- 1Department of Neurosurgery, The First Affiliated Hospital of Henan Medical University, Xinxiang, Henan, China
- 2Center for Surgical Oncology, Xinjiang Medical University Affiliated Cancer Hospital, Xinjiang, China
Background: The incidence of postoperative complications following cranioplasty (CP) procedures remains relatively high, which has a significant impact on patient prognosis. While current research on predictive factors for complications has focused primarily on patient demographics, the timing of surgery and material selection, the association between skin flap shift and complications has yet to be systematically evaluated.
Objective: To investigate the correlation between skin flap shift and postoperative complications following CP.
Methods: A cohort of patients undergoing CP was enrolled and categorized into postoperative complication and no-complication groups. First, we conducted a univariate analysis on the following variables: age; gender; medical history; and surgical variables. Variables with a p-value of ≤0.2 in the univariate analysis were included in the multivariate logistic regression analysis. For the continuous variables, ROC curves were used to determine the optimal cut-off values for predicting complications. These values were then converted into binary variables for the multivariate analysis.
Results: Univariate analysis demonstrated that the differences in the materials utilized for repair, intraoperative blood loss, and skin flap shift between the two groups were statistically significant. The optimal cutoff values for intraoperative blood loss and skin flap shift, as determined by ROC curve analysis, were identified as 175 mL and 13.55 mm, respectively. Multivariate logistic regression analysis identified skin flap shift to be independently associated with postoperative complications after CP. (OR: 3.239, 95% CI: [1.450–7.237], p = 0.004). The area under the curve for predicting postoperative complications based on skin flap shift was 0.719 (95%CI: 0.646–0.797).
Conclusion: Skin flap shift was independently associated with postoperative complications following CP surgery. Patients with flap displacements exceeding 13.55 mm are at an increased risk of experiencing such complications.
1 Introduction
Craniectomy decompression is currently one of the primary neurosurgical interventions for the alleviation of elevated intracranial pressure and the saving of patients’ lives (1). This procedure, involving the excision of a cranial bone flap and craniotomy, is employed in patients with severe traumatic brain injury and significant cerebral oedema or swelling (2). The treatment is effective in reducing intracranial pressure and preventing further neurological damage, with a reported high success rate (3–5). However, it must be noted that the treatment inevitably results in a cranial defect. Cranioplasty (CP) is defined as the surgical procedure of filling and repairing the defect left after decompressive craniectomy using various reconstructive materials. It is currently one of the most routine procedures in neurosurgery. A growing body of evidence indicates that cranial reconstruction not only restores the morphology of the cranial cavity, achieving aesthetic restoration, but also plays a significant role in the recovery of the patient’s neurological function (6).
Although the surgical technique for cranioplasty is well-established and the optimal timing for repair is widely accepted, postoperative complications can vary significantly. These include superficial or deep surgical site infections, poor wound healing, tissue non-union, seroma or hematoma formation beneath the galea aponeurotica or bone flap, cranial bone resorption, exposure of the graft material, various delayed haematomas, seromas, cerebral oedema or cerebral infarction, and new-onset epilepsy (7). Severe complications necessitate reoperation or removal of the bone flap followed by re-repair, while systemic complications may lead to poor patient prognosis or even death (8).
Recent studies on predictive factors for cranial repair complications have predominantly focused on patient demographics, comorbidities, timing of surgery, defect size, implant material selection, and intraoperative techniques (9). Nevertheless, systematic research on the relationship between skin flap shift and postoperative complications remains limited. Research has indicated that bone window depression is an independent risk factor for complications arising in the context of cranial repair (10). Abnormal flap displacement has been demonstrated to be closely associated with postoperative dead space formation, local haemodynamic impairment, and altered biomechanical conditions (11). This has been shown to become a significant precipitating factor for complications such as subcutaneous fluid accumulation and infection. The present study utilized a cohort of 148 CP patients to investigate the relationship between skin flap shift and postoperative complications following CP (12).
2 Materials and methods
2.1 Sample sources
Patients treated at the Department of Neurosurgery at the First Affiliated Hospital of Henan Medical University between July 2018 and June 2025 were selected for this study. Patients who met the following inclusion criteria were deemed eligible for participation: (i) patients who underwent elective titanium mesh CP after first receiving decompressive craniectomy; (ii) possession of comprehensive preoperative thin-slice cranial CT three-dimensional reconstruction imaging data, enabling precise measurement of skin flap shift; (iii) complete clinical documentation; (iiii) signed informed consent. Exclusion criteria are as follows: (i) presence of systemic diseases severely impairing healing (immunodeficiency disorders, long-term immunosuppressant or glucocorticoid use, connective tissue diseases); (ii) pre-existing neurological deficits or infection; (iii) active infection in the surgical area, excessive flap tension, or significant vascular compromise prior to surgery; (iiii) refusal of follow-up by the patient or their family. Based on these criteria, 148 patients were ultimately included: The cohort consisted of 110 males and 38 females, with a median age of 52 years (interquartile range [IQR], 42–58) (Figure 1).

Figure 1. Diagram of the selected patients with cranioplasty from July 2018 and June 2025 included for analysis.
2.2 Variables
The medical records system collects patient age, gender, medical history (diabetes, hypertension) and the clinical context for craniectomy (trauma, stroke). Surgical records were used to retrieve data on craniectomy characteristics (choice of CP material, size of cranial defect) and the presence and reasons for reintervention. The duration of surgery was determined based on hospital surgical records. The cranioplasty interval was defined as the difference between the cranioplasty date and the date of decompressive craniectomy.
In our study, all patients underwent a standardized three-month postoperative clinical follow-up. This is consistent with commonly used short-term outcome windows in cranioplasty research, where early complications such as infection, hematoma, subgaleal effusion, wound breakdown, and hydrocephalus typically occur within the first 1–3 months after surgery (7). Complications that occurred within this predefined three-month period were recorded and included in the analysis. The complication group was defined by the development of any complication during follow-up, whereas the no-complication group included patients who remained free of all complications.
The measurement of skin flap shift was conducted at the image plane that exhibited the most pronounced deviation. All images employed for the measurement of skin flap shift were obtained 7 days prior to surgery and measured by two associate chief physicians, with excellent inter-observer reliability indicated by an intraclass correlation coefficient (ICC) of 0.923 (95% CI: 0.894–0.945, p < 0.001). Initially, the bony platform at the cranial defect site that was furthest horizontally from the midline was selected. A straight line parallel to the midline reference line was drawn from the centre of the defect margin. The skin flap shift was determined by measuring the distance between this line and the flap position at the midpoint of the craniectomy axis. In instances where the flap measurement point did not reach this vertical line (Figure 2A, +23.61 mm), it was deemed positive. Conversely, if the flap measurement point lay lateral to the reference line (Figure 2B, −13.38 mm), it was negative. Cerebral displacement was the underlying factor that resulted in the non-utilisation of the contralateral side as a reference benchmark.
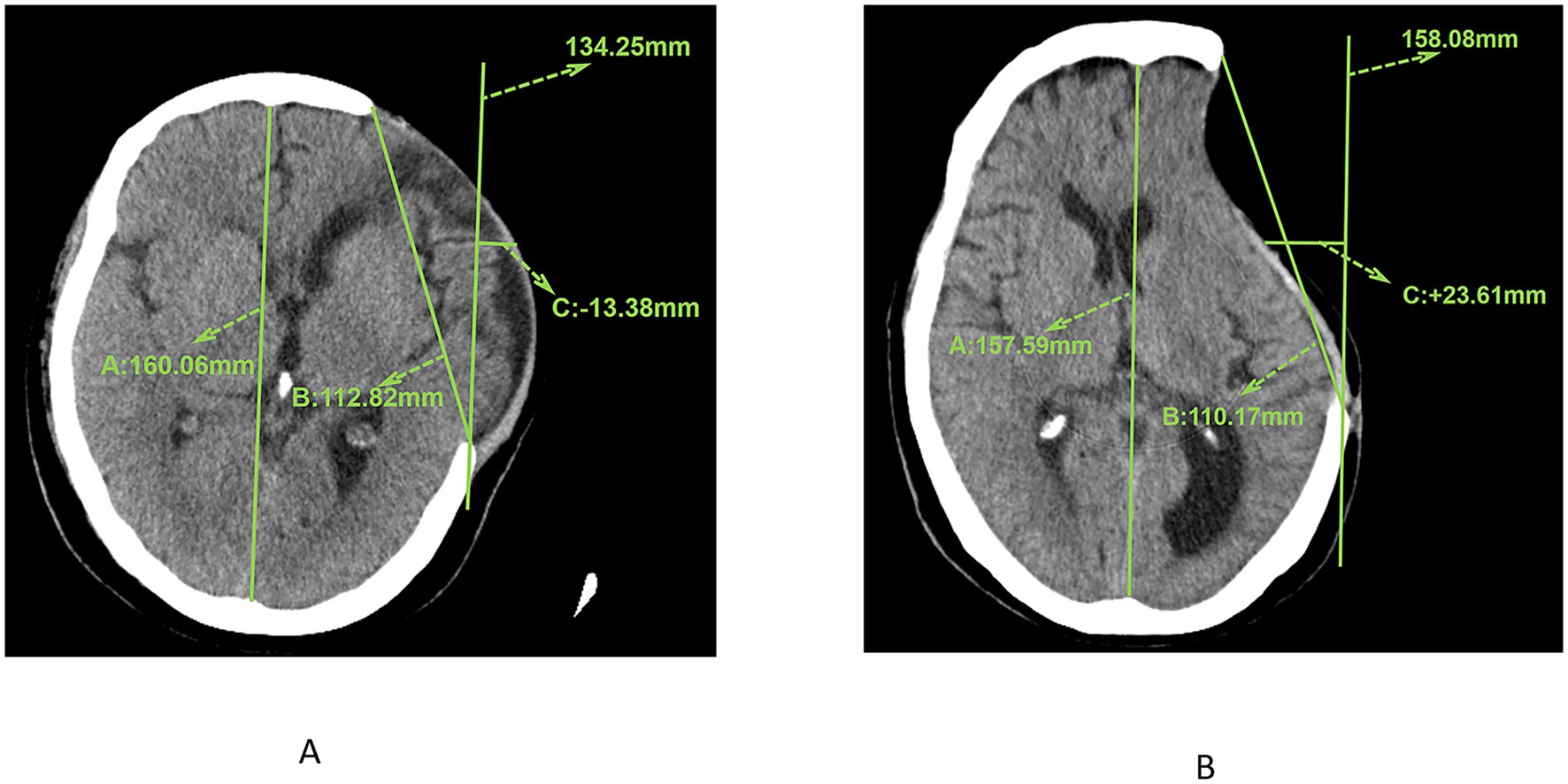
Figure 2. (A) CT image showing the measurement results of the maximum axial craniectomy size (110.17 mm) and skin flap shift (23.61 mm). (B) CT image showing the measurement results of the maximum axial craniectomy size (112.82 mm) and skin flap shift (13.38 mm).
2.3 Statistical analysis
Statistical analyses were conducted using SPSS version 26 (IBM Corp, Chicago, USA). Continuous variables were expressed as mean ± standard deviation and analyzed with Student’s t-test, while categorical data were summarized as frequencies and compared with the chi-square test. The normality of data distribution was assessed with the Kolmogorov–Smirnov test. For non-normally distributed continuous variables or ordinal data, the rank-sum test was applied. Single factor analysis with p ≤ 0.2 was included in the multi-factor analysis. In the context of continuous variables, the Receiver Operating Characteristic (ROC) curves were utilized to ascertain the optimal cutoff values, which were subsequently converted into binary variables. A p-value of less than 0.05 was considered statistically significant.
3 Results
3.1 Information on post-CP complications
Among the enrolled patients, 35.1% (52/148) experienced postoperative complications. The cases included one instance of scalp infection, three cases of new-onset hydrocephalus, one instance of epilepsy, one instance of subcutaneous effusion with epilepsy, two instances of cerebral haemorrhage, one instance of cerebral haemorrhage with subdural haemorrhage, one instance of cerebral infarction, one instance of hydrocephalus with subdural effusion, six instances of subcutaneous effusion, one instance of subcutaneous effusion with epidural haematoma, one instance of subcutaneous effusion with subdural haematoma, two instances of subcutaneous effusion with both epidural and subdural haematoma, one instance of subcutaneous effusion with subdural haematoma, three instances of subcutaneous effusion with subdural effusion, nine instances of epidural haematoma with subdural haematoma, and one instance of epidural haematoma. As illustrated in Table 1, the following cases were observed: five cases of hematoma, two cases of epidural effusion, seven cases of subdural haematoma, and three cases of subdural effusion.
3.2 Univariate analysis of complications after CP
No statistically significant differences were observed between the postoperative complication group and the non-complication group with regard to age, gender, epilepsy, hypertension, diabetes, repair interval duration, skin flap shift, displacement direction, or cranial bone resection size. Statistically significant differences were observed in relation to the type of repair material used, the volume of blood loss, and the skin flap shift (Table 2).
3.3 Multifactorial analysis of post-CP complications
Multivariate analysis incorporated epilepsy, graft material, skin flap shift, displacement direction, and displacement distance. Continuous variables underwent ROC curve analysis to determine optimal cut-off values, yielding 13.55 mm for displacement distance and 175 mL for blood loss. Prior to the final analysis, the transformed variables were examined for multicollinearity. The results, presented in Table 3, indicated that multicollinearity was not a concern in the model. After conversion into categorical variables for multivariate logistic regression, and following confirmation of adequate model fit by a non-significant Hosmer-Lemeshow test (p = 0.941), skin flap shift was independently associated with postoperative complications following CP (Table 4).
3.4 Assessment of the predictive value of skin flap shift based on ROC curve for complications after CP
Based on the multi-factor analysis, skin flap shift was classified into two categories, using the occurrence of complications after CP as the predictive indicator, and analyzed using the ROC curve. It was found that the area under curve was 0.719 (95%CI: 0.646–0.797) (Figure 3).
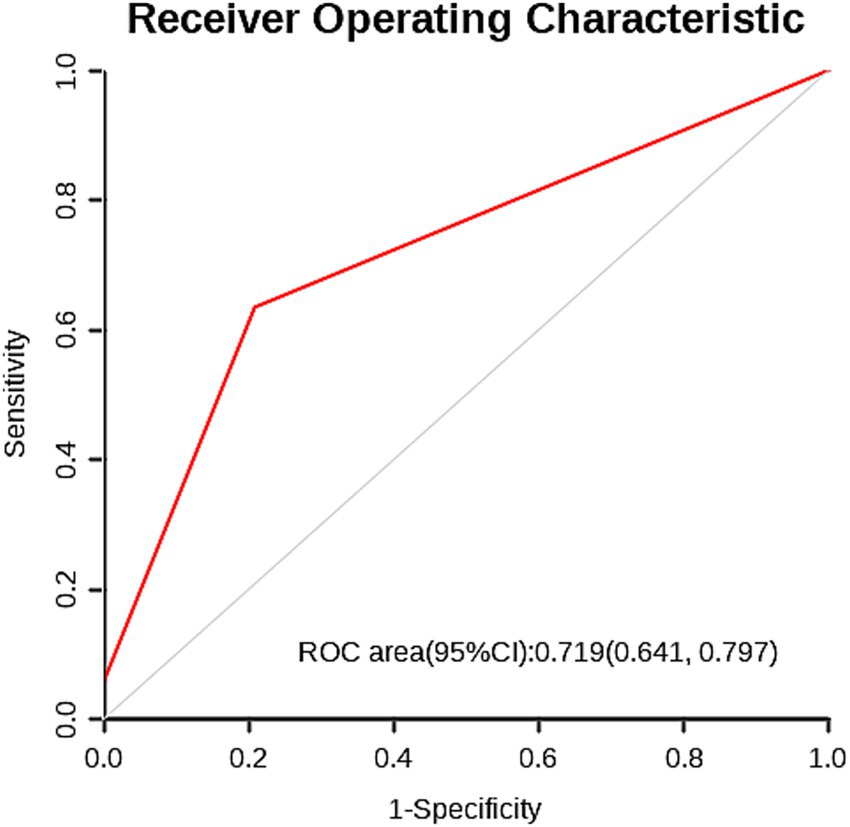
Figure 3. The ROC curve for predicting complications after CP based on flap displacement (cut-off value: 13.55 mm) shows an area under the curve of 0.719 (95% confidence interval: 0.641–0.797).
4 Discussion
Cranial reconstruction not only prevents secondary brain tissue damage caused by cranial defects but also alleviates the psychological burden patients experience due to such defects (13). Although certain postoperative complications following cranioplasty may be managed through pharmacological or conservative treatment without severe consequences, some patients remain susceptible to adverse effects despite active intervention (14). Consequently, the precise preoperative prediction of complication risks and the development of personalized treatment plans for high-risk patients hold significant clinical importance.
Postoperative skin flap shift following CP is not merely a morphological alteration, but rather the core mechanical initiating factor triggering a series of complications (15). Its key hazards lie in not only potentially compromising the anatomical integrity of the surgical site, leading to localized blood supply insufficiency or even tissue necrosis, but also increasing the risk of infection, fluid accumulation, and neurological dysfunction. This study’s univariate analysis first established an association between flap displacement and postoperative complications following CP (16). Subsequently, a ROC curve determined the optimal cutoff value for skin flap shift. This value was then converted into a binary variable and incorporated into a multivariate logistic regression model, ultimately confirming that skin flap shift is independently associated with postoperative complications following CP.
These observations align with previous literature showing that reduced flap perfusion, excessive tension, and impaired venous drainage significantly increase infection risk and poor wound healing. Studies using laser-Doppler flowmetry and skin-perfusion imaging have demonstrated that even modest increases in flap tension can reduce microcirculatory flow by 20–40%, promoting tissue breakdown and fluid accumulation (17). Although such physiological data were unavailable in the present study, the displacement distance observed on preoperative CT may serve as a practical surrogate for tension-induced perfusion impairment.
The objective of ideal CP is to achieve precise alignment between the flap and bone window margins, thereby restoring cranial cavity integrity (18). However, any displacement of the flap, whether concave or convex, inevitably results in the creation of dead space. This area of dead space is susceptible to accumulation of blood, serous fluid, and inflammatory exudate (19). The consequence of this is twofold: firstly, it provides a favorable environment for bacterial proliferation, and secondly, it disrupts the normal fluid dynamics within the cranial cavity (19). It has been hypothesized that this may result in impaired cerebrospinal fluid circulation and absorption, which may consequently increase the risk of postoperative infection and subcutaneous fluid/haematoma accumulation (20).
Beyond the absolute displacement distance, the biomechanical behavior of the scalp flap may vary depending on the pattern of movement. In the domain of reconstructive surgery, the deformation of flaps can entail advancement, rotation, or transposition components. These variations in deformation result in distinct vectors of tension and vascular stretch (21). Although routine cranial CT cannot fully delineate these dynamics, certain radiologic features may provide indirect clues. Findings such as asymmetric thickening, contour bulging, or directional drift toward the defect can suggest altered biomechanical behavior. Intra-operatively, most displaced flaps demonstrated an advancement-dominant deformation pattern. Rotation or transposition type displacement was observed less frequently. These variations may influence factors such as perfusion, deep-tissue shear stress, and dead space formation. As a result, they may contribute to postoperative fluid accumulation or infection (22). Future imaging based biomechanical analyses are warranted to determine whether specific displacement patterns confer differential risk.
The initial hypothesis that the direction of skin flap shift would correlate with postoperative complications was not supported by the data. Specifically, in the univariate analysis, no significant association was found between the direction of shift and the incidence of complications (χ2 = 1.81, p = 0.179). Similarly, in the multivariate logistic regression analysis that adjusted for potential confounders related to patients, yielded a non-significant odds ratio for the direction of shift (OR = 1.65, 95% CI: 0.75–3.64, p = 0.207). These results indicate that, based on the current dataset, the direction of flap shift does not appear to be a significant predictor of postoperative complications. However, this lack of significance may be attributable to study limitations. Firstly, the limited sample size precluded further subtyping of postoperative CP complications, potentially masking direction-specific effects. Secondly, specific skin flap shift directions may correlate with particular subtypes, which could not be adequately explored. Thirdly, current definitions and measurement methods for skin flap shift direction require refinement to improve precision and reproducibility. It is recommended that future studies be conducted to explore the association between flap displacement direction and postoperative CP complications. Such studies would be improved by the expansion of sample sizes and subtyping of complications. In subsequent work, our team will undertake a more thorough validation of this hypothesis.
The present study incorporated two additional elements into the analysis. Firstly, the direction of skin flap shift was taken into consideration. Secondly, the absolute value of skin flap shift, measured by CT, was incorporated. The findings of the study indicated a substantial correlation with postoperative complications following CP: patients with skin flap shift distances exceeding 13.55 mm demonstrated a 3.239-fold higher incidence of postoperative complications compared to those with displacement distances ≤13.55 mm. From a clinical standpoint, these findings carry several important technical implications. A tension free scalp closure is essential to prevent vascular congestion and reduce postoperative fluid accumulation. Preserving the vascular pedicle during flap elevation is also critical, as excessive manipulation may impair perfusion. In addition, meticulous hemostasis before closure remains a key strategy in minimizing complications, particularly in patients with marked preoperative flap displacement. The use of three-dimensional navigation systems and patient specific implants may further enhance contour matching, reduce dead space, and decrease mechanical stress on the flap. Collectively, these approaches may help lower complication rates in patients with pronounced flap shift. Future large scale quantitative studies are needed to develop risk-stratification models for flap displacement across different brain regions. Such research will help generate more precise evidence and support the standardization of CP surgical procedures.
Furthermore, the study identified several additional variables that exhibited no statistically significant association with complications following cranioplasty. These significant negative findings necessitate further investigation. In univariate analysis, no significant differences were demonstrated between the complication group and the non-complication group with respect to age or pre-existing conditions. This finding is in contrast with the results of previous studies which suggested an increased surgical risk with advancing age, a phenomenon that may be attributable to age-related declines in tissue repair capacity and immune function (23–25). However, the median age of the cohort was 52 years, with a narrow interquartile range (42–58), indicating minimal variability in age-related risk factors that may have obscured potential associations.
There was no significant association with comorbidities or complications such as epilepsy, hypertension, or diabetes. This finding is somewhat unexpected, given the widely recognized impact of diabetes on wound healing and increased infection risk in surgical populations (26). Hypertension and epilepsy, on the other hand, are associated with broader cardiovascular and neurological vulnerability (27, 28). A potential explanation for this phenomenon may be found in the study’s inclusion criteria. Patients with systemic diseases that severely impair healing were excluded from the study, potentially homogenizing the comorbidity burden within the cohort and thereby diminishing the impact of these conditions. Furthermore, rigorous perioperative management may have mitigated these risks, underscoring the role of clinical interventions in counteracting the harms associated with comorbidities.
The present study is subject to the following limitations: Firstly, the sample size was relatively small, and the conclusions drawn require further validation in large-scale cohort studies. Secondly, given that all research data originated from a single center, there is a possibility that selection bias may have been introduced; the representativeness of the data requires assessment through multicenter prospective validation with standardized imaging-based measurements and stratified analyses across different displacement patterns. Thirdly, the present study was limited to the inclusion of short-term complications. As late bone flap resorption or delayed infection may manifest several months to years following cranioplasty (25), further long-term studies are warranted to explore the correlation between the uniqueness of skin flap shift and long-term complications.
5 Conclusion
Our retrospective analysis reveals an independent association between the distance of skin flap shift and postoperative complications after CP surgery, positioning it as a potential novel predictor. This observation implies a correlation that merits further investigation in preoperative assessments. Consequently, it is hypothesized that for patients with a skin flap shift greater than 13.55 mm, enhanced perioperative management might be considered, with the aim of potentially improving outcomes.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by The First Affiliated Hospital of Henan Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
SL: Investigation, Project administration, Writing – original draft. RD: Methodology, Resources, Writing – review & editing. YL: Investigation, Project administration, Writing – original draft. LM: Investigation, Resources, Writing – original draft. WZ: Conceptualization, Funding acquisition, Project administration, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declared that financial support was not received for this work and/or its publication.
Acknowledgments
We express our gratitude to patients who participated in the study for their patience and cooperation. We also thank Bullet Edits Limited for the language editing assistance.
Conflict of interest
The author(s) declared that this work was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declared that Generative AI was not used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Hutchinson, PJ, Adams, H, Mohan, M, Devi, BI, Uff, C, Hasan, S, et al. Decompressive craniectomy versus craniotomy for acute subdural hematoma. N Engl J Med. (2023) 388:2219–29. doi: 10.1056/NEJMoa2214172,
2. Beez, T, Munoz-Bendix, C, Steiger, HJ, and Beseoglu, K. Decompressive craniectomy for acute ischemic stroke. Crit Care. (2019) 23:209. doi: 10.1186/s13054-019-2490-x,
3. Timofeev, I, Czosnyka, M, Nortje, J, Smielewski, P, Kirkpatrick, P, Gupta, A, et al. Effect of decompressive craniectomy on intracranial pressure and cerebrospinal compensation following traumatic brain injury. J Neurosurg. (2008) 108:66–73. doi: 10.3171/JNS/2008/108/01/0066,
4. Oertel, M, Kelly, DF, Lee, JH, McArthur, DL, Glenn, TC, Vespa, P, et al. Efficacy of hyperventilation, blood pressure elevation, and metabolic suppression therapy in controlling intracranial pressure after head injury. J Neurosurg. (2002) 97:1045–53. doi: 10.3171/jns.2002.97.5.1045,
5. Olivecrona, M, Rodling-Wahlström, M, Naredi, S, and Koskinen, LO. Effective ICP reduction by decompressive craniectomy in patients with severe traumatic brain injury treated by an ICP-targeted therapy. J Neurotrauma. (2007) 24:927–35. doi: 10.1089/neu.2005.356E,
6. Yang, J, Sun, T, Yuan, Y, Li, X, Yu, H, and Guan, J. Evaluation of titanium cranioplasty and polyetheretherketone cranioplasty after decompressive craniectomy for traumatic brain injury: a prospective, multicenter, non-randomized controlled trial. Medicine. (2020) 99:e21251. doi: 10.1097/MD.0000000000021251,
7. Ferreira, A, Viegas, V, Cerejo, A, and Silva, PA. Predictive factors for cranioplasty complications - a decade's experience. Brain Spine. (2024) 4:102925. doi: 10.1016/j.bas.2024.102925,
8. Sahoo, NK, Tomar, K, Thakral, A, and Rangan, NM. Complications of cranioplasty. J Craniofac Surg. (2018) 29:1344–8. doi: 10.1097/SCS.0000000000004478,
9. Bader, ER, Kobets, AJ, Ammar, A, and Goodrich, JT. Factors predicting complications following cranioplasty. J Cranio Maxillo Facial Surg. (2022) 50:134–9. doi: 10.1016/j.jcms.2021.08.001,
10. Yao, S, Zhang, Q, Mai, Y, Yang, H, Li, Y, Zhang, M, et al. Outcome and risk factors of complications after cranioplasty with polyetheretherketone and titanium mesh: a single-center retrospective study. Front Neurol. (2022) 13:926436. doi: 10.3389/fneur.2022.926436,
11. Bura, V, Visrodia, P, Bhosale, P, Faria, SC, Pintican, RM, Sharma, S, et al. MRI of surgical flaps in pelvic reconstructive surgery: a pictorial review of normal and abnormal findings. Abdom Radiol. (2020) 45:3307–20. doi: 10.1007/s00261-019-02211-z,
12. von Elm, E, Altman, DG, Egger, M, Pocock, SJ, Gøtzsche, PC, and Vandenbroucke, JP. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. (2007) 370:1453–7. doi: 10.1016/S0140-6736(07)61602-X,
13. Li, J, Li, N, Jiang, W, and Li, A. The impact of early cranioplasty on neurological function, stress response, and cognitive function in traumatic brain injury. Medicine. (2024) 103:e39727. doi: 10.1097/MD.0000000000039727,
14. Klinger, DR, Madden, C, Beshay, J, White, J, Gambrell, K, and Rickert, K. Autologous and acrylic cranioplasty: a review of 10 years and 258 cases. World Neurosurg. (2014) 82:e525–30. doi: 10.1016/j.wneu.2013.08.005,
15. Goel, A, Dindorkar, K, Desai, K, and Muzumdar, D. Migration of craniotomy flap: an unusual complication. J Postgrad Med. (1997) 43:17–8.
16. Pedroza Gómez, S, Gómez Ortega, V, Tovar-Spinoza, Z, and Ghotme, KA. Scalp complications of craniofacial surgery: classification, prevention, and initial approach: an updated review. Eur J Plast Surg. (2023) 46:315–25. doi: 10.1007/s00238-022-02008-2,
17. Chung, J, Lee, S, Park, JC, Ahn, JS, and Park, W. Scalp thickness as a predictor of wound complications after cerebral revascularization using the superficial temporal artery: a risk factor analysis. Acta Neurochir. (2020) 162:2557–63. doi: 10.1007/s00701-020-04500-9,
18. Xie, BS, Wang, FY, Zheng, SF, Lin, YX, Kang, DZ, and Fang, WH. A novel titanium cranioplasty technique of marking the coronal and Squamosoparietal sutures in three-dimensional titanium mesh as anatomical positioning markers to increase the surgical accuracy and reduce postoperative complications. Front Surg. (2021) 8:754466. doi: 10.3389/fsurg.2021.754466,
19. Joo, JK, Choi, JI, Kim, CH, Lee, HK, Moon, JG, and Cho, TG. Initial dead space and multiplicity of bone flap as strong risk factors for bone flap resorption after cranioplasty for traumatic brain injury. Korean J Neurotrauma. (2018) 14:105–11. doi: 10.13004/kjnt.2018.14.2.105,
20. Paredes, I, Lagares, A, San-Juan, R, Castaño-León, AM, Gómez, PA, Jimenez-Roldán, L, et al. Reduction in the infection rate of cranioplasty with a tailored antibiotic prophylaxis: a nonrandomized study. Acta Neurochir. (2020) 162:2857–66. doi: 10.1007/s00701-020-04508-1,
21. Tepole, AB, Gosain, AK, and Kuhl, E. Computational modeling of skin: using stress profiles as predictor for tissue necrosis in reconstructive surgery. Comput Struct. (2014) 143:32–9. doi: 10.1016/j.compstruc.2014.07.004,
22. Machado, P, Le, T, Rozen, WM, Hunter-Smith, DJ, and Niumsawatt, V. An optimal scalp rotation flap design: mathematical and bio-mechanical analysis. JPRAS Open. (2025) 43:251–64. doi: 10.1016/j.jpra.2024.10.007,
23. Göttsche, J, Fritzsche, F, Kammler, G, Sauvigny, T, Westphal, M, and Regelsberger, J. A comparison between Pediatric and adult patients after cranioplasty: aseptic bone resorption causes earlier revision in children. J Neurol Surg Part A Central Europ Neurosurg. (2020) 81:227–32. doi: 10.1055/s-0039-1698391,
24. Armstrong, RE, and Ellis, MF. Determinants of 30-day morbidity in adult cranioplasty: an ACS-NSQIP analysis of 697 cases. Plast Reconstr Surg Glob Open. (2019) 7:e2562. doi: 10.1097/GOX.0000000000002562,
25. Li, A, Azad, TD, Veeravagu, A, Bhatti, I, Long, C, Ratliff, JK, et al. Cranioplasty complications and costs: a national population-level analysis using the MarketScan longitudinal database. World Neurosurg. (2017) 102:209–20. doi: 10.1016/j.wneu.2017.03.022,
26. Alfonso, AR, Kantar, RS, Ramly, EP, Daar, DA, Rifkin, WJ, Levine, JP, et al. Diabetes is associated with an increased risk of wound complications and readmission in patients with surgically managed pressure ulcers. Wound Repair Regenerat. (2019) 27:249–56. doi: 10.1111/wrr.12694,
27. Nam, HH, Ki, HJ, Lee, HJ, and Park, SK. Complications of cranioplasty following decompressive craniectomy: risk factors of complications and comparison between autogenous and artificial bones. Korean J Neurotrauma. (2022) 18:238–45. doi: 10.13004/kjnt.2022.18.e40,
Keywords: skin flap shift, cranioplasty, post-operative complications, predictive factors, cohort study
Citation: Liu S, Dang R, Li Y, Ma L and Zhou W (2025) Skin flap shift is associated with postoperative complications after cranioplasty: a retrospective cohort study. Front. Neurol. 16:1714893. doi: 10.3389/fneur.2025.1714893
Edited by:
Julius Höhne, Paracelsus Medical Private University, Nuremberg, GermanyReviewed by:
Daniel Dubinski, University Hospital Rostock, GermanyKemel A. Ghotme, Universidad de La Sabana, Colombia
Yu Chi Wang, Kaohsiung Medical University, Taiwan
Copyright © 2025 Liu, Dang, Li, Ma and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenke Zhou, emhvdXdlbmtlMTk5OUAxNjMuY29t
†These authors have contributed equally to this work and share first authorship
 Shangshuo Liu1†
Shangshuo Liu1† Wenke Zhou
Wenke Zhou


