- 1Research Center for Quality of Life and Applied Psychology, Key Laboratory for Quality of Life and Psychological Assessment and Intervention, School of Humanities and Management, Guangdong Medical University, Dongguan, China
- 2Central Hospital of Guangdong Nongken, The six wards of Medical Oncology, Zhanjiang, China
Objective: To determine the minimal clinically important differences (MCIDs) for the breast cancer scale QLICP-BR (V2.0) among the Quality of Life Instruments system for cancer patients (QLICP), which consist of the general module of 32 items classifying into 4 domains and the specific module of 10 items.
Methods: According to the scoring rule of QLICP-BR (V2.0), the scores of each domain and the overall scale were calculated. The MCIDs of this scale were established by anchor-based and distribution-based methods. The anchor method used the Q29 item in the EORTC QLQ-C30 scale as anchors and defined the treatment effectiveness of the anchor-based method using criteria A (one level improvement after treatment) and B (at least one level improvement after treatment), while methods of effect size (ES), standard error of measurement (SEM), and reliability change index (RCI) were used in distribution-based methods.
Results: Using the anchor-based method, according to standard A, the MCIDs of the physical domain (PHD), psychological domain (PSD), social domain (SOD), common symptoms and side effect domain (SSD), core/general module (CGD), specific domain (SPD), and the total score (TOT) were 16.24, 11.37, 11.31, 12.07, 11.49, 10.69, and 11.23 respectively; according to standard B, the MCIDs of PHD, PSD, SOD, SSD, CGD, SPD, and TOT were 18.88, 15.14, 14.10, 14.50, 13.93, 12.17, and 14.23 respectively. In the distribution-based MCID study, when ES = 0.8, the MCID values of each domain and the total score of the scale were 9.14, 10.34, 8.34, 10.54, 6.79, 9.73, and 6.96 respectively. The MCIDs calculated when a SEM of 1.96 was used as the intermediary index were 8.38, 11.04, 8.67, 10.00, 7.44, 9.83, and 7.81. The MCIDs calculated when a RCI of 1.96 was used as the intermediary index were 11.84, 15.61, 12.27, 14.14, 10.52, 13.90, and 11.05. Additionally, the MCID value calculated by the two standards of the anchor method was similar to 0.8 ES, 1.96 SEM, and 1.96 RCI.
Conclusion: Using the anchor-based method, 0.8ES, 1.96SEM, and 1.96RCI have a better effect on the minimal clinically important difference of breast cancer scale and were recommended to be the preferred methods for establishing MCID.
Introduction
Breast cancer is one of the most common malignant tumors in women and a leading cause of cancer-related deaths. The age of breast cancer diagnosis is between 40 and 60 years old. Each year more than 1.7 million women are diagnosed and more than 500 000 die from breast cancer, making it the leading cause of cancer death among women globally (1, 2). There are more than 1.67 million new cases of breast cancer in women worldwide in 2012, ranking first place in the incidence of female malignant tumors (3–5). In 2018, breast cancer accounted for 11.6% of all cancer deaths worldwide (6). In China, the incidence of breast cancer has increased in recent years with the development of society and economy, changes in lifestyle, and ecological environment. From 2003 to 2008, the standardized incidence of breast cancer in Chinese women increased from 21.17/100,000 to 26.26/100,000, an increase of 17.65%. The incidence of breast cancer declined slightly in 2009, but from 2010 to 2012, it rose sharply to 30.43/100,000, an increase of 43.74% compared with 2003. The average annual rate of change in breast cancer mortality over the past 10 years was 3.87 percent (7). It can be seen from this that the incidence and mortality of breast cancer in China as a whole show a trend of a gradual increase, and the burden of disease is also increasing.
Along with the increasing number of breast cancer patients and the longer survival due to early detection programs and advancement in medical technology, accurately assessing the health-related quality of life (HRQOL) of breast cancer patients is crucial (8–10). By far, the more popular HRQOL tools for breast cancer are the European Organization for Research and Treatment (EORTC) Quality of Life Questionnaire-C30 (QLQ-C30) and the breast cancer-specific module QLQ-BR23, and the American Breast Cancer Quality of Life Measurement Scale FACT-B (10–12).
However, these scales are not fully applicable to the assessment of the quality of life of Chinese people. Therefore, Wan’s QOL team developed the Chinese instrument for the breast cancer quality of life measurement QLICP-BR (including the first (13) and second editions), which is one scale of the system of Quality of Life Instruments for cancer patients (QLICP). The second version QLICP-BR (V2.0) is composed of a general module QLICP-GM (V2.0) and a breast cancer-specific module. Among them, QLICP-GM (V2.0) includes 32 items from 10 facets grouped into four domains: physical function (8 items), psychological function (9 items), social function (8 items), and common symptoms and side effects (7 items) (14). The breast cancer-specific module consists of 3 facets and 10 items. The whole scale consists of 5 domains, 13 facets, and 42 items (15). The QLICP-BR (V2.0) has been verified in Mainland China and has good reliability, validity, and responsiveness after testing. It can be used for the determination of the quality of life in patients with breast cancer during the period of onset, treatment, and rehabilitation (15).
In order to reasonably explain the clinical significance of questionnaire measurements and scale scores, as early as 1987, Guyatt et al. proposed to use the Minimal Clinically Important Difference (MCID) as an appropriate benchmark for evaluating important changes in the responsiveness of the scale. The researchers did not define MCID and acknowledged the difficulty of quantifying MCID, indicating that the changes caused by interventions with known efficacy can provide a preliminary estimate. Two years later, Jaeschke, Singer, and Guyatt defined MCID as “the smallest change in the score of the questionnaire dimension recognized by the patient without considering side effects and costs (16).” Thus, clinicians need a systematic approach to assess the perceived benefit of a treatment based on the individual patient’s improvement in cost and risk of complications. Ideally, MCID will provide a specific threshold as a treatment target and has been widely used in this regard.
The QLICP-BR (V2.0) has been widely used in China, but MCID has not been developed, so it is not convenient for further applications. The purpose of this study is to use the QLICP-BR (V2.0) scale to develop the minimal clinically important difference (MCID) for breast cancer.
Materials and Methods
Subjects and Data Collection
This study was based on inpatients with a clinical breast cancer who were diagnosed by pathological examination in the Affiliated Hospital of Guangdong Medical University and the Central Hospital of Guangdong Nongken. The investigator appeared as a doctor and briefly explained the content and purpose of the investigation. After obtaining the consent of the patient and signing the informed consent form, the investigator sent the QLICP-BR (V2.0) to the patients to fill in by themselves. In total, 246 patients were included in the study. The inclusion and exclusion criteria are as follows:
Inclusion criteria: (1) Patients with a clear diagnosis, that is, those diagnosed with breast cancer by pathological examination; (2) Good reading and presentation skills, able to fill out questionnaires by themselves; (3) Volunteer to participate in the survey, no mental illness or disturbance of consciousness.
Exclusion criteria: (1) Cognitive and consciousness dysfunction; (2) Those who refuse to participate in the research or those with a low degree of cooperation; (3) At the end of life, combined with other primary cancers, other serious diseases, mental illnesses, etc.; (4) Malignant tumors that frequently metastasize.
In terms of sample size, we use the sample size calculation formula:
p is the effective rate of treatment, q=1-p, d represents the allowable error, and Zα is the statistical quantity of the significance test. When d = 0.1p and α =0.05, . According to experience, p =0.67,q=0.33, So n is equal to 197. In addition, according to the empirical method, the sample size should be 5-10 times that of the variable. The number of items in this scale is 42, and the sample size is suitable for 210-420 cases. The sample size of this investigation is 246 cases, which can meet the statistical requirements of sample size.
MCIDs of the Anchor-Based Method
The anchor-based method is used to clarify the meaning of the scale’s score change by examining the relationship between the scale and the score of another independent measurement tool or other indicators. Anchor-based approaches assess the extent to which changes in measurement instruments correspond to a minimally important change defined by external indicators (17). Anchors are divided into cross-sectional anchors and longitudinal anchors (18). This article is used to compare the efficacy before and after treatment, so the longitudinal anchor was selected. First, the RS (raw score) was scored based on the number of questions contained in each domain and the patient’s options. Then, linear transformation was performed using the range method to convert the raw score into a standardized score (SS) with a value between 0 and 100. The formula for calculating the score in each domain is as follows (19):
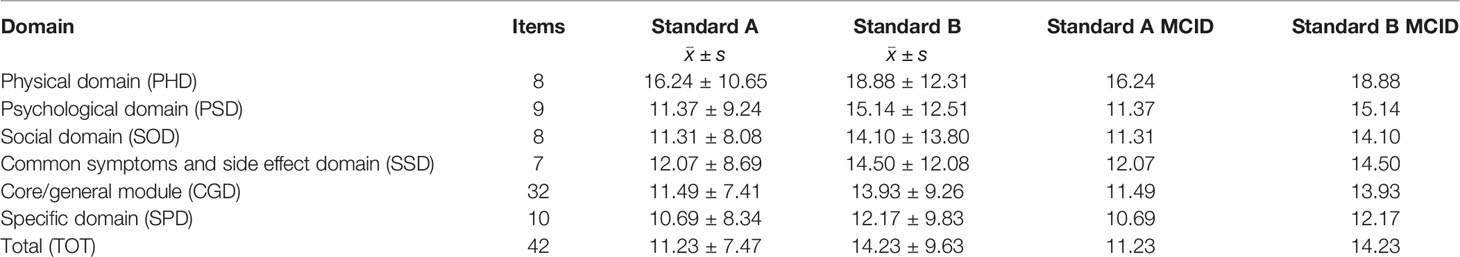
The Q29 item “ How would you rate your overall health during the past week?” in the EORTC QLQ-C30 scale (20) was taken as the anchor and used to calculate the correlation coefficient between Q29 and the QLICP-BR (V2.0) total scale score. Then, patients who differed by one grade (criterion A) and at least one grade (criterion B) in Q29 before and after treatment were screened out. The score difference in each domain before and after treatment was calculated, and the mean of the absolute value of the difference was recorded as MCID. The calculation formula is shown in Table 1.
MCIDs of the Distribution-Based Method
The distribution-based method uses the evaluation tool sample data distribution (variation) to determine the MCID from a statistical point of view. Commonly used indicators for calculating variation include effect size (ES), standard error of measurement (SEM), and reliability change index (RCI). The calculation formula and corresponding MCID calculation are as follows:
X0is the baseline score of the respondent;
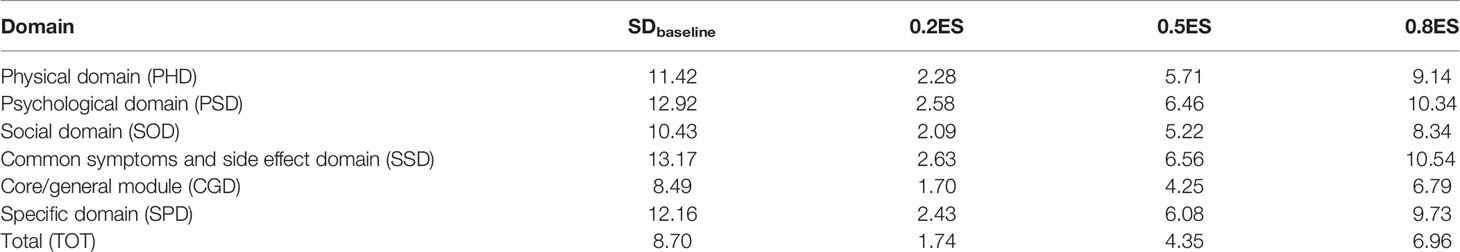
is the mean baseline score of the respondent; SDbaseline is the standard deviation of the baseline score of the respondent; is the mean score of the respondent after treatment; and n is the sample size (21–23). In the health-related quality of life assessments, the ES is currently a relatively recognized parameter in determining the importance of group or individual changes. There is also an accepted standard for ES judgment: 0.2 is a small effect; 0.5 is a medium effect; 0.8 or greater is a large effect (23).
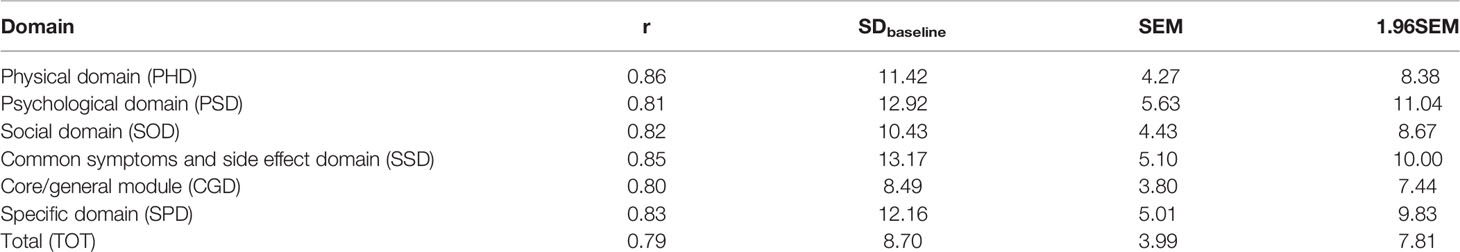
Where r is the reliability coefficient of the evaluation questionnaire and the test-retest reliability coefficient is generally used. If the test-retest reliability coefficient is unknown, the Cronbach coefficient can be used instead. X can be 1 (small effect), 1.96 (medium effect), or 2.77 (large effect) (22–24).
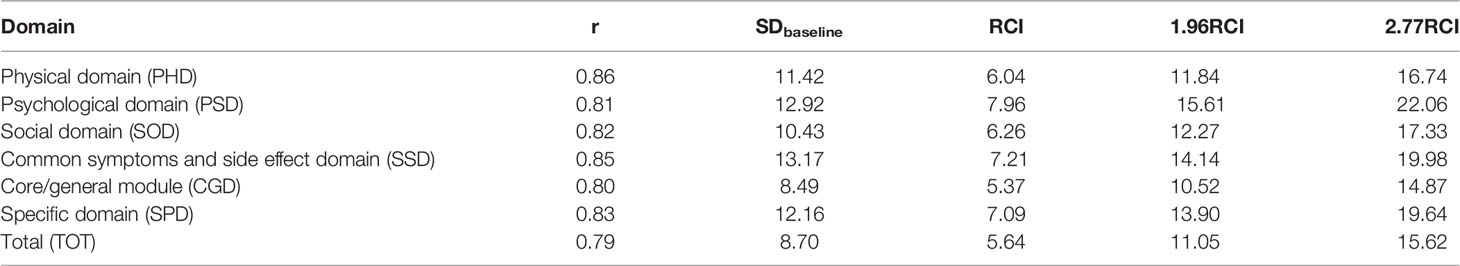
RCI, which is the change in questionnaire score divided by the square root of the standard measurement error (25, 26).
Statistical Software
This survey used Epidata3.1 software to input data and SPSS 25.0 software to organize and analyze the data. The scores of various domains and the total score of the scale and the MCID value of breast cancer were calculated.
Results
Social-Demographic and Clinical Characteristics of Breast Cancer Patients
A total of 246 breast cancer patients were investigated in this study, all of which were women. The age distribution was between 17-77 years old. The average age was 50.07 ± 10.25. Among the patients, 5.3% were under 30 years old, 10.2% were between 30 and 40 years old, 37.0% were between 40 and 50 years old, 29.3% were between 50 and 60 years old, and 18.3% were over 60 years old. The household economy was mostly medium, accounting for 67.9% of the total population. The occupations were mostly workers and farmers, with 20 workers (8.1%) and 112 farmers (45.5.0%), respectively. The majority of patients were married, accounting for 97.2%. The most common ethnicity was Han, accounting for 97%. The distribution of educational level was mostly in Middle school or High school, accounting for 60.2%. Medical insurance was mostly used in medical forms, accounting for 91.9%. Medical treatment insurance included medical insurance for urban residents, medical insurance for urban workers, cooperative medical treatment, and commercial medical insurance. TNM stages were distributed between I-IV stages, with 53 being stage I (21.5%), 86 stage II (35.0%), 54 stage III (22.0%), and 27 stage IV (11.0%), respectively. See Table 2 in detail.
MCIDs of the Anchor-Based Method
The correlation coefficient between the Q29 and QLICP-BR (V2.0) score in the EORTC QLQ-C30 scale was calculated using the Q29 item “How do you evaluate your total health condition in the past week” as an anchor, and r = 0.651 was obtained.
According to standard A, patients with a difference of one grade on Q29 before and after treatment were screened, 116 cases were effective, and the QLICP-BR (V2.0) scores in each domain and the total scale score difference before and after treatment were calculated.
In regard to socio-demographic and clinical characteristics of the sample based on Standard A, 116 (100%) patients were female, 78 (67.2%) were aged between 40 and 60 years old. Most of them had medium family income (69%) and had middle school education (64.7%). 54 (46.6%) were farmers, 100 (94.8%) were married, 112 (96.6%) were Han nationality, 107 (92.2%) had medical insurance. On clinical stages, the cases distributed in the four stages of TNM I, II, III, IV were 29, 38, 21, 14, respectively.
According to standard B, patients with at least one grade difference in Q29 before and after treatment were screened. A total of 166 patients were effective. The scores of QLICP-BR (V2.0) in each domain and the total scale score difference before and after treatment were calculated.
In terms of socio-demographic and clinical characteristics of the sample based on Standard B, all of the 166 patients were female, and 112 (67.5%) were aged between 40 and 60 years old. Most of them had medium family income (67.5%) and middle school education (64.4%). 81(48.8%) were farmers, 160 (96.4%) were married, 162 (97.6%) were Han nationality, and 155(93.4%) had medical insurance. On clinical stages, the cases distributed in the four stages of TNM I, II, III, IV were 38, 59, 31, 22, respectively.
Then, the mean and standard deviation of the difference between the scores of each domain and the total scale under the two standards were calculated and the mean of the difference as MCID was recorded. The results are shown in Table 3. According to standard A, the MCID values of physical domain (PHD), psychological domain (PSD), social domain (SOD), common symptoms and side effect domain (SSD), core/general module (CGD), specific domain (SPD) and the total scale (TOT) were 16.24, 11.37, 11.31, 12.07, 11.49, 10.69 and 11.23, respectively. According to standard B, the MCID values of PHD, PSD, SOD, SSD, CGD, SPD and TOT were 18.88, 15.14, 14.10, 14.50, 13.93, 12.17, and 14.23, respectively.
MCIDs of the Distribution-Based Methods
The distribution-based method estimates MCID based on the observed distribution of score changes. The MCID results of breast cancer were calculated using three variation indexes: ES, SEM, and RCI. We calculated the MCID results using the three indicators respectively as shown in Tables 4–6.
As can be seen from Table 4, when ES = 0.2, the MCID values for each domain and the total scale were 2.28, 2.58, 2.09, 2.63, 1.70, 2.43 and 1.74, respectively. When ES = 0.5, the MCID values of each domain and the total scale were 5.71, 6.46, 5.22, 6.56, 4.25, 6.08 and 4.35, respectively. When ES = 0.8, the MCID values of each domain and the total scale were 9.14, 10.34, 8.34, 10.54, 6.79, 9.73 and 6.96, respectively.
The MCIDs calculated for above domains/the total when SEM was used as an intermediary index were 4.27, 5.63, 4.43, 5.10, 3.80, 5.01 and 3.99, respectively. The MCIDs calculated for above domains/the total when 1.96SEM was used as the intermediary index were 8.38, 11.04, 8.67, 10.00, 7.44, 9.83 and 7.81, respectively.
The MCIDs calculated for above domains/the total when RCI was used as the intermediary index were 6.04, 7.96, 6.26, 7.21, 5.37, 7.09 and 5.64, respectively. The MCIDs calculated for above domains/the total when 1.96RCI was used as the intermediary index were 11.84, 15.61, 12.27, 14.14, 10.52, 13.90 and 11.05, respectively. The MCIDs calculated for above domains/the total when 2.77RCI was used as the intermediary index were 16.74, 22.06, 17.33, 19.98, 14.87, 19.64, and 15.62, respectively.
Discussions
MCID is a difference score that is sufficient to reflect the effect on patients after clinical treatment, and its main function is to help clinical and research personnel to determine whether changes in the score of the scale have clinical significance. Clearly, clinicians also need a systematic way to assess the perceived benefit of a certain treatment based on individual patient improvement relative to both cost and risk of complications (27). At the same time, it can also further explain the score of the scale, so as to provide a scientific basis for clinical and scientific researchers to deal with patients of different degrees more specifically (28). Therefore, it is critical that the MCID score is a valid and stable measure. A low MCID may result in overestimating the positive effects of treatment, whereas a high MCID value may incorrectly classify patients as failing to respond to treatment when in fact the treatment was beneficial (26).
In clinical studies, a lot of methodological approaches have been reported to calculate MCID such as the anchor-based method, distribution-based method, expert opinion method, literature analysis method, etc (29). In general, methodological approaches can be classified into two broad groups: anchor-based and distribution-based (29–32). The two kinds of methods can get a variety of results from different angles, which is conducive to comparison and user selection according to the situation. But each method has its advantages and disadvantages. An anchor-based approach relies on external calibration, which must be easy to interpret and strong in relation to the quality of life. But choosing different anchors will result in different MCID values. The distribution-based method takes into account the measurement error and also has a specific calculation formula, which is easy to implement, but easily affected by sample size (33, 34). Therefore, most scholars recommend applying multiple methods where the anchoring method is used as the main method and the distribution method is used as a supplement to determine MCID.
The EORTC-QLQ-C30 is a 30-item health-related quality of life questionnaire for cancer patients. Q30(How would you rate your overall quality of life during the past week)? and Q29 (How would you rate your overall health during the past week)? in the scale are comprehensive items and are often used as anchors. We calculated the correlation coefficients of Q29, Q30 and QLICP-BR (V2.0) scales respectively. The correlation coefficients were 0.651 and 0.588, respectively. Obviously, the correlation coefficient between Q29 and scale score is larger. At the same time, the content of Q29 is easier to be understood by patients. Therefore, we choose Q29 as the anchor. In this study, using the anchor-based method, according to standard A, the MCIDs of PHD, PSD, SOD, SSD, CGD, SPD and TOT were 16.24, 11.37, 11.31, 12.07, 11.49, 10.69 and 11.23, respectively; ranging from 10.69 to 16.24 points. According to standard B, the MCIDs of PHD, PSD, SOD, SSD, CGD, SPD and TOT were 18.88, 15.14, 14.10, 14.50, 13.93, 12.17 and 14.23, respectively, ranging from 12.17 to 18.88 points. It can be seen that according to criterion A, patients are more likely to obtain meaningful clinical change values; in other words, the quality of life of breast cancer patients is more likely to be judged as improved.
However, the selected anchor Q29 and the total scale score correlation coefficient r was 0.651, which is not too high and may affect the reliability of MCID. In addition, the selected anchor is relatively single, which may cause unstable results. So, we continued to use the distribution-based method and calculated the MCID under the three variation indicators of ES, SEM, and RCI.
In the distribution-based MCID study, When ES = 0.2, ES = 0.5, and ES = 0.8 were used as intermediary indicators, the MCID values of the scores for each domain and the overall scale were ranged from 1.70 to 2.63 points, 4.25 to 6.56 points, and 6.79 to 10.54 points, respectively. The MCIDs of the scores for each domain and the overall scale calculated with SEM and 1.96SEM as the intermediary indicators were ranged from 3.80 to 5.63 points and 7.44 to 11.04 points, respectively. When RCI, 1.96RCI, and 2.77RCI were used as the intermediary index, the calculated MCIDs of the scores for each domain and the overall scale were ranged from 5.37 to 7.96 points, 10.52 to 15.61 points, and 14.87 to 22.06 points, respectively.
In the evaluation of clinical efficacy, the clinicians and researchers can judge clinical significance by these MCIDs. Taking 0.5ES method as an example, the overall QOL of patients can be evaluated as clinical significance when the QOL change before and after treatment is over 4.35 points for its MCID is 4.35. Similarly, the MCID of the MFSI-SF identified by Chan A et al. ranged from 4.50 to 10.79 points, and can be used to interpret the clinical significance of fatigue deterioration in patients with breast cancer (35). Quinten C et al. evaluated the short and long-term effects of chemotherapy on the reported quality of life (QOL) and patient-clinician symptom reporting in older breast cancer patients with an MCID of 10. Results showed that symptom burden and diminished QOL in an older breast cancer population receiving adjuvant TC chemotherapy are short-lived and disappear after a while with no long-term differences compared to a similar population not receiving chemotherapy (36).
In this study, anchor method and distribution method were used to calculate MCID of breast cancer. When the MCID calculated by anchor method is smaller than that calculated by distribution method or the correlation coefficient between the selected anchor and the measured scale is small (the absolute value r at least 0.5 is currently recommended), the results of the distribution method are recommended as the MCIDs of breast cancer (37). In the calculation of MCID, the anchor method is generally preferred. However, the distribution method is also considered comprehensively if there is no good anchor or the number of patients is not large.
In summary, the MCID value calculated by the two standards of the anchor method was similar to 0.8ES, 1.96SEM, and 1.96RCI. Therefore, these indicators should be given priority in the development of MCID for breast cancer.
Similar results have also been obtained in developing MCID for breast cancer in foreign countries. Alexandre Chan et al. used the anchor-based method, distribution-based method, and ROC curve method to develop the MCID of breast cancer, and the results showed that the MCID of the MFSI-SF identified by all ranged from 4.50 to 10.79 points (38). Yin Ting Cheung et al. used the anchor-based method, distribution-based method, and ROC curve method to develop MCID for breast cancer, and the results were as follows: the estimates of 6.9-10.6 points as MCID can facilitate the interpretation of patient-reported cognitive weakness and sample size in future studies (39). These results are similar to the result of our study, but the MCID value of breast cancer we developed was relatively higher than that of the foreign results. This may be caused by the use of different scales whose scores ranging from 0 to 100 and cultural differences between China and foreign countries.
From the results obtained, we conclude that the MCID value calculated by the two standards of the anchor-based method was similar to 0.8ES, 1.96SEM, and 1.96RCI of the distribution-based methods. Therefore, when selecting the minimal clinical important difference for breast cancer patients, the results of the anchor-based method can be used preferentially, also 0.8ES, 1.96SEM, 1.96RCI of distribution-based methods can be used.
Of course, this study has certain limitations. The methods used in this study include the anchor-based method and the distribution-based method. Other methods can also be used to formulate MCID such as the ROC curve method and the response cumulative distribution function method. Additionally, the sample size of this subject research can be expanded to make the MCID more stable. In future studies, the sample size of breast cancer patients will be expanded, and multiple methods will be used to jointly develop MCID for breast cancer, so as to ensure more stable results.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The study protocol and the informed consent form were approved by the IRB (Institutional Review Board) of the affiliated hospital of Guangdong Medical University (PJ2012052, YJYS2019010). The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
CW designed the study. FL, JZ, and HC performed the data collection. FL,JT, and YL performed data analyses and drafted the manuscript. CW revised the manuscript deeply. All authors contributed to interpreting the data, and have read and approved the final manuscript.
Funding
This study is supported by the National Natural Science Foundation of China (71974040, 81273185), Dongguan Science and Technology of Social Development Program (20211800905102).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
In carrying out this research project, we have received substantial assistance from Prof. Gary Lyman from Hutchinson Institute for Cancer Outcomes Research, and Prof. David Cella, Benjamin J. Arnold and Hiramatsu Toshiko at the Center on Outcomes, Research, and Education (CORE), and many staffs at the Central Hospital of Guangdong Nongken. We sincerely acknowledge all the support.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.753729/full#supplementary-material
Abbreviations
MCID, minimal clinically important differences; QLICP-BR, Quality of Life Instruments system in breast cancer patients; EORTC, European Organization for Research and Treatment; ES, effect size; SEM, standard error of measurement; RCI, reliability change index; PHD, physical domain; PSD, psychological domain; SOD, social domain; SSD, common symptoms and side effect domain; CGD, core/general module; SPD, specific domain; TOT, total; HRQOL, health-related quality of life; ROC curve, receiver operating characteristic.
References
1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global Cancer Statistics, 2012. CA Can J Clin (2015) 65:87–108. doi: 10.3322/caac.21262
2. El-Hashimi D, Gorey KM. Yoga-Specific Enhancement of Quality of Life Among Women With Breast Cancer: Systematic Review and Exploratory Meta-Analysis of Randomized Controlled Trials. J Evid Base Integr Med (2019) 24:1–9. doi: 10.1177/2515690X19828325
3. Shi J, Liang D, Li DJ. Epidemiological Status of Global Female Breast Cancer. China Cancer (2017) 26(09):683–90. doi: 10.11735/j.issn.1004-0242.2017.09.A006
4. Qing C, Shunping L, Min W, Liu L, Gang C, Abdelaziz M, et al. Health-Related Quality of Life Among Women Breast Cancer Patients in Eastern China. BioMed Res Int (2018) 2018:1452635. doi: 10.1155/2018/1452635
5. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer Incidence and Mortality Worldwide: Sources, Methods and Major Patterns in GLOBOCAN 2012. Int J Cancer (2015) 136:E359–86. doi: 10.1002/ijc.29210
6. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin (2018) 68(6):394–424. doi: 10.3322/caac.21492
7. He MY. Analysis of Epidemic Trend With Breast Cancer and Research on the Effect of Postoperative Radiotherapy. Hengyang, Hunan Province: University of South China (2019).
8. Yusuf A, Ahmad Z, Keng SL. Quality of Life in Malay and Chinese Women Newly Diagnosed With Breast Cancer in Kelantan, Malaysia. Asian Pac J Cancer Prev (2013) 14(1):435–40. doi: 10.7314/APJCP.2013.14.1.435
9. Perry S, Kowalski TL, Chang CH. Quality of Life Assessment in Women With Breast Cancer: Benefits, Acceptability and Utilization. Health Qual Life Outcome (2017) 5:24. doi: 10.1186/1477-7525-5-24
10. Chen Q, Li S, Wang M, Liu L, Chen G. Health-Related Quality of Life Among Women Breast Cancer Patients in Eastern China. BioMed Res Int (2018) 2018:1452635. doi: 10.1155/2018/1452635
11. Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A Quality-of-Life Instrument for Use in International Clinical Trials in Oncology. J Natl Cancer Inst (1993) 85(5):365–76. doi: 10.1093/jnci/85.5.365
12. Wan C, Zhang D, Yang Z, Tu X, Tang W, Feng C, et al. Validation of the Simplified Chinese Version of the FACT-B for Measuring Quality of Life for Patients With Breast Cancer. Breast Cancer Res Treat (2007) 106(3):413–8. doi: 10.1007/s10549-007-9511-1
13. Wan C, Yang Z, Tang X, Zou T, Chen D, Zhang D, et al. Development and Validation of the System of Quality of Life Instruments for Cancer Patients: Breast Cancer (QLICP-Br). Sup Care Cancer (2009) 17(4):359–66. doi: 10.1007/s00520-008-0478-1
14. Wu J, Hu L, Zhang G, Liang Q, Meng Q, Wan C. Development and Validation of the Nasopharyngeal Cancer Scale Among the System of Quality of Life Instruments for Cancer Patients (QLICP-NA V2.0): Combined Classical Test Theory and Generalizability Theory. Qual Life Res (2016) 25(8):2087–100. doi: 10.1007/s11136-016-1251-4
15. Wan C. Research Status on the Second Version of the System of Quality of Life Instruments for Cancer Patients QLICP (V2.0). J Guangdong Med Univ (2020) 38(05):511–7. doi: 10.3969/j.issn.1005-4057.2020.05.001
16. King MT. A Point of Minimal Important Difference (MID): A Critique of Terminology and Methods. Expert Rev Pharmacoecon Outcome Res (2011) 11(2):171–84. doi: 10.1586/erp.11.9
17. Huang IC, Liu JH, Wu AW, Wu MY, Leite W, Hwang CC. Evaluating the Reliability, Validity and Minimally Important Difference of the Taiwanese Version of the Diabetes Quality of Life (DQOL) Measurement. Health Qual Life Outcome (2008) 6(1):87. doi: 10.1186/1477-7525-6-87
18. Crosby RD, Kolotkin RL, Williams GR. Defining Clinically Meaningful Change in Health-Related Quality of Life. J Clin Epidemiol (2003) 56(5):395–407. doi: 10.1016/S0895-4356(03)00044-1
19. Wan CH, Yu YL, Tan JF, Meng Q, Huang XP. Introductions on Quality of Life Research-Measurements·Assessments·Improvements. Beijing: Science Press (2016).
20. Zawisza K, Tobiasz-Adamczyk B, Nowak W, Kulig J, Jedrys J. Validity and Reliability of the Quality of Life Questionnaire (EORTC QLQ C30) and its Breast Cancer Module (EORTC QLQ Br23). Ginekol Pol (2010) 81(4):262–7.
21. Jaeschke R, Singer J, Guyatt GH. Measurement of Health Status. Ascertaining the Minimal Clinically Important Difference. Control Clin Trial (1989) 10(4):407–15. doi: 10.1016/0197-2456(89)90005-6
22. Sprangers MA, Moinpour CM, Moynihan TJ, Patrick DL, Revicki DA. Clinical Significance Consensus Meeting Group. Assessing Meaningful Change in Quality of Life Over Time: A Users' Guide for Clinicians. Mayo Clin Proc (2002) 77(6):561–71. doi: 10.4065/77.6.561
23. Xue HH, Yang Z, Wan CH, Xu QA, Xu CZ, Chen Y. The Method of Making Minimum Difference of Clinical Importance (MCID) Based on Scale Score. Chin J Health Stat (2019) 36(3):436–40. doi: CNKI: SUN: ZGWT.0.2019-03-035
24. Osoba D, Rodrigues G, Myles J, Zee B, Pater J. Interpreting the Significance of Changes in Health-Related Quality-of-Life Scores. J Clin Oncol (1998) 16(1):139–44. doi: 10.1200/JCO.1998.16.1.139
25. Guyatt GH, Osaba D, Wu AW, Wyrwich KW, Norman GR. Methods to Explain the Clinical Significance of Health Status Measures. Mayo Clin Proc (2002) 77(4):371–83. doi: 10.4065/77.4.371
26. Jacobson NS, Truax P. Clinical Significance: A Statistical Approach to Defining Meaningful Change in Psychotherapy Research. J Consult Clin Psychol (1991) 59(1):12–9. doi: 10.1037/0022-006X.59.1.12
27. Copay AG, Subach BR, Glassman SD, Polly DW Jr, Schuler TC. Understanding the Minimum Clinically Important Difference: A Review of Concepts and Methods. Spine J (2007) 7(5):541–6. doi: 10.1016/j.spinee.2007.01.008
28. Yost KJ, Cella D, Chawla A, Holmgren E, Eton DT, Ayanian JZ, et al. Minimally Important Differences Were Estimated for the Functional Assessment of Cancer Therapy-Colorectal (FACT-C) Instrument Using a Combination of Distribution- and Anchor-Based Approaches. J Clin Epidemiol (2005) 58(12):1241–51. doi: 10.1016/j.jclinepi.2005.07.008
29. Wright A, Hannon J, Hegedus EJ, Kavchak AE. Clinimetrics Corner: A Closer Look at the Minimal Clinically Important Difference (MCID). J Man Manipulat Ther (2012) 20(3):160–6. doi: 10.1179/2042618612Y.0000000001
30. Rai SK, Yazdany J, Fortin PR, Aviña-Zubieta JA. Approaches for Estimating Minimal Clinically Important Differences in Systemic Lupus Erythematosus. Arthritis Res Ther (2015) 17(1):143. doi: 10.1186/s13075-015-0658-6
31. Rodrigues JN, Mabvuure NT, Nikkhah D, Shariff Z, Davis TR. Minimal Important Changes and Differences in Elective Hand Surgery. J Handb Surg Eur Vol (2015) 40(9):900–12. doi: 10.1177/1753193414553908
32. Engel L, Beaton DE, Touma Z. Minimal Clinically Important Difference: A Review of Outcome Measure Score Interpretation. Rheum Dis Clin North Am (2018) 44(2):177–88. doi: 10.1016/j.rdc.2018.01.011
33. Hu GQ, Huang QF, Huang ZN, Sun ZQ. Methods to Determine Minimal Clinically Important Difference. J Cent South Univ (Medical Science) (2009) 34(11):1058–62. doi: 10.3321/j.issn:1672-7347.2009.11.002
34. Wright A, Hannon J, Hegedus EJ, Kavchak AE. Clinimetrics Corner: A Closer Look at the Minimal Clinically Important Difference (MCID). J Man Manipulat Ther (2012) 20(3):160–6. doi: 10.1179/2042618612Y.0000000001
35. Chan A, Yo TE, Wang XJ, Ng T, Chae JW, Yeo HL, et al. Minimal Clinically Important Difference of the Multidimensional Fatigue Symptom Inventory-Short Form (MFSI-SF) for Fatigue Worsening in Asian Breast Cancer Patients. J Pain Symptom Manage (2018) 55(3):992–7.e2. doi: 10.1016/j.jpainsymman.2017.10.014
36. Quinten C, Kenis C, Hamaker M, Coolbrandt A, Brouwers B, Dal Lago L, et al. The Effect of Adjuvant Chemotherapy on Symptom Burden and Quality of Life Over Time; A Preliminary Prospective Observational Study Using Individual Data of Patients Aged ≥70 With Early Stage Invasive Breast Cancer. J Geriatr Oncol (2018) 9(2):152–62. doi: 10.1016/j.jgo.2017.10.001
37. Wyrwich KW, Norquist JM, Lenderking WR, Acaster S. Methods for Interpreting Change Over Time in Patient-Reported Outcome Measures. Qual Life Res (2013) 22(3):475–83. doi: 10.1007/s11136-012-0175-x
38. Chan A, Yo TE, Wang XJ, Ng T, Chae JW, Yeo HL, et al. Minimal Clinically Important Difference of the Multidimensional Fatigue Symptom Inventory-Short Form (MFSI-SF) for Fatigue Worsening in Asian Breast Cancer Patients. J Pain Symptom Manage (2018) 55(3):992–7. doi: 10.1016/j.jpainsymman.2017.10.014
Keywords: breast cancer, quality of life, minimal clinically important difference, anchor-based method, distribution-based method
Citation: Li F, Liu Y, Wan C, Zhou J, Tan J and Chen H (2022) Establishing Minimal Clinically Important Differences for the Quality of Life Instrument in Patients With Breast Cancer QLICP-BR (V2.0) Based on Anchor-Based and Distribution-Based Methods. Front. Oncol. 12:753729. doi: 10.3389/fonc.2022.753729
Received: 05 August 2021; Accepted: 04 April 2022;
Published: 02 May 2022.
Edited by:
Julio de la Torre, Comillas Pontifical University, SpainReviewed by:
Tsuguo Iwatani, National Cancer Centre, JapanAgnieszka Guzik, University of Rzeszow, Poland
Copyright © 2022 Li, Liu, Wan, Zhou, Tan and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chonghua Wan, wanchh@hotmail.com
†These authors have contributed equally to this work and share first authorship
 Fei Li
Fei Li Yuxi Liu
Yuxi Liu Chonghua Wan
Chonghua Wan Jiali Zhou1
Jiali Zhou1