- 1School of Natural Sciences, Bangor University, Bangor Gwynedd, United Kingdom
- 2Faculty of Natural and Environmental Sciences, University of Southampton, Southampton, United Kingdom
Global forecasts for prevalence of Alzheimer’s Disease (AD) estimate that 152.8 million people will have dementia in 2050, a sharp rise from 57.4 million in 2019 (GBD 2019). This rise can be attributable to increases in population growth and aging, but in the absence of disease-modifying therapies it poses a huge societal challenge that must be addressed urgently. One way to combat this challenge is to explore the utility of holistic treatments that may protect against AD, including traditional herbs, spices and other nutraceuticals that are pharmacologically safe, inexpensive and readily available. In this light, the spice turmeric, and its active ingredient curcumin, has been investigated as a potential holistic treatment for AD over the past 2 decades; however, promising results with animal studies have not translated to success in clinical trials. One issue is that most animal models examining the effects of curcumin and curcumin derivatives in AD have been done with a focus at ameliorating amyloid pathology. Due to the limited success of Amyloid-β-based drugs in recent clinical trials, tau-focused therapeutics provide a promising alternative. In this article, we aim to provide a clearer picture of what is currently known about the effectiveness of curcumin and curcumin derivatives to ameliorate tau pathology. Tau focused studies may help inform more successful clinical studies by placing greater emphasis on the development and optimised delivery of curcumin derivatives that more effectively target tau pathology.
Introduction
Alzheimer’s disease (AD) is the most common form of dementia, characterised clinically by progressive cognitive decline, and neuropathologically by amyloid plaques and neurofibrillary tau tangles (NFT). Global forecasts for prevalence of AD estimate that 152.8 million people will have dementia in 2050, a sharp rise from 57.4 million in 2019 (GBD 2019). Although this rise can be attributable to increases in population growth and aging, in the absence of disease-modifying therapies it poses a huge societal challenge we must urgently address. One approach is to implement public health measures that scale up modifiable lifestyle factors known to influence risk of AD (GBD 2019) (Livingston et al., 2020). Another is to explore the utility of holistic treatments that may protect against AD, including traditional herbs, spices and other nutraceuticals that are pharmacologically safe, inexpensive and readily available. The case for repurposing such nutraceuticals for dementia treatment first arose from the observation that the prevalence of dementia in countries like India, where such spices are regularly consumed, is remarkably low when compared to high income countries where these spices are not common (Kannappan et al., 2011). Although other factors, including population ageing differences, have since been put forward to explain at least some of this differential dementia prevalence, there have been significant efforts to assess the disease-modifying potential of nutraceuticals in cellular and animal models of AD. Such research reveals that common Indian herbs and spices such as turmeric, garlic, ginger, black pepper, coriander and others, contain phytochemicals that display a range of neuroprotective properties (Kannappan et al., 2011). They have been shown to be antioxidant, anti-inflammatory, anti-amyloidogenic and also to upregulate protective cellular responses like heat shock responses (Forouzanfar et al., 2019). As misfolding and aggregation of tau and amyloid proteins, oxidative stress and aberrant inflammation are all implicated in AD, it is not surprising that these spice-derived nutraceuticals have shown some disease-modifying potential in AD models. This review summarises the research done to investigate the protective effects of the spice, curcumin, against tau pathology in AD.
Curcumin and Alzheimer’s Disease
The medicinal properties of the nutraceutical, turmeric, have been known for centuries, prompting its widespread use in holistic traditional medicines in India and China. It is derived from the rhizome of Curcuma longa, a member of the ginger family indigenous to South and Southeast Asia. The active ingredient in turmeric is the phytochemical, curcumin ((1E, 6E)-1, 7-bis (4-hydroxy-3-methoxyphenyl)-1, 6-heptadiene-3, 5-dione). As a key ingredient in Asian cooking, turmeric was first considered for prevention or treatment of AD because of reports that prevalence of AD in India was significantly lower than in the West where this spice is not routinely consumed (Kannappan et al., 2011). Although more recent studies have begun to show that several other lifestyle related factors modulate AD risk, and that prevalence may be increasing in Asian countries and decreasing in the West (Livingston et al., 2020), these original observations prompted research into the disease-modifying potential of turmeric and its active ingredient, curcumin. Further, as evidence mounts for the importance of diet on wellbeing and disease prevention, curcumin becomes a more attractive medicinal agent providing a cost-effective, easily accessible solution to promote resilience to dementia. This will have even greater significance for developing countries.
Curcumin has a wide variety of neuroprotective properties: it inhibits nuclear factor kB-mediated transcription of inflammatory cytokines (Singh and Aggarwal 1995), it behaves as an anti-oxidant by scavenging reactive oxygen species (ROS) (Scapagnini et al., 2010)and neutralising NO-based free radicals (Sreejayan and Rao 1997), it is capable of disrupting amyloids displaying anti-amyloidogenic and anti-fibrillogenic behaviour (Garcia-Alloza et al., 2007), and interacts with homeostatic pathways such as the unfolded protein response (UPR) and chaperones like Hsp70 to facilitate correct protein folding (Mukherjee et al., 2021). Curcumin has also been shown to play a protective role in aging and age-related disorders including atherosclerosis, diabetes and cancer (Olszanecki et al., 2005; He et al., 2015). One striking observation is the ability of dietary curcumin to extend life span in fly, nematode and mouse models (Suckow and Suckow 2006; Kitani et al., 2007; Lee et al., 2010; Liao et al., 2011). Therefore, it is not surprising that curcumin and its derivatives have been found to suppress phenotypes in multiple models of neurodegenerative disease in which aging is a substantive risk factor, including Huntington’s Disease (Chongtham and Agrawal 2016; Labanca et al., 2021), Parkinson’s Disease (Chetty et al., 2021; Nebrisi 2021)), and AD (Reddy et al., 2018; Chainoglou and Hadjipavlou-Litina 2020). The observation that curcumin treatment leads to suppression of phenotypes caused by a wide range of conditions, from aging-related to several different misfolded proteins characterising distinct proteinopathies, suggests that its disease-modifying actions may be affected through key overlapping mechanisms such as oxidative stress and neuroinflammation.
The Status Quo
In the absence of a cure for AD, there are four drugs in general use for the symptomatic treatment of AD. Three of these drugs—donepezil, galantamine and rivastigmine—are acetylcholinesterase (AChE) inhibitors, developed on the cholinergic hypothesis which proposed AD was due to preferential loss of cholinergic neurons (Bartus 2000). The fourth drug, memantine, is a N-methyl-D-aspartate (NMDA) receptor antagonist, which suppresses the neuronal excitotoxicity noted in AD caused by excess glutamate binding of NMDA receptors (Reisberg et al., 2003). More recently, the Federal Drug Administration (USA) granted accelerated approval to the drug, aducanumab, which works by reducing amyloid deposits in the brain that may slow the progression of AD; however, it’s effectiveness in halting the progression of cognitive decline or dementia has yet to be clinically demonstrated.
Tau-directed treatments have also been investigated, and over the years a number of different tau-centric agents have entered clinical trials with limited success. (Panza et al., 2016). These include kinase inhibitors (Lovestone et al., 2015; Maqbool et al., 2016), phosphatase stimulators (Parekh et al., 2013), aggregation inhibitors like methylene blue (Wischik et al., 1996), tau vaccines (d'Abramo et al., 2015; Sankaranarayanan et al., 2015), and microtubule stabilising agents (Brunden et al., 2011; Quraishe et al., 2016). However, in the absence of approved tau-modifying treatments or in fact any disease modifying treatments, current medical treatments arguably provide small benefits at the cost of side effects and lack general accessibility. It is here that holistic medicines, such as curcumin, can play a key role.
To date, numerous animal studies have been conducted, mainly in rodent models, with promising results for the effectiveness of curcumin for therapeutic purposes (see comprehensive review of animal and clinical studies in Voulgaropoulou et al. (2019). Unfortunately, this has not translated to similar success in human clinical trials where study results are inconsistent, with some studies reporting a beneficial effect of curcumin on cognitive function (Cox et al., 2015; Rainey-Smith et al., 2016; Small et al., 2018) and others reporting none (Baum et al., 2008; Ringman et al., 2012). Since so few clinical studies have actually been done, this may not be surprising. However, many factors may contribute to the observed discrepancy in translation with the first being issues with curcumin supplementation, which include low bioavailability of the compound, rapid metabolism in gut, and low penetration across the blood brain barrier (Anand et al., 2007). There are a number of ways in which these issues have been, and are currently being, addressed with the development of curcumin derivatives with greater bioavailability and optimisation of delivery methods, including isomerisation, liposomes, micelles, phospholipids, nanotechnology, vaporising and intravenous injection.
Coupled with the above issue is that much of the work examining the effects of curcumin in AD in animal models has been done with a focus at ameliorating amyloid pathology. However, the limited success of Amyloid-beta (Aβ)-based drugs in clinical trials suggests a shift in focus is needed. This is where tau-targeted therapeutics provide a promising alternative. Currently, few studies have directly examined the effectiveness of curcumin and curcumin derivatives in tauopathy models or to ameliorate tau pathology in animal and clinical studies. Here we provide a snapshot of this field with a view that tau-focused animal studies may help inform more successful clinical studies by emphasising development of curcumin derivatives and delivery mechanisms that more effectively target tau pathology.
Animal Models Examining Curcumin and Tau Pathology
Tauopathies are a class of over 20 degenerative disorders, including AD, marked by neuronal aggregation of abnormally phosphorylated forms of the protein tau, a microtubule associated protein (MAPT) essential for microtubule assembly and stability ((Grundke-Iqbal et al., 1986; Williams 2006; Spillantini and Goedert 2013). In pathological scenarios, there is evidence of both toxic loss of function (LOF) as well as gain of toxic functions (GOF) by pathogenic tau. Hyperphosphorylated tau detaches from microtubules and causes their destabilisation, consequently leading to disruption of cytoskeletal integrity, axonal transport and synaptic transmission (Mudher et al., 2004; Chee et al., 2005; Cowan et al., 2010; Hoover et al., 2010). Further, these abnormally hyperphosphorylated tau proteins aggregate to form higher order structures from soluble oligomers to paired helical filaments (PHFs) to insoluble neurofibrillary tangles (NFTs) ((Spillantini and Goedert 2013; von Bergen et al., 2000). While PHFs have historically been thought of as the main pathological species in tauopathies, more recent studies have suggested that it is their precursors, soluble tau oligomers, that are the toxic species spurring disease pathogenesis and progression through a variety of mechanisms including oxidative stress (Stamer et al., 2002; Cowan et al., 2021), mitochondrial dysfunction and transport (Ittner et al., 2008), nuclear dysfunction (Andorfer et al., 2005; Khurana et al., 2012), prion-like conversion and propagation of pathology (Holmes et al., 2014), inflammation (Nagele et al., 2004; Ismail et al., 2020), and induction of ER stress (Hoozemans et al., 2009) (Götz et al., 2019). Thus, finding ways to target toxic tau species is one solution for developing disease-modifying treatments that ameliorate or prevent these LOF and GOF pathogenic effects.
While the vast majority of animal studies with curcumin have been done in models of amyloid pathology, there are a handful that have examined the effects of curcumin in ameliorating tau pathology (Table 1, Figure 1). Using a nematode model of tauopathy, Miyasaka et al. (Miyasaka et al., 2016) expressed wild-type human tau isoform 0N4R (hTau0N4R) and hTau with the R406W (hTauR406W) mutation associated with frontotemporal lobe dementia (FTD). Worms were grown for 3 days on growth medium plates with or without curcumin (at concentrations of 0, 3, 30 uM) before collection of behavioural data. They found that htau-expressing worms grown on 30 uM of curcumin showed significant improvement in behavioural abnormalities (e.g., uncoordinated movement and touch sense). In addition, curcumin significantly decreased the number of morphological abnormalities (i.e., kinks and protrusions) noted in neurons expressing hTau. Interestingly, curcumin treatment had no effect on the phosphorylation of tau (AT100, AT8, AT180, pS262, PHF-1). However, further analysis found that curcumin significantly increased the levels of acetylated alpha-tubulin in worms. Since acetylation of alpha-tubulin occurs in association with microtubule stabilization, one possible mechanism for the therapeutic effects of curcumin is that it enhances microtubule stability.
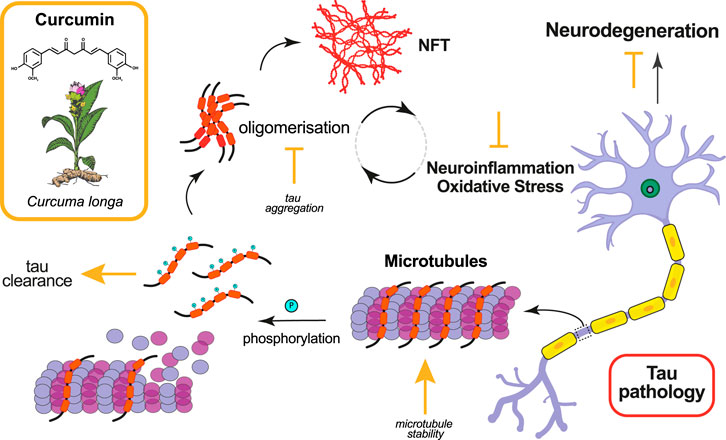
FIGURE 1. Effects of curcumin in ameliorating tau pathology. Curcumin and its derivatives have been shown to ameliorate tau pathology in animal models by targeting various molecular mechanisms. The mode of action of curcumin, whether promotive (arrow) or inhibitive (falsum), is indicated in yellow next to the process it modifies.
The more common animal model for evaluating curcumin on tau pathology has been the mouse. Ma et al. (Ma et al., 2013) used a transgenic mouse model expressing human wild-type Tau (hTau) on a mouse-Tau knockout background, where synaptic and cognitive deficits and NFTs are present at 11–12 months and neuron loss occurs by 17 months. The mice used in this 4-months study were 15–16 months old at study commencement and were placed on diets without or with 500 ppm Longvida© (Verdure Sciences, Indianapolis, IN) curcumin, solid lipid curcumin particles showing higher bioavailability of free curcumin in the brain compared to other curcumin formulations. This study found that curcumin reduced the level of soluble tau dimers, reversed the disruption noted in htau-expressing mice in expression of the molecular chaperones, HSP90, HSP70, and HSP72, and improved cognitive performance in some behavioural assays. More specifically, they found that curcumin treatment improved spatial learning and memory, as measured using a Morris Water Maze (MWM), when compared to untreated htau mice. Also, curcumin treated htau mice showed normalisation of recognition memory toward wildtype measures using the Novel Object Recognition test (NORT).
Another study also using Longvida© examined abnormal accumulation of NFT’s and Aβ in a p25 transgenic mouse model (Sundaram et al., 2017). The authors found that curcumin treatment significantly reduced p25-mediated tau hyperphosphorylation (AT8) and Aβ1-42 immunostaining in curcumin-treated p25Tg mice compared to their non-treated counterparts. One explanation for this reduction is the suppressed expression of neuroinflammatory cytokines. The authors found that the expression levels of the pro-inflammatory cytokines MIP-1α (macrophage inflammatory protein-1α), TNF-α (tumour necrosis factor-alpha), and IL-1β (Interleukin-1α) were significantly reduced in curcumin treated p25Tg mice. Further, behavioural studies using an 8-arm radial maze showed improved spatial and working memory with curcumin treatment.
Other labs have both developed and tested the effectiveness of curcumin derivatives in mouse models. Yanagisawa et al. (Yanagisawa et al., 2018) examined a novel curcumin derivative synthesised by the group called Shiga-Y5 (SY5), which they had previously found to be effective in inhibiting cognitive impairment and amyloid deposition in an AD mouse model (Yanagisawa et al., 2015). In this study, control and SY5-containing (500 ppm) chow diets were fed for 4 months to rTg4510 mice, a mouse tauopathy model expressing human four-repeat tau with P301L mutation in the forebrain at levels 13-fold higher than endogenous mouse tau (Ramsden et al., 2005). Behavioural tests were conducted from 5.5 months of age and mice sacrificed at 6 months. They found no significant differences in behavioural performance between rTg4510 mice fed SY5 or a control diet. Further, histological and biochemical analyses found no significant changes in tau accumulation following curcumin treatment. Given that SY5 was first designed as a fluorine-19 magnetic resonance imaging probe to detect amyloid deposition, it’s effectiveness against tau accumulation may not be optimal (Yanagisawa et al., 2011).
Okuda et al. (2017) designed, synthesized and evaluated the efficacy of the curcumin derivative, PE859, a dual inhibitor of tau and Aβ aggregation, in a senescence-accelerated mouse prone 8 (SAMP8) model. In this 9-weeks study, SAMP8 mice were given oral administrations via gastric tube of PE859 at three concentrations: 0, 1 mg/k/day, 3 mg/kg/day, from 2 to 4 months of age. They reported a significant improvement in cognitive deficits in spatial working memory (i.e., using Y-maze tests), but not for any other behavioural measure. They did however note that PE859 reduced the amount of aggregated tau and Aβ1-40 in SAMP8 mice. It is possible that a more robust behavioural response would have been noted had curcumin treatment been extended.
Clinical Studies Examining Curcumin and Tau Pathology
Conclusions on the efficacy of curcumin and its derivatives has been hindered by the limited number of clinical trials, and inconsistent reports on the effectiveness of these compounds in improving cognitive deficits (see Voulgaropoulou et al., 2019). The studies that have directly examined tau pathology in clinical studies have also reported mixed results. Ringman et al. (Ringman et al., 2012) evaluated the efficacy of the Curcumin 3 Complex® (Sabinsa Corporation, Piscataway, NJ), a curcuminoid mixture of curcumin, bisdemethoxycurcumin and demethoxycurcumin, in a population with mild to moderate AD. The compound was administered in three doses (placebo, 2 g/day and 4 g/day curcumin) for 24 weeks. However, Curcumin C3 Complex® did not improve cognitive deficits or reduce tau and Aβ levels in plasma and cerebrospinal fluid (CSF). In this study, low bioavailability of the compound was noted in plasma and patient dropout due to gastrointestinal complains was reported.
In contrast, Small et al. (Small et al., 2018) evaluated the effects of Theracurmin®, a form of curcumin with increased intestinal endothelium penetrability, and reported positive effects. In this study, non-demented adults between 51–84 years of age were given Theracurmin® or placebo twice a day for 18 months. The authors found that Theracurmin® improved verbal and visual memory and attention. Further, 2-(1-ethylidene) malononitrile positron emission tomography (FDDNP-PET) scans performed pre- and post-treatment found a reduction in tau and amyloid accumulation in the amygdala of the curcumin treated group compared to placebo. In the hypothalamus, the curcumin treated group showed no change, while the placebo group showed an increase. Together, this indicates that curcumin can reduce pathogenic protein accumulation in the brain. The success of this latter study may be due to 1) it’s length, indicating that extended curcumin usage may be key for improved cognitive function, and 2) it’s use of non-demented adults, suggesting a more preventative than curative role for curcumin in allaying cognitive decline in human populations. For a stronger grasp on curcumin’s effectiveness in treating AD, more clinical studies are necessary, especially in more severely affected populations.
Future Directions
The general success of curcumin and curcumin derivatives in ameliorating AD pathology in animal studies is encouraging. Although, there has been limited success in clinical trials this may simply be attributable to the small number of such studies having been attempted and published to date. Given that curcumin provides a cost-effective, easily accessible solution to promote resilience to dementia, and that such a holistic treatment for AD will have even greater significance for developing countries, there is an urgent need to level the gap between animal and clinical studies. First, more clinical trials are required, especially with a focus on understanding the ability of curcumin to ameliorate cognitive deficits in severely affected populations and on the benefits of extended curcumin treatment. Other avenues for improvement include finding ways to increase the bioavailability of curcumin in the brain and considering dose dependent effects of curcumin in clinical studies (Voulgaropoulou et al., 2019).
Yet another approach of placing greater emphasis on tau-focused development of curcumin derivatives and subsequent validation in animal models may provide a more fruitful strategy. For example, a number of in vitro studies have demonstrated the effectiveness of curcumin derivatives in attenuating the formation of toxic tau oligomers, including tau-targeting biomimetic nanoparticles that reduce phospho-tau levels (Gao et al., 2020) and sugar-curcumin conjugates that suppress tau aggregation more efficiently at lower concentrations than curcumin (Dolai et al., 2011). A study by Cascio et al. (Lo Cascio et al., 2019) screened a small library of novel curcumin derivatives against pre-formed tau oligomers and identified six compounds that interacted with toxic tau species to further promote aggregation. Interestingly, these larger tau structures had decreased toxicity in human neuroblastoma SH-SY5Y cell lines and cultured primary cortical neurons. A subsequent paper examining these compounds and disease-relevant brain-derived tau oligomers (BDTOs) isolated from brain tissues of different tauopathies reported similar results but identified compound CL3 as effective in the formation of larger, less toxic tau aggregates with decreased seeding propensity (Lo Cascio et al., 2020). A thorough validation in animal and clinical studies will reveal if any of these tau-directed curcumin derivatives are more effective in reducing cognitive deficits caused by AD. In summary, the work summarised here highlights a great potential for curcumin as a therapeutic treatment for AD. With a continued push towards development and testing of more effective curcumin derivatives, future studies may finally bridge the gap in translation.
Author Contributions
LS ands AM cowrote this manuscript.
Funding
This work was supported by the Salifu Dagarti Foundation, and by funding to LS from the Alzheimers Society United Kingdom [grant number 315 (AS-JF-16-004)].
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Anand, P., Kunnumakkara, A. B., Newman, R. A., and Aggarwal, B. B. (2007). Bioavailability of Curcumin: Problems and Promises. Mol. Pharm. 4 (6), 807–818. doi:10.1021/mp700113r
Andorfer, C., Acker, C. M., Kress, Y., Hof, P. R., Duff, K., and Davies, P. (2005). Cell-cycle Reentry and Cell Death in Transgenic Mice Expressing Nonmutant Human Tau Isoforms. J. Neurosci. 25 (22), 5446–5454. doi:10.1523/JNEUROSCI.4637-04.2005
Bartus, R. T. (2000). On Neurodegenerative Diseases, Models, and Treatment Strategies: Lessons Learned and Lessons Forgotten a Generation Following the Cholinergic Hypothesis. Exp. Neurol. 163 (2), 495–529. doi:10.1006/exnr.2000.7397
Baum, L., Lam, C. W., Cheung, S. K., Kwok, T., Lui, V., Tsoh, J., et al. (2008). Six-month Randomized, Placebo-Controlled, Double-Blind, Pilot Clinical Trial of Curcumin in Patients with Alzheimer Disease. J. Clin. Psychopharmacol. 28 (1), 110–113. doi:10.1097/jcp.0b013e318160862c
Brunden, K. R., Yao, Y., Potuzak, J. S., Ferrer, N. I., Ballatore, C., James, M. J., et al. (2011). The Characterization of Microtubule-Stabilizing Drugs as Possible Therapeutic Agents for Alzheimer's Disease and Related Tauopathies. Pharmacol. Res. 63 (4), 341–351. doi:10.1016/j.phrs.2010.12.002
Chainoglou, E., and Hadjipavlou-Litina, D. (2020). Curcumin in Health and Diseases: Alzheimer's Disease and Curcumin Analogues, Derivatives, and Hybrids. Int. J. Mol. Sci. 21 (6), 1975. doi:10.3390/ijms21061975
Chee, F. C., Mudher, A., Cuttle, M. F., Newman, T. A., MacKay, D., Lovestone, S., et al. (2005). Over-expression of Tau Results in Defective Synaptic Transmission in Drosophila Neuromuscular Junctions. Neurobiol. Dis. 20 (3), 918–928. doi:10.1016/j.nbd.2005.05.029
Chetty, D., Abrahams, S., van Coller, R., Carr, J., Kenyon, C., and Bardien, S. (2021). Movement of Prion-like α-synuclein along the Gut-Brain axis in Parkinson's Disease: A Potential Target of Curcumin Treatment. Eur. J. Neurosci. 54 (2), 4695–4711. doi:10.1111/ejn.15324
Chongtham, A., and Agrawal, N. (2016). Curcumin Modulates Cell Death and Is Protective in Huntington's Disease Model. Sci. Rep. 6, 18736. doi:10.1038/srep18736
Cowan, C. M., Bossing, T., Page, A., Shepherd, D., and Mudher, A. (2010). Soluble Hyper-Phosphorylated Tau Causes Microtubule Breakdown and Functionally Compromises Normal Tau In Vivo. Acta Neuropathol. 120 (5), 593–604. doi:10.1007/s00401-010-0716-8
Cowan, C. M., Sealey, M. A., and Mudher, A. (2021). Suppression of Tau-Induced Phenotypes by Vitamin E Demonstrates the Dissociation of Oxidative Stress and Phosphorylation in Mechanisms of Tau Toxicity. J. Neurochem. 157 (3), 684–694. doi:10.1111/jnc.15253
Cox, K. H., Pipingas, A., and Scholey, A. B. (2015). Investigation of the Effects of Solid Lipid Curcumin on Cognition and Mood in a Healthy Older Population. J. Psychopharmacol. 29 (5), 642–651. doi:10.1177/0269881114552744
d'Abramo, C., Acker, C. M., Jimenez, H., and Davies, P. (2015). Passive Immunization in JNPL3 Transgenic Mice Using an Array of Phospho-Tau Specific Antibodies. PLoS One 10 (8), e0135774. doi:10.1371/journal.pone.0135774
Dolai, S., Shi, W., Corbo, C., Sun, C., Averick, S., Obeysekera, D., et al. (2011). "Clicked" Sugar-Curcumin Conjugate: Modulator of Amyloid-β and Tau Peptide Aggregation at Ultralow Concentrations. ACS Chem. Neurosci. 2 (12), 694–699. doi:10.1021/cn200088r
Forouzanfar, F., Barreto, G., Majeed, M., and Sahebkar, A. (2019). Modulatory Effects of Curcumin on Heat Shock Proteins in Cancer: A Promising Therapeutic Approach. Biofactors 45 (5), 631–640. doi:10.1002/biof.1522
Gao, C., Chu, X., Gong, W., Zheng, J., Xie, X., Wang, Y., et al. (2020). Neuron Tau-Targeting Biomimetic Nanoparticles for Curcumin Delivery to Delay Progression of Alzheimer's Disease. J. Nanobiotechnology 18 (1), 71. doi:10.1186/s12951-020-00626-1
Garcia-Alloza, M., Borrelli, L. A., Rozkalne, A., Hyman, B. T., and Bacskai, B. J. (2007). Curcumin Labels Amyloid Pathology In Vivo, Disrupts Existing Plaques, and Partially Restores Distorted Neurites in an Alzheimer Mouse Model. J. Neurochem. 102 (4), 1095–1104. doi:10.1111/j.1471-4159.2007.04613.x
Götz, J., Halliday, G., and Nisbet, R. M. (2019). Molecular Pathogenesis of the Tauopathies. Annu. Rev. Pathol. 14, 239–261. doi:10.1146/annurev-pathmechdis-012418-012936
Grundke-Iqbal, I., Iqbal, K., Tung, Y. C., Quinlan, M., Wisniewski, H. M., and Binder, L. I. (1986). Abnormal Phosphorylation of the Microtubule-Associated Protein Tau (Tau) in Alzheimer Cytoskeletal Pathology. Proc. Natl. Acad. Sci. U. S. A. 83 (13), 4913–4917. doi:10.1073/pnas.83.13.4913
He, Y., Yue, Y., Zheng, X., Zhang, K., Chen, S., and Du, Z. (2015). Curcumin, Inflammation, and Chronic Diseases: How Are They Linked? Molecules 20 (5), 9183–9213. doi:10.3390/molecules20059183
Holmes, B. B., Furman, J. L., Mahan, T. E., Yamasaki, T. R., Mirbaha, H., Eades, W. C., et al. (2014). Proteopathic Tau Seeding Predicts Tauopathy In Vivo. Proc. Natl. Acad. Sci. U. S. A. 111 (41), E4376–E4385. doi:10.1073/pnas.1411649111
Hoover, B. R., Reed, M. N., Su, J., Penrod, R. D., Kotilinek, L. A., Grant, M. K., et al. (2010). Tau Mislocalization to Dendritic Spines Mediates Synaptic Dysfunction Independently of Neurodegeneration. Neuron 68 (6), 1067–1081. doi:10.1016/j.neuron.2010.11.030
Hoozemans, J. J., van Haastert, E. S., Nijholt, D. A., Rozemuller, A. J., Eikelenboom, P., and Scheper, W. (2009). The Unfolded Protein Response Is Activated in Pretangle Neurons in Alzheimer's Disease hippocampus. Am. J. Pathol. 174 (4), 1241–1251. doi:10.2353/ajpath.2009.080814
Ismail, R., Parbo, P., Madsen, L. S., Hansen, A. K., Hansen, K. V., Schaldemose, J. L., et al. (2020). The Relationships between Neuroinflammation, Beta-Amyloid and Tau Deposition in Alzheimer's Disease: a Longitudinal PET Study. J. Neuroinflammation 17 (1), 151. doi:10.1186/s12974-020-01820-6
Ittner, L. M., Fath, T., Ke, Y. D., Bi, M., van Eersel, J., Li, K. M., et al. (2008). Parkinsonism and Impaired Axonal Transport in a Mouse Model of Frontotemporal Dementia. Proc. Natl. Acad. Sci. U. S. A. 105 (41), 15997–16002. doi:10.1073/pnas.0808084105
Kannappan, R., Gupta, S. C., Kim, J. H., Reuter, S., and Aggarwal, B. B. (2011). Neuroprotection by Spice-Derived Nutraceuticals: You Are what You Eat! Mol. Neurobiol. 44 (2), 142–159. doi:10.1007/s12035-011-8168-2
Khurana, V., Merlo, P., DuBoff, B., Fulga, T. A., Sharp, K. A., Campbell, S. D., et al. (2012). A Neuroprotective Role for the DNA Damage Checkpoint in Tauopathy. Aging Cell. 11 (2), 360–362. doi:10.1111/j.1474-9726.2011.00778.x
Kitani, K., Osawa, T., and Yokozawa, T. (2007). The Effects of Tetrahydrocurcumin and Green Tea Polyphenol on the Survival of Male C57BL/6 Mice. Biogerontology 8 (5), 567–573. doi:10.1007/s10522-007-9100-z
Labanca, F., Ullah, H., Khan, H., Milella, L., Xiao, J., Dajic-Stevanovic, Z., et al. (2021). Therapeutic and Mechanistic Effects of Curcumin in Huntington's Disease. Curr. Neuropharmacol. 19 (7), 1007–1018. doi:10.2174/1570159x18666200522201123
Lee, K. S., Lee, B. S., Semnani, S., Avanesian, A., Um, C. Y., Jeon, H. J., et al. (2010). Curcumin Extends Life Span, Improves Health Span, and Modulates the Expression of Age-Associated Aging Genes in Drosophila melanogaster. Rejuvenation Res. 13 (5), 561–570. doi:10.1089/rej.2010.1031
Liao, V. H., Yu, C. W., Chu, Y. J., Li, W. H., Hsieh, Y. C., and Wang, T. T. (2011). Curcumin-mediated Lifespan Extension in Caenorhabditis elegans. Mech. Ageing Dev. 132 (10), 480–487. doi:10.1016/j.mad.2011.07.008
Livingston, G., Huntley, J., Sommerlad, A., Ames, D., Ballard, C., Banerjee, S., et al. (2020). Dementia Prevention, Intervention, and Care: 2020 Report of the Lancet Commission. Lancet 396 (10248), 413–446. doi:10.1016/S0140-6736(20)30367-6
Lo Cascio, F., Garcia, S., Montalbano, M., Puangmalai, N., McAllen, S., Pace, A., et al. (2020). Modulating Disease-Relevant Tau Oligomeric Strains by Small Molecules. J. Biol. Chem. 295 (44), 14807–14825. doi:10.1074/jbc.RA120.014630
Lo Cascio, F., Puangmalai, N., Ellsworth, A., Bucchieri, F., Pace, A., Palumbo Piccionello, A., et al. (2019). Toxic Tau Oligomers Modulated by Novel Curcumin Derivatives. Sci. Rep. 9 (1), 19011. doi:10.1038/s41598-019-55419-w
Lovestone, S., Boada, M., Dubois, B., Hüll, M., Rinne, J. O., Huppertz, H. J., et al. (2015). A Phase II Trial of Tideglusib in Alzheimer's Disease. J. Alzheimers Dis. 45 (1), 75–88. doi:10.3233/JAD-141959
Ma, Q. L., Zuo, X., Yang, F., Ubeda, O. J., Gant, D. J., Alaverdyan, M., et al. (2013). Curcumin Suppresses Soluble Tau Dimers and Corrects Molecular Chaperone, Synaptic, and Behavioral Deficits in Aged Human Tau Transgenic Mice. J. Biol. Chem. 288 (6), 4056–4065. doi:10.1074/jbc.M112.393751
Maqbool, M., Mobashir, M., and Hoda, N. (2016). Pivotal Role of Glycogen Synthase Kinase-3: A Therapeutic Target for Alzheimer's Disease. Eur. J. Med. Chem. 107, 63–81. doi:10.1016/j.ejmech.2015.10.018
Miyasaka, T., Xie, C., Yoshimura, S., Shinzaki, Y., Yoshina, S., Kage-Nakadai, E., et al. (2016). Curcumin Improves Tau-Induced Neuronal Dysfunction of Nematodes. Neurobiol. Aging 39, 69–81. doi:10.1016/j.neurobiolaging.2015.11.004
Mudher, A., Shepherd, D., Newman, T. A., Mildren, P., Jukes, J. P., Squire, A., et al. (2004). GSK-3beta Inhibition Reverses Axonal Transport Defects and Behavioural Phenotypes in Drosophila. Mol. Psychiatry 9 (5), 522–530. doi:10.1038/sj.mp.4001483
Mukherjee, S., Mishra, A. K., Peer, G. D. G., Bagabir, S. A., Haque, S., Pandey, R. P., et al. (2021). The Interplay of the Unfolded Protein Response in Neurodegenerative Diseases: A Therapeutic Role of Curcumin. Front. Aging Neurosci. 13, 767493. doi:10.3389/fnagi.2021.767493
Nagele, R. G., Wegiel, J., Venkataraman, V., Imaki, H., Wang, K. C., and Wegiel, J. (2004). Contribution of Glial Cells to the Development of Amyloid Plaques in Alzheimer's Disease. Neurobiol. Aging 25 (5), 663–674. doi:10.1016/j.neurobiolaging.2004.01.007
Nebrisi, E. E. (2021). Neuroprotective Activities of Curcumin in Parkinson's Disease: A Review of the Literature. Int. J. Mol. Sci. 22 (20), 11248. doi:10.3390/ijms222011248
Okuda, M., Fujita, Y., Hijikuro, I., Wada, M., Uemura, T., Kobayashi, Y., et al. (2017). PE859, A Novel Curcumin Derivative, Inhibits Amyloid-β and Tau Aggregation, and Ameliorates Cognitive Dysfunction in Senescence-Accelerated Mouse Prone 8. J. Alzheimers Dis. 59 (1), 313–328. doi:10.3233/JAD-161017
Olszanecki, R., Jawień, J., Gajda, M., Mateuszuk, L., Gebska, A., Korabiowska, M., et al. (2005). Effect of Curcumin on Atherosclerosis in apoE/LDLR-Double Knockout Mice. J. Physiol. Pharmacol. 56 (4), 627–635.
Panza, F., Solfrizzi, V., Seripa, D., Imbimbo, B. P., Lozupone, M., Santamato, A., et al. (2016). Tau-Centric Targets and Drugs in Clinical Development for the Treatment of Alzheimer's Disease. Biomed. Res. Int. 2016, 3245935. doi:10.1155/2016/3245935
Parekh, K. D., Dash, R. P., Pandya, A. N., Vasu, K. K., and Nivsarkar, M. (2013). Implication of Novel Bis-Imidazopyridines for Management of Alzheimer's Disease and Establishment of its Role on Protein Phosphatase 2A Activity in Brain. J. Pharm. Pharmacol. 65 (12), 1785–1795. doi:10.1111/jphp.12149
Quraishe, S., Sealey, M., Cranfield, L., and Mudher, A. (2016). Microtubule Stabilising Peptides Rescue Tau Phenotypes In-Vivo. Sci. Rep. 6, 38224. doi:10.1038/srep38224
Rainey-Smith, S. R., Brown, B. M., Sohrabi, H. R., Shah, T., Goozee, K. G., Gupta, V. B., et al. (2016). Curcumin and Cognition: a Randomised, Placebo-Controlled, Double-Blind Study of Community-Dwelling Older Adults. Br. J. Nutr. 115 (12), 2106–2113. doi:10.1017/S0007114516001203
Ramsden, M., Kotilinek, L., Forster, C., Paulson, J., McGowan, E., SantaCruz, K., et al. (2005). Age-dependent Neurofibrillary Tangle Formation, Neuron Loss, and Memory Impairment in a Mouse Model of Human Tauopathy (P301L). J. Neurosci. 25 (46), 10637–10647. doi:10.1523/JNEUROSCI.3279-05.2005
Reddy, P. H., Manczak, M., Yin, X., Grady, M. C., Mitchell, A., Tonk, S., et al. (2018). Protective Effects of Indian Spice Curcumin against Amyloid-β in Alzheimer's Disease. J. Alzheimers Dis. 61 (3), 843–866. doi:10.3233/JAD-170512
Reisberg, B., Doody, R., Stöffler, A., Schmitt, F., Ferris, S., Möbius, H. J., et al. (2003). Memantine in Moderate-To-Severe Alzheimer's Disease. N. Engl. J. Med. 348 (14), 1333–1341. doi:10.1056/NEJMoa013128
Ringman, J. M., Frautschy, S. A., Teng, E., Begum, A. N., Bardens, J., Beigi, M., et al. (2012). Oral Curcumin for Alzheimer's Disease: Tolerability and Efficacy in a 24-week Randomized, Double Blind, Placebo-Controlled Study. Alzheimers Res. Ther. 4 (5), 43. doi:10.1186/alzrt146
Sankaranarayanan, S., Barten, D. M., Vana, L., Devidze, N., Yang, L., Cadelina, G., et al. (2015). Passive Immunization with Phospho-Tau Antibodies Reduces Tau Pathology and Functional Deficits in Two Distinct Mouse Tauopathy Models. PLoS One 10 (5), e0125614. doi:10.1371/journal.pone.0125614
Scapagnini, G., Caruso, C., and Calabrese, V. (2010). Therapeutic Potential of Dietary Polyphenols against Brain Ageing and Neurodegenerative Disorders. Adv. Exp. Med. Biol. 698, 27–35. doi:10.1007/978-1-4419-7347-4_3
Singh, S., and Aggarwal, B. B. (1995). Activation of Transcription Factor NF-Kappa B Is Suppressed by Curcumin (Diferuloylmethane) [corrected]. J. Biol. Chem. 270 (42), 24995–25000. doi:10.1074/jbc.270.42.24995
Small, G. W., Siddarth, P., Li, Z., Miller, K. J., Ercoli, L., Emerson, N. D., et al. (2018). Memory and Brain Amyloid and Tau Effects of a Bioavailable Form of Curcumin in Non-demented Adults: A Double-Blind, Placebo-Controlled 18-Month Trial. Am. J. Geriatr. Psychiatry 26 (3), 266–277. doi:10.1016/j.jagp.2017.10.010
Spillantini, M. G., and Goedert, M. (2013). Tau Pathology and Neurodegeneration. Lancet Neurol. 12 (6), 609–622. doi:10.1016/S1474-4422(13)70090-5
Sreejayan, R., and Rao, M. N. (1997). Nitric Oxide Scavenging by Curcuminoids. J. Pharm. Pharmacol. 49 (1), 105–107. doi:10.1111/j.2042-7158.1997.tb06761.x
Stamer, K., Vogel, R., Thies, E., Mandelkow, E., and Mandelkow, E. M. (2002). Tau Blocks Traffic of Organelles, Neurofilaments, and APP Vesicles in Neurons and Enhances Oxidative Stress. J. Cell. Biol. 156 (6), 1051–1063. doi:10.1083/jcb.200108057
Suckow, B. K., and Suckow, M. A. (2006). Lifespan Extension by the Antioxidant Curcumin in Drosophila melanogaster. Int. J. Biomed. Sci. 2 (4), 402–405.
Sundaram, J. R., Poore, C. P., Sulaimee, N. H. B., Pareek, T., Cheong, W. F., Wenk, M. R., et al. (2017). Curcumin Ameliorates Neuroinflammation, Neurodegeneration, and Memory Deficits in P25 Transgenic Mouse Model that Bears Hallmarks of Alzheimer's Disease. J. Alzheimers Dis. 60 (4), 1429–1442. doi:10.3233/JAD-170093
von Bergen, M., Friedhoff, P., Biernat, J., Heberle, J., Mandelkow, E. M., and Mandelkow, E. (2000). Assembly of Tau Protein into Alzheimer Paired Helical Filaments Depends on a Local Sequence Motif ((306)VQIVYK(311)) Forming Beta Structure. Proc. Natl. Acad. Sci. U. S. A. 97 (10), 5129–5134. doi:10.1073/pnas.97.10.5129
Voulgaropoulou, S. D., van Amelsvoort, T. A. M. J., Prickaerts, J., and Vingerhoets, C. (2019). The Effect of Curcumin on Cognition in Alzheimer's Disease and Healthy Aging: A Systematic Review of Pre-clinical and Clinical Studies. Brain Res. 1725, 146476. doi:10.1016/j.brainres.2019.146476
Williams, D. R. (2006). Tauopathies: Classification and Clinical Update on Neurodegenerative Diseases Associated with Microtubule-Associated Protein Tau. Intern Med. J. 36 (10), 652–660. doi:10.1111/j.1445-5994.2006.01153.x
Wischik, C. M., Edwards, P. C., Lai, R. Y., Roth, M., and Harrington, C. R. (1996). Selective Inhibition of Alzheimer Disease-like Tau Aggregation by Phenothiazines. Proc. Natl. Acad. Sci. U. S. A. 93 (20), 11213–11218. doi:10.1073/pnas.93.20.11213
Yanagisawa, D., Amatsubo, T., Morikawa, S., Taguchi, H., Urushitani, M., Shirai, N., et al. (2011). In Vivo detection of Amyloid β Deposition Using ¹⁹F Magnetic Resonance Imaging with a ¹⁹F-Containing Curcumin Derivative in a Mouse Model of Alzheimer's Disease. Neuroscience 184, 120–127. doi:10.1016/j.neuroscience.2011.03.071
Yanagisawa, D., Hamezah, H. S., Durani, L. W., Taguchi, H., and Tooyama, I. (2018). Study of Tau Pathology in Male rTg4510 Mice Fed with a Curcumin Derivative Shiga-Y5. PLoS One 13 (12), e0208440. doi:10.1371/journal.pone.0208440
Yanagisawa, D., Ibrahim, N. F., Taguchi, H., Morikawa, S., Hirao, K., Shirai, N., et al. (2015). Curcumin Derivative with the Substitution at C-4 Position, but Not Curcumin, Is Effective against Amyloid Pathology in APP/PS1 Mice. Neurobiol. Aging 36 (1), 201–210. doi:10.1016/j.neurobiolaging.2014.07.041
Keywords: turmeric (Curcuma longa L), curcumin, Alzheheimer’s disease, tauopathy, animal models, holistic medicine
Citation: Sivanantharajah L and Mudher A (2022) Curcumin as a Holistic Treatment for Tau Pathology. Front. Pharmacol. 13:903119. doi: 10.3389/fphar.2022.903119
Received: 23 March 2022; Accepted: 02 May 2022;
Published: 19 May 2022.
Edited by:
Shijun Xu, Chengdu University of Traditional Chinese Medicine, ChinaReviewed by:
Ali Mohammad Pourbagher-Shahri, Birjand University of Medical Sciences, IranCopyright © 2022 Sivanantharajah and Mudher. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lovesha Sivanantharajah, l.sivanantharajah@bangor.ac.uk
 Lovesha Sivanantharajah
Lovesha Sivanantharajah Amritpal Mudher
Amritpal Mudher