulation (TMS), transcranial direct current stimulation (tDCS), and vagus nerve stimulation (VNS), aiming to provide references and insights for developing more effective, safer, and personalized therapeutic strategies in the future.
2 Materials and methods
2.1 Literature search and selection
This work is a structured narrative review with a systematic literature search (2010–2025); quantitative meta-analysis was not performed. A systematic literature search was performed on June 30, 2025, in PubMed (n = 753), Web of Science (n = 866), and the Cochrane Library (n = 296) to identify studies published between January 1, 2010 and June 30, 2025. We combined three concept blocks in title/abstract fields:
Insomnia terms: “insomnia” OR “chronic insomnia” OR “primary insomnia” OR “sleep disturbance” OR “sleep disorder” OR “sleep quality” OR PSQI.
Intervention terms: “acupuncture” OR “electroacupuncture” OR “auricular acupuncture” OR “scalp acupuncture” OR TMS OR “transcranial magnetic stimulation” OR rTMS OR tDCS OR tACS OR VNS OR tVNS OR taVNS OR neuromodulation.
Mechanistic terms: “Heart rate variability (HRV) “OR cortisol OR melatonin OR “Gamma-aminobutyric acid (GABA)” OR “functional connectivity (fMRI/EEG)” OR plasticity OR “hypothalamic–pituitary–adrenal(HPA) axis”.
The initial search returned a total of 1,915 records; following deduplication, 1,650 unique entries remained. Two independent reviewers then screened titles and abstracts, excluding 1,557 records that did not meet pre-specified inclusion criteria, and assessed the full text of 93 articles—all of which were included in the final analysis. Any discrepancies were resolved by consensus.
References 18-40—covering benzodiazepines, dual orexin receptor antagonists, melatonin, and antihistamines—were identified separately via manual citation tracking of authoritative clinical practice guidelines and targeted PubMed searches; these were not part of the primary systematic search. Supplementary citations (e.g., 45, 46, 102) that discuss key neurophysiological mechanisms or the clinical relevance of acupuncture/neuromodulation were also manually retrieved to provide foundational mechanistic context.
2.2 Inclusion and exclusion criteria
Studies were selected according to the following criteria to ensure methodological rigor and thematic focus on behavioral therapies, acupuncture, and neuromodulation for chronic insomnia.
2.2.1 Inclusion criteria
Population: Adult patients (≥18 years) diagnosed with chronic insomnia by DSM-5 or ICSD-3 criteria.
2.2.1.1 Interventions (at least one)
Behavioral/Cognitive Therapies: CBT-I and its components (sleep hygiene, sleep restriction, stimulus control, relaxation training, cognitive restructuring), exercise interventions (e.g., Tai Chi), bright light therapy, music therapy.
Acupuncture Modalities: Manual acupuncture, electroacupuncture, auricular acupuncture, scalp acupuncture, acupoint embedding.
Neuromodulation Techniques: Repetitive/transcranial magnetic stimulation (rTMS/TMS), transcranial direct current stimulation (tDCS), transcranial alternating current stimulation (tACS), transcutaneous or auricular vagus nerve stimulation (tVNS/taVNS), or combined protocols.
Comparators: Sham or placebo interventions, usual care, active controls (e.g., CBT-I alone), or no-treatment controls.
2.2.1.2 Outcomes
Clinical efficacy: PSQI, sleep latency, total sleep time, sleep efficiency, and adverse events.
Mechanistic endpoints: HRV, serum cortisol or melatonin levels, GABA concentration, fMRI/EEG, HPA-axis markers, and neural plasticity indicators.
2.2.1.3 Study design
Randomized controlled trials and nonrandomized clinical trials.
Systematic reviews and meta-analyses that (a) report a reproducible search strategy and (b) focus on RCTs of behavioral, acupuncture, or neuromodulation interventions for insomnia. (c) Mechanistic studies conducted in chronic insomnia patients or validated insomnia animal models.
Publication Date: January 1, 2010 to June 30, 2025, with priority given to studies published from January 1, 2020 onward.
Language: English full-text publications.
2.2.2 Exclusion criteria
We excluded case reports, narrative reviews lacking a defined search strategy, study protocols, letters, and conference abstracts without full data. Basic science studies were excluded unless they were conducted in validated insomnia animal models with clear translational endpoints directly mapping to clinical phenotypes (e.g., sleep latency/efficiency, HPA-axis markers); such studies, when retained, were used solely to contextualize mechanisms and were not used to grade clinical efficacy (and were rated as low certainty in our qualitative framework).
Pediatric populations (<18 years), non-insomnia sleep disorders, mixed cohorts without separable insomnia data, and non-English publications were excluded.
2.3 Evidence appraisal and certainty rating
Two reviewers qualitatively appraised study limitations across standard domains (randomization/allocation, blinding, missing data, outcome measurement, and selective reporting) and summarized the certainty of evidence for key outcomes using four categories—high, moderate, low, and very low. This was a qualitative synthesis; no formal domain-level tool scoring or quantitative meta-analysis was performed. Data extraction focused on study design, sample size, intervention parameters, and the direction and magnitude of primary sleep outcomes as reported (e.g., Pittsburgh Sleep Quality Index (PSQI) changes and p values). In practice, the included literature mapped to the Moderate or Low categories; no topic achieved High certainty (owing to small sample sizes, heterogeneity, and imprecision), and none met our threshold for Very low certainty (because evidence was primarily clinical rather than indirect or severely limited).
3 Conventional treatments for chronic insomnia
3.1 Pharmacological treatments
The primary categories of medications currently used for insomnia treatment each have distinct advantages and disadvantages:
3.1.1 Benzodiazepines and benzodiazepine receptor agonists
Common benzodiazepines include diazepam and lorazepam, while benzodiazepine receptor agonists include zolpidem, zaleplon, and eszopiclone. These medications are characterized by rapid onset, effectively addressing difficulty initiating sleep and nighttime awakenings in the short term (18, 19). However, studies have shown that long-term use can lead to drug tolerance, dependence, and withdrawal symptoms (20). Additionally, these medications may impair cognitive functions (21, 22), cause nighttime confusion, and increase the risk of falls (23, 24). Consequently, most guidelines recommend their use only for short durations and advocate strict control over treatment duration (6, 25). Some scholars even suggest avoiding these medications altogether due to their significant adverse effects (26).
3.1.2 Low-dose sedating antidepressants
Commonly used medications such as trazodone and low-dose doxepin can improve sleep quality and alleviate mild emotional disturbances, making them suitable for insomnia patients with comorbid emotional disorders (27, 28). However, their effectiveness exhibits considerable individual variation, and they can cause adverse effects such as falls (29), urinary retention, and dry mouth (28).
3.1.3 Dual orexin receptor antagonists
Representative medications include suvorexant, lemborexant, and daridorexant. DORA improves sleep by inhibiting orexin A/B neuropeptides, thus reducing excessive central wakefulness (30). Overall, this drug category is associated with lower dependence and better safety profiles (31). The most commonly reported side effects include somnolence, nasopharyngitis, and headache (32). Drawbacks of these medications include high treatment costs and limited current clinical data; consequently, only some drugs within this category are recommended by clinical guidelines for treating insomnia (33, 34).
3.1.4 Melatonin/melatonin receptor agonists
Melatonin and its receptor agonists (such as ramelteon) regulate circadian rhythms and have been widely used to treat insomnia, particularly in patients aged 55 years and older. Nevertheless, recent literature reviews have produced conflicting conclusions regarding the effectiveness of melatonin and its receptor agonists (35–38). Thus, clinical guidelines frequently exhibit ambiguity regarding their recommendation for routine use in treating insomnia (25).
3.1.5 Antihistamines
Antihistamines commonly used for insomnia treatment are typically over-the-counter medications such as diphenhydramine and doxylamine. Despite clinical use, these drugs are generally not recommended due to potential side effects and possible drug tolerance (39). Recent studies have also suggested an association between these medications and increased mortality, warranting caution in their use (40).
3.1.6 Herbal/botanical therapies
Although some reviews have summarized the use of herbal and botanical therapies for insomnia relief (41, 42), there is a relative lack of randomized controlled trial data. While these therapies are practiced clinically in certain countries and regions, guidelines do not recommend herbal and botanical remedies as standard treatments for insomnia (6).
Overall, although some commonly used medications in clinical practice can rapidly alleviate insomnia symptoms, issues such as cognitive impairment, impaired consciousness, and insufficient clinical evidence leading to medication misuse remain significant concerns. These issues highlight the fact that while pharmacological treatments are among the most widely utilized methods, numerous factors may adversely impact patient treatment outcomes and overall health during their clinical application.
3.2 Non-pharmacological treatments
3.2.1 Cognitive behavioral therapy for insomnia
CBT-I is a structured, evidence-based, non-pharmacological therapy currently recommended as a first-line intervention for chronic insomnia (43). It primarily works by altering unhealthy sleep behaviors and modifying negative cognitive patterns, thereby helping patients establish effective sleep patterns and improving sleep quality (44). CBT-I comprises several key components, including psychoeducation and sleep hygiene (SH), relaxation therapy (RT), sleep restriction therapy (SRT), stimulus control therapy (SCT), and cognitive therapy (CT).
3.2.1.1 Psychoeducation and SH
Psychoeducation is frequently utilized as an adjunct therapy for various psychological disorders (45, 46), mainly serving educational and advisory roles (45). In insomnia treatment, psychoeducation typically includes basic information regarding the role and functions of sleep, age-related changes, and the circadian rhythm regulation, exemplified by the classical two-process model of sleep regulation (47, 48). In the practical application of CBT-I, psychoeducation is generally integrated across therapeutic components rather than presented independently. Sleep hygiene is a central component of CBT-I. Although the academic community has not reached a consensus on the precise definition of SH, it is broadly considered a set of behavioral and environmental recommendations aimed at promoting healthy sleep (49). These recommendations typically include regular exercise, noise reduction during sleep, and maintaining consistent sleep schedules (50). The efficacy of SH was once questioned (51), but with accumulating evidence, recent research acknowledges SH as having a positive impact on sleep outcomes (52, 53). Overall, the lack of consensus on the precise components of SH complicates the establishment of universally quantifiable and objective standards in clinical practice (49). Although some studies advocate personalized SH interventions tailored to different populations (54, 55), large-scale research remains limited.
3.2.1.2 RT
RT employs various relaxation techniques, such as progressive muscle relaxation, deep breathing exercises, meditation, or guided imagery, to help patients reduce physical and mental tension and alleviate anxiety, thereby facilitating sleep onset (56). Progressive muscle relaxation is widely applied; Mehdi Harorani et al. (57), for example, employed progressive muscle relaxation therapy and found significant improvements in anxiety and sleep quality among burn patients in the intervention group. This therapy also positively impacts insomnia in patients with hip fractures (58). Karuna Datta et al. demonstrated through clinical experiments that yoga nidra practice improves N3 sleep, total wake time, and subjective sleep quality among patients with insomnia (59). Despite its frequent clinical application, some meta-analyses suggest that RT may have limited overall effectiveness or even counterproductive outcomes (60).
3.2.1.3 SRT
SRT aims to increase sleep drive by initially restricting the patient’s “time in bed” to their average total sleep duration, thereby promoting more concentrated and continuous sleep. Initially, patients schedule their bedtime based on actual sleep duration, gradually increasing time spent in bed as sleep efficiency improves, ultimately enhancing sleep quality (61). SRT has a long history of clinical use and is relatively mature, demonstrating positive short-term effects on various insomnia severity indicators such as sleep latency and sleep efficiency (62). Recent experimental studies have also confirmed good long-term efficacy and flexible implementation through telephone-guided interventions (63). However, deliberate sleep deprivation during initial treatment stages often leads to adverse effects including extreme fatigue, daytime sleepiness, headaches, mood fluctuations, decreased energy, and reduced motivation (64). These side effects may negatively influence patient adherence and thus compromise intervention outcomes (65).
3.2.1.4 SCT
SCT involves altering patients’associations with the bed and bedroom, reestablishing a positive connection between bed and sleep. Specific measures include going to bed only when sleepy and, if unable to fall asleep within 20 min, leaving the bed to engage in relaxing activities until feeling sleepy again (66). Clinical trials indicate that SCT effectively alleviates insomnia symptoms and reduces pre-sleep cognitive activation (67). However, the overall quality of existing experimental studies on SCT is relatively low, and further rigorous research is necessary to clearly evaluate its efficacy and underlying mechanisms (68).
3.2.1.5 CT
CT focuses on identifying and challenging irrational beliefs and negative thoughts related to sleep, such as “I will definitely sleep poorly” or “Not getting enough sleep will affect tomorrow,” employing cognitive restructuring to reduce sleep-related anxiety and excessive worry (60). Clinical trials exclusively employing CT have been relatively infrequent in recent years. Rikard Sunnhed et al. (69), however, found that internet-delivered CT demonstrated favorable efficacy as a standalone therapy for insomnia.
In summary, CBT-I, as a preferred non-pharmacological treatment for insomnia, effectively improves sleep quality without pharmacological side effects and offers good long-term benefits with some of its components (63). It also positively influences patients with comorbid psychiatric conditions (9). Nonetheless, CBT-I presents certain limitations: SH, as a component, lacks consensus (49) and has even been used as a placebo in randomized controlled trials (70); RT may potentially exacerbate insomnia symptoms (60); and SRT might impair daytime energy (64). Furthermore, CBT-I requires prolonged treatment periods, high patient motivation, and sustained adherence, all of which substantially impact therapeutic efficacy (65). Limited availability of standardized CBT-I services due to a shortage of qualified therapists further constrains treatment accessibility in certain regions (71). Therefore, although CBT-I is a first-line therapy for insomnia, further clinical validation and refinement are necessary, alongside the development of novel therapeutic methods.
3.2.2 Exercise, bright light therapy, and music therapy
3.2.2.1 Exercise interventions
EI enhances deep sleep regulation by increasing biosynthesis of melatonin precursors in the brain (72). Preliminary studies suggest exercise may also improve sleep–wake rhythms by modulating hypothalamic–pituitary–adrenal axis activity (73). Clinical interventions typically involve running or stepping exercises (72); recent studies indicate Tai Chi can also alleviate insomnia (74). Additional research (75) found that incorporating exercise into CBT-I can help sustain cognitive therapy effectiveness.
However, EI presents limitations including substantial variability in exercise prescriptions (intensity, frequency, timing), inadequate adherence assessment, and insufficient individualized recommendations for varying age groups and comorbidities (76, 77). Despite supportive evidence, high-quality research remains insufficient.
3.2.2.2 Bright light therapy
BLT involves exposure to intense light during morning or evening to suppress delayed melatonin secretion and reset the circadian rhythm regulated by the suprachiasmatic nucleus, reducing sleep latency and enhancing sleep efficiency (78, 79). Qin Wang et al. (78), through a randomized controlled trial, showed that daily 30-min morning exposures to 7,500-lux white light for 2 weeks improved PSQI from 12.4 ± 3.1 to 7.2 ± 2.8 (Δ = −5.2; p < 0.001). Another trial (80) using 10,000-lux morning exposure for 30 min/day over 2 weeks improved sleep efficiency and reduced daytime sleepiness, fatigue, and mood disturbance (between-group p < 0.05).
Despite accumulating evidence supporting BLT’s efficacy, significant heterogeneity in intervention parameters—light intensity (2,000–10,000 lux), wavelengths (white vs. blue), exposure duration (15–60 min), and timing (morning vs. evening)—hinders high-quality meta-analyses, greatly affecting comparability and clinical implementation (81). Other research suggests moderate intensity (900–6,000 lux) and longer duration (≥1 h) nighttime exposure is more effective for extending total sleep time, though efficacy varies across parameter combinations (82). Consequently, standardized BLT protocols require further development.
3.2.2.3 Music therapy
MT involves pre-sleep listening to slow-paced, soothing music to activate parasympathetic responses, reduce cortisol and anxiety levels, alleviate tension, and promote sleep (83). Helle Nystrup Lund et al. (84) found significant improvements in PSQI scores after 4 weeks of MT in patients with insomnia and depression (from 14.1 ± 3.2 to 8.3 ± 2.5, p < 0.001), shorter sleep latency (p < 0.01), and enhanced subjective well-being compared to controls. Li Chang et al. (85) demonstrated that MT combined with aerobic exercise significantly improved PSQI scores compared to controls (by 5.3 points, p < 0.001), benefiting multiple sleep dimensions. However, significant variability in music type (classical vs. nature sounds), duration (15–60 min), delivery methods (headphones vs. speakers), and individual music preferences (86, 87), challenges in blinding, reliance on subjective evaluations, and lack of objective physiological metrics (e.g., polysomnography) limit robust conclusions (88). Additionally, some studies suggest limited evidence for MT’s sleep-improving effects (89).
4 From single therapies to synergistic strategies: acupuncture + neuromodulation
4.1 Acupuncture
Acupuncture, an essential component of Traditional Chinese Medicine (TCM), involves the insertion of needles at specific acupoints (e.g., Shenmen [HT7], Baihui [GV20]), accompanied by manual or electrical stimulation, to unblock meridians and balance Yin and Yang (90). However, recent studies indicate that its clinical application is not guided solely by TCM theory; anatomical and other biomedical theories are also widely utilized. For example, acupuncture can restore autonomic and endocrine homeostasis (91, 92). Currently, acupuncture is primarily regarded as a therapeutic modality rather than a distinct medical discipline. Numerous empirical studies support acupuncture’s efficacy in treating insomnia (93). Wang et al. (94) demonstrated acupuncture’s effectiveness in alleviating insomnia symptoms, observing enhanced efficacy with appropriate acupoint combinations. Weng et al. (95), through systematic review and meta-analysis, concluded that acupuncture significantly improves PSQI scores in breast cancer patients experiencing insomnia. Besides traditional acupuncture, Yin et al. (96) applied electroacupuncture (EA) at TCM-specific points for 8 weeks, significantly reducing PSQI scores from 16.1 ± 3.5 at baseline to 9.9 ± 2.7 post-treatment, outperforming both sham EA (11.0 ± 3.0) and controls (13.5 ± 3.2) (both p < 0.001), with lasting effects. Other acupuncture-derived techniques or combined approaches also yield promising results for insomnia; for instance, Lu et al. (97) found moderate evidence for the effectiveness of acupoint embedding therapy and auricular acupuncture combined with traditional acupuncture, although additional robust evidence is needed.
Current mechanistic studies of acupuncture for insomnia primarily focus on three areas. First, acupuncture modulates autonomic function by enhancing vagal tone and increasing HRV. Meira do Valle et al. (98) suggested acupuncture effectively reduces sympathetic stress, possibly by activating the vagus nerve, thereby increasing HRV and coherence. Li et al. (99) reviewed two decades of literature, consistently identifying the autonomic nervous system as a primary acupuncture target. Second, acupuncture exerts endocrine regulatory effects, reducing serum cortisol and promoting melatonin secretion rhythms. Li et al. (100) observed significant improvements in sleep quality and reduced daytime fatigue in insomnia patients, with elevated plasma melatonin and reduced cortisol levels, attenuating HPA axis hyperactivation. Huang et al. (101), utilizing the traditional TCM “Ziwu Liuzhu” acupuncture method, noted significantly higher melatonin levels in acupuncture-treated rats compared to medication-treated counterparts. Third, acupuncture reshapes central neural networks, directly impacting sleep-related cortical–limbic circuits. Wang et al. (94), using resting-state functional magnetic resonance imaging (RS-fMRI), reported enhanced prefrontal-hippocampal connectivity and increased electroencephalographic slow-wave amplitude following multi-acupoint manual acupuncture compared to sham. Jin et al. (102) further confirmed enhanced functional connectivity between default mode network and cognitive control network structures following manual stimulation at Zusanli (ST36) in healthy subjects (p < 0.01). Collectively, these studies indicate acupuncture’s cortical–limbic network remodeling capacity significantly enhances slow-wave sleep generation and maintenance.
Acupuncture exhibits several notable advantages for insomnia treatment. Firstly, as a non-pharmacological therapy, it eliminates medication dependence (103) or central adverse effects (104), thus offering high safety. Secondly, acupuncture’s therapeutic benefits have proven durability, with multiple clinical trials demonstrating effects persisting several months after treatment completion (96, 105). Lastly, acupuncture synergistically integrates with therapies such as CBT-I (106), exercise interventions (107), and neuromodulation (16), enhancing overall treatment efficacy. However, acupuncture has limitations, including lengthy treatment courses with associated discomfort (94), potentially affecting patient adherence and tolerance. Additionally, lack of standardized consensus regarding acupoint selection, needle insertion depth, retention duration, and stimulation intensity introduces considerable variability, complicating efficacy comparisons and reproducibility (108).
4.2 Neuromodulation techniques
Neurostimulation techniques—transcranial magnetic stimulation (TMS), transcranial direct current stimulation (tDCS), transcranial alternating current stimulation (tACS), and vagus nerve stimulation (VNS)—constitute an emerging class of non-pharmacological interventions that modulate central or peripheral nervous system activity to rebalance network excitation and inhibition (109). Collins et al. (110) reported that rTMS treatment significantly improved mood and sleep quality in patients with severe depression and comorbid insomnia, independently of age and medication factors, accompanied by notable reductions in PSQI scores. Sun et al. (111), through systematic analysis of previous studies, concluded that rTMS is a safe and effective treatment for insomnia, significantly improving PSQI scores whether applied as primary or adjunctive therapy.
Transcranial electrical stimulation primarily encompasses tDCS and tACS, with increasing clinical trials conducted in recent years. Bakhshayesh Eghbali et al. (112) demonstrated through clinical trials that active tDCS significantly improved sleep quality in patients experiencing insomnia after traumatic brain injury, exhibiting greater reductions in PSQI scores compared to sham tDCS. Additionally, tDCS has shown efficacy in improving sleep quality among patients with depression (113). Regarding tACS, Wang et al. (114, 115) validated its ability to significantly reduce PSQI scores, shorten sleep latency, and increase total sleep duration. Other researchers (116), applying alpha-frequency tACS stimulation over the medial parietal cortex for chronic insomnia, reported notably enhanced and sustained improvements in PSQI scores and sleep quality.
Clinical studies of VNS currently include transcutaneous vagus nerve stimulation (tVNS) and transcutaneous auricular vagus nerve stimulation (taVNS). Zhang et al. (117) reported positive effects of tVNS on sleep quality improvement in patients with insomnia induced by high-altitude conditions. Zhang et al. (118) demonstrated that taVNS significantly reduced the severity of insomnia symptoms, with sustained effects lasting beyond 20 weeks. Current research on neuromodulation techniques continues to expand, including the exploration of combined neuromodulation methods. For instance, Zhou et al. (119) found that combining tDCS and rTMS achieved significant therapeutic benefits within 2 weeks of treatment, with sustained efficacy observed in subsequent follow-up periods. Some researchers (120) have proposed the potential clinical value of combining taVNS with slow-paced breathing exercises for treating insomnia.
The mechanisms underlying neuromodulation primarily involve three aspects. First is autonomic regulation, predominantly via modulation of vagus nerve function. Butt et al. (121), through detailed anatomical and neural tracing studies, demonstrated that taVNS directly activates vagal afferent branches located in the auricular concha, transmitting signals to the nucleus tractus solitarius and vagal nuclei within the brainstem, thus enhancing parasympathetic tone and improving heart rate variability (HRV). Similarly, tDCS enhances parasympathetic function and HRV via cortical-brainstem pathways (122), whereas rTMS may improve control over cardiovascular autonomic regulation by modulating functional connectivity between the left dorsolateral prefrontal cortex (DLPFC) and central autonomic networks (123). Second is endocrine modulation; rTMS treatment for insomnia was shown to significantly reduce serum cortisol, adrenocorticotropic hormone (ACTH), high-sensitivity thyroid-stimulating hormone, and free T3/T4 levels alongside improvements in insomnia symptoms. This modulation likely involves the DLPFC’s regulatory influence over the hypothalamic–pituitary–adrenal (HPA) and hypothalamic–pituitary-thyroid axes (124). Furthermore, other studies suggest rTMS alleviates insomnia symptoms through elevated GABA levels (125). The third aspect involves modulation of network synchronization and neural plasticity. For example, taVNS may enhance functional connectivity between the insula and medial prefrontal cortex, improving the dynamic balance between interoceptive awareness and cognitive experiences. This regulatory effect potentially affects mind–body interactions, elucidating taVNS’s therapeutic mechanism (126). Additional studies (121) found unilateral tVNS increased the negative compatibility effect, a GABA-related behavioral marker, promoting regional neural plasticity changes. Similarly, tDCS facilitates sleep by activating glutamatergic projections from the infralimbic cortex (IL) to the ventrolateral preoptic nucleus (VLPO) (127).
Neuromodulation technologies demonstrate clear advantages in insomnia treatment. Firstly, these methods exhibit high safety and tolerability; numerous randomized controlled trials involving insomnia or related populations have reported only minor, transient adverse effects, such as mild headaches or local discomfort, without serious adverse events (115, 117, 119). Secondly, treatments such as rTMS often achieve rapid improvements in sleep quality and architecture within a short period (111). Finally, neuromodulation devices offer adjustable parameters and precise targeting capabilities. For example, tDCS devices feature adjustable current intensity, polarity configurations, and multi-array electrodes capable of precisely stimulating critical sleep–wake centers such as frontoparietal or dorsolateral prefrontal cortices. However, mechanistic understanding of neuromodulation remains limited, with some experiments still in preliminary stages (128). Furthermore, certain researchers suggest that the efficacy of neuromodulation techniques requires further exploration; some studies (129) indicate tACS does not show significantly greater therapeutic effects compared to sham treatments for insomnia symptoms. Overall, Krone et al. (115), in a systematic review, concluded that neuromodulation therapies warrant additional rigorous verification.
4.2.1 Comparative overview
To facilitate direct comparison across major neuromodulation approaches in chronic insomnia, Table 1 summarizes key attributes of rTMS, tDCS/tACS, and tVNS/taVNS, including their proposed mechanisms, stimulation targets and parameters, typical clinical efficacy (PSQI change), common adverse events, and overall evidence level.
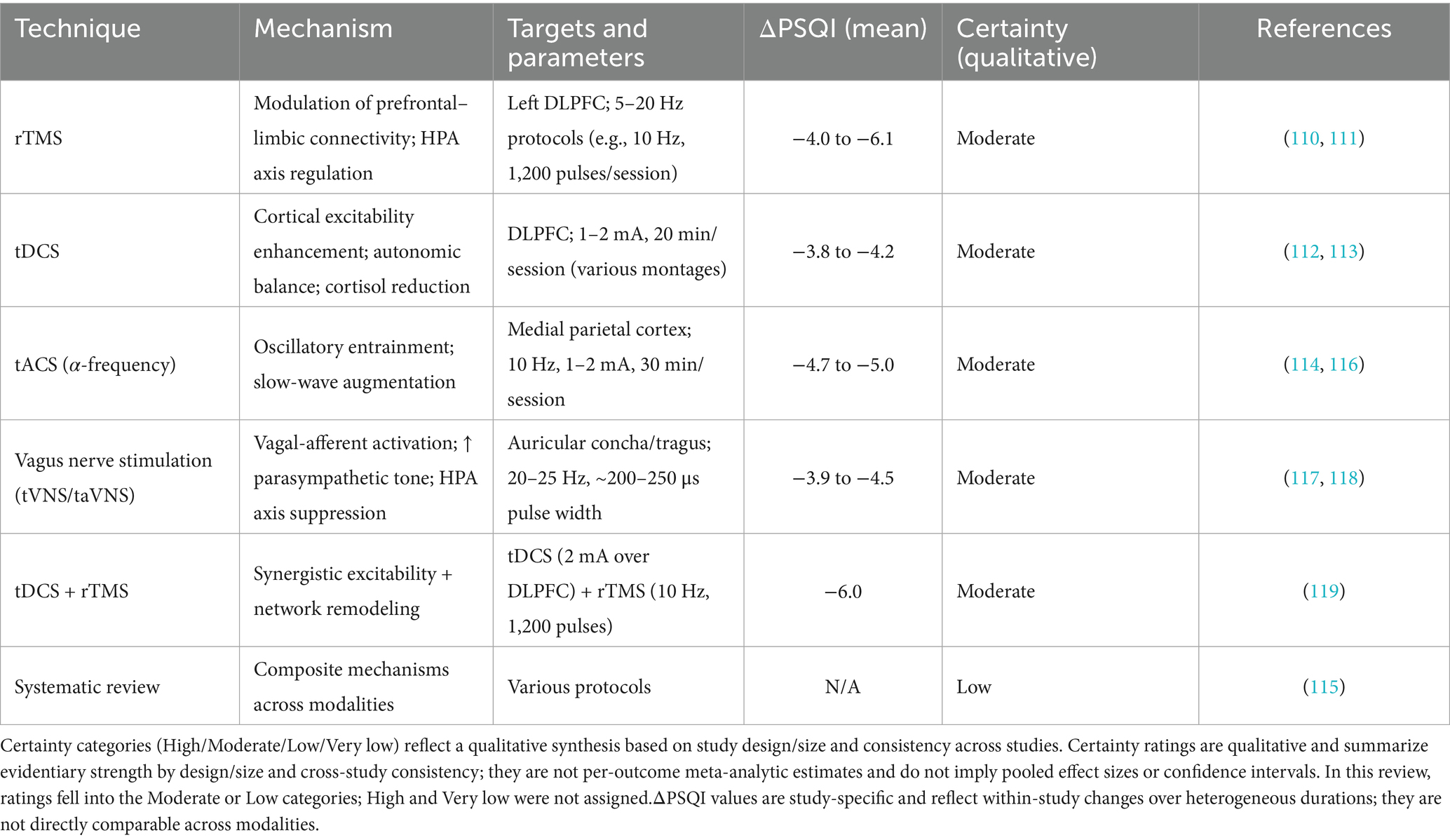
Table 1. Comparative summary of neuromodulation techniques in chronic insomnia (ΔPSQI indicates mean change where reported).
4.3 Synergistic strategy: acupuncture + neuromodulation
Recent trials have begun to demonstrate the clinical benefit of combining acupuncture with neuromodulation. Zhang et al. (17) compared low-frequency rTMS alone to rTMS plus manual acupuncture in patients with chronic insomnia and observed a significantly greater reduction in PSQI score in the combination group (p < 0.05). More recently, Zhou et al. (119) reported that a two-week adjunctive protocol of tDCS plus rTMS produced faster and more durable improvements in sleep efficiency than either modality alone, with superiority maintained at four-week follow-up.
These results provide empirical support for a synergistic clinical effect. However, to date there are no published studies directly probing the combined neurophysiological and autonomic mechanisms of acupuncture and neuromodulation. Therefore, the following hypotheses remain highly exploratory and require empirical validation in future trials.
Accordingly, drawing upon the mechanistic insights presented in Sections 3.1 and 3.2, together with findings from aforementioned RCTs, we propose two specific, testable hypotheses:
(i) Vagal-adrenal priming of infralimbic to ventrolateral preoptic (IL-VLPO) plasticity:
Acupuncture-induced enhancement of vagal-adrenal axis activity may create a permissive neurochemical environment (e.g., increased GABA (125), reduced cortisol (100)) that amplifies tDCS-mediated synaptic plasticity within the IL-VLPO sleep-promoting pathway.
(ii) Prefrontal-limbic recalibration with HPA-axis suppression:
rTMS-driven remodeling of dorsolateral prefrontal–limbic connectivity may synergize with acupuncture-mediated suppression of HPA-axis hyperactivity to more effectively attenuate the chronic stress responses underpinning insomnia (124).
These hypotheses remain speculative and await direct empirical testing through rigorous experimental and clinical studies in future. Well-powered, factorial-designed trials—incorporating multimodal neuroimaging (fMRI, EEG), autonomic (HRV), and endocrine (cortisol, melatonin) biomarkers—are needed to test differential effects across insomnia subtypes.
4.4 Limitations and prospects
Preliminary studies demonstrate combined acupuncture-neuromodulation efficacy (16, 17), but related research remains nascent. Considering rising insomnia prevalence, limited treatment options, and inadequate CBT-I availability, alternative therapies merit exploration (130). However, combined therapies present complexity in training, implementation costs, and the current paucity of large-scale randomized controlled trials (RCTs). Future studies should employ interdisciplinary teams (acupuncturists plus neuromodulation operators), large-sample multi-center randomized double-blind trials, objective-subjective endpoints, and prolonged follow-up to validate safety and efficacy comprehensively.
4.5 Safety, adverse effects, and contraindications
Although the preceding sections have emphasized the potential benefits of acupuncture and neuromodulation techniques in chronic insomnia, the safety profiles and possible adverse events of these interventions also warrant thorough discussion. Clinical studies have reported that rTMS treatment is often accompanied by transient headaches or scalp discomfort (131); moreover, a very small number of patients may face a risk of seizure (132), necessitating cautious evaluation or avoidance in individuals with a prior history of epilepsy or severe brain injury. The adverse effects of tDCS/tACS typically include mild tingling or erythema at the stimulation site; adverse events were predominantly mild and transient, and serious events were rare in included trials (133). Similarly, tVNS/taVNS procedures should be performed under monitored conditions in individuals with high-risk arrhythmias or severe cardiopulmonary insufficiency (134). By contrast, acupuncture, as a low-risk non-pharmacological therapy, primarily causes minor local adverse events such as bruising, infection, or needling discomfort; however, the incidence rate is extremely low, and serious adverse events are rare and mostly related to improper operation (135, 136). Future research should incorporate multi-center, large-sample randomized controlled trials with concurrent monitoring of adverse events and safety outcomes to establish a more comprehensive and balanced risk–benefit assessment framework.
5 Conclusion and future directions
Despite diverse treatments, significant gaps in current sleep physiology and insomnia pathophysiology models remain (115). The complementary integration of acupuncture and neuromodulation provides an innovative, personalized therapeutic approach, warranting further standardized, multi-center RCTs to optimize procedures, confirm sustained efficacy and safety, and explore differential responses across insomnia subtypes.
Author contributions
WMe: Writing – original draft, Writing – review & editing. WMi: Writing – original draft, Writing – review & editing. ZJ: Writing – original draft. PB: Data curation, Writing – original draft. WQ: Supervision, Writing – original draft, Writing – review & editing. WC: Funding acquisition, Resources, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The study was supported by the CACMS Innovation Project: CI2021B001, YZX-202345 and YZX-202313.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that Gen AI was used in the creation of this manuscript. The author(s) verify and take full responsibility for the use of generative AI in the preparation of this manuscript. Generative AI was used for partial translation of manuscript sections (ChatGPT, model: OpenAI o4-mini).
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Morin, CM, and Jarrin, DC. Epidemiology of insomnia: prevalence, course, risk factors, and public health burden. Sleep Med Clin. (2022) 17:173–91. doi: 10.1016/j.jsmc.2022.03.003
2. Sejbuk, M, Mironczuk-Chodakowska, I, and Witkowska, AM. Sleep quality: a narrative review on nutrition, stimulants, and physical activity as important factors. Nutrients. (2022) 14:1912. doi: 10.3390/nu14091912
3. Sweetman, A, Lack, L, Crawford, M, and Wallace, DM. Comorbid insomnia and sleep apnea: assessment and management approaches. Sleep Med Clin. (2022) 17:597–617. doi: 10.1016/j.jsmc.2022.07.006
4. Ostovar-Kermani, T, Arnaud, D, Almaguer, A, Garcia, I, Gonzalez, S, Mendez Martinez, YH, et al. Painful sleep: insomnia in patients with chronic pain syndrome and its consequences. Folia Med (Plovdiv). (2020) 62:645–54. doi: 10.3897/folmed.62.e50705
5. Wickwire, EM, Shaya, FT, and Scharf, SM. Health economics of insomnia treatments: the return on investment for a good night’s sleep. Sleep Med Rev. (2016) 30:72–82. doi: 10.1016/j.smrv.2015.11.004
6. Riemann, D, Espie, CA, Altena, E, Arnardottir, ES, Baglioni, C, Bassetti, CLA, et al. The European insomnia guideline: an update on the diagnosis and treatment of insomnia 2023. J Sleep Res. (2023) 32:e14035. doi: 10.1111/jsr.14035
7. Dubovsky, SL, and Marshall, D. Benzodiazepines remain important therapeutic options in psychiatric practice. Psychother Psychosom. (2022) 91:307–34. doi: 10.1159/000524400
8. Chapoutot, M, Peter-Derex, L, Bastuji, H, Leslie, W, Schoendorff, B, Heinzer, R, et al. Cognitive behavioral therapy and acceptance and commitment therapy for the discontinuation of long-term benzodiazepine use in insomnia and anxiety disorders. Int J Environ Res Public Health. (2021) 18:222. doi: 10.3390/ijerph181910222
9. Hertenstein, E, Trinca, E, Wunderlin, M, Schneider, CL, Zust, MA, Feher, KD, et al. Cognitive behavioral therapy for insomnia in patients with mental disorders and comorbid insomnia: a systematic review and meta-analysis. Sleep Med Rev. (2022) 62:101597. doi: 10.1016/j.smrv.2022.101597
10. Zhang, J, and Yuan, C. Status of health economics research on cognitive behavioral therapy for insomnia. Sichuan Da Xue Xue Bao Yi Xue Ban. (2023) 54:263–7. doi: 10.12182/20230360102
11. Morin, CM, Bei, B, Bjorvatn, B, Poyares, D, Spiegelhalder, K, and Wing, YK. World sleep society international sleep medicine guidelines position statement endorsement of "behavioral and psychological treatments for chronic insomnia disorder in adults: an American academy of sleep medicine clinical practice guidelines". Sleep Med. (2023) 109:164–9. doi: 10.1016/j.sleep.2023.07.001
12. Dibben, GO, Martin, A, Shore, CB, Johnstone, A, McMellon, C, Palmer, V, et al. Adolescents’ interactive electronic device use, sleep and mental health: a systematic review of prospective studies. J Sleep Res. (2023) 32:e13899. doi: 10.1111/jsr.13899
13. Lefaucheur, J, Antal, A, Ayache, SS, Benninger, DH, Brunelin, J, Cogiamanian, F, et al. Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS). Clin Neurophysiol. (2017) 128:56–92. doi: 10.1016/j.clinph.2016.10.087
14. Woods, AJ, Antal, A, Bikson, M, Boggio, PS, Brunoni, AR, Celnik, P, et al. A technical guide to tDCS, and related non-invasive brain stimulation tools. Clin Neurophysiol. (2016) 127:1031–48. doi: 10.1016/j.clinph.2015.11.012
15. Lin, J, Kotha, P, and Chen, Y. Understandings of acupuncture application and mechanisms. Am J Transl Res. (2022) 14:1469–81.
16. Yan, L, Zhou, P, Lai, M, Wu, M, Zhang, Y, Tang, R, et al. Effect of acupuncture combined with low frequency rTMS on comorbid mild-to-moderate depressive disorder and insomnia: a randomized controlled trial. Zhongguo Zhen Jiu. (2023) 43:374–8. doi: 10.13703/j.0255-2930.20220730-k0001
17. Zhang, Y, Liao, W, and Xia, W. Effect of acupuncture cooperated with low-frequency repetitive transcranial magnetic stimulation on chronic insomnia: a randomized clinical trial. Curr Med Sci. (2018) 38:491–8. doi: 10.1007/s11596-018-1905-2
18. Wick, JY. The history of benzodiazepines. Consult Pharm. (2013) 28:538–48. doi: 10.4140/TCP.n.2013.538
19. Soni, A, Thiyagarajan, A, and Reeve, J. Feasibility and effectiveness of deprescribing benzodiazepines and z-drugs: systematic review and meta-analysis. Addiction. (2023) 118:7–16. doi: 10.1111/add.15997
20. Kobayashi, C, Kitanaka, N, Nakai, M, Hall, FS, Tomita, K, Igarashi, K, et al. Protein phosphatase 2a inhibitors: a possible pharmacotherapy for benzodiazepine dependence. J Pharm Pharmacol. (2025) 77:335–40. doi: 10.1093/jpp/rgae136
21. Barker, MJ, Greenwood, KM, Jackson, M, and Crowe, SF. Persistence of cognitive effects after withdrawal from long-term benzodiazepine use: a meta-analysis. Arch Clin Neuropsychol. (2004) 19:437–54. doi: 10.1016/S0887-6177(03)00096-9
22. Stranks, EK, and Crowe, SF. The acute cognitive effects of zopiclone, zolpidem, zaleplon, and eszopiclone: a systematic review and meta-analysis. J Clin Exp Neuropsychol. (2014) 36:691–700. doi: 10.1080/13803395.2014.928268
23. Treves, N, Perlman, A, Kolenberg Geron, L, Asaly, A, and Matok, I. Z-drugs and risk for falls and fractures in older adults-a systematic review and meta-analysis. Age Ageing. (2018) 47:201–8. doi: 10.1093/ageing/afx167
24. Rapoport, MJ, Lanctot, KL, Streiner, DL, Bedard, M, Vingilis, E, Murray, B, et al. Benzodiazepine use and driving: a meta-analysis. J Clin Psychiatry. (2009) 70:663–73. doi: 10.4088/JCP.08m04325
25. Sateia, MJ, Buysse, DJ, Krystal, AD, Neubauer, DN, and Heald, JL. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: an American Academy of sleep medicine clinical practice guideline. J Clin Sleep Med. (2017) 13:307–49. doi: 10.5664/jcsm.6470
26. Matheson, EM, Brown, BD, and DeCastro, AO. Treatment of chronic insomnia in adults. Am Fam Physician. (2024) 109:154–60.
27. Shah, YD, Stringel, V, Pavkovic, I, and Kothare, SV. Doxepin in children and adolescents with symptoms of insomnia: a single-center experience. J Clin Sleep Med. (2020) 16:743–7. doi: 10.5664/jcsm.8338
28. Cuomo, A, Ballerini, A, Bruni, AC, Decina, P, Di Sciascio, G, Fiorentini, A, et al. Clinical guidance for the use of trazodone in major depressive disorder and concomitant conditions: pharmacology and clinical practice. Riv Psichiatr. (2019) 54:137–49. doi: 10.1708/3202.31796
29. Coin, A, Malara, A, Noale, M, Trevisan, C, Devita, M, Abbatecola, AM, et al. Real-world use of trazodone in older persons in long term care setting: a retrospective study. Int J Geriatr Psychiatry. (2024) 39:e70009. doi: 10.1002/gps.70009
30. Muehlan, C, Roch, C, Vaillant, C, and Dingemanse, J. The orexin story and orexin receptor antagonists for the treatment of insomnia. J Sleep Res. (2023) 32:e13902. doi: 10.1111/jsr.13902
31. Kunz, D, Dauvilliers, Y, Benes, H, Garcia-Borreguero, D, Plazzi, G, Seboek Kinter, D, et al. Long-term safety and tolerability of daridorexant in patients with insomnia disorder. CNS Drugs. (2023) 37:93–106. doi: 10.1007/s40263-022-00980-8
32. Rocha, RB, Bomtempo, FF, Nager, GB, Cenci, GI, and Telles, JPM. Dual orexin receptor antagonists for the treatment of insomnia: systematic review and network meta-analysis. Arq Neuropsiquiatr. (2023) 81:475–83. doi: 10.1055/s-0043-1768667
33. Wu, X, Xue, T, Chen, Z, Wang, Z, and Chen, G. Orexin receptor antagonists and insomnia. Curr Psychiatry Rep. (2022) 24:509–21. doi: 10.1007/s11920-022-01357-w
34. Mignot, E, Mayleben, D, Fietze, I, Leger, D, Zammit, G, Bassetti, CLA, et al. Safety and efficacy of daridorexant in patients with insomnia disorder: results from two multicentre, randomised, double-blind, placebo-controlled, phase 3 trials. Lancet Neurol. (2022) 21:125–39. doi: 10.1016/S1474-4422(21)00436-1
35. De Crescenzo, F, D’Alo, GL, Ostinelli, EG, Ciabattini, M, Di Franco, V, Watanabe, N, et al. Comparative effects of pharmacological interventions for the acute and long-term management of insomnia disorder in adults: a systematic review and network meta-analysis. Lancet. (2022) 400:170–84. doi: 10.1016/S0140-6736(22)00878-9
36. Maruani, J, Reynaud, E, Chambe, J, Palagini, L, Bourgin, P, and Geoffroy, PA. Efficacy of melatonin and ramelteon for the acute and long-term management of insomnia disorder in adults: a systematic review and meta-analysis. J Sleep Res. (2023) 32:e13939. doi: 10.1111/jsr.13939
37. Yue, J, Chang, X, Zheng, J, Shi, L, Xiang, Y, Que, J, et al. Efficacy and tolerability of pharmacological treatments for insomnia in adults: a systematic review and network meta-analysis. Sleep Med Rev. (2023) 68:101746. doi: 10.1016/j.smrv.2023.101746
38. Poza, JJ, Pujol, M, Ortega-Albas, JJ, and Romero, O. Melatonin in sleep disorders. Neurologia (Engl Ed). (2022) 37:575–85. doi: 10.1016/j.nrleng.2018.08.004
39. Panula, P. Histamine receptors, agonists, and antagonists in health and disease. Handb Clin Neurol. (2021) 180:377–87. doi: 10.1016/B978-0-12-820107-7.00023-9
40. Oyekan, PJ, Gorton, HC, and Copeland, CS. Antihistamine-related deaths in England: are the high safety profiles of antihistamines leading to their unsafe use? Br J Clin Pharmacol. (2021) 87:3978–87. doi: 10.1111/bcp.14819
41. Borras, S, Martinez-Solis, I, and Rios, JL. Medicinal plants for insomnia related to anxiety: an updated review. Planta Med. (2021) 87:738–53. doi: 10.1055/a-1510-9826
42. Kenda, M, Kocevar Glavac, N, Nagy, M, and Sollner Dolenc, M. Medicinal plants used for anxiety, depression, or stress treatment: an update. Molecules. (2022) 27:21. doi: 10.3390/molecules27186021
43. Riemann, D, Benz, F, Dressle, RJ, Espie, CA, Johann, AF, Blanken, TF, et al. Insomnia disorder: state of the science and challenges for the future. J Sleep Res. (2022) 31:e13604. doi: 10.1111/jsr.13604
44. Perlis, ML, Posner, D, Riemann, D, Bastien, CH, Teel, J, and Thase, M. Insomnia. Lancet. (2022) 400:1047–60. doi: 10.1016/S0140-6736(22)00879-0
45. Rabelo, JL, Cruz, BF, Ferreira, JDR, Viana, BDM, and Barbosa, IG. Psychoeducation in bipolar disorder: a systematic review. World J Psychiatr. (2021) 11:1407–24. doi: 10.5498/wjp.v11.i12.1407
46. Galvez-Sanchez, CM, and Montoro, CI. Psychoeducation for fibromyalgia syndrome: a systematic review of emotional, clinical and functional related-outcomes. Behav Sci (Basel). (2023) 13:415. doi: 10.3390/bs13050415
48. Borbely, A. The two-process model of sleep regulation: beginnings and outlook. J Sleep Res. (2022) 31:e13598. doi: 10.1111/jsr.13598
49. De Pasquale, C, El Kazzi, M, Sutherland, K, Shriane, AE, Vincent, GE, Cistulli, PA, et al. Sleep hygiene - what do we mean? A bibliographic review. Sleep Med Rev. (2024) 75:101930. doi: 10.1016/j.smrv.2024.101930
50. Baranwal, N, Yu, PK, and Siegel, NS. Sleep physiology, pathophysiology, and sleep hygiene. Prog Cardiovasc Dis. (2023) 77:59–69. doi: 10.1016/j.pcad.2023.02.005
51. Irish, LA, Kline, CE, Gunn, HE, Buysse, DJ, and Hall, MH. The role of sleep hygiene in promoting public health: a review of empirical evidence. Sleep Med Rev. (2015) 22:23–36. doi: 10.1016/j.smrv.2014.10.001
52. Edinger, JD, Arnedt, JT, Bertisch, SM, Carney, CE, Harrington, JJ, Lichstein, KL, et al. Behavioral and psychological treatments for chronic insomnia disorder in adults: an American academy of sleep medicine systematic review, meta-analysis, and GRADE assessment. J Clin Sleep Med. (2021) 17:263–98. doi: 10.5664/jcsm.8988
53. Takano, Y, Iwano, S, Aoki, S, Nakano, N, and Sakano, Y. A systematic review of the effect of sleep interventions on presenteeism. Biopsychosoc Med. (2021) 15:21. doi: 10.1186/s13030-021-00224-z
54. Shriane, AE, Rigney, G, Ferguson, SA, Bin, YS, and Vincent, GE. Healthy sleep practices for shift workers: consensus sleep hygiene guidelines using a delphi methodology. Sleep. (2023) 46:182. doi: 10.1093/sleep/zsad182
55. Rampling, CM, Gupta, CC, Shriane, AE, Ferguson, SA, Rigney, G, and Vincent, GE. Does knowledge of sleep hygiene recommendations match behaviour in Australian shift workers? A cross-sectional study. BMJ Open. (2022) 12:e059677. doi: 10.1136/bmjopen-2021-059677
56. Toussaint, L, Nguyen, QA, Roettger, C, Dixon, K, Offenbacher, M, Kohls, N, et al. Effectiveness of progressive muscle relaxation, deep breathing, and guided imagery in promoting psychological and physiological states of relaxation. Evid Based Complement Alternat Med. (2021) 2021:5924040. doi: 10.1155/2021/5924040
57. Harorani, M, Davodabady, F, Masmouei, B, and Barati, N. The effect of progressive muscle relaxation on anxiety and sleep quality in burn patients: a randomized clinical trial. Burns. (2020) 46:1107–13. doi: 10.1016/j.burns.2019.11.021
58. Mashhadi-Naser, S, Shirvani, S, and Vasli, P. A randomized controlled trial to evaluate the progressive muscle relaxation technique in hip fracture patients. Sci Rep. (2024) 14:13534. doi: 10.1038/s41598-024-64516-4
59. Datta, K, Tripathi, M, Verma, M, Masiwal, D, and Mallick, HN. Yoga nidra practice shows improvement in sleep in patients with chronic insomnia: a randomized controlled trial. Natl Med J India. (2021) 34:143–50. doi: 10.25259/NMJI_63_19
60. Furukawa, Y, Sakata, M, Yamamoto, R, Nakajima, S, Kikuchi, S, Inoue, M, et al. Components and delivery formats of cognitive behavioral therapy for chronic insomnia in adults: a systematic review and component network meta-analysis. JAMA Psychiatr. (2024) 81:357–65. doi: 10.1001/jamapsychiatry.2023.5060
61. Maurer, LF, Espie, CA, Omlin, X, Reid, MJ, Sharman, R, Gavriloff, D, et al. Isolating the role of time in bed restriction in the treatment of insomnia: a randomized, controlled, dismantling trial comparing sleep restriction therapy with time in bed regularization. Sleep. (2020) 43:96. doi: 10.1093/sleep/zsaa096
62. Maurer, LF, Schneider, J, Miller, CB, Espie, CA, and Kyle, SD. The clinical effects of sleep restriction therapy for insomnia: a meta-analysis of randomised controlled trials. Sleep Med Rev. (2021) 58:101493. doi: 10.1016/j.smrv.2021.101493
63. Looman, MI, Blanken, TF, Schoenmakers, TM, Reesen, JE, Effting, M, Linnebank, FE, et al. Telephone-guided sleep restriction for insomnia: a randomized sleep diary-controlled trial. Psychother Psychosom. (2025) 94:147–61. doi: 10.1159/000545138
64. Steinmetz, L, Simon, L, Feige, B, Riemann, D, Akram, U, Crawford, MR, et al. Adherence to sleep restriction therapy - an evaluation of existing measures. J Sleep Res. (2023) 32:e13975. doi: 10.1111/jsr.13975
65. Mellor, A, Kavaliotis, E, Mascaro, L, and Drummond, SPA. Approaches to the assessment of adherence to CBT-i, predictors of adherence, and the association of adherence to outcomes: a systematic review. Sleep Med Rev. (2022) 63:101620. doi: 10.1016/j.smrv.2022.101620
66. Jansson-Frojmark, M, Nordenstam, L, Alfonsson, S, Bohman, B, Rozental, A, and Norell-Clarke, A. Stimulus control for insomnia: a systematic review and meta-analysis. J Sleep Res. (2024) 33:e14002. doi: 10.1111/jsr.14002
67. Goodhines, PA, Svingos, AM, Gerish, S, Park, A, and Gellis, LA. Randomized controlled trial of cognitive refocusing versus stimulus control treatment for college insomnia: feasibility of a brief, electronic-based, and peer-led approach. J Am Coll Heal. (2024) 72:2229–41. doi: 10.1080/07448481.2022.2109031
68. Verreault, MD, Granger, E, Neveu, X, Delage, JP, Bastien, CH, and Vallieres, A. The effectiveness of stimulus control in cognitive behavioural therapy for insomnia in adults: a systematic review and network meta-analysis. J Sleep Res. (2024) 33:e14008. doi: 10.1111/jsr.14008
69. Sunnhed, R, Hesser, H, Andersson, G, Carlbring, P, Morin, CM, Harvey, AG, et al. Comparing internet-delivered cognitive therapy and behavior therapy with telephone support for insomnia disorder: a randomized controlled trial. Sleep. (2020) 43:245. doi: 10.1093/sleep/zsz245
70. Bjorvatn, B, Fiske, E, and Pallesen, S. A self-help book is better than sleep hygiene advice for insomnia: a randomized controlled comparative study. Scand J Psychol. (2011) 52:580–5. doi: 10.1111/j.1467-9450.2011.00902.x
71. Cheng, P, Santarossa, S, Kalmbach, D, Sagong, C, Hu, K, and Drake, C. Patient perspectives on facilitators and barriers to equitable engagement with digital CBT-i. Sleep Health. (2023) 9:571–8. doi: 10.1016/j.sleh.2023.07.003
72. Kim, N, Ka, S, and Park, J. Effects of exercise timing and intensity on physiological circadian rhythm and sleep quality: a systematic review. Phys Act Nutr. (2023) 27:52–63. doi: 10.20463/pan.2023.0029
73. De Nys, L, Anderson, K, Ofosu, EF, Ryde, GC, Connelly, J, and Whittaker, AC. The effects of physical activity on cortisol and sleep: a systematic review and meta-analysis. Psychoneuroendocrinology. (2022) 143:105843. doi: 10.1016/j.psyneuen.2022.105843
74. Han, D, Cheng, J, Qu, J, Wen, X, Liu, X, Chen, Y, et al. Effectiveness of taijiquan in treating insomnia: a systematic review and meta-analysis of randomized controlled studies. Front Psych. (2022) 13:892453. doi: 10.3389/fpsyt.2022.892453
75. Dekker, K, Benjamins, JS, Maksimovic, T, Filardi, M, Hofman, WF, van Straten, A, et al. Combined internet-based cognitive-behavioral and chronobiological intervention for insomnia: a randomized controlled trial. Psychother Psychosom. (2020) 89:117–8. doi: 10.1159/000503570
76. Drozd, C, Curtit, E, Gillet, V, Jacquinot, Q, Meneveau, N, and Mougin, F. Exercise intervention on insomnia in patients with a cancer: a systematic review of the literature. Cancers (Basel). (2024) 16:241. doi: 10.3390/cancers16122241
77. Drozd, C, Curtit, E, Jacquinot, Q, Marquine, C, Mansi, L, Chaigneau, L, et al. A randomized trial to evaluate the effects of a supervised exercise program on insomnia in patients with non-metastatic breast cancer undergoing chemotherapy: design of the FATSOMCAN study. BMC Cancer. (2023) 23:449. doi: 10.1186/s12885-023-10902-6
78. Wang, Q, Wu, S, Luo, Z, Pu, L, Wang, X, Guo, M, et al. Effects of light therapy on sleep and circadian rhythm in older type 2 diabetics living in long-term care facilities: a randomized controlled trial. Front Endocrinol (Lausanne). (2024) 15:1307537. doi: 10.3389/fendo.2024.1307537
79. Pun, TB, Phillips, CL, Marshall, NS, Comas, M, Hoyos, CM, D’Rozario, AL, et al. The effect of light therapy on electroencephalographic sleep in sleep and circadian rhythm disorders: a scoping review. Clocks Sleep. (2022) 4:358–73. doi: 10.3390/clockssleep4030030
80. Kim, W, Joa, K, Kim, C, Lee, H, Kang, S, Jung, H, et al. The effect of bright light therapy on sleep and quality of life in patients with poststroke insomnia. Psychosom Med. (2022) 84:123–30. doi: 10.1097/PSY.0000000000001014
81. Chambe, J, Reynaud, E, Maruani, J, Fraih, E, Geoffroy, PA, and Bourgin, P. Light therapy in insomnia disorder: a systematic review and meta-analysis. J Sleep Res. (2023) 32:e13895. doi: 10.1111/jsr.13895
82. Zhao, C, Li, N, Miao, W, He, Y, and Lin, Y. A systematic review and meta-analysis on light therapy for sleep disorders in shift workers. Sci Rep. (2025) 15:134. doi: 10.1038/s41598-024-83789-3
83. Ding, J, Huang, T, Hu, J, and Yuan, F. Effectiveness and safety of music therapy for insomnia disorder patients: a protocol for systematic review and meta-analysis. Medicine (Baltimore). (2021) 100:e26399. doi: 10.1097/MD.0000000000026399
84. Lund, HN, Pedersen, IN, Heymann-Szlachcinska, AM, Tuszewska, M, Bizik, G, Larsen, JI, et al. Music to improve sleep quality in adults with depression-related insomnia (MUSTAFI): randomized controlled trial. Nord J Psychiatry. (2023) 77:188–97. doi: 10.1080/08039488.2022.2080254
85. Chang, L, Wang, Y, Zhang, J, Zhao, W, Li, X, and Yang, L. Effect of music therapy combined with aerobic exercise on sleep quality among breast cancer patients undergoing chemotherapy after a radical mastectomy: a randomized controlled trial. BMC Womens Health. (2024) 24:408. doi: 10.1186/s12905-024-03241-6
86. Gou, Q, Li, M, Wang, X, Yuan, X, Yang, M, Li, J, et al. Meta-narrative review: the impact of music therapy on sleep and future research directions. Front Neurol. (2024) 15:1433592. doi: 10.3389/fneur.2024.1433592
87. Saskovets, M, Liang, Z, Piumarta, I, and Saponkova, I. Effects of sound interventions on the mental stress response in adults: protocol for a scoping review. JMIR Res Protoc. (2024) 13:e54030. doi: 10.2196/54030
88. Jespersen, KV, Otto, M, Kringelbach, M, Van Someren, E, and Vuust, P. A randomized controlled trial of bedtime music for insomnia disorder. J Sleep Res. (2019) 28:e12817. doi: 10.1111/jsr.12817
89. Jespersen, KV, Pando-Naude, V, Koenig, J, Jennum, P, and Vuust, P. Listening to music for insomnia in adults. Cochrane Database Syst Rev. (2022) 8:CD010459. doi: 10.1002/14651858.CD010459.pub3
90. Mao, Y, and Yang, L. Clinical application of electroacupuncture in enhanced recovery after surgery. Front Rehabil Sci. (2023) 4:1135618. doi: 10.3389/fresc.2023.1135618
91. Wang, M, Liu, W, Ge, J, and Liu, S. The immunomodulatory mechanisms for acupuncture practice. Front Immunol. (2023) 14:1147718. doi: 10.3389/fimmu.2023.1147718
92. Liu, S, Wang, Z, Su, Y, Qi, L, Yang, W, Fu, M, et al. A neuroanatomical basis for electroacupuncture to drive the vagal-adrenal axis. Nature. (2021) 598:641–5. doi: 10.1038/s41586-021-04001-4
93. Zhao, F, Fu, Q, Kennedy, GA, Conduit, R, Zhang, W, Wu, W, et al. Can acupuncture improve objective sleep indices in patients with primary insomnia? A systematic review and meta-analysis. Sleep Med. (2021) 80:244–59. doi: 10.1016/j.sleep.2021.01.053
94. Wang, Y, Li, T, Ha, L, Lv, Z, Wang, F, Wang, Z, et al. Effectiveness and cerebral responses of multi-points acupuncture for primary insomnia: a preliminary randomized clinical trial and fMRI study. BMC Complement Med Ther. (2020) 20:254. doi: 10.1186/s12906-020-02969-6
95. Weng, Y, Ren, X, Zu, Z, Xiao, L, and Chen, M. Efficacy and safety of acupuncture for the treatment of insomnia in breast cancer patients: a systematic review and meta-analysis. Complement Ther Med. (2024) 86:103087. doi: 10.1016/j.ctim.2024.103087
96. Yin, X, Li, W, Liang, T, Lu, B, Yue, H, Li, S, et al. Effect of electroacupuncture on insomnia in patients with depression: a randomized clinical trial. JAMA Netw Open. (2022) 5:e2220563. doi: 10.1001/jamanetworkopen.2022.20563
97. Lu, Y, Zhu, H, Wang, Q, Tian, C, Lai, H, Hou, L, et al. Comparative effectiveness of multiple acupuncture therapies for primary insomnia: a systematic review and network meta-analysis of randomized trial. Sleep Med. (2022) 93:39–48. doi: 10.1016/j.sleep.2022.03.012
98. Meira Do Valle, SS, and Hong, H. Acupuncture treatment for generalized anxiety disorder by activating the vagus nerve and improving heart-rate variability and heart-rhythm coherence, a case-series study. Med Acupunct. (2024) 36:21–6. doi: 10.1089/acu.2023.0036
99. Li, Y, Li, W, Wang, S, Gong, Y, Dou, B, Lyu, Z, et al. The autonomic nervous system: a potential link to the efficacy of acupuncture. Front Neurosci. (2022) 16:1038945. doi: 10.3389/fnins.2022.1038945
100. Li, J, Wu, W, Liu, C, Wang, X, Qin, S, Zhao, Y, et al. Effect of tiaoshen needling on plasma melatonin and cortisol in patients with chronic insomnia. Zhen Ci Yan Jiu. (2021) 46:690–4. doi: 10.13702/j.1000-0607.201009
101. Huang, A, Xiao, G, Chen, Y, Hu, Z, Lee, P, Huang, Y, et al. Ziwuliuzhu acupuncture modulates clock mRNA, bmal1 mRNA and melatonin in insomnia rats. J Acupunct Meridian Stud. (2023) 16:109–18. doi: 10.51507/j.jams.2023.16.3.109
102. Li, B, Deng, S, Sang, B, Zhu, W, Zhuo, B, Zhang, M, et al. Revealing the neuroimaging mechanism of acupuncture for poststroke aphasia: a systematic review. Neural Plast. (2022) 2022:5635596. doi: 10.1155/2022/5635596
103. Wang, J, Zhao, H, Shi, K, and Wang, M. Treatment of insomnia based on the mechanism of pathophysiology by acupuncture combined with herbal medicine: a review. Medicine (Baltimore). (2023) 102:e33213. doi: 10.1097/MD.0000000000033213
104. Zhang, J, Zhang, Z, Huang, S, Qiu, X, Lao, L, Huang, Y, et al. Acupuncture for cancer-related insomnia: a systematic review and meta-analysis. Phytomedicine. (2022) 102:154160. doi: 10.1016/j.phymed.2022.154160
105. Verma, K, Singh, D, and Srivastava, A. The impact of complementary and alternative medicine on insomnia: a systematic review. Cureus. (2022) 14:e28425. doi: 10.7759/cureus.28425
106. Kutana, S, Mao, JJ, and Garland, SN. Acupuncture as an adjunct treatment to cognitive-behavioral therapy for insomnia. Sleep Med Clin. (2023) 18:113–22. doi: 10.1016/j.jsmc.2022.10.005
107. Ferreira, WS, Santana, MG, Youngstedt, SD, de Assis, DE, de Assis, BP, Cerqueira, DP, et al. Effects of exercise training and exercise plus acupuncture on chronic insomnia: a feasibility study. Sleep Sci. (2022) 15:288–96. doi: 10.5935/1984-0063.20220053
108. Hein, M, Hubain, P, Linkowski, P, and Loas, G. Support for insomnia: recommendations for practice in general medicine. Rev Med Brux. (2016) 37:235–41.
109. San-Juan, D, Davila-Rodriguez, DO, Jimenez, CR, Gonzalez, MS, Carranza, SM, Hernandez Mendoza, JR, et al. Neuromodulation techniques for status epilepticus: a review. Brain Stimul. (2019) 12:835–44. doi: 10.1016/j.brs.2019.04.005
110. Collins, AR, Cheung, J, Croarkin, PE, Kolla, BP, and Kung, S. Effects of transcranial magnetic stimulation on sleep quality and mood in patients with major depressive disorder. J Clin Sleep Med. (2022) 18:1297–305. doi: 10.5664/jcsm.9846
111. Sun, N, He, Y, Wang, Z, Zou, W, and Liu, X. The effect of repetitive transcranial magnetic stimulation for insomnia: a systematic review and meta-analysis. Sleep Med. (2021) 77:226–37. doi: 10.1016/j.sleep.2020.05.020
112. Bakhshayesh Eghbali, B, Ramezani, S, Sedaghat Herfeh, S, Emir Alavi, C, Najafi, K, Esmaeeli Lipaei, P, et al. ¬Transcranial direct current stimulation improves sleep quality in patients with insomnia after traumatic brain injury. Brain Inj. (2023) 37:63–73. doi: 10.1080/02699052.2022.2145363
113. Zhou, Q, Yu, C, Yu, H, Zhang, Y, Liu, Z, Hu, Z, et al. The effects of repeated transcranial direct current stimulation on sleep quality and depression symptoms in patients with major depression and insomnia. Sleep Med. (2020) 70:17–26. doi: 10.1016/j.sleep.2020.02.003
114. Wang, H, Wang, L, Zhang, W, Xue, Q, Peng, M, Sun, Z, et al. Effect of transcranial alternating current stimulation for the treatment of chronic insomnia: a randomized, double-blind, parallel-group, placebo-controlled clinical trial. Psychother Psychosom. (2020) 89:38–47. doi: 10.1159/000504609
115. Krone, LB, Feher, KD, Rivero, T, and Omlin, X. Brain stimulation techniques as novel treatment options for insomnia: a systematic review. J Sleep Res. (2023) 32:e13927. doi: 10.1111/jsr.13927
116. Wang, L, Chen, Y, Piao, Z, Gu, X, Liu, H, Wang, D, et al. Medial parietal alpha-frequency transcranial alternating current stimulation for chronic insomnia: a randomized sham-controlled trial. Psychol Med. (2025) 55:e102. doi: 10.1017/S0033291725000625
117. Zhang, L, Jin, Y, Zhang, Q, Liu, H, Chen, C, Song, L, et al. Transcutaneous vagus nerve stimulation for insomnia in people living in places or cities with high altitudes: a randomized controlled trial. Brain Sci. (2023) 13:985. doi: 10.3390/brainsci13070985
118. Zhang, S, Zhao, Y, Qin, Z, Han, Y, He, J, Zhao, B, et al. Transcutaneous auricular vagus nerve stimulation for chronic insomnia disorder: a randomized clinical trial. JAMA Netw Open. (2024) 7:e2451217. doi: 10.1001/jamanetworkopen.2024.51217
119. Zhou, Q, Liu, Z, Yu, C, Wang, Q, Zhuang, W, Tang, Y, et al. Effect of combined treatment with transcranial direct current stimulation and repetitive transcranial magnetic stimulation compared to monotherapy for the treatment of chronic insomnia: a randomised, double-blind, parallel-group, controlled trial. BMC Med. (2024) 22:538. doi: 10.1186/s12916-024-03751-y
120. Szulczewski, MT. Transcutaneous auricular vagus nerve stimulation combined with slow breathing: speculations on potential applications and technical considerations. Neuromodulation. (2022) 25:380–94. doi: 10.1111/ner.13458
121. Butt, MF, Albusoda, A, Farmer, AD, and Aziz, Q. The anatomical basis for transcutaneous auricular vagus nerve stimulation. J Anat. (2020) 236:588–611. doi: 10.1111/joa.13122
122. Ko, D, Lee, H, Kim, D, Park, Y, and Kang, N. Transcranial direct current stimulation improves heart rate variability: a systematic review and meta-analysis. Prog Neuro-Psychopharmacol Biol Psychiatry. (2024) 134:111072. doi: 10.1016/j.pnpbp.2024.111072
123. Lee, H, Lee, JH, Hwang, M, and Kang, N. Repetitive transcranial magnetic stimulation improves cardiovascular autonomic nervous system control: a meta-analysis. J Affect Disord. (2023) 339:443–53. doi: 10.1016/j.jad.2023.07.039
124. Jiang, C, Zhang, T, Yue, F, Yi, M, and Gao, D. Efficacy of repetitive transcranial magnetic stimulation in the treatment of patients with chronic primary insomnia. Cell Biochem Biophys. (2013) 67:169–73. doi: 10.1007/s12013-013-9529-4
125. Zhang, H, Huang, X, Wang, C, and Liang, K. Alteration of gamma-aminobutyric acid in the left dorsolateral prefrontal cortex of individuals with chronic insomnia: a combined transcranial magnetic stimulation-magnetic resonance spectroscopy study. Sleep Med. (2022) 92:34–40. doi: 10.1016/j.sleep.2022.03.003
126. Zhang, Y, Lin, P, Wang, R, Zhou, J, Xu, X, Jiang, W, et al. Insula-medial prefrontal cortex functional connectivity modulated by transcutaneous auricular vagus nerve stimulation: an fMRI study. IEEE J Biomed Health Inform. (2024) 28:5962–70. doi: 10.1109/JBHI.2024.3423019
127. Su, Y, Yi, P, and Chang, F. Transcranial direct current stimulation (tDCS) ameliorates stress-induced sleep disruption via activating infralimbic-ventrolateral preoptic projections. Brain Sci. (2024) 14:105. doi: 10.3390/brainsci14010105
128. Zhou, H, Tang, X, Wang, D, Huang, Z, Zeng, Y, Liu, S, et al. Neuroregulatory and clinical efficacy of auricular vagus nerve stimulation in elderly patients with chronic insomnia comorbid with functional dyspepsia: protocol for a randomized controlled trial. Front Med (Lausanne). (2025) 12:1537515. doi: 10.3389/fmed.2025.1537515
129. Lee, M, Hong, JK, Lee, Y, and Yoon, I. Transcranial alternating current stimulation in subjects with insomnia symptoms: a randomized, double-blind and controlled study. J Psychiatr Res. (2025) 186:129–36. doi: 10.1016/j.jpsychires.2025.04.020
130. Ell, J, Schmid, SR, Benz, F, and Spille, L. Complementary and alternative treatments for insomnia disorder: a systematic umbrella review. J Sleep Res. (2023) 32:e13979. doi: 10.1111/jsr.13979
131. Rossi, S, Antal, A, Bestmann, S, Bikson, M, Brewer, C, Brockmöller, J, et al. Safety and recommendations for TMS use in healthy subjects and patient populations, with updates on training, ethical and regulatory issues: expert guidelines. Clin Neurophysiol. (2021) 132:269–306. doi: 10.1016/j.clinph.2020.10.003
132. Trapp, NT, Purgianto, A, Taylor, JJ, Singh, MK, Oberman, LM, Mickey, BJ, et al. Consensus review and considerations on TMS to treat depression: a comprehensive update endorsed by the National Network of depression centers, the clinical TMS Society, and the International Federation of Clinical Neurophysiology. Clin Neurophysiol. (2025) 170:206–33. doi: 10.1016/j.clinph.2024.12.015
133. Bjekić, J, Živanović, M, Stanković, M, Paunović, D, Konstantinović, U, and Filipović, SR. The subjective experience of transcranial electrical stimulation: a within-subject comparison of tolerability and side effects between tDCS, tACS, and otDCS. Front Hum Neurosci. (2024) 18:1468538. doi: 10.3389/fnhum.2024.1468538
134. Liu, C, Chen, S, Zhang, Y, Wu, X, and Liu, J. Transcutaneous auricular vagus nerve stimulation (taVNS) for insomnia disorder: a narrative review of effectiveness, mechanisms and recommendations for clinical practice. Nat Sci Sleep. (2025) 17:1327–44. doi: 10.2147/NSS.S515809
135. Duan, Y, Zhao, P, Liu, S, Wu, S, Deng, Y, Xu, Z, et al. Patient-reported outcomes and acupuncture-related adverse events are overlooked in acupuncture randomised controlled trials: a cross-sectional meta-epidemiological study. BMJ Evid Based Med. (2025) 2025:497. doi: 10.1136/bmjebm-2024-113497
Keywords: chronic insomnia, acupuncture, neuromodulation, autonomic nervous system, corticolimbic functional connectivity, synergistic therapy, nonpharmacological intervention
Citation: Meng W, Mingqiang W, Junyang Z, Bin P, Qiong W and Chao W (2025) Synergistic acupuncture and neuromodulation for chronic insomnia: a structured narrative review with systematic search and future directions. Front. Neurol. 16:1663585. doi: 10.3389/fneur.2025.1663585
Edited by:
Jason H. Huang, Baylor Scott and White Health, United StatesReviewed by:
Jieying Zhang, First Teaching Hospital of Tianjin University of Traditional Chinese Medicine, ChinaLi Sheng, Southwest Medical University, China
Copyright © 2025 Meng, Mingqiang, Junyang, Bin, Qiong and Chao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wu Qiong, d3VqdW5lMjAyMUAxMjYuY29t; Wang Chao, d2FuZ2NoYW8xOTg5MTIxNkAxNjMuY29t
†These authors have contributed equally to this work and share first authorship
 Wang Meng
Wang Meng Wang Mingqiang1†
Wang Mingqiang1†