- 1State Key Laboratory of Cancer Biology, Department of Pathology, Xijing Hospital and School of Basic Medicine, Fourth Military Medical University (Air Force Medical University), Xi’an, China
- 2Department of Cardiology, Xijing Hospital, Fourth Military Medical University (Air Force Medical University), Xi’an, China
EWSR1::SMAD3-rearranged fibroblastic tumor is a recently described entity that mostly occurs in acral locations. Only 15 cases have been reported in the English literature, with a wide age range and marked female predominance. The most common sites are the foot, followed by the hand and the distal lower leg. There are four cases that recurred locally during 5–120 months of follow-up, with no metastases to date. Herein, we presented a case of EWSR1::SMAD3-rearranged fibroblastic tumor that recurred twice in a 20-year-old man. The patient presented with a second recurrent painful nodule in the left plantar of the second toe. Grossly, the lesion was pale solid and well-defined, measuring 9 × 8 × 9 mm in size. Histological examination revealed a monomorphic spindle cell tumor composed of cellular fascicles of bland fibroblasts in a collagenous to myxoid stroma with low mitotic activity, which evoked a wide spectrum of differential diagnoses. Immunohistochemically, the tumor cells were diffusely and strongly positive for ERG while negative for S100, α-SMA, CD34, and other vascular markers. An unbalanced rearrangement of EWSR1 was demonstrated by fluorescence in situ hybridization (FISH), and a gene fusion between EWSR1 exon 7 and SMAD3 exon 6 was confirmed by RT-PCR and Sanger sequencing. This case recurred twice within 6 years with no sign of further relapse and metastasis at another 9-month follow-up since the last surgery, indicating that this tumor was benign but prone to local recurrence. Nevertheless, more cases and further studies are needed to better interpret the biological behavior of this new entity.
Introduction
EWSR1::SMAD3-rearranged fibroblastic tumor is currently classified as an emerging entity in the fifth edition of the WHO classification of Tumors of Soft Tissue and Bone (1). Only 15 cases have been reported in the English literature (2–6). The tumor mostly occurs in superficial soft tissue at the extremities, with a marked female predominance. They appear to be benign tumors but are prone to local recurrence. The differential diagnoses are challenging, including a series of benign, borderline, and malignant fibroblastic lesions at acral sites. Herein, we present a male case of EWSR1::SMAD3-rearranged fibroblastic tumor with twice recurrence in the left plantar of the second toe. The clinical, histopathological, and molecular characteristics of this tumor along with differential diagnoses are summarized to increase awareness of this new entity among pathologists.
Case description
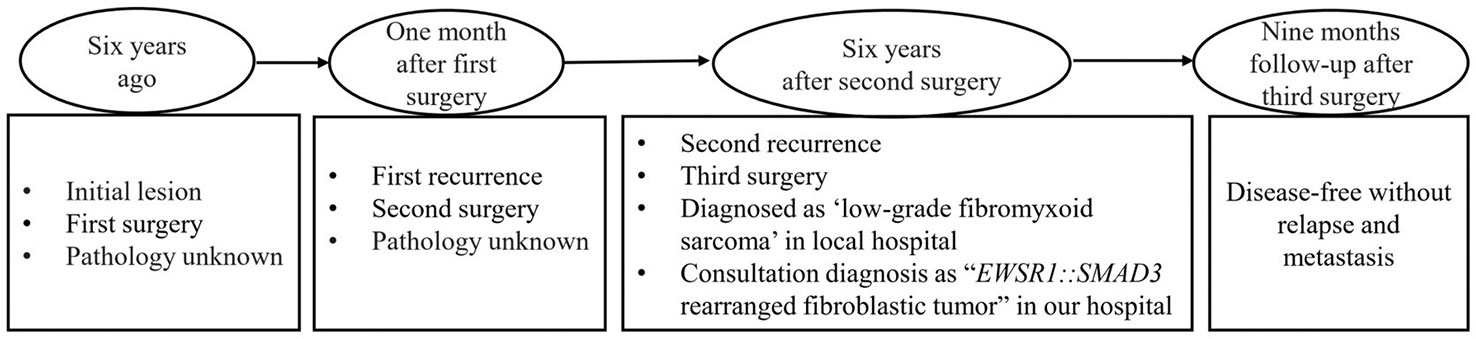
The patient was a 20-year-old man of Chinese descent presenting with a painful nodule in the left plantar of the second toe for 1 month. For past history, the patient had surgery twice on the same site of the toe 6 years ago and relapsed 1 month later, and there was no other treatment except surgery. Unfortunately, the pathological data were not available, and the operating margin was not known. For this time, the nodule was subcutaneous and palpable. The third surgical resection was performed, and a diagnosis of “low-grade fibromyxoid sarcoma” was made in a local hospital. For further medical attention, the pathological materials were referred to our department for consultation. The clinical follow-up period was 9 months since the last surgery. The timeline is summarized in Figure 1.
Diagnostic assessment
Clinicopathological characteristics
Grossly, the lesion was pale solid, and well-defined measuring 9 × 8 × 9 mm in size. Histological examination revealed a relatively well-defined and nodular tumor without a capsule (Figure 2A). The tumor was composed of short fascicles of uniform spindle cells in a collagenous to myxoid stroma. The tumor cells had pale eosinophilic cytoplasm and elongated nuclei with finely dispersed chromatin and small inconspicuous nucleoli (Figures 2B, C). Mitotic figures were rare.
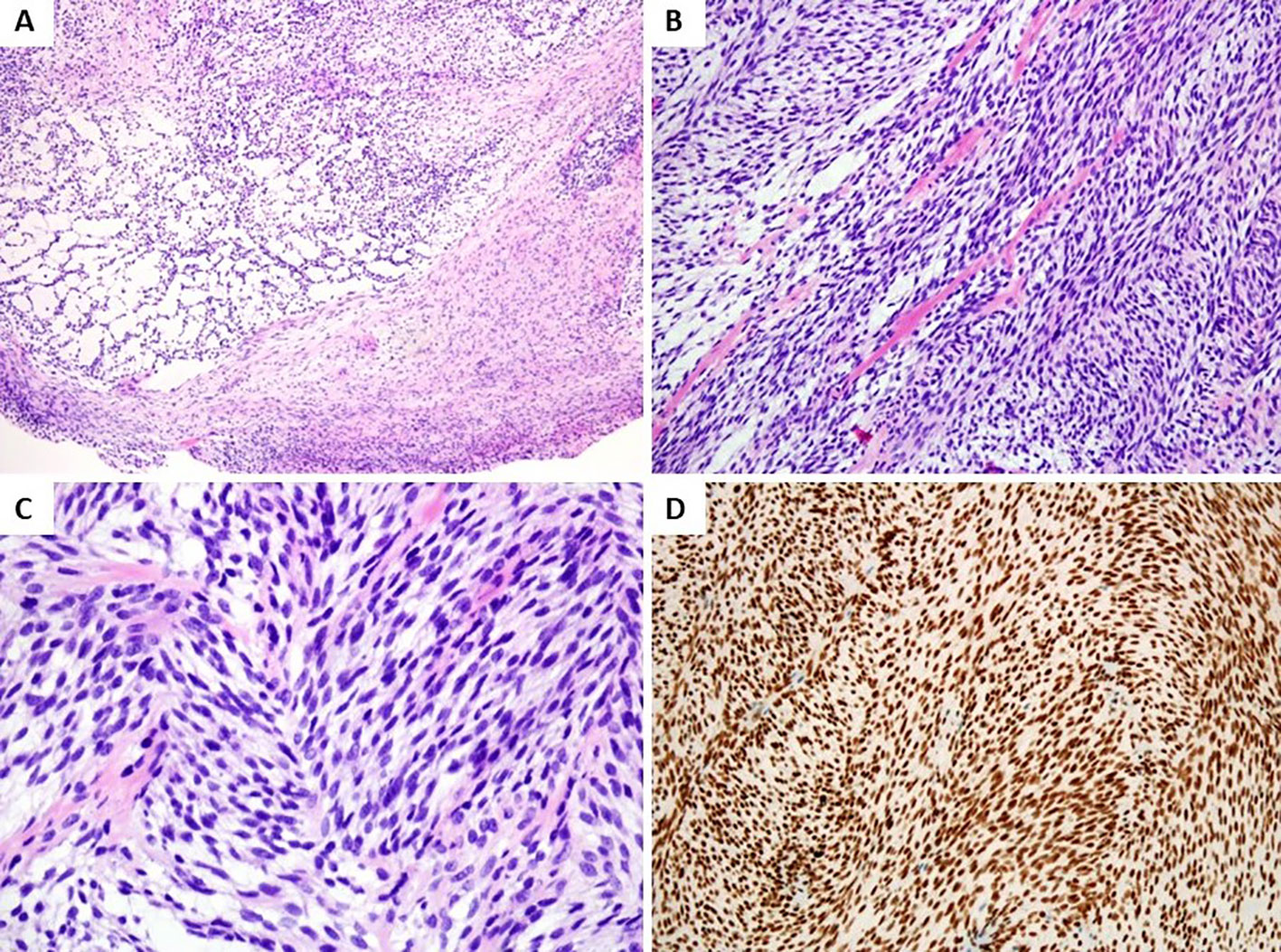
Figure 2 Histologic and immunohistochemical features. (A) Histological examination revealed a relatively well-defined, nodular tumor without capsule (H&E, low magnification). (B) Monomorphic spindle cell tumor composed of cellular fascicles of bland fibroblasts with hyalinization and mucoid degeneration (H&E, medium magnification). (C) Spindle cells showed elongated nuclei with finely dispersed chromatin and small inconspicuous nucleoli (H&E, high magnification). (D) Tumor cells showed diffuse nuclear staining for ERG (medium magnification).
Immunohistochemistry
Immunohistochemical staining was performed on 4-µm formalin-fixed, paraffin-embedded sections, which were immersed in a 10-mM sodium citrate buffer (pH 6) for 20 min at 97°C for antigen retrieval. The following antibodies were used: pankeratin (AE1/AE3, 1:50; Dako, Glostrup, Denmark), epithelial membrane antigen (EMA) (E29, 1:200; Dako), bcl-2 (clone 124, 1:100; Dako), CD34 (QB End 10, 1:100; Dako), α-smooth muscle actin (α-SMA) (1A4, 1:200; Dako), desmin (clone D33, 1:200; Dako), S100 protein (polyclonal, 1:800; Dako), SOX10 (polyclonal, 1:200; Santa Cruz Biotechnology, Dallas, TX, USA), H3K27me3 (RM175, 1:2,000, RevMAb, South San Francisco, CA, USA), MUC4 (8G7, 1:100; Abcam, Cambridge, UK), ERG (EPR3864, prediluted; Roche, Basel, Switzerland), SATB2 (SATBA4B10, 1:100, ZSGB), and Ki67 (MIB1, 1:150; Dako).The positive, blank, and negative controls were also performed in parallel.
By immunohistochemistry, the spindle tumor cells showed diffusely and strongly nuclear positive for ERG (Figure 2D), while S100, α-SMA, CD34, desmin, SATB2, EMA, MUC4, and other spindle cell tumor and vascular markers were negative. Ki67 index was about 15% in tumors.
Molecular genetic studies
Fluorescence in situ hybridization
According to the manufacturer’s instructions, fluorescence in situ hybridization (FISH) was performed on formalin-fixed, paraffin-embedded tissue sections. Unstained 4-µm sections were placed on electrostatically charged slides and then evaluated using an EWSR1 (22q12) rearrangement in a dual-color, break-apart probe and an EWSR1 (22q12, green) and SMAD3 (15q22, red) fusion probe (LBP, Guangzhou, China). Under fluorescence microscopy, signals from 200 non-overlapping nuclei were counted. The cutoff level for the score as positive was when at least 20% of the nuclei showed a break-apart signal and/or loss of telomeric part or fusion signal (yellow).
The FISH assessment demonstrated an unbalanced rearrangement of EWSR1 with loss of the telomeric part (Figure 3A), combined with tumor cells, diffuse nuclear staining for ERG the diagnosis of EWSR1::SMAD3-rearranged fibroblastic tumor was considered. To identify the fusion partner, EWSR1 and SMAD3 fusion was detected by FISH (Figure 3B), and the fusion was confirmed by RT-PCR and Sanger sequencing between exon 7 of EWSR1 and exon 6 of SMAD3 (Figure 3C).
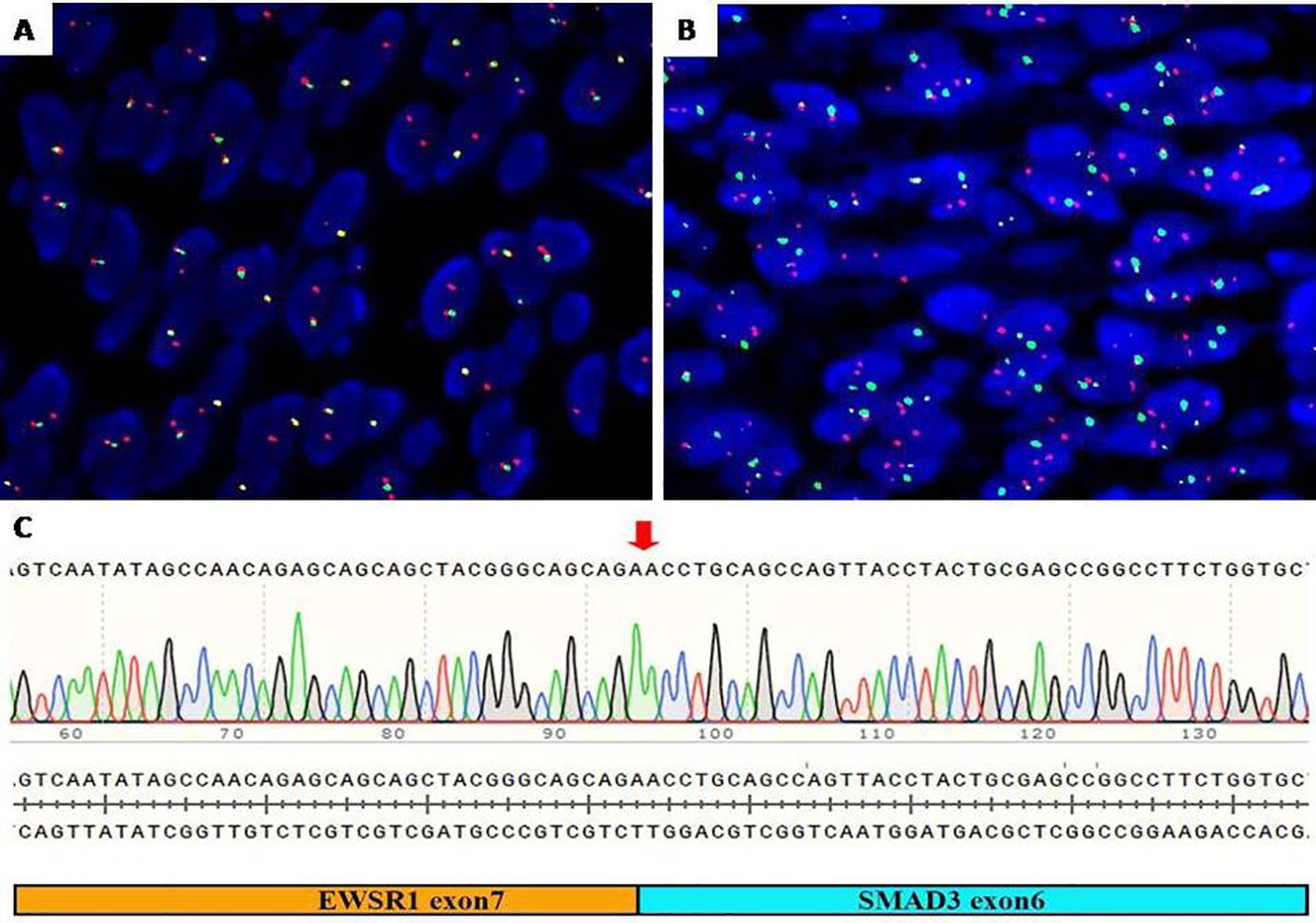
Figure 3 Molecular detection. (A) FISH assay showed an unbalanced rearrangement of EWSR1 with loss of the telomeric part (green). (B) FISH assay showed EWSR1 (green) and SMAD3 (red) fusion signal (yellow). (C) RT-PCR identified exon 7 of EWSR1 and exon 6 of SMAD3 gene fusion. FISH, fluorescence in situ hybridization.
Follow-up study
Based on the morphology, immunohistochemistry, and gene analysis, the final diagnosis was EWSR1::SMAD3-rearranged fibroblastic tumor. Treatment of the tumor was wide excision. To date, the patient is disease-free without relapse and metastasis for 9 months after the last surgery.
Discussion
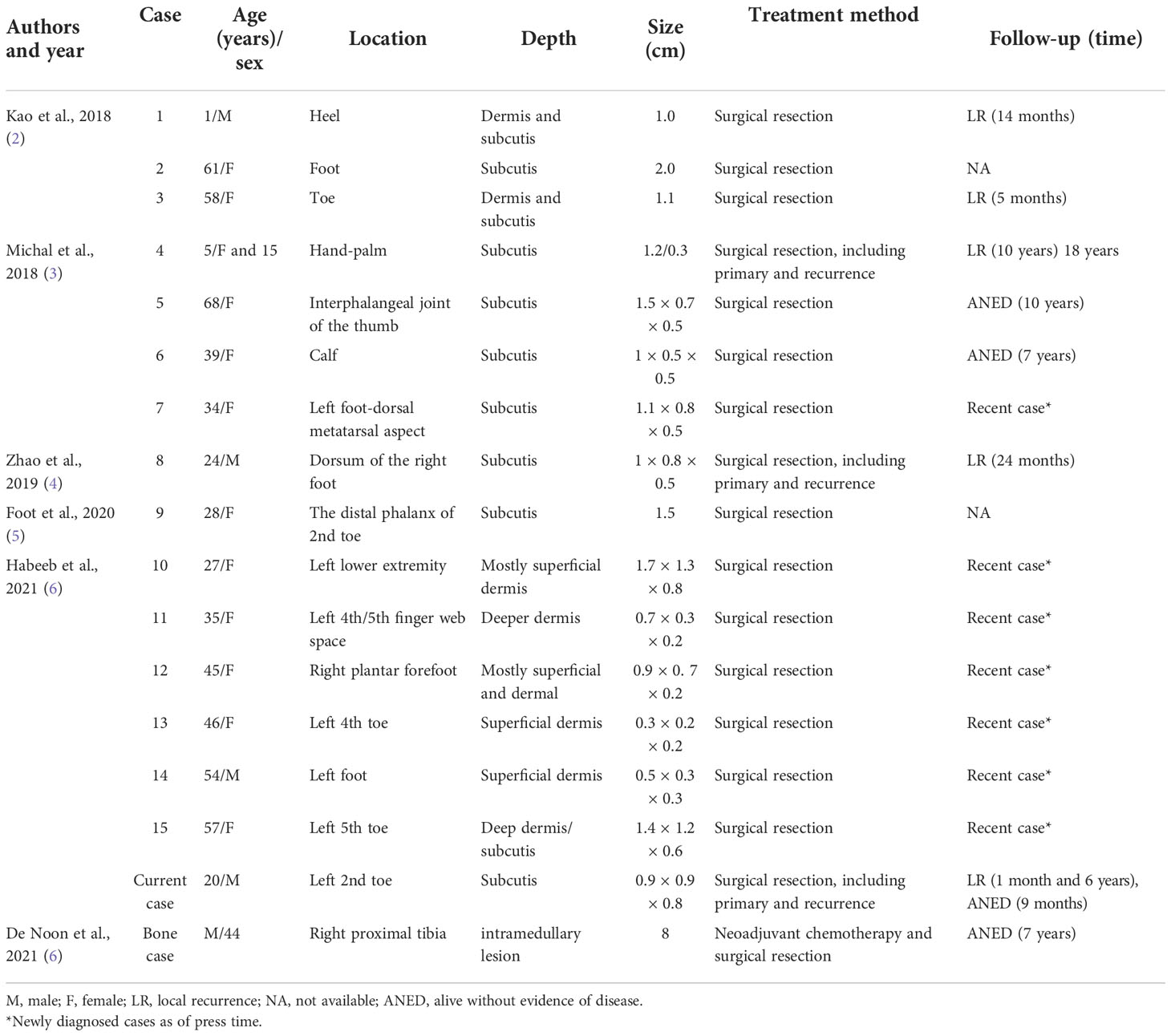
EWSR1::SMAD3-rearranged fibroblastic tumor is a new entity that mostly occurs at the extremities, initially reported in the foot of a 1-year-old infant, presenting with an ill-defined dermal and subcutaneous nodule (2). Thereafter, another series described similar cases and suggested the name EWSR1::SMAD3-rearranged fibroblastic tumor (3). However, only 15 cases have been reported in the English literature to our knowledge, of which the median age was 39 years (range 1–68 years) with a female predilection (10/15, 67%). The most common affected site was the foot (10 cases), and the other three cases were in the hand and two in the distal lower leg. Clinically, all tumors presented as a dermal or subcutaneous nodule or mass ranging in size from 1 to 2 cm (mean, 1.13 cm; median, 1.1 cm). Four cases recurred locally (4/6, 67%) during 5–120 months of follow-up mostly due to incomplete excision, but without metastases to date (2–6). Our case recurred twice within 6 years, indicating that a long-term follow-up needs to be warranted. The series of cases have long-term follow-up information in only seven cases, including what we report in this case, five cases of recurrence, and only two cases without relapse in the follow-up of 7 and 10 years later. The remaining six cases with follow-up information were newly reported, and more cases of follow-up information need to be collected on the biological behavior of this group of tumors. The data are summarized in Table 1.
Recently, another EWSR1::SMAD3-rearranged fibroblastic tumor reported in the bone was supported by imaging and histopathological features (7). The tumor was relatively large (8 cm) with aggressive radiological features and initially diagnosed as an unusual cartilaginous tumor, raising the possibility of chondrosarcoma (possibly dedifferentiated or mesenchymal). Histologically, focal cytological atypia and necrosis were observed. Immunohistochemically, the tumor cells showed very focal ERG nuclear expression. The lesion was treated with complete excision and has shown no signs of relapse over a 7-year follow-up period. Although EWSR1::SMAD3-rearranged fibroblastic tumor is currently considered benign in soft tissue, the biological behavior of the bone lesion cannot be inferred from a single case (8).
The cellularity and fascicular morphology of EWSR1::SMAD3-rearranged fibroblastic tumor may lead to confusion with other histologic mimics of both benign and malignant soft tissue tumors (9), including fibromatosis, cellular schwannoma, low-grade malignant peripheral nerve sheath tumor (MPNST), monophasic synovial sarcoma, and low-grade fibromyxoid sarcoma. This neoplasm was composed of uniform fibroblastic spindle cells without nuclear atypia, pleomorphism, prominent nucleoli, and mitotic activity. In some cases, a distinctive zonation pattern with acellular central hyalinization and peripheral areas of cellular spindle cell fascicles was present, especially in adults. Stippled dystrophic calcification was found in one-part cases (2, 4). In addition, our case also showed an alternative myxoid change of the stroma, which could be misdiagnosed as a low-grade fibromyxoid sarcoma and low-grade MPNST. Immunohistochemically, the tumor characteristically showed diffuse and strong nuclear staining of ERG and focal and weak expression of SATB2 in a small number of cases but was usually negative for S100, SMA, CD34, and other vascular markers, excluding the endothelial, neurogenic, and smooth muscle differentiation of neoplastic cells. The ubiquitous staining of ERG in the tumor is considered to be attributed to the overexpression of ERG, which was revealed by mRNA expression that was significantly upregulated at even higher levels than vascular neoplasms (2, 10).
SMAD3 is an important signal transducer in the TGF-β/SMAD signaling pathway, which is involved in extracellular matrix synthesis by fibroblasts (2, 11). EWSR1 gene rearrangement has been found in many bone and soft tissue tumors (12, 13), including Ewing or Ewing-like sarcoma, low-grade fibromyxoid sarcoma, and spindle cell rhabdomyosarcoma, and EWSR1::SMAD3-rearranged fibroblastic tumor should be added to the list. EWSR1 gene rearrangement was most common in Ewing or Ewing-like sarcoma; the tumor cells were uniformly small and round and expressed CD99, ERG, NKX2.2, and most differently, the fusion partners with EWSR1 including FLI-1, ERG, and other candidate genes. Low-grade fibromyxoid sarcoma may have been an EWSRI-CREB3L1 fusion, but MUC4 is highly sensitive and specific immunohistochemistry. Spindle cell rhabdomyosarcoma sometimes appeared in EWSR1-TFCP2 fusion, combined with immunohistochemical markers such as desmin, myogenin, and MyoD1; the diagnosis can be confirmed.
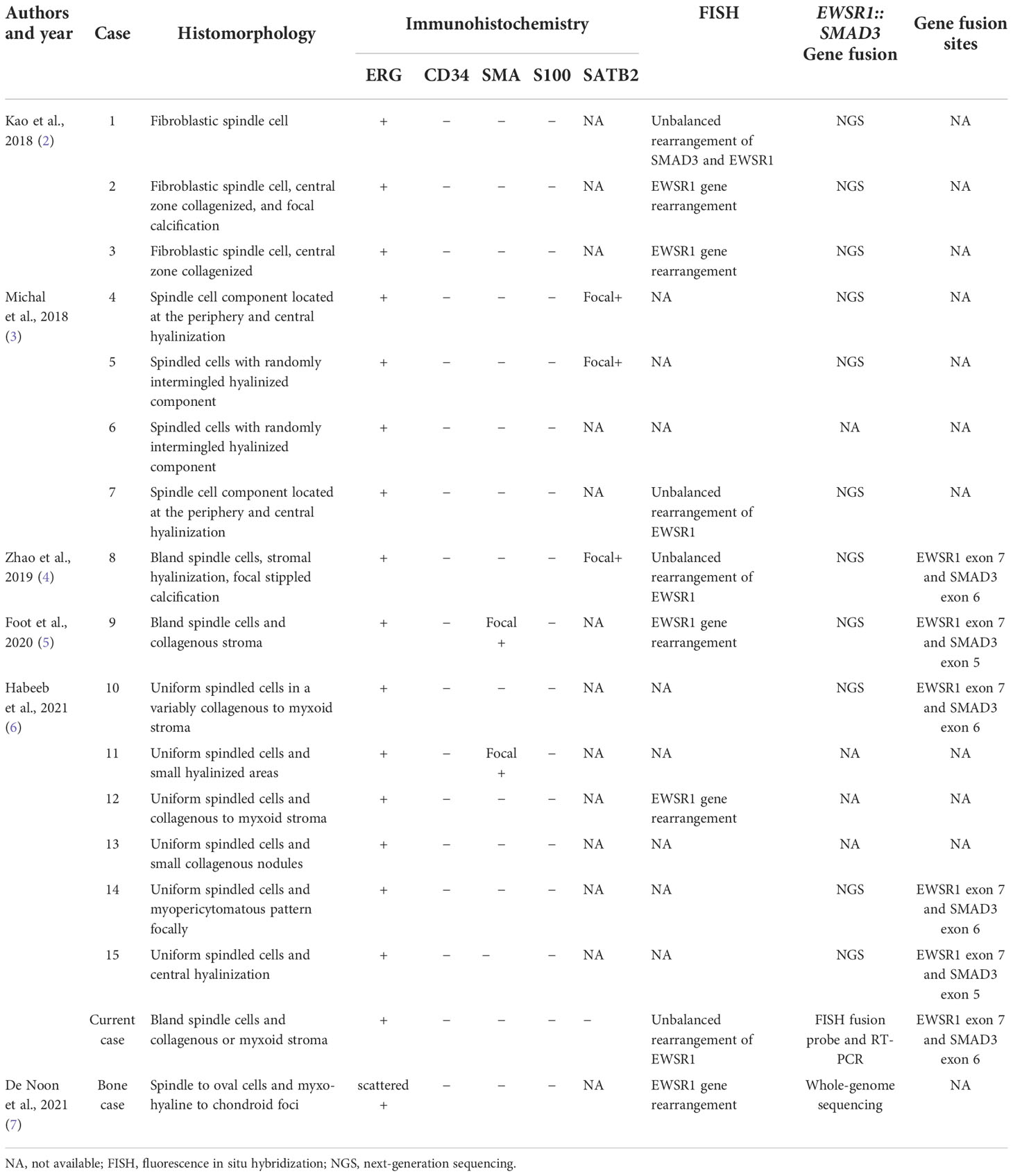
Identification of EWSR1::SMAD3-rearranged fibroblastic tumor is critical to provide prognostic information and guide appropriate therapeutic decisions and most importantly avoid overtreatment (9, 14, 15). When encountered with uniform spindle cells in a collagenous to myxoid stroma, diffuse and strong ERG expression will render a clue for EWSR1::SMAD3-rearranged fibroblastic tumor. EWSR1 rearrangement by FISH would help to give a correct interpretation. The data on the morphology, immunohistochemistry, and molecular genetic characteristics are summarized in Table 2.
In conclusion, EWSR1::SMAD3-rearranged fibroblastic tumor should be taken into consideration for the differential diagnosis when encountered with spindle cell lesions in acral sites with strong ERG expression only, and further molecular studies should be employed to confirm the diagnosis. More cases and further studies are needed to further understand the nature of this tumor.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by the Institutional Review Board Committee of Xijing Hospital. Written informed consent for participation was not required for this study in accordance with the institutional requirements.
Author contributions
LY and LF wrote the initial draft of the manuscript. ZY analyzed the clinical aspects of the case. HC and ZW co-designed the study, collected the histopathological and molecular studies, interpreted the findings, and edited the manuscript. YL conducted the FISH analysis and interpreted the findings. DZ performed RT-PCR/direct sequencing. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by grants from the National Natural Science Foundation of China (No. 82072654 and 81870185).
Acknowledgments
The authors are grateful to Anbiping Laboratory, Guangzhou, China, for the FISH analysis.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Antonescu CR, Suurmeijer AJH. EWSR1-SMAD3-positive fibroblastic tumour (emerging). WHO classification of tumours, 5th edition. In: Soft tissue and bone tumours. Lyon, France: IARC Press (2020). p. 76–7.
2. Kao YC, Flucke U, Eijkelenboom A, Zhang L, Sung YS, Suurmeijer AJH, et al. Novel EWSR1-SMAD3 gene fusions in a group of acral fibroblastic spindle cell neoplasms. Am J Surg Pathol (2018) 42(4):522–8. doi: 10.1097/pas.0000000000001002
3. Michal M, Berry RS, Rubin BP, Kilpatrick SE, Agaimy A, Kazakov DV, et al. EWSR1-SMAD3-rearranged fibroblastic tumor: An emerging entity in an increasingly more complex group of Fibroblastic/Myofibroblastic neoplasms. Am J Surg Pathol (2018) 42(10):1325–33. doi: 10.1097/pas.0000000000001109
4. Zhao L, Sun M, Lao IW, Yu L, Wang J. EWSR1-SMAD3 positive fibroblastic tumor. Exp Mol Pathol (2019) 110:104291. doi: 10.1016/j.yexmp.2019.104291
5. Foot O, Hallin M, Jones RL, Sumathi VP, Thway K. EWSR1-SMAD3-Positive fibroblastic tumor. Int J Surg Pathol (2021) 29(2):179–81. doi: 10.1177/1066896920938124
6. Habeeb O, Korty KE, Azzato EM, Astbury C, Farkas DH, Ko JS, et al. EWSR1-SMAD3 rearranged fibroblastic tumor: Case series and review. J Cutan Pathol (2021) 48(2):255–62. doi: 10.1111/cup.13870
7. De Noon S, Flanagan AM, Tirabosco R, O'Donnell P, Amary F. EWSR1-SMAD3 fibroblastic tumour of bone: expanding the clinical spectrum. Skeletal Radiol (2021) 50(2):445–50. doi: 10.1007/s00256-020-03563-0
8. Lam SW, Silva TM, Bovée J. New molecular entities of soft tissue and bone tumors. Curr Opin Oncol (2022) 34(4):354–61. doi: 10.1097/cco.0000000000000844
9. Papke DJ Jr., Hornick JL. Recent advances in the diagnosis, classification and molecular pathogenesis of cutaneous mesenchymal neoplasms. Histopathology (2022) 80(1):216–32. doi: 10.1111/his.14450
10. Friedman BJ. Pitfall regarding expression of ETS-related gene (ERG) in fibrohistiocytic neoplasms. J Cutan Pathol (2021) 48(7):1003–4. doi: 10.1111/cup.14024
11. Ooshima A, Park J, Kim SJ. Phosphorylation status at Smad3 linker region modulates transforming growth factor-β-induced epithelial-mesenchymal transition and cancer progression. Cancer Sci (2019) 110(2):481–8. doi: 10.1111/cas.13922
12. Flucke U, van Noesel MM, Siozopoulou V, Creytens D, Tops BBJ, van Gorp JM, et al. EWSR1-the most common rearranged gene in soft tissue lesions, which also occurs in different bone lesions: An updated review. Diagnostics (Basel) (2021) 11(6):1093. doi: 10.3390/diagnostics11061093
13. Thway K, Fisher C. Mesenchymal tumors with EWSR1 gene rearrangements. Surg Pathol Clin (2019) 12(1):165–90. doi: 10.1016/j.path.2018.10.007
14. Armstrong SM, Demicco EG. What's new in fibroblastic tumors? Virchows Arch (2020) 476(1):41–55. doi: 10.1007/s00428-019-02682-x
Keywords: EWSR1, SMAD3, fibroblastic tumor, ERG, case report
Citation: Yang L, Fan L, Yin Z, Liu Y, Zhao D, Wang Z and Cheng H (2022) EWSR1::SMAD3-rearranged fibroblastic tumor: A case with twice recurrence and literature review. Front. Oncol. 12:1017310. doi: 10.3389/fonc.2022.1017310
Received: 11 August 2022; Accepted: 25 November 2022;
Published: 15 December 2022.
Edited by:
Chien-Feng Li, National Health Research Institutes, TaiwanReviewed by:
Guoqing Ru, Zhejiang Provincial People’s Hospital, ChinaMing Zhao, Zhejiang Provincial People’s Hospital, China
Josephine Dermawan, Cleveland Clinic, United States
Copyright © 2022 Yang, Fan, Yin, Liu, Zhao, Wang and Cheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hong Cheng, chenghongfmmu85@yahoo.com; Zhe Wang, zhwang@fmmu.edu.cn
†These authors have contributed equally to this work
 Li Yang
Li Yang Linni Fan
Linni Fan Zhiyong Yin2†
Zhiyong Yin2† Zhe Wang
Zhe Wang Hong Cheng
Hong Cheng