- 1Key Laboratory of Breast Cancer in Shanghai, Department of Breast Surgery, Fudan University Shanghai Cancer Center, Shanghai, China
- 2Department of Oncology, Shanghai Medical College, Fudan University, Shanghai, China
- 3Institutes of Biomedical Sciences, Fudan University, Shanghai, China
Background: Little is known regarding the clinicopathologic characteristics, oncologic outcomes, and treatment strategies that could be ascribed to BRCA mutation in early-onset triple-negative breast cancer (eTNBC).
Methods: eTNBC patients who underwent BRCA genetic testing were derived from our clinical database between 2012 and 2018. Differences in clinical features and pathologic characteristics were examined in groups divided by BRCA mutation status, and the contribution of germline mutations in conjunction with treatment modalities to survival outcomes was determined.
Results: Of the 355 qualifying eTNBC patients, 67 (18.87%) were BRCA mutated and 288 (81.13%) were BRCA wild. Overall, median age at diagnosis was 34 years (range, 24–40 years) in the BRCA mutated subgroup and 35 years (range, 21–40 years) in BRCA wild. The majority of clinicopathologic parameters were parallel; however, tumor size (P = 0.07) and nuclear grade (P =0.08) tend to be more aggressive in the BRCA mutated subgroup. Compared with BRCA wild patients, BRCA mutated patients had a higher likelihood of receiving anthracyclines and taxane-based combination chemotherapy (P = 0.04) and tend to be lower tumor burden (P =0.01). After approximately 5-year median follow-up, the overall survival (OS) (P = 0.021) and breast cancer-specific survival (BCSS) (P = 0.004) in BRCA mutated patients were superior to those in their BRCA wild counterparts. Intriguingly, the clinical outcomes were comparable in patients with breast conserving surgery (BCS) regardless of BRCA mutations and in patients with BRCA mutations in spite of surgical schedules.
Conclusions: These results suggest that eTNBC patients with BRCA mutations are prone to better OS and BCSS, which might be largely attributed to more benefit from anthracyclines and taxane-based chemotherapy. The BCS procedure could be a safe alternative surgical option for eTNBC patients with BRCA mutations. Future studies with substantial numbers of participants are urgently needed to validate whether BRCA mutation eTNBC patients are more sensitive to chemotherapy.
Introduction
Breast cancer incidence increases with age (1). Young age breast cancer, diagnosed before the age of 40 years, is a unique biological and clinical entity and currently represents a top biomedical research priority. Epidemiologically, it is now established knowledge that the proportion of women diagnosed with breast cancer before the age of 35 in the West and before 40 in the East is about 4 and 13%, respectively (1, 2). Accumulating evidence suggests that young age breast cancer is the leading cause of cancer-related deaths of women under the age of 45 years and has been listed as the paramount health burden in developing countries compared with their developed counterparts (3).
Generally, early age of breast cancer onset is considered an indicator of cancer susceptibility genes (4). A substantial proportion of hereditary breast cancer can be attributed to mutations in one of two genes, BRCA1 or BRCA2 (5). The literature has documented that women who inherited a deleterious BRCA mutation suffer a high lifetime risk of developing breast cancer (6–8), with a large-sized prospective study estimating a cumulative incidence of 66 and 61% for BRCA1 and BRCA2 up to the age of 70 years, respectively (6). BRCA mutations are the most common genetic variabilities in breast cancer and closely associated with aggressive clinical and biologic course of breast cancer, especially the triple-negative breast cancer (TNBC) subtype (9, 10). It is estimated that 10% of patients with TNBC present with deleterious germline mutations in BRCA1 or BRCA2 (11). Around 60 to 80% patients carrying a BRCA1 germline mutation are characterized by TNBC phenotype (12), and 15 to 25% TNBC patients of Ashkenazi ethnicity have a BRCA1 mutation (13, 14). Intriguingly, in contrast to BRCA2, BRCA1 mutations are thought to contribute to more cases of early onset breast cancer (15).
BRCA mutation cancers possess a deficiency in homologous recombination repair of DNA double-strand breaks (DSBs), thus causing genomic instability (16, 17). Drugs that induce DSB have shown sensitivity to and promise for BRCA-associated TNBC in a series of clinical trials (18). Many other pathway-specific inhibitors have been investigated to overcome the drawbacks of current treatment options for TNBC in recent years (19). The current screening, recommendations, therapeutic strategies, and even surveillance of BRCA-associated TNBC are in reference to sporadic TNBC. Despite this intensive investigation of the penetrance of BRCA mutations in early onset TNBC, significant knowledge gaps exist. Robust evidence shows that young age at breast cancer diagnosis indicates a distinct entity; however, the prevalence, oncologic outcomes, and treatment modalities of young age breast cancer vary and remain controversial.
We conducted this population-based study of eTNBC with BRCA genetic testing results in an attempt to better define the therapeutic schedule of BRCA-associated eTNBC and the effect of germline mutations on the clinicopathologic features and outcomes of these tumors.
Materials and Methods
Study Population and Ethical Statement
A retrospective review was conducted to identify patients with unilateral invasive eTNBC (age at diagnosis ≤40 years) who underwent surgery at Fudan University Shanghai Cancer Center between 2012 and 2018. The following variables were collected: genetic data (BRCA genetic test results), clinicopathologic data (age at diagnosis, family history of breast cancer or ovarian cancer (FH of BC or OC) in first- or second-degree relatives, parity, body mass index (BMI), histopathology, nuclear grade, tumor size, lymph node involvement and proliferative index), and treatment data (surgical type and adjuvant systemic therapy according to local protocols). Patients with a previous invasive breast cancer or ductal carcinoma in situ or bilateral breast cancer were excluded. This study was approved by the Ethical Committee of the Shanghai Cancer Center of Fudan University.
BRCA Mutation Analysis
Briefly, genomic DNA extracted from peripheral blood was subjected to next-generation sequencing (NGS) according to the manufacturer’s instruction. All mutations considered disease-associated were confirmed through Sanger sequencing. The details of procedures of NGS and interpretation of the mutations were described in our previous study (20), and the genetic testing results were available before decision-making.
Outcome Measures and Statistical Analysis
Overall survival (OS) was defined as the time from surgery to death from any cause. Disease-free survival (DFS) was defined as the interval from definitive surgery to any recurrence, contralateral breast cancer, distant metastasis, or death irrespective of cause. Breast cancer-specific survival (BCSS) was defined as the interval of survival time from surgery to death caused by breast cancer. Survivals were estimated using the Kaplan–Meier method. The log-rank test was adopted to compare survival outcomes between different conditions of patients. Categorical variables were compared using Pearson’s chi-squared test or Fisher’s exact test, and continuous variables were compared using independent t-test, as appropriate. Hazard ratios (HRs) and 95% confidential intervals (CIs) for univariate and multivariate analyses were calculated using Cox proportional hazards models. All tests were two-sided, and P <0.05 was considered statistically significant. Statistical analysis was performed using SPSS for Windows (version 23.0, SPSS Inc., Chicago, IL, USA).
Results
Patient Demographics and Clinicopathologic Characteristics
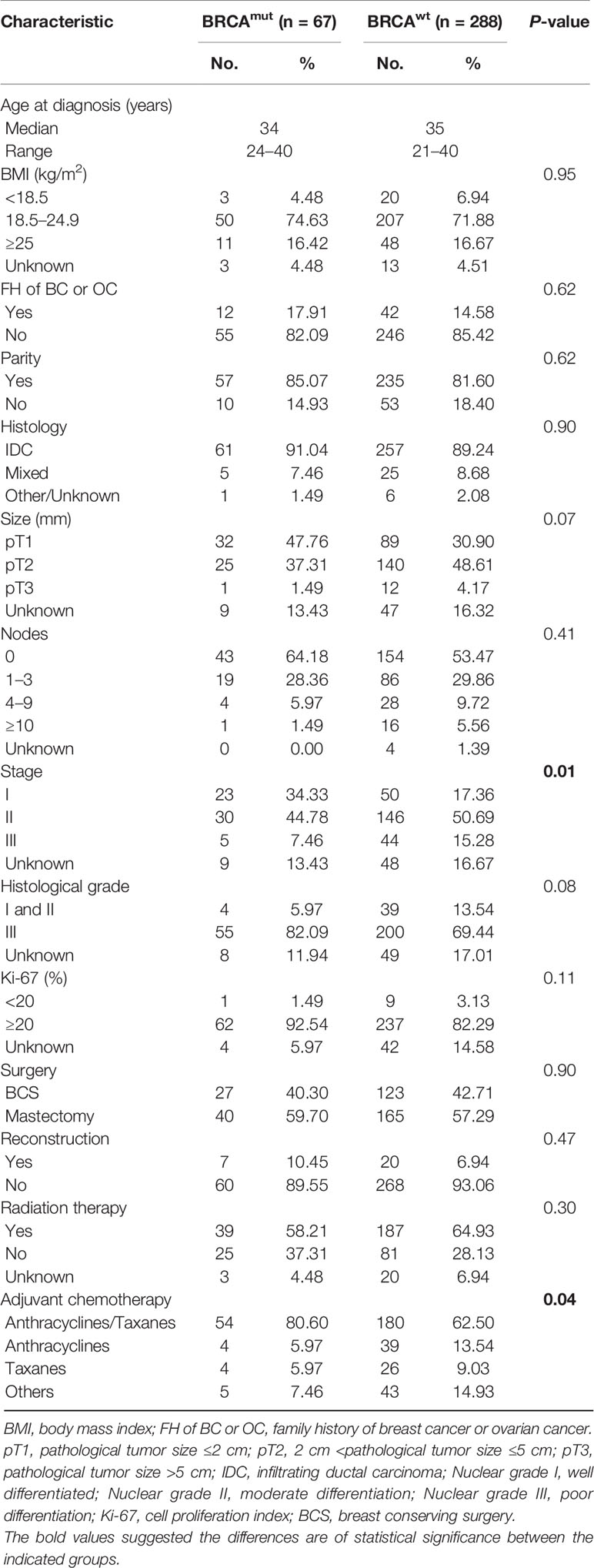
A total of 355 eTNBC patients were eligible and subjected to this analysis, of whom 67 (18.87%) patients were BRCA mutated and 288 (81.13%) patients were BRCA wild. Of the 67 patients with BRCA mutations, 58 (86.57%) had BRCA1 mutations and nine (13.43%) BRCA2 (data not shown). The prevalence of BRCA mutations with respect to patient demographics and clinicopathologic characteristics is presented in Table 1. Median age at diagnosis was 34 years (range, 24–40 years) and 35 years (range, 21–40 years) for BRCA mutated and BRCA wild eTNBC patients, respectively. No statistical significance in the proportion of FH of BC or OC in first- or second-degree relatives was exhibited (17.91 and 14.58% of BRCA mutated and BRCA wild subgroups, respectively). BMI, full-term pregnancy, lymph node involvement, and proliferative index were not predictive of BRCA mutation status.
Predictably, the vast majority of participants had infiltrating ductal carcinoma (IDC), followed by mixed pathological pattern, including IDC with invasive lobular carcinoma, IDC with medullary carcinoma, and IDC with ductal carcinoma in situ; all of those were similar between the two subgroups. Although there was a trend for BRCA mutated tumors to have higher histological grade than BRCA wild tumors, this did not reach statistical significance (P = 0.08). BRCA mutated tumors were more frequently treated with anthracyclines and taxane-based combination chemotherapy (P = 0.04). However, similar likelihoods of receiving radiation therapy and breast reconstruction were manifested in both subgroups.
Survival Estimates
Median follow-up of the study cohort was 56.5 months. A total of 38 deaths, 32 breast cancer-specific events, and 68 deaths or recurrences were observed. The estimated OS was significantly better in BRCA mutated eTNBC patients than BRCA wild ones (P = 0.021). Although limitations of the retrospective study and some missing values of variables existed, the Cox regression analyses indicated that only BRCA status [HR = 0.22, 95%CI(0.05–0.91)] was the independent factor contributing the difference of OS outcome (data not shown). The same tendency was demonstrated with regard to BCSS (P = 0.004). As to DFS, there was no significant difference between BRCA mutated patients and BRCA wild ones (P = 0.355). The Kaplan–Meier plots for OS, DFS, and BCSS by mutational status are shown in Figure 1A–C. Though no statistical significance was obtained in landmark analysis at the 5-year time point, the trend was absolutely reversed.
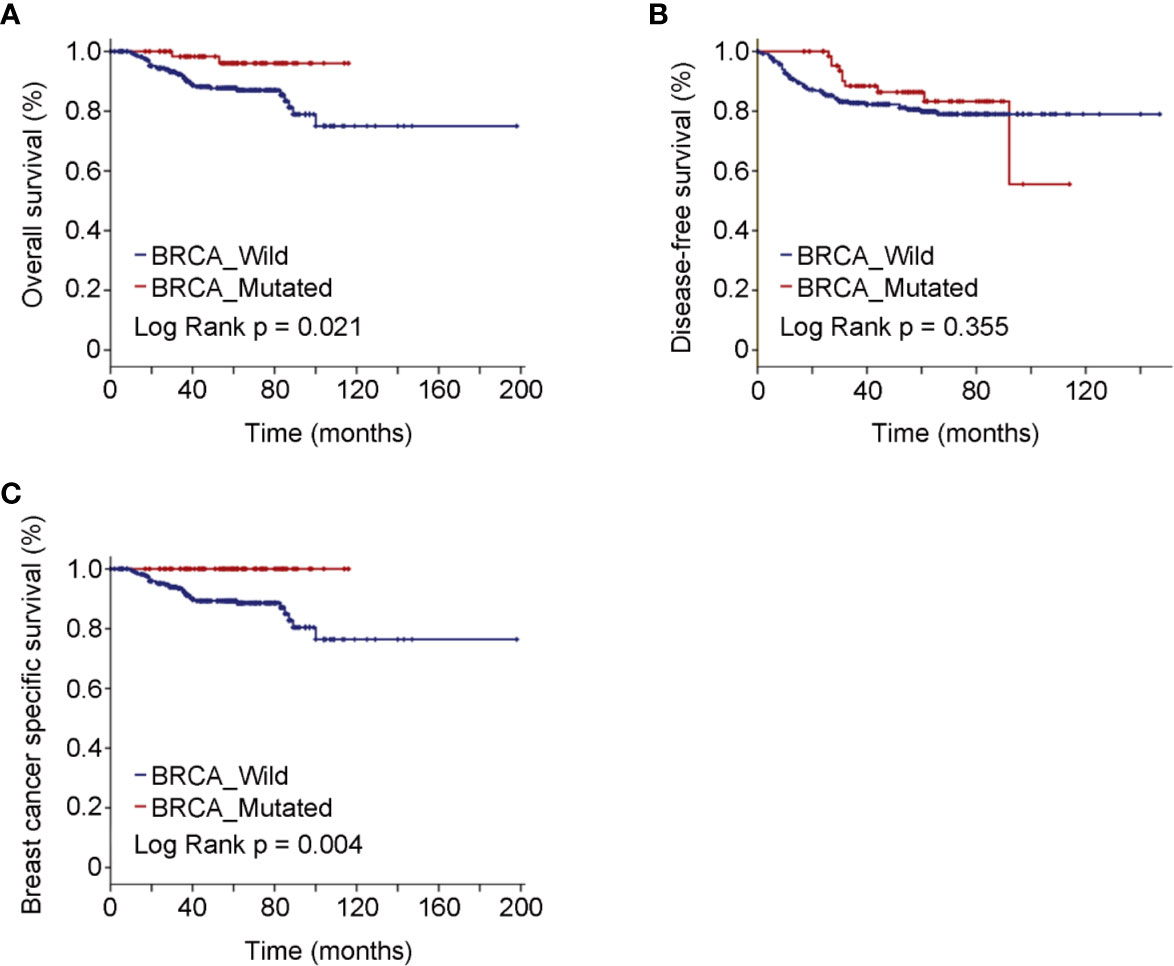
Figure 1 Oncologic outcomes for all patients enrolled in the study cohort by BRCA mutation status. (A) Kaplan–Meier estimates of OS. (B) Kaplan–Meier estimates of DFS. (C) Kaplan–Meier estimates of BCSS.
Treatment Interventions
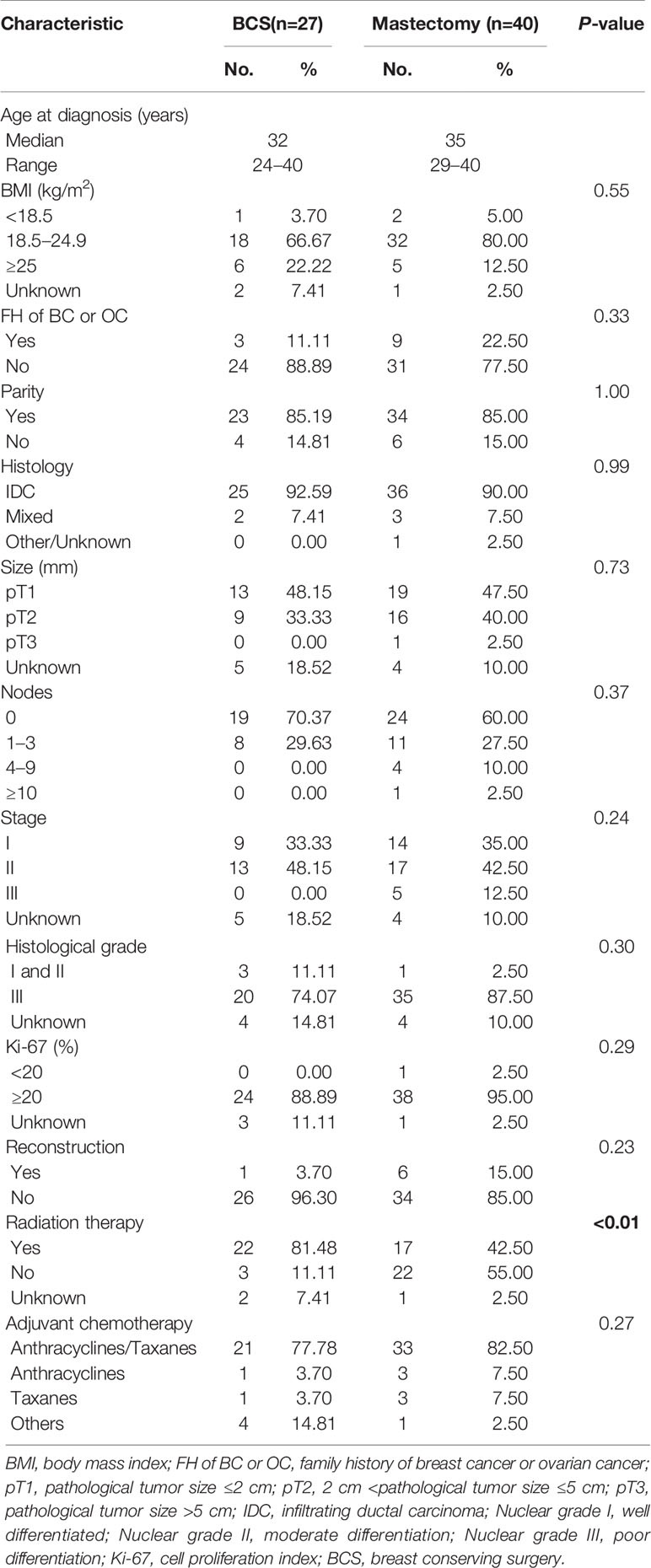
The most frequently used chemotherapy regimen was anthracyclines with or without taxanes, but quite remarkably, no participant was receiving platinum-containing regimen (Table 1). Breast-conserving surgery (BCS) was performed in 40.30% BRCA mutated eTNBC patients and 42.71% BRCA wild patients. The clinicopathologic features of eTNBC patients who underwent BCS were parallel between subgroups divided by BRCA mutation status (Table 2). Clinical outcomes, whether OS, DFS, or BCSS, did not significantly differ between the two subgroups (Figure 2).
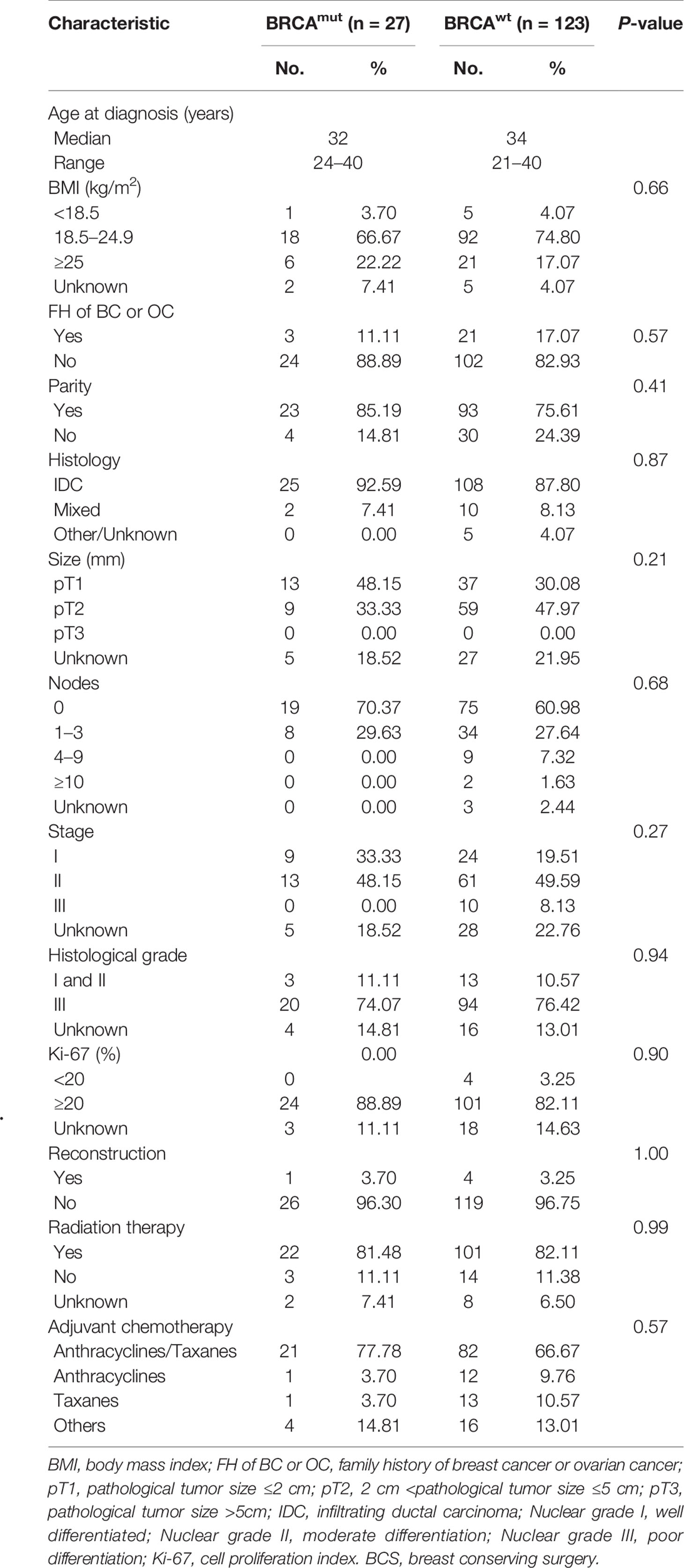
Table 2 Clinicopathologic features of eTNBC patients underwent BCS grouped by germline BRCA1 status.
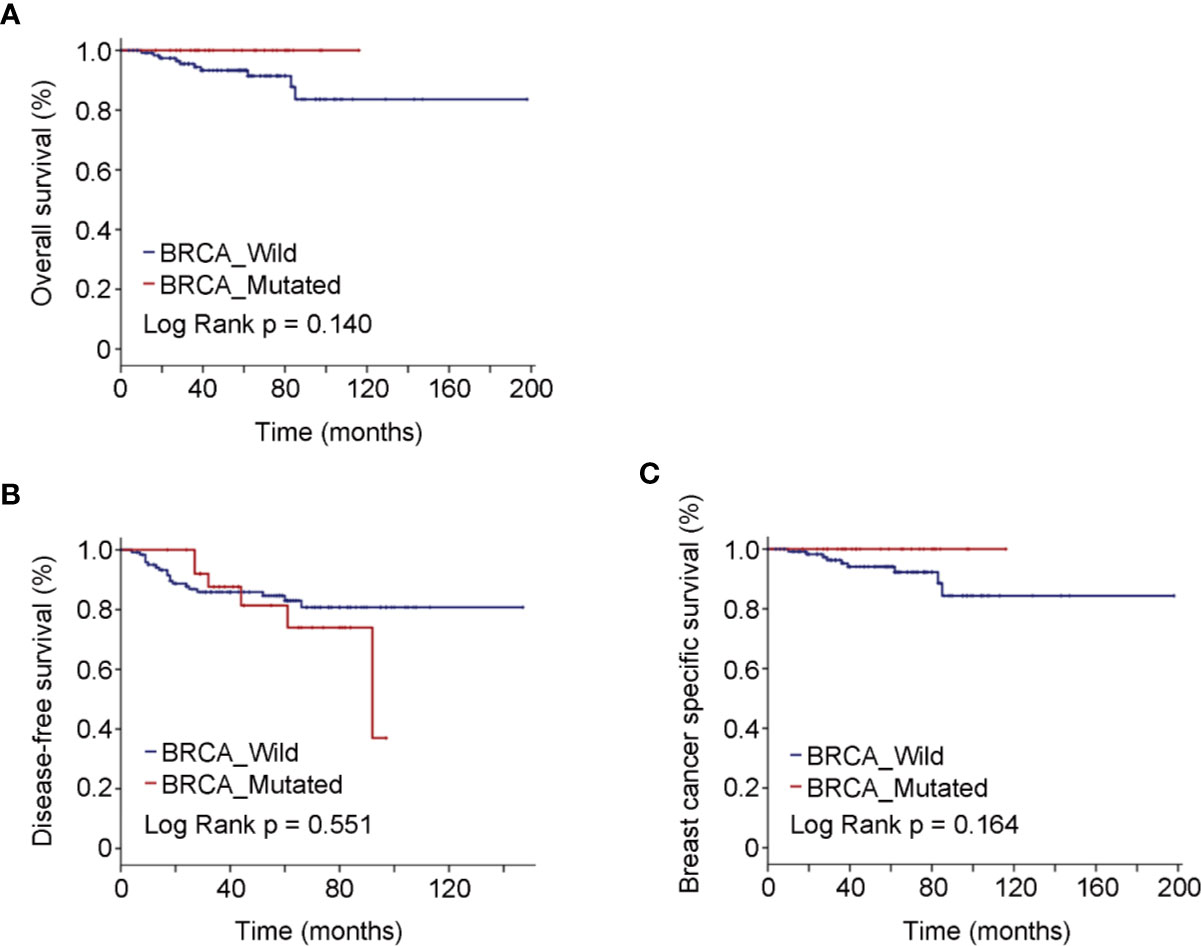
Figure 2 Oncologic outcomes for patients who underwent BCS by BRCA mutation status. (A) Kaplan–Meier estimates of OS. (B) Kaplan–Meier estimates of DFS. (C) Kaplan–Meier estimates of BCSS.
As to the 67 BRCA mutated eTNBC patients grouped by surgical treatment, 27 underwent BCS, and 40 chose mastectomy. Unsurprisingly, radiation therapy was significantly more frequent in those who underwent BCS. Beyond that, basic characteristics were comparable between the two subgroups, as shown in Table 3. Likewise, OS and DFS were not significantly different between the two subgroups (Figure 3). BCSS was not analyzed because of few events and unrepresented statistical power.
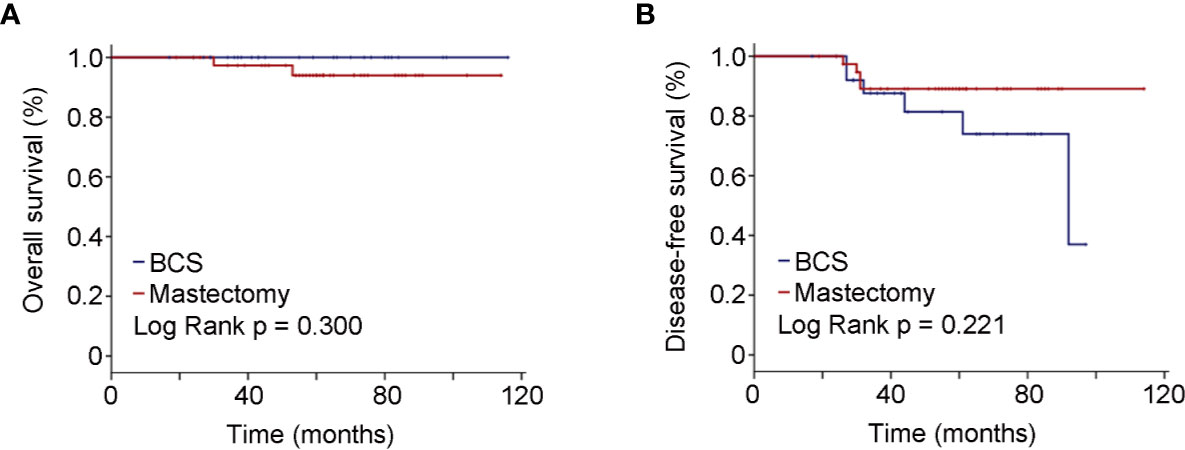
Figure 3 Oncologic outcomes for patients with BRCA positivity by surgical schedules. (A) Kaplan–Meier estimates of OS. (B) Kaplan–Meier estimates of DFS.
Discussion
In this study, we investigated the clinical and pathological characteristics as well as survival outcomes in a cohort of unselected women with eTNBC patients and to what extent these phenotypes could be contributed to BRCA mutation. The prevalence of BRCA mutation in this study was 18.87%, consistent with previous reports mainly based on institutional- or hospital-based samples (21–26). Published reports have indicated that a high BMI was protective against breast cancer risk in premenopausal women (27), and no significant differences in BMI, warning that a higher BMI may be favorable in BRCA mutated eTNBC patients. It was worth noting that FH of BC or OC seemed lower in the current study as opposed to previous reports (22, 25), which suggested 19% or even 90% in young age breast cancer. Generally, BRCA mutated patients were more likely to have a FH of BC or OC than BRCA wild patients (28–30). The fact that patients self-reported family history might not be accurate, resulting in underrepresentation. Additionally, ethnicity and geographical distribution should be taken into consideration.
As to disease characteristics, of note, in agreement with most previous studies (21–23, 25), the tumor burden in BRCA mutated eTNBC patients was comparable with that in BRCA wild ones. Recently, a prospective large cohort study showed no significant difference in tumor size between BRCA mutated and BRCA wild young-onset breast cancer (26). More recently, a retrospective study conducted in Chinese early-onset breast cancer with more than 80% TNBC also failed to demonstrate a significant difference of tumor size (31). Herein, the other clinicopathologic features were in broad agreement with previous reports (23, 32–34), indicating that eTNBC tends to be aggressive irrespective of BRCA mutation.
Although the prognosis of eTNBC patients with BRCA mutation was inclusive, numerous studies failed to document an inferior survival outcome of eTNBC patients carrying BRCA mutation compared with their counterparts (35, 36). Confirming the previous studies (26, 37, 38), OS and BCSS of eTNBC patients with BRCA mutation were superior to those of their BRCA wild counterparts; however, DFS was not significantly different between the two subgroups. It should be noted that greater sensitivity to adjuvant systemic therapy was probable the key factor resulting in better survival outcomes, under the circumstance of equality of disease characteristics and stage at diagnosis in the current study. Up to now, the optimal treatment for eTNBC patients with BRCA mutation was largely unknown and remained a matter of debate. Interestingly, in view of patients underwent BCS, the survival outcomes were similar between the two subgroups with similar baseline traits. Simultaneously, the same tendency was revealed in patients with BRCA mutated eTNBC, no matter what surgical procedures were performed. In recent decades, although the risk for recurrent breast cancer or contralateral breast cancer was higher in BRCA mutated TNBC, advances in biology and systemic therapy have decreased the risk to an acceptable level (39–42). Thus, it was rational to propose that BCS was a safe and feasible option for patients with BRCA-associated eTNBC if systemic therapy was available and tolerable.
The current study has some strengths. BRCA genetic testing was performed in all participants to avoid referral bias, and the testing results were available to physicians in decision-making treatment. Furthermore, the comprehensive details of demographic and clinicopathologic characteristics were derived from medical records to reduce the potential bias induced by using questionnaires. One limitation of this study was that the FH was obtained from individual report rather than medical records. Another limitation of this study was that the total number of the study cohort was small, especially of the BRCA2 positive subgroup, meaning we were unable to distinguish the effect of BRCA1 on prognosis from that of BRCA2. Besides, the contribution of other factors, such as neoadjuvant therapies, prophylactic surgeries, on the outcomes were unavailable. Future studies that recruit larger sample sizes with precise FH are needed to offer an extrapolative conclusion.
In conclusion, our results suggested that eTNBC patients with BRCA mutations tend to have better OS and BCSS, which might be attributed to more benefit from systemic therapy. The BCS procedure would be a safe alternative surgical option to early-stage BRCA mutation eTNBC patients on condition that systemic therapy was available and tolerable. Future studies with large size and comprehensive clinicopathologic details are urged to validate whether BRCA mutation patients are more sensitive to chemotherapy.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethical Committee of the Shanghai Cancer Center of Fudan University. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
AC conceptualized and designed the study. FY and AC provided the study patients and methods. FY, MH, LH, GL, XH, ZS, GD and AC collected the data. FY and AC analyzed and interpreted the data. FY and AC wrote the manuscript. FY, MH, LH, GL, XH, ZS, GD and AC gave the final approval of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
The authors are grateful for the financial support of the Shanghai Science and Technology Innovation Action Plan (20ZR1412000).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Gabriel CA, Domchek SM. Breast cancer in young women. Breast Cancer Res (2010) 12(5):212. doi: 10.1186/bcr2647
2. Lee H-B, Han W. Unique features of young age breast cancer and its management. J Breast Cancer (2014) 17(4):301–7. doi: 10.4048/jbc.2014.17.4.301
3. Anastasiadi Z, Lianos GD, Ignatiadou E, Harissis HV, Mitsis M. Breast cancer in young women: an overview. Updates Surg (2017) 69(3):313–7. doi: 10.1007/s13304-017-0424-1
4. Claus EB, Risch NJ, Thompson WD. Age at onset as an indicator of familial risk of breast cancer. Am J Epidemiol (1990) 131(6):961–72. doi: 10.1093/oxfordjournals.aje.a115616
6. Kuchenbaecker KB, Hopper JL, Barnes DR, Phillips K-A, Mooij TM, Roos-Blom M-J, et al. Risks of Breast, Ovarian, and Contralateral Breast Cancer for BRCA1 and BRCA2 Mutation Carriers. JAMA (2017) 317(23):2402–16. doi: 10.1001/jama.2017.7112
7. Chen S, Parmigiani G. Meta-analysis of BRCA1 and BRCA2 penetrance. J Clin Oncol (2007) 25(11):1329–33. doi: 10.1200/JCO.2006.09.1066
8. Antoniou A, Pharoah PDP, Narod S, Risch HA, J Eyfjord JE, Hopper JL, et al. Average risks of breast and ovarian cancer associated with BRCA1 orBRCA2 mutations detected in case Series unselected for family history: a combined analysis of 22 studies. Am J Hum Genet (2003) 72(5):1117–30. doi: 10.1086/375033
9. Armes JE, Trute L, White D, Southey MC, Hammet F, Tesoriero A, et al. Distinct molecular pathogeneses of early-onset breast cancers in BRCA1 and BRCA2 mutation carriers: a population-based study. Cancer Res (1999) 59(8):2011–7.
10. Perou CM, Sørlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, et al. Molecular portraits of human breast tumours. Nature (2000) 406(6797):747–52. doi: 10.1038/35021093
11. Hartman A-R, Kaldate RR, Sailer LM, Painter L, Grier CE, Endsley RR, et al. Prevalence of BRCA mutations in an unselected population oftriple-negative breast cancer. Cancer (2012) 118(11):2787–95. doi: 10.1002/cncr.26576
12. Atchley DP, Albarracin CT, Lopez A, Valero V, Amos CI, Gonzalez-Angulo AM, et al. Clinical and pathologic characteristics of patients withBRCA-positive and BRCA-negative breast cancer. J Clin Oncol (2008) 26(26):4282–8. doi: 10.1200/jco.2008.16.6231
13. Foulkes WD, Stefansson IM, Chappuis PO, Bégin LR, Goffin JR, Wong N, et al. Germline BRCA1 mutations and a basal epithelial phenotype in breastcancer. J Natl Cancer Inst (2003) 95(19):1482–5. doi: 10.1093/jnci/djg050
14. Laakso M, Loman N, Borg A, Isola J. Cytokeratin 5/14-positive breast cancer: true basal phenotypeconfined to BRCA1 tumors. Mod Pathol (2005) 18(10):1321–8. doi: 10.1038/modpathol.3800456
15. Krainer M, Silva-Arrieta S, FitzGerald MG, Shimada A, Ishioka C, Kanamaru R, et al. Differential contributions of BRCA1 and BRCA2 to early-onset breastcancer. N Engl J Med (1997) 336(20):1416–21. doi: 10.1056/nejm199705153362003
16. Moynahan ME, Chiu JW, Koller BH, Jasin M. Brca1 controls homology-directed DNA repair. Mol Cell (1999) 4(4):511–8. doi: 10.1016/S1097-2765(00)80202-6
17. Tutt A, Bertwistle D, Valentine J, Gabriel A, Swift S, Ross G. Mutation in Brca2 stimulates error-prone homology-directed repair of DNA double-strand breaks occurring between repeated sequences. EMBO J (2001) 20(17):4704–16. doi: 10.1093/emboj/20.17.4704
18. Farmer H, McCabe N, Lord CJ, Tutt ANJ, Johnson DA, Richardson TB, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature (2005) 434(7035):917–21. doi: 10.1038/nature03445
19. Yadav BS, Sharma SC, Chanana P, Jhamb S. Systemic treatment strategies for triple-negative breast cancer. World J Clin Oncol (2014) 5(2):125–33. doi: 10.5306/wjco.v5.i2.125
20. Lang G-T, Shi J-X, Hu X, Zhang C-H, Shan L, Song C-G, et al. The spectrum of BRCA mutations and characteristics of BRCA-associated breast cancers in China: Screening of 2,991 patients and 1,043 controls by next-generation sequencing. Int J Cancer (2017) 141(1):129–42. doi: 10.1002/ijc.30692
21. Pal T, Bonner D, Kim J, Monteiro ANA, Kessler L, Royer R, et al. Early onset breast cancer in a registry-based sample of African-american women: BRCA mutation prevalence, and other personal and system-level clinical characteristics. Breast J (2013) 19(2):189–92. doi: 10.1111/tbj.12083
22. Robson M, Gilewski T, Haas B, Levin D, Borgen P, Hirschaut PRY, et al. BRCA-associated breast cancer in young women. J Clin Oncol (1998) 16(5):1642–9. doi: 10.1200/JCO.1998.16.5.1642
23. Musolino A, Bella MA, Bortesi B, Michiara M, Naldi N, Zanelli P, et al. BRCA mutations, molecular markers, and clinical variables in early-onset breast cancer: a population-based study. Breast (Edinburgh Scotland) (2007) 16(3):280–92. doi: 10.1016/j.breast.2006.12.003
24. Pal T, Bonner D, Cragun D, Monteiro ANA, Phelan C, Servais L, et al. A high frequency of BRCA mutations in young black women with breast cancer residing in Florida. Cancer (2015) 121(23):4173–80. doi: 10.1002/cncr.29645
25. Bayraktar S, Amendola L, Gutierrez-Barrera AM, Hashmi SS, Amos C, Gambello M, et al. Clinicopathologic characteristics of breast cancer in BRCA-carriers and non-carriers in women 35 years of age or less. Breast (Edinburgh Scotland) (2014) 23(6):770–4. doi: 10.1016/j.breast.2014.08.010
26. Copson ER, Maishman TC, Tapper WJ, Cutress RI, Greville-Heygate S, Altman DG, et al. Germline BRCA mutation and outcome in young-onset breast cancer (POSH): a prospective cohort study. Lancet Oncol (2018) 19(2):169–80. doi: 10.1016/S1470-2045(17)30891-4
27. Gonzalez-Angulo AM, Broglio K, Kau S-W, Eralp Y, Erlichman J, Valero V, et al. Women age < or = 35 years with primary breast carcinoma: disease features at presentation. Cancer (2005) 103(12):2466–72. doi: 10.1002/cncr.21070
28. Kenen R, Ardern-Jones A, Eeles R. Family stories and the use of heuristics: women from suspected hereditary breast and ovarian cancer (HBOC) families. Sociol Health Illn (2003) 25(7):838–65. doi: 10.1046/j.1467-9566.2003.00372.x
29. Narod SA. Modifiers of risk of hereditary breast cancer. Oncogene (2006) 25(43):5832–6. doi: 10.1038/sj.onc.1209870
30. Frank TS, Deffenbaugh AM, Hulick M, Gumpper K. Hereditary susceptibility to breast cancer: significance of age of onset in family history and contribution of BRCA1 and BRCA2. Dis Markers (1999) 15:89–92. doi: 10.1155/1999/291023
31. Chen L, Fu F, Huang M, Lv J, Zhang W, Wang C, et al. The spectrum of BRCA1 and BRCA2 mutations and clinicopathological characteristics in Chinese women with early-onset breast cancer. Breast Cancer Res Treat (2020) 180(3):759–66. doi: 10.1007/s10549-020-05573-x
32. Ryu JM, Choi HJ, Kim I, Nam SJ, Kim SW, Yu J, et al. Prevalence and oncologic outcomes of BRCA 1/2 mutations in unselected triple-negative breast cancer patients in Korea. Breast Cancer Res Treat (2019) 173(2):385–95. doi: 10.1007/s10549-018-5015-4
33. Li W-F, Hu Z, Rao N-Y, Song C-G, Zhang B, Cao M-Z, et al. The prevalence of BRCA1 and BRCA2 germline mutations in high-risk breast cancer patients of Chinese Han nationality: two recurrent mutations were identified. Breast Cancer Res Treat (2008) 110(1):99–109. doi: 10.1007/s10549-007-9708-3
34. Shannon C, Smith IE. Breast cancer in adolescents and young women. Eur J Cancer (Oxford Engl 1990) (2003) 39(18):2632–42. doi: 10.1016/S0959-8049(03)00669-5
35. Turchetti D, Cortesi L, Federico M, Bertoni C, Mangone L, Ferrari S, et al. BRCA1 mutations and clinicopathological features in a sample of Italian women with early-onset breast cancer. Eur J Cancer (Oxford Engl 1990) (2000) 36(16):2083–9. doi: 10.1016/S0959-8049(00)00287-2
36. Porter DE, Dixon M, Smyth E, Steel CM. Breast cancer survival in BRCA1 carriers. Lancet (London England) (1993) 341(8838):184–5. doi: 10.1016/0140-6736(93)90052-I
37. Gonzalez-Angulo AM, Timms KM, Liu S, Chen H, Litton JK, Potter J, et al. Incidence and outcome of BRCA mutations in unselected patients with triple receptor-negative breast cancer. Clin Cancer Res (2011) 17(5):1082–9. doi: 10.1158/1078-0432.CCR-10-2560
38. Bayraktar S, Gutierrez-Barrera AM, Liu D, Tasbas T, Akar U, Litton JK, et al. Outcome of triple-negative breast cancer in patients with or without deleterious BRCA mutations. Breast Cancer Res Treat (2011) 130(1):145–53. doi: 10.1007/s10549-011-1711-z
39. Valachis A, Nearchou AD, Lind P. Surgical management of breast cancer in BRCA-mutation carriers: a systematic review and meta-analysis. Breast Cancer Res Treat (2014) 144(3):443–55. doi: 10.1007/s10549-014-2890-1
40. Shi Y, Jin J, Ji W, Guan X. Therapeutic landscape in mutational triple negative breast cancer. Mol Cancer (2018) 17(1):99. doi: 10.1186/s12943-018-0850-9
41. Eskiler GG, Cecener C, Egeli U, Tunca B. Triple negative breast cancer: new therapeutic approaches and BRCA status. APMIS Acta pathologica microbiologica immunologica Scandinavica (2018) 126(5):371–9. doi: 10.1111/apm.12836
Keywords: early-onset, triple-negative, breast cancer, breast cancer type 1 susceptibility protein, mutation
Citation: Ye F, He M, Huang L, Lang G, Hu X, Shao Z, Di G and Cao A (2021) Insights Into the Impacts of BRCA Mutations on Clinicopathology and Management of Early-Onset Triple-Negative Breast Cancer. Front. Oncol. 10:574813. doi: 10.3389/fonc.2020.574813
Received: 21 June 2020; Accepted: 24 November 2020;
Published: 11 January 2021.
Edited by:
Marilena Valeria Iorio, Istituto Nazionale dei Tumori (IRCCS), ItalyReviewed by:
Agostina Stradella, Catalan Institute of Oncology, SpainBruce Kimler, University of Kansas Medical Center, United States
Copyright © 2021 Ye, He, Huang, Lang, Hu, Shao, Di and Cao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ayong Cao, caoayong0309@sina.com
 Fugui Ye1
Fugui Ye1 Xin Hu
Xin Hu Zhimin Shao
Zhimin Shao Ayong Cao
Ayong Cao