- 1Department of Internal Medicine, Medical Oncology, King Hussein Cancer Center, Amman, Jordan
- 2Department of Internal Medicine, Hematology, Oncology and Blood and Marrow Transplantation, University of Iowa, Iowa City, IA, United States
- 3Department of Radiation Oncology, King Hussein Cancer Center, Amman, Jordan
- 4Department of Surgery, Thoracic Surgery, King Hussein Cancer Center, Amman, Jordan
- 5Department of Radiology, King Hussein Cancer Center, Amman, Jordan
- 6Department of Nuclear Medicine, King Hussein Cancer Center, Amman, Jordan
- 7Department of Pathology, King Hussein Cancer Center, Amman, Jordan
- 8Department of Internal Medicine, Pulmonary and Critical Care Medicine, King Hussein Cancer Center, Amman, Jordan
- 9Operative Research Unit of Medical Oncology, Fondazione Policlinico Universitario Campus Bio-Medico, Rome, Italy
- 10Department of Surgery and Cancer, Imperial College London, London, United Kingdom
- 11Grossman School of Medicine, New York University, New York, NY, United States
- 12School of Medicine, University of Jordan, Amman, Jordan
Background: This study aims to evaluate real-world (rw) outcomes of immunotherapy (IO) for advanced stage NSCLC at King Hussein Cancer Center (KHCC) in Jordan.
Methods: Advanced stage NSCLC patients who received IO at KHCC between 2017 and 2022 were included. The data were retrospectively collected. PFS and OS were estimated for patients with ECOG performance status (ECOG PS) 0-1. Cox regression analyzed predictors of OS in first-line (1L) IO, regardless of performance status.
Results: The total number of patients included was 244. Out of those, 160 (65%), 67 (28%), and 17 (7%) patients received IO as 1L, second-line (2L), or third-line or beyond (3L or beyond), respectively. The median age for all patients was 59 years. Male were 88%, and 77% were smokers. The median follow-up time was 12.5 months. The median PFS and OS for 1L IO were 7 [95% CI 5.8 – 10.3] and 11.8 [95% CI 8.8 – 14.4], months, respectively. In the first 3 months after starting 1L IO, 34/160 (21%) patients had died. For those who survived beyond 3 months after starting 1L IO, the median PFS and OS were 11.3 [95% CI 8.3 – 16.5] and 15.4 [95% CI 13.2 – 21] months, respectively. In the Cox regression model of 1L IO patients with any performance status, ECOG PS 2 was predictive of worse OS compared to ECOG PS 0-1 (p= 0.005).
Conclusion: This real-world study of advanced-stage NSCLC patients treated with immunotherapy at KHCC reveals outcomes that fall short of those anticipated from clinical trials. The inclusion of Middle Eastern patients in lung cancer trials is essential to ensure adequate representation of various ethnicities in clinical research.
Introduction
Lung cancer ranks as the fourth most common cancer in Jordan, comprising 6.5% of all annual cancer cases. In males, it is the second most prevalent type of cancer and the primary cause of cancer-related mortality, accounting for 22.4% of all cancer deaths among men (1). The smoking prevalence in Jordan reaches up to 51% (2) and the average age for lung cancer diagnosis was reported as 63.8 years (3).
Immunotherapy (IO) has revolutionized the treatment of advanced-stage non-small cell lung cancer (NSCLC). Utilizing the patient’s immune system to combat cancer, IO has demonstrated significant efficacy in these patients. The advent of checkpoint inhibitors, including PD-1 and PD-L1 inhibitors, has enhanced clinical outcomes, yielding improved tumor response rates and extending survival compared to conventional cytotoxic chemotherapy (4). The 5 year overall survival estimate for stage IV NSCLC patients with PD-L1 expression of ≥50% who received single agent pembrolizumab, a PD-1 inhibitor, was 32% (5).
Clinical trials have underscored the importance of PD-L1 immunohistochemistry as a crucial biomarker for assessing the effectiveness of immunotherapy (IO) in treating NSCLC (6). This finding emerged from extensive research on single-agent IO (4), IO-chemotherapy combinations (7, 8), and IO-IO combinations (9). Consequently, evaluating PD-L1 expression levels in advanced-stage NSCLC has become a critical aspect of treatment planning. The PD-L1 expression in a lung cancer patient population from the Middle East was found to be similar to published literature (10).
The objective of this study is to delineate the clinical characteristics and treatment outcomes of advanced-stage NSCLC patients treated with IO at King Hussein Cancer Center (KHCC) in Jordan, particularly since the introduction of pembrolizumab at KHCC in 2017. This research holds significant global implications, as it addresses a notable gap in survival outcomes data for NSCLC patients receiving immunotherapy in the Middle East, a region often underrepresented in international clinical trials (11). The findings of this study contribute to a better understanding of the outcomes of IO across diverse patient populations.
Materials and methods
Study design and patient population
This retrospective study investigates the outcomes of advanced-stage NSCLC patients with a favorable performance status (ECOG PS 0-1) who underwent immunotherapy (IO) at King Hussein Cancer Center (KHCC) in Jordan. Throughout the study, data confidentiality and privacy were rigorously upheld in line with the Health Insurance Portability and Accountability Act (HIPAA) standards. Adherence to the ethical principles outlined in the Declaration of Helsinki was ensured. The study protocol received the requisite approval from the Institutional Review Board (IRB) at KHCC (IRB number: 23 KHCC 020), and the IRB granted a waiver for informed consent.
The study population included patients ≥18 years old diagnosed with advanced stage NSCLC who had received at least one IO treatment between December 2017 and February 2022. For analyses, only patients with ECOG PS 0-1 were included, except for the univariate and multivariate analysis where patients with any performance status (ECOG PS 0-4) were included. The total number of patients with ECOG PS 2-4 was 67. Excluding patients with poor ECOG PS from the PFS and OS survival analysis aimed to ensure a cohort resembling NSCLC patients in clinical trials, facilitating more meaningful comparisons of survival figures. Then, ECOG PS 2-4 cases were added to the univariate and multivariate analysis to identify the impact of poor ECOG PS, among other variables, on survival specifically in our patient population. The first approved IO at KHCC was pembrolizumab. Patients received IO as monotherapy or with chemotherapy. Demographic and clinical characteristics were categorized by IO line of treatment (1L, 2L, 3L or beyond).
Data sources
A structured database was built from patient information in the VISTA CPRS Electronic Medical Record (EMR) using Fileman. Data cleaning and analysis were performed with Microsoft Excel, Python, and R. To ensure accuracy, a random sample underwent manual verification by cross-checking with original VISTA CPRS EMR records to identify discrepancies or errors.
Study variables and outcome measures
We gathered patient data, including demographics, clinical features, and treatment specifics. Key time points, such as diagnosis dates, treatment initiation, post-immunotherapy (IO) progression, last known alive status, and death dates, were recorded for outcome assessment. To prevent biases, we used the date of IO initiation, not the diagnosis date, as the reference point for survival analysis.
The study’s primary endpoints were progression-free survival (PFS) and overall survival (OS). For OS, the defining event was death. In contrast, for PFS, the event was identified as either the date of radiographic progression as determined by the official radiology read, the date of starting subsequent line of treatment following IO, or death, whichever occurred first. The most recent survival date was established based on the latest of several criteria: the last known inpatient admission, the most recent emergency room visit, the date of the last recorded vital signs, or the date of the last clinic visit.
Statistical analysis
Descriptive statistical methods were employed to evaluate baseline characteristics, stratified by the line of immunotherapy (IO) treatment. We presented categorical data as frequencies and continuous variables as medians with their respective ranges. To discern significant differences between groups, we applied Wilcoxon Rank Sum tests for continuous variables, Pearson’s Chi-Squared tests, and Fisher’s Exact tests for categorical data. In our survival analysis, the Kaplan-Meier method was utilized to estimate overall survival (OS) and progression-free survival (PFS), employing log-rank tests to determine statistical significance. Additionally, a Cox proportional hazards model was employed in the multivariate analysis to evaluate the effects of various covariates on survival outcomes, with results reported as hazard ratios (HRs) and 95% confidence intervals (CIs). All statistical analyses were conducted using R software, version 4.2.2.
Results
Patients and treatments
Of 244 patients with advanced stage NSCLC, 160 (65%), 67 (28%), and 17 (7%) received 1L, 2L, or 3L or beyond IO, respectively. Patients with known EGFR or ALK alterations were excluded. Patients and disease characteristics are listed in Table 1. The median age for all patients was 59 (range: 26 -86) years. Patients who received 3L or beyond were more likely to have younger age (median age was 60 years in 1L, 58 years in 2L, and 51 years in 3L or beyond, p = 0.004). Most of the patients were men (87%), and were smokers (75%) or ex-smokers (13%). All patients included had ECOG PS of 0-1 (100%). Numerically, more patients received 3L or beyond IO who had stage III NSCLC upon initial diagnosis compared to 1L and 2L patients (29% vs 24% and 7.5%, p = 0.054) and more who had received CCRT (29% vs 21% and 4.5%, p < 0.002), respectively. There was no significant difference between the small numbers of patients who received neoadjuvant or adjuvant chemotherapy upon initial diagnosis of NSCLC. The most common NSCLC histology was non-squamous (67%). The patients who received 1L IO were more likely to have a PD-L1 ≥50% (p < 0.001) and to receive IO and chemotherapy combination (p < 0.001). All patients received pembrolizumab except for 1 patient who received nivolumab.
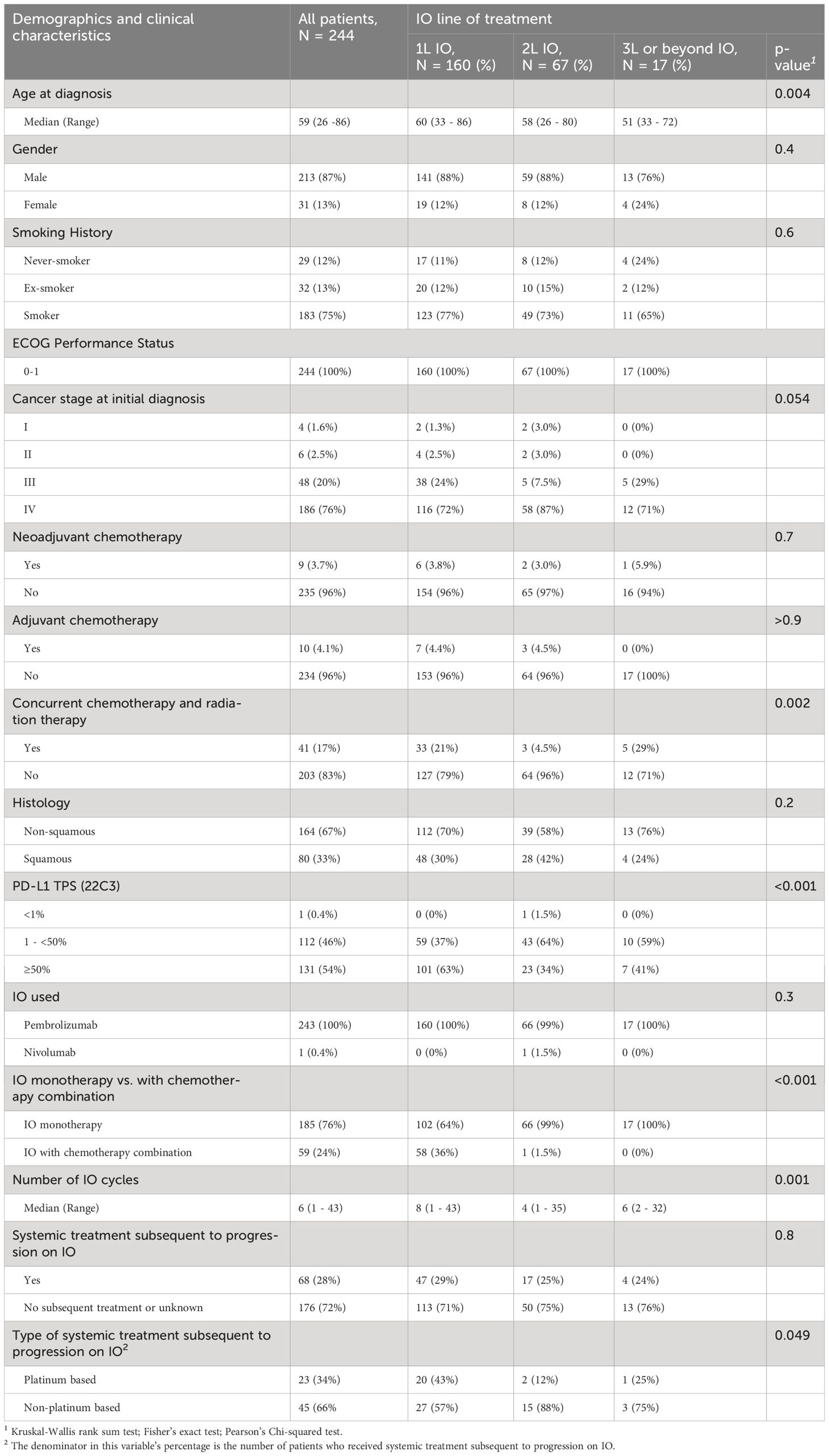
Table 1 Demographic and clinical characteristics of advanced stage NSCLC patients (ECOG PS 0 -1) who received first line, second line, or third line or beyond IO.
The median number of IO cycles received was 6 (range, 1-43) cycles. The patients who received 1L IO had significantly higher median number of IO treatments, 8 (range 1-43) cycles compared to 2L IO and 3L or beyond IO patients who received 4(range 1-35) and 6(range 2-32) cycles, respectively (p = 0.001). Around one quarter (28%) of all patients who were treated with IO received subsequent systemic treatment upon progression on IO. Around half of the 1L IO patients (43%) received platinum based chemotherapy upon progression on IO.
Sequential introduction of IO into the advanced stage NSCLC treatment lines at KHCC
KHCC added IO for treating advanced stage NSCLC at different times. Pembrolizumab was approved on December 13, 2017, as 1L treatment if PD-L1 was ≥ 50%, and on January 10, 2018, as 2L treatment if PD-L1 was ≥ 1%. Approval was expanded on November 10, 2019, for non-squamous NSCLC with PD-L1 ≥1%, combining IO and chemotherapy as 1L treatment. On January 25, 2023, advanced squamous NSCLC with PD-L1 ≥1% became eligible for pembrolizumab and chemotherapy. This staggered introduction explains differences in 1L IO patients, more likely to have PD-L1 ≥50% (p < 0.001) and receive IO and chemotherapy combination (p < 0.001). Formulary decisions at KHCC are guided by cost-effectiveness analyses.
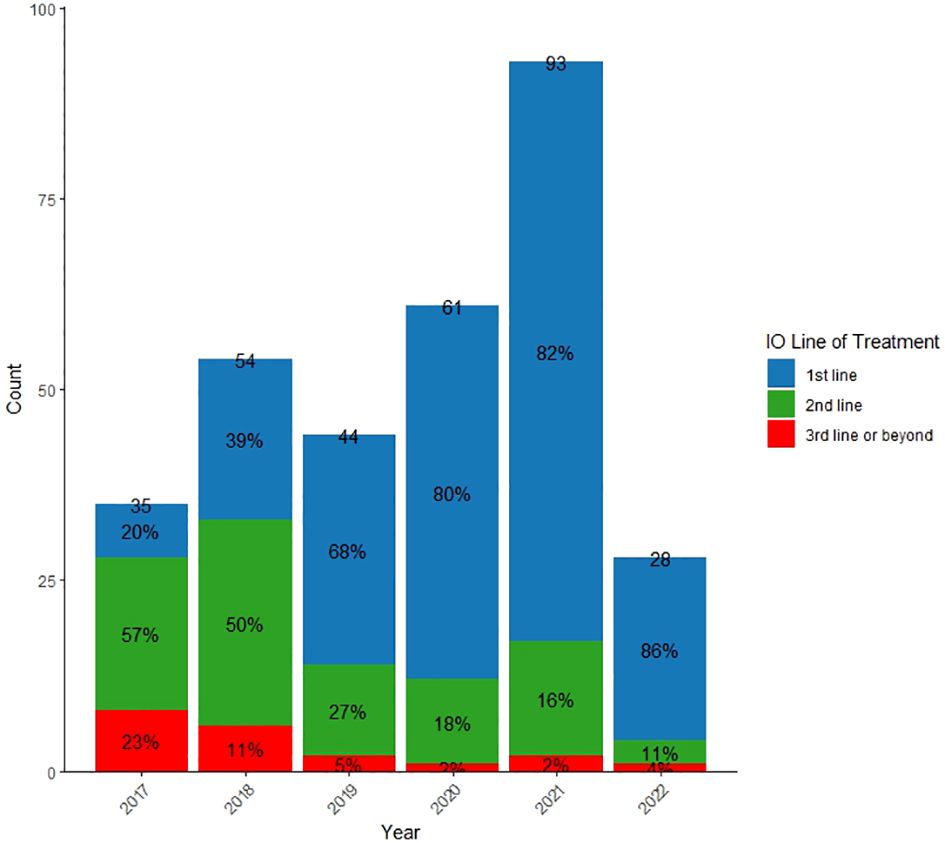
Figure 1 shows the evolving usage of IO in the treatment of NSCLC at KHCC from 2017 to 2022. The unique NSCLC patients receiving IO at KHCC increased from 44 in 2019 to 93 in 2021. In the 1L setting, there was a consistent rise, reaching 82% in 2021, compared to 39% in 2018. Conversely, the use of IO as 2L treatment decreased from 50% in 2018 to 16% in 2021, and only 2% of patients received IO as 3L or beyond in 2021.
Survival outcomes
Survival for patients after 1L, 2L, and 3L or beyond IO
The median follow up time for all patients was 12.1 months. The median follow up time for 1L, 2L and 3L or beyond IO was 12.6, 5.8, and 10.9 months, respectively. Progression-free survival (PFS) and overall survival (OS) for 1L, 2L and 3L or beyond IO are shown in Figure 2. Median PFS was the longest for patients who received 1L IO, 7 [95% CI 5.8 – 10.3] months. The median PFS for 2L and 3L or beyond IO was 3.4 [95% CI 2.9 – 5.2] months and 6.6 [95% CI 3.9 – 12.8] months, respectively. Median OS was the longest for patients who received 1L IO, which was 11.8 [95% CI 8.8 – 14.4] months. Median OS for 2L and 3L or beyond IO was 5.3 [95% CI 3.8 – 7.5] months and 10.5 [95% CI 4.5 – 16.2] months, respectively. Survival for 1L, 2L and 3L or beyond IO at 36 months since initiation of IO was 20%, 9%, and 18%, respectively.
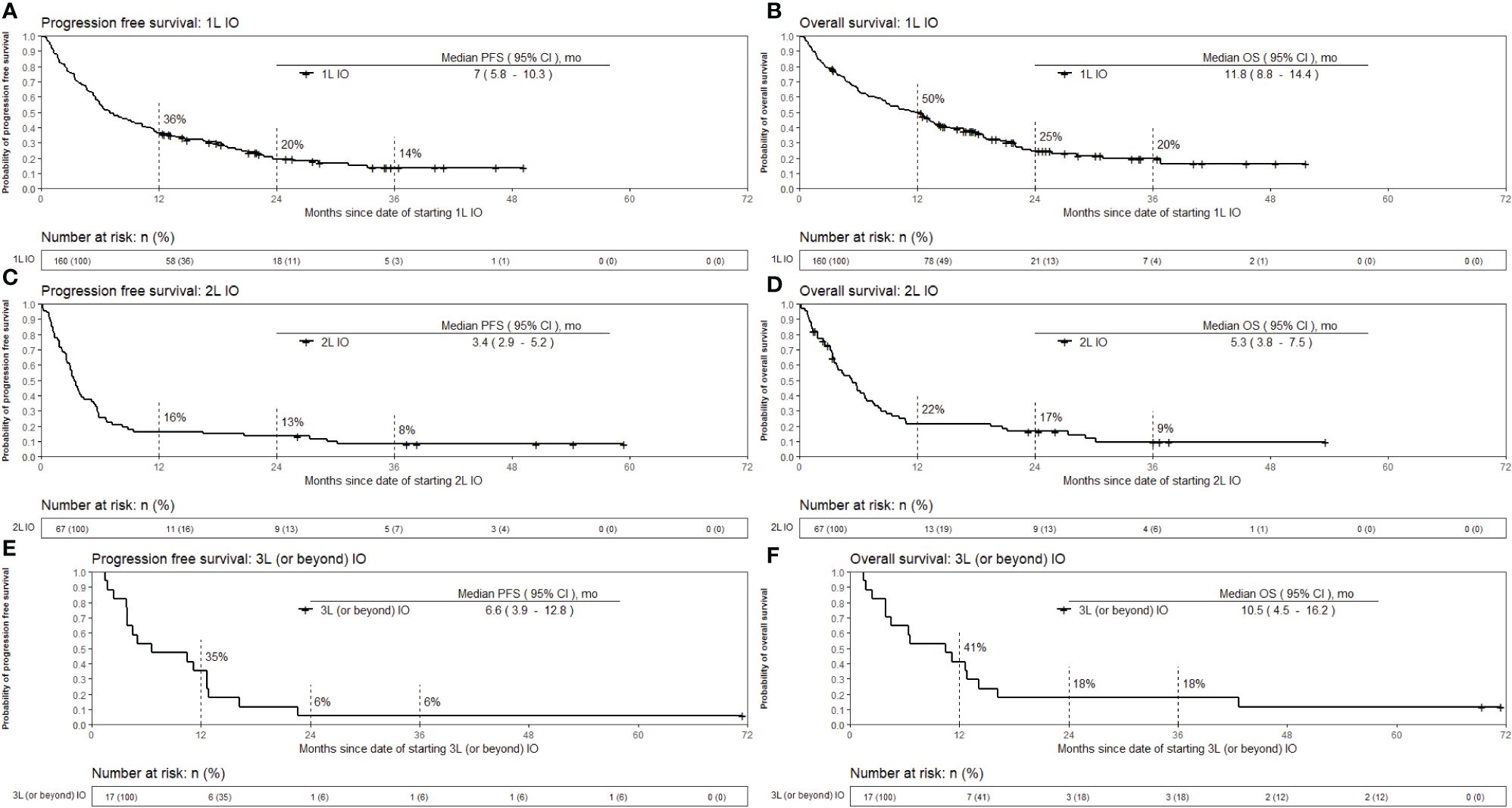
Figure 2 Kaplan-Meier estimates of overall survival and progression free survival from the date of starting immunotherapy. The patients with EGFR mutation in exon 19 or 21, ALK positivity by IHC or rearrangement by FISH, or ROS1 rearrangement by FISH were excluded from the survival analysis. (A) OS for 1L IO (B) OS for 2L IO (C) OS for 3L or beyond IO (D) PFS for 1L IO (E) PFS for 2L IO (F) PFS for 3L or beyond IO. OS, Overall survival; PFS, Progression free survival; IO, Immunotherapy; 1L, First line; 2L, Second line; 3L, Third line; mo, months.
Survival for patients who received 1L IO and survived ≥ 3 months after starting IO
Out of the 160 patients who received 1L IO, 34 (21%) died within 3 months of initiating IO. The survival outcomes for the patients who received 1L IO and survived 3 months or more after starting IO are shown in Figure 3. The median PFS was 11.3 [95% CI 8.3 – 16.5] months, and the median OS was 15.4 [95% CI 13.2 – 21] months. Survival at 36 months was 25%. Table 2 shows the characteristics of the patients who received 1L IO and survived <3 versus ≥ 3 months. Those who survived ≥ 3 months were more likely to receive subsequent systemic treatment after progression on IO (p < 0.001). The median number of IO cycles administered for the patients who survived ≥ 3 months was 11 (range 1-43).
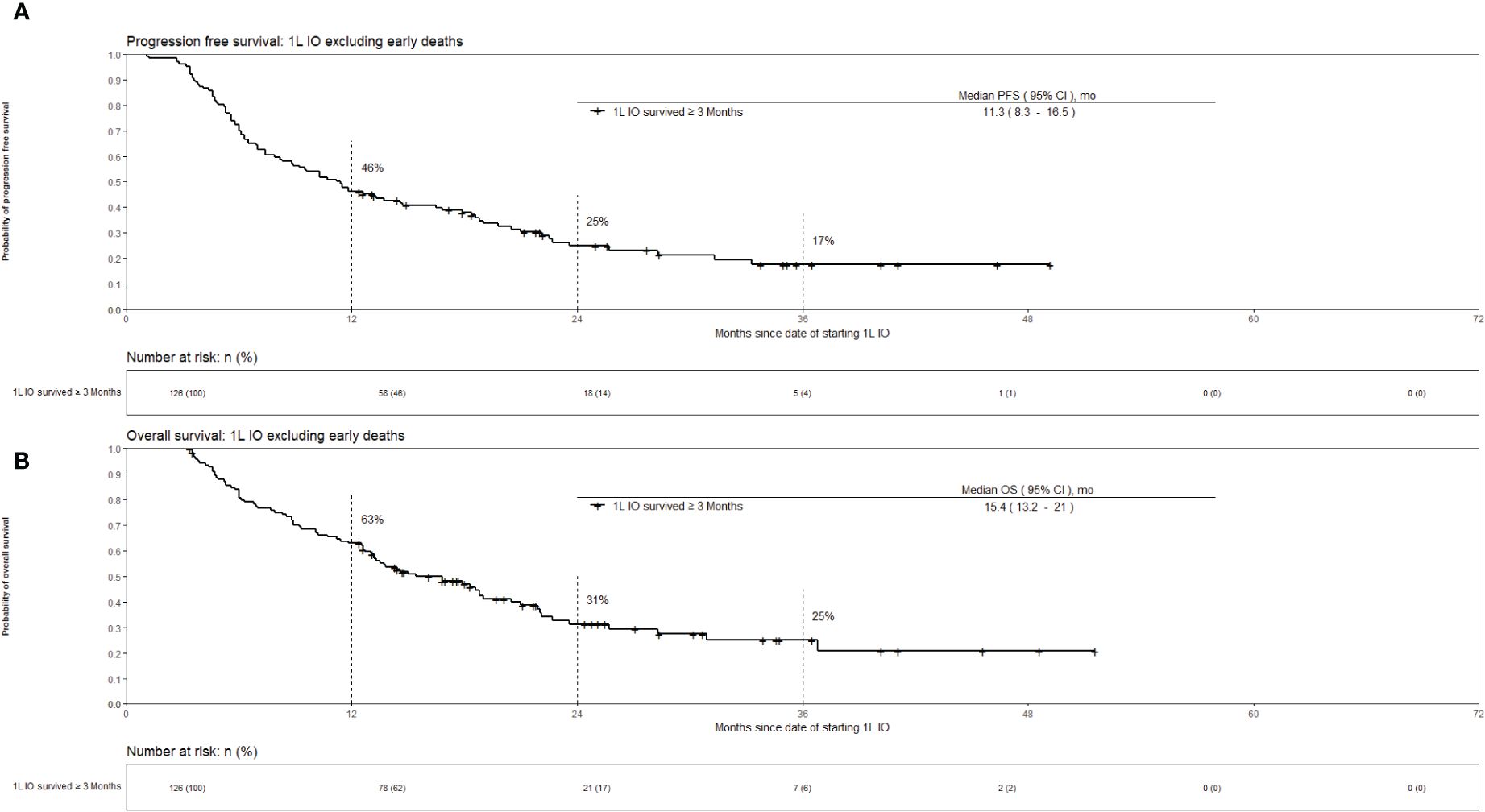
Figure 3 Kaplan-Meier estimates of overall survival for advanced stage NSCLC who received 1L IO excluding early deaths (survival <3 months). Part (A) progression free survival. Part (B) overall survival.
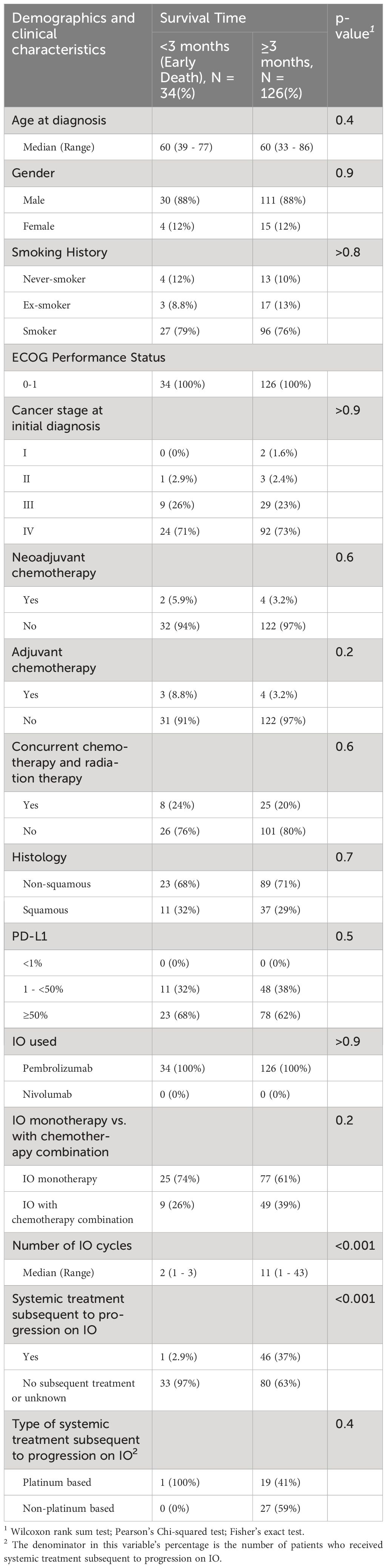
Table 2 Characteristics of advanced stage NSCLC patients who received first line according to survival time (<3 vs ≥3, months).
Survival of patients based on receiving IO monotherapy versus IO in combination with chemotherapy, and the PD-L1 status
Survival for 1L IO patients categorized by IO monotherapy versus IO with chemotherapy combination, and PD-L1 expression were also explored. The patients who received IO in combination with chemotherapy had better overall survival compared to those who received IO monotherapy in the 1L setting; however, the difference in survival was not statistically significant [HR 0.81 (95% CI 0.55 – 1.2), p = 0.3]. Regarding PD-L1 expression, the patients who received 1L IO had better survival in the first 12 months after starting IO if PD-L1 was 1 - < 50% compared to ≥50%; however, the overall survival over the follow up period for the study was not significantly different based on the PD-L1 status [HR 1.07 (95% CI 0.73 – 1.56), p = 0.74]. There was no significant overall survival difference for the 2L patients based on PD-L1 level of expression [HR 1.13 (95% CI 0.69 – 1.84), p = 0.64]. However, the 3L or beyond IO patients who had PD-L1 expression ≥50% survived longer compared to those with PD-L1 of 1 - < 50% [HR 0.36 (95% CI 0.13 – 1.01), p = 0.05].
Cox Proportional hazard model for predictors of overall survival
The univariate and multivariate Cox Proportional Hazard models for predictors of overall survival are presented in Table 3. These hazard models included patients who received 1L only and had ECOG PS 0-4. The total number of patients included was 207. The only variable that was predictive of overall survival in both the univariate and multivariate analyses was the ECOG PS. Compared to the reference ECOG PS of 0-1, those with ECOG PS 2-4 had worse overall survival on multivariate analysis. [HR 1.83 (95% CI 1.24 – 2.7), p = 0.002].
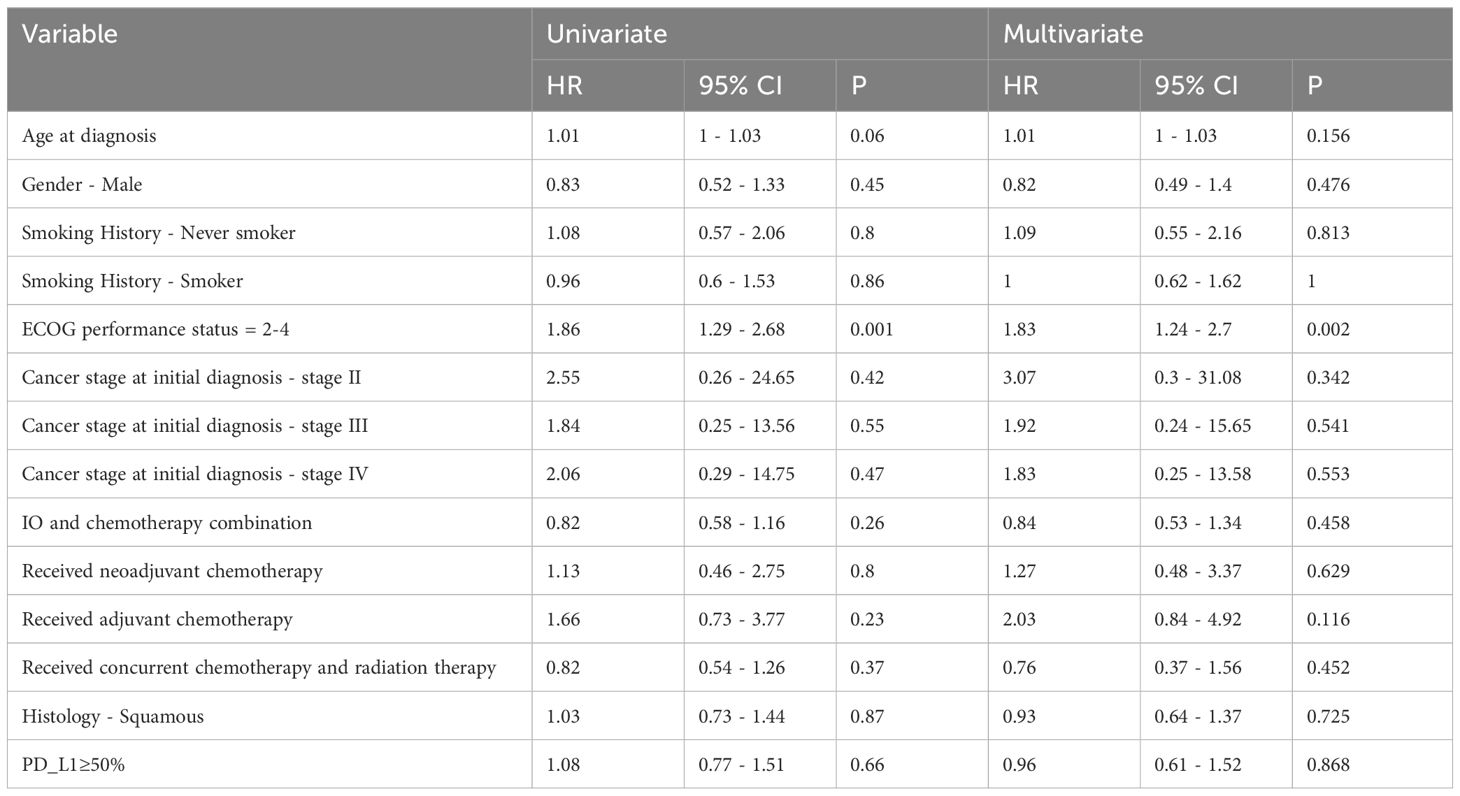
Table 3 Univariate and multivariate analysis of overall survival for advanced stage NSCLC patients who received first line IO.
Discussion
This rw study from KHCC-Jordan provides a critical examination of IO outcomes in advanced stage NSCLC in a patient population that is under-represented in clinical trials. In the advanced stage NSCLC patients with good performance status who received 1L IO at KHCC, our findings reveal a median OS of 11.8 months, extending to 15.4 months when excluding those who survived less than three months post IO initiation. These figures fall short of the anticipated outcomes from randomized NSCLC IO clinical trials (4, 7, 8), underscoring the necessity of inclusive and diverse patient representation in global lung cancer studies.
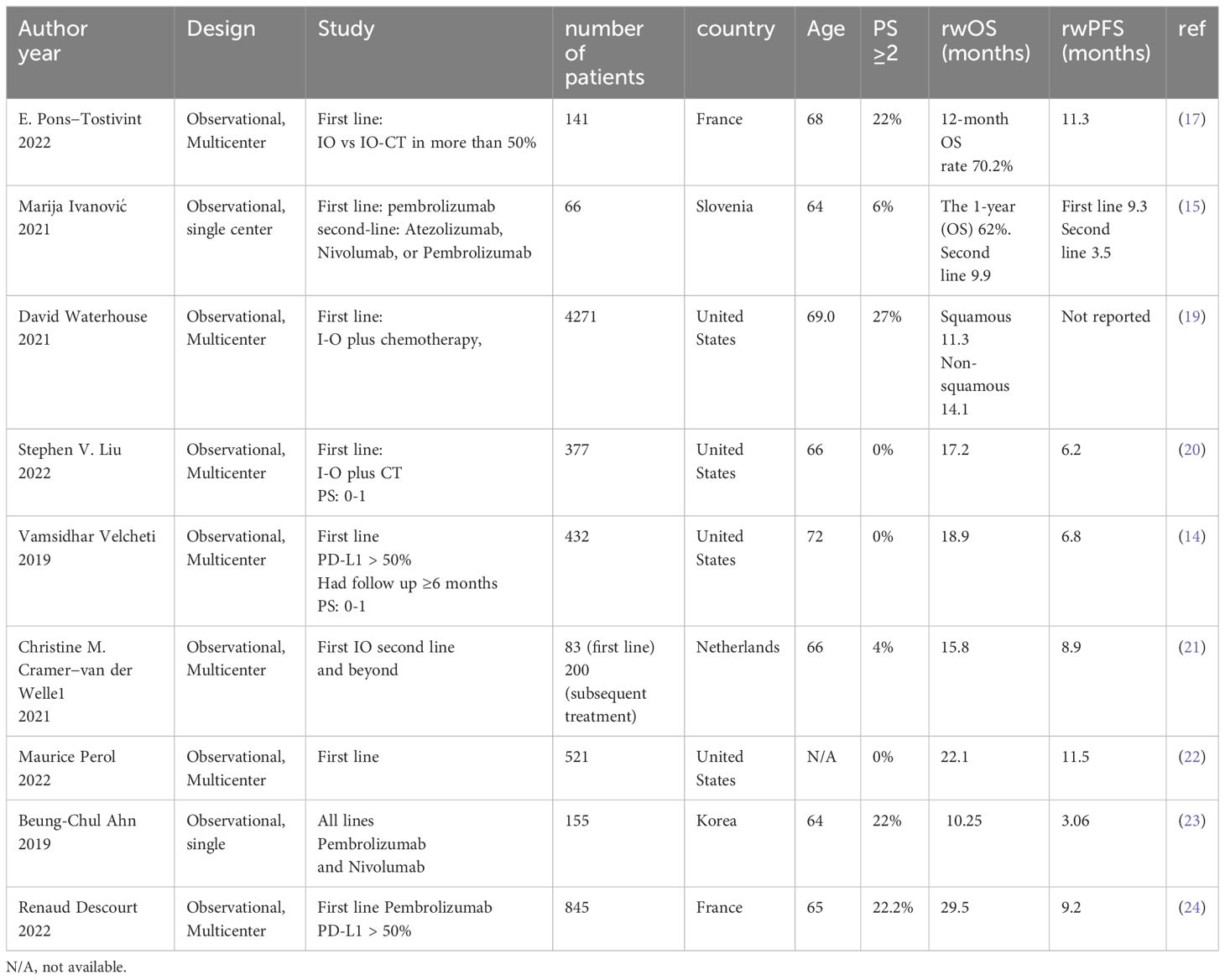
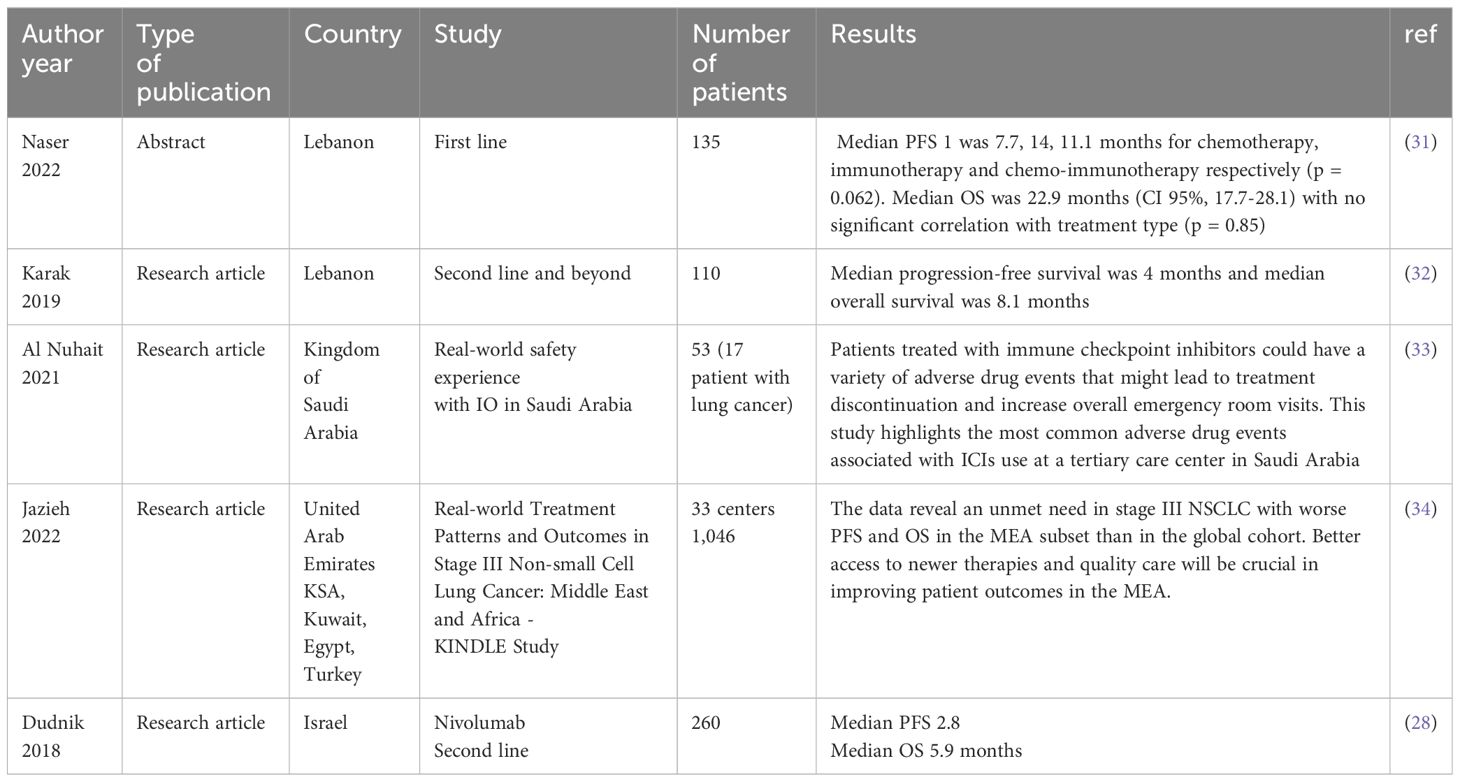
Real-world data on outcomes of IO as a 1L treatment of advanced stage NSCLC has been reported in several publications (12–15). Khozin et al. (12) utilized Flatiron Health to study a total of 5257 NSCLC patients who received IO. The median OS for those who received 1L IO was 10.8 months (12). Another study by Velcheti et al. which included patients who received 1L IO, survived at least 6 months and had an ECOG PS of 0-1 showed a median OS of 19.6 months (16). Another retrospective study showed the survival was better for the NSCLC patients who were alive at 12 weeks after starting single agent pembrolizumab compared to the whole cohort; however, the median OS was not reached when the data was published (17). The frequency of death at 12 weeks was 11% for those who received single agent pembrolizumab and 15.2% for the pembrolizumab and chemotherapy combination group (17). In our study, 21% of the patients died within 3 months of starting IO. Deaths in the first 3 months after enrollment in four of the major 1L IO clinical trials in NSCLC were less than what we report in our study (4, 7, 8, 18). This observed early mortality rate after initiation of IO further accentuates the need for prudent patient selection to optimize treatment outcomes and minimize early mortality risks. In Tables 4–6, we summarize survival outcomes from rw data studies including figures from data from the Middle East.
The influence of ECOG PS on treatment outcomes is a significant finding in our study. Patients with ECOG PS 0-1 showed better survival outcomes compared to those with higher PS scores [HR 1.83 (95% CI 1.24 – 2.7), echoing the global literature’s emphasis on the importance of comprehensive patient evaluation before IO initiation. Studies showed that careful consideration is needed when using IO in NSCLC patients with poor PS, higher comorbidity score, and older age (35–37). In a metanalysis of Pembrolizumab as monotherapy or in combination with chemotherapy of 11 randomized clinical trials and 26 retrospective real-world (rw) studies, multivariate analysis showed that an ECOG PS of 0-1 was an independent predictor of longer OS (38).
In our patient population, PD-L1 expression in the 1L IO treatment setting showed an unusual pattern: those with PD-L1 expression 1 - < 50% exhibited improved survival within the initial 12 months of receiving IO compared to those with PD-L1 expression of ≥50%. However, this survival advantage diminished over subsequent follow-up periods. This finding contrasts with the prevailing literature, which typically associates higher PD-L1 expression with better survival outcomes (18). While the exact cause of this observation remains unclear, it’s important to note that the limited sample size may hinder definitive conclusions. Nonetheless, one plausible explanation could be linked to heavy smoking habits, prevalent in Jordan, potentially contributing to elevated PD-L1 expression levels (39) and more smoking-related comorbidities, possibly diluting the benefits of IO among patients with high PD-L1 expression.
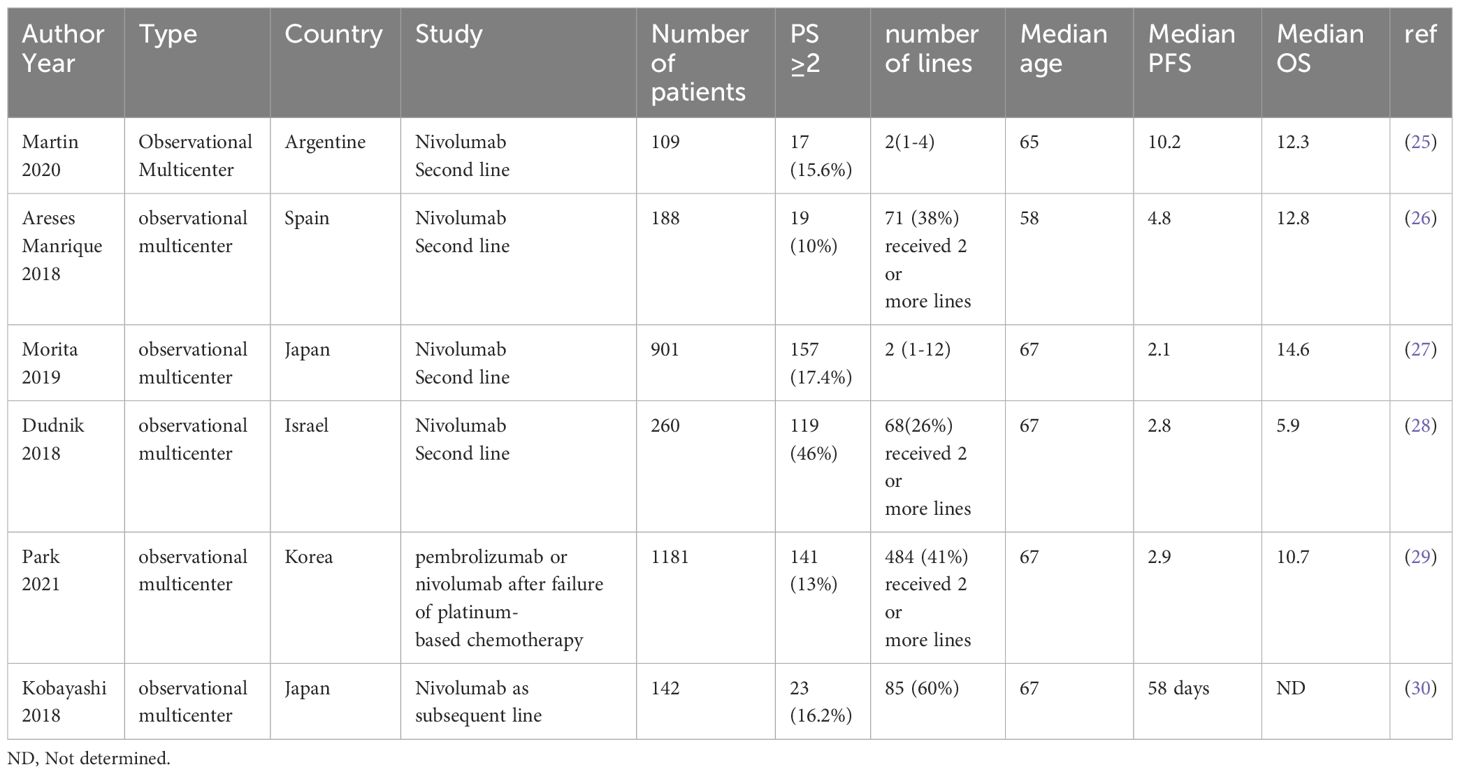
The benefit of IO in the 2L setting and beyond in rw data is also of interest. In our study, the patients who received 2L IO had a median PFS and OS of 3.4 [95% CI 2.9 – 5.2] months and 5.3 [95% CI 3.8 – 7.5] months, respectively. In the 2L NSCLC treatment trial, KEYNOTE-010, pembrolizumab showed a survival benefit over docetaxel with a median PFS and OS of 3·9 and 10.4 months, respectively (40). In our study, when compared to KEYNOTE-010, the PD-L1 expression was ≥50% in 34% of the cases who received 2L IO, as opposed to 42% in the latter. In rw data, Khozin et al. reported on the survival of patients who received 2L pembrolizumab showing a median PFS and OS of 3.7 (2.9-4.1) months and 12.0 (9.3-14.7) months, respectively (12). Juergens et al. also published the rw data on using nivolumab as 2L IO in Canada showing a median PFS and OS of 3.5 and 12 months, respectively (41). In a metanalysis that included 32 rw studies of 2L IO in NSCLC, safety and efficacy of IO in the rw data were comparable to the figures from randomized clinical trials with a median PFS and OS of 3.35 months and 9.98 months, respectively (42). Although the PFS in our data is similar to the published figures in clinical trials and rw data, the lower OS in our study could be due to comorbid conditions that were not captured in our study. Regarding the patients who received 3L or beyond IO, our study had a total of 17 patients. Their median PFS and OS since initiating IO were 6.6 and 10.5 months, respectively. Higher PD-L1 expression was associated with longer OS in this subgroup of patients. However, we need to exercise caution when interpreting survival figures for these patients given the small numbers and possibly selection bias of the healthiest to receive more cancer treatments.
Our study brings into focus the critical gap in the representation of Middle Eastern patients in global clinical trials. Ethnic and genetic diversity can significantly influence the efficacy and safety profile of IO therapies. Pharmacogenomics variations, lifestyle and environmental factors, prevalent comorbidities, and healthcare access disparities can modulate treatment responses (43, 44). In Jordan, the high prevalence of smoking, particularly among males (2), and the younger age at lung cancer diagnosis may result in distinct disease profiles, influencing treatment outcomes. Our study showed that 87% of the patients were males, 88% were smokers or ex-smokers, and the median age of lung cancer diagnosis was 59. Therefore, including more ethnically diverse populations in clinical trials is essential to ensure that the findings are reflective of a global patient population.
Limitations of our study include the retrospective nature of the data, which comes from a single cancer center in the Middle East and the relatively short median follow up time of 12.1 months. The authors recognize the importance of a larger sample size that includes a more diverse representation of Middle Eastern countries to improve generalizability of the findings to the region. This is especially important because different Middle Eastern countries have different ethnicities, healthcare practices and access to IO.
In conclusion, our study showed shorter survival in advanced stage NSCLC patients with good PS receiving 1L IO compared to randomized clinical trials. Even after excluding early deaths (21% of patients), survival remained suboptimal. This study serves as a clarion call for more inclusive and diverse clinical research, advocating for further participation of patients from the Middle East and other parts of the world. It stresses the importance of careful patient selection for IO therapy in NSCLC and underscores the need to account for ethnic diversity to enhance the generalizability and applicability of clinical trial outcomes. Our real-world research, alongside others, invites the establishment of real-world benchmarks for IO outcomes, complementing the outcomes of clinical trials. Such benchmarks could offer valuable insights for researchers and regulatory authorities evaluating the effectiveness of IO in lung cancer across diverse global populations.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by King Hussein Cancer Center, IRB No: 23 KHCC 20, IRB Approval Date: February 26, 2023. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
TA: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. KA: Data curation, Writing – original draft, Writing – review & editing, Methodology, Software, Validation. RAH: Validation, Writing – review & editing, Data curation. SA: Data curation, Methodology, Writing – review & editing. TA-B: Validation, Writing – review & editing. SY: Writing – review & editing. HA: Writing – review & editing. JK: Writing – review & editing. AA: Writing – review & editing. IM: Writing – review & editing. RAJ: Writing – review & editing. AAS: Writing – review & editing. AG: Writing – review & editing. MA: Writing – review & editing. AA-I: Writing – review & editing. HH: Writing – review & editing. NM: Writing – review & editing. SO: Writing – review & editing. MA-J: Writing – review & editing. MF: Writing – review & editing. AC: Writing – review & editing. VV: Writing – review & editing. KA-R: Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We want to acknowledge the great contributions made by our Thoracic Oncology Clinics Coordinators, Fathi Abdel Al-AL, Amneh Khashashneh, Rahaf Saadeh, Reem Younis, Subhia Al Yakry, Rozan Rizeq. Also, to thank Haitham Arian for helping with data extraction, and The Office of Scientific Affairs and Research (OSAR) and the Research Council at KHCC for logistical support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
2. Alkouri O, Khader Y, Al-Bashaireh AM. Prevalence of cigarettes and waterpipe smoking among Jordanians, refugees, and migrants in Jordan and its associated factors: A secondary data analysis. Int J Environ Res Public Health. (2022) 20(1):82. doi: 10.3390/ijerph20010082
3. Alqudah MA, Alfaqih MA, Hamouri S, Al-Shaikh AF, Haddad HK, Al-Quran WY, et al. Epidemiology and histopathological classification of lung cancer: A study from Jordan, retrospective observational study. Ann Med Surg (Lond). (2021) 65:102330. doi: 10.1016/j.amsu.2021.102330
4. Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. (2016) 375:1823–33. doi: 10.1056/NEJMoa1606774
5. Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp, et al. Five-year outcomes with pembrolizumab versus chemotherapy for metastatic non–small-cell lung cancer with PD-L1 tumor proportion score ≥ 50%. J Clin Oncol. (2021) 39:2339–49. doi: 10.1200/jco.21.00174
6. Memmott RM, Wolfe AR, Carbone DP, Williams TM. Predictors of response, progression-free survival, and overall survival in patients with lung cancer treated with immune checkpoint inhibitors. J Thorac Oncol. (2021) 16:1086–98. doi: 10.1016/j.jtho.2021.03.017
7. Paz-Ares L, Luft A, Vicente D, Tafreshi A, Gümüş M, Mazières J, et al. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med. (2018) 379:2040–51. doi: 10.1056/NEJMoa1810865
8. Gandhi L, Rodríguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. (2018) 378:2078–92. doi: 10.1056/NEJMoa1801005
9. Paz-Ares L, Ciuleanu TE, Cobo M, Schenker M, Zurawski B, Menezes J, et al. First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer (CheckMate 9LA): an international, randomised, open-label, phase 3 trial. Lancet Oncol. (2021) 22:198–211. doi: 10.1016/s1470-2045(20)30641-0
10. Jazieh AR, Bounedjar A, Bamefleh H, Alfayea T, Almaghraby HQ, Belarabi A, et al. Expression of immune response markers in arab patients with lung cancer. JCO Glob Oncol. (2020) 6:1218–24. doi: 10.1200/go.20.00107
11. Al-Shamsi HO, Abu-Gheida I, Sameh K, Tahoun NE, Musallam KM. Arab countries and oncology clinical trials: A bibliometric analysis. Cancers (Basel). (2023) 15(18):4428. doi: 10.3390/cancers15184428
12. Khozin S, Miksad RA, Adami J, Boyd M, Brown NR, Gossai A, et al. Real-world progression, treatment, and survival outcomes during rapid adoption of immunotherapy for advanced non-small cell lung cancer. Cancer. (2019) 125:4019–32. doi: 10.1002/cncr.32383
13. Velcheti V, Hu X, Yang L, Pietanza MC, Burke T. Long-term real-world outcomes of first-line pembrolizumab monotherapy for metastatic non-small cell lung cancer with ≥50% Expression of programmed cell death-ligand 1. Front Oncol. (2022) 12:834761. doi: 10.3389/fonc.2022.834761
14. Velcheti V, Chandwani S, Chen X, Pietanza MC, Piperdi B, Burke T. Outcomes of first-line pembrolizumab monotherapy for PD-L1-positive (TPS ≥50%) metastatic NSCLC at US oncology practices. Immunotherapy. (2019) 11:1541–54. doi: 10.2217/imt-2019-0177
15. Ivanović M, Knez L, Herzog A, Kovačević M, Cufer T. Immunotherapy for metastatic non-small cell lung cancer: real-world data from an academic central and Eastern European center. Oncologist. (2021) 26:e2143–50. doi: 10.1002/onco.13909
16. Velcheti V, Hu X, Piperdi B, Burke T. Real-world outcomes of first-line pembrolizumab plus pemetrexed-carboplatin for metastatic nonsquamous NSCLC at US oncology practices. Sci Rep. (2021) 11:9222. doi: 10.1038/s41598-021-88453-8
17. Pons-Tostivint E, Hulo P, Guardiolle V, Bodot L, Rabeau A, Porte M, et al. Real-world multicentre cohort of first-line pembrolizumab alone or in combination with platinum-based chemotherapy in non-small cell lung cancer PD-L1 ≥ 50. Cancer Immunol Immunother. (2023) 72(6):1881–90. doi: 10.1007/s00262-022-03359-2
18. Mok TSK, Wu YL, Kudaba I, Kowalski DM, Cho BC, Turna HZ, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. (2019) 393:1819–30. doi: 10.1016/s0140-6736(18)32409-7
19. Waterhouse D, Lam J, Betts KA, Yin L, Gao S, Yuan Y, et al. Real-world outcomes of immunotherapy-based regimens in first-line advanced non-small cell lung cancer. Lung Cancer (2021) 156:41–9. doi: 10.1016/j.lungcan.2021.04.007
20. Liu SV, Hu X, Li Y, Zhao B, Burke T, Velcheti V. Pembrolizumab-combination therapy for previously untreated metastatic nonsquamous NSCLC: Real-world outcomes at US oncology practices. Front Oncol. (2022) 12:999343. doi: 10.3389/fonc.2022.999343
21. Cramer-van der Welle CM, Verschueren MV, Tonn M, Peters BJM, Schramel FMNH, Klungel OH, et al. Real-world outcomes versus clinical trial results of immunotherapy in stage IV non-small cell lung cancer (NSCLC) in the Netherlands. Sci Rep. (2021) 11:6306. doi: 10.1038/s41598-021-85696-3
22. Pérol M, Felip E, Dafni U, Polito L, Pal N, Tsourti Z, et al. Effectiveness of PD-(L)1 inhibitors alone or in combination with platinum-doublet chemotherapy in first-line (1L) non-squamous non-small-cell lung cancer (Nsq-NSCLC) with PD-L1-high expression using real-world data. Ann Oncol. (2022) 33:511–21. doi: 10.1016/j.annonc.2022.02.008
23. Ahn BC, Pyo KH, Xin CF, Jung D, Shim HS, Lee CY, et al. Comprehensive analysis of the characteristics and treatment outcomes of patients with non-small cell lung cancer treated with anti-PD-1 therapy in real-world practice. J Cancer Res Clin Oncol. (2019) 145:1613–23. doi: 10.1007/s00432-019-02899-y
24. Descourt R, Greillier L, Perol M, et al. First-line single-agent pembrolizumab for PD-L1-positive (tumor proportion score >/= 50%) advanced non-small cell lung cancer in the real world: impact in brain metastasis: a national French multicentric cohort (ESCKEYP GFPC study). Cancer Immunol Immunother. (2023) 72:91–9. doi: 10.1007/s00262-022-03232-2
25. Martin C, Lupinacci L, Perazzo F, Bas C, Carranza O, Puparelli C, et al. Efficacy and safety of nivolumab in previously treated patients with non-small-cell lung cancer: real world experience in Argentina. Clin Lung Cancer. (2020) 21:e380–7. doi: 10.1016/j.cllc.2020.02.014
26. Areses Manrique MC, Mosquera Martinez J, Garcia Gonzalez J, Afonso Afonso FJ, Lázaro Quintela M, Fernández Núñez N, et al. Real world data of nivolumab for previously treated non-small cell lung cancer patients: a Galician lung cancer group clinical experience. Transl Lung Cancer Res. (2018) 7:404–15. doi: 10.21037/tlcr.2018.04.03
27. Morita R, Okishio K, Shimizu J, Saito H, Sakai H, Kim YH, et al. Real-world effectiveness and safety of nivolumab in patients with non-small cell lung cancer: A multicenter retrospective observational study in Japan. Lung Cancer. (2020) 140:8–18. doi: 10.1016/j.lungcan.2019.11.014
28. Dudnik E, Moskovitz M, Daher S, Shamai S, Hanovich E, Grubstein A, et al. Effectiveness and safety of nivolumab in advanced non-small cell lung cancer: The real-life data. Lung Cancer. (2018) 126:217–23. doi: 10.1016/j.lungcan.2017.11.015
29. Park JH, You GL, Ahn MJ, Kim SW, Hong MH, Han JY, et al. Real-world outcomes of anti-PD1 antibodies in platinum-refractory, PD-L1-positive recurrent and/or metastatic non-small cell lung cancer, and its potential practical predictors: first report from Korean Cancer Study Group LU19-05. J Cancer Res Clin Oncol. (2021) 147:2459–69. doi: 10.1007/s00432-021-03527-4
30. Kobayashi K, Nakachi I, Naoki K, Satomi R, Nakamura M, Inoue T, et al. Real-world efficacy and safety of nivolumab for advanced non-small-cell lung cancer: A retrospective multicenter analysis. Clin Lung Cancer. (2018) 19:e349–58. doi: 10.1016/j.cllc.2018.01.001
31. Nasr L, Diab S, Ghoche A, Yehia I, Nasr FL. Real-world outcomes of immunotherapy regimens in first line non small cell lung cancer in lebanese patients. J Clin Oncol. (2022) 40:e18741–1. doi: 10.1200/JCO.2022.40.16_suppl.e18741
32. El Karak F, Gh Haddad F, Eid R, Al Ghor M, El Rassy E, Ahmadieh N, et al. Lung cancer and immunotherapy: a real-life experience from second line and beyond. Future Oncol. (2019) 15:3025–32. doi: 10.2217/fon-2019-0144
33. Al Nuhait M, Bajnaid E, Al Otaibi A, Al Shammari A, Al Awlah Y. Real-world safety experience with immune checkpoint inhibitors in Saudi Arabia. Sci Prog. (2021) 104:36850421997302. doi: 10.1177/0036850421997302
34. Jazieh AR, Sağlam EK, Önal HC, Abdelkader Y, Gaafar R, Dawoud E, et al. Real-world treatment patterns and outcomes in stage III non-small cell lung cancer: Middle East and Africa - KINDLE study. Clin Lung Cancer. (2022) 23:364–73. doi: 10.1016/j.cllc.2022.02.002
35. Petrillo LA, El-Jawahri A, Nipp RD, Lichtenstein MRL, Durbin SM, Reynolds KL, et al. Performance status and end-of-life care among adults with non-small cell lung cancer receiving immune checkpoint inhibitors. Cancer. (2020) 126:2288–95. doi: 10.1002/cncr.32782
36. Friedlaender A, Banna GL, Buffoni L, Addeo A. Poor-performance status assessment of patients with non-small cell lung cancer remains vague and blurred in the immunotherapy era. Curr Oncol Rep. (2019) 21:107. doi: 10.1007/s11912-019-0852-9
37. Kano H, Ichihara E, Harada D, Inoue K, Kayatani H, Hosokawa S, et al. Utility of immune checkpoint inhibitors in non-small-cell lung cancer patients with poor performance status. Cancer Sci. (2020) 111:3739–46. doi: 10.1111/cas.14590
38. Yang B, Wang B, Chen Y, Wan N, Xie F, Yang N, et al. Effectiveness and safety of pembrolizumab for patients with advanced non-small cell lung cancer in real-world studies and randomized controlled trials: A systematic review and meta-analysis. Front Oncol. (2023) 13:1044327. doi: 10.3389/fonc.2023.1044327
39. Calles A, Liao X, Sholl LM, Rodig SJ, Freeman GJ, Butaney M, et al. Expression of PD-1 and its ligands, PD-L1 and PD-L2, in smokers and never smokers with KRAS-mutant lung cancer. J Thorac Oncol. (2015) 10:1726–35. doi: 10.1097/jto.0000000000000687
40. Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. (2016) 387:1540–50. doi: 10.1016/s0140-6736(15)01281-7
41. Juergens RA, Mariano C, Jolivet J, Finn N, Rothenstein J, Reaume MN, et al. Real-world benefit of nivolumab in a Canadian non-small-cell lung cancer cohort. Curr Oncol. (2018) 25:384–92. doi: 10.3747/co.25.4287
42. Mencoboni M, Ceppi M, Bruzzone M, Taveggia P, Cavo A, Scordamaglia F, et al. Effectiveness and safety of immune checkpoint inhibitors for patients with advanced non small-cell lung cancer in real-world: review and meta-analysis. Cancers (Basel). (2021) 13(6):1388. doi: 10.3390/cancers13061388
43. Castrillon JA, Eng C, Cheng F. Pharmacogenomics for immunotherapy and immune-related cardiotoxicity. Hum Mol Genet. (2020) 29:R186–r196. doi: 10.1093/hmg/ddaa137
Keywords: Jordan, KHCC, immunotherapy, NSCLC, real-world
Citation: Abu Hejleh T, AlSawalha K, Abdel Hafiz S, Al-Batsh T, Abu Hejleh R, Yaser S, Abu Jazar H, Khader J, Alnsour A, Mohamad I, Abdel Jalil R, Abu-Shanab A, Gharaibeh A, Abu Shattal M, Alibraheem A, Haddad H, Mahmoud N, Obeidat S, Al-Jaghbeer MJ, Furqan M, Cortellini A, Velcheti V and Al-rabi K (2024) Beyond clinical trials: real-world impact of immunotherapy on NSCLC in Jordan. Front. Oncol. 14:1369126. doi: 10.3389/fonc.2024.1369126
Received: 11 January 2024; Accepted: 08 April 2024;
Published: 30 April 2024.
Edited by:
Andrea Camerini, Azienda Usl Toscana Nord Ovest, ItalyReviewed by:
Magdalena Knetki-Wróblewska, Maria Sklodowska-Curie National Research Institute of Oncology, PolandTeodora Alexa-Stratulat, Grigore T. Popa University of Medicine and Pharmacy, Romania
Copyright © 2024 Abu Hejleh, AlSawalha, Abdel Hafiz, Al-Batsh, Abu Hejleh, Yaser, Abu Jazar, Khader, Alnsour, Mohamad, Abdel Jalil, Abu-Shanab, Gharaibeh, Abu Shattal, Alibraheem, Haddad, Mahmoud, Obeidat, Al-Jaghbeer, Furqan, Cortellini, Velcheti and Al-rabi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kamal Al-rabi, KA.10798@KHCC.JO
 Taher Abu Hejleh
Taher Abu Hejleh Karim AlSawalha
Karim AlSawalha Sufian Abdel Hafiz1
Sufian Abdel Hafiz1 Issa Mohamad
Issa Mohamad Akram Alibraheem
Akram Alibraheem Muhammad Furqan
Muhammad Furqan Alessio Cortellini
Alessio Cortellini Vamsidhar Velcheti
Vamsidhar Velcheti Kamal Al-rabi
Kamal Al-rabi