- Department of Radiation Oncology, The First Affiliated Hospital of Guangxi Medical University, Nanning, China
Background: Stage IIIC1p cervical cancer is characterized by marked heterogeneity and considerable variability in the postoperative prognosis. This study aimed to identify the clinical and pathological characteristics affecting the survival of patients diagnosed with stage IIIC1p cervical cancer.
Methods: We retrospectively analyzed patients diagnosed with stage IIIC1p cervical cancer who underwent radical hysterectomy and lymph node dissection between March 2012 and March 2022. Overall survival (OS) was estimated using Kaplan-Meier survival curves. Univariate and multivariate Cox proportional hazards models were used to evaluate prognostic factors for OS and forest plots were used to visualize these findings. Nomogram charts were created to forecast survival rates at 3 and 5 years, and the accuracy of predictions was evaluated using Harrell’s concordance index (C-index) and calibration curves.
Results: The study cohort comprised 186 women diagnosed with stage IIIC1p cervical cancer. The median follow-up duration was 51.1 months (range, 30-91 months), and the estimated 5-year OS rate was 71.5%. Multivariate analysis revealed that concurrent chemoradiotherapy plus adjuvant chemotherapy (CCRT + AC), monocyte-lymphocyte ratio (MLR), ratio of lymph node metastasis (LNM), and squamous cell carcinoma antigen (SCCA) levels independently predicted OS.
Conclusions: Significant prognostic disparities exist among patients diagnosed with stage IIIC1p cervical cancer. MLR, ratio of LNM, and SCCA were associated with poor OS. In contrast, the CCRT + AC treatment regimen appeared to confer a survival advantage.
1 Introduction
Cervical cancer is one of the most prevalent malignancies of the female reproductive system. According to 2020 worldwide cancer data, approximately 604,000 new cases and 342,000 fatalities occur annually, positioning it as the primary cause of non-breast gynecological malignancies (1). The occurrence and fatality rates of cervical cancer in China account for 18.3% and 17.6%, respectively, of the worldwide total. Despite a decreasing trend in incidence due to widespread early screening, incidence rates among younger patients are still on the rise (1–3).
Lymph node metastasis (LNM) significantly affects the cervical cancer prognosis. Radical hysterectomy and lymph node dissection are crucial for patients during the early stages. However, based on existing randomized controlled trials, we found insufficient evidence to suggest that hysterectomy improves the survival of women with locally advanced cervical cancer treated with either radiotherapy or chemoradiotherapy (4). The chances of pelvic LNM in cervical cancer stages IB2, IIA, and IIB were 11%, 13%, and 16%, respectively (5–7). After the 2018 International Federation of Gynecology and Obstetrics (FIGO) update, patients who were pathologically confirmed to have pelvic LNM were specifically labeled as stage IIIC1p. According to the US SEER Program, patients with stage IIIC1 disease have a higher disease-specific survival rate than patients with stage IIIA-IIIB disease. Additionally, the survival rate within the stage IIIC1 group decreased as the T stage increased (5-year overall survival [OS] rates were 74.8% for T1, 58.7% for T2, and 39.3% for T3) (8). This suggests that the outlook for individuals with cervical cancer and pelvic LNM varies owing to factors beyond the status of the lymph nodes. Hence, a more in-depth examination of the clinical and pathological characteristics of individuals diagnosed with stage IIIC1p cervical cancer may improve prognostic evaluation and personalized supplementary therapy.
Several studies have suggested that parameters such as the number of LNM, ratio of LNM (proportion of positive to total lymph nodes removed), and number of LNM sites are correlated with OS (9–11). Furthermore, the prognosis of patients with IIIC1p was found to be associated with pathological type (12, 13), pT2b (12), and neutrophil-to-lymphocyte ratio (NLR) > 3.8 (9). Nevertheless, elucidating which factors are more effective in assessing the outlook of individuals with IIIC1p cervical cancer remains unresolved. Therefore, the objective of this study was to identify the risk factors impacting prognosis, providing a basis for accurate prognostic assessment, and tailored therapeutic strategies to improve the survival and quality of life of patients with stage IIIC1p cervical cancer.
2 Materials and methods
2.1 Patients
A retrospective analysis was conducted on 186 patients with cervical cancer who had undergone radical hysterectomy and lymph node dissection at the First Affiliated Hospital of Guangxi Medical University, spanning the period from March 2012 to March 2022. Following the 2018 FIGO criteria, all patients with positive pelvic lymph nodes were re-staged to cervical cancer stage IIIC1p and underwent postoperative concurrent chemoradiotherapy (CCRT). The following criteria were used for exclusion (1): chemotherapy before surgery or radiotherapy (2), LNM in the para-aortic region (3), concurrent presence of other tumors (4), distant metastasis, and (5) no follow-up data. In this study, we collected data from patients with stage IIIC1p cervical cancer regarding LNM (including common iliac lymph node, number of LNM sites, the number and ratio of LNM), para-aortic lymph nodes (PALN) resection, vaginal brachytherapy utilization, type of radicality, postoperative high-risk factors (surgical margins and parametrial infiltration), intermediate-risk factors (tumor size, lymph-vascular space invasion [LVSI], depth of stromal invasion [DSI]), preoperative tumor markers (squamous cell carcinoma antigen [SCCA] and carcinoembryonic antigen [CEA]), adjuvant treatment regimens (CCRT and CCRT+ adjuvant chemotherapy [AC]), monocyte-to-lymphocyte ratio (MLR), and chemotherapy protocol. According to the literature, we classified LVSI positivity into focal and diffuse. Focal was defined as a single focus of LVSI recognized around a tumor, and diffuse was defined as diffuse LVSI (more than 1) recognized around the tumor (14). The study was approved by the institutional ethics review committee and was conducted in compliance with the principles of the Declaration of Helsinki (approval number: 2023-E724-01). Follow-up was concluded on October 10, 2023, via telephone interviews or outpatient medical records.
2.2 Treatment
All patients underwent radical hysterectomy and systematic bilateral pelvic lymphadenectomy. If common iliac lymph nodes were identified as being positive by the intraoperative frozen section or the PALN were identified as suspicious by visualization and palpation, para-aortic lymphadenectomy was also performed during radical surgeries. After surgery, external-beam radiation therapy to the pelvis was conducted on all patients. Extended field radiation with the upper margin up to T12-L1 was performed in common iliac lymph node-positive patients. The dose to the whole pelvis was 45-50.4 Gy at 25-28 fractions delivered five times per week. Patients with positive vaginal margins also received intracavitary brachytherapy with a dose of 30 Gy delivered in five fractions twice per week. The concurrent chemotherapeutic drugs included cisplatin, 5-fluorouracil, and paclitaxel. AC drugs included cisplatin, docetaxel, and paclitaxel.
2.3 Statistical analysis and study endpoints
The data were analyzed using SPSS 26.0, and R version 4.3.1. Quantitative variables were summarized using medians (P25, P75) or means ± standard deviations, and intergroup comparisons were made using the independent sample t-test or Mann-Whitney U test. The chi-square test was used to compare categorical variables, which were expressed as counts (%). To determine the prognostic factors for OS, we utilized the R programming language and conducted Cox regression analysis with the assistance of the ‘survival’ and ‘survminer’ packages. The results were presented as forest plots. Nomograms were constructed, and their predictive accuracies were assessed using Harrell’s concordance index (C-index) and 1,000 bootstrap resampling for the calibration curves.
The primary outcome measure was OS, which was defined as the period from the surgical procedure until either death or the most recent follow-up available.
3 Results
3.1 Patient clinicopathological characteristics
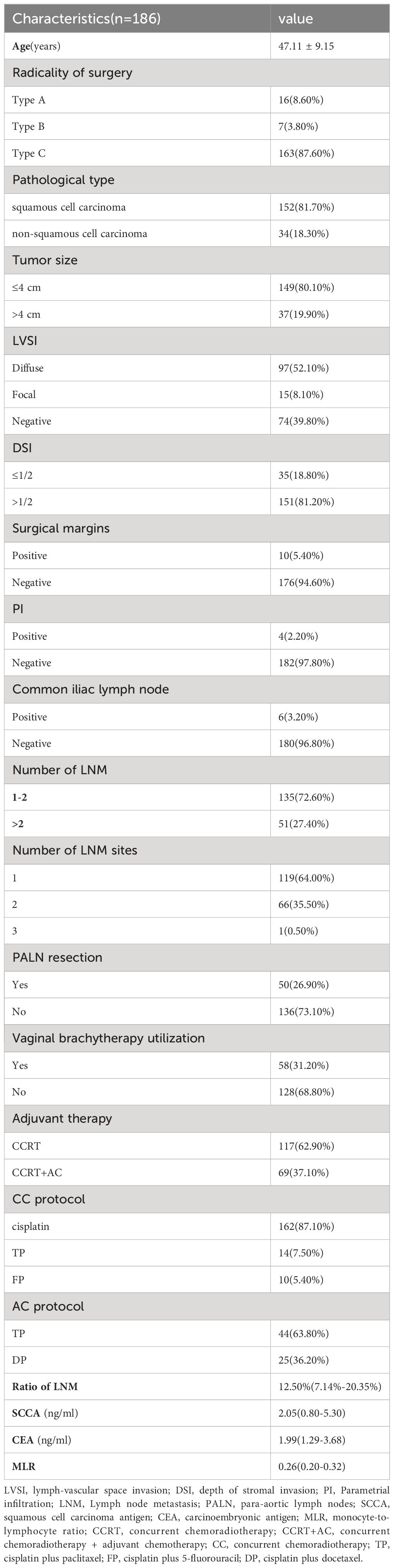
Our study included 186 patients diagnosed with stage IIIC1p cervical cancer. Table 1 shows the clinicopathological characteristics of these patients. The mean patient age was 47 years. The vast majority of patients (87.60%) underwent a type C radical hysterectomy. The most prevalent histologic type was squamous cell carcinoma, accounting for 81.70% of cases. Non-squamous cell carcinomas included adenocarcinomas, adenosquamous carcinomas, and neuroendocrine carcinomas. Most cases showed tumor sizes ≤ 4 cm (80.10%), diffuse positive LVSI (52.10%), DSI >1/2 (81.20%), negative resection margins (94.60%), negative parametrial invasion (97.80%), negative common iliac lymph node (96.80%), 1-2 LNM (72.60%), LNM in one site (64.00%), no PALN resection (73.10%), and no vaginal brachytherapy utilization (68.80%).
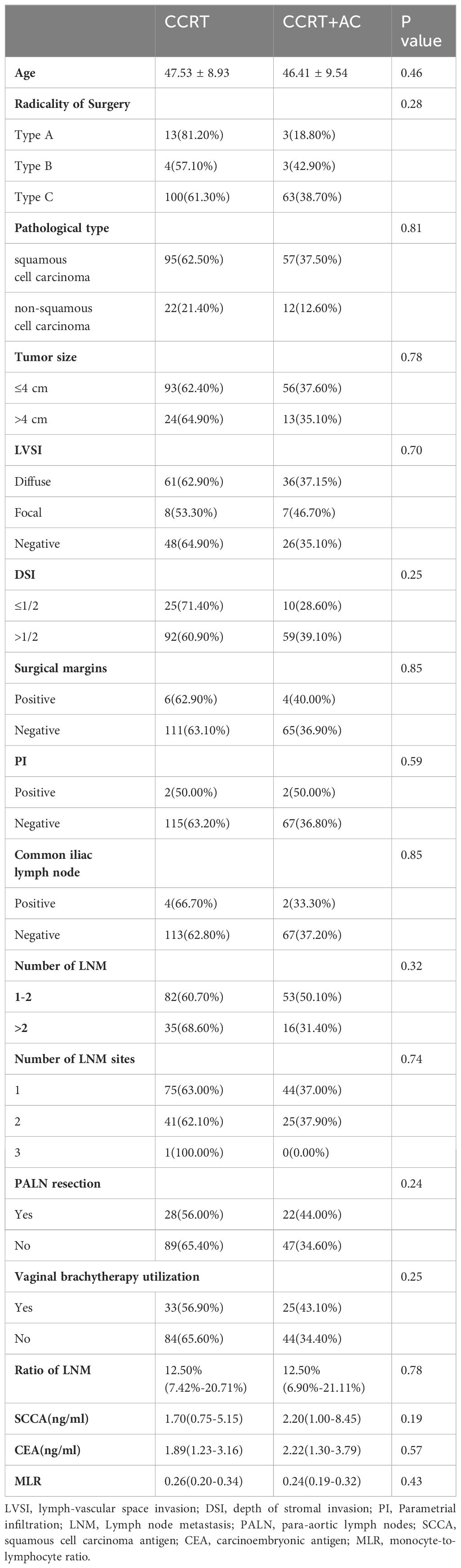
For non-normally distributed data, the median values for the number of LNM, ratio of LNM, SCCA, CEA, and MLR were 1, 12.50%, 2.05 ng/mL, 1.99 ng/mL, and 0.26, respectively. All patients received CCRT postoperatively, and 37.10% (n=69) received AC. In the concurrent chemotherapy regimens, weekly cisplatin, TP (cisplatin plus paclitaxel), and FP (cisplatin plus 5-fluorouracil) accounted for 87.1%, 7.5%, and 5.4% of treatments, respectively. For the AC regimen, TP and DP (cisplatin plus docetaxel) accounted for 63.8% and 36.2% of the treatments, respectively. No statistically significant differences in the patient characteristics between the CCRT and CCRT+AC treatment groups were observed (Table 2).
3.2 Univariate and multivariate analyses for OS
We conducted univariate Cox regression analysis of the factors that could affect the outlook of individuals diagnosed with stage IIIC1p cervical cancer. These findings indicated that the combination of CCRT + AC, number of LNM >2, LNM in two site, PALN were resected, MLR, ratio of LNM, and SCCA were strongly linked to OS in individuals diagnosed with stage IIIC1p cervical cancer (p<0.05) (Figure 1). Multivariate Cox regression analysis further delineated CCRT + AC (hazard ratio [HR] =0.37, 95% confidence interval [CI] 0.18-0.75, p=0.006), MLR (HR=2.81, 95% CI 1.24-6.35, p=0.013), ratio of LNM (HR=5.64, 95% CI 1.12-28.35, p=0.036), and SCCA (HR=1.04, 95% CI 1.01-1.06, p=0.001) as independent prognostic factors for OS (Figure 1). Based on multivariate analysis, the Kaplan-Meier survival curves indicated that the CCRT + AC treatment regimen resulted in a notably increased 5-year OS (HR=0.53, 95% CI=0.27-1.01) and OS (HR=0.46, 95% CI=0.24-0.88) compared to CCRT alone (Figures 2A, B). However, no significant difference existed in the 2-year OS (HR=0.93, 95% CI=0.37-2.33) (Figure 2C).
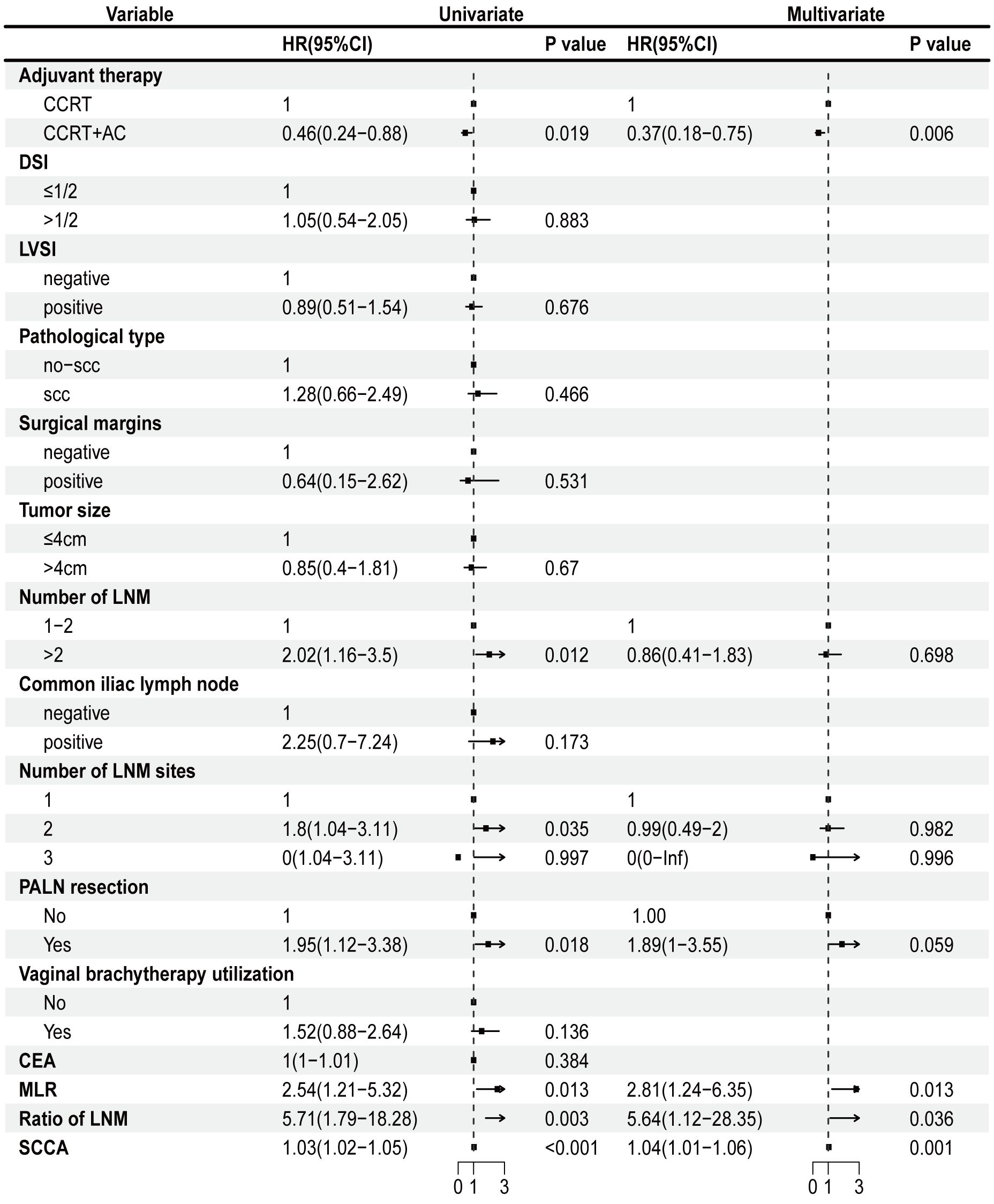
Figure 1 Univariate Cox regression analysis and multivariate Cox regression regarding OS. CCRT, concurrent chemoradiotherapy; CCRT+AC, concurrent chemoradiotherapy + adjuvant chemotherapy; DSI, depth of stromal invasion; LVSI, lymph-vascular space invasion; scc, squamous cell carcinoma; CEA, carcinoembryonic antigen; MLR, monocyte-to-lymphocyte ratio; LNM, Lymph node metastasis; PALN, para-aortic lymph nodes; SCCA, squamous cell carcinoma antigen; OS, overall survival.
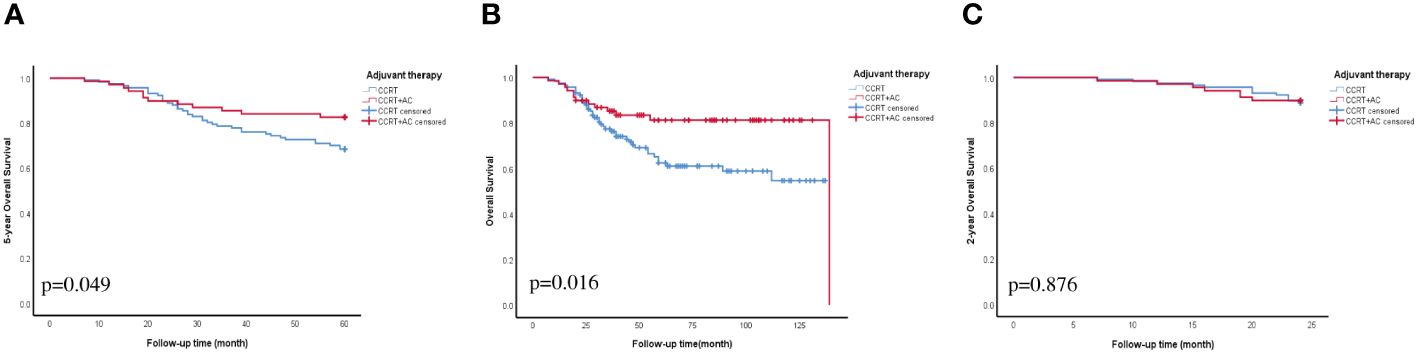
Figure 2 OS curve for adjuvant therapy. (A) 5-year OS. (B) OS. (C) 2-year OS. Abbreviations: CCRT, concurrent chemoradiotherapy; CCRT+AC, concurrent chemoradiotherapy + adjuvant chemotherapy; OS, overall survival.
3.3 Nomogram
To better predict the OS of patients with stage IIIC1p cervical cancer, nomograms were created using four factors: ratio of LNM, SCCA, MLR, and postoperative adjuvant therapy (CCRT or CCRT + AC) (Figure 3A). The C-index of the nomogram was 0.75, demonstrating strong concordance, as evidenced by the calibration curves for the 3 and 5-year periods depicted in Figures 3B, C.
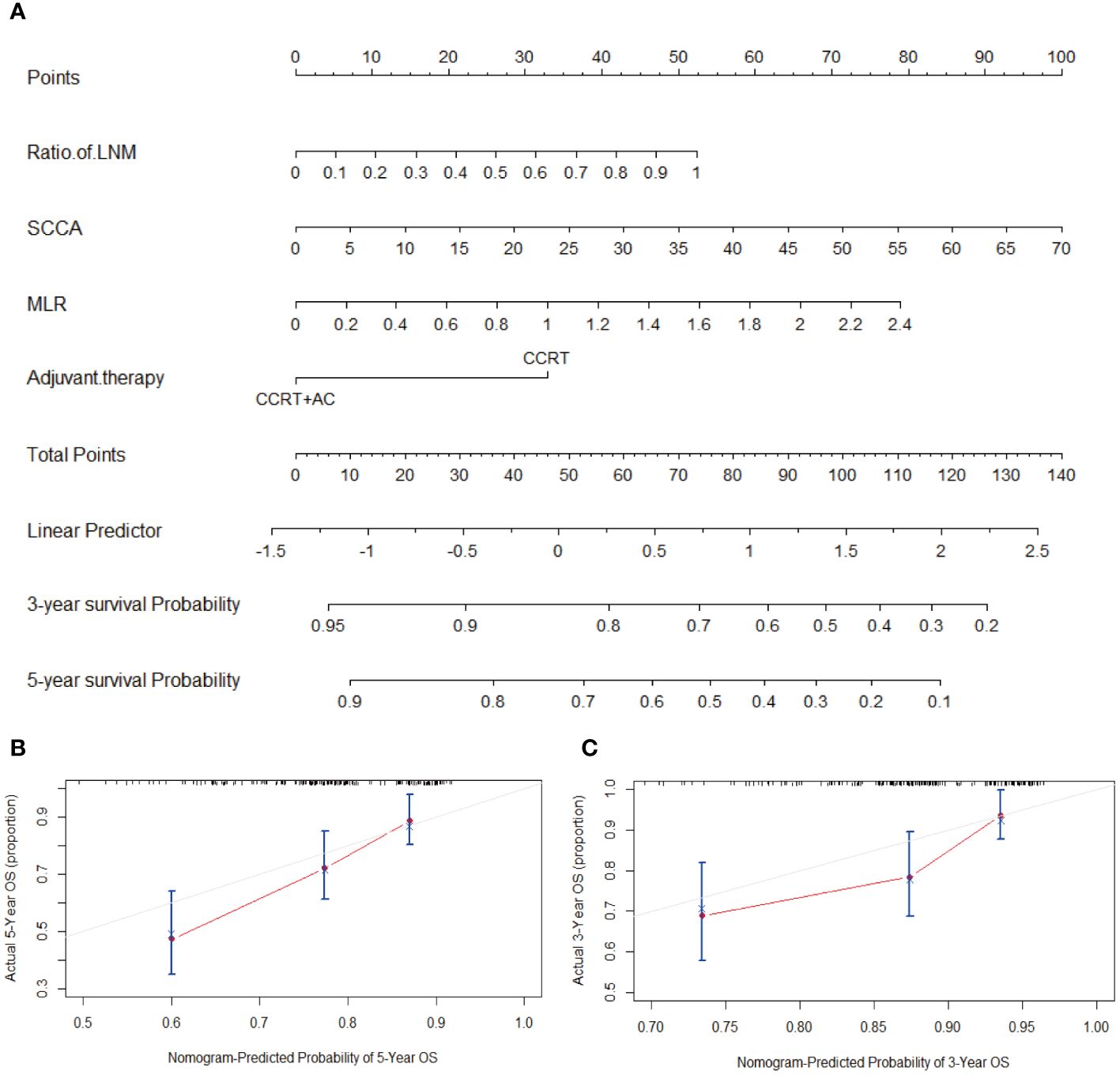
Figure 3 (A) Nomogram for predicting OS, which had a C-index of 0.75. (B) Calibration curve to predict 5-year OS. (C) Calibration curve to predict 3-year OS. Abbreviations: LNM, Lymph node metastasis; SCCA, squamous cell carcinoma antigen; MLR, monocyte-to-lymphocyte ratio; CCRT, concurrent chemoradiotherapy; CCRT+AC, concurrent chemoradiotherapy + adjuvant chemotherapy; OS, overall survival; C-index, concordance index.
4 Discussion
Cervical cancer primarily spreads through LNM, sequentially dispersing to the para-uterine, obturator, internal and external iliac, and common iliac lymph nodes. Approximately 10-20% of individuals diagnosed with cervical cancer in its early stages experience the spread of cancer cells to the lymph nodes (15). This study found that patients with postoperative pelvic LNM had a 5-year overall survival rate of 71.5%, which is consistent with the findings of previous studies. The 2018 FIGO staging system emphasizes the significance of lymph node status in prognosis and the recognition of diverse prognostic outcomes within this group.
The National Comprehensive Cancer Network (NCCN) guidelines recommend that patients with stage IB1- IIA2 cervical cancer with LNM receive platinum-based CCRT after surgery (16). Studies have shown that the postoperative recurrence and distant metastasis rates of patients with cervical cancer featuring LNM are significantly higher than those of patients without LNM (17). Fan et al. found that no postoperative adjuvant therapy was an independent risk factor for poor OS and DFS in patients with stage IIIC1p cervical cancer (9). Simultaneous dual-platinum-based chemotherapy and pelvic radiation therapy could be a potential approach to enhance results in these patients (18). Another potential strategy, augmenting CCRT with AC, did not show a significant difference in 5-year OS in the “Outback” trial (19). This is contrary to our findings where significant OS improvement was evident in the CCRT + AC group versus CCRT alone (p<0.05). These divergent outcomes could stem from the unique prognostic heterogeneity of stage patients with IIIC1p, which was not separately analyzed in the “Outback” trial. Therefore, our findings indicate that the CCRT + AC protocol could potentially enhance OS in individuals diagnosed with stage IIIC1p cervical cancer, highlighting the significance of this therapeutic strategy.
Several studies have confirmed that tumor-associated inflammatory cells are involved in tumor initiation and progression (20, 21). Fan et al. found that NLR>3.8 were independent risk factors for OS and DFS in patients with stage IIIC1p cervical cancer (9). Increased MLR indicated an increased in monocytes and a decrease in lymphocytes. This reflects a state of enhanced inflammation and reduced immunity, which favors tumor progression. Studies have shown that MLR is a valuable marker for the diagnosis and prognosis of various cancers including colorectal (22), breast (23), and gastric (24) cancers. According to our research, the MLR serves as a separate predictive element for individuals diagnosed with stage IIIC1p cervical cancer, and a higher preoperative MLR value corresponds to a poorer prognosis.
Yan et al. showed that the 5-year PFS and OS rates of patients with stage IIIC1p cervical cancer and > 2 LNM were significantly lower than those of patients with only 1-2 LNM (11). Our findings are similar to theirs. Our result shows that the p-value of > 2 LNM is significant in univariate but not in multivariate analysis. Theoretically, the number of LNM is directly influenced by the number of lymph nodes extracted. However, the ideal count for lymph node removals remains contentious (10, 25), and we believe that the quantity of LNM is an inadequate factor for forecasting the outlook of individuals diagnosed with stage IIIC1p cervical cancer. Fan et al. and Li et al. both analyzed the total number of lymph nodes resected, number of pelvic LNM, location of pelvic LNM, and ratio of LNM in patients with stage IIIC1p cervical cancer (9, 10). They identified the ratio of LNM >0.3 or the ratio of LNM ≥0.08 as an independent risk factor for OS and DFS in patients with stage IIIC1p cervical cancer, respectively, and conducted a more detailed stratified analysis of the ratio of LNM in the Li et al. study. The ratio of LNM has been acknowledged as a distinct prognostic indicator of different types of cancers (26–29). Some researchers have suggested that a higher ratio of LNM, is indicative of a higher intranodal tumor burden and may increase the likelihood of tumor cell spillage during lymphadenectomy, contributing to poorer outcomes (9). This hypothesis was consistent with our results. Therefore, we believe that the ratio of LNM holds significance in assessing the outlook of individuals diagnosed with stage IIIC1p cervical cancer.
Tumor markers are common indicators observed in patients with cancer to evaluate and monitor changes in their condition (30). Our research indicates that preoperative SCCA measurement serves as a stand-alone predictor in patients with stage IIIC1p cervical cancer. The nomogram showed that when the preoperative SCCA level was 70 ng/mL, the corresponding single score was 100. Furthermore, our study findings indicated that no significant correlation was evident between CEA levels and the prognosis of patients with stage IIIC1p cervical cancer. Studies have shown that CEA levels are an effective indicator of the prognosis of cervical adenocarcinoma (31, 32). We believe that this result is related to the fact that 81.7% of the pathologies in the collected samples were squamous cell carcinomas.
The incidence of common iliac LNM is lower in pelvic LNM of cervical cancer. Yan et al. found that the 5-year OS of patients with common iliac LNM was significantly lower than in those without common iliac LNM (11). However, common iliac LNM has no significant effect on OS in our study, and we believe that fewer patients with common iliac LNM is one of the reasons. There was research assessed the impact of the site of LNM in cervical cancer survival. Yan et al. found that > 2 LNM sites were predictive factors of poor survival in stage IIIC1p cervical cancer patients (11). Our result shows that LNM in two sites was strongly linked to OS. We think the difference in this result is related to our different classifications. In addition, we have an interesting finding that for patients who underwent PALN resection, although they did not have PALN metastasis, it still had an impact on the OS of stage IIIC1p cervical cancer patients. This is worth further research in the future.
Our study has several limitations. First, this was a retrospective study with all inherent limitations of this form of research. Second, the patients’ chemotherapy regimens were not uniform, and prospective studies are required to support our findings. Third, this study was conducted at a single medical facility with a limited number of participants, necessitating validation of the findings across numerous medical centers in future studies.
5 Conclusion
This study illustrates the heterogeneity in the prognosis of patients with stage IIIC1p cervical cancer. The ratio of LNM, SCCA, MLR, and CCRT + AC are independent prognostic predictors in patients with stage IIIC1p cervical cancer. In clinical practice, comprehensive evaluation of these factors is valuable for clinicians to treat and manage patients more proactively.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by First Affiliated Hospital of Guangxi Medical University Ethical Review Committee. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because this is a retrospective study that only collects clinical information from patients.
Author contributions
TG: Writing – original draft. ZY: Writing – original draft. LW: Formal analysis, Writing – original draft. XT: Formal analysis, Writing – original draft. SM: Formal analysis, Writing – original draft. LJ: Formal analysis, Writing – original draft. YZ: Writing – original draft. FW: Funding acquisition, Supervision, Validation, Writing – review & editing, Writing – original draft.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Development and Application of Appropriate Medical and Health Technologies in Guangxi (Nos. S2018025), and Scientific Research projects of Health Commission in Guangxi (Nos. Z20211395).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer J Clin. (2018) 68:394–424. doi: 10.3322/caac.21492
3. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA: A Cancer J Clin. (2015) 65:87–108. doi: 10.3322/caac.21262
4. Kokka F, Bryant A, Olaitan A, Brockbank E, Powell M, Oram D. Hysterectomy with radiotherapy or chemotherapy or both for women with locally advanced cervical cancer. Cochrane Database Systematic Rev. (2022) 2022:CD010260–332. doi: 10.1002/14651858.CD010260.pub3
5. Hughes RR, Brewington KC, Hanjani P, Photopulos G, Dick D, Votava C, et al. Extended field irradiation for cervical cancer based on surgical staging. Gynecol Oncol. (1980) 9:153–61. doi: 10.1016/0090-8258(80)90022-0
6. Heller PB, Malfetano JH, Bundy BN, Barnhill DR, Okagaki T. Clinical-pathologic study of stage IIB, III, and IVA carcinoma of the cervix: extended diagnostic evaluation for paraaortic node metastasis—a gynecologic oncology group study. Gynecol Oncol. (1990) 38:425–30. doi: 10.1016/0090-8258(90)90085-Y
7. Oh J, Seol KH, Lee HJ, Choi YS, Park JY, Bae JY. Prophylactic extended-field irradiation with concurrent chemotherapy for pelvic lymph node-positive cervical cancer. Radiat Oncol J. (2017) 35:349–58. doi: 10.3857/roj.2017.00367
8. Matsuo K, Machida H, Mandelbaum RS, Konishi I, Mikami M. Validation of the 2018 FIGO cervical cancer staging system. Gynecol Oncol. (2019) 152:87–93. doi: 10.1016/j.ygyno.2018.10.026
9. Fan X, Wang Y, Yang N, Zhu P. Prognostic analysis of patients with stage IIIC1p cervical cancer treated by surgery. World J Surg Oncol. (2023) 21:186–97. doi: 10.1186/s12957-023-03076-9
10. Li A, Wang L, Jiang Q, Wu W, Huang B, Zhu H. Risk stratification based on metastatic pelvic lymph node status in stage iiic1p cervical cancer. Cancer Manag Res. (2020) 12:6431–9. doi: 10.2147/CMAR.S253522
11. Yan DD, Tang Q, Tu YQ, Chen JH, Lv XJ. A comprehensive analysis of the factors of positive pelvic lymph nodes on survival of cervical cancer patients with 2018 FIGO stage IIIC1p. Cancer Manag Res. (2019) 11:4223–30. doi: 10.2147/CMAR.S204154
12. Shigeta S, Shimada M, Tsuji K, Watanabe Z, Tanase Y, Matsuo K, et al. Surgically treated cervical cancer in a high-risk group in the era of the 2018 FIGO staging schema: A nationwide study. Sci Rep. (2023) 13:12020–30. doi: 10.1038/s41598-023-39014-8
13. Cho WK, Kim YJ, Kim H, Kim YS, Park W. Significance of para-aortic lymph node evaluation in patients with FIGO IIIC1 cervical cancer. Japan J Clin Oncol. (2020) 50:1150–6. doi: 10.1093/jjco/hyaa091
14. Ronsini C, Anchora LP, Restaino S, Fedele C, Arciuolo D, Teodorico E, et al. The role of semiquantitative evaluation of lympho-vascular space invasion in early stage cervical cancer patients. Gynecol Oncol. (2021) 162:299–307. doi: 10.1016/j.ygyno.2021.06.002
15. Tantari M, Bogliolo S, Morotti M, Balaya V, Bouttitie F, Buenerd A, et al. Lymph node involvement in early-stage cervical cancer: is lymphangiogenesis a risk factor? Results from the microcol study. Cancers. (2022) 14:212–26. doi: 10.3390/cancers14010212
16. Abu-rustum NR, Yashar CM, Arend R, et al. NCCN Clinical Practice Guidelines in Oncology, Cervical Cancer. Version 2 (2024). Available online at: https://www.nccnChina.org.cn/guide/detail/554.
17. Aslan K, Meydanli MM, Oz M, Tohma YA, Haberal A, Ayhan A. The prognostic value of lymph node ratio in stage IIIC cervical cancer patients triaged to primary treatment by radical hysterectomy with systematic pelvic and para-aortic lymphadenectomy. J Gynecol Oncol. (2020) 31:e1–13. doi: 10.3802/jgo.2020.31.e1
18. Mabuchi S, Isohashi F, Yokoi T, Takemura M, Yoshino K, Shiki Y, et al. A phase II study of postoperative concurrent carboplatin and paclitaxel combined with intensity-modulated pelvic radiotherapy followed by consolidation chemotherapy in surgically treated cervical cancer patients with positive pelvic lymph nodes. Gynecol Oncol. (2016) 141:240–6. doi: 10.1016/j.ygyno.2016.02.011
19. Mileshkin LR, Moore KN, Barnes EH, Gebski V, Narayan K, King MT, et al. Adjuvant chemotherapy following chemoradiotherapy as primary treatment for locally advanced cervical cancer versus chemoradiotherapy alone (Outback): an international, open-label, randomised, phase 3 trial. Lancet Oncol. (2023) 24:468–82. doi: 10.1016/S1470-2045(23)00147-X
20. Hinshaw DC, Shevde LA. The tumor microenvironment innately modulates cancer progression. Cancer Res. (2019) 79:4557–66. doi: 10.1158/0008-5472.Can-18-3962
21. Albini A, Bruno A, Noonan DM, Mortara L. Contribution to tumor angiogenesis from innate immune cells within the tumor microenvironment: implications for immunotherapy. Front Immunol. (2018) 9:527. doi: 10.3389/fimmu.2018.00527
22. Lisanti C, Basile D, Garattini SK, Parnofiello A, Corvaja C, Cortiula F, et al. The saffo study: sex-related prognostic role and cut-off definition of monocyte-to-lymphocyte ratio (MLR) in metastatic colorectal cancer. Cancers. (2022) 15:175–86. doi: 10.3390/cancers15010175
23. Tiainen S, Rilla K, Hämäläinen K, Oikari S, Auvinen P. The prognostic and predictive role of the neutrophil-to-lymphocyte ratio and the monocyte-to-lymphocyte ratio in early breast cancer, especially in the HER2+ Subtype. Breast Cancer Res Treat. (2020) 185:63–72. doi: 10.1007/s10549-020-05925-7
24. An S, Eo W, Lee S, Lee Y-J. Monocyte-to-lymphocyte ratio as a determinant of survival in patients with gastric cancer undergoing gastrectomy: A cohort study. Medicine. (2023) 102:e33930–7. doi: 10.1097/md.0000000000033930
25. Zhou J, Zhang W-W, Wu S-G, He Z-Y, Sun J-Y, Wang Y, et al. The impact of examined lymph node count on survival in squamous cell carcinoma and adenocarcinoma of the uterine cervix. Cancer Manage Res. (2017) 9:315–22. doi: 10.2147/cmar.S141335
26. Jin C, Li J, Zou C, Qiao X, Ma P, Hu D, et al. Lymph node ratio predicts prognosis in patients with surgically resected invasive pancreatic cystic neoplasms. Trans Cancer Res. (2020) 9:5843–56. doi: 10.21037/tcr-20-1355
27. Supsamutchai C, Wilasrusmee C, Jirasiritham J, Rakchob T, Phosuwan S, Chatmongkonwat T, et al. Recurrence outcome of lymph node ratio in gastric cancer after underwent curative resection: a retrospective cohort study. Ann Med Surg. (2020) 54:57–61. doi: 10.1016/j.amsu.2020.04.002
28. Sakin A, Aldemir MN. Lymph node ratio predicts long-term survival in lymph node-positive breast cancer. Eur J Breast Health. (2020) 16:270–5. doi: 10.5152/ejbh.2020.5809
29. Polterauer S, Hefler L, Seebacher V, Rahhal J, Tempfer C, Horvat R, et al. The impact of lymph node density on survival of cervical cancer patients. Br J Cancer. (2010) 103:613–6. doi: 10.1038/sj.bjc.6605801
30. Kato H, Torigoe T. Radioimmunoassay for tumor antigen of human cervical squamous cell carcinoma. Cancer. (1977) 40:1621–8. doi: 10.1002/(ISSN)1097-0142
31. Kubota H, Tsujino K, Sulaiman NS, Sekii S, Matsumoto Y, Ota Y, et al. Comparison of salvage therapies for isolated para-aortic lymph node recurrence in patients with uterine cervical cancer after definitive treatment. Radiat Oncol. (2019) 14:236–43. doi: 10.1186/s13014-019-1442-6
Keywords: cervical cancer, stage IIIC1p, overall survival, adjuvant chemotherapy, lymph node metastasis
Citation: Gao T, Yang Z, Wei L, Tang X, Ma S, Jiang L, Zhang Y and Wu F (2024) Prognostic analysis of stage IIIC1p cervical cancer patients. Front. Oncol. 14:1362281. doi: 10.3389/fonc.2024.1362281
Received: 28 December 2023; Accepted: 12 April 2024;
Published: 25 April 2024.
Edited by:
Robert Fruscio, University of Milano Bicocca, ItalyReviewed by:
Carlo Ronsini, Università degli Studi della Campania “Luigi Vanvitelli”, ItalyAhmed I. Ghanem, Henry Ford Health System, United States
Copyright © 2024 Gao, Yang, Wei, Tang, Ma, Jiang, Zhang and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fang Wu, wufang@gxmu.edu.cn; Yong Zhang, zhangyonggxmu@163.com
†These authors have contributed equally to this work
 Ting Gao†
Ting Gao† Yong Zhang
Yong Zhang Fang Wu
Fang Wu