- 1Department of Radiation Oncology, Affiliated Cancer Hospital and Institute of Guangzhou Medical University, Guangzhou, China
- 2Department of Oncology, First affiliated Hospital of Anhui Medical University, Hefei, China
- 3Department of Radiation Oncology, Sun Yat-sen University Cancer Center, Sun Yat-sen Medical University, Guangzhou, China
Background: ALK inhibitors have shown positive advance in the treatment of ALK+ NSCLC. They have achieved better results in prolonging the progression free survival and improving quality of life in comparison to chemotherapy. We have assembled the evidence related to the efficacy and safety of these agents in the treatment of ALK positive NSCLC.
Materials and Methods: A comprehensive search was conducted using electronic databases of PubMed, Medline and Cochrane Library to identify the studies involving comparison of ALK inhibitors to chemotherapy and Next generation ALK inhibitors to crizotinib. PFS was the primary outcome while other outcomes like ORR, adverse events, quality of life and OS were also analyzed and compared. Hazard ratios and odds ratios obtained were analyzed using fixed effect or random effects model in Review Manager Software.
Results: A total of 12 studies (n = 3,297) met the criteria for inclusion in this review and meta-analysis. ALK inhibitors including crizotinib, ceritinib and alectinib revealed significantly better PFS (HR 0.42 [0.35, 0.50; p < 0.00001]), ORR (Overall OR 6.59 [4.86, 8.94; p < 0.00001] as compared to chemotherapy in the first line as well as second line treatment settings. Intracranial response rate was better with ALK inhibitors (ceritinib and alectinib) as compared to chemotherapy OR 6.51 [2.86, 14.83; p < 0.00001]. No significant increase in grade 3 or 4 adverse events was observed with crizotinib (OR 1.21 [0.82, 1.77; p = 0.34]) or ceritinib (OR 1.49 [0.86, 2.57; p = 0.17]) when compared to chemotherapy individually. Quality of life indicators assessed were significantly improved with ALK inhibitors. Next generation agents (ceritinib, alectinib and brigatinib) revealed significant improvement in PFS (HR 0.50 [0.43, 0.57; p < 0.00001]), ORR (OR 1.57 [1.21, 2.04; p = 0.0006]) in comparison to crizotinib. Next generation agents (Alectinib and brigatinib) yielded better response intra-cranially than crizotinib in terms of objective response rate (OR 5.87 [3.49, 9.87; p < 0.00001]) and time to CNS progression (HR 0.25 [0.13, 0.46; p < 0.0001]). Alectinib by far resulted in fewer adverse events than chemotherapy or crizotinib.
Conclusions: Overall ALK inhibitors are safe and effective treatment option in ALK+ non-small cell lung cancer. Of the ALK inhibitors, Next generation agents in particular alectinib and brigatinib are safer and more effective intra-cranially and can be preferred as first option.
Introduction
Lung cancer is the cause of 1.5 million deaths every year with < 20% of 5 year-OS for newly diagnosed patients (1). Based upon the microscopic appearance of tumor cells, lung cancers are classified into two main types: small cell lung cancer (15–20%) and non-small cell Lung cancer (80–85%) (2). NSCLC are further subdivided into three main types: adenocarcinoma (50%), squamous cell carcinomas (30%) and large cell carcinomas. This classification is based upon the types of cells found in the tumor (3). Molecular and biological targets involved in cancer growth and survival (gene mutations, proteins and signaling pathways) have been identified with progress being made in the understanding of tumor biology (4). Gene mutations like EGFR gene mutation (10–15% nsclc), KRAS mutations (10–15% nsclc) and ALK gene rearrangement (5% nsclc); Proteins like Epidermal growth factor receptor (EGFR), abnormal ALK protein; are some of the targets in NSCLC that essentially have modernized the concept of personalized medicine (5). These newer developments have essentially lead to modern molecular classification of NSCLC particularly the histology cell type adenocarcinoma.
ALK gene alterations have been well reported to play a key role in the pathogenesis of several cancers (inflammatory myofibroblastic tumors and neuroblastomas) after it was first discovered in anaplastic large cell lymphoma and hence the name anaplastic lymphoma kinase (6, 7). In 2007, ALK gene rearrangement was discovered in NSCLC: for the first time in solid tumors (8, 9). This gene alteration resulted from inter-chromosomal inversions within the short arm of chromosome 2 [Inv(2)(p21p23)] joining the exons 1–13 of the echinoderm microtubule-associated protein-like 4 (EML4) gene to exons 20–29 of ALK gene. The resulting EML4-ALK protein, novel to NSCLC, contains N-terminal portion encoded by (EML4) gene and a C-terminal portion (intracellular signaling portion of the receptor tyrosine kinase) encoded by (ALK) gene (10).
Anaplastic lymphoma kinase gene rearrangement is present in 3–5% of the NSCLC. Clinical features associated with this distinct NSCLC subgroup included young age, non-smoking history and adenocarcinoma histology (11). Standard chemotherapy was used as the first line of therapy before EML4-ALK discovery. Upon discovery, Crizotinib, a tyrosine kinase inhibitor targeting MET, ROS1 and ALK entered phase I trial which reported 72% 6-month progression-free survival and overall response of 57% in ALK positive NSCLC (12). This trial had led to conditional approval by the FDA. A phase II study of Crizotinib in ALK positive NSCLC also reported a similar encouraging positive results (ORR 53% (95% CI: 47–60) & median PFS 8.5 months (95% CI: 6.2–9.9) (13). On the other hand, standard single agent chemotherapies generally have produced PFS of merely 2–3 months and a 10% response rates. Consequently, a phase III study reported comparative results of Crizotinib to single agent chemotherapy (premetexed or docetaxel) in previously treated NSCLC patients with former being significantly superior in median PFS (7.7–3.0 months; P < 0.001) and ORR (65–20%; P < 0.001) (14). Crizotinib has shown significant PFS and ORR in a phase III study compared to pemetrexed plus platinum chemotherapy in the first line setting as well (15).
Crizotinib however is reported with issues of resistance and relapse owing ALK dependent and independent mechanisms (16). Ceritinib, another oral ATP-competitive, a second-generation ALK tyrosine kinase inhibitor, is similar in action to Crizotinib without MET inhibiting ability. Initially indicated on progression of disease or resistance after Crizotinib use (17, 18). However, lately Ceritinib has been compared to chemotherapy and proven superior in first line as well as second line setting (19, 20). Alectinib, yet another ALK inhibitor, is a more potent ALK inhibitor with proven activity against crizotinib resistance ALK mutations. One important aspect of alectinib is its penetration of CNS. Alectinib has also shown its superiority over chemotherapy and crizotinib in recently concluded studies (21–23). A recently concluded study has also added brigatinib as a potential ALK inhibitor option to the list (24). Ceritinib, alectinib and brigatinib are termed as Next-generation agents. Newer agents are being developed which will ever expand the treatment options for ALK positive NSCLC.
The aim of this study was to assemble the available evidence of ALK inhibitors' efficacy and safety in the treatment of ALK positive NSCLC in order to provide clinicians and practitioners a better clinical picture. As well as, review and elaborate various therapeutic aspects of these agents.
Results
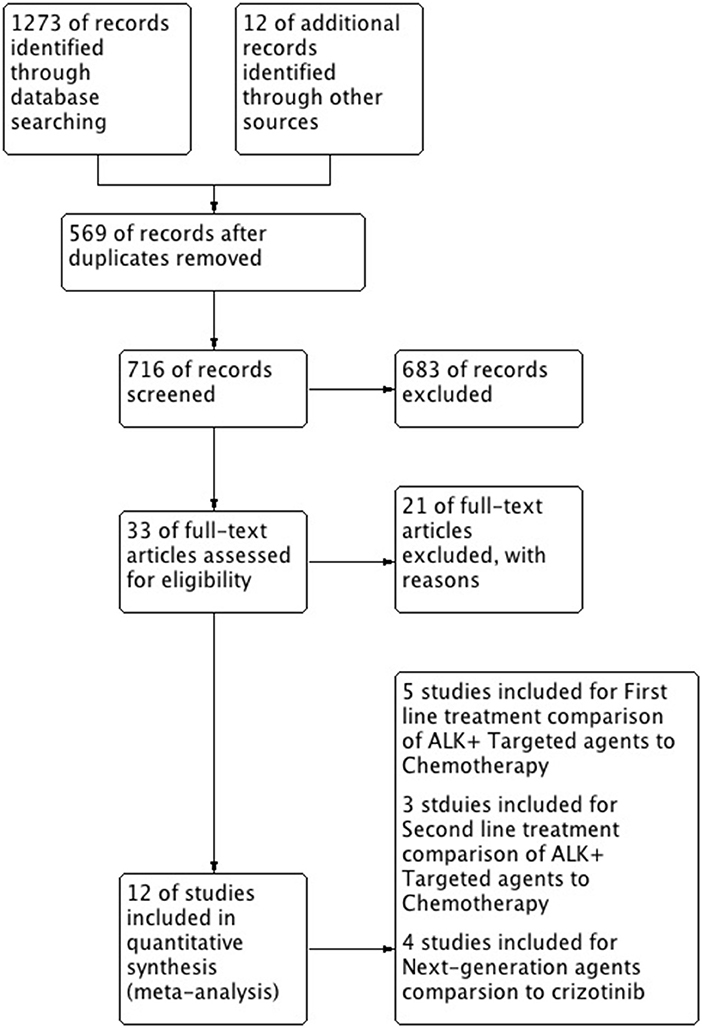
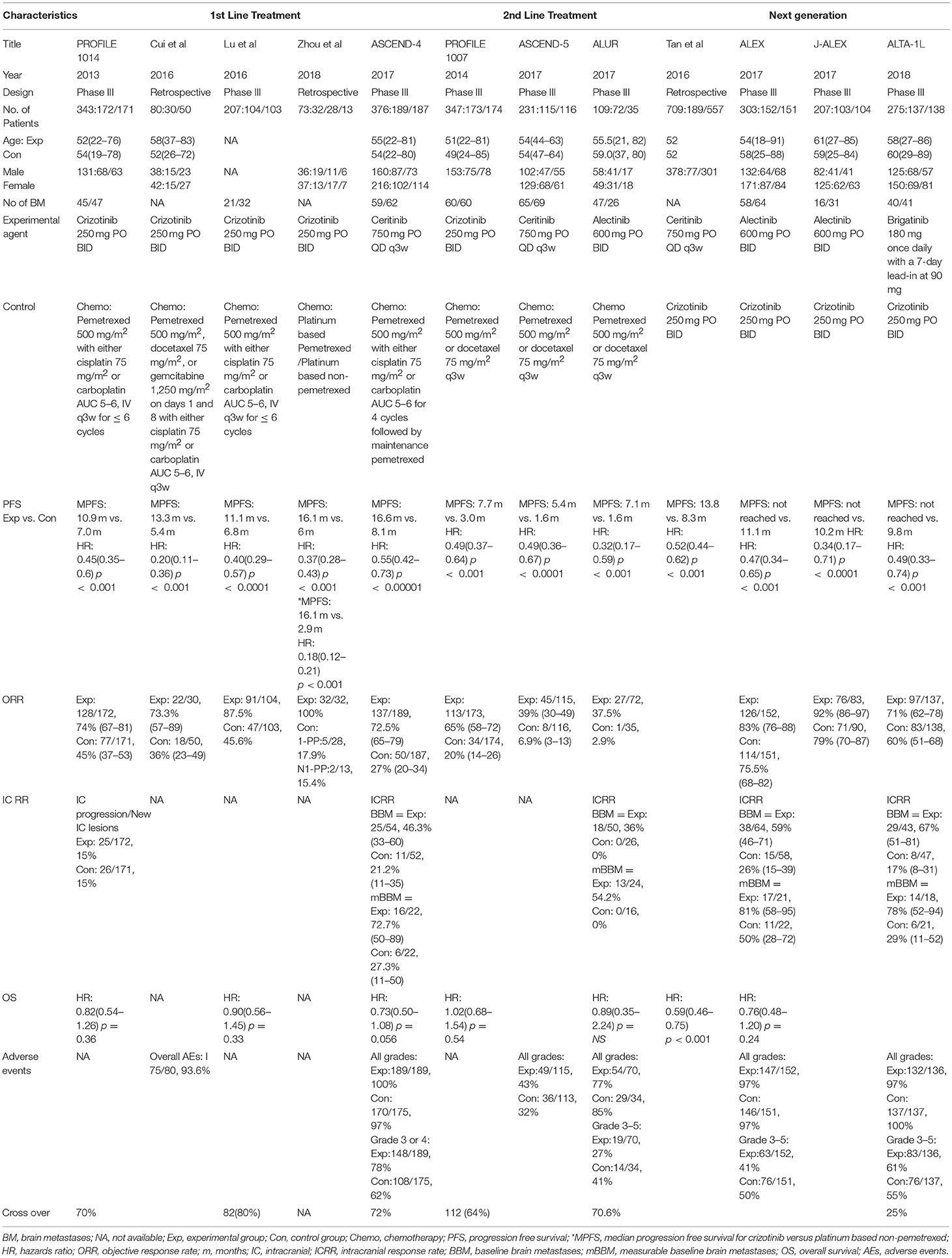
A total of 12 studies were included in this review and meta-analysis involving 3,297 patients (14, 15, 19–28). Results of research strategy and study selection is shown in Figure 1. First line comparison of ALK inhibitors vs. chemotherapy was based on 5 studies (n = 1,079, 4 studies included crizotinib/1 study of ceritinib vs. chemo) (15, 19, 25–27) while second line comparison was based on 3 studies (n = 687) (14, 20, 21). Furthermore, next generation agents (ceritinib, alectinib and brigatinib) were compared to crizotinib. Comparison was based on 4 studies involving 1,531 patients. One study was included for comparison of ceritinib to crizotinib (n = 746, a comparative study based on the data taken from five RCTs) (28). Two phase-III trials were identified for alectinib comparison to crizotinib and one study for comparison of brigatinib to crizotinib (22–24).
Overall, highly significant PFS was achieved with ALK inhibitors compared to chemotherapy (HR 0.42 [0.35, 0.50; p < 0.00001]) (Figure 2). Significant PFS with ALK inhibitors in the first line setting was based on 5 studies comprising 3 phase III trials and 2 retrospective studies. The Progression-free survival hazards ratio for ALK inhibitors (crizotinib 4 + ceritinib 1) to chemotherapy was 0.38 [0.29, 0.50; p < 0.00001]. Meta-analysis of ALK inhibitors in the second line setting also revealed significant PFS based on 3 phase III trials (crizotinib 1+ ceritinib1+ alectinib 1). Hazard to progression was 0.47 [0.39, 0.57; p < 0.00001]. Significant heterogeneity was observed and hence randome effects model was applied. Patients derived better progression free survival with next generation agents in comparison to crizotinib (HR 0.50 [0.43, 0.57; p < 0.00001]) (Figure 3). No heterogeneity was revealed among the studies.
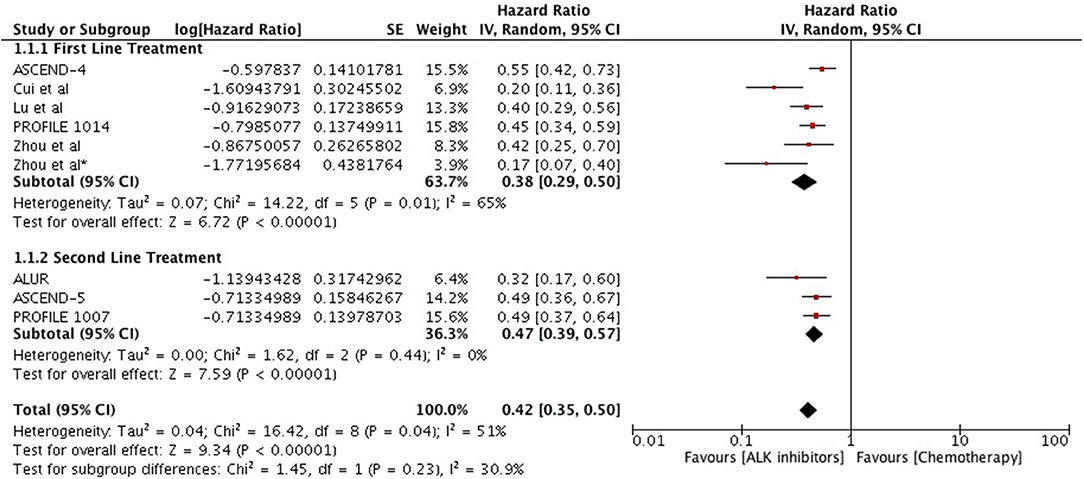
Figure 2. Forest plot of meta-analysis of the progression-free survival (PFS) showing comparison of ALK inhibitors to chemotherapy in ALK positive NSCLC.
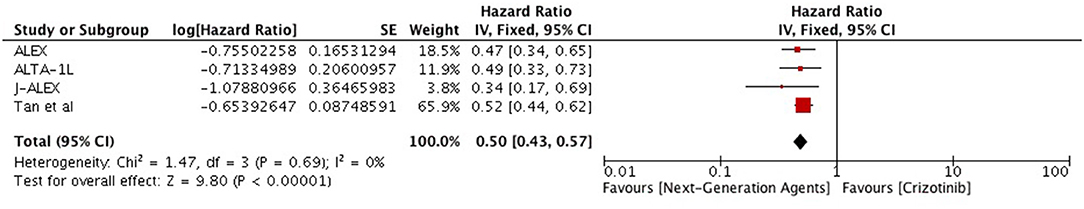
Figure 3. Forest plot of meta-analysis of the progression-free survival (PFS) showing comparison of Next generation agents to crizotinib in ALK positive NSCLC.
Objective response was higher as well with ALK inhibitors in the first line as well as second line treatment in comparison to chemotherapy (Overall OR 6.59 [4.86, 8.94; p < 0.00001] (Figure 4) with no significant heterogeneity. As first line treatment, odds of achieving objective response with ALK inhibitors were significantly higher (OR 6.72 [4.24, 10.63; p < 0.00001]. Results are based on data from 5 studies. A similar odds ratio was observed in the second line treatment comparison (OR 7.12 [4.82, 10.53; p < 0.00001]. Odds of achieving objective response were significantly higher with next generation agents compared to crizotinib (OR 1.57 [1.21, 2.04; p = 0.0006]) (Figure 5) with heterogeneity at 0%.
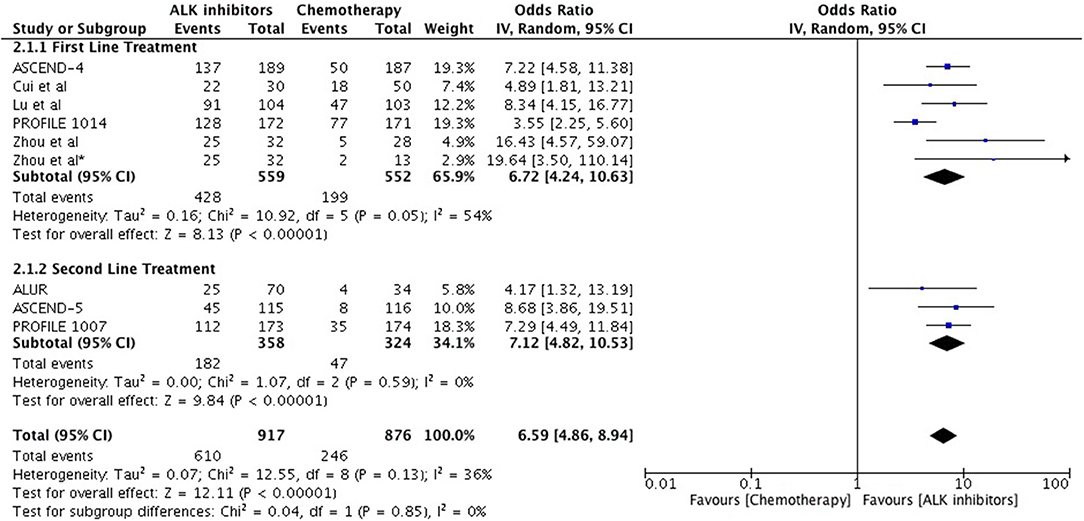
Figure 4. Forest plot of meta-analysis of the objective response rate (ORR) showing comparison of ALK inhibitors to chemotherapy in ALK positive NSCLC.
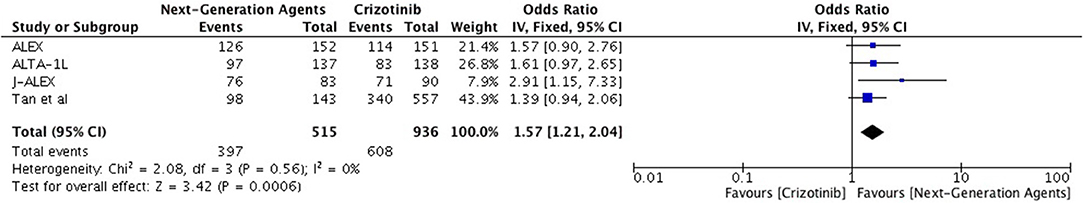
Figure 5. Forest plot of meta-analysis of the objective response rate (ORR) showing comparison of Next generation agents to crizotinib in ALK positive NSCLC.
Overall intracranial response was mainly obtained from studies comprising ceritinib and alectinib to chemotherapy. Patients responded better to ALK inhibitors in comparison to chemotherapy. A significant odds ratio was achieved without any heterogeneity among the studies (OR 6.51 [2.86, 14.83; p < 0.00001] (Figure 6). Indicators of response in CNS were in favor of alectinib and brigatinib in comparison to crizotinib. Odds of achieving intracranial response was significantly higher with these two agents (OR 5.87 [3.49, 9.87; p < 0.00001]) (Figure 7). HR for CNS progression was HR 0.25 [0.13, 0.46; p < 0.0001] for treatment difference in intention-to-treat population (Figure 8). Time to progression of brain metastatic lesion for patients with CNS disease at baseline was shorter (HR 0.25 [0.15, 0.42; p < 0.00001]) in comparison to no baseline CNS disease (HR 0.16 [0.07, 0.33; p < 0.00001]).
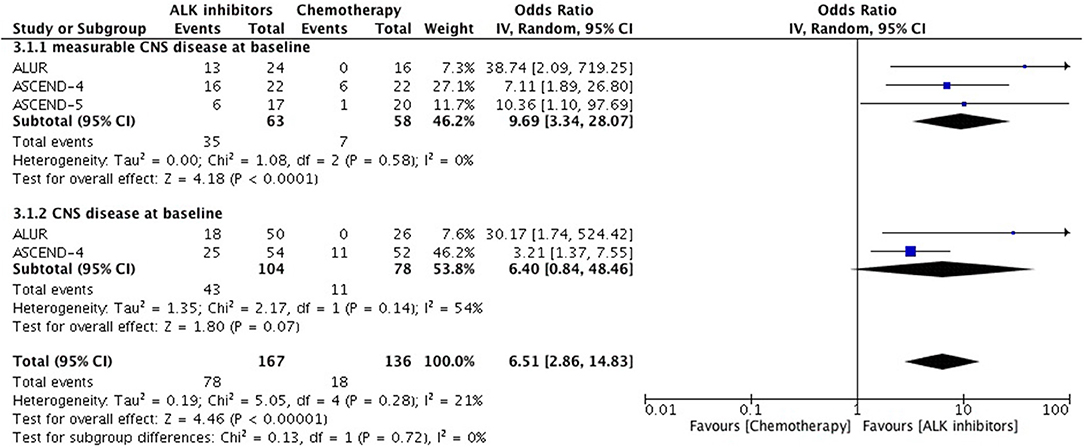
Figure 6. Forest plot of meta-analysis of the Intra-cranial response rate (ICRR) showing comparison of ALK inhibitors to chemotherapy in ALK positive NSCLC.
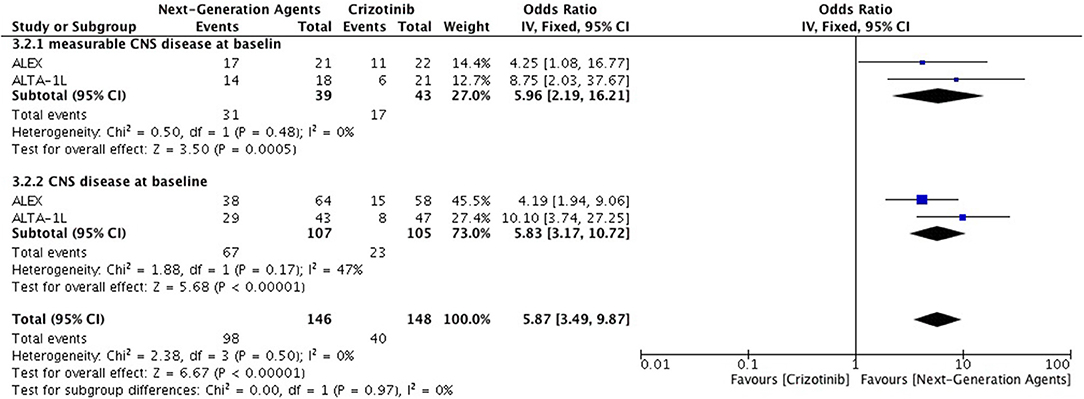
Figure 7. Forest plot of meta-analysis of the Intra-cranial response rate (ICRR) showing comparison of Next generation agents to crizotinib in ALK positive NSCLC.
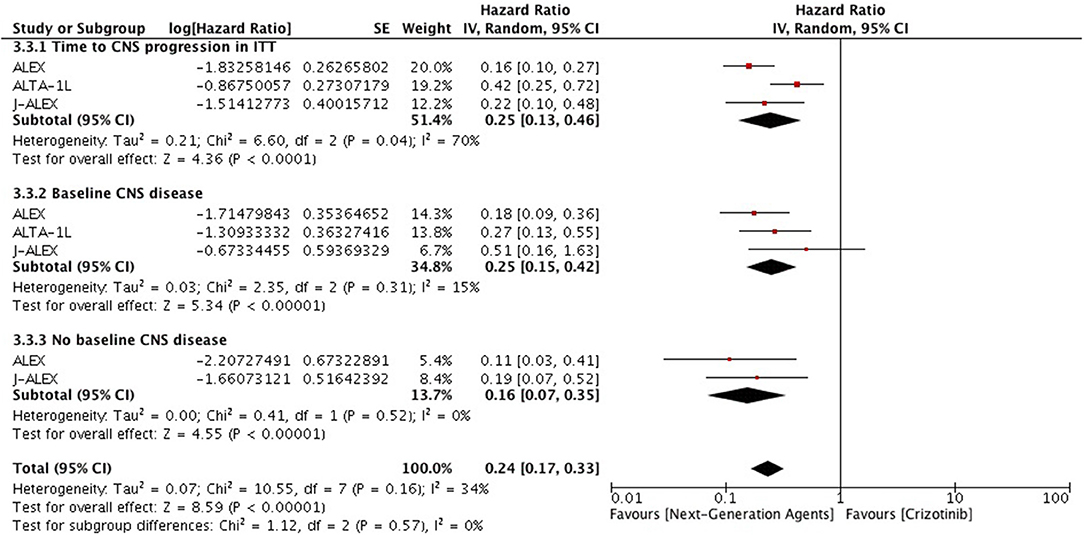
Figure 8. Forest plot of meta-analysis of the Time to CNS progression showing comparison of Next generation agents to crizotinib in ALK positive NSCLC.
Overall survival analysis revealed only numerical advantage over chemotherapy (HR 0.89 [0.74, 1.32; p = 0.19]) particularly in first line comparison (HR 0.80 [0.63, 1.02; p = 0.08] (Figure 9). Next generation ALK inhibitors revealed significant improvement in overall survival, however, result was based only on two studies (HR 0.62 [0.50, 0.77; p < 0.0001]) (Figure 10).
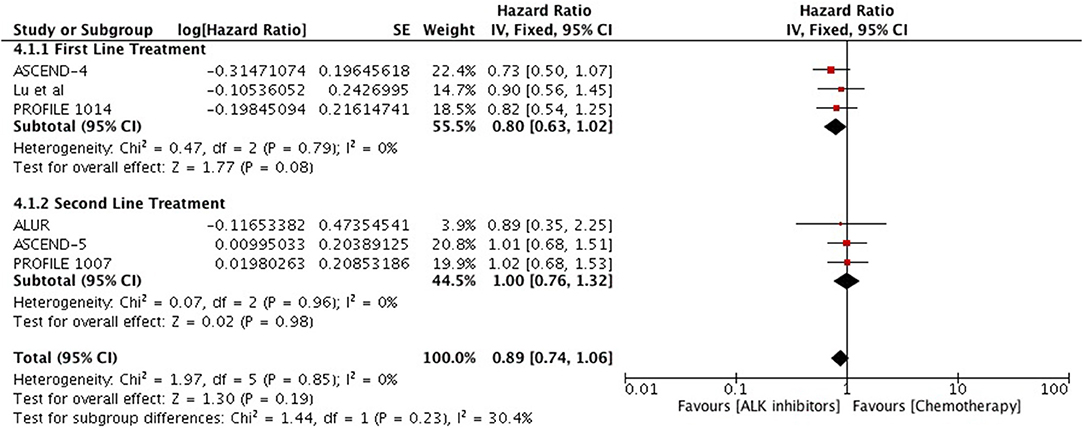
Figure 9. Forest plot of meta-analysis of the overall survival (OS) showing comparison of ALK inhibitors to chemotherapy in ALK positive NSCLC.
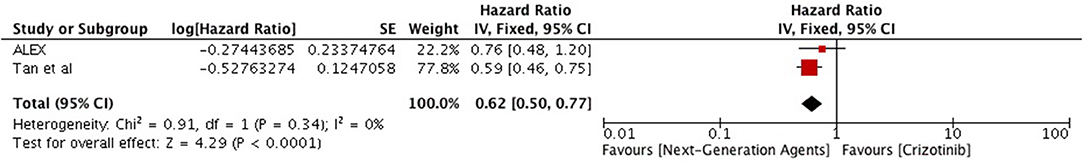
Figure 10. Forest plot of meta-analysis of the overall survival (OS) showing comparison of Next generation agents to crizotinib in ALK positive NSCLC.
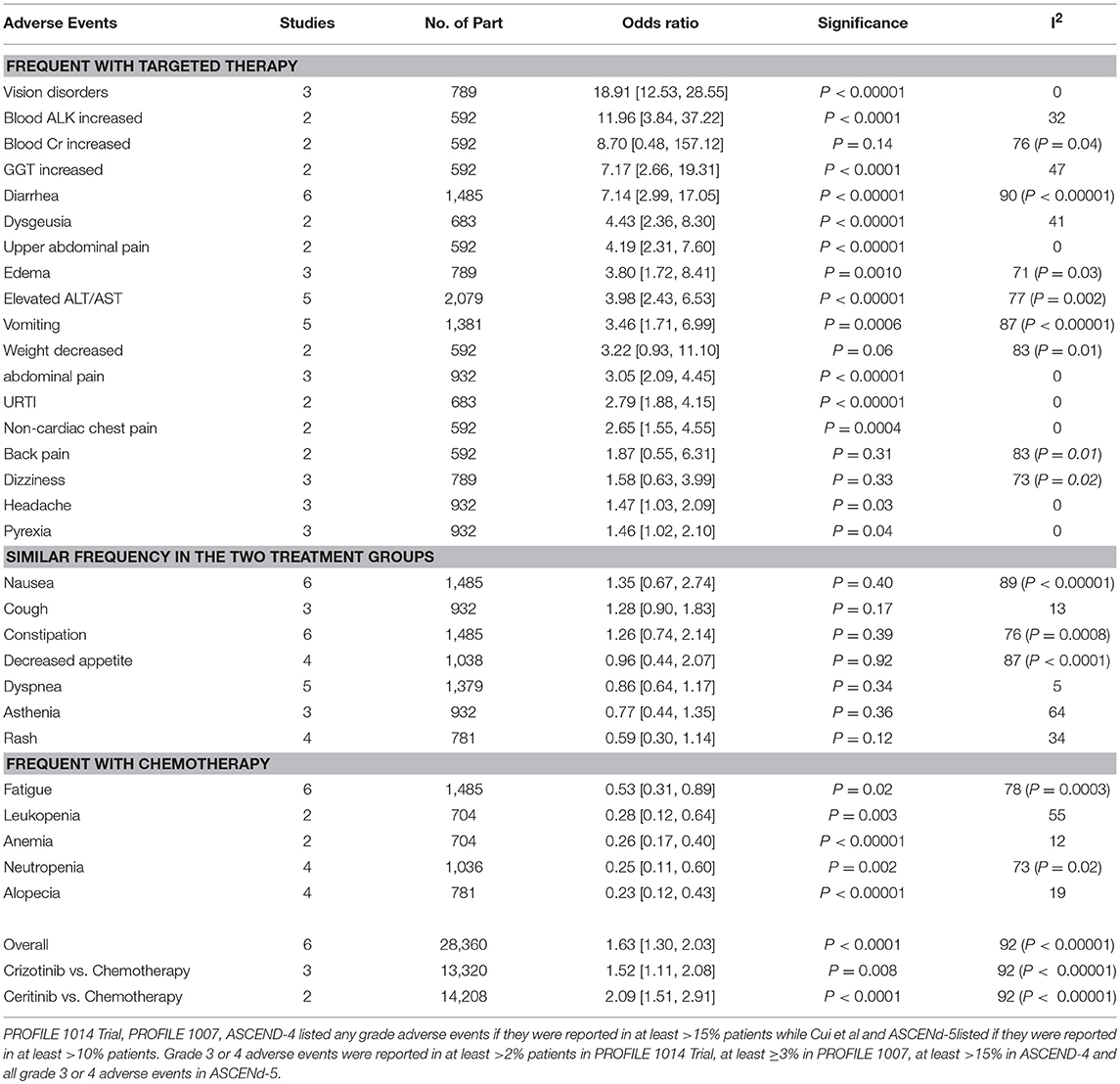
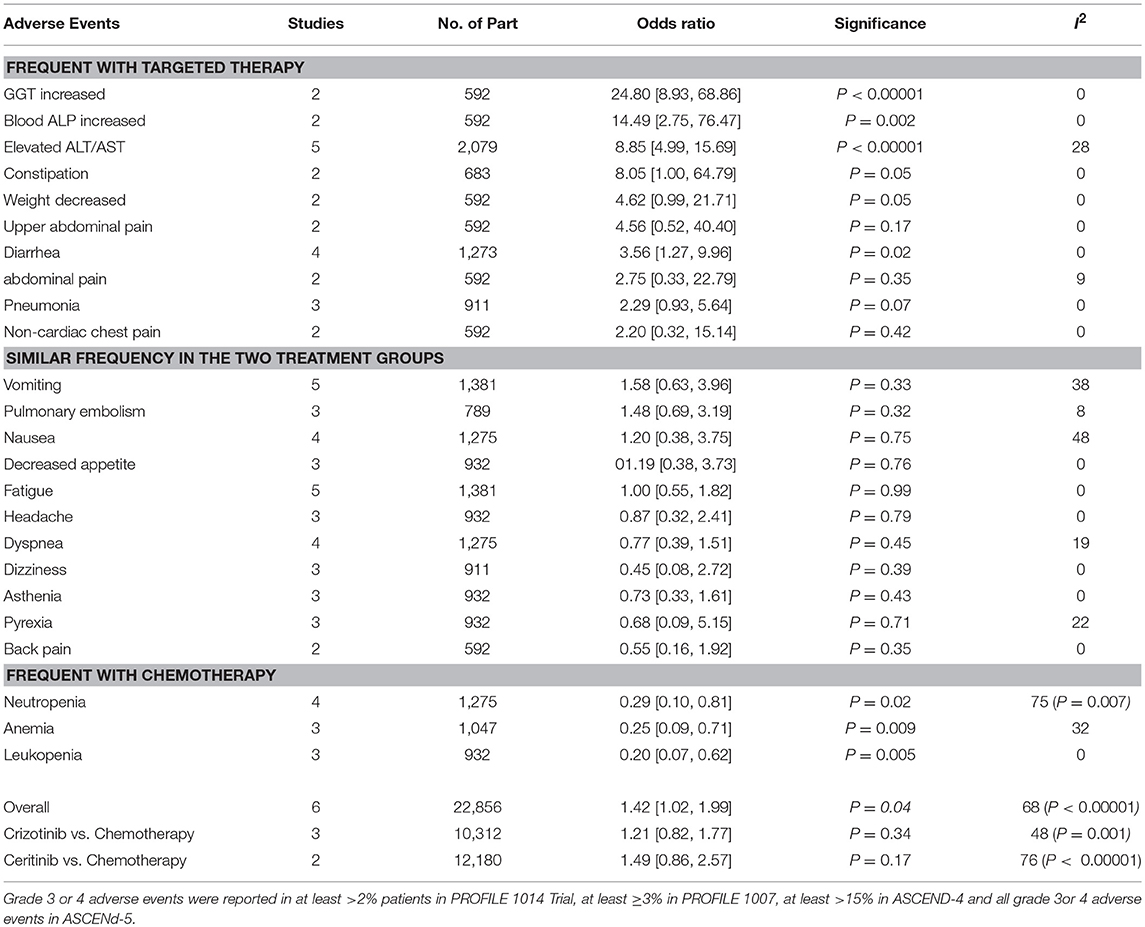
Patients receiving ALK inhibitors reported significant increase in adverse events of any grade (HR 1.63 [1.30, 2.03; p < 0.0001]) as well as grade 3 or 4 adverse events (HR 1.42 [1.02, 1.99; p = 0.04]) in comparison to chemotherapy. Significant difference was maintained even when analyses were restricted to single agent alone. Crizotinib as well as ceritinib had lead to significant increase in causing any grade adverse events individually (Table 2). Crizotinib reported a hazard ratio of 1.52 [1.11, 2.08; p = 0.008] while ceritinib reported a HR 2.09 [1.51, 2.91; p < 0.0001]. However, there was no difference in the crizotinib and ceritinib in comparison to chemotherapy when only grade 3 or 4 adverse events were considered for analysis (Table 3). Alectinib, on the other hand, was associated with least adverse events and caused comparatively less grade 3 or 4 adverse events compared to chemotherapy. Discontinuation of therapy from adverse events was significantly higher with chemotherapy (HR 0.55 [0.36, 0.83; p<0.005]) (Figure 11). Next generation agents mainly alectinib and brigatinib have shown overall reduction in frequency of any as well as grade 3 or 4 adverse events. However, there was no significant difference for the treatment difference in causing adverse events (Figure 12).
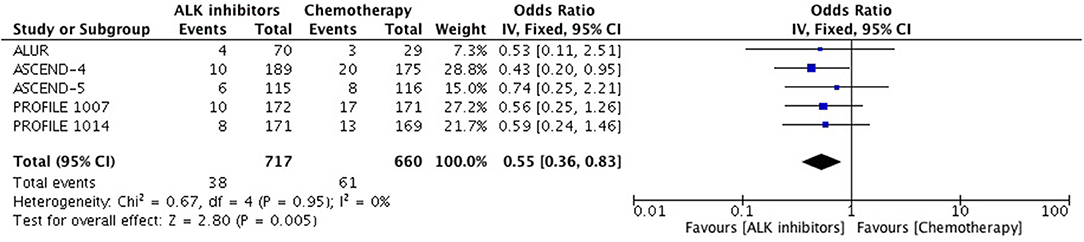
Figure 11. Forest plot of meta-analysis of discontinuation due to adverse events showing comparison of ALK inhibitors to chemotherapy in ALK positive NSCLC.
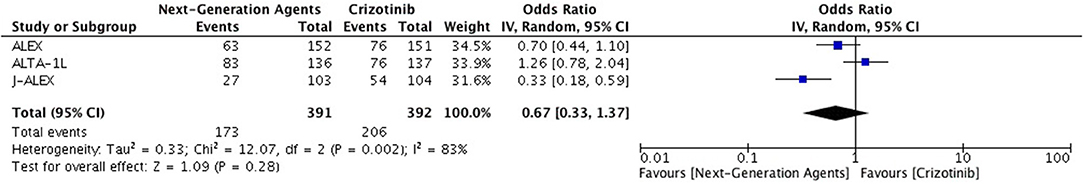
Figure 12. Forest plot of meta-analysis of the Grade 3 or 4 adverse events (AEs) showing comparison of Next generation agents to crizotinib in ALK positive NSCLC.
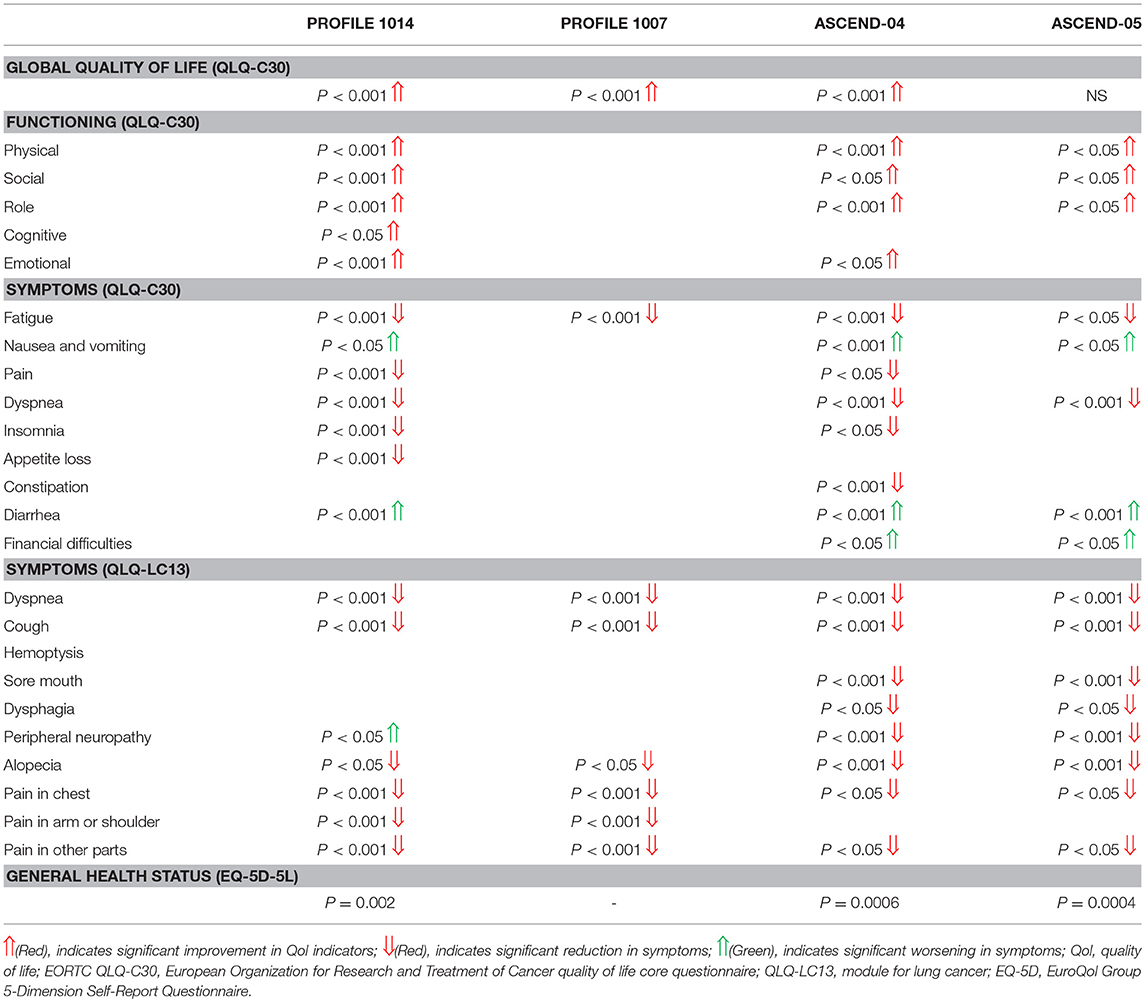
Quality of life was assessed in 4 studies comprising 2 studies (14, 15) comparing crizotinib and 2 studies comparing ceritinib to chemotherapy (19, 20). QLQ-C30, QLQ-C13 and EuroQol (ED-5D-5L) questionnaires were used to assess the quality of life. Global quality of life was significantly improved with crizotinib and ceritinib (p < 0.001). Functioning outcomes were also improved specifically physical, social and role functioning across studies. Response to QLQ-C30 Symptoms questionnaire revealed significant decrease in symptoms relief with crizotinib particularly in first line setting and ceritinib in both treatment lines as compared to chemotherapy (Table 4). These symptoms included fatigue, pain, dyspnea, insomnia and appetite loss. Significant reduction in symptoms such as dyspnea, cough, alopecia, chest pain and pain in other parts as per response to QLQ-C13 questionnaire were also reported in all the 4 studies.
Time to deterioration for the composite endpoint of Lung Cancer Specific Symptoms (LCSS) was significantly prolonged with crizotinib and ceritinib in comparison to chemotherapy. Meta-analysis of the hazard ratios revealed significant delay in time to deterioration (HR 0.51 [0.44, 0.60; p < 0.00001]) (Figure 13).
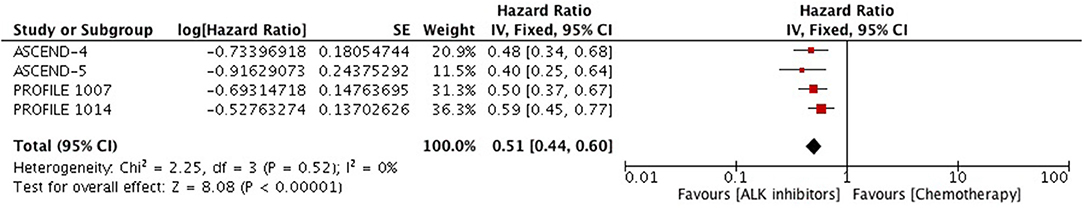
Figure 13. Forest plot of meta-analysis of the time to deterioration with respect to a composite end point of three symptoms–cough, dyspnea or chest pain showing comparison of ALK inhibitors to chemotherapy in ALK positive NSCLC.
Discussion
Overall these agents had been successful in controlling the progression of the disease as compared to chemotherapy. Progression free survival had been significantly improved with crizotinib and next generation ALK inhibitors as compared to chemotherapy in first line as well as second line treatment setting. Moreover, each drug individually had been proven to be effective in its comparison to chemotherapy in terms of PFS. Objective response rate as defined by combination of complete response and partial response was reported across all studies favoring targeted therapeutic agents. Patients responded in significantly high number to targeted therapies. All the studies were consistent in their results in terms of PFS and ORR favoring targeted agents. Next generation agents including ceritinib, alectinib and brigatinib were superior in terms of PFS and ORR to crizotinib.
Brain metastases are common with ALK+ NSCLC substantiating patients' symptoms (fatigue, headaches and depression), treatment cost, outpatient visits and inpatient stays (29). Chemotherapy as well as crizotinib is limited in their ability to penetrate CNS (30, 31) and hence in majority of the cases disease progression site is CNS particularly when baseline brain metastases are present which is deemed as worst prognostic factor (27). A similar intracranial response was reported in PROFILE 1,014 trial between the treatment groups (15% each) (15). However, Solomon et al. reported a non-significant improvement in Intra-cranial time to progression (HR, 0.45; P = 0.063) and significant improvement in intra-cranial disease control rate with crizotinib at 12 weeks (85 vs. 45%, respectively; P < 0.001) and at 24 weeks (56 vs. 25%, respectively; P = 0.006) as compared to chemotherapy in a population of stable treated
brain metastases (32). In contrast, next generation inhibitors, ceritinib and alectinib, has been more potential CNS penetrant (33). Our meta-analysis showed a significantly better intra-cranial response with ceritinib and alectinib in comparison to chemotherapy. Next generation agents have shown a greater potency in the brain overall. Alectinib and brigatinib have reported significantly better response intra-cranially. These agents also have significantly delayed CNS disease progression in patients with or without baseline CNS disease as well as with or without prior radiotherapy which has been associated with better response from targeted therapy (34, 35).
Survival analysis must be interpreted carefully as either the data was immature or high crossover was reported. PROFILE 1,014 reported no significant survival difference with about 70% crossover from chemotherapy to crizotinib group. Rank-preserving structural failure time model was used for adjusting the cross-over which revealed a better overall survival with crizotinib as calculated with Wilcoxon test (HR 0.60 [0.27, 1.42; p < 0.00001]) and log-rank test (HR 0.67 [0.28, 1.48; p < 0.00001]). This outcome suggested that cross over might have confounded the overall survival analysis (15). Final OS analysis of PROFILE 1,014 reported better OS achieved with crizotinib after adjustment for crossover (HR 0.346 [0.081, 0.718]) (36). Lu et al. survival analysis was based only on 35% of OS events while 82 (80%) patients had crossed over to crizotinib (26). Despite such a huge crossover in first line comparison, a numerical advantage close to being statistically significant was yet achieved. A similar trend of crossover has also been reported in each individual study comprising second line treatment comparison (14, 20, 21). The improvement in progression free survival might have been resulted in significant OS given the maturity of the study was reached and high crossover was prevented. Duruisseaux et al. (37) reported a better median OS of 16.6 months with crizotinib in unselected ALK+ NSCLC patients. It also reported that the line of treatment was not associated with survival outcome.
ALK inhibitors (except for alectinib) had been shown to cause significantly higher number of grade 1 and 2 adverse events. Vision disorders, diarrhea, edema, vomiting, elevated aminotransferases, cough, back pain, upper abdominal pain, weight decrease, blood alkaline phosphatase increase, blood creatinine increase, gamma-glutamyltransferase (GGT) increase and non-cardiac chest pain were reported predominantly in ALK inhibitors group. While patients receiving chemotherapy reported fatigue, alopecia, anemia, neutropenia, leukopenia. A number of adverse events were reported similar in frequency in both treatment groups. These included cough, nausea, dizziness, dyspnea, constipation, decreased appetite, asthenia and rash. Several adverse events were unique to each agent individually. Vision disorders, dizziness, dysgeusia and edema were only reported with crizotinib. Ceritinib also reported a number of distinct adverse events such as back pain, upper abdominal pain, weight decrease, blood alkaline phosphatase increase, blood creatinine increase, gamma-glutamyltransferase (GGT) increase and non-cardiac chest pain. Analysis was not adjusted for the duration of treatment as duration of crizotinib treatment was comparatively longer as compared to chemotherapy. Median duration of treatment was 10.6 months and 31 weeks in crizotinib group compared to 4.1 months and 21 weeks in chemotherapy group in the PROFILE 1,014 and 1,007 trial, respectively. A similar longer duration was also reported with ceritinib as well.
Grade 3 and 4 adverse events were comparatively similar in frequency in both treatments. There was no difference between the treatments when each single agent (ceritinib or crizotinib) was compared to chemotherapy in causing grade 3 or 4 adverse events. Total 6 patients receiving crizotinib qualified for Hy's law criteria leading to discontinuation of treatment (5 patients were reported in PROFILE 1,014 and 1 from PROFILE 1,007). Three of the 6 patients were with grade 2 or 3 elevated aminotransferases and one with drug-induced hepatic injury. No patient was reported in ceritinib group meeting Hy's criteria. Overall adverse events leading to permanent discontinuation of treatment were comparatively higher in chemotherapy group compared to crizotinib and ceritinib. Most of the adverse events were managed with dose adjustments, interruptions or delays.
Quality of life was assessed with changes from baseline on the European Organization for Research and Treatment of Cancer (EORTC) quality of life core questionnaire (QLQ-C30), module for lung cancer (QLQ-LC13) and EuroQol Group 5-Dimension Self-Report Questionnaire (EQ-5D) scales. Ceritinib showed a decrease in peripheral neuropathy while crizotinib was associated with an increase. Ceritinib also lead to significant decrease in symptoms like sore mouth and dysphagia. Pain in arm or shoulder was significantly reduced with crizotinib therapy and no significant difference was reported with ceritinib in comparison to chemotherapy. Overall a first line use reported better control of these agents in symptoms reduction and improvement of quality of life as compared to second line treatment setting.
There are several limitations to this study. Not all the studies included were of high quality. Phase III trials were not blinded. Two of the studies included were of retrospective nature with high chance of incurring selection bias (25, 27). Results of one study comparing ceritinib to crizotinib based on data derived from 5 different trials involving the two agents could be confounding due to non-randomization, study level data incorporation and inherent limitations of the involved studies (28). A high crossover has been reported with all the 6 studies comprising survival analysis and might have confounded the survival outcome. In PROFILE 1014, maintenance therapy with premetexed was not continued after initially planned six cycles of premetexed-plus-platinum, which might have affected the progression free survival in chemotherapy group as reported in PARAMOUNT study, however, ever so slightly (38).
Main aim of the study was to highlight the progress being made in ALK inhibitors treatment of ALK positive NSCLC. Since a number of agents being approved with next-generation agents more efficacious in CNS metastatic disease, it is debatable to choose an agent as initial choice. Crizotinib is usually given as first line treatment choice. Relapse is more common with crizotinib. Both next generation agents have been proven efficacious in crizotinib resistant disease (35, 36). On other hand, ceritinib and particularly alectinib is more efficacious in controlling the progression of the disease compared to crizotinib and more active in CNS metastatic lesions which is a main progression site in crizotinib treated patients. Alectinib has a strong safety profile when compared to crizotinib and ceritinib. Newer agents include brigatinib, lorlatinib, ensartinib and entrectinib are making their way to enter the treatment paradigm of ALK+ NSCLC. Brigatinib has already been approved and lorlatinib has also shown good efficacy (39, 40). The spot for first choice of treatment dimension is being changed over time with the addition of brigatinib showing comparability to alectinib in all aspects of treatment efficacy and safety (24). Currently a clinical trial (NCT03596866) is undergoing comparing brigatinib to alectinib in advanced ALK positive NSCLC patients who have progressed on crizotinib (41). Ensartinib and entrectinib are under investigational stages.
Conclusions
ALK inhibitors collectively and individually have shown significant improvement in PFS, ORR, Quality of life without any increase in toxicity (grade 3,4 adverse events) compared to chemotherapy. First line as well as second line treatment comparison revealed a similar prominent picture of ALK inhibitors' efficacy. ALK inhibitors clearly represent a better choice of treatment and could be recommended and preferred over chemotherapy. Alectinib has shown all positive indicators to be first choice of ALK positive NSCLC treatment as it has reported its superiority over chemotherapy as well as crizotinib in terms of PFS, ORR, and intracranial efficacy and by far safer to other agents including ceritinib. Recently, alectinib is being compared to brigatinib for superiority. Dimension of ALK positive NSCLC treatment is undergoing fast development with the addition of newer ALK inhibitors.
Materials and Methods
Search Strategy and Study Selection
PubMed, Medline and Cochrane Library were searched comprehensively until Sep 2018 using a wide range of terms including “ALK+ NSCLC” OR “ALK positive non-small cell lung cancer” AND “ALK inhibitors” OR “ALK” OR “crizotinib” OR “ceritinib” OR “alectinib” OR “brigatinib” OR “Next generation ALK inhibitors” AND “Chemotherapy.” Titles and abstracts of the retrieved studies were screened for eligibility. Full texts screening was done to include studies qualifying for inclusion according to eligibility criteria.
Eligibility Criteria
Published in English studies comparing the “ALK inhibitors with chemotherapy” and “next generation inhibitors to crizotinib” in the treatment of ALK+ non-small cell lung cancer. Comparisons of interests were: crizotinib vs. chemotherapy; ceritinib vs. chemotherapy; alectinib vs. chemotherapy; ceritinib vs. crizotinib and alectinib vs. crizotinib.
Outcomes of Interest and Data Extraction
Progression-free survival was the primary outcome of interest. Secondary outcome of interests included objective response rate, overall survival, intracranial efficacy, adverse events and quality of life. Data was extracted from all the studies included general characteristics of the trial, patient's characteristics and main outcomes of interest (Table 1).
Quality Assessment
Quality of the RCTs was assessed by Jaded et al. method (42). Jaded et al.'s method is a 3 questions based method. These questions addresses whether or not the trial has reported the following three items: appropriate randomization (0–2 points); blinding (0–2 points); and withdrawals and dropouts (0–1 point). A trial achieving < 3 points was considered low quality while ≥3 points was considered high quality. All the RCTs included in this meta-analysis achieved a score of 3 points (42). Newcastle-Ottawa Scale was used to assess the quality of the retrospective studies (43).
Statistical Analysis
Hazard ratios were extracted from the studies for time to event data (44). Hazard ratio for some outcomes was calculated from the time to event graphs in case there was no direct mention of hazard ratio in the study. Odds ratio with 95% CI were calculated for dichotomous data. Obtained Hazard ratios with 95% CI and odds ratio were pooled using the Review Manager (RevMan, V5.3) software provided by Cochrane Collaboration Tools. Heterogeneity was assessed with χ2 (Chi-square) and I2 statistic. >50% I2 statistic value was considered significant heterogeneity as well as a p < 0.05. Fixed effects model was used in case no significant heterogeneity was present otherwise a random effects model was applied.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Funding
This work was supported by the National Natural Science Foundation Of China Grants (81572964, 81773354, 81502342 and 81502194) and Guangzhou Key Medical Discipline Construction Project.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
ALK, Anaplastic lymphoma kinase; NSCLC, Non-small cell lung cancer; OS, Overall survival; ORR, Objective response rate; PFS, Progression free survival; HR, Hazard ratio; OR, Odds ratio; RCT, Randomized controlled trial; EGFR, Epidermal growth factor receptor; KRAS, Kirsten RAt Sarcoma; EML4, Echinoderm microtubule-associated protein-like 4; MET, mesenchymal-epithelial transition factor (MET); ROS1, Proto-oncogene tyrosine-protein kinase ROS; CNS, Central nervous system; AEs, Adverse events; BBM, Baseline brain metastases; mBBM, Measurable baseline brain metastases; SE, Standard error; CI, Confidence interval; X2, Chi square test.
References
1. Rolfo C, Caglevic C, Santarpia M, Araujo A, Giovannetti E, Gallardo CD, et al. Immunotherapy in NSCLC: a promising and revolutionary weapon. Adv Exp Med Biol. (2017) 995:97–125. doi: 10.1007/978-3-319-53156-4_5
2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. (2018) 68:7–30. doi: 10.3322/caac.21442
3. Petersen I. The morphological and molecular diagnosis of lung cancer. Deutsch Arztebl Int. (2011) 108:525–31. doi: 10.3238/arztebl.2011.0525
4. Giovannetti EA., Rodriguez J. Targeted therapies in cancer: where are we going? Cancer Drug Resist. (2018) 1:82–6. doi: 10.20517/cdr.2018.05
5. Travis WD, Brambilla E, Noguchi M, Nicholson AG, Geisinger KR, Yatabe Y, et al. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol. (2011) 6:244–85. doi: 10.1097/JTO.0b013e318206a221
6. Morris SW, Kirstein MN, Valentine MB, Dittmer KG, Shapiro DN, Saltman DL, et al. Fusion of a kinase gene, ALK, to a nucleolar protein gene, NPM, in non-Hodgkin's lymphoma. Science (1994) 263:1281–4. doi: 10.1126/science.8122112
7. Chiarle R, Voena C, Ambrogio C, Piva R, Inghirami G. The anaplastic lymphoma kinase in the pathogenesis of cancer. Nat Rev Cancer (2008) 8:11–23. doi: 10.1038/nrc2291
8. Soda M, Choi YL, Enomoto M, Takada S, Yamashita Y, Ishikawa S, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. (2007) 448:561–6. doi: 10.1038/nature05945
9. Rikova K, Guo A, Zeng Q, Possemato A, Yu J, Haack H, et al. Global survey of phosphotyrosine signaling identifies oncogenic kinases in lung cancer. Cell (2007) 131:1190–203. doi: 10.1016/j.cell.2007.11.025
10. Horn L, Pao W. EML4-ALK: honing in on a new target in non–small-cell lung cancer. J Clin Oncol. (2009) 27:4232–5. doi: 10.1200/JCO.2009.23.6661
11. Sasaki T, Rodig SJ, Chirieac LR, Jänne PA. The biology and treatment of EML4-ALK non-small cell lung cancer. Eur J Cancer (2010) 46:1773–80. doi: 10.1016/j.ejca.2010.04.002
12. Kwak EL, Bang YJ, Camidge DR, Shaw AT, Solomon B, Maki RG, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. (2010) 363:1693–703. doi: 10.1056/NEJMoa1006448
13. Kim D-W, Ahn M-J, Shi Y, De Pas TM, Yang P-C, Riely GJ, et al. Results of a global phase II study with crizotinib in advanced ALK-positive non-small cell lung cancer (NSCLC). J Clin Oncol. (2012) 30(Suppl. 15):7533. doi: 10.1200/jco.2012.30.15
14. Shaw AT, Kim D-W, Nakagawa K, Seto T, Crinó L, Ahn M-J. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med. (2015) 373:1582. doi: 10.1056/NEJMx150036
15. Solomon BJ, Mok T, Kim DW, Wu YL, Nakagawa K, Mekhail T, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med. (2014) 371:2167–77. doi: 10.1056/NEJMoa1408440
16. Nix NM, Brown KS. Ceritinib for ALK-rearrangement–positive non–small cell lung cancer. J Adv Pract Oncol. (2015) 6:156–60.
17. Shaw AT, Kim D-W, Mehra R, Tan DSW, Felip E, et al. Ceritinib in ALK-rearranged non–small-cell lung cancer. N Engl J Med. (2014) 370:1189–97. doi: 10.1056/NEJMoa1311107
18. Mok T, Spigel D, Felip E, deMarinis F, Ahn M-J, Groen HJM, et al. ASCEND-2: a single-arm, open-label, multicenter phase II study of ceritinib in adult patients (pts) with ALK-rearranged (ALK+) non-small cell lung cancer (NSCLC) previously treated with chemotherapy and crizotinib (CRZ). J Clin Oncol. (2015) 33(Suppl. 15):8059. doi: 10.1200/jco.2015.33.15
19. Soria JC, Tan DSW, Chiari R, Wu YL, Paz-Ares L, Wolf J, et al. First-line ceritinib versus platinum-based chemotherapy in advanced ALK-rearranged non-small-cell lung cancer (ASCEND-4): a randomised, open-label, phase 3 study. Lancet (2017) 389:917–29. doi: 10.1016/S0140-6736(17)30123-X
20. Shaw AT, Kim TM, Crinò L, Gridelli C, Kiura K, Liu G, et al. Ceritinib versus chemotherapy in patients with ALK-rearranged non-small-cell lung cancer previously given chemotherapy and crizotinib (ASCEND-5): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. (2017) 18:874–86. doi: 10.1016/S1470-2045(17)30339-X
21. Novello S, Mazières J, Oh IJ, de Castro J, Migliorino MR, Helland Å, et al. Alectinib versus chemotherapy in crizotinib-pretreated anaplastic lymphoma kinase (ALK)-positive non-small-cell lung cancer: results from the phase III ALUR study. Ann Oncol. (2018) 29:1409–16. doi: 10.1093/annonc/mdy121
22. Shaw AT, Peters S, Mok T, Gadgeel SM, Ahn JS, Ignatius Ou S-H, et al. Alectinib versus crizotinib in treatment-naive advanced ALK-positive non-small cell lung cancer (NSCLC): primary results of the global phase III ALEX study. J Clin Oncol. (2017) 35(Suppl. 18):LBA9008. doi: 10.1200/JCO.2017.35.15_suppl.LBA9008
23. Hida T, Nokihara H, Kondo M, Kim YH, Azuma K, Seto T, et al. Alectinib versus crizotinib in patients with ALK-positive non-small-cell lung cancer (J-ALEX): an open-label, randomised phase 3 trial. Lancet (2017) 390:29–39. doi: 10.1016/S0140-6736(17)30565-2
24. Camidge DR, Kim HR, Ahn MJ, Yang JC, Han JY, Lee JS, et al. Brigatinib versus crizotinib in ALK-positive non-small-cell lung cancer. N Engl J Med. (2018). doi: 10.1056/NEJMoa1810171. [Epub ahead of print].
25. Cui S, Zhao Y, Dong L, Gu A, Xiong L, Qian J, et al. Is there a progression-free survival benefit of first-line crizotinib versus standard chemotherapy and second-line crizotinib in ALK-positive advanced lung adenocarcinoma? A retrospective study of Chinese patients. Cancer Med. (2016) 5:1013–21. doi: 10.1002/cam4.659
26. Lu S, Mok T, Lu Y, Zhou J, Shi Y, Sriuranpong V, et al. Phase 3 study of first-line crizotinib vs pemetrexed–cisplatin/carboplatin (PCC) in East Asian patients (pts) with ALK+ advanced non-squamous non-small cell lung cancer (NSCLC). J Clin Oncol. (2016) 34(Suppl. 15):9058. doi: 10.1200/JCO.2016.34.15_suppl.9058
27. Zhou J, Zheng J, Zhang X, Zhao J, Zhu Y, Shen Q, et al. Crizotinib in patients with anaplastic lymphoma kinase-positive advanced non-small cell lung cancer versus chemotherapy as a first-line treatment. BMC Cancer. (2018) 18:10. doi: 10.1186/s12885-017-3720-8
28. Tan DS, Araújo A, Zhang J, Signorovitch J, Zhou ZY, Cai X, et al. Comparative efficacy of ceritinib and crizotinib as initial ALK-targeted therapies in previously treated advanced NSCLC: an adjusted comparison with external controls. J Thorac Oncol. (2016) 11:1550–7. doi: 10.1016/j.jtho.2016.05.029
29. Guérin A, Sasane M, Zhang J, Culver KW, Dea K, Nitulescu R, et al. Brain metastases in patients with ALK+ non-small cell lung cancer: clinical symptoms, treatment patterns and economic burden. J Med Econom. (2015) 18:312–22. doi: 10.3111/13696998.2014.1003644
30. Deeken JF, Löscher W. The blood-brain barrier and cancer: transporters, treatment, and trojan horses. Clin Cancer Res. (2007) 13:1663–74. doi: 10.1158/1078-0432.CCR-06-2854
31. Yoshida T, Oya Y, Tanaka K, Shimizu J, Horio Y, Kuroda H, et al. Clinical impact of crizotinib on central nervous system progression in ALK-positive non-small lung cancer. Lung Cancer (2016) 97:43–7. doi: 10.1016/j.lungcan.2016.04.006
32. Solomon BJ, Cappuzzo F, Felip E, Blackhall FH, Costa DB, Kim DW, et al. Intracranial efficacy of crizotinib versus chemotherapy in patients with advanced ALK-positive non-small-cell lung cancer: results from PROFILE 1014. J Clin Oncol. (2016) 34:2858–65. doi: 10.1200/JCO.2015.63.5888
33. Klempner SJ, Ou SH. Anaplastic lymphoma kinase inhibitors in brain metastases from ALK+ non-small cell lung cancer: hitting the target even in the CNS. Chin Clin Oncol. (2015) 4:20. doi: 10.3978/j.issn.2304-3865.2015.05.03
34. Solomon BJ, Kim DW, Wu YL, Nakagawa K, Mekhail T, Felip E, et al. Final overall survival analysis from a study comparing first-line crizotinib versus chemotherapy in ALK-mutation-positive non-small-cell lung cancer. J Clin Oncol. (2018) 36:2251–8. doi: 10.1200/JCO.2017.77.4794
35. Nishio M, Nakagawa K, Mitsudomi T, Yamamoto N, Tanaka T, Kuriki H, et al. Analysis of central nervous system efficacy in the J-ALEX study of alectinib versus crizotinib in ALK-positive non-small-cell lung cancer. Lung Cancer (2018) 121:37–40. doi: 10.1016/j.lungcan.2018.04.015
36. Gadgeel S, Peters S, Mok T, Shaw AT, Kim DW, Ou SI, et al. Alectinib versus crizotinib in treatment-naive anaplastic lymphoma kinase-positive (ALK+) non-small-cell lung cancer: CNS efficacy results from the ALEX study. Ann Oncol. (2018). doi: 10.1093/annonc/mdy405. [Epub ahead of print].
37. Duruisseaux M, Besse B, Cadranel J, Pérol M, Mennecier B, Bigay-Game L, et al. Overall survival with crizotinib and next-generation ALK inhibitors in ALK-positive non-small-cell lung cancer (IFCT-1302 CLINALK): a French nationwide cohort retrospective study. Oncotarget (2017) 8:21903–17. doi: 10.18632/oncotarget.15746
38. Paz-Ares L, de Marinis F, Dediu M, Thomas M, Pujol JL, Bidoli P, et al. Maintenance therapy with pemetrexed plus best supportive care versus placebo plus best supportive care after induction therapy with pemetrexed plus cisplatin for advanced non-squamous non-small-cell lung cancer (PARAMOUNT): a double-blind, phase 3, randomised controlled trial. Lancet Oncol. (2012) 13:247–55. doi: 10.1016/S1470-2045(12)70063-3
39. Gettinger SN, Bazhenova LA, Langer CJ, Salgia R, Gold KA, Rosell R, et al. Activity and safety of brigatinib in ALK-rearranged non-small-cell lung cancer and other malignancies: a single-arm, open-label, phase 1/2 trial. Lancet Oncol. (2016) 17:1683–96. doi: 10.1016/S1470-2045(16)30392-8
40. Shaw AT, Felip E, Bauer TM, Besse B, Navarro A, Postel-Vinay S, et al. Lorlatinib in non-small-cell lung cancer with ALK or ROS1 rearrangement: an international, multicentre, open-label, single-arm first-in-man phase 1 trial. Lancet Oncol. (2017) 18:1590–9. doi: 10.1016/S1470-2045(17)30680-0
41. Clinicaltrials.gov. An Efficacy Study Comparing Brigatinib Versus Alectinib in Advanced Anaplastic Lymphoma Kinase-Positive Non-Small-Cell Lung Cancer Participants Who Have Progressed on Crizotinib - Full Text View - ClinicalTrials.gov. (2018). Available online at: https://clinicaltrials.gov/ct2/show/NCT03596866 (Accessed October 29, 2018).
42. Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. (1996) 17:1–12. doi: 10.1016/0197-2456(95)00134-4
43. Wells G, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. NewCastle-Ottawa Quality Assessment Scale –Cohort Studies[EB/OL]. (2018). Available online at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (Accessed May 06, 2012).
Keywords: anaplastic lymphoma kinase (ALK), non-small cell lung cancer (NSCLC), molecular targeted agents, chemotherapy, progression free survival (PFS), quality of life (Qol)
Citation: Khan M, Lin J, Liao G, Tian Y, Liang Y, Li R, Liu M and Yuan Y (2019) ALK Inhibitors in the Treatment of ALK Positive NSCLC. Front. Oncol. 8:557. doi: 10.3389/fonc.2018.00557
Received: 16 October 2018; Accepted: 09 November 2018;
Published: 09 January 2019.
Edited by:
Alfredo Addeo, Geneva University Hospitals (HUG), SwitzerlandReviewed by:
Giulio Metro, Ospedale Santa Maria della Misericordia di Perugia, ItalyPetra Jana Jankowska, Taunton and Somerset, NHS Foundation Trust, United Kingdom
Antonio Passaro, Istituto Europeo di Oncologia s.r.l., Italy
Copyright © 2019 Khan, Lin, Liao, Tian, Liang, Li, Liu and Yuan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yawei Yuan, yuanyawei2015@outlook.com
Mengzhong Liu, liumengzhong@126.com
†Co-first authorship
‡These authors have contributed equally to this work
 Muhammad Khan
Muhammad Khan Jie Lin
Jie Lin Guixiang Liao
Guixiang Liao Yunhong Tian
Yunhong Tian Yingying Liang
Yingying Liang Rong Li
Rong Li Mengzhong Liu
Mengzhong Liu Yawei Yuan
Yawei Yuan