- Department of Pharmacy, Daping Hospital, Army Medical University, Chongqing, China
Background: Proton pump inhibitors (PPIs) are usually prescribed to prevent gastrointestinal (GI) complications in patients receiving dual antiplatelet therapy (DAPT). This systematic review and meta-analysis aimed to explore the efficacy and safety of the concomitant use of PPIs with aspirin-clopidogrel DAPT in patients with Coronary heart disease (CHD).
Method: The PubMed, Embase, Cochrane Library, and Web of Science databases were searched from inception to August 2022 for eligible studies. The adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated to evaluate the clinical outcomes. Subgroup analysis was conducted according to different PPI subtypes, populations, follow-up times and study types. This study was registered on PROSPERO (CRD42022332195).
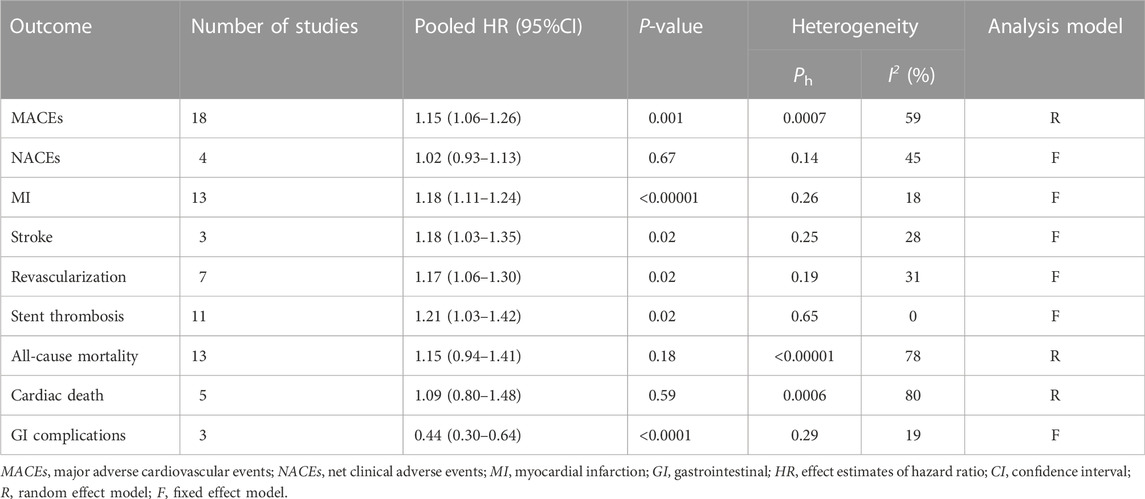
Results: A total of 173,508 patients from 18 studies [2 randomized controlled trials (RCTs), 3 post hoc analyses of RCTs, and 13 cohort studies] were included in this study. Pooled data revealed that coadministration of PPIs significantly increased the risk of major adverse cardiovascular events (MACEs) (HR = 1.15, 95% CI = 1.06–1.26, p = .001) and reduced the risk of gastrointestinal (GI) complications (HR = 0.44, 95% CI = 0.30–0.64, p < .0001). Subgroup analysis results showed that the esomeprazole users and patients with coronary stenting in the PPI group were associated with an increased risk of MACEs compared with the non-PPI group. The occurrence of MACEs in PPI users was more common than that in non-PPI users in long-term follow-up (≥12 months) studies and in the observational studies. There was no significant differences in the incidences of net clinical adverse events (NACEs), all-cause mortality, or cardiac death between the two groups.
Conclusion: In patients with CHD, the concomitant use of PPIs with aspirin and clopidogrel was associated with a reduced risk of GI complications but could increase the rates of MACEs (particularly in patients receiving esomeprazole or with coronary stenting). There was no clear evidence of an association between PPI use and NACEs, all-cause mortality, or cardiac death. The results could have been affected by the follow-up time and study type. Further large-scale RCTs with long-term follow-up are needed.
1 Introduction
Coronary heart disease (CHD) is one of the most common chronic illnesses and is the leading cause of death worldwide (Zhou et al., 2019; Voutilainen, et al., 2022). Dual antiplatelet therapy (DAPT) with aspirin plus clopidogrel is recommended for patients with CHD to reduce the risk of ischemic cardiovascular events while increasing the risk of bleeding compared with either of the regimens alone (Diener et al., 2004; Benavente et al., 2012; Valgimigli et al., 2018). Gastrointestinal (GI) bleeding accounts for a significant proportion of bleeding complications in DAPT, which can lead to DAPT cessation and has been identified as an independent risk factor for poor prognosis. (Capodanno et al., 2018). Because aspirin damages the gastric mucosa by suppressing the synthesis of prostaglandins (PGs) (Nishida et al., 2011), the antiangiogenic effects of clopidogrel could impair the healing of gastric erosions (Luo et al., 2016). These patients are frequently prescribed proton pump inhibitors (PPIs) to minimize GI complications (involving ulcers and bleeding) (Levine et al., 2016; Valgimigli et al., 2018). However, previous studies have indicated that coadministration of PPIs with aspirin-clopidogrel DAPT could be associated with adverse drug-drug interactions.
Aspirin is mainly absorbed in the acidic environment. PPIs inhibit gastric acid production and increase gastric pH, resulting in poor aspirin absorption (Gesheff et al., 2014; Vaduganathan and Bhatt, 2016). Clopidogrel is a prodrug that depends on cytochrome P450 isoenzyme (mainly CYP2C19) to metabolize into an active form. PPIs are also metabolized by CYP enzymes and thus could inhibit the conversion of clopidogrel into its active metabolite (Furuta et al., 2017). Furthermore, mounting clinical data have shown that long-term intake of PPIs increases the susceptibility of patients to serious adverse events, including cardiovascular events and damage to the lower GI tract (Lue and Lanas, 2016; Xie et al., 2019; Marlicz et al., 2022; Zhai et al., 2022). However, the existing clinical studies of the association between cardiovascular events and the concomitant use of PPIs with aspirin-clopidogrel DAPT in CHD have been conflicting (Ben Ghezala et al., 2022). Some meta-analyses were conducted to assess the clinical significance of this interaction and found that coadministration of PPIs could increase the rates of major adverse cardiovascular events (MACEs), stroke, revascularization, and stent thrombosis (ST) but not myocardial infarction (MI), all-cause mortality, or cardiac death (Guo et al., 2021; Hu et al., 2018; Li et al., 2021; Melloni et al., 2015; Sherwood et al., 2015). Interestingly, two recent clinical studies reported that the rates of MI, all-cause mortality and cardiac death were significantly increased in PPI users compared to non-users (Maret-Ouda et al., 2021; Mohammed et al., 2021). In these studies, hazard ratios (HRs) containing the status of event occurrence and the time when events happened were calculated by multivariable Cox proportional hazards regression models (Spruance et al., 2004). Moreover, net clinical adverse events (NACEs) are also an important clinical outcome in CHD (Chandrasekhar et al., 2017). However, to the best of our knowledge, no meta-analyses of previous studies have reported NACEs outcomes. In addition, the length of follow-up is vital for the clinical outcome evaluation of PPI coadministration, but it has rarely been considered in previous meta-analyses. Furthermore, the results of clinical studies have also been inconsistent regarding the protective effects of PPIs in the GI tract.
Therefore, we performed this meta-analysis to evaluate the efficacy and safety of the combination treatment of PPIs with aspirin-clopidogrel DAPT for CHD patients by extracting adjusted HRs to provide a theoretical basis for clinical, individualized practice. Furthermore, subgroup analysis was conducted according to different PPI subtypes, populations, follow-up times and study types to analyze the heterogeneity.
2 Methods
2.1 Search strategy
This study was conducted in adherence to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines. The study was registered on PROSPERO (CRD42022332195). We searched the PubMed, Embase, the Cochrane Library, and Web of Science databases for relevant studies published in English from inception to August 2022. The following Medical Subject Headings (MeSH) and keywords were used for the literature retrieval: “proton pump inhibitors (PPIs),” “dual antiplatelet therapy (DAPT),” “aspirin,” “clopidogrel,” “acute coronary syndrome (ACS),” “myocardial infarction (MI),” “percutaneous coronary intervention (PCI),” and “coronary stenting.” We also searched conference abstracts and reviewed reference lists of relevant review articles to provide additional citations.
2.2 Study selection
Studies were selected based on the following inclusion criteria: 1) subjects: patients with ACS, PCI, or coronary stenting receiving aspirin-clopidogrel DAPT; 2) exposure intervention: the experimental group was treated with PPIs, whereas the control group was treated with a placebo or no PPIs; 3) outcome measures: the primary outcome was MACEs, and the secondary outcomes were NACEs, MI, stroke, revascularization, ST, all-cause mortality, cardiac death, and GI complications (involving ulcers and bleeding events); and 4) study design: randomized, controlled trials (RCTs) and observational studies.
Studies were excluded if they met the following exclusion criteria: 1) patients receiving aspirin or clopidogrel alone; 2) control group patients receiving H2 receptor antagonists; 3) effect estimates of adjusted HRs and corresponding 95% confidence intervals (CIs) was not provided; 4) different reports of the same trial or duplicate data; and 5) case reports.
2.3 Data extraction and quality assessment
Two reviewers independently extracted data from eligible studies, including authors, publication year, region, population, type of PPI, follow-up time, study endpoints, study design, and sample size. The Jadad Scale and Newcastle-Ottawa Scale (NOS) scoring methods were used to assess the quality of RCTs and observational studies, respectively. Post hoc analyses of RCTs were regarded as observational studies to evaluate study quality. Discrepancies in data extraction and quality assessment, if necessary, were resolved by consultation with a third reviewer.
2.4 Statistical analysis
Review Manager software, version 5.3, was used for the analysis of adjusted HRs. Between-study heterogeneity was calculated with Higgins’s I2 test: I2 > 50% could represent substantial heterogeneity, and a random-effect model was applied; otherwise, a fixed-effect model was used. Subgroup analysis was conducted based on the PPI subclass, follow-up time, population, and study type. We estimated publication bias through a visual inspection of funnel plots.
3 Results
3.1 Search results and quality evaluation
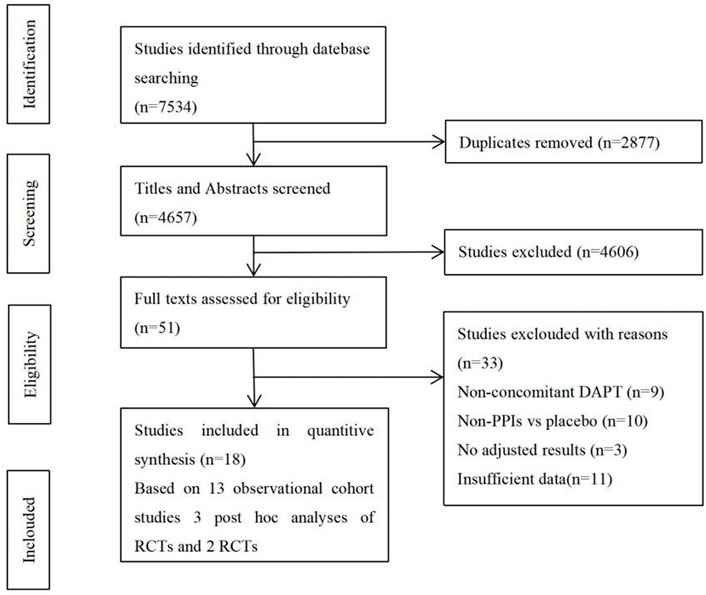
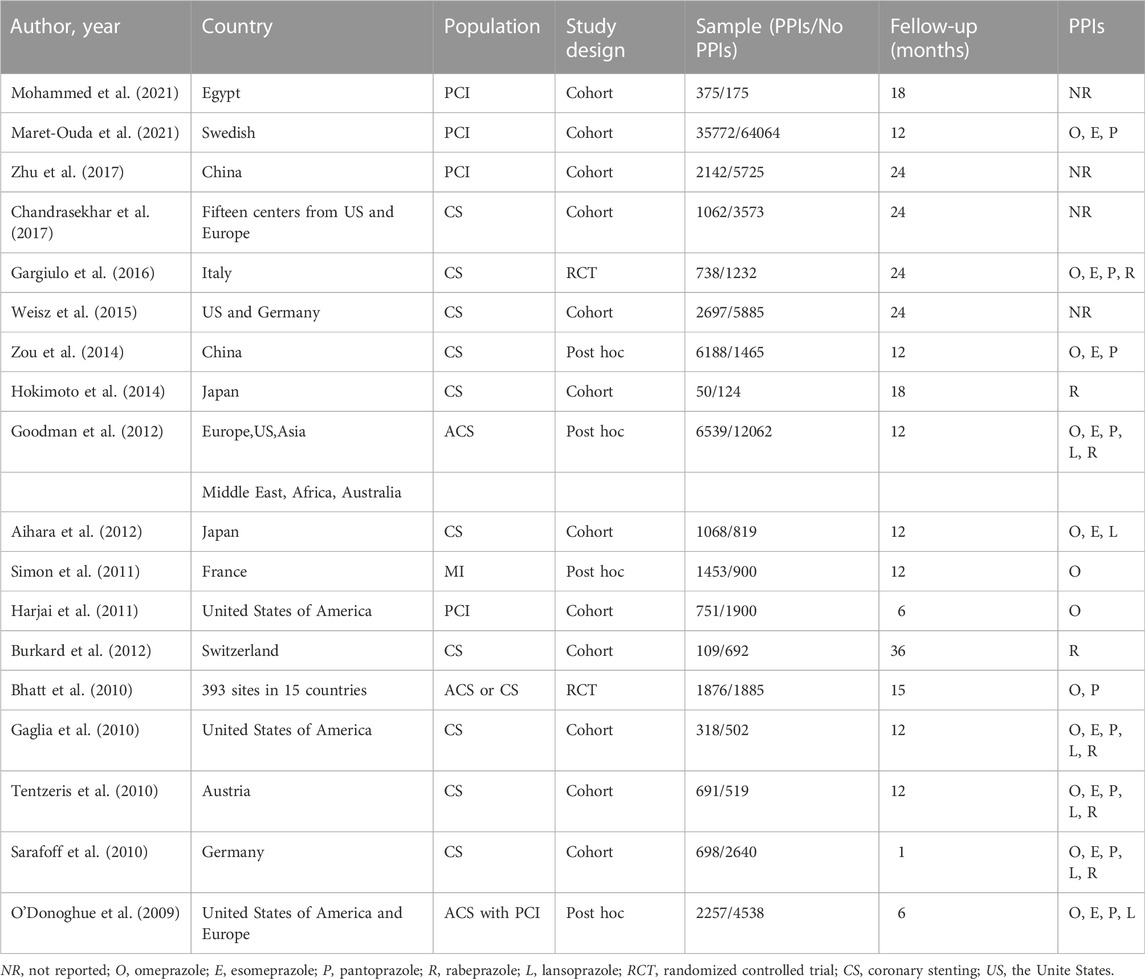
The flowchart of study selection is shown in Figure 1. There were 7,534 studies identified in the preliminary electronic database search. After tiered screening, a total of 18 articles were selected for the quantitative analysis, including 2 RCTs (Bhatt et al., 2010; Gargiulo et al., 2016), 3 post hoc analyses of RCTs (Goodman, 2012; O'Donoghue, 2009; Simon, 2011), and 13 cohort studies (Aihara, 2012; Burkard, 2012; Chandrasekhar, 2017; Gaglia, 2010; Harjai, 2011; Hokimoto, 2014; Maret-Ouda, 2021; Mohammed, 2021; Sarafoff, 2010; Tentzeris, 2010; Weisz, 2015; Zhu, 2017; Zou, 2014). PPIs were used by 64,784 of the 173,508 patients (37.34%), and 108,700 patients did not use PPIs. Table 1 presents the main characteristics of the included studies, and the results of the quality evaluation are listed in Supplementary Tables S1, S2.
3.2 Quantitative synthesis
3.2.1 The primary outcome
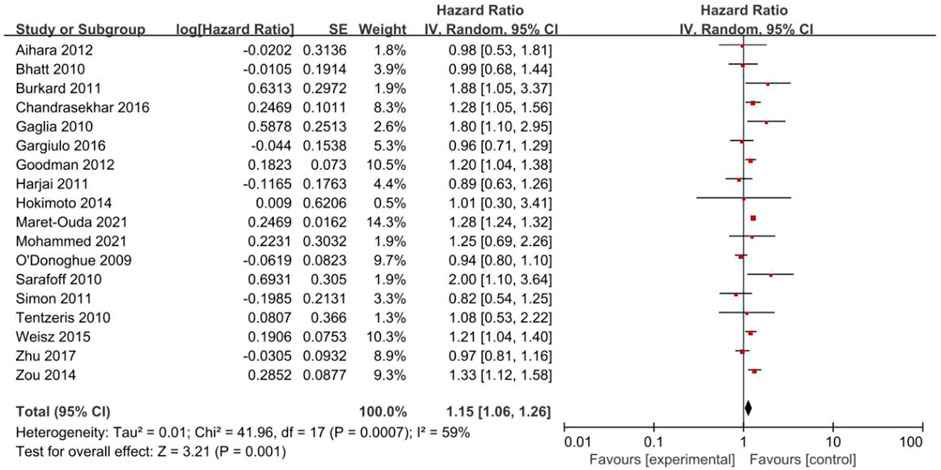
Eighteen studies reported MACEs (Figure 2). The results indicated that PPIs significantly increased the occurrence of MACEs (HR = 1.15, 95% CI = 1.06–1.26; p = .001) with a random-effect model (P = .0007, I2 = 59%).
Subgroup analyses of PPI subclasses, populations, follow-up times and study types were performed (Table 2).
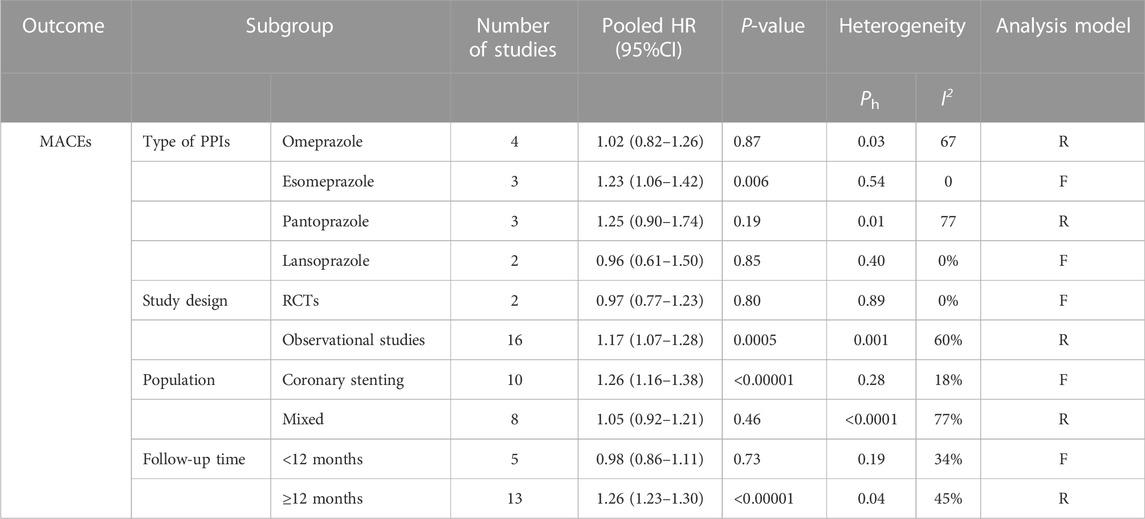
With regard to the types of PPIs, the use of esomeprazole (HR = 1.23, 95% CI = 1.06–1.42; p = .006) was associated with a significant increase in the risk of MACEs but not omeprazole (HR = 1.02, 95% CI = 0.82–1.26; p = .87), pantoprazole (HR = 1.25, 95% CI = 0.90–1.74; p = .19), or lansoprazole (HR = 0.96, 95% CI = 0.61–1.50; p = .85). Only one study reported MACEs in patients treated with rabeprazole; therefore, subgroup analysis was not possible for rabeprazole users.
In the stratification analyses by population, we found that the occurrence of MACEs was higher in the coronary stenting group administered PPIs (HR = 1.26, 95% CI = 1.16–1.38; p < .00001) but not in the mixed group (HR = 1.05, 95% CI = 0.92–1.21; p = .46).
When stratified by length of follow-up, there was no significant difference in the incidences of MACEs between PPI users and non-PPI users in the short-term follow-up (<12 months) group (HR = 0.98, 95% CI = 0.86–1.11; p = .73). However, in the long-term follow-up (≥12 months) group, the occurrence of MACEs was higher in the patients administered PPIs (HR = 1.26, 95% CI = 1.23–1.30; p < .00001).
Subgroup analysis of observational studies (HR = 1.17, 95% CI = 1.07–1.28; p = .0005) showed that PPIs increased the occurrence of MACEs, while analysis of the RCTs (HR = 0.97, 95% CI = 0.77–1.23; p = .80) did not demonstrate statistical significance.
3.2.2 The secondary outcomes
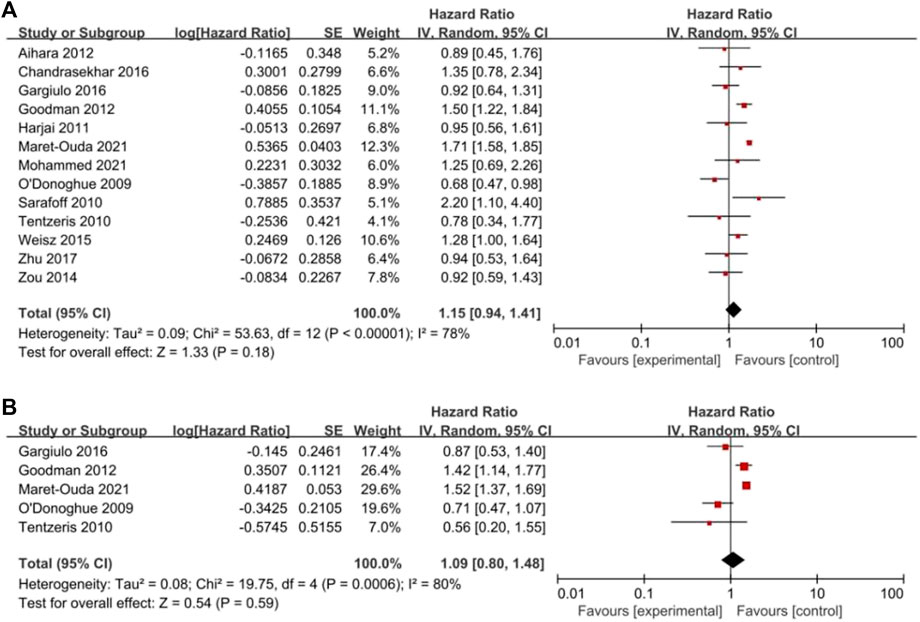
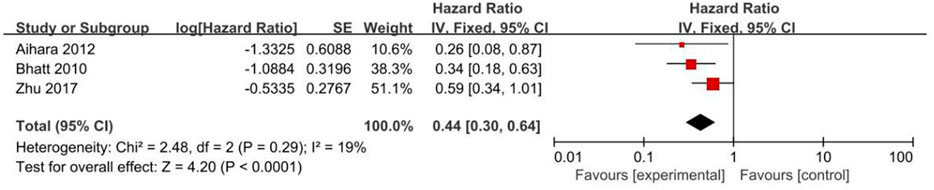
All clinical outcomes are shown in Table 3. PPIs were associated with a significant increase in the risk of MI (HR = 1.18, 95% CI = 1.11–1.24; p < .00001, I2 = 18%, Figure 3B), stroke (HR = 1.18, 95% CI = 1.03–1.35; p = .02, I2 = 28%, Figure 3C), revascularization (HR = 1.17, 95% CI = 1.06–1.30; p = .02, I2 = 31%, Figure 3D), and ST (HR = 1.21, 95% CI = 1.03–1.42; p = .02, I2 = 0%, Figure 3E) but not NACEs (HR = 1.02, 95% CI = 0.93–1.13; p = .67, I2 = 45%, Figure 3A), all-cause mortality (HR = 1.15, 95% CI = 0.94–1.41; p = .18, I2 = 78%, Figure 4A) or cardiac death (HR = 1.09, 95% CI = 0.80–1.48; p = .59, I2 = 80%, Figure 4B). However, PPIs significantly reduced the risk of GI complications (HR = 0.44, 95% CI = 0.30–0.64; p < .0001, I2 = 19%, Figure 5).
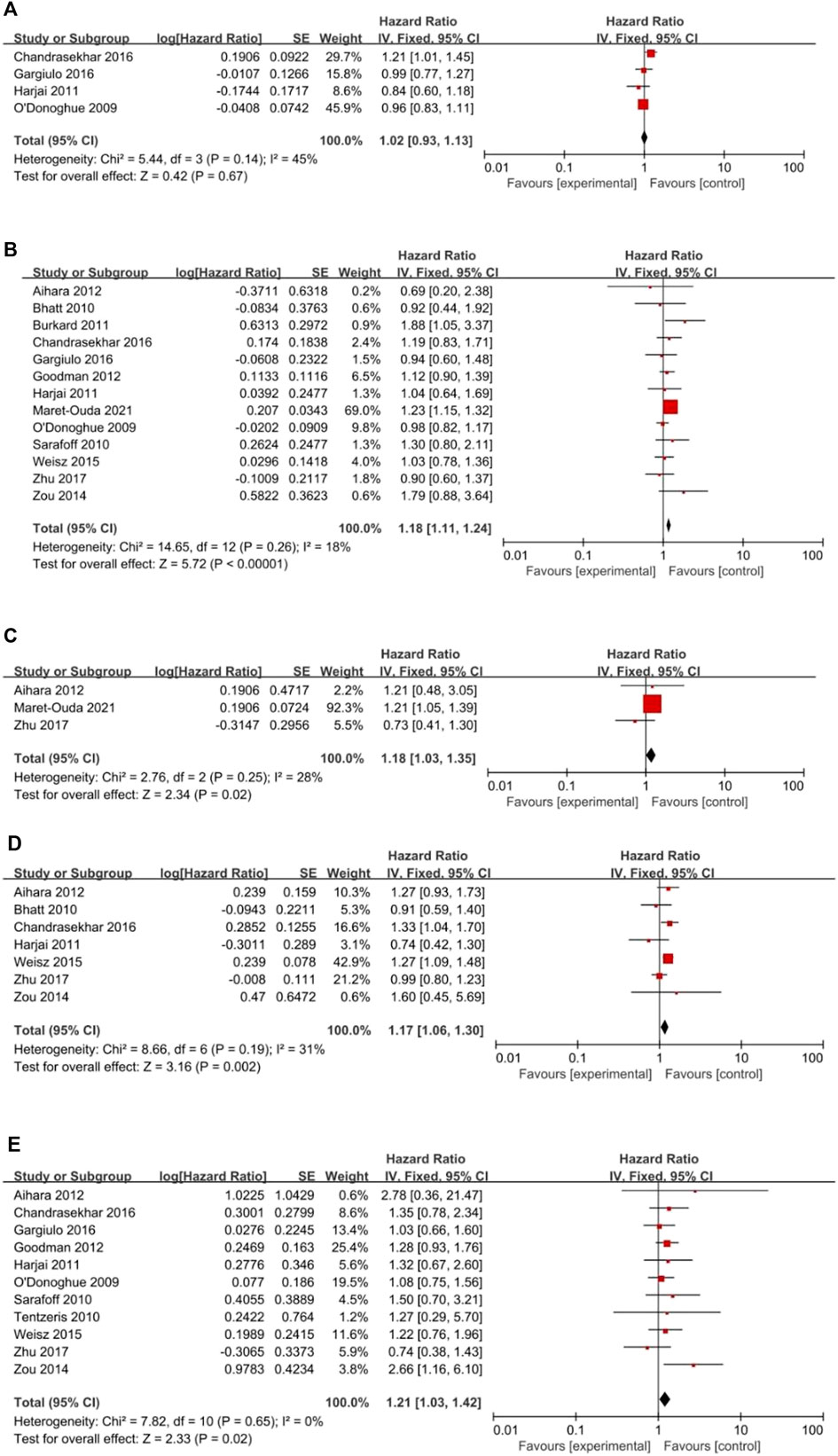
FIGURE 3. (Continued). Forest plots of (A) NACEs, (B) MI, (C) Stroke, (D) Revascularization and (E) Stent thrombosis.
3.3 Publication bias
A funnel plot was drawn for the primary outcome, and it showed symmetry on visual inspection, indicating that publication bias was not large (Supplementary Figure S1).
3.4 Sensitivity analysis
No single study markedly altered the overall effect in the sensitivity analysis, suggesting that the pooled HR of MACEs was stable.
4 Discussion
This systematic review and meta-analysis evaluated the efficacy and safety of the concomitant use of PPIs with aspirin-clopidogrel DAPT in CHD. The results showed that PPI coadministration decreased the risk of GI complications but could increase the rates of MACEs, stroke, revascularization and ST, in line with previous studies (Melloni et al., 2015; Hu et al., 2018). There were also no significant differences in the risks of all-cause mortality and cardiac death (Hu et al., 2018; Li et al., 2021; Melloni et al., 2015).
Interestingly, the incidence of MI in the PPI group was significantly increased in our study, inconsistent with previous meta-analysis results (Hu et al., 2018; Li et al., 2021). Several reasons account for this outcome. On the one hand, PPIs could decrease the effects of aspirin and clopidogrel on platelet aggregation (Zuern et al., 2010). On the other hand, it has been reported that PPIs could augment cardiovascular risk via platelet-independent biological pathways. One suggested mechanism is that PPIs inhibit the enzyme activity of dimethylarginine dimethylaminohydrolase (DDAH), thereby blocking the degradation of endothelial asymmetrical dimethylarginine (ADMA), an endogenous and competitive inhibitor of nitric oxide synthase. Excess ADMA in turn leads to impaired endothelial nitric oxide (NO) generation and reduced vascular function (Ghebremariam et al., 2013; Nolde et al., 2021; Zhai et al., 2022). In addition, a study investigating the long-term effect of PPIs on endothelial dysfunction found that chronic exposure to PPIs could expedite endothelial aging, which might explain the increased cardiovascular events (Yepuri et al., 2016). Therefore, the benefits for GI should be weighed against the recurrent ischemic cardiovascular events. Moreover, we found that there was no significant difference in the risk of NACEs between the two groups, although the risk was higher in the PPI group than in the non-PPI group.
Most interestingly, the elevated risk of MACEs for PPI users might be affected by the PPI subtype and population. In vitro studies suggested that different types of PPIs could affect CYP2C19 differently. Based on drug-drug interaction studies, the clinically relevant interaction tendency was the greatest for omeprazole and esomeprazole, with a moderate probability for lansoprazole and the lowest for pantoprazole and rabeprazole (Li et al., 2004; Norgard et al., 2009; Valgimigli et al., 2018). These results prompted our subgroup analyses of PPI subclasses. In agreement with the findings by Sherwood (Sherwood et al., 2015), the use of esomeprazole was associated with an increased risk of MACEs. Physicians should consider the potential risks with different PPIs when prescribing them for individual patients taking aspirin and clopidogrel. Because PCI with stent implantation is the most common interventional treatment for patients with coronary disease, we further performed stratification analyses of the population. In patients following coronary stenting, the occurrence of MACEs was higher in PPI users than in non-PPI users, which could have been driven by the significantly increased risk of ST (Zou et al., 2014).
In addition, we found that the length of follow-up time was quite different among our included studies, with a certain impact on the evaluation of MACEs (Harjai et al., 2011; Maret-Ouda et al., 2021). No previous meta-analyses were performed to evaluate such a difference. The incidence of MACEs varied according to different follow-up times in our study. When the follow-up periods were shorter than 12 months, there was no significant difference between the two groups, while the incidence of MACEs in the PPI group was significantly higher than that in the non-PPI group with longer follow-up (≥12 months). Consequently, long-term follow-up seems to be necessary for cardiovascular event investigations. Furthermore, the results were also inconsistent in different types of studies. The data from observational studies revealed that the use of PPIs increased the risk of MACEs, while the limited data from RCTs showed no significant difference.
There were several limitations to this study. First, most of our included articles were observational studies, and selection bias, along with unmeasured confounding, could account for these findings. Although we extracted the adjusted HRs, our results might still be biased by residual confounding. Second, a small number of RCTs (2 eligible for meta-analysis) were included, and the sample size of some subgroups might have been too small to indicate statistical significance and limit the representativeness of the results, again prompting more RCTs to assess the clinically relevant interactions. Third, we excluded many studies due to the inability to extract data, resulting in some bias. Fourth, subgroup analysis was conducted according to different PPI subtypes, populations, follow-up times and study types to analyze the heterogeneity in our study; however, clinical details, including the duration of DAPT and PPIs, type of stent, CYP2C19 genotypes, and concomitant diseases (such as diabetes), were insufficient in some articles, also potentially leading to heterogeneity among studies. Moreover, the included literature did not stratify the participants by the risk of cardiovascular events, and GI bleeding limited the evaluation of clinical outcomes. Thus, further studies regarding the efficacy and safety of concomitant use of PPIs with aspirin-clopidogrel DAPT should consider these limitations.
5 Conclusion
The concomitant use of PPIs was associated with a reduced risk of GI complications, while it could increase the rates of MACEs (particularly in patients receiving esomeprazole or with coronary stenting), MI, stroke, revascularization, and ST in CHD patients receiving aspirin-clopidogrel DAPT. There was no clear evidence of associations between PPI use and NACEs, all-cause mortality, or cardiac death. These results could have been affected by the follow-up time and study type. In light of the limitations of the current systematic review and meta-analysis, large-scale RCTs with longer-term follow-up are warranted to evaluate the safety and efficacy of PPIs with DAPT.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
XL, HX, XY and PZ contributed to the conception or design of the study. XL and SH contributed to the study selection, data extraction, quality assessment and statistical analysis. XY and YZ contributed to data acquisition and data interpretation. XL, MH and HX drafted the manuscript or substantively revised it. All the authors have final approval of the submitted manuscript and reached agreement to be accountable for all aspects of the work.
Funding
This work was supported in part by grants from the Training Program of Clinical Medical Researcher of Army Medical University (NO. 2018XLC3072). This project was also supported by the grants from the Humanities and Social Sciences Foundation of Army Medical University (NO. 2019XRW24) and Chongqing Clinical Pharmacy Key Specialties Construction Project.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.1021584/full#supplementary-material
References
Aihara, H., Sato, A., Takeyasu, N., Nishina, H., Hoshi, T., Akiyama, D., et al. (2012). Effect of individual proton pump inhibitors on cardiovascular events in patients treated with clopidogrel following coronary stenting: Results from the ibaraki cardiac assessment study registry. Catheter Cardiovasc Interv. 80 (4), 556–563. doi:10.1002/ccd.23327
Ben Ghezala, I., Luu, M., and Bardou, M. (2022). An update on drug-drug interactions associated with proton pump inhibitors. Expert Opin. Drug Metab. Toxicol. 18, 337–346. doi:10.1080/17425255.2022.2098107
Benavente, O. R., Hart, R. G., McClure, L. A., Szychowski, J. M., Coffey, C. S., Pearce, L. A., et al. (2012). Effects of clopidogrel added to aspirin in patients with recent lacunar stroke. N. Engl. J. Med. 367 (9), 817–825. doi:10.1056/NEJMoa1204133
Bhatt, D. L., Cryer, B. L., Contant, C. F., Cohen, M., Lanas, A., Schnitzer, T. J., et al. (2010). Clopidogrel with or without omeprazole in coronary artery disease. N. Engl. J. Med. 363 (20), 1909–1917. doi:10.1056/NEJMoa1007964
Burkard, T., Kaiser, C. A., Brunner-La Rocca, H., Osswald, S., Pfisterer, M. E., Jeger, R. V., et al. (2012). Combined clopidogrel and proton pump inhibitor therapy is associated with higher cardiovascular event rates after percutaneous coronary intervention: A report from the BASKET trial. J. Intern Med. 271 (3), 257–263. doi:10.1111/j.1365-2796.2011.02423.x
Capodanno, D., Alfonso, F., Levine, G. N., Valgimigli, M., and Angiolillo, D. J. (2018). ACC/AHA versus ESC guidelines on dual antiplatelet therapy: JACC guideline comparison. J. Am. Coll. Cardiol. 72, 2915–2931. doi:10.1016/j.jacc.2018.09.057
Chandrasekhar, J., Bansilal, S., Baber, U., Sartori, S., Aquino, M., Farhan, S., et al. (2017). Impact of proton pump inhibitors and dual antiplatelet therapy cessation on outcomes following percutaneous coronary intervention: Results from the PARIS Registry. Catheter Cardiovasc Interv. 89 (7), E217–e225. doi:10.1002/ccd.26716
Diener, H. C., Bogousslavsky, J., Brass, L. M., Cimminiello, C., Csiba, L., Kaste, M., et al. (2004). Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high-risk patients (MATCH): Randomised, double-blind, placebo-controlled trial. Lancet 364 (9431), 331–337. doi:10.1016/s0140-6736(04)16721-4
Furuta, T., Sugimoto, M., Kodaira, C., Nishino, M., Yamade, M., Uotani, T., et al. (2017). Influence of low-dose proton pump inhibitors administered concomitantly or separately on the anti-platelet function of clopidogrel. J. Thromb. Thrombolysis 43 (3), 333–342. doi:10.1007/s11239-016-1460-2
Gaglia, M. A., Torguson, R., Hanna, N., Gonzalez, M. A., Collins, S. D., Syed, A. I., et al. (2010). Relation of proton pump inhibitor use after percutaneous coronary intervention with drug-eluting stents to outcomes. Am. J. Cardiol. 105 (6), 833–838. doi:10.1016/j.amjcard.2009.10.063
Gargiulo, G., Costa, F., Ariotti, S., Biscaglia, S., Campo, G., Esposito, G., et al. (2016). Impact of proton pump inhibitors on clinical outcomes in patients treated with a 6- or 24-month dual-antiplatelet therapy duration: Insights from the PROlonging Dual-antiplatelet treatment after Grading stent-induced Intimal hyperplasia studY trial. Am. Heart J. 174, 95–102. doi:10.1016/j.ahj.2016.01.015
Gesheff, M. G., Franzese, C. J., Bliden, K. P., Contino, C. J., Rafeedheen, R., Tantry, U. S., et al. (2014). Review of pharmacokinetic and pharmacodynamic modeling and safety of proton pump inhibitors and aspirin. Expert Rev. Clin. Pharmacol. 7 (5), 645–653. doi:10.1586/17512433.2014.945428
Ghebremariam, Y. T., LePendu, P., Lee, J. C., Erlanson, D. A., Slaviero, A., Shah, N. H., et al. (2013). Unexpected effect of proton pump inhibitors: Elevation of the cardiovascular risk factor asymmetric dimethylarginine. Circulation 128 (8), 845–853. doi:10.1161/circulationaha.113.003602
Goodman, S. G., Clare, R., Pieper, K. S., Nicolau, J. C., Storey, R. F., Cantor, W. J., et al. (2012). Association of proton pump inhibitor use on cardiovascular outcomes with clopidogrel and ticagrelor: Insights from the platelet inhibition and patient outcomes trial. Circulation 125 (8), 978–986. doi:10.1161/circulationaha.111.032912
Guo, H., Ye, Z., and Huang, R. (2021). Clinical outcomes of concomitant use of proton pump inhibitors and dual antiplatelet therapy: A systematic review and meta-analysis. Front. Pharmacol. 12, 694698. doi:10.3389/fphar.2021.694698
Harjai, K. J., Shenoy, C., Orshaw, P., Usmani, S., Boura, J., and Mehta, R. H. (2011). Clinical outcomes in patients with the concomitant use of clopidogrel and proton pump inhibitors after percutaneous coronary intervention: An analysis from the guthrie health off-label stent (GHOST) investigators. Circ. Cardiovasc Interv. 4 (2), 162–170. doi:10.1161/circinterventions.110.958884
Hokimoto, S., Mizobe, M., Akasaka, T., Arima, Y., Kaikita, K., Nakagawa, K., et al. (2014). Impact of CYP2C19 polymorphism and proton pump inhibitors on platelet reactivity to clopidogrel and clinical outcomes following stent implantation. Thromb. Res. 133 (4), 599–605. doi:10.1016/j.thromres.2014.01.003
Hu, W., Tong, J., Kuang, X., Chen, W., and Liu, Z. (2018). Influence of proton pump inhibitors on clinical outcomes in coronary heart disease patients receiving aspirin and clopidogrel: A meta-analysis. Med. Baltim. 97 (3), e9638. doi:10.1097/md.0000000000009638
Levine, G. N., Bates, E. R., Bittl, J. A., Brindis, R. G., Fihn, S. D., Fleisher, L. A., et al. (2016). 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: A report of the American college of cardiology/American heart association task force on clinical practice guidelines: An update of the 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention, 2011 ACCF/AHA guideline for coronary artery bypass graft Surgery, 2012 ACC/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease, 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction, 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes, and 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac Surgery. Circulation 134 (10), e123–e155. doi:10.1161/CIR.0000000000000404
Li, X. Q., Andersson, T. B., Ahlström, M., and Weidolf, L. (2004). Comparison of inhibitory effects of the proton pump-inhibiting drugs omeprazole, esomeprazole, lansoprazole, pantoprazole, and rabeprazole on human cytochrome P450 activities. Drug Metab. Dispos. 32 (8), 821–827. doi:10.1124/dmd.32.8.821
Li, Y., Ren, X., and Fang, Z. (2021). Systematic review and meta-analysis: The effects of prophylactic proton pump inhibitor treatment in patients with coronary heart disease receiving dual antiplatelet therapy. J. Cardiovasc Pharmacol. 77 (6), 835–861. doi:10.1097/fjc.0000000000001014
Lué, A., and Lanas, A. (2016). Protons pump inhibitor treatment and lower gastrointestinal bleeding: Balancing risks and benefits. World J. Gastroenterol. 22 (48), 10477–10481. doi:10.3748/wjg.v22.i48.10477
Luo, J. C., Peng, Y. L., Chen, T. S., Huo, T. I., Hou, M. C., Huang, H. C., et al. (2016). Clopidogrel inhibits angiogenesis of gastric ulcer healing via downregulation of vascular endothelial growth factor receptor 2. J. Formos. Med. Assoc. 115 (9), 764–772. doi:10.1016/j.jfma.2015.07.022
Maret-Ouda, J., Santoni, G., Xie, S., Rosengren, A., and Lagergren, J. (2021). Proton pump inhibitor and clopidogrel use after percutaneous coronary intervention and risk of major cardiovascular events. Cardiovasc Drugs Ther. 36, 1121–1128. doi:10.1007/s10557-021-07219-6
Marlicz, W., Loniewski, I., and Koulaouzidis, G. (2022). Proton pump inhibitors, dual antiplatelet therapy, and the risk of gastrointestinal bleeding. Mayo Clin. Proc. 97 (4), 648–651. doi:10.1016/j.mayocp.2022.02.023
Melloni, C., Washam, J. B., Jones, W. S., Halim, S. A., Hasselblad, V., Mayer, S. B., et al. (2015). Conflicting results between randomized trials and observational studies on the impact of proton pump inhibitors on cardiovascular events when coadministered with dual antiplatelet therapy: Systematic review. Circ. Cardiovasc Qual. Outcomes 8 (1), 47–55. doi:10.1161/CIRCOUTCOMES.114.001177
Mohammed, S., Arabi, A., Bensmail, N., Moursi, A., Sharabash, M., El-Menyar, A., et al. (2021). TCTAP A-033 the impact of concomitant use of proton pump inhibitors and clopidogrel on patient mortality and outcome post angioplasty. J. Am. Coll. Cardiol. 77 (14), S20–S21. doi:10.1016/j.jacc.2021.03.061
Nishida, U., Kato, M., Nishida, M., Kamada, G., Ono, S., Shimizu, Y., et al. (2011). Evaluation of gastrointestinal injury and blood flow of small bowel during low-dose aspirin administration. J. Clin. Biochem. Nutr. 48 (3), 245–250. doi:10.3164/jcbn.10-112
Nolde, M., Bahls, M., Friedrich, N., Dörr, M., Dreischulte, T., Felix, S. B., et al. (2021). Association of proton pump inhibitor use with endothelial function and metabolites of the nitric oxide pathway: A cross-sectional study. Pharmacotherapy 41 (2), 198–204. doi:10.1002/phar.2504
Norgard, N. B., Mathews, K. D., and Wall, G. C. (2009). Drug-drug interaction between clopidogrel and the proton pump inhibitors. Ann. Pharmacother. 43 (7), 1266–1274. doi:10.1345/aph.1M051
O'Donoghue, M. L., Braunwald, E., Antman, E. M., Murphy, S. A., Bates, E. R., Rozenman, Y., et al. (2009). Pharmacodynamic effect and clinical efficacy of clopidogrel and prasugrel with or without a proton-pump inhibitor: An analysis of two randomised trials. Lancet 374 (9694), 989–997. doi:10.1016/s0140-6736(09)61525-7
Sarafoff, N., Sibbing, D., Sonntag, U., Ellert, J., Schulz, S., Byrne, R. A., et al. (2010). Risk of drug-eluting stent thrombosis in patients receiving proton pump inhibitors. Thromb. Haemost. 104 (3), 626–632. doi:10.1160/th09-11-0800
Sherwood, M. W., Melloni, C., Jones, W. S., Washam, J. B., Hasselblad, V., and Dolor, R. J. (2015). Individual proton pump inhibitors and outcomes in patients with coronary artery disease on dual antiplatelet therapy: A systematic review. J. Am. Heart Assoc. 4 (11), e002245. doi:10.1161/jaha.115.002245
Simon, T., Steg, P. G., Gilard, M., Blanchard, D., Bonello, L., Hanssen, M., et al. (2011). Clinical events as a function of proton pump inhibitor use, clopidogrel use, and cytochrome P450 2C19 genotype in a large nationwide cohort of acute myocardial infarction: Results from the French registry of acute ST-elevation and non-ST-elevation myocardial infarction (FAST-MI) registry. Circulation 123 (5), 474–482. doi:10.1161/circulationaha.110.965640
Spruance, S. L., Reid, J. E., Grace, M., and Samore, M. (2004). Hazard ratio in clinical trials. Antimicrob. Agents Chemother. 48 (8), 2787–2792. doi:10.1128/aac.48.8.2787-2792.2004
Tentzeris, I., Jarai, R., Farhan, S., Brozovic, I., Smetana, P., Geppert, A., et al. (2010). Impact of concomitant treatment with proton pump inhibitors and clopidogrel on clinical outcome in patients after coronary stent implantation. Thromb. Haemost. 104 (6), 1211–1218. doi:10.1160/th10-04-0218
Vaduganathan, M., and Bhatt, D. L. (2016). Aspirin and proton-pump inhibitors: Interpreting the interplay. Eur. Heart J. Cardiovasc Pharmacother. 2 (1), 20–22. doi:10.1093/ehjcvp/pvv038
Valgimigli, M., Bueno, H., Byrne, R. A., Collet, J. P., Costa, F., Jeppsson, A., et al. (2018). 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS. Eur. J. Cardiothorac. Surg. 53 (1), 34–78. doi:10.1093/ejcts/ezx334
Voutilainen, A., Brester, C., Kolehmainen, M., and Tuomainen, T. P. (2022). Epidemiological analysis of coronary heart disease and its main risk factors: Are their associations multiplicative, additive, or interactive? Ann. Med. 54 (1), 1500–1510. doi:10.1080/07853890.2022.2078875
Weisz, G., Smilowitz, N. R., Kirtane, A. J., Rinaldi, M. J., Parvataneni, R., Xu, K., et al. (2015). Proton pump inhibitors, platelet reactivity, and cardiovascular outcomes after drug-eluting stents in clopidogrel-treated patients: The ADAPT-DES study. Circ. Cardiovasc Interv. 8 (10), e001952. doi:10.1161/circinterventions.114.001952
Xie, Y., Bowe, B., Yan, Y., Xian, H., Li, T., and Al-Aly, Z. (2019). Estimates of all cause mortality and cause specific mortality associated with proton pump inhibitors among US veterans: Cohort study. Bmj 365, l1580. doi:10.1136/bmj.l1580
Yepuri, G., Sukhovershin, R., Nazari-Shafti, T. Z., Petrascheck, M., Ghebre, Y. T., and Cooke, J. P. (2016). Proton pump inhibitors accelerate endothelial senescence. Circ. Res. 118 (12), e36–e42. doi:10.1161/circresaha.116.308807
Zhai, Y., Ye, X., Hu, F., Xu, J., Guo, X., Lin, Z., et al. (2022). Updated insights on cardiac and vascular risks of proton pump inhibitors: A real-world pharmacovigilance study. Front. Cardiovasc Med. 9, 767987. doi:10.3389/fcvm.2022.767987
Zhou, M., Wang, H., Zeng, X., Yin, P., Zhu, J., Chen, W., et al. (2019). Mortality, morbidity, and risk factors in China and its provinces, 1990-2017: A systematic analysis for the global burden of disease study 2017. Lancet 394 (10204), 1145–1158. doi:10.1016/s0140-6736(19)30427-1
Zhu, P., Gao, Z., Tang, X. F., Xu, J. J., Zhang, Y., Gao, L. J., et al. (2017). Impact of proton-pump inhibitors on the pharmacodynamic effect and clinical outcomes in patients receiving dual antiplatelet therapy after percutaneous coronary intervention: A propensity Score analysis. Chin. Med. J. Engl. 130 (24), 2899–2905. doi:10.4103/0366-6999.220304
Zou, J. J., Chen, S. L., Tan, J., Lin, L., Zhao, Y. Y., Xu, H. M., et al. (2014). Increased risk for developing major adverse cardiovascular events in stented Chinese patients treated with dual antiplatelet therapy after concomitant use of the proton pump inhibitor. PLoS One 9 (1), e84985. doi:10.1371/journal.pone.0084985
Zuern, C. S., Geisler, T., Lutilsky, N., Winter, S., Schwab, M., and Gawaz, M. (2010). Effect of comedication with proton pump inhibitors (PPIs) on post-interventional residual platelet aggregation in patients undergoing coronary stenting treated by dual antiplatelet therapy. Thromb. Res. 125 (2), e51–e54. doi:10.1016/j.thromres.2009.08.016
Keywords: proton pump inhibitors, aspirin, clopidogrel, coronary heart disease, medication interaction, meta-analysis
Citation: Luo X, Hou M, He S, Yang X, Zhang P, Zhao Y and Xing H (2023) Efficacy and safety of concomitant use of proton pump inhibitors with aspirin-clopidogrel dual antiplatelet therapy in coronary heart disease: A systematic review and meta-analysis. Front. Pharmacol. 13:1021584. doi: 10.3389/fphar.2022.1021584
Received: 17 August 2022; Accepted: 16 December 2022;
Published: 10 January 2023.
Edited by:
Linan Zeng, McMaster University, CanadaReviewed by:
Naida Bulaeva, Bakulev Scientific Center for Cardiovascular Surgery Russian Academy of Medical Sciences, RussiaHelge Waldum, Norwegian University of Science and Technology, Norway
Copyright © 2023 Luo, Hou, He, Yang, Zhang, Zhao and Xing. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Haiyan Xing, haiyanxing@aliyun.com
 Xiaofeng Luo
Xiaofeng Luo Min Hou
Min Hou Shuangshuang He
Shuangshuang He