- 1Department of Integrative Oncology (Rehabilitation Technology), Sichuan Nursing Vocational College, Chengdu, China
- 2Department of Thoracic Surgery, West China Hospital, Sichuan University, Chengdu, China
- 3Department of Urology, Feinberg School of Medicine, Northwestern University, Chicago, IL, United States
- 4Robert H. Lurie Comprehensive Cancer Center, Northwestern University, Chicago, IL, United States
- 5Department of Breast Surgery, Chengdu Seventh People’s Hospital, Chengdu, China
Background: Myeloid sarcoma (MS) is a rare hematological malignancy characterized by the formation of a solid mass of myeloblasts outside the bone marrow, such as in the lymph nodes, skin, or bone. MS may arise de novo or concurrently with acute myeloid leukemia (AML), myeloproliferative neoplasm (MPN), or myelodysplastic syndrome (MDS). MS accounts for less than 1% of extramedullary acute myeloid leukemia cases. Phyllodes tumors (PTs) are a rare fibroepithelial breast tumor that can be benign, malignant, or borderline, and account for less than 1% of all breast cancers.
Case presentation: We present a unique case of a 50-year-old woman with both breast MS and borderline PT with malignant features, which presented a diagnostic challenge. The patient initially presented with a mass in her right breast, and the initial fine-needle biopsy revealed the presence of immature myeloperoxidase (MPO)+ myeloid cells consistent with MS. Subsequent pathological analysis of tumor tissues after neoadjuvant radiotherapy and chemotherapy showed a borderline PT with malignant features. Following excision of the tumor, the patient experienced a local recurrence, which was also surgically removed. At 8 months post-surgery, the patient remains free of recurrence under close follow-up.
Conclusion: This case highlights the importance of considering the possibility of concurrent malignancies in the differential diagnosis of complex breast masses and underscores the challenges involved in diagnosing and managing such cases. Additionally, we also emphasize the value of neoadjuvant radiotherapy and chemotherapy in MS.
1 Introduction
Myeloid sarcoma (MS) is a rare and distinct form of hematological malignancy that is characterized by the extramedullary accumulation of myeloblasts. Also known as chloroma, granulocytic sarcoma, or extramedullary myeloid tumor, MS can manifest in various organs, including the skin, lymph nodes, soft tissues, liver, spleen, and bones. There are three types of MS based on the type of cell and degree of differentiation involved: granulocytic sarcoma (GS) type, primitive mononuclear cell sarcoma type, and triple hematopoietic cell myeloid sarcoma type. MS may arise de novo or concurrently with AML, myeloproliferative neoplasm (MPN), or myelodysplastic syndrome (MDS), and can even be the initial presentation of AML or a manifestation of AML recurrence (1). The diagnosis of MS can be clinically challenging. Breast phyllodes tumors are a rare type of breast tumor, comprising only 0.3-1.0% of all breast tumors (2), and are classified into benign, borderline, and malignant subtypes. In this case report, we present a unique case of a female patient diagnosed with concurrent breast MS and borderline phyllodes tumor (borderline PT) with malignant features, which, to our knowledge, has not been previously reported.
2 Case presentation
A 50-year-old woman presented to the 7th People’s Hospital of Chengdu with an erythematous breast mass that was painful and had central necrosis in the upper outer quadrant of her right breast (Figures 1A–D). Physical examination revealed an immobile mass in the upper outer quadrant of her right breast, with an orange-peel appearance, but no signs of nipple retraction were noted. The patient had a medical history of well-controlled hypertension, which was being treated with felodipine and enalapril. She denied any history of smoking, alcohol or substance abuse, and had no family history of cancer. Her physical examination, aside from the breast mass, was unremarkable.
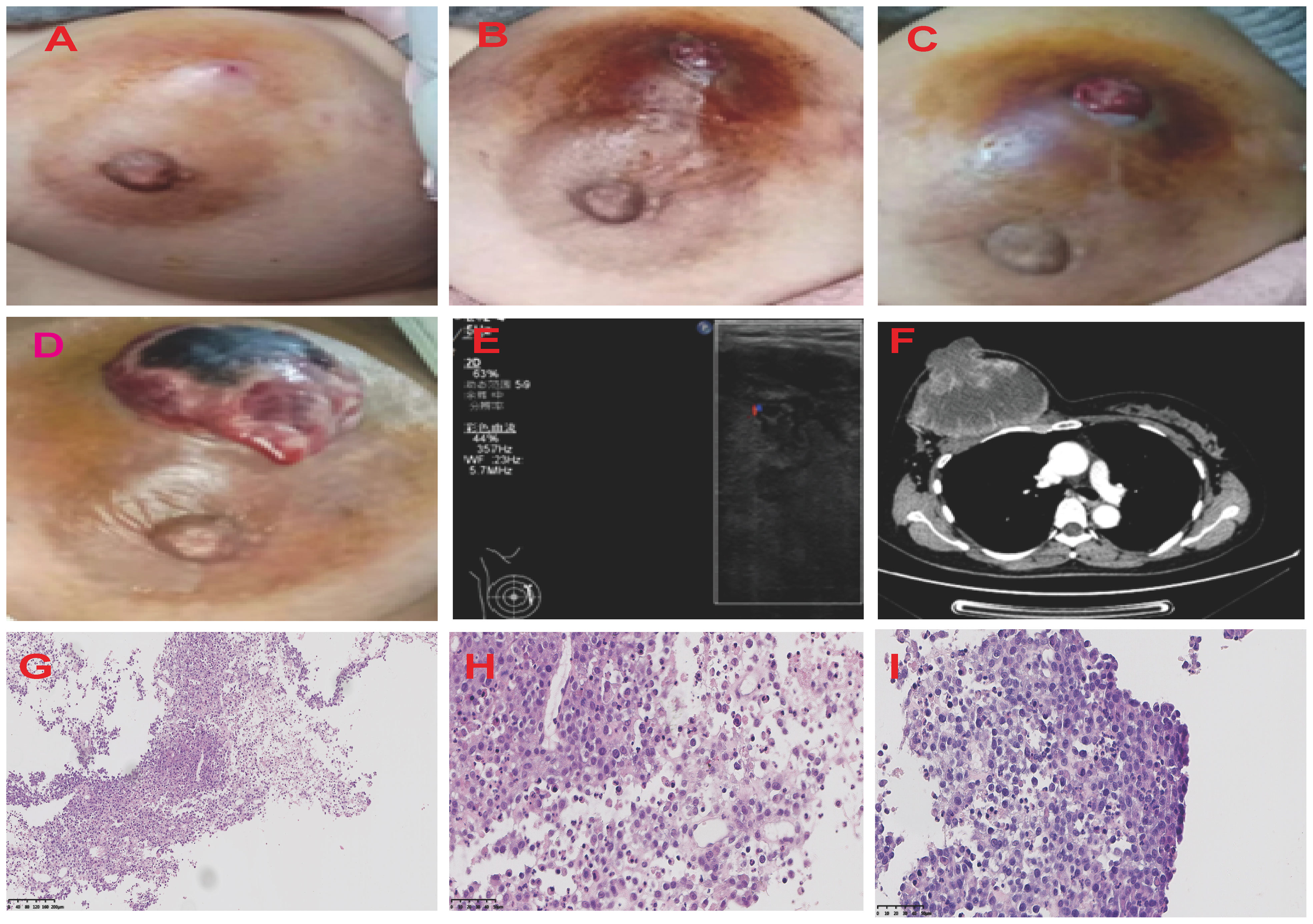
Figure 1 Graphic Representation of Breast Lesion Progression Over Time. The series of four images chronicle the evolution of the right breast lesion from March to April 2021. (A) The image of the breast lesion on [5/3/2021]. The patient initially presented with marked breast swelling and tenderness. (B) The image of the breast lesion on [5/4/2021]. (C) The image of the breast lesion on [23/4/2021]. (D) The image of the breast lesion on [25/4/2021]. The breast lesion has evolved into a mass with profound erythema and sinus tract. The ultrasound and CT images of the patient’s right breast (E, F). The H&E staining of MS. (G) The description of myeloblasts and lymphoid H&E×100. (H) The description of myeloblasts and lymphoid H&E×400. (I) The description of tumor cells H&E×400. H&E, hematoxylin and eosin.
Breast ultrasound revealed an 8.8cm mass with mixed solid echogenic and cystic anechoic components. (Figure 1E). The contrast-enhanced CT scan showed an 11.7×8.9cm mass occupying a substantial portion of the right breast, accompanied by right axillary lymphadenopathy (Figure 1F). An ultrasound-guided fine needle biopsy revealed the presence of immature myeloid cells (Figures 1G–I). Immunohistochemistry (IHC) analysis demonstrated strong expression of CD68KP1 (Figure 2A), CD45 (Figure 2B), CD33, Cyclin D1 and CD4, with weak positivity for myeloperoxidase (MPO), and a high level of Ki67 expression (80%). The cells were negative for CD34 (Figure 2C), CD3 (Figure 2D), CD20 (Figure 2E), CD79a (Figure 2F), CD14, CD117, and terminal deoxynucleotidyl transferase (TdT). A bone marrow biopsy was performed, and there was no evidence of systemic hematological malignancies such as AML, MPD, or MDS. Therefore, the patient was diagnosed with isolated myeloid sarcoma (MS).
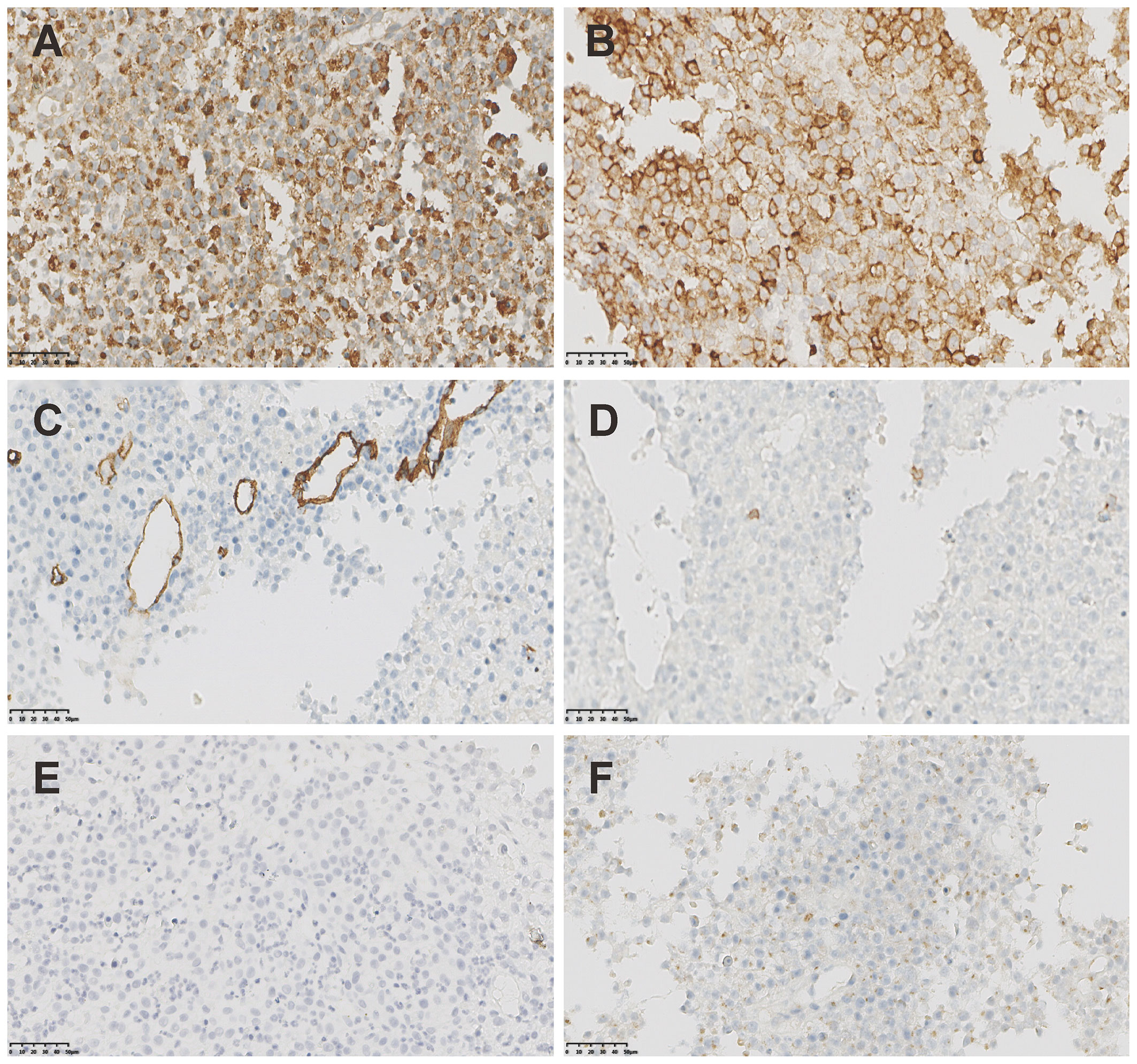
Figure 2 The IHC staining of MS. (A) Strong positivity of CD68KP1 (×200). (B) Strong positivity of CD45(×200). (C) Negative staining of CD34(×200). (D) Negative staining of CD3(×200). (E) Negative staining of CD20(×200). (F) Negative staining of CD79a(×200).
The patient underwent neoadjuvant radiotherapy and chemotherapy, which included cytarabine and daunorubicin. After undergoing two cycles of chemotherapy, which included cytarabine at a dose of 100 mg/m2 administered over days 1-7, and daunorubicin at a dose of 60 mg/m2 over days 1-3, a follow-up CT scan revealed a significant reduction in the size of the breast mass (4cm×3cm) and a ground-glass appearance in the upper lobes of both lungs (Figures 3A–D). The patient presented with newly onset of dry cough and dyspnea. Empirical treatment with cefoperazone-tazobactam was initiated, but did not result in significant improvement of the patient’s condition. A fungal infection was suspected, and she was switched to voriconazole, which resolved her respiratory symptoms within 2 weeks. However, the patient declined subsequent cycles of chemotherapy due to adverse effects. Three months after completing chemotherapy, the patient underwent a right mastectomy. Histological analysis of the surgical specimen revealed the presence of both epithelial and mesenchymal components. Benign epithelial components are illustrated in Figure 3E. The spindle-shaped hyperproliferative fibroblasts, representative of the malignant components, are shown in Figure 3F. The cartilage region is considered to be heterogenous mesenchyme, which is associated with tumor invasion. (Figure 3G). Notably, these malignant interstitial cells are characterized by the permeative border (Figure 3H), heteromorphism and abundant mitosis, with a visible nuclear division rate of 11/10HPF (Figures 3I, J). In summary, the pathological analysis aligns with a diagnosis of borderline phyllodes tumor with malignant features. The IHC analysis demonstrated strong expression of ER (Figure 4A), PR, SMA and SATB2, with a moderate level of Ki67 expression (25-30%) (Figure 4B) and weak positivity for CD117 (Figure 4C) and Blc2 (Figure 4D). The tissues were negative for P63 (Figure 4E), CD34 (Figure 4F). CD10 (Figure 4G), desmin, cytokeratin-5/6 (Figure 4H).
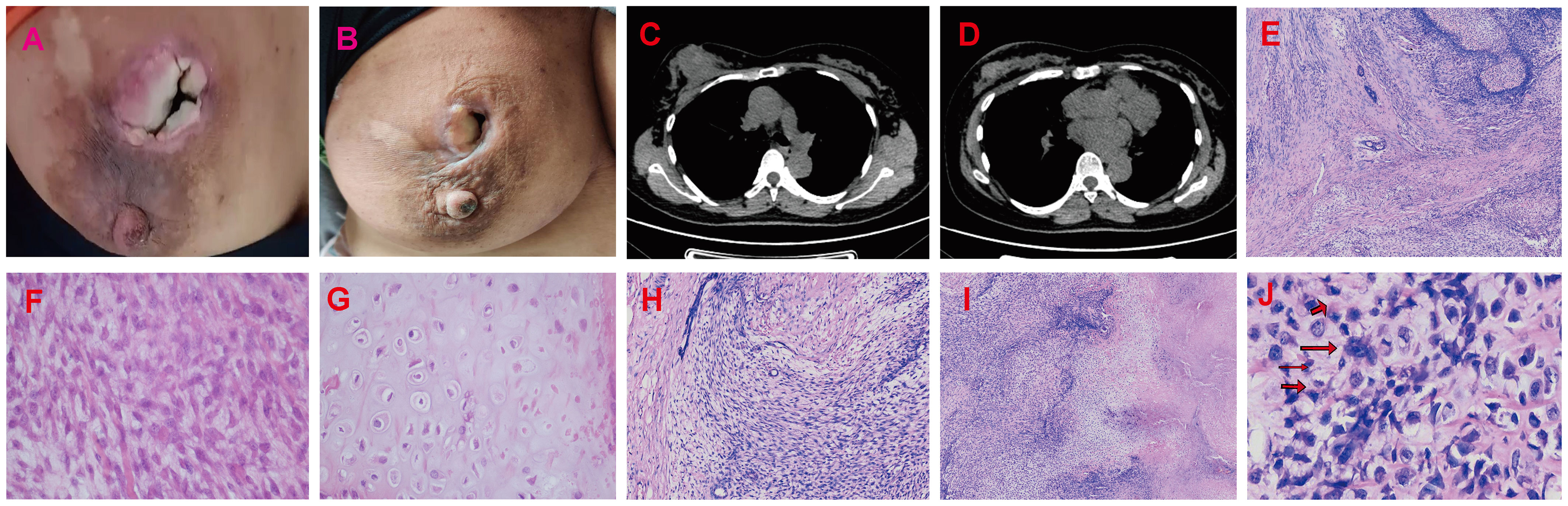
Figure 3 Radiotherapy and chemotherapy significantly reduced the size of the breast mass. (A, B) Graphic representation of breast lesion after radiotherapy and chemotherapy. (C, D) The CT images of the patient’s right breast after radiotherapy and chemotherapy. The H&E staining of Borderline Phyllodes Tumor with malignant features. (E) The benign epithelial components H&E×4. (F) The staining depicting of the spindle cell region H&E×400. (G) The staining illustrating the cartilage region H&E×400. (H) The permeative border of the borderline PT with malignant features H&E×100. (I) The interstitial overgrowth of borderline PT with malignant features H&E×40. (J) The nuclear division and pathological nuclear division H&E×400. H&E = hematoxylin and eosin.
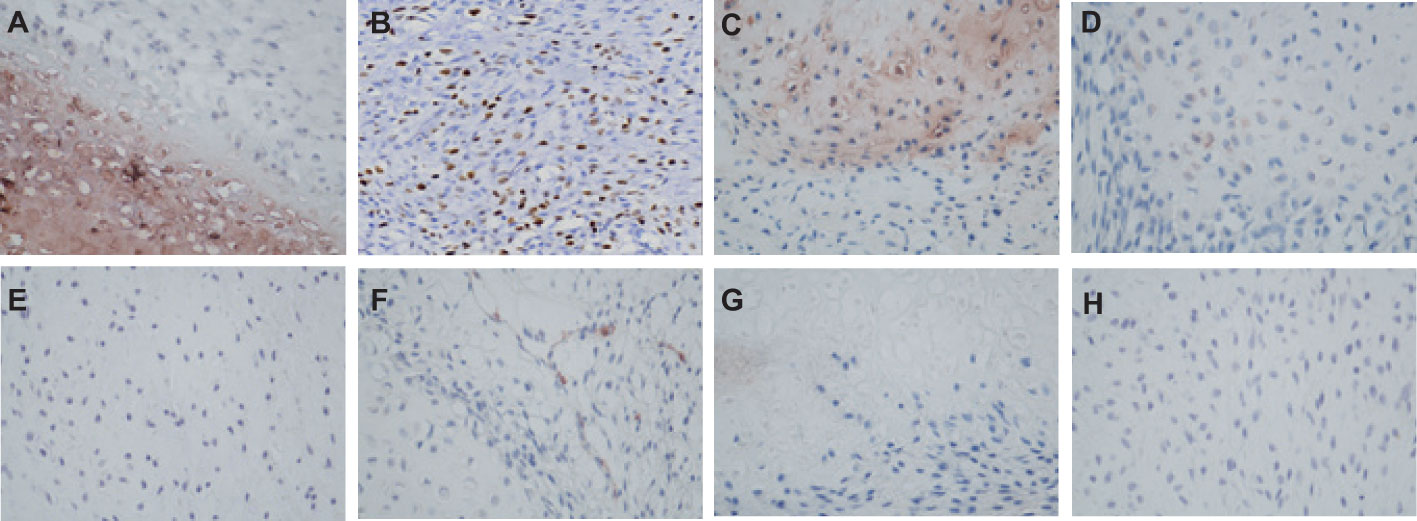
Figure 4 The IHC staining of Borderline Phyllodes Tumor with malignant features. (A) Strong positivity of ER×400. (B) A moderate level of Ki67 expression×200. (C) Weakly positivity of CD117 ×400. (D) Weakly positivity of P63 ×400. (E) Weakly positivity of Bcl2×400. (F) Negative staining of CD34×400. (G) Negative staining of CD10×400. (H) Negative staining of CK5/6 ×400. IHC, Immunohistochemistry.
The patient developed a local recurrence at the site of the surgical incision 4 months after the initial mastectomy and underwent another surgical excision to remove the new lesion. The subsequent pathological analysis confirmed the presence of borderline PT with malignant features. The patient has been closely monitored every 2 months since the second surgery and has remained disease-free for the past 8 months.
3 Discussion
MSs are rare tumors of immature myeloid cells that can occur outside the bone marrow, including the skin, lymph nodes, testes, intestines, bones, and central nervous system (3–7). Although MSs often develop concurrently with or after the diagnosis of myeloid malignancies (1, 8–10), cases of primary MS without any blood or bone marrow involvement (11–13), like our case, have also been reported. The rarity of MS can make diagnosing it challenging, leading to a higher incidence of misdiagnosis. Differential diagnoses of MS include various hematological malignancies such as anaplastic large-cell lymphoma and diffuse large B-cell lymphoma, as well as solid tumors such as breast carcinoma and melanoma (5, 13). Thus, a precise diagnosis of MS requires extensive immunophenotyping to confirm the myeloid origin of the malignancy. In our case, we observed the typical profile of myeloid malignant cells in the initial biopsy, which showed immunoreactivity to markers such as MPO (5) and CD33. Due to the rarity of myeloid sarcoma, there is currently no established consensus on the optimal treatment approach. Some previous studies have suggested that a combination of radiotherapy and chemotherapy may be beneficial for MS patients (14, 15), which is similar to our case. However, there is conflicting evidence, as another study reported no survival benefit in patients with isolated MS who were treated with radiation and chemotherapy (4).
PTs are considered an uncommon fibroepithelial neoplasms of the breast (16), which are classified into three subtypes: benign, borderline and malignant. The benign PTs are more common, An article analyzing 170 cases found that benign, borderline and malignant PTs incidence are 54.1%, 11.2% and 34.7% respectively (17). PTs pose diagnostic challenges, as they can be difficult to distinguish from fibroadenoma (18, 19). Histologically, PTs are characterized by a leaflike architecture resulting from an enhanced intracanalicular growth pattern, cleft-like spaces lined by epithelium, and hypercellular stroma. Malignant PT is characterized by marked stromal cellularity and nuclear pleomorphism, stromal overgrowth, and more than 10 mitoses per 10 HPF. The presence of heterologous sarcomatous elements (liposarcoma, chondrosarcoma, and osteosarcoma) alone qualifies a PT as malignant (20). In our case, the histological analysis revealed spindle cell and cartilage regions, with notable heteromorphism and mitosis in the spindle cell area. Additionally, there are reports indicating that the stromal cells of phyllodes tumors can express p63, cytokeratins and CD117 (21–23). Notably, CD117 expression in the stroma has been linked to predicting disease recurrence. In our patient, there were observed expressions of ER, PR, CD117, SMA, and SATB2, aligning with the previously documented trend as she experienced local recurrence.
To our knowledge, this is the first reported case of co-existing breast PT and MS. The co-occurrence posed further diagnostic challenges but also provides insights into the management of isolated MS. There is no definitive consensus regarding the optimal treatment and follow-up of primary extramedullary MS. Our patient benefited from neoadjuvant radiotherapy and chemotherapy, which led to a significant reduction in the breast mass and subsequent surgical excision with a clean margin. To our surprise, the repeated analysis of the surgical specimen revealed borderline PT with malignant features, without evidence of co-existing metastatic disease. We believe that the neoadjuvant therapies led to MS remission. This case highlights the importance of a comprehensive diagnostic approach and individualized treatment plan for patients with rare co-existing malignancies, such as PT and MS.
4 Conclusion
To our knowledge, this marks the inaugural report of a simultaneous occurrence of MS and PT in the breast. A prior study unveiled the coexistence of PT with anaplastic ependymoma (19), emphasizing the intricate nature of PT diagnosis when intertwined with other malignancies. Our case emphasizes the significance of adopting a comprehensive diagnostic approach to ensure precision, particularly in complex cases. The effective management of our patient’s condition with neoadjuvant radiotherapy and chemotherapy additionally underscores the potential advantages of this treatment strategy for individuals with myeloid sarcoma.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
LC: Resources, Writing – original draft. ZZ: Writing – review & editing, Methodology. QG: Writing – review & editing, Methodology. YH: Data curation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
The authors would like to express their sincere gratitude to the patient and their family for their invaluable support, cooperation, and willingness to participate in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
MS, Myeloid Sarcoma; AML, Acute myeloid leukemia; MPN, Myeloproliferative neoplasm; MDS, Myelodysplastic syndrome; PTs, Phyllodes tumors; IHC, Immunohistochemistry.
References
1. Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood (2016) 127(20):2391–405. doi: 10.1182/blood-2016-03-643544
2. Guerrero MA, Ballard BR, Grau AM. Malignant phyllodes tumor of the breast: review of the literature and case report of stromal overgrowth. Surg Oncol (2003) 12(1):27–37. doi: 10.1016/s0960-7404(03)00005-7
3. Goyal G, Bartley AC, Patnaik MM, Litzow MR, Al-Kali A, Go RS. Clinical features and outcomes of extramedullary myeloid sarcoma in the United States: analysis using a national data set. Blood Cancer J (2017) 7(8):e592. doi: 10.1038/bcj.2017.79
4. Lontos K, Yabes JG, Farah RJ, Boyiadzis M. Impact of upfront chemotherapy on overall survival in isolated myeloid sarcoma. Leukemia (2021) 35(4):1193–6. doi: 10.1038/s41375-020-01017-z
5. Pileri SA, Ascani S, Cox MC, Campidelli C, Bacci F, Piccioli M, et al. Myeloid sarcoma: clinico-pathologic, phenotypic and cytogenetic analysis of 92 adult patients. Leukemia (2007) 21(2):340–50. doi: 10.1038/sj.leu.2404491
6. Valbuen JR, Joan H, Admirand M, Gabriela Gualco ML, Jeffrey Medeiros M. Myeloid sarcoma involving the breast. Arch Pathol Lab Med (2005) 129:32–8. doi: 10.5858/2005-129-32-MSITB
7. Zhao H, Dong Z, Wan D, Cao W, Xing H, Liu Z, et al. Clinical characteristics, treatment, and prognosis of 118 cases of myeloid sarcoma. Sci Rep (2022) 12(1):6752. doi: 10.1038/s41598-022-10831-7
8. Miyazato H, Sono H, Naiki Y, Miyatake J, Nozaki Y, Yamaguchi T, et al. Detection of myeloperoxidase gene expression by in situ hybridization in a case of granulocytic sarcoma associated with AML-M0. Leukemia (2001) 15(11):1797–9. doi: 10.1038/sj.leu.2402270
9. Marra A, Martino G. Placental myeloid sarcoma. Blood (2020) 135(16):1410. doi: 10.1182/blood.2020004975
10. Wilson CS, Medeiros LJ. Extramedullary manifestations of myeloid neoplasms. Am J Clin Pathol (2015) 144(2):219–39. doi: 10.1309/AJCPO58YWIBUBESX
11. Qian X, Gigantelli JW, Abromowitch M, Morgan LA, Suh DW. Myeloid sarcoma in the orbit. J Pediatr Ophthalmol Strabismus (2016) 53:e64–8. doi: 10.3928/01913913-20161102-01
12. Artinyan A, Guzman E, Maghami E, Al-Sayed M, D’Apuzzo M, Wagman L, et al. Metastatic parathyroid carcinoma to the liver treated with radiofrequency ablation and transcatheter arterial embolization. J Clin Oncol (2008) 26(24):4039–41. doi: 10.1200/JCO.2007.15.9038
13. Menasce LP, Banerjee SS, Beckett E, Harris M. Extra-medullary myeloid tumor (granulocytic sarcoma) is often misdiagnosed: a study of 26 cases. Histopathology (1999) 34(5):391–8. doi: 10.1038/sj.leu.2404491
14. Zuo X, Wang Y, Zhou Y, Luo L, Liu M. Primary granulocytic sarcoma of cervix (PGSC): review and analysis of 13 cases. Blood (2014) 124(21):5271–1. doi: 10.1182/blood.V124.21.5271.5271
15. Frietsch JJ, Hunstig F, Wittke C, Junghanss C, Franiel T, Scholl S, et al. Extra-medullary recurrence of myeloid leukemia as myeloid sarcoma after allogeneic stem cell transplantation: impact of conditioning intensity. Bone Marrow Transplant (2021) 56(1):101–9. doi: 10.1038/s41409-020-0984-4
16. Rayzah M. Phyllodes tumors of the breast: A literature review. Cureus (2020) 12(9):e10288. doi: 10.7759/cureus.10288
17. Reinfuss M, Mitus J, Duda K, Stelmach A, Rys J, Smolak K. The treatment and prognosis of patients with phyllodes tumor of the breast: an analysis of 170 cases. Cancer (1996) 77(5):910–6. doi: 10.1002/(sici)1097-0142(19960301)77:5<910::aid-cncr16>3.0.co;2-6
18. Kazakov DV, Spagnolo DV, Stewart CJ, Thompson J, Agaimy A, Magro G, et al. Fibroadenoma and phyllodes tumors of anogenital mammary-like glands: A series of 13 neoplasms in 12 cases, including mammary-type juvenile fibroadenoma, fibroadenoma with lactation changes, and neurofibromatosis-associated pseudoangiomatous stromal hyperplasia with multinucleated giant cells. Am J Surg Pathol (2010) 34:95–103. doi: 10.1097/PAS.0b013e3181c6e5c5
19. Aribas BK, Sahin G, Uluoglu O, Bozgul M, Barit G, Torun E. Subcutaneous sacrococcygeal anaplastic ependymoma with Malignant cystosarcoma phylloides of the breast. J Neurological Sci (Turkish) (2012) 29:402–9.
20. Zhang Y, Kleer CG. Phyllodes tumor of the breast: histopathologic features, differential diagnosis, and molecular/genetic updates. Arch Pathol Lab Med (2016) 140(7):665–71. doi: 10.5858/arpa.2016-0042-RA
21. Cimino-Mathews A, Sharma R, Illei PB, Vang R, Argani P. A subset of Malignant phyllodes tumors express p63 and p40: a diagnostic pitfall in breast core needle biopsies. Am J Surg Pathol (2014) 38(12):1689–96. doi: 10.1097/PAS.0000000000000301
22. Chia Y, Thike AA, Cheok PY, Yong-Zheng Chong L, Man-Kit Tse G, Tan PH. Stromal keratin expression in phyllodes tumours of the breast: a comparison with other spindle cell breast lesions. J Clin Pathol (2012) 65(4):339–47. doi: 10.1136/jclinpath-2011-200377
Keywords: breast cancer, myeloid sarcoma (MS), borderline phyllodes tumor with malignant features, neoadjuvant radiotherapy, neoadjuvant chemotherapy
Citation: Chun L, Zeng Z, Guo Q and He Y (2024) Case report: The first case of concurrent breast myeloid sarcoma and borderline phyllodes tumor with malignant features. Front. Oncol. 13:1268617. doi: 10.3389/fonc.2023.1268617
Received: 28 July 2023; Accepted: 28 December 2023;
Published: 19 January 2024.
Edited by:
Xiaowei Qi, Army Medical University, ChinaReviewed by:
Bilgin Kadri Aribas, Bülent Ecevit University, TürkiyeArmando Pérez Torres, National Autonomous University of Mexico, Mexico
Copyright © 2024 Chun, Zeng, Guo and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Li Chun, 1306839492@qq.com
 Li Chun
Li Chun Zhen Zeng2
Zhen Zeng2