- 1Department of Craniofacial and Regenerative Biology, King's College London, London, United Kingdom
- 2Department of Experimental Immunobiology, School of Immunology and Microbial Sciences, King's College London, London, United Kingdom
A commentary on
Neuronal regulation of type 2 innate lymphoid cells via neuromedin U
by Cardoso, V., Chesné, J., Ribeiro, H., García-Cassani, B., Carvalho, T., Bouchery, T., et al. (2017). Nature 549, 277–281. doi: 10.1038/nature23469
The links between the intestine and the nervous system have gained renewed attention since diverse factors such as diet, the microbiota, inflammation, and drugs are now known to not only influence the function of this gut-brain axis, but also have systemic effects. Recent studies add a new contributor to this mix, revealing that mucosal neurons can sense helminth infections and directly activate group 2 innate lymphoid cells (ILCs) opening the door to new therapeutic strategies to tackle intestinal dysregulation (Figure 1).
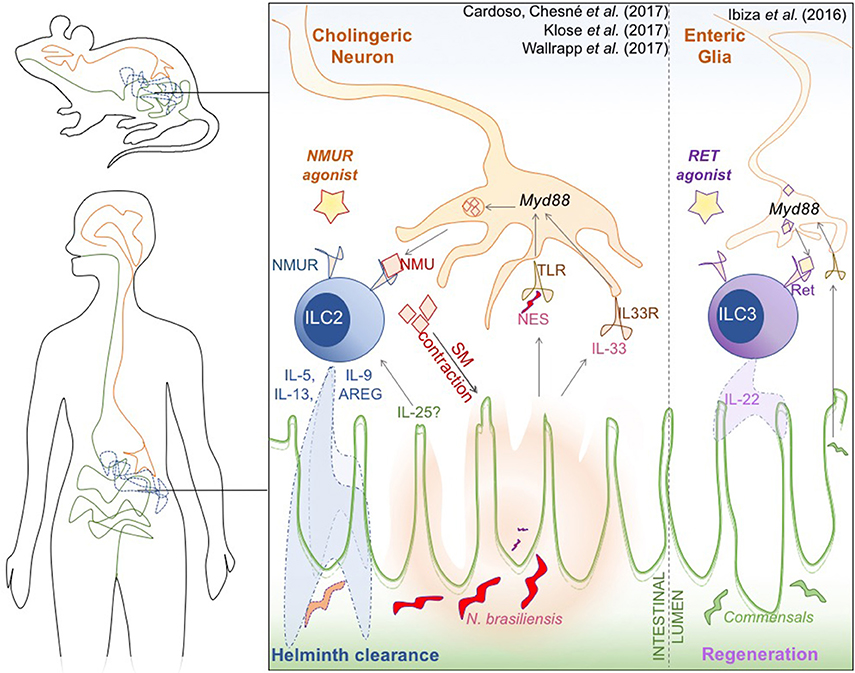
Figure 1. Novel neuro-immune interactions in the mammalian gut. Cholinergic neural cells, type 2 innate lymphoid cells (ILC), and the intestinal epithelium orchestrate an innate response to N. brasiliensis worm infection (Left). Glial cells sense alarmins in the form of the helminth N. brasiliensis excretory/secretory products (NES) and the cytokine IL-33, that is secreted by damaged epithelium. This process is mediated by Myd88, and triggers the production and secretion of Neuromedin U (NMU). NMU induces smooth muscle contractions around the epithelium, which is hypothesized to help physically expulse the helminth infection, but is also recognized by the NMURI receptor specifically expressed on ILC2. NMU induces Ki67 mediated proliferation and activation of ILC2, which secrete type-2 cytokines [IL-5, IL-9, Il-13, amphiregulin (AREG)] to promote clearance of helminthic infection. A similar Myd88-dependent neuronal activation of ILC3 occurs when commensal microbiota induce neuronal activation of Ref-expressing ILC3 (Right), triggering the characteristic release of cytokine IL-22 to promote epithelial regeneration. Both receptors could be attractive therapeutic targets to modulate intestinal inflammation in IBD.
Innate lymphoid cells (ILCs) are highly abundant in mucosal tissues and have attracted interest due to their role in controlling bacterial and parasitic infections, in amplifying immune signals, and in maintaining intestinal barrier integrity. For example, group 2 ILC (ILC2) have a key role in the immune response to helminth infection and group 3 ILC (ILC3) are important for intestinal epithelial regeneration (Britanova and Diefenbach, 2017). Furthermore, this seems to be a bidirectional relationship, as epithelial Tuft-cell derived IL-25 appears to be crucial for ILC homeostasis, particularly of ILC2 (von Moltke et al., 2015; Gerbe et al., 2016; Howitt et al., 2016). Despite these protective roles, ILC can also promote intestinal inflammation, as evidenced by the accumulation of group 1 ILC (ILC1) in the gut of patients with inflammatory bowel disease (IBD) (Bernink et al., 2013). The intestinal epithelium and ILC thus clearly exist in a delicate balance, and together with the luminal commensal microbiota form a trinity that is essential maintaining intestinal homeostasis. Recent publications in Nature reveal that neural cells are also crucial for this homeostatic balance, not only through interactions with the epithelium and the microbiota, but by directly engaging ILC2.
Cardoso et al. (2017) demonstrate that both murine and human ILC2 express a receptor that recognizes the neuropeptide neuromedin U (NMU). NMU is a highly conserved 33 amino-acid peptide secreted by cholinergic neurons that mediate intestinal peristalsis and modulate the sense of satiety (Minamino et al., 1985; Martinez and O'Driscoll, 2015). The authors have shown that within immune cells, the expression of NMU-receptor NMUR1 is enriched in ILC2, and that NMUR1-expressing ILC2 closely associate with NMU-expressing neurons both in the lung and in the gut. Both in vitro and in vivo stimulation of ILC2 with recombinant NMU led to the release of amphiregulin (AREG) and IL-5 and IL-13 cytokines which are associated with immune defense against helminths (Fallon et al., 2006). Thus, to probe the physiological role of NMU-driven activation of ILC2, the authors challenged mice with the helminth Nippostrongylus brasiliensis. Notably, infection resulted in increased expression of NMU in wild-type mice, and both NMUR1 KO mice or chimeric mice in which ILC2 do not express NMUR1 were significantly less able to clear N. brasiliensis.
Finally, the group also shed light on the mechanism underlying activation of this neuron-immune axis during infection. Using enteric neuronal organoids, they found that neuronal expression of Nmu in neurons was triggered by both IL-33 and N. brasiliensis excretory-secretory products (NES), and was dependent on Myd88. On the ILC2, NMUR1 activation triggered ERK1/2 phosphorylation and a Ca2+-calcineurin–NFAT cascade that results in cytokine production. The authors propose that Myd88 is a signaling adaptor of both TLR (which could sense NES) and the IL-33R (ST2) expressed by neurons, allowing these cells to directly sense infection, thereby triggering NMU secretion and ILC2 activation. The same group had previously demonstrated that enteric glial cells can also sense commensal products in a Myd88 dependent manner and that ILC3 express the neuro-receptor Ret, secreting IL-22 in response to glial signaling, thereby mediating epithelial repair, a mechanism similar to that of NMU-NMUR(ILC2) pathway (Ibiza et al., 2016).
Klose et al. independently recapitulated many aspects of the neuron-ILC2 axis, including NMUR1 expression on ILC2, co-localization between ILC2 and neurons, secretion of IL-5, IL-9, and IL-13 by ILC2 upon NMU activation and increased worm burden in NMUR1 KO mice. These effects were eosinophil independent as shown by NMU activation of ILC2 in eosinophil-deficient animals (Klose et al., 2017).
In a third paper, the role of IL-25 for NMU function in ILC2 was addressed by showing that together these signals augment allergic inflammation in the lung in response to house dust mites (Wallrapp et al., 2017). Since IL-25 is secreted by intestinal Tuft cells and is important for ILC2 homeostasis in the intestine, it will be interesting to investigate whether this synergistic effect also occurs in the gut. Future studies should also address if NMU's roles in ILC2 activation and smooth muscle contraction are synergistic, allowing mucosal tissues to physically expel pathogens, as suggested by Cardoso et al. (2017).
Collectively, these papers illustrate why enteric neuronal cells and their involvement in regulating the intestinal milieu have become of interest to immunologists. However, it is important to keep in mind that the gut also signals back to the brain. For example, enterochromaffin enteroendocrine cells (EEC) in the intestinal epithelium are capable of sensing commensal bacterial products and trigger the secretion of serotonin into the synaptic gap between these EEC and neurons (Bellono et al., 2017). Therefore, it will be exciting to determine whether upon neuronal-mediated activation during infection, the gut signals back to the brain, and its' consequences. More importantly, it will be key to dissect if the gut-brain axis, the epithelial-ILC axis, and the neuro-immune axis can be tied together, elucidating whether these connections operate redundantly, in parallel, or perhaps sequentially.
This is a fast-moving field, and combining these axes to better understand ILC activation in mucosal tissues could open many pharmacological doors. For example, it is plausible that other ILC subsets, including pathogenic IFNγ-secreting ILC1, are similarly triggered by neuropeptides, which would provide a source of therapeutic targets for IBD (Bernink et al., 2013). NMUR and RET also provide interesting targets for agonists that could help locally alleviate inflammatory signals. With the constant improvements in organoid culture systems and transcriptomic techniques, systems could be established in the near future to screen for novel molecules that act on targets in human neural, intestinal, and immune cells, leading to new treatments for IBD.
Author Contributions
GJ: Wrote and revised the commentary and executed the figure; JN: Designed, wrote, and revised the commentary and the figure.
Funding
GJ is funded by a Ph.D. fellowship from the Wellcome Trust (203757/Z/16/A). JN is funded by a RCUK/UKRI Innovation fellowship (MR/R024812/1) and a Seed Award in Science from the Wellcome Trust (204394/Z/16/Z).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank Dr. Cláudio Nunes-Alves and Dr. Luke Roberts for critically reading this commentary.
References
Bellono, N. W., Bayrer, J. R., Leitch, D. B., Castro, J., Zhang, C., O'Donnell, T. A., et al. (2017). Enterochromaffin cells are gut chemosensors that couple to sensory neural pathways. Cell 170, 185.e16–198.e16. doi: 10.1016/j.cell.2017.05.034
Bernink, J. H., Peters, C. P., Munneke, M., te Velde, A. A., Meijer, S. L., Weijer, K., et al. (2013). Human type 1 innate lymphoid cells accumulate in inflamed mucosal tissues. Nat. Immunol. 14, 221–229. doi: 10.1038/ni.2534
Britanova, L., and Diefenbach, A. (2017). Interplay of innate lymphoid cells and the microbiota. Immunol. Rev. 279, 36–51. doi: 10.1111/imr.12580
Cardoso, V., Chesné, J., Ribeiro, H., García-Cassani, B., Carvalho, T., Bouchery, T., et al. (2017). Neuronal regulation of type 2 innate lymphoid cells via neuromedin U. Nature 549, 277–281. doi: 10.1038/nature23469
Fallon, P. G., Ballantyne, S. J., Mangan, N. E., Barlow, J. L., Dasvarma, A., Hewett, D. R., et al. (2006). Identification of an interleukin (IL)-25–dependent cell population that provides IL-4, IL-5, and IL-13 at the onset of helminth expulsion. J. Exp. Med. 203, 1105–1116. doi: 10.1084/jem.20051615
Gerbe, F., Sidot, E., Smyth, D. J., Ohmoto, M., Matsumoto, I., Dardalhon, V., et al. (2016). Intestinal epithelial tuft cells initiate type 2 mucosal immunity to helminth parasites. Nature 529, 226–230. doi: 10.1038/nature16527
Howitt, M. R., Lavoie, S., Michaud, M., Blum, A. M., Tran, S. V., Weinstock, J. V., et al. (2016). Tuft cells, taste-chemosensory cells, orchestrate parasite type 2 immunity in the gut. Science 351, 1329–1333. doi: 10.1126/science.aaf1648
Ibiza, S., García-Cassani, B., Ribeiro, H., Carvalho, T., Almeida, L., Marques, R., et al. (2016). Glial-cell-derived neuroregulators control type 3 innate lymphoid cells and gut defence. Nature 535, 440–443. doi: 10.1038/nature18644
Klose, C. S. N., Mahlakõiv, T., Moeller, J. B., Rankin, L. C., Flamar, A. L., Kabata, H., et al. (2017). The neuropeptide neuromedin U stimulates innate lymphoid cells and type 2 inflammation. Nature 549, 282–286. doi: 10.1038/nature23676
Martinez, V. G., and O'Driscoll, L. (2015). Neuromedin U: a multifunctional neuropeptide with pleiotropic roles. Clin. Chem. 61, 471–482. doi: 10.1373/clinchem.2014.231753
Minamino, N., Kangawa, K., and Matsuo, H. (1985). Neuromedin U-8 and U-25: novel uterus stimulating and hypertensive peptides identified in porcine spinal cord. Biochem. Biophys. Res. Commun. 130, 1078–1085. doi: 10.1016/0006-291X(85)91726-7
von Moltke, J., Ji, M., Liang, H. E., and Locksley, R. M. (2015). Tuft-cell-derived IL-25 regulates an intestinal ILC2–epithelial response circuit. Nature 529, 221–225. doi: 10.1038/nature16161
Keywords: innate lymphoid cells, neuromedin U, IBD, mucosal epithelia, neuroimmunology
Citation: Jowett GM and Neves JF (2018) Commentary: Neuronal regulation of type 2 innate lymphoid cells via neuromedin U. Front. Pharmacol. 9:230. doi: 10.3389/fphar.2018.00230
Received: 23 December 2017; Accepted: 28 February 2018;
Published: 14 March 2018.
Edited by:
Julio Galvez, University of Granada, SpainReviewed by:
Jose Garrido-Mesa, Barts and The London School of Medicine and Dentistry, Queen Mary University of London, United KingdomSzilvia Benkő, University of Debrecen, Hungary
Copyright © 2018 Jowett and Neves. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Joana F. Neves, am9hbmEucGVyZWlyYV9kYXNfbmV2ZXNAa2NsLmFjLnVr
 Geraldine M. Jowett
Geraldine M. Jowett Joana F. Neves
Joana F. Neves