- 1Pharmaceutical Care Research Group, University of Basel, Basel, Switzerland
- 2Department of Health Research Methods, Evidence and Impact, McMaster University, Hamilton, ON, Canada
Background: In pharmacotherapy, the achievement of a target clinical outcome requires a certain level of medication intake or adherence. Based on Haynes's early empirical definition of sufficient adherence to antihypertensive medications as taking ≥80% of medication, many researchers used this threshold to distinguish adherent from non-adherent patients. However, we propose that different diseases, medications and patient's characteristics influence the cut-off point of the adherence rate above which the clinical outcome is satisfactory (thereafter medication adherence threshold). Moreover, the assessment of adherence and clinical outcomes may differ greatly and should be taken into consideration. To our knowledge, very few studies have defined adherence rates linked to clinical outcomes. We aimed at investigating medication adherence thresholds in relation to clinical outcomes.
Method: We searched for studies that determined the relationship between adherence rates and clinical outcomes in the databases PubMed, EmbaseⓇ and Web of Science™ until December 2017, limited to English-language. Our outcome measure was any threshold value of adherence. The inclusion criteria of the retrieved studies were (1) any measurement of medication adherence, (2) any assessment of clinical outcomes, and (3) any method to define medication adherence thresholds in relation to clinical outcomes. We excluded articles considered as a tutorial. Two authors (PB and IA) independently screened titles and abstracts for relevance, reviewed full-texts, and extracted items. The results of the included studies are presented qualitatively.
Result: We analyzed 6 articles that assessed clinical outcomes linked to adherence rates in 7 chronic disease states. Medication adherence was measured with Medication Possession Ratio (MPR, n = 3), Proportion of Days Covered (PDC, n = 1), both (n = 1), or Medication Event Monitoring System (MEMS). Clinical outcomes were event free episodes, hospitalization, cortisone use, reported symptoms and reduction of lipid levels. To find the relationship between the targeted clinical outcome and adherence rates, three studies applied logistic regression and three used survival analysis. Five studies defined adherence thresholds between 46 and 92%. One study confirmed the 80% threshold as valid to distinguish adherent from non-adherent patients.
Conclusion: The analyzed studies were highly heterogeneous, predominantly concerning methods of calculating adherence. We could not compare studies quantitatively, mostly because adherence rates could not be standardized. Therefore, we cannot reject or confirm the validity of the historical 80% threshold. Nevertheless, the 80% threshold was clearly questioned as a general standard.
Introduction
With pharmacotherapy, the achievement of the targeted clinical outcome (e.g., control of high blood pressure or HIV viral load suppression) requires a certain level of medication intake or adherence (Maggiolo et al., 2007; Jung et al., 2013). Adherence to medication is defined as “the extent to which a patient's behavior matches the agreed recommendations from a healthcare provider”(Sabaté, 2003). Individual patient's adherence is usually reported as percentage of the actual medication taken over a defined period of time (i.e., adherence rate) and varies from 0% to over 100% in literature (DiMatteo, 2004; Briesacher et al., 2008; Fischer et al., 2010; Nieuwlaat et al., 2014; Huurne et al., 2015). By using a threshold, patients can be dichotomized in persons who take their medications as prescribed (i.e., adherers) and those who deviate from the recommendations in any way (i.e., non-adherers). Based on Haynes's early empirical definition of sufficient adherence to antihypertensive medications as taking ≥80% of medication (Haynes et al., 1980), many researchers used this threshold to distinguish adherent from non-adherent patients (Caro et al., 2004; Doro et al., 2005; Hansen et al., 2010). In Haynes's study, the 80% threshold was supported by a regression analysis indicating that diastolic blood-pressure only fell systematically above this level of adherence. Unsurprisingly, in most other studies the 80% threshold has been used with no clinical rationale (Steiner and Prochazka, 1997; Doro et al., 2005; Hansen et al., 2010). The misconception of using 80% as universal threshold for good adherence is one remaining myth in 40 years of adherence science (Gellad et al., 2017). We propose that the disease, medication and patient's characteristics influence the cut-off point of the adherence rate above which the clinical outcome is satisfactory (thereafter medication adherence threshold). Moreover, the assessment of adherence and clinical outcomes may differ greatly and should be taken into consideration. Some recent theoretical approaches exist to determine adherence thresholds with computer models such as using simulated pharmacodynamic and pharmacokinetic parameters of statins to simulate the adherence rate needed to reach a LDL-C value below 70 mg/dL (Stauffer et al., 2017). However, to our knowledge, very few studies have defined adherence thresholds according to clinical outcomes. We aimed at defining medication adherence thresholds in relation to clinical outcomes.
Methods
We searched for studies that determined medication adherence thresholds in relation to clinical outcomes. We conducted a systematic literature search according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) Guidelines (Moher et al., 2009).
Eligibility Criteria
To be included, a study had to describe (1) any measurement of medication adherence, (2) any assessment of clinical outcomes, and (3) any method to define medication adherence thresholds in relation to clinical outcomes. Citations of the type book chapter, conference proceedings, and dissertations were excluded. We excluded articles considered as a tutorial. We deliberately avoided to restrict our search to a target population, disease, or medication because of the universality of adherence behavior.
Search Strategy and Information Sources
We developed our strategy utilizing the terms “adherence” and synonyms, and “threshold” and synonyms in the title of publications. The databases PubMed, EmbaseⓇ and Web of Science™ were searched covering the time period from inception to 31st December 2017, limiting to English-language publications. The search strategy for each database is shown in Supplementary Material.
Study Selection
After we removed all duplications, the retrieved citations were screened based on the title and abstract, then on the full text. Two investigators assessed eligibility (PB, IA). Any disparity was resolved by consensus. All work was performed in Endnote™ (Clarivate Analytics, Version X8).
Data Collecting Process
Data extraction was performed by one investigator (PB) and a second investigator (IA) checked the worktable for completeness and accuracy. Disagreements were resolved by consensus.
Data Items
We collected the following variables in the included studies: disease; medication class or medication; population; medication adherence measurement; clinical outcomes; study design; method for threshold determination.
Summary Measure
Measures of interest were: mean medication adherence rate with standard deviation; medication adherence threshold value; probability to reach the targeted clinical outcome with the medication adherence threshold (expressed as odds ratio or hazard ratio); percentage of patients below the threshold.
Result
Study Selection
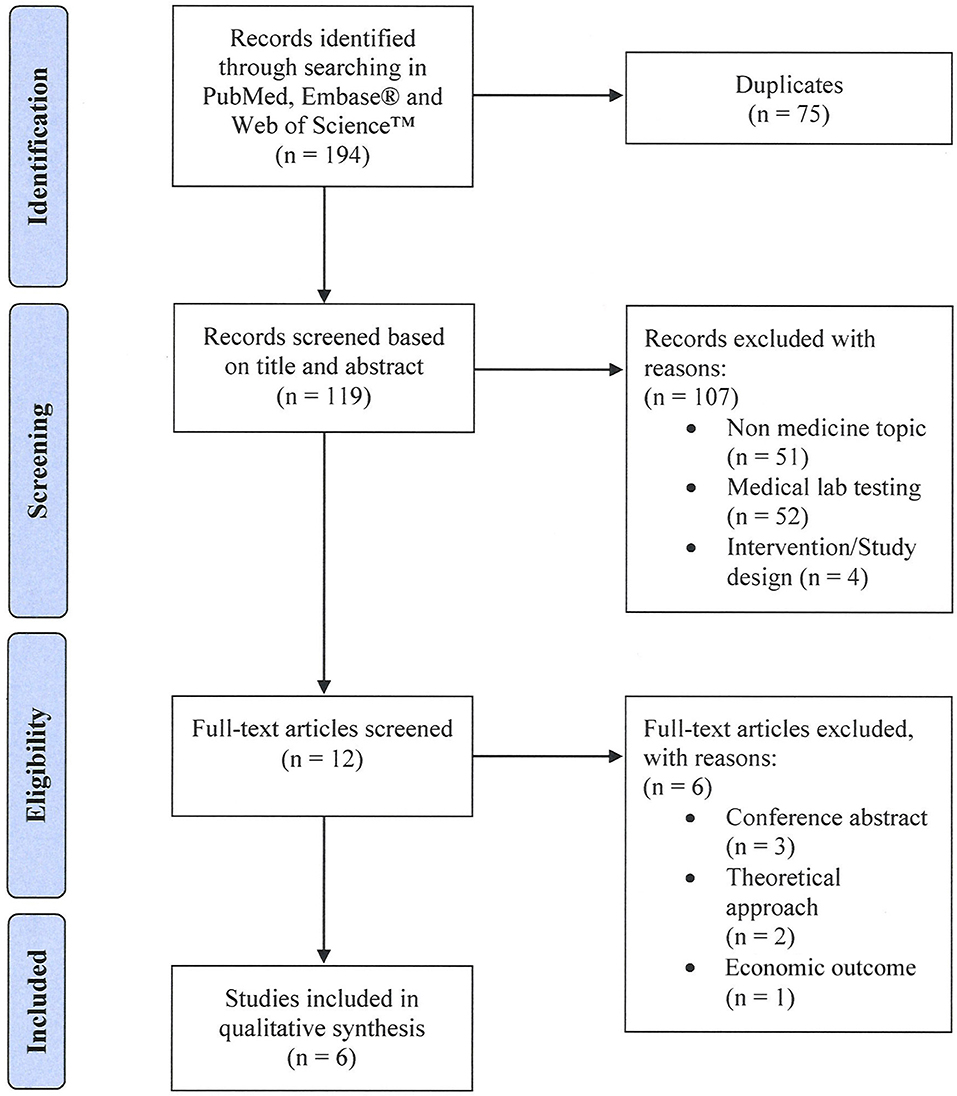
The systematic literature search yielded 194 records. After removal of duplicates, 119 unique citations were screened based on title and abstract. We excluded 107 articles that were not in the field of medicine (n = 51), investigated medical lab testing (n = 52), or were discussing adherence interventions (n = 4). Of the remaining twelve articles that were assessed for eligibility in full text, 6 articles were excluded [conference abstracts (n = 3), focusing on economic outcome (n = 1), discussing a theoretical approach (n = 2)]. Six articles met all set eligibility criteria and were included in our qualitative synthesis (see Figure 1).
Study Characteristics
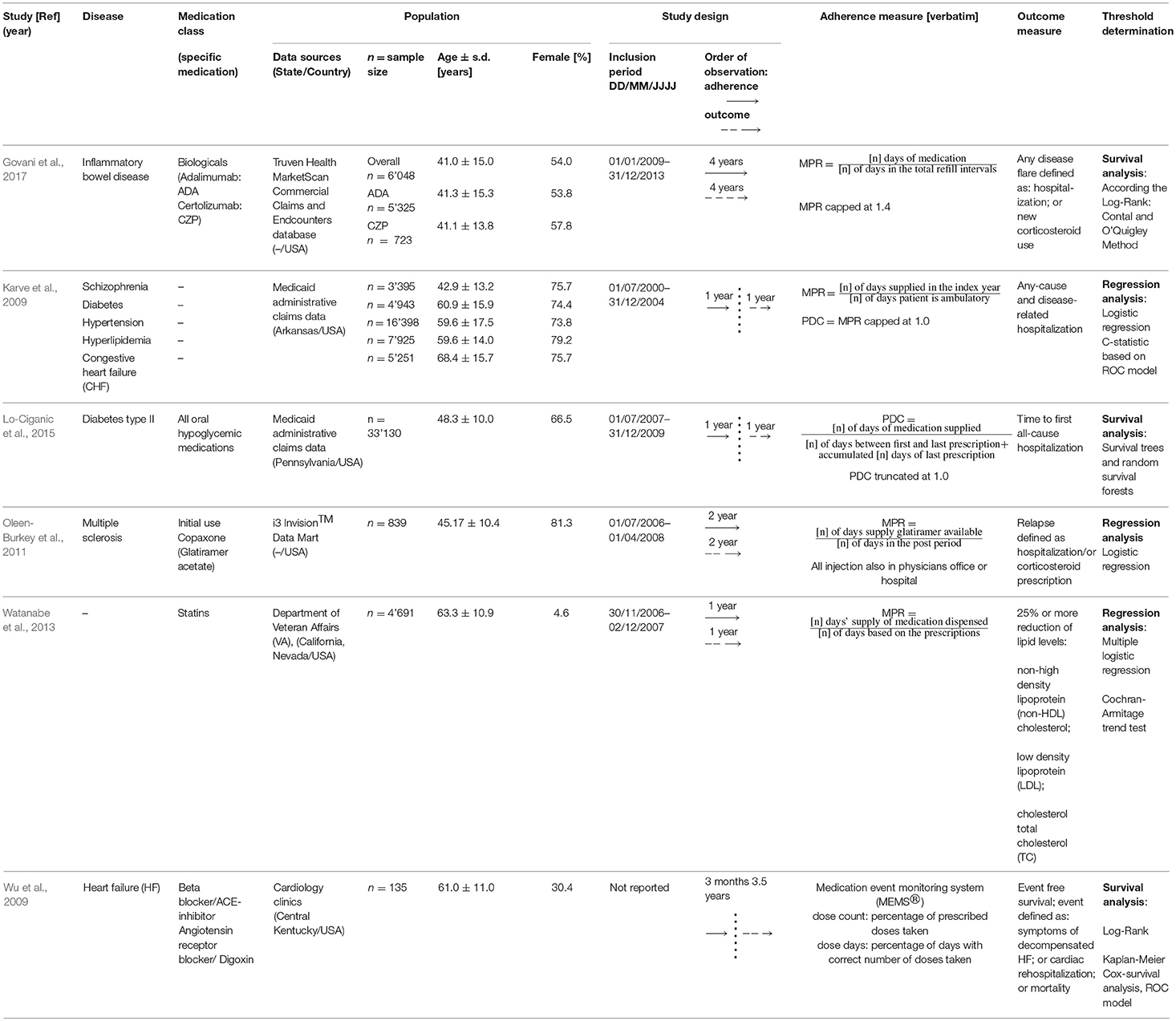
The 6 identified studies were published between 2009 (Karve et al., 2009; Wu et al., 2009) and 2017 (Govani et al., 2017) and were all conducted in the USA (Karve et al., 2009; Wu et al., 2009; Oleen-Burkey et al., 2011; Watanabe et al., 2013; Lo-Ciganic et al., 2015; Govani et al., 2017). Data originated from insurance services covering patients throughout the USA (Oleen-Burkey et al., 2011; Govani et al., 2017), from a Medicaid program of a state (Karve et al., 2009; Lo-Ciganic et al., 2015), the Department of Veterans affairs (Watanabe et al., 2013) or cardiology clinics in Central Kentucky (Wu et al., 2009). Average age of patients ranged from 41 (Govani et al., 2017) to 68.4 years (Karve et al., 2009), the percentage of females 4.6 (Watanabe et al., 2013) to 81.29% (Oleen-Burkey et al., 2011). The study population ranged from 135 (Wu et al., 2009) to 37,912 patients (Karve et al., 2009). Fives studies (Wu et al., 2009; Oleen-Burkey et al., 2011; Watanabe et al., 2013; Lo-Ciganic et al., 2015; Govani et al., 2017) were focusing on a single chronic disease, while one (Karve et al., 2009) included patients with one out of five chronic disease states (schizophrenia, diabetes, hypertension, hyperlipidemia, and congestive heart failure). Medication adherence was calculated to a single medication in five studies (Karve et al., 2009; Wu et al., 2009; Oleen-Burkey et al., 2011; Watanabe et al., 2013; Govani et al., 2017), or to all hypoglycemic agents in one study (Lo-Ciganic et al., 2015). Two studies (Oleen-Burkey et al., 2011; Watanabe et al., 2013) focused on new medication users, and four studies (Karve et al., 2009; Wu et al., 2009; Lo-Ciganic et al., 2015; Govani et al., 2017) included patients on the medication of interest without further explanations.
Study Design
Five studies were retrospective with pharmacy claims data (Karve et al., 2009; Oleen-Burkey et al., 2011; Watanabe et al., 2013; Lo-Ciganic et al., 2015; Govani et al., 2017), one study was designed as a prospective study using an electronic medication bottle (MEMSⓇ) (Wu et al., 2009). The observation period ranged from 1 (Karve et al., 2009; Watanabe et al., 2013; Lo-Ciganic et al., 2015) to 4 years (Govani et al., 2017). Three studies observed adherence and clinical outcome simultaneously (Oleen-Burkey et al., 2011; Watanabe et al., 2013; Govani et al., 2017) while three studies assessed sequentially first adherence, followed by the targeted clinical outcome (Karve et al., 2009; Wu et al., 2009; Lo-Ciganic et al., 2015). The period during which medication adherence was measured ranged from 3 months (Wu et al., 2009) to 4 years (Govani et al., 2017). The occurrence of the targeted clinical outcome was assessed over 1 year (Karve et al., 2009; Watanabe et al., 2013; Lo-Ciganic et al., 2015) up to 4 years (Govani et al., 2017).
Medication Adherence Measures
Retrospective database studies measured adherence by calculating the Medication Possession Ratio (MPR; this measure assesses the proportion of time with adequate supply over a predefined observation period) (Oleen-Burkey et al., 2011; Watanabe et al., 2013; Govani et al., 2017), or the Proportion of Days Covered (PDC; this measure represents the proportion of days a patient has a medication available in a given period of time, mostly a calendar year) (Lo-Ciganic et al., 2015) or both (Karve et al., 2009). Different definitions and operationalization of the MPR and PDC were used (See Table 1). In the MEMSⓇ study (Wu et al., 2009), adherence rates were defined as the percentage of prescribed doses taken (dose count) and percentage of days with correct number of doses taken (dose day). Adherence outliers were truncated at 100 (Karve et al., 2009; Lo-Ciganic et al., 2015), at 140% (Govani et al., 2017), or not (Wu et al., 2009; Oleen-Burkey et al., 2011; Watanabe et al., 2013).
Clinical Outcomes
Five studies used event free survival as clinical outcome (Karve et al., 2009; Wu et al., 2009; Oleen-Burkey et al., 2011; Lo-Ciganic et al., 2015; Govani et al., 2017) and one study used the reduction of lipid levels (Watanabe et al., 2013). Events were defined as mortality (Wu et al., 2009), hospitalization (Karve et al., 2009; Wu et al., 2009; Oleen-Burkey et al., 2011; Lo-Ciganic et al., 2015; Govani et al., 2017), cortisone use (Govani et al., 2017), cortisone prescription (Oleen-Burkey et al., 2011), and reported symptoms (Wu et al., 2009). Studies using hospitalization as clinical outcome were either including all-cause hospitalization (Oleen-Burkey et al., 2011; Lo-Ciganic et al., 2015; Govani et al., 2017), disease specific hospitalization (Wu et al., 2009) or both (Karve et al., 2009).
Threshold Determination
Two methods were applied to link the targeted clinical outcome and adherence rates: logistic regression [i.e., correlating the independent variable “adherence” with the dependent dichotomized variable “outcome” (Karve et al., 2009; Oleen-Burkey et al., 2011; Watanabe et al., 2013)] and survival analysis [i.e., comparing different adherence rate groups in regard to time to event rates (Wu et al., 2009; Lo-Ciganic et al., 2015; Govani et al., 2017)]. Studies using logistic regression determined the optimal threshold based on Receiver Operating Characteristic curve (i.e., a method that plots sensitivity/specificity values to a particular decision threshold) (Karve et al., 2009); or compared the odds ratio of different adherence rate groups for a relapse (Oleen-Burkey et al., 2011) or for achieving a therapeutic goal (Watanabe et al., 2013). For survival analysis, maximized log rank statistics generated two adherence groups that separated most significantly either by shifting the threshold and comparing the resulting dichotomized adherence groups (Wu et al., 2009) or using a macro (Contal and O'Quigley, 1999) that calculates log rank statistics for all possible thresholds (Govani et al., 2017) or a special approach developing a random survival forest model for predictor of hospitalization with adherence being one of fifteen predictors for hospitalization (Lo-Ciganic et al., 2015).
Adherence Thresholds and Clinical Outcomes
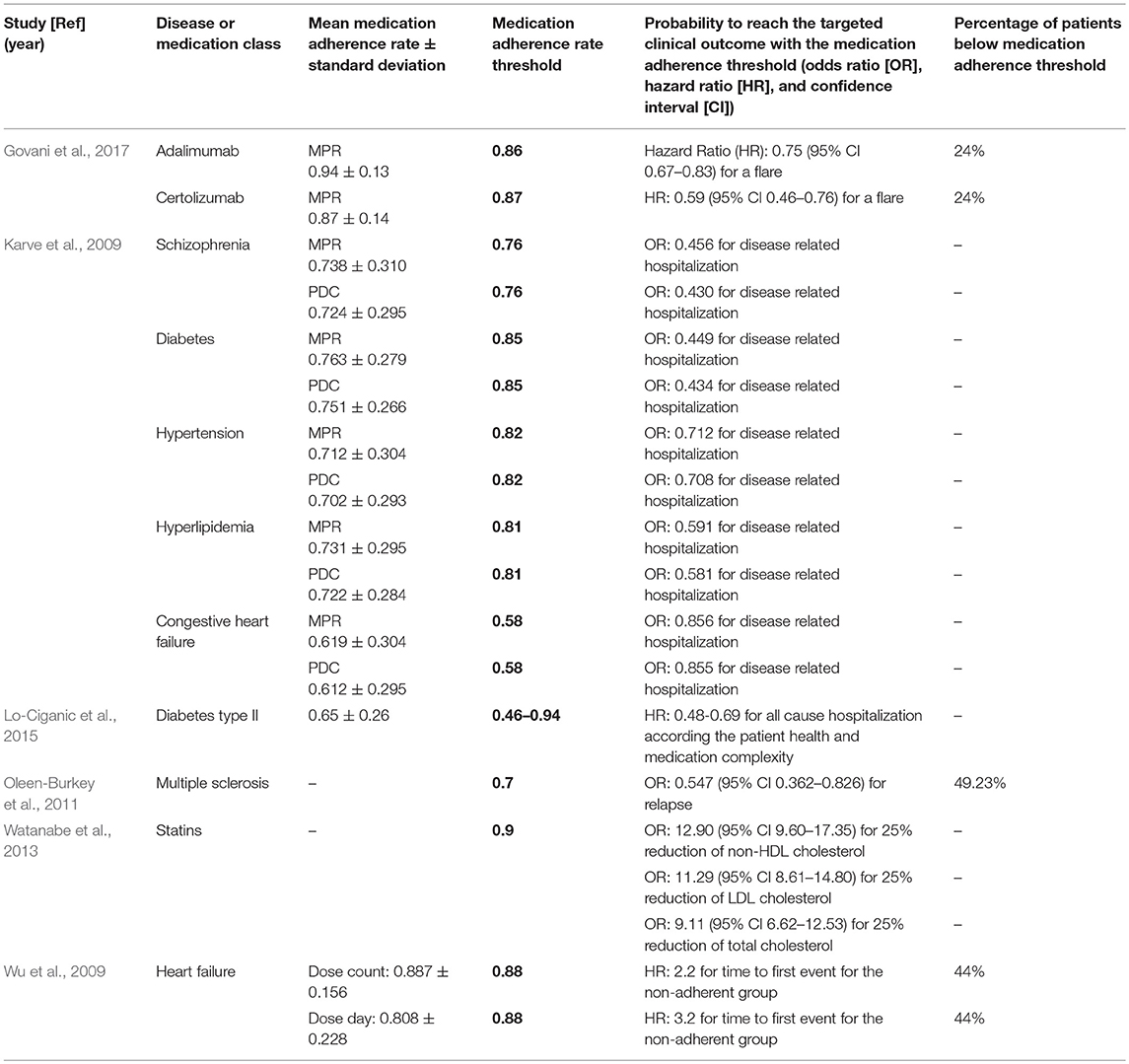
Four studies reported mean adherence rates (Karve et al., 2009; Lo-Ciganic et al., 2015; Govani et al., 2017) between 61% in congestive heart failure (Karve et al., 2009) and 94% in patients with inflammatory bowel disease (Govani et al., 2017). Adherence rate thresholds linked to the targeted clinical outcome ranged from 63% for congestive heart failure (Karve et al., 2009) to 90% for statins (Watanabe et al., 2013). In the study with diabetes type II, threshold values were determined depending on other predictors of hospitalization (such as prior hospitalization, number of monthly prescriptions, insulin use), and ranged from 46 to 92% (Lo-Ciganic et al., 2015). In the retrieved studies, the relationships between the medication adherence thresholds and the clinical outcomes were expressed as odds ratio (Karve et al., 2009; Oleen-Burkey et al., 2011; Watanabe et al., 2013) or hazard ratio (Wu et al., 2009; Lo-Ciganic et al., 2015; Govani et al., 2017). For example, the hazard ratio for a flare was 0.75 for patients achieving an MPR of 0.86 (i.e., patients who reached a Medication Possession Ratio of 86% had a 25% lower risk to have a flare) (Govani et al., 2017). For all values, see Table 2.
Discussion
To our knowledge, this is the first systematic review that aimed at defining medication adherence threshold in relationship to a targeted clinical outcome, and shed light on the historical 80% threshold. Six studies published in the past 9 years met our eligibility criteria and demonstrate the low interest in the question or the complexity of the task. Five studies critically questioned the commonly used 80% adherence threshold as being suboptimal. However, studies were highly heterogeneous predominately concerning study design, clinical outcomes, number of included patients and underlying diseases. Further, various methods exist for the assessment of medication adherence and for its calculation, according to the research setting. Therefore, we were unable to standardize the adherence rates of the different measures, and could not compare the included studies quantitatively. A general agreement to reject or confirm the historical 80% threshold cannot be given due to the low number and the high diversity of the included studies. However, we could summarize some findings to guide future research.
Medications Under Investigation
Three studies investigated one medication as surrogate for multiple treatments in the disease of interest (Karve et al., 2009; Wu et al., 2009; Watanabe et al., 2013). This was done with the rationale that a single medication suffices to detect the medication intake behaviors of a patient. However, medication adherence is known to be negatively influenced by a large number of medications or the complexity of treatment (Marcum and Gellad, 2012). In diseases with simple or limited drug regimens, such as hyperlipidemia or multiple sclerosis, it is possible to choose medications as a surrogate with similar properties out of a chemical subgroup [such as HMG-CoA reductase inhibitors (Watanabe et al., 2013)] or even special chemical substance [such as glatiramer acetate (Oleen-Burkey et al., 2011)]. In progressive diseases complex drug regimens are common. As for example, according to the European Society Cardiology (ESC) guidelines, treatments for congestive heart failure (Ponikowski et al., 2016) consist of up to four simultaneous medications with different mechanisms of action. Thus, selecting one single medication as a surrogate for a complex treatment needs clear ground, especially when adherence parameters will be extrapolated from a lead medication to the entire regimen. Therefore, we recommend to include all concerned medications when investigating the intake behaviors of a patient.
Clinical Outcome and Observation Period
Ideally, there are two types of outcome markers available for analysis, intermediary outcomes (surrogate measures such as blood pressure, lipids, glucose), and patient-important outcomes [e.g., death, stroke, myocardial infarction, hospitalization (Yordanov et al., 2018)]. The latter would require much larger and longer studies—but they would answer the key question of whether the adherence level makes a clinically important difference. Five studies (Karve et al., 2009; Oleen-Burkey et al., 2011; Watanabe et al., 2013; Lo-Ciganic et al., 2015; Govani et al., 2017) used hospitalization as outcome marker for a various diseases such as diabetes (Wu et al., 2009), congestive heart failure (Wu et al., 2009), schizophrenia (Karve et al., 2009), and hyperlipidemia (Watanabe et al., 2013); and for various medications such as adalimumab (Govani et al., 2017) and galtiramer acetate (Oleen-Burkey et al., 2011). Surrogate markers were seldom described (Watanabe et al., 2013). However, the observation periods were mostly 1 year (Karve et al., 2009; Watanabe et al., 2013; Lo-Ciganic et al., 2015), which is short to observe hard endpoints such as hospitalization. Even if hospitalization is easy to document and allows dichotomization for statistical analysis, many cofactors influence the probability of hospitalization in a year such as number of monthly prescriptions, prior hospitalization and disease severity (Lo-Ciganic et al., 2015). As a comparison, the follow-up period of randomized controlled trials with statin therapy and patient important outcome measures (major coronary event, stroke, death) was at least 3 years (Cheung et al., 2004). Consequently, for smaller studies with short observation periods, fast reacting surrogate measures such as blood pressure seem more suitable endpoints to link adherence level with single medication. Thus, researchers should select a specific clinical endpoint and an observation period long enough to catch the full effect of medication adherence on the target clinical outcome.
Calculation of Medication Adherence
Even without a gold standard (Lam and Fresco, 2015), any mathematical method used to compute medication adherence needs to be clearly defined (Arnet et al., 2016). Many studies demonstrated that medication possession ratio (MPR) is highly influenced by the observation period (Kozma et al., 2013; Sperber et al., 2017) and oversupply (Martin et al., 2009). Thus, it is surprising that the four retrieved studies that used MPR (Karve et al., 2009; Oleen-Burkey et al., 2011; Watanabe et al., 2013; Govani et al., 2017) present four different formulas with poor specification. Consequently, each study is a standalone and direct comparison is impossible.
Further, according to a new adherence taxonomy (Vrijens et al., 2012), behaviors differ whether patients are initiating, implementing, or discontinuing their treatment. Calculating adherence rate from claims data delivers an aggregate estimate of a patient's medication possession. MPR and PDC are summary measures and cannot differentiate between implementation and discontinuation, mainly because pharmacy claims data do not allow to define precisely the time point of discontinuation. Currently, no method to calculate medication adherence from claims data seems adequate to deliver values for each phase of medication adherence. Researchers need to be aware of the prerequisites of the calculation measure they plan to use.
Dichotomizing Continuous Data
To determine medication adherence thresholds, the authors of the studies categorized the population in two groups that vary significantly. Dichotomizing is commonly used in medicine, because it makes data summarization more efficient and offers a simple risk classification in populations for clinicians. However, statisticians advise against dichotomizing continuous data such as medication adherence data, because a substantial loss of information can occur (Streiner, 2002; Royston et al., 2006). Further, replicates of thresholds are made impossible in subsequent studies. As a consequence, the continuous variable “medication adherence” should be described with a distribution plot to present the entire data. In the retrieved studies, only mean adherence value and standard deviation (as indicator of homogeneity) were given to describe the data (Karve et al., 2009; Wu et al., 2009; Lo-Ciganic et al., 2015; Govani et al., 2017). These two values are insufficient to describe the distribution of the data. A graphic such as a histogram of the medication adherence values could deliver additional and comprehensive information covering the distribution.
Adherence Threshold in Context of the Clinical Relevance
The novelty of the retrieved studies was not to try to distinguish adherent from non-adherent patients, but to express the clinical benefit obtained by patients reaching a certain level of engagement in their dosing regimens. Thus, categorizing patients in arbitrary groups such as “good” and “poor” adherer is misleading. On the contrary, to indicate the degree of execution of a treatment in form of a medication adherence threshold represents valuable and concrete information for clinicians. Surprisingly, only half of the studies (Wu et al., 2009; Oleen-Burkey et al., 2011; Govani et al., 2017) presented the percentage of the population below their medication adherence threshold. Thus, this information combined with the adherence distribution (mean value, standard deviation, graphic representation) should enable healthcare providers and policy makers to target patients with low adherence that would clearly clinically benefit reaching a certain level of adherence.
Limitations
We acknowledge some limitations. First, we may have missed articles that did not contain our search words in their title. However, it is likely that such articles have mentioned adherence threshold in a subsidiary content and then would not have filled our inclusion criteria. For example, a recently published study investigating the adherence to antihypertensive medications and the risk of cardiovascular disease among older adults did not define an unambiguous threshold (Yang et al., 2017). Second, the search was limited to English-language. Third, all included studies were performed in the US-population with inherent specificities such as the underrepresentation of women [US veterans with a percentage 4.6% women (Watanabe et al., 2013)], population with lower income patients [Medicaid enrollees (Karve et al., 2009; Lo-Ciganic et al., 2015)] or a small locally defined population (Wu et al., 2009). Consequently, our results cannot be generalized to other populations. Fourth, due to the diversity of studies, the quantitative comparison of adherence thresholds was not possible.
Conclusions
This study revealed a large research gap in determining medication adherence thresholds in relationship to clinical outcomes. The authors of the included studies must be complimented for their attempt to question the historical 80% threshold. We were able to extract five recommendations for future research in this field:
1. Include all medications prescribed for a disease to estimate the medication intake behavior;
2. Select an observation period sufficiently long to detect the targeted clinical outcome; orientate to the length of the observation period used in high quality studies;
3. Define the adherence measurement; calculations have to be replicable;
4. Select statistical methods for the threshold determination carefully, in order to avoid loss of information;
5. Put the adherence threshold in context to clinical relevance.
Based on this new knowledge, further studies are needed to define adherence thresholds linked to the targeted clinical outcome in order to deliver high quality and comparable results to ultimately guide healthcare professionals.
Author Contributions
PB designed the review protocol, carried out the literature search, extracted data from selected studies, and drafted the manuscript. IA participated in the literature search. RH, KH, and IA revised the manuscript critically for intellectual content. All authors read and approved the final manuscript.
Funding
The study was funded by the Pharmaceutical Care Research Group.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2018.01290/full#supplementary-material
References
Arnet, I., Kooij, M. J., Messerli, M., Hersberger, K. E., Heerdink, E. R., and Bouvy, M. (2016). Proposal of standardization to assess adherence with medication records: methodology matters. Ann. Pharmacother. 50, 360–368. doi: 10.1177/1060028016634106
Briesacher, B. A., Andrade, S. E., Fouayzi, H., and Chan, K. A. (2008). Comparison of drug adherence rates among patients with seven different medical conditions. Pharmacotherapy 28, 437–443. doi: 10.1592/phco.28.4.437
Caro, J. J., Ishak, K. J., Huybrechts, K. F., Raggio, G., and Naujoks, C. (2004). The impact of compliance with osteoporosis therapy on fracture rates in actual practice. Osteoporos. Int. 15, 1003–1008. doi: 10.1007/s00198-004-1652-z
Cheung, B. M., Lauder, I. J., Lau, C. P., and Kumana, C. R. (2004). Meta-analysis of large randomized controlled trials to evaluate the impact of statins on cardiovascular outcomes. Br. J. Clin. Pharmacol. 57, 640–651. doi: 10.1111/j.1365-2125.2003.02060.x
Contal, C., and O'Quigley, J. (1999). An application of changepoint methods in studying the effect of age on survival in breast cancer. Comput. Stat. Data Anal. 30, 253–270. doi: 10.1016/S0167-9473(98)00096-6
DiMatteo, M. R. (2004). Variations in patients' adherence to medical recommendations: a quantitative review of 50 years of research. Med. Care 42, 200–209. doi: 10.1097/01.mlr.0000114908.90348.f9
Doró, P., Benko, R., Kosik, E., Matuz, M., Tóth, K., and Soós, G. (2005). Utilization of oral antihyperglycemic drugs over a 7-year period (1998-2004) in a Hungarian population and adherence to drug therapy. Eur. J. Clin. Pharmacol. 61, 893–897. doi: 10.1007/s00228-005-0031-9
Fischer, M. A., Stedman, M. R., Lii, J., Vogeli, C., Shrank, W. H., Brookhart, M. A., et al. (2010). Primary medication non-adherence: analysis of 195,930 electronic prescriptions. J. Gen. Intern. Med. 25, 284–290. doi: 10.1007/s11606-010-1253-9
Gellad, W. F., Thorpe, C. T., Steiner, J. F., and Voils, C. I. (2017). The myths of medication adherence. Pharmacoepidemiol. Drug Saf. 26, 1437–1441. doi: 10.1002/pds.4334
Govani, S. M., Noureldin, M., Higgins, P. D. R., Heisler, M., Saini, S. D., Stidham, R. W., et al. (2017). Defining an optimal adherence threshold for patients taking subcutaneous anti-TNFs for inflammatory bowel diseases. Am. J. Gastroenterol. 113, 276–282. doi: 10.1038/ajg.2017.438
Hansen, R. A., Farley, J. F., Droege, M., and Maciejewski, M. L. (2010). A retrospective cohort study of economic outcomes and adherence to monotherapy with metformin, pioglitazone, or a sulfonylurea among patients with type 2 diabetes mellitus in the United States from 2003 to 2005. Clin. Ther. 32, 1308–1319. doi: 10.1016/j.clinthera.2010.07.011
Haynes, R. B., Taylor, D. W., Sackett, D. L., Gibson, E. S., Bernholz, C. D., and Mukherjee, J. (1980). Can simple clinical measurements detect patient noncompliance? Hypertension 2, 757–764.
Jung, O., Gechter, J. L., Wunder, C., Paulke, A., Bartel, C., Geiger, H., et al. (2013). Resistant hypertension? Assessment of adherence by toxicological urine analysis. J. Hypertens. 31, 766–774. doi: 10.1097/HJH.0b013e32835e2286
Karve, S., Cleves, M. A., Helm, M., Hudson, T. J., West, D. S., and Martin, B. C. (2009). Good and poor adherence: optimal cut-point for adherence measures using administrative claims data. Curr. Med. Res. Opin. 25, 2303–2310. doi: 10.1185/03007990903126833
Koehorst-ter Huurne, K., Movig, K., van der Valk, P., van der Palen, J., and Brusse-Keizer, M. (2015). Differences in adherence to common inhaled medications in COPD. COPD 12, 643–648. doi: 10.3109/15412555.2014.995292
Kozma, C. M., Dickson, M., Phillips, A. L., and Meletiche, D. M. (2013). Medication possession ratio: implications of using fixed and variable observation periods in assessing adherence with disease-modifying drugs in patients with multiple sclerosis. Pat. Prefer. Adherence 7, 509–516. doi: 10.2147/PPA.S40736
Lam, W. Y., and Fresco, P. (2015). Medication adherence measures: an overview. Biomed Res. Int. 2015:217047. doi: 10.1155/2015/217047
Lo-Ciganic, W. H., Donohue, J. M., Thorpe, J. M., Perera, S., Thorpe, C. T., Marcum, Z. A., et al. (2015). Using machine learning to examine medication adherence thresholds and risk of hospitalization. Med. Care 53, 720–728. doi: 10.1097/MLR.0000000000000394
Maggiolo, F., Airoldi, M., Kleinloog, H. D., Callegaro, A., Ravasio, V., Arici, C., et al. (2007). Effect of adherence to HAART on virologic outcome and on the selection of resistance-conferring mutations in NNRTI- or PI-treated patients. HIV Clin. Trials 8, 282–292. doi: 10.1310/hct0805-282
Marcum, Z. A., and Gellad, W. F. (2012). Medication adherence to multi-drug regimens. Clin. Geriatr. Med. 28, 287–300. doi: 10.1016/j.cger.2012.01.008
Martin, B. C., Wiley-Exley, E. K., Richards, S., Domino, M. E., Carey, T. S., and Sleath, B. L. (2009). Contrasting measures of adherence with simple drug use, medication switching, and therapeutic duplication. Ann. Pharmacother. 43, 36–44. doi: 10.1345/aph.1K671
Moher, D., Liberati, A., Tetzlaff, J., and Altman, D. G. (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 339:b2535. doi: 10.1136/bmj.b2535
Nieuwlaat, R., Wilczynski, N., Navarro, T., Hobson, N., Jeffery, R., Keepanasseril, A., et al. (2014). Interventions for enhancing medication adherence. Cochrane Database Syst. Rev. 2:CD000011. doi: 10.1002/14651858.CD000011.pub4
Oleen-Burkey, M. A., Dor, A., Castelli-Haley, J., and Lage, M. J. (2011). The relationship between alternative medication possession ratio thresholds and outcomes: evidence from the use of glatiramer acetate. J. Med. Econ. 14, 739–747. doi: 10.3111/13696998.2011.618517
Ponikowski, P., Voors, A. A., Anker, S. D., Bueno, H., Cleland, J. G., Coats, A. J., et al. (2016). 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. J. Heart. Fail. 18, 891–975. doi: 10.1002/ejhf.592
Royston, P., Altman, D. G., and Sauerbrei, W. (2006). Dichotomizing continuous predictors in multiple regression: a bad idea. Stat. Med. 25, 127–141. doi: 10.1002/sim.2331
Sabaté, E. (2003). Adherence to Long-Term Therapies: Evidence for Action. Geneva: World Health Organization.
Sperber, C. M., Samarasinghe, S. R., and Lomax, G. P. (2017). An upper and lower bound of the medication possession ratio. Pat. Prefer. Adherence 11, 1469–1478. doi: 10.2147/PPA.S136890
Stauffer, M. E., Hutson, P., Kaufman, A. S., and Morrison, A. (2017). The adherence rate threshold is drug specific. Drugs R D 17, 645–653. doi: 10.1007/s40268-017-0216-6
Steiner, J. F., and Prochazka, A. V. (1997). The assessment of refill compliance using pharmacy records: methods, validity, and applications. J. Clin. Epidemiol. 50, 105–116. doi: 10.1016/S0895-4356(96)00268-5
Streiner, D. L. (2002). Breaking up is hard to do: the heartbreak of dichotomizing continuous data. Can. J. Psychiatry 47, 262–266. doi: 10.1177/070674370204700307
Vrijens, B., De Geest, S., Hughes, D. A., Przemyslaw, K., Demonceau, J., Ruppar, T., et al. (2012). A new taxonomyfor describing and defining adherence to medications. Br. J. Clin. Pharmacol. 73, 691–705. doi: 10.1111/j.1365-2125.2012.04167.x
Watanabe, J. H., Bounthavong, M., and Chen, T. (2013). Revisiting the medication possession ratio threshold for adherence in lipid management. Curr. Med. Res. Opin. 29, 175–180. doi: 10.1185/03007995.2013.766164
Wu, J. R., Moser, D. K., De Jong, M. J., Rayens, M. K., Chung, M. L., Riegel, B., et al. (2009). Defining an evidence-based cutpoint for medication adherence in heart failure. Am. Heart J. 157, 285–291. doi: 10.1016/j.ahj.2008.10.001
Yang, Q., Chang, A., Ritchey, M. D., and Loustalot, F. (2017). Antihypertensive medication adherence and risk of cardiovascular disease among older adults: a population-based cohort study. J. Am. Heart Assoc. 6:e006056. doi: 10.1161/JAHA.117.006056
Keywords: medication adherence (MeSH), patient compliance, threshold, systematic (literature) review, clinical outcome, adherence measurement methods, adherence metric, adherence methodologies
Citation: Baumgartner PC, Haynes RB, Hersberger KE and Arnet I (2018) A Systematic Review of Medication Adherence Thresholds Dependent of Clinical Outcomes. Front. Pharmacol. 9:1290. doi: 10.3389/fphar.2018.01290
Received: 30 July 2018; Accepted: 22 October 2018;
Published: 20 November 2018.
Edited by:
Brian Godman, Karolinska Institutet (KI), SwedenReviewed by:
Joseph O. Fadare, Ekiti State University, NigeriaDan Kibuule, University of Namibia, Namibia
Copyright © 2018 Baumgartner, Haynes, Hersberger and Arnet. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pascal C. Baumgartner, pascal.baumgartner@unibas.ch
 Pascal C. Baumgartner
Pascal C. Baumgartner R. Brian Haynes
R. Brian Haynes Kurt E. Hersberger
Kurt E. Hersberger Isabelle Arnet
Isabelle Arnet