- 1Department of Pathology, Second Affiliated Hospital of Jiujiang University, Jiujiang, China
- 2Department of Clinical Laboratory, Wuhan Jinyintan Hospital, Wuhan, China
- 3Department of General Surgery, Second Affiliated Hospital of Jiujiang University, Jiujiang, China
- 4Department of Pathology, Jiujiang First People’s Hospital, Jiujiang, China
- 5Department of Pathology, Yangpu Hospital, School of Medicine, Tongji University, Shanghai, China
Cases of mixed neuroendocrine-non-neuroendocrine neoplasms (MiNENs) of the urinary system are rare, and reports of primary MiNENs in the ureter are lacking. Herein, we present the case of a 71-year-old man who presented with painless gross hematuria and weight loss. Contrast-enhanced abdominal computed tomography (CT) revealed a tumor, comprising small cell neuroendocrine carcinoma (SCNEC) and adenocarcinomatous components, attached to the ureter. The SCNEC components were strongly positive for synaptophysin, CD56 and INSM1 and adenocarcinomatous components were strongly positive for CDX2 and cytokeratin 20, respectively. Four weeks post-surgery, the patient received four cycles of cisplatin-based chemotherapy; the 7-month follow-up CT confirmed that he was healthy without disease recurrence. The occurrence of MiNEN in the ureter with SCNEC and adenocarcinomatous components is extremely rare, wherein histopathological and immunohistochemical features aid in the diagnosis MiNEN. With its aggressive nature, MiNEN can only be effectively treated by early diagnosis and radical surgery.
Introduction
Mixed neuroendocrine-non-neuroendocrine neoplasms (MiNENs) are characterized by the presence of two major neuroendocrine and non-neuroendocrine morphological components, each accounting for >30% of the total lesion (1). MiNENs have been commonly detected in the gastrointestinal tract and recently in many other organs including the cervix, bladder, and tongue (2, 3). Urothelial carcinoma represents >90% of all malignancies in the urinary system. However, MiNEN that originate in the ureter, including neuroendocrine carcinomas (NECs) and urothelial carcinomas, are rare (4). Although the developmental mechanism closely relates to the genetic events in tumor cells, the biological behavior, prognosis, and molecular origin of MiNENs remain unclear.
Here, we report a case of a patient with a primary MiNEN of the ureter that comprised small cell neuroendocrine carcinoma (SCNEC) and adenocarcinomatous components. Additionally, we have summarized the relevant literature, to improve the medical community’s understanding of this rare type of tumor.
Case description
A 71-year-old man presented with a 3-month history of painless gross hematuria and a 2-month history of weight loss. The patient had a 32-year and 43-year history of smoking and alcohol abuse, respectively. The patient was previously fit and had no specific life history. The physical examination findings were unremarkable. Laboratory investigations revealed that the routine blood, electrolytes, liver function, and serum amylase levels were within the reference ranges, urinalysis revealed red blood cells (3+), and negative levels of the serum tumor markers PSA, BTA and AFP. The urine cytological analysis revealed malignant epithelial neoplastic cells. Computed tomography (CT) revealed a 25-mm extensive heterogeneously-enhanced mass in the middle and lower part of his left ureteral segment and left hydronephrosis (Figure 1A). A subsequent ureteroscopic biopsy revealed poorly differentiated tumors. Positron emission tomography-CT and gastrointestinal endoscopy confirmed the lack of primary or metastatic lesions in other organs. The patient underwent laparoscopic left nephroureterectomy with bladder cuff resection and retroperitoneal lymph node removal. The patient has been followed up with CT postoperatively without ultrasound. Cystoscopy was normal and PET CT showed no other lesions. Postoperative laboratory indicators: all indicators are still normal, urine routine and test hematuria returned to normal. Four weeks post-discharge, the patient received four cycles of cisplatin and etoposide chemotherapy. At his 7-month follow-up, no recurrence or metastasis was noted; follow-up care is continued.
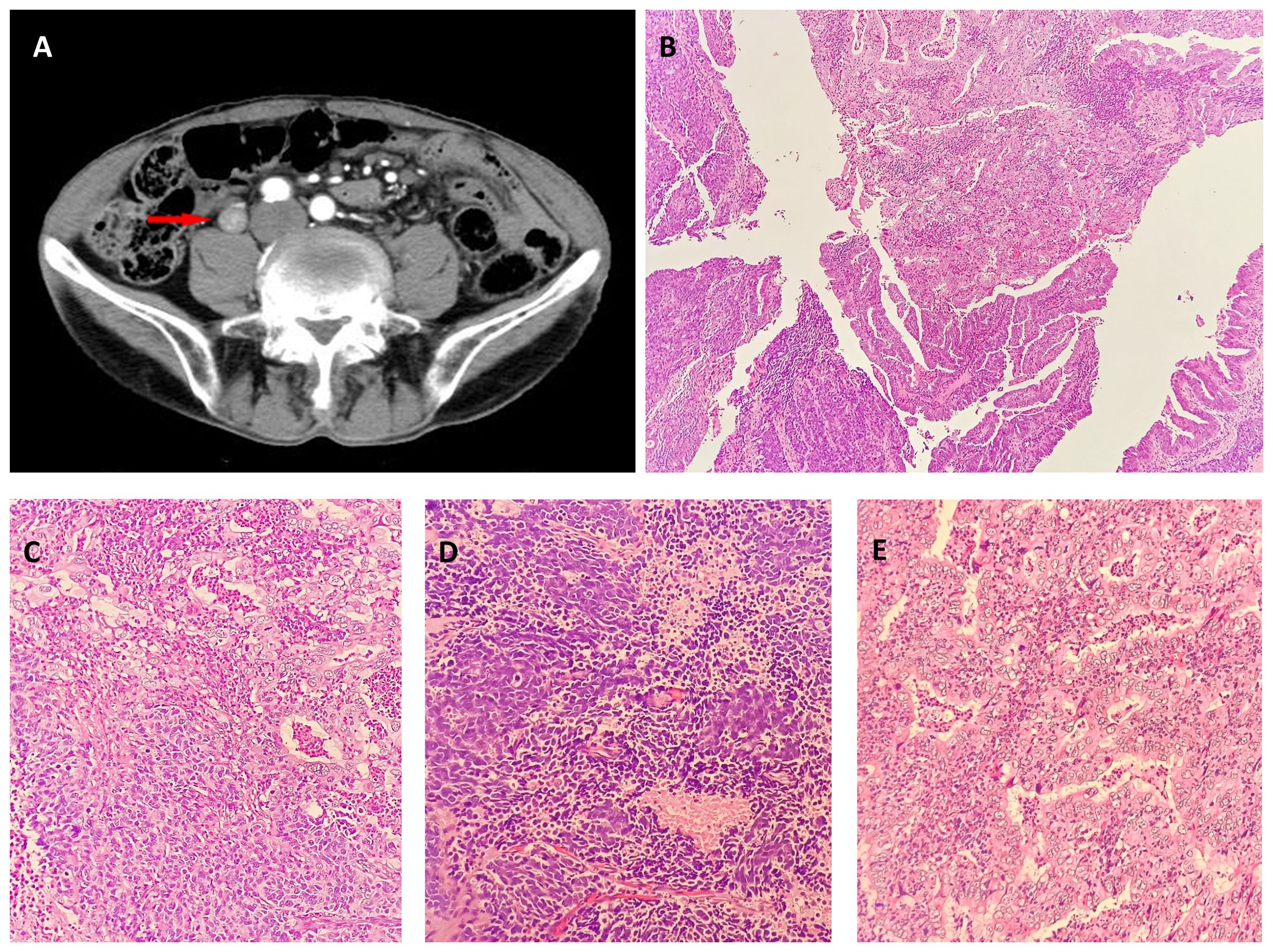
Figure 1 (A) Contrast-enhanced computerized tomography (CECT) of the left ureteral segment and left hydronephrosis. CECT revealed a 20-mm lesion on the left ureteral segment. Neuroendocrine carcinoma and adenocarcinoma in biopsy. Low ((B), hematoxylin-eosin, × 40) and intermediate [(C), hematoxylin-eosin, × 200] magnification showing adenocarcinoma and Neuroendocrine carcinoma. Neuroendocrine in biopsy: High magnification [(D), hematoxylin-eosin, × 400]. Adenocarcinoma in biopsy: High magnification [(E), hematoxylin-eosin, × 400].
The immunohistochemical study was performed on 3-μm-thick sections using prediluted ready-to-use vials of the antibodies with an automated immunostainer.
The tissue sent for examination was a specimen of the left kidney, ureter, and part of the bladder. The ureter was 26 cm in length, and 9 cm from the opening of the ureter, a 2.5 cm × 2 cm ×1 cm mass was visible that was grayish-white in color on the section, brittle, with unclear borders, and without necrosis. Microscopically, the tumor protruding into the lumen comprised two intimately admixed morphological tumor cells (Figures 1B, C). Most cells (55%) exhibited solid and trabecular growth, were small, and had round or spindle-shapes, scant cytoplasm, dark staining, inconspicuous nucleoli, extensive necrosis, and a mitotic index of 60/10 high-power fields (HPFs) (Figures 1D). The other 45% of tumor cells exhibited glandular lumens and papillary formation, with larger tumor cells, columnar and cubic, abundant cytoplasm, conspicuous nucleoli, enteric and mucinous differentiation, and a mitotic index of 4/10 HPFs (Figures 1E). Conventional urothelial carcinoma components were not detected, and the tumor invaded the deep muscle. Lymph node dissection information: abdominal lymph nodes, common iliac lymph nodes, external iliac lymph nodes, obturator lymph nodes, and internal iliac lymph nodes, 0.2-1 cm in diameter. However, renal parenchymal, arteriovenous, periureteral tissue, and lymph node involvement were absent. The overall pathological stage at diagnosis was pT2N0M0.
Immunohistochemistry showed positive staining in different histological components (Figures 2A–J). The tumor cells in the solid components were positive for synaptophysin, CD56 and INSM1, partly positive for TTF-1 and negative for SSTR2. Staining with chromogranin A was very weak, and staining for CDX2, SATB2, and cytokeratin (CK)20 was negative. The tumor cells in the glandular components were positive for CDX2 and CK20 and focally positive for SATB2. The cells tested negative for synaptophysin, CD56, chromogranin A, and TTF1. Both tumor cell components were positive for CKpan, p53, MLH1, PMS2, MSH2, and MSH6 and were negative for GATA3, LCA, and HER2. The Ki-67 indices of tumor cells in the solid and glandular components were approximately 80% and 30%, respectively.
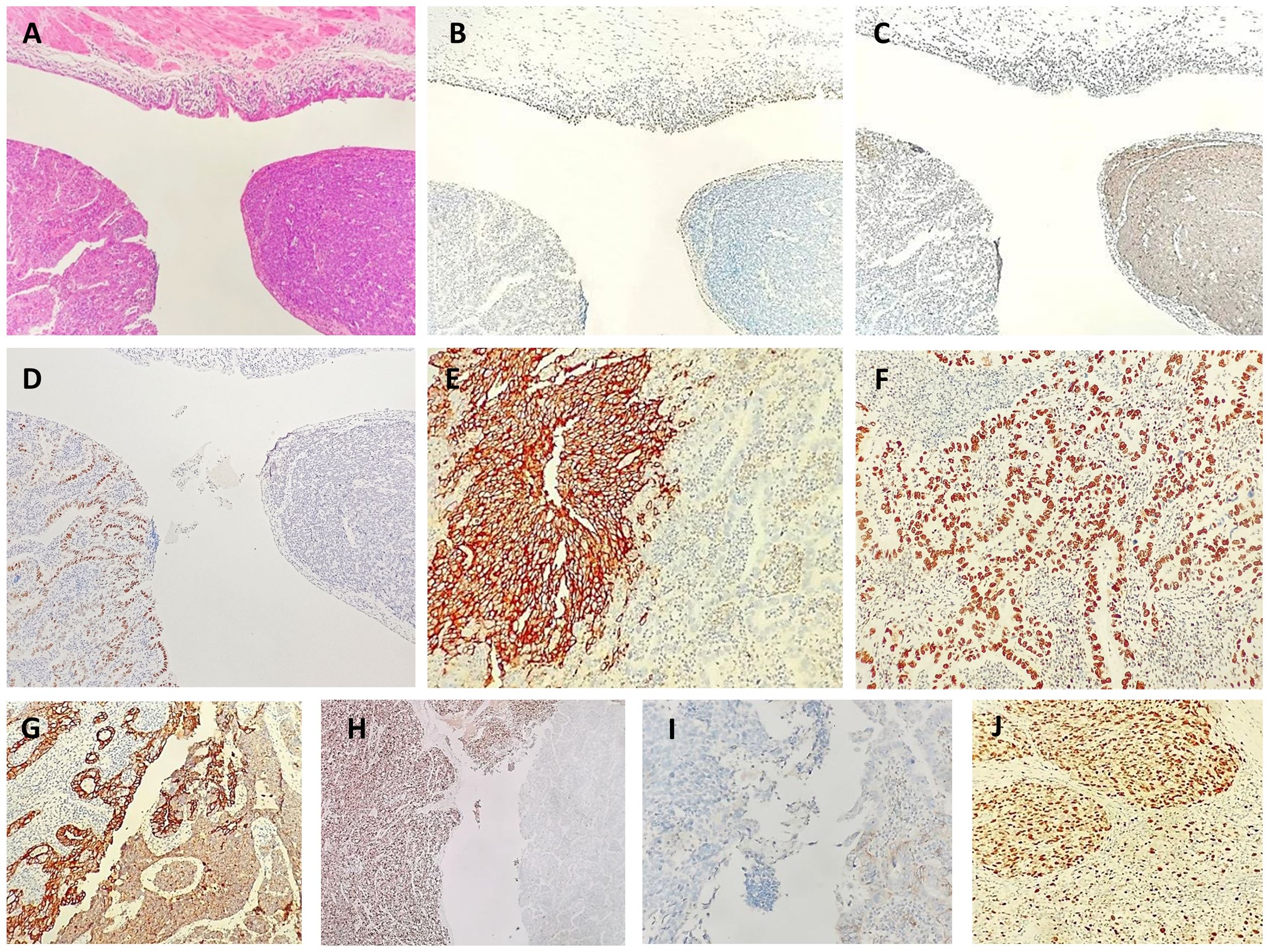
Figure 2 Neuroendocrine carcinoma and adenocarcinoma in biopsy. Low [(A), hematoxylin-eosin, × 40]. Immunohistochemical staining showed positivity for a urothelial marker [(GATA3 (B)], neuroendocrine marker [synaptophysin (C)], adenocarcinoma marker [CDX2 (D)], a neuroendocrine marker [CD56 (E)], adenocarcinoma marker [CDX2 (F), epithelial marker (CKpan (G)], a neuroendocrine marker [INSM1 (H)], a neuroendocrine marker [SSTR2 (I)] and proliferation index marker [Ki67 (J)].
Finally, we diagnosed the patient with primary MiNEN of the ureter, with poorly differentiated neuroendocrine carcinoma (SCNEC, 55%) and moderately and highly differentiated non-neuroendocrine carcinoma (adenocarcinoma, 45%).
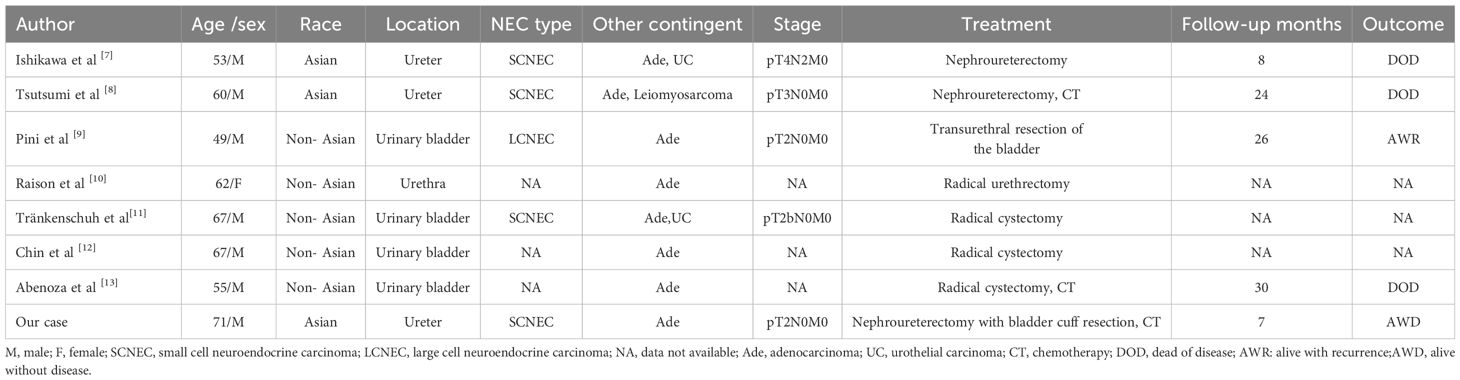
Discussion
NECs of the urinary system include SCNECs, large cell neuroendocrine carcinoma (LCNEC), well-differentiated neuroendocrine tumors, and paragangliomas and account for <0.5% of all urothelial neoplasms. Over half of the primary urinary NECs are mixed with other histological components (5, 6), and more than 90% of these reports of non-neuroendocrine components were of urothelial carcinoma, followed by squamous cell carcinoma, lymphoma, sarcoma, and adenocarcinoma, in order of frequency. We summarized published cases of mixed neuroendocrine and adenocarcinomatous tumor of the urinary system between 1990 and 2023 (Table 1) (7–13). The male-to-female ratio was 7:1, with a median of 61 years, and it can occur in the bladder, ureter and urethra. Specifically, SCNEC is the most common NEC type, while the non-neuroendocrine components can be simple adenocarcinoma or mixed with other components. Unfortunately, the proportion of components has not been reported in most cases. In World Health Organization (WHO) classification 5th edition (2022) of urogenital tumors, mixed neuroendocrine neoplasms are still not defined as MiNEN with 30% component criteria in the manner of gastrointestinal tumors, possibly because of evidence that only a small portion of neuroendocrine carcinoma has a prognostic impact in urinary tract tumors (14). Nevertheless, in order to better understanding of this rare case of Ureter MiNEN, especially when they are morphological high-grade, more detailed clinicopathological data and percentages of each component are necessary, because they still may influence prognosis.
Xu et al. (15) evaluated 17 patients and reported that ureteral collision carcinoma, especially those occurring in the lower right ureter, were common in older Asian men. Concordantly, Zhong et al. (16) evaluated 32 patients with ureteral SCNECs and suggested that the cancers may be related to genetic, environmental, and lifestyle habits in Asians. This may be attributed to a higher incidence of smoking exposure, drug abuse (Chinese herbal medicine, painkillers), occupational exposure to certain aromatic amines, and lithiasis concomitant infections among Asians (4, 17). Our patient was an older Asian man with a 32-year history of smoking, consistent with the literature reports; however, the lesion was in the middle and lower left ureter, unlike previous literature reports that it is more common in the lower right ureter.
The preoperative diagnosis of ureteral MiNEN is confounded by uncharacteristic symptoms, including painless gross hematuria and a ureter mass on imaging; additionally, the accuracy of preoperative biopsy remains controversial. Therefore, final confirmation requires complete surgical excision for examination (18). Combining the clinical history and imaging findings to exclude gastrointestinal and bladder metastases is crucial for the primary diagnosis. In our case, the histomorphological features were comprehensively evaluated, and the differential diagnosis was based on immunohistochemical positivity for synaptophysin, chromogranin A, CD56, neuron-specific enolase, and characteristic markers of the corresponding non-neuroendocrine components. Furthermore, urinary cytology and biopsy pathology revealed malignancy; therefore, we completely surgically resected the tumor. Combined with the medical history, negative imaging, and spectroscopic nodes to exclude metastasis, we diagnosed the patient with primary ureteral MiNEN.
The histogenesis of the primary ureteral MiNEN remains unclear, but has been discussed extensively. At present, many scholars support the theory of MiNEN is hypothesized to originate from monoclonal pluripotent stem cells that differentiate bidirectionally when stimulated with certain factors, which could explain the mixed histological features in urogenital NECs (19). Tränkenschuh et al. (11) reported the case of a patient with primary adenoneuroendocrine carcinoma of the bladder combined with in situ urothelial carcinoma and observed significant similarities in the molecular features between these carcinomas, thereby highlighting their common clonal origin. Another postulated theory is that ureteral MiNEN may be caused by conversion of non-neuroendocrine to neuroendocrine components during tumor progression, including originating from residual urachus epithelium, or cystitis glandularis and intestinal anisocytosis, which would seem to explain the mix of adenocarcinoma components in MiNEN. Eckstein et al. (20) described the case of a patient with adenoneuroendocrine carcinoma of the bladder, with significant cystitis glandularis and intestinal anisocytosis surrounding the tumor. The investigators suggested that this carcinoma developed from primary adenocarcinoma of the bladder. In our case, epithelial neuroendocrine cells transformation such as glandular or intestinal epithelial chemotaxis components in the mucosa surrounding the ureteral MiNEN were not detected. In fact, almost half of urogenital NECs patients had other tumor components such as UC, squamous cell carcinoma, adenocarcinoma. Meanwhile, the conversion of non-neuroendocrine components to neuroendocrine components is rarely reported, thus further buttresses the hypothesis of the origin of multipotent stem cells.
At present, the genetic characteristics of MiNENs containing adenocarcinoma components have been investigated in the digestive system, and it is suggested that CCNE1 gain and FAT1 loss might promote the tumorigenesis of MiNENs partially through regulating cell cycle G1/S checkpoint signaling. Moreover, loss of MAPK1 and alterations in the MAPK signaling pathway, specifically occurred in the NEC component, which might contribute to the neuroendocrine differentiation (21). However, due to its rare molecular changes in the urinary system have not been reported, the relevant studies of single components can provide some references. In a report about urinary molecular typing of bladder SCNEC, a significant proportion of these tumors expressed the c-kit, TP53 and RB1 gene mutations (22, 23). Furthermore, whole exome sequencing of urinary system adenocarcinoma has found that prevalent T>A substitutions were observed among somatic mutations, and major trinucleotide contexts included 5’-CTC-3’ and 5’-CTG-3’ (24). Lu et al (25). Explore the genomic alterations of ureteral hepatoid adenocarcinoma, driver somatic mutations of TP53 and KMT2D genes were found, indicating that ureteral hepatoid adenocarcinoma has similar mutational characteristics to urothelial carcinoma. These genetic characteristics have certain potential significance for future targeted therapy and prognosis judgment, and need to be confirmed by further studies on large samples.
We possess limited treatment experience owing to the rarity of ureteral MiNEN; thus, nephroureterectomy with bladder cuff resection is preferred (4, 15). Furthermore, postoperative platinum-based adjuvant chemotherapy for highly malignant SCNECs components significantly improves prognosis compared with surgical resection alone (5, 26). Qing et al. (27) attempted to treat primary ureteral SCNECs with a combination of PD-L1 ICIs and radiotherapy. Despite the unsatisfactory outcome, the report provided new insights into various treatment strategies. Furthermore, the prognosis of MiNENs is driven by their high-grade neuroendocrine components, especially in the digestive system; therefore, their prognosis is comparable with that of pure neuroendocrine cancers (28, 29). However, another large sample of urinary MiNENs was considered to be better than that of pure SCNECs (30). Pini et al. (9) reported that case of a patient with MiNEN (comprising LCNEC and adenocarcinoma) of the bladder and observed that mucinous adenocarcinoma was the most aggressive component. These findings suggest that non-neuroendocrine carcinomas are likely crucial in determining patient quality of life and prognosis. The prognostic factors of urinary MiNEN are closely related to the pathological stage; however, their association with sex remains controversial (5, 30). This disparity may be related to the rarity of the case, diverse sample sources or limited case numbers. In future work, increasing case collection and pooled case reports can make prognosis clearer. Anyhow, ureter MiNENs have a poor prognosis, which have an overall survival of approximately 15 months and a 3-year survival rate of less than 30%, with metastases occurring within 13 months in most patients. Furthermore, the most common target organs are the lungs and brain (5). In our patient, we performed a laparoscopic left nephroureterectomy with bladder cuff resection, followed by cisplatin and etoposide chemotherapy. We observed no recurrence during the 7-month follow-up. This outcome may relate to the T2 pathological stage, increased proportion of well-differentiated adenocarcinoma components, and shorter follow-up time. And a timeline figure summarizing the case diagnosis and treatment pathway was shown in Figure 3.
In conclusion, we report a rare case of a patient with ureteral MiNEN with SCNECs and adenocarcinoma components, whose clinical imaging presentation was mostly atypical. Characteristic histological patterns and immunohistochemical markers facilitated the accurate diagnosis. Although we lack standard MiNEN treatment options owing to its rarity, radical ureteral resection and postoperative chemotherapy can help improve prognosis, as in the cases of ureteral small cell carcinoma and adenocarcinoma.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by ethics committee of Second Affiliated Hospital of Jiujiang University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
BZ: Conceptualization, Data curation, Investigation, Visualization, Writing – original draft. XG: Conceptualization, Investigation, Writing – original draft. XL: Investigation, Visualization, Writing – original draft. LP: Methodology, Writing – original draft. HH: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Project administration, Supervision, Visualization, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by Climb Plan of Tongji University Affiliated Yangpu Hospital (No.Ye2202105) and Project of College-level Key Discipline of Tongji University Affiliated Yangpu Hospital (No.2023YJXK01).
Acknowledgments
The authors would like to acknowledge the Department of Pathology of Jiujiang First People’s Hospital for their assistance with providing research materials and digitizing the slides in this research. We thank Editage (www.editage.cn) for its linguistic assistance during the preparation of this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, et al. The 2019 who classification of tumours of the digestive system. Histopathology. (2020) 76:182–8. doi: 10.1111/his.13975
2. Robinson L, Schouwstra CM, van Heerden WFP. Oropharyngeal mixed neuroendocrine-nonneuroendocrine neoplasm (Minen): A case report and literature review. Head Neck Pathol. (2021) 15:1415–20. doi: 10.1007/s12105-021-01312-w
3. Maragliano R, Libera L, Carnevali I, Pensotti V, De Vecchi G, Testa M, et al. Mixed neuroendocrine/non-neuroendocrine neoplasm (Minen) of the ovary arising from endometriosis: Molecular pathology analysis in support of a pathogenetic paradigm. Endocrine Pathol. (2022) 33:400–10. doi: 10.1007/s12022-021-09689-8
4. Jang H, Yuk SM, Kim JO, Han DS. A rare case of primary Malignant small cell carcinoma combined with urothelial cell carcinoma in the ureter. World J Surg Oncol. (2013) 11:181. doi: 10.1186/1477-7819-11-181
5. Nakasato T, Hiramatsu A, Matsui Y, Unoki T, Shimoyama H, Oshinomi K, et al. Systematic review and two new cases of primary upper urinary tract neuroendocrine carcinomas. Cancer Treat Res Commun. (2018) 17:23–30. doi: 10.1016/j.ctarc.2018.10.003
6. Ouzzane A, Ghoneim TP, Udo K, Verhasselt-Crinquette M, Puech P, Betrouni N, et al. Small cell carcinoma of the upper urinary tract (Uut-scc): report of a rare entity and systematic review of the literature. Cancer Treat Rev. (2011) 37:366–72. doi: 10.1016/j.ctrv.2010.12.005
7. Ishikawa S, Koyama T, Kumagai A, Takeuchi I, Ogawa D. [a case of small cell carcinoma of the ureter with siadh-like symptoms]. Nihon Hinyokika Gakkai zasshi Japanese J Urol. (2004) 95:725–8. doi: 10.5980/jpnjurol1989.95.725
8. Tsutsumi M, Kamiya M, Sakamoto M, Tobisu K, Kakizoe T. A ureteral small cell carcinoma mixed with Malignant mesodermal and ectodermal elements: A clinicopathological, morphological and immunohistochemical study. Japanese J Clin Oncol. (1993) 23:325–9.
9. Pini GM, Uccella S, Corinti M, Colecchia M, Pelosi G, Patriarca C. Primary minen of the urinary bladder: An hitherto undescribed entity composed of large cell neuroendocrine carcinoma and adenocarcinoma with a distinct clinical behavior : Description of a case and review of the pertinent literature. Virchows Archiv an Int J Pathol. (2021) 479:69–78. doi: 10.1007/s00428-021-03023-7
10. Raison N, McGovern U, Hines J, Volanis D. Mixed adenoneuroendocrine carcinoma of the urethra. BMJ Case Rep. (2019) 12(3):e227948. doi: 10.1136/bcr-2018-227948
11. Tränkenschuh W, Biesdorf AS, Papadimas N, Samara S, Hefty R, Stahl PR. Mixed adenocarcinomatous and neuroendocrine tumor of the urinary bladder with concomitant carcinoma in situ: A case report with a comprehensive immunohistochemical analysis and review of the literature. Int J Surg Pathol. (2019) 27:684–92. doi: 10.1177/1066896919839781
12. Chin NW, Marinescu AM, Fani K. Composite adenocarcinoma and carcinoid tumor of urinary bladder. Urology. (1992) 40:249–52. doi: 10.1016/0090-4295(92)90484-E
13. Abenoza P, Manivel C, Sibley RK. Adenocarcinoma with neuroendocrine differentiation of the urinary bladder. Clinicopathologic, immunohistochemical, and ultrastructural study. Arch Pathol Lab Med. (1986) 110:1062–6.
14. Netto GJ, Amin MB, Berney DM, Compérat EM, Gill AJ, Hartmann A, et al. The 2022 world health organization classification of tumors of the urinary system and male genital organs-part B: Prostate and urinary tract tumors. Eur Urol. (2022) 82:469–82. doi: 10.1016/j.eururo.2022.07.002
15. Xu S, Xu L, Cao P, Yao S, Wu T, Hu X, et al. Collision carcinoma involving small cell neuroendocrine carcinoma and squamous cell carcinoma of the ureter: A case report and review of the literature. Front Oncol. (2021) 11:663119. doi: 10.3389/fonc.2021.663119
16. Zhong W, Lin R, Zhang L, Jin C, Li X, He Q, et al. Clinicopathologic characteristics, therapy and outcomes of patients with primary ureteral small cell carcinoma: A case series and systematic review of the literature. OncoTargets Ther. (2017) 10:4105–11. doi: 10.2147/ott.s138769
17. Zhu J, Feng Y, Fan M, He X, Wang L. Primary small cell neuroendocrine carcinoma combined with squamous carcinoma of the ureter after renal transplantation: A rare case report. Int J Clin Exp Pathol. (2019) 12:1357–62.
18. Luo HL, Kang CH, Chen YT, Chuang YC, Lee WC, Cheng YT, et al. Diagnostic ureteroscopy independently correlates with intravesical recurrence after nephroureterectomy for upper urinary tract urothelial carcinoma. Ann Surg Oncol. (2013) 20:3121–6. doi: 10.1245/s10434-013-3000-z
19. Cheng L, Jones TD, McCarthy RP, Eble JN, Wang M, MacLennan GT, et al. Molecular genetic evidence for a common clonal origin of urinary bladder small cell carcinoma and coexisting urothelial carcinoma. Am J Pathol. (2005) 166:1533–9. doi: 10.1016/s0002-9440(10)62369-3
20. Eckstein M, Rasper B, Busche J, Stachetzki U, Wistuba DE, Agaimy A, et al. [Primary mixed adeno-neuroendocrine carcinoma (Manec) of the urinary bladder: A rare entity]. Aktuelle Urologie. (2017) 48:350–5. doi: 10.1055/s-0043-110088
21. Sun L, Zhang J, Wang C, Zhao S, Shao B, Guo Y, et al. Chromosomal and molecular pathway alterations in the neuroendocrine carcinoma and adenocarcinoma components of gastric mixed neuroendocrine-nonneuroendocrine neoplasm. Modern Pathol an Off J United States Can Acad Pathology Inc. (2020) 33:2602–13. doi: 10.1038/s41379-020-0579-z
22. Pan CX, Yang XJ, Lopez-Beltran A, MacLennan GT, Eble JN, Koch MO, et al. C-kit expression in small cell carcinoma of the urinary bladder: prognostic and therapeutic implications. Modern Pathol an Off J United States Can Acad Pathology Inc. (2005) 18:320–3. doi: 10.1038/modpathol.3800318
23. Warrick JI, Knowles MA, Yves A, van der Kwast T, Grignon DJ, Kristiansen G, et al. Report from the international society of urological pathology (Isup) consultation conference on molecular pathology of urogenital cancers. Ii. Molecular pathology of bladder cancer: progress and challenges. Am J Surg Pathol. (2020) 44:e30–46. doi: 10.1097/pas.0000000000001453
24. Yang G, Shahatiaili A, Bai S, Wang L, Jin D, Cao M, et al. Mutational signature and prognosis in adenocarcinoma of the bladder. J Pathol. (2024) 262:334–46. doi: 10.1002/path.6239
25. Lu M, Li Y, Hu D, Yu J, Zheng H, Liu T. Hepatoid adenocarcinoma in ureter with next-generation sequencing: A case report and literature review. BMC Med Genomics. (2024) 17:7. doi: 10.1186/s12920-023-01776-5
26. Mukesh M, Cook N, Hollingdale AE, Ainsworth NL, Russell SG. Small cell carcinoma of the urinary bladder: A 15-year retrospective review of treatment and survival in the anglian cancer network. BJU Int. (2009) 103:747–52. doi: 10.1111/j.1464-410X.2008.08241.x
27. Qing D, Peng L, Cen F, Huang X, Wei Q, Lu H. Hyperprogression after immunotherapy for primary small cell neuroendocrine carcinoma of the ureter: A case report. Front Oncol. (2021) 11:696422. doi: 10.3389/fonc.2021.696422
28. La Rosa S, Sessa F, Uccella S. Mixed neuroendocrine-nonneuroendocrine neoplasms (Minens): unifying the concept of a heterogeneous group of neoplasms. Endocrine Pathol. (2016) 27:284–311. doi: 10.1007/s12022-016-9432-9
29. Quek ML, Nichols PW, Yamzon J, Daneshmand S, Miranda G, Cai J, et al. Radical cystectomy for primary neuroendocrine tumors of the bladder: the university of Southern California experience. J Urol. (2005) 174:93–6. doi: 10.1097/01.ju.0000162085.20043.1f
Keywords: ureter, mixed neuroendocrine-non-neuroendocrine neoplasm, MiNEN, immunohistochemistry, diagnosis
Citation: Zhou B, Gan X, Li X, Peng L and Hao H (2024) Ureter mixed neuroendocrine-non-neuroendocrine neoplasm: a case report and literature review. Front. Oncol. 14:1390350. doi: 10.3389/fonc.2024.1390350
Received: 23 February 2024; Accepted: 13 May 2024;
Published: 31 May 2024.
Edited by:
Angelo Naselli, MultiMedica Holding SpA (IRCCS), ItalyReviewed by:
Qi-Xing Gong, First Affiliated Hospital of Nanjing Medical University, ChinaKhaled Murshed, Hamad Medical Corporation, Qatar
Copyright © 2024 Zhou, Gan, Li, Peng and Hao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hua Hao, haohua410@tongji.edu.cn
 Bing Zhou
Bing Zhou Xing Gan2
Xing Gan2 Hua Hao
Hua Hao