- 1Department of Pathology, China-Japan Union Hospital, Jilin University, Changchun, China
- 2School of Medicine, Tongji University Cancer Center, Shanghai Tenth People’s Hospital of Tongji University, Tongji University, Shanghai, China
Lung cancer is one of the common malignant cancers worldwide. Immune checkpoint inhibitor (ICI) therapy has improved survival of lung cancer patients. However, ICI therapy leads to adaptive immune resistance and displays resistance to PD-1/PD-L1 blockade in lung cancer, leading to less immune response of lung cancer patients. Tumor microenvironment (TME) is an integral tumor microenvironment, which is involved in immunotherapy resistance. Nanomedicine has been used to enhance the immunotherapy in lung cancer. In this review article, we described the association between TME and immunotherapy in lung cancer. We also highlighted the importance of TME in immunotherapy in lung cancer. Moreover, we discussed how nanoparticles are involved in regulation of TME to improve the efficacy of immunotherapy, including Nanomedicine SGT-53, AZD1080, Nanomodulator NRF2, Cisplatin nanoparticles, Au@PG, DPAICP@ME, SPIO NP@M-P, NBTXR3 nanoparticles, ARAC nanoparticles, Nano-DOX, MS NPs, Nab-paclitaxel, GNPs-hPD-L1 siRNA. Furthermore, we concluded that targeting TME by nanoparticles could be helpful to overcome resistance to PD-1/PD-L1 blockade in lung cancer.
Introduction
Lung cancer is one of the common malignant cancers worldwide (Lee and Kazerooni, 2022). There are about 236,740 new cases of lung cancer and about 130,180 deaths from this disease in the United States (Siegel et al., 2022). In the United States, the 5-year relative survival rates for lung cancer is 22% (Siegel et al., 2022). Cigarette smoking is one key reason for lung cancer development. Lung cancer has three types: non-small cell lung cancer (NSCLC, 82%), small cell lung cancer (SCLC, 14%) and unspecified histology (3%) (Miller et al., 2022). Patients with stage I or II lung cancer often undergo surgery, and patients with stage III lung cancer undergo surgery, chemotherapy and/or radiation (Guo et al., 2022a). However, the lung cancer patients often obtain drug resistance during targeted therapy, chemotherapy and radiotherapy (Wang et al., 2022a; Wu and Lin, 2022).
Immune checkpoints belong to the immune system, which prevent an immune reaction to impair healthy cells (Yu et al., 2022a; Johnson et al., 2022; Song et al., 2022). Immune checkpoint proteins often exist on the surface of T immune cells and tumor cells. After immune checkpoint proteins of T cells bind to other partner proteins of tumor cells, T cells are blocked from impairing the tumor cells (Ma et al., 2021; Hassanian et al., 2022; Korman et al., 2022). For example, immune checkpoint protein PD-1 of T cells can bind with PD-L1 of tumor cells, leading to impairment of killing tumor cells in the body (Hu et al., 2021a; Hou et al., 2023). Immune checkpoint blockade (ICB) with PD-1 antibody or PD-L1 antibody can block the binding between PD-1 and PD-L1, and allow the T cells to destroy tumor cells (Hu et al., 2021b; Jiang et al., 2021; Archilla-Ortega et al., 2022). Immune checkpoint inhibitors (ICIs) often impair the interaction between checkpoint proteins (such as PD-1) and their partner proteins (PD-L1), which allows the T cells to eradicate tumor cells (Havel et al., 2019). For example, one ICI ipilimumab blocks CTLA-4, pembrolizumab and nivolumab blocks PD-1, and atezolizumab, avelumab and durvalumab blocks PD-L1 (Huang and Zappasodi, 2022; Tison et al., 2022). Therefore, ICI therapy has been used in various cancer types, including lung cancer (Fang and Su, 2022; Hao et al., 2022; Mussafi et al., 2022; Punekar et al., 2022; Zulfiqar et al., 2022).
Tumor microenvironment (TME) is an integral tumor microenvironment, which is involved in tumorigenesis, malignant progression and drug resistance (Li and Qiao, 2022; Shi et al., 2022). TME includes complex cellular components, such as tumor-associated macrophages (TAMs), T cells and other immunocytes, blood vessels, fibroblast and extracellular matrix (Liu et al., 2022a). TAMs have been revealed to promote tumor initiation and progression via promotion of T cell dysfunction, invasive activity, migratory capacity and angiogenesis (Cao et al., 2022). TAMs can regulate tumor immune microenvironment (TIME) and suppress cytotoxic T lymphocyte (CTL) reaction, resulting in impeding activity of ICIs (Petroni et al., 2022).
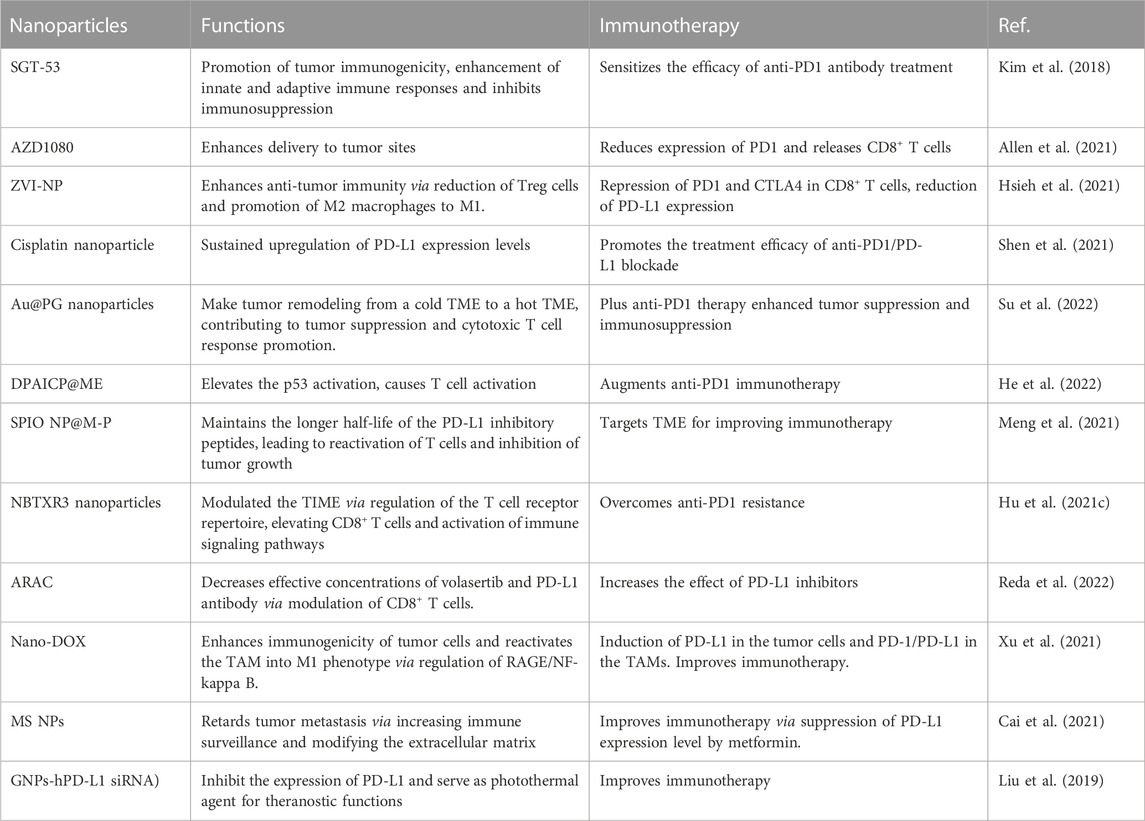
Recently, nanomedicine has been widely used to enhance the immunotherapy in lung cancer (Cheng and Santos, 2022). In this review article, we discussed the relationship between TME and immunotherapy in lung cancer. Moreover, we discussed how nanoparticles are involved in regulation of TME to improve the efficacy of immunotherapy. Furthermore, we verified that nanoparticles might target TME to improve immunotherapy efficacy and overcome anti-PD1/PD-L1 therapeutic resistance in lung cancer (Table 1).
TME and immunotherapy
TME has been identified to be associated with lung cancer development and progression (Zhao et al., 2022a; Liu et al., 2022b; Madeddu et al., 2022; Mansouri et al., 2022; Peters et al., 2022). TIME is associated with molecular heterogeneity of immunotherapy efficacy in NSCLC patients (Graves et al., 2010; Jin et al., 2020; Belluomini et al., 2021; Genova et al., 2021). In this section, we discuss the association of TME and immunotherapy.
Signaling pathways and TME in immunotherapy
Cellular signaling pathways play a prominent role in regulation of TME in lung cancer. For example, depletion of Periostin modulated TME and suppressed bone metastasis via repressing integrin signaling pathway in lung cancer (Che et al., 2017). Vav1 was found to modulate TME in Ras-driven lung cancer (Shalom et al., 2022). Vav1 instigated TME by fibrosis enhancement and reduction in tumor infiltrating macrophage, which was due to cross-talking with colony-stimulating factor 1 (CSF1) pathway, favoring lung cancer growth (Sebban et al., 2014). Blockade of IL6 regulated TME and limited the lung cancer development and progression via inactivation of STAT3 pathway (Caetano et al., 2016). Indoleamine 2,3-dioxygenase (IDO) pathway governed antitumor immunity via metabolic reprogramming of immune cells in TME through regulating AMPK pathway in lung cancer (Schafer et al., 2016). TRAF4 facilitated lung cancer malignant aggressiveness by regulating TME in normal fibroblasts via NF-κB pathway-induced ICAM1 upregulation (Kim et al., 2017). One study showed that low dose of IFNγ endowed tumor stemness in TME of NSCLC via regulation of ICAM1/PI3K/AKT/Notch-1 pathway, whereas high dose of IFNγ induced apoptosis of NSCLC cells via activation of JAK1/STAT1/caspase pathway (Song et al., 2019). Fibronectin in TME activated inflammatory response via regulation of TLR4/NF-κB signaling pathway in lung cancer cells (Cho et al., 2020).
Cyclophosphamide, a cytotoxic agent for cancer treatment, modulated TME via TGF-β signaling pathway in a mouse model of Lewis lung cancer (Zhong et al., 2020). Low dose cyclophosphamide increased CD4 + /CD8 + T cells and reduced Treg cells as well as reduced myofibroblasts, which was accompanied with upregulation of E-cadherin and downregulation of N-cadherin (Zhong et al., 2020). Dietary restriction attenuated tumor growth and improved TME and inhibited angiogenesis in NSCLC xenografts through regulation of PI3K/AKT and NF-κB/COX2/iNOS pathways (Lin et al., 2013). The diffusible gas carbon monoxide altered TME and impeded tumor growth via regulating MAPK/Erk1/2 pathway, Notch-1 pathway, HO-1 and CD86 expressions in lung cancer (Nemeth et al., 2016). Neobavaisoflavone nanoemulsion was reported to block tumor progression via targeting TME of lung cancer through repressing TGF-β/SMADs pathway (Ye et al., 2020). A small nucleolar RNA SNORA38B was reported to promote oncogenesis and reduce immunotherapy effects via remodeling the TME through targeting GAB2/AKT/mTOR signaling pathway (Zhuo et al., 2022). SNORA38B recruited the CD4+FOXP3 + Treg cells in TME, contributing to poorer immune efficacy in NSCLC (Zhuo et al., 2022). Evidence has suggested that simultaneous targeting of tumor-initiating cells and signaling pathways in the TME led to effective immunotherapy in lung cancer (Dubinett and Sharma, 2009).
EGFR and ALK mutations affect TME in immunotherapy
EGFR-mutant tumors and ALK-rearranged tumors exhibited a poor response to anti-PD-1/PD-L1 immunotherapy (Jin et al., 2020). TIME was associated with oncogenic manners in NSCLC patients because KRAS mutations and EGFR L858R mutation play a critical role in inflammatory response and immune resistance in tumor microenvironment (Jin et al., 2020). In addition, EGFR-mutant and ALK-rearranged tumors displayed more resting memory CD4+ T cells and less CD8+ T cells and activated memory CD4+ T cells (Jin et al., 2020). Another study also confirmed that targeting the PD-1/PD-L1 did not benefit the EGFR-mutant or ALK-translocated NSCLC patients (Bylicki et al., 2017).
One study reported that CD25+; CD4+ T cells with high expression of PD-L1 (PD-L1 high Treg) were increased in TME of NSCLC patients (Wu et al., 2018). PD-L1 high Treg was positively linked to PD-1 + CD8 in Treg in NSCLC. Moreover, PD-1/PD-L1 pathway promoted the effect of TILs by anti-PD-1/PD-L1 immunotherapy (Wu et al., 2018). These NSCLC patients with PD-L1 expression exhibited a better prognosis. Hence, the density of PD-L1 +; CD4+; CD25+ Tregs in TME could be a diagnostic predictor and immunotherapy response markers in NSCLC (Wu et al., 2018). Another study implied that lymphocyte activing 3 (LAG-3) expression was associated with TILs, PD-1, PD-L1 and survival in NSCLC (He et al., 2017). LAG-3 was expressed on TILs in NSCLC patients’ tumor tissues, which was associated with the expression of PD-1 and PD-L1 in NSCLC. NSCLC patients with LAG-3 positivity or both PD-L1 and LAG-3 positivity had an early recurrence and poor prognosis (He et al., 2017).
Low expression of PD-1 in cytotoxic CD8+ TILs indicated a privileged TIME in NSCLC, which suggested a predictive and prognostic values (Mazzaschi et al., 2018). NSCLC patients with low PD-1 expression in CD8+ cells after nivolumab treatment displayed a prolonged survival, indicating that CD8+; PD-1 low was a prediction factor for response to immunotherapy in NSCLC (Mazzaschi et al., 2018). Similarly, one tissue microarray showed that CD8+ cells, especially PD-L1 negative tumors lacking PD-1 + TILs had a big prognostic value. PD-L1 positive tumors with CD8+ lymphocytes can promote the survival in NSCLC (Munari et al., 2021). In addition, PD-L1 overexpression had an unfavorable prognosis and high CD8+ TILs had a favorable prognosis in NSCLC patients (Rashed et al., 2017). Zhang et al. reported that PD-L1 plus CD8+ TILs established an immunosuppressive TME with high mutation burden in NSCLC (Zhang et al., 2021). NSCLC patients with high PD-L1+; CD8+ TILs had a better response to the anti-PD-1 immunotherapy (Zhang et al., 2021). Consistently, differential TIME determined immunotherapy efficacy in NSCLC patients with advanced stages (Shirasawa et al., 2021).
Four types of groups from 228 NSCLC patients were identified: type I, 73 patients with PD-L1 high/TIL high; type II: 70 cases with PD-L1 low/TIL low; type III: 37 patients with PD-L1 high/TIL low; type IV: 48 cases with PD-L1 low/TIL high. Each type patients had a different survival: Type I tumors had good prognosis compared with type III tumors (Shirasawa et al., 2021). Yang et al. also observed that PD-L1 expression in combination with CD8+ TILs showed a prognostic value in patients with NSCLC after surgery (Yang et al., 2018). A retrospective study defined that PD-L1 expression plus CD8+ TILs density is useful for prediction of disease-free survival in lung squamous cell carcinoma after surgery (Cheng et al., 2022). In addition, PD-L1 expression and TIL infiltration appeared in brain metastasis of small cell lung cancer. PD-L1 TILs and CD45RO+ memory T cells were linked to favorable survival (Berghoff et al., 2016). Lung cancer patients with intracranial resection of brain metastases had a better outcomes if the patients had PD-L1 positivity and a high intraepithelial CD8+ T cell infiltration (Li et al., 2022a). Zhou et al. discovered that paired primary NSCLC and brain metastatic lesions in NSCLC have a difference for PD-L1 expression and CD8+ TILs (Zhou et al., 2018). There were a fewer CD8+ TILs in brain metastatic tissues versus primary lung tumor samples, which was associated with shorter overall survival versus high CD8+ TILs density (Zhou et al., 2018). However, Batur et al. found that PD-L1 expression and CD8+ TIL intensity had a concordance between brain metastases and NSCLC (Batur et al., 2020). Hence, further investigation is required to determine the role of PD-L1, CD8+ TIL and TIME in immunotherapy of NSCLC patients (Vilarino et al., 2020). Notably, seven randomized controlled trials had uncovered that PD-1/PD-L1 inhibitors exhibited a treatment efficacy in brain metastases of NSCLC, reducing risk of disease progression and death in NSCLC patients with brain metastases (Li et al., 2022b). Strikingly, TME, including spatial and temporal discordance of TILs and PD-L1 expression, was discovered between lung primary lesions and brain metastases in lung cancer (Mansfield et al., 2016). Together, PD-1, PD-L1 expression and TIL status can predict the response of anti-PD-1/PD-L1 treatment in NSCLC (Nakagawa and Kawakami, 2022).
TME and CCRT in NSCLC patients
TME has been known to affect concurrent chemoradiation therapy (CCRT) in NSCLC patients (Shirasawa et al., 2020). Next, we summarize how TME regulates CCRT via CD8+ TILs and PD-L1 in NSCLC. A retrospective research showed that chemoradiation therapy can change the expression of PD-L1 and CD8+ TILs (Choe et al., 2019). NSCLC patients with PD-L1 expression had a short survival after concurrent chemoradiation therapy (CCRT). Patients with an upregulation of CD8+ TILs after CCRT displayed a longer overall survival (Choe et al., 2019). Moreover, Tokito et al. defined predictive relevance of PD-L1 plus CD8+ TIL density in patients with stage III NSCLC after CCRT (Tokito et al., 2016). PD-L1+/CD8 low patients had the worst overall survival, whereas PD-L1-/CD8 high patients had the best prognosis (Tokito et al., 2016). PD-L1+ tumor cells were reduced after CCRT in NSCLC patients. Modulation of PD-L1 expression was linked to prognosis in locally advanced NSCLC patients after CCRT (Fujimoto et al., 2017). Similarly, evidence showed that PD-L1 expression was linked to high tumor grade and low density of CD8 TILs (El-Guindy et al., 2018). PD-L1-/CD8 high patients had a good overall survival, while PD-L1+/CD8 low patients often had advantage tumor stage and the poorest overall survival (El-Guindy et al., 2018).
One clinical trial also confirmed the alteration in tumoral PD-L1 expression and stromal CD8+ TILs in NSCLC patients after CCRT (Yoneda et al., 2019). PD-L1 expression was increased in NSCLC patients after CCRT, and stromal CD8+ TIL was elevated after CCRT. Higher CD8+ TILs supported a favorable prognosis. CCRT-induced PD-L1 expression in NSCLC after CCRT suggested that PD-L1 blockade in combination with CCRT is necessary to improve survival in NSCLC (Yoneda et al., 2019). PD-L1-/CD8 low NSCLC patients after CCRT had the longest overall survival, while PD-L1+/CD8 low patients with locally advanced NSCLC after CCRT had the shortest overall survival (Gennen et al., 2020). One study also discovered that PD-L1 expression was changed after CCRT, the density of CD8+ TILs was upregulated after CCRT. Moreover, locally advanced NSCLC patients after CCRT had a good response to anti-PD-1/PD-L1 therapy (Shirasawa et al., 2020).
In lung cancer mouse models
To better understand the role of TME in PD-1 blockade resistance in lung cancer, one group used genetically engineered lung cancer mouse models (Martinez-Usatorre et al., 2021). The mouse models included KrasG12D/+; p53−/− (KP) mice, KrasG12D/+; p53−/−; Msh2-/- (KPM) mice and KrasG12D/+; p53−/−; ovalbumin (KPO) mice. Blockade of ANGPT2 and VEGFA by a bispecific antibody A2V retarded progression and metastasis of KP lung tumors via development of a favorable TME and modulation of the immune cell composition of KP tumors (Martinez-Usatorre et al., 2021). Specifically, A2V treatment was correlated with reprogramming of the TIME through increased T cells and decreased TAMs (Martinez-Usatorre et al., 2021). Interestingly, inhibition of PD-1 by its antibody failed to improve tumor response to A2V treatment in KP mice. However, the PD-1 antibody affected PD-1+ Tregs in KP tumors (Martinez-Usatorre et al., 2021). Moreover, TAMs interacted with Tregs in KP tumors. Furthermore, CSF1R suppression in combination with cisplatin inhibited TAMs and enhanced the efficacy of anti-angiogenic immunotherapy (Martinez-Usatorre et al., 2021). Another study used the KrasLSL−G12D/+Tp53fl/fl (KP) and the KrasLSL−G12D/+Lkb1fl/fl (KL) NSCLC mouse models to determine the immunotherapy efficacy and TME of NSCLC (Zhang et al., 2020a). This work showed that CCL7 enhanced anti-PD-1 therapy in these mouse models via promoting conventional DC 1 into TME, leading to T cell expansion. In NSCLC tissues, CCL7 expression was elevated, and it was associated with conventional DC 1 infiltration and overall survival in NSCLC patients (Zhang et al., 2020a). In KP mouse model, depletion of CCL7 destroyed the conventional DC 1 infiltration in the TME and promoted expansion of CD4+ and CD8+ T cells in TME, resulting in tumor development. Upregulation of CCL7 in lungs retarded tumor development and increased the survival of KP and KL mice. Thus, CCL7 could act a biomarker for anti-PD-1 therapy of NSCLC (Zhang et al., 2020a).
Nanomedicine
Nanomedicine is a novel unique branch of medicine using nano-size technology for exploration of underlying mechanisms of disease development and progression and for the prevention, diagnosis and therapy of various diseases (de Lazaro and Mooney, 2021; Stater et al., 2021). Nanomedicine has been used for cancer diagnosis and therapy via merging the physical, biological, chemical and digital technologies together (Ahmad et al., 2023; Li et al., 2023). In recent years, nanomedicine has exhibited a potent effect in immunotherapy for cancer patients (Irvine and Dane, 2020; Guo et al., 2022b; Wang et al., 2022b; Yang et al., 2023).
Nanomedicine enhances immunotherapy in lung cancer
Evidence has suggested that nanomedicine could enhance immunotherapy in NSCLC (Seshadri and Ramamurthi, 2018; Garcia-Fernandez et al., 2020; Pu et al., 2022). For example, TME component-targeted nanomedicine delivery facilitated the efficacy of immune checkpoint inhibitors (Kim et al., 2021). In addition, nanoparticle-based ICI therapy elevated the local dose of ICIs and reduced the side effects of ICIs, leading to boosting the anti-tumor immunity in several types of cancers, including lung cancer (Sanaei et al., 2021).
Nanomedicine SGT-53 enhances anti-PD1 antibody immunotherapy
One group designed SGT-53, a nanomedicine carrying a p53 plasmid, to detect whether SGT-53 can augment immune checkpoint inhibitor therapy (Kim et al., 2018). This group used three mouse models, including a glioblastoma, a NSCLC and a breast cancer. SGT-53 sensitized the efficacy of anti-PD1 antibody treatment via promotion of tumor immunogenicity, enhancement of innate and adaptive immune responses and inhibited immunosuppression in a breast tumor model (Kim et al., 2018). STG-53 in combination of an anti-PD1 antibody was stronger than each agent individually in suppression of tumor growth and metastasis. STG-53 blocked fatal xenogeneic hypersensitivity after anti-PD1 therapy in breast cancer model (Kim et al., 2018). This work indicated that nanomedicine SGT-53 restored p53 biological function and caused anti-tumor immunity to increase sensitization of anti-PD1 treatment in human cancer.
Nanoparticle AZD1080 enhances delivery to tumor sites
One study used the remote loading of GSK3 inhibitor AZD1080 into nanoparticles coated with a lipid bilayer. Intravenous injection of AZD1080 nanoparticles enhanced biodistribution and drug delivery to cancer site and reduced the expression of PD1 and released CD8+ T cells (Allen et al., 2021). Encapsulated AZD1080 reduced tumor growth in CT26 colorectal tumor, KPC pancreatic cancer and LLC lung cancer models without treatment toxicity. Hence, nano drug delivery of AZD1080 could be used in combination with immunotherapy or chemotherapy in human cancer (Allen et al., 2021).
Nanomodulator NRF2 induces an immunostimulatory TME
It has been reported that zero-valent-iron nanoparticle (ZVI-NP) triggered anticancer immunity and cancer-specific cytotoxicity in lung cancer (Hsieh et al., 2021). ZVI-NP induced ferroptotic death via regulation of lipid peroxidation, mitochondria dysfunction and ROS in lung tumor cells. Furthermore, β-TrCP-induced NRF2 destruction via AMPK/mTOR pathway was increased in this kind of ferroptosis. ZVI-NP suppressed angiogenesis-associated genes and reduced the self-renewal capacity. ZVI-NP enhanced anti-tumor immunity via reduction of Treg cells and promotion of M2 macrophages to M1, and repression of PD1 and CTLA4 in CD8+ T cells, as well as reduction of PD-L1 expression in tumor cells. Strikingly, ZVI-NP mainly stayed in lung tissues and tumor sites, resulting in inhibition of tumor metastasis and growth (Hsieh et al., 2021). Taken together, NRF2 nanomodulator stimulated lung cancer ferroptosis and maintained an immunostimulatory TME.
Cisplatin nanoparticles sensitize PD1/PD-L1 inhibitors
Cisplatin nanoparticles improved the PD1/PD-L1 inhibitor therapeutic outcomes because cisplatin nanoparticles increased the expression of PD-L1 levels (Shen et al., 2021). Cisplatin nanoparticles in combination with PD1/PD-L1 inhibitors, BMS-202 and anti-PD1 antibody, caused a superior inhibition of tumor growth. Cisplatin nanoparticles plus anti-PD1 antibody displayed a stronger tumor inhibition than cisplatin plus anti-PD1 antibody in the LLC tumor model. Altogether, cisplatin nanoparticle promoted the treatment efficacy of anti-PD1/PD-L1 blockade through sustained upregulation of PD-L1 expression levels (Shen et al., 2021).
Au@PG nanoparticles improve immunotherapy
Polyaniline-based glyco-condensation on Au nanoparticles have been found to promote immunotherapy in lung cancer (Su et al., 2022). Au@PG nanoparticles can make tumor remodeling from a cold TME to a hot TME, contributing to tumor suppression and cytotoxic T cell response promotion. Au@PG nanoparticles plus anti-PD1 therapy enhanced tumor suppression and immunosuppression and improved cytokine secretion (Su et al., 2022). Moreover, the size of Au@PG nanoparticles made a decision for the switch from M2 to M1 macrophages: the smaller Au@PG nanoparticles exhibited better functions than larger ones. Au@PG nanoparticles caused endoplasmic reticulum stress and spleen tyrosine kinase activation and macrophage polarization in lung cancer (Su et al., 2022).
DPAICP@ME augments anti-PD1 immunotherapy
A chiral-peptide supramolecular (DPAICP) interacting with the membrane from milk-derived extracellular vesicles (ME) was constructed (He et al., 2022). DPAICP@ME was found to be stable in blood circulation after gastrointestinal absorption, and displayed tumor accumulation by oral medication. Oral DPAICP@ME elevated the p53 activation for treating cancer in LLC lung cancer orthotopic model and PDOX mice of colon cancer and B16F10 homograft melanoma model (He et al., 2022). Oral DPAICP@ME caused T cell activation and led to enhancement of anti-PD1 immunotherapy. Hence, DPAICP@ME could boost the anti-PD1 immunotherapy in human cancer (He et al., 2022).
SPIO NP@M-P targets TME for immunotherapy
Superparamagnetic iron oxide nanoparticles (SPIO NPs) were combined with lung cancer H460 cell membranes, PD-L1 inhibitory peptide (TPP1) and MMP2 substrate peptide (PLGLLG), which were named as SPIO NP@M-P (Meng et al., 2021). The TPP1 peptide with homotypic effect of cancer cell membrane was entered to the TME and digested by MMP2 enzyme. Therefore, SPIO NP@M-P maintained the longer half-life of the PD-L1 inhibitory peptides, leading to reactivation of T cells and inhibition of tumor growth (Meng et al., 2021). SPIO NP@M-P could be a useful platform for cancer therapy and tumor diagnosis.
NBTXR3 nanoparticles overcomes anti-PD1 resistance
To overcome anti-PD1 resistance in lung cancer, one group combined radiation with NBTXR3 nanoparticles and anti-PD1 therapy (Hu et al., 2021c). This group reported that the triple combination (anti-PD1, localized radiation and NBTXR3) reduced growth of irradiated and unirradiated tumors in 344SQP anti-PD1-sensitive lung cancer cells and 344SQR anti-PD1-resistant lung cancer cells (Hu et al., 2021c). Moreover, NBTXR3 modulated the TIME of unirradiated tumors via regulation of the T cell receptor repertoire, elevating CD8+ T cells and activation of immune signaling pathways in 344SQR tumor model. NBTXR3 nanoparticles could be helpful for treating metastatic lung cancer patients regardless of immunotherapeutic resistance or sensitivity (Hu et al., 2021c). Later, this group combined NBTXR3 with three inhibitors of checkpoint receptors: PD1, TIGIT and LAG3 (Hu et al., 2022). The nanoparticle-involved combination treatment reduced the growth of irradiated and unirradiated tumors due to activation of the immune response and increased immune cells (Hu et al., 2022). Furthermore, a triple-combination therapy includes NBTXR3, high-dose radiation (HDXRT) for primary tumors and low-dose radiation (LDXRT) for a secondary tumor, and ICIs (Hu et al., 2021d). This triple-combination therapy displayed remarkable anticancer activity and improve the survival in mice of anti-PD1-resistant lung cancer. NBTXR3+HDXRT + LDXRT reduced the number of Treg cells and promoted CD8 T cell infiltration. NBTXR3 nanoparticle plus radioimmunotherapy enhance antitumor immune response and promote the survival (Hu et al., 2021d).
ARAC nanoparticles target PD-L1
A nanoparticle-based treatment named antigen release agent and checkpoint inhibitor (ARAC) was discovered to increase the effect of PD-L1 inhibitors (Reda et al., 2022). ARAC nanoparticle co-delivered PLK1 inhibitor (volasertib) and PD-L1 antibody. Because PLK1 was often upregulated in lung cancer and promoted tumor growth, suppression of PLK1 could reduce cancer growth. ARAC decreased effective concentrations of volasertib and PD-L1 antibody in LLC tumor model via modulation of CD8+ T cells. ARAC exhibited therapy efficacy in KLN-205 lung tumor model (Reda et al., 2022).
Nano-DOX improve immunotherapy
Nanodiamond-doxorubicin conjugates (Nano-DOX) in combination with anti-PD-L1 agent BMS-1 synergistically enhanced tumor suppression (Xu et al., 2021). Nano-DOX enhanced immunogenicity of tumor cells and reactivated the TAM into M1 phenotype via regulation of RAGE/NF-κB pathway and induction of PD-L1 in the tumor cells and PD-1/PD-L1 in the TAMs. Nano-DOX increased the cytokine HMGB1 via targeting RAGE/NF-κB pathway (Xu et al., 2021). BMS-1 promoted M1 activation of TAMs that was induced by Nano-DOX via reducing PD-L1 in the TAMs and impairing the interaction between PD1 and PD-L1, contributing to inhibition of tumor growth due to killing tumor cells. Nano-DOX enhanced BMS-1 efficacy on tumor growth in a TAM-mediated manner (Xu et al., 2021).
MS NPs enhance chemo-immunotherapy
A nanodrug (MS NPs) was developed to combine immunoadjuvant metformin with 7-ethyl-10-hydroxycamptothecin (SN38) via electrostatic interactions (Cai et al., 2021). MS NPs improved immunotherapy via suppression of PD-L1 expression level by metformin. MS NPs were also found to retard tumor metastasis via increasing immune surveillance and modifying the extracellular matrix (Cai et al., 2021). Importantly, MS NPs increased mouse survival with no obvious toxicity. MS NPs increased the efficacy of ICIs, indicating that MS NPs could open a new window for the development of novel anti-PD1/PD-L1 therapy (Cai et al., 2021).
Other nanoparticles improve immunotherapy
One group designed nanoarchitecture using anti-PD-L1 antibody and magnetic-nanoparticle-attached YFCD for the separation and identification of PD-L1-expressing exosomes (Pramanik et al., 2022). Different lung cancer cell lines had a different amount of PD-L1+ exosomes. H460 lung cancer cells expressed huge PD-L1+ exosomes, and A549 cancer cells expressed low PD-L1+ exosomes, while normal skin HaCaT cells did not express PD-L1+ exosomes (Pramanik et al., 2022). The nanoarchitectures with YFCDs and anti-PD-L1 antibody separated and tracked PD-L1+ exosomes, suggesting that this nanoarchitecture could be used for clinical application to analyze PD-L1+ exosomes, which can help immunotherapy (Pramanik et al., 2022). Nanoparticle albumin-bound (Nab)-paclitaxel had improved the survival of older patients with stage IV NSCLC after disease progression with platinum-based doublet chemotherapy (Weiss et al., 2020). A gold nanoprism-assisted human PD-L1 siRNA (GNPs-hPD-L1 siRNA) was designed to inhibit the expression of PD-L1 and serve as photothermal agent for theranostic functions in lung cancer (Liu et al., 2019).
Conclusion
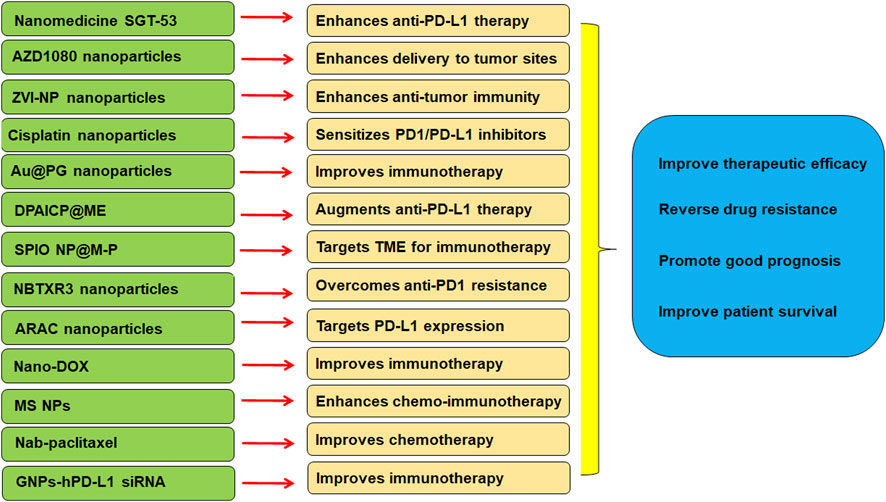
In conclusion, there is a close association between TME and immunotherapy in lung cancer. Targeting TME could be a strategy for overcoming resistance to PD-1/PD-L1 blockade in lung cancer. ICIs have demonstrated the therapeutic benefits in NSCLC. But ICIs have several side effects, such as ICI-related pneumonitis (Conroy and Naidoo, 2022; Hao et al., 2022). Altogether, nanoparticle-based ICI therapy can boost the anti-tumor immunity in lung cancer (Figure 1).
Several issues need to be highlighted in lung cancer therapy. For example, mRNA vaccine development to recognize immune-associated tumor antigens and immune subtypes in lung cancer (Wang et al., 2021; Zhao et al., 2022b; Zhao et al., 2022c; Xu et al., 2022; Zhou et al., 2022). Similar mRNA vaccines have been reported in other cancers, such as glioma and melanoma (Zhong et al., 2021; Sittplangkoon et al., 2022). Moreover, lipid nanoparticle-based mRNA cancer vaccines displayed advantages in cancer therapy ((Persano et al., 2017; Chen et al., 2022a; Huang et al., 2022a)). In addition, non-coding RNAs have been revealed to participate in tumorigenesis in various cancer types ((Chen et al., 2022b; Yu et al., 2022b; Liu and Shang, 2022)). RNA nanotechnology has emerged in cancer therapy in recent years [(Guo, 2010), (Huang et al., 2022b)]. It is important to use nanomedicine to treat cancer cells via modulation of non-coding RNAs in lung cancer.
Recently, there is an association between lung cancer, COVID-19 and vaccines [(Trivanovic et al., 2022), (Mao et al., 2021)]. Lung cancer patients have an increased risk from COVID-19 infection and exhibit poor outcomes [(Oldani et al., 2022), (Aramini et al., 2022)]. In addition, genomics, transcriptomics, proteomics, lipidomics and metabolomics are important to be used to determine the mechanisms of disease development and carcinogenesis [(Zhou et al., 2019; Zhang et al., 2020b; Boys et al., 2022)]. The role of multi-omics has been described in lung cancer early detection and therapy [(Abbasian et al., 2022), (Ling et al., 2022)]. The multi-omics should be applied for exploration of TME and immunotherapy in lung cancer to overcome the immunotherapy resistance. Lastly, it must be mentioned that nanomaterials could have side-effects, such as toxicity, and delivery problems. The degradation byproducts from the nanomaterials could have toxicity for host cells. Hence, it is critical to solve these disadvantages of nanomaterials when they are used for cancer therapy to improve immunotherapeutic efficacy in lung cancer.
Author contributions
XZ and XW wrote the manuscript; LH, ZX, and, YL edited manuscript and made the figures and tables. XW edited and revised the manuscript. All authors have read and approved for the final version of the manuscript.
Funding
This work is supported by the Young Scientists Fund of the National Natural Science Foundation of China (grant No. 81700198), the Science and Technology Development Project of Jilin Province (grant No. 20190701064GH).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ALK, anaplastic lymphoma kinase; EGFR, epidermal growth factor receptor; GAB2, GRB2-associated-binding protein 2; ICAM1, intercellular adhesion molecule-1; ICIs, immune checkpoint inhibitors; KRAS, Kirsten rat sarcoma; mTOR, mammalian target of rapamycin; NSCLC, non-small cell lung carcinoma; OS, overall survival; PD-1, programmed cell death-1; PD-L1, programmed cell death ligand-1; ROS1, receptor tyrosine kinase c-ros oncogene 1; TME, tumor microenvironment; TIME, tumor immune microenvironment; YFCD, yellow fluorescent carbon dot.
References
Abbasian, M. H., Ardekani, A. M., Sobhani, N., and Roudi, R. (2022). The role of genomics and proteomics in lung cancer early detection and treatment. Cancers (Basel) 14 (20), 5144. Epub 20221020. doi:10.3390/cancers14205144
Ahmad, A., Rashid, S., Chaudhary, A. A., Alawam, A. S., Alghonaim, M. I., Raza, S. S., et al. (2023). Nanomedicine as potential cancer therapy via targeting dysregulated transcription factors. Semin. Cancer Biol. 89, 38–60. Epub 20230118. doi:10.1016/j.semcancer.2023.01.002
Allen, S. D., Liu, X., Jiang, J., Liao, Y. P., Chang, C. H., Nel, A. E., et al. (2021). Immune checkpoint inhibition in syngeneic mouse cancer models by a silicasome nanocarrier delivering a GSK3 inhibitor. Biomaterials 269, 120635. Epub 20201228. doi:10.1016/j.biomaterials.2020.120635
Aramini, B., Masciale, V., Samarelli, A. V., Tonelli, R., Cerri, S., Clini, E., et al. (2022). Biological effects of COVID-19 on lung cancer: Can we drive our decisions. Front. Oncol. 12, 1029830. Epub 20221010. doi:10.3389/fonc.2022.1029830
Archilla-Ortega, A., Domuro, C., Martin-Liberal, J., and Munoz, P. (2022). Blockade of novel immune checkpoints and new therapeutic combinations to boost antitumor immunity. J. Exp. Clin. Cancer Res. 41 (1), 62. Epub 20220214. doi:10.1186/s13046-022-02264-x
Batur, S., Dulger, O., Durak, S., Yumuk, P. F., Caglar, H. B., Bozkurtlar, E., et al. (2020). Concordance of PD-L1 expression and CD8+ TIL intensity between NSCLC and synchronous brain metastases. Bosn. J. Basic Med. Sci. 20 (3), 329–335. Epub 20200803. doi:10.17305/bjbms.2019.4474
Belluomini, L., Dodi, A., Caldart, A., Kadrija, D., Sposito, M., Casali, M., et al. (2021). A narrative review on tumor microenvironment in oligometastatic and oligoprogressive non-small cell lung cancer: A lot remains to be done. Transl. Lung Cancer Res. 10 (7), 3369–3384. doi:10.21037/tlcr-20-1134
Berghoff, A. S., Ricken, G., Wilhelm, D., Rajky, O., Widhalm, G., Dieckmann, K., et al. (2016). Tumor infiltrating lymphocytes and PD-L1 expression in brain metastases of small cell lung cancer (SCLC). J. Neurooncol 130 (1), 19–29. Epub 20160719. doi:10.1007/s11060-016-2216-8
Boys, E. L., Liu, J., Robinson, P. J., and Reddel, R. R. (2022). Clinical applications of mass spectrometry-based proteomics in cancer: Where are we? Proteomics, e2200238. Epub 20220815. doi:10.1002/pmic.202200238
Bylicki, O., Paleiron, N., Margery, J., Guisier, F., Vergnenegre, A., Robinet, G., et al. (2017). Targeting the PD-1/PD-L1 immune checkpoint in EGFR-mutated or ALK-translocated non-small-cell lung cancer. Target Oncol. 12 (5), 563–569. doi:10.1007/s11523-017-0510-9
Caetano, M. S., Zhang, H., Cumpian, A. M., Gong, L., Unver, N., Ostrin, E. J., et al. (2016). IL6 blockade reprograms the lung tumor microenvironment to limit the development and progression of K-ras-Mutant lung cancer. Cancer Res. 76 (11), 3189–3199. Epub 20160401. doi:10.1158/0008-5472.CAN-15-2840
Cai, S., Chen, Z., Wang, Y., Wang, M., Wu, J., Tong, Y., et al. (2021). Reducing PD-L1 expression with a self-assembled nanodrug: An alternative to PD-L1 antibody for enhanced chemo-immunotherapy. Theranostics 11 (4), 1970–1981. Epub 20210101. doi:10.7150/thno.45777
Cao, H., Gao, S., Jogani, R., and Sugimura, R. (2022). The tumor microenvironment reprograms immune cells. Cell Reprogr. 24 (6), 343–352. Epub 20221026. doi:10.1089/cell.2022.0047
Che, J., Shen, W. Z., Deng, Y., Dai, Y. H., Liao, Y. D., Yuan, X. L., et al. (2017). Effects of lentivirus-mediated silencing of Periostin on tumor microenvironment and bone metastasis via the integrin-signaling pathway in lung cancer. Life Sci. 182, 10–21. 10.1016/j.lfs.2017.05.030.
Chen, J., Ye, Z., Huang, C., Qiu, M., Song, D., Li, Y., et al. (2022). Lipid nanoparticle-mediated lymph node-targeting delivery of mRNA cancer vaccine elicits robust CD8(+) T cell response. Proc. Natl. Acad. Sci. U. S. A. 119 (34), e2207841119. Epub 20220815. doi:10.1073/pnas.2207841119
Chen, T., Liu, J., Zhang, H., Li, J., and Shang, G. (2022). Long intergenic noncoding RNA00265 enhances cell viability and metastasis via targeting miR-485-5p/USP22 Axis in osteosarcoma. Front. Oncol. 12, 907472. Epub 20220526. doi:10.3389/fonc.2022.907472
Cheng, R., and Santos, H. A. (2022). Smart nanoparticle-based platforms for regulating tumor microenvironment and cancer immunotherapy. Adv. Healthc. Mater 2022, e2202063. Epub 20221207. doi:10.1002/adhm.202202063
Cheng, X., Wang, L., and Zhang, Z. (2022). Prognostic significance of PD-L1 expression and CD8(+) TILs density for disease-free survival in surgically resected lung squamous cell carcinoma: A retrospective study. J. Thorac. Dis. 14 (6), 2224–2234. doi:10.21037/jtd-22-630
Cho, C., Horzempa, C., Longo, C. M., Peters, D. M., Jones, D. M., and McKeown-Longo, P. J. (2020). Fibronectin in the tumor microenvironment activates a TLR4-dependent inflammatory response in lung cancer cells. J. Cancer 11 (11), 3099–3105. Epub 20200304. doi:10.7150/jca.39771
Choe, E. A., Cha, Y. J., Kim, J. H., Pyo, K. H., Hong, M. H., Park, S. Y., et al. (2019). Dynamic changes in PD-L1 expression and CD8(+) T cell infiltration in non-small cell lung cancer following chemoradiation therapy. Lung Cancer 136, 30–36. Epub 20190801. doi:10.1016/j.lungcan.2019.07.027
Conroy, M., and Naidoo, J. (2022). Immune-related adverse events and the balancing act of immunotherapy. Nat. Commun. 13 (1), 392. Epub 20220119. doi:10.1038/s41467-022-27960-2
de Lazaro, I., and Mooney, D. J. (2021). Obstacles and opportunities in a forward vision for cancer nanomedicine. Nat. Mater 20 (11), 1469–1479. Epub 20210705. doi:10.1038/s41563-021-01047-7
Dubinett, S., and Sharma, S. (2009). Towards effective immunotherapy for lung cancer: Simultaneous targeting of tumor-initiating cells and immune pathways in the tumor microenvironment. Immunotherapy 1 (5), 721–725. in: Pubmed; PMID 20636013. doi:10.2217/imt.09.56
El-Guindy, D. M., Helal, D. S., Sabry, N. M., and Abo El-Nasr, M. (2018). Programmed cell death ligand-1 (PD-L1) expression combined with CD8 tumor infiltrating lymphocytes density in non-small cell lung cancer patients. J. Egypt Natl. Canc Inst. 30 (4), 125–131. Epub 20181015. doi:10.1016/j.jnci.2018.08.003
Fang, Y., and Su, C. (2022). Research progress on the microenvironment and immunotherapy of advanced non-small cell lung cancer with liver metastases. Front. Oncol. 12, 893716. Epub 20220728. doi:10.3389/fonc.2022.893716
Fujimoto, D., Uehara, K., Sato, Y., Sakanoue, I., Ito, M., Teraoka, S., et al. (2017). Alteration of PD-L1 expression and its prognostic impact after concurrent chemoradiation therapy in non-small cell lung cancer patients. Sci. Rep. 7 (1), 11373. Epub 20170912. doi:10.1038/s41598-017-11949-9
Garcia-Fernandez, C., Fornaguera, C., and Borros, S. (2020). Nanomedicine in non-small cell lung cancer: From conventional treatments to immunotherapy. Cancers (Basel) 12 (6). doi:10.3390/cancers12061609
Gennen, K., Kasmann, L., Taugner, J., Eze, C., Karin, M., Roengvoraphoj, O., et al. (2020). Prognostic value of PD-L1 expression on tumor cells combined with CD8+ TIL density in patients with locally advanced non-small cell lung cancer treated with concurrent chemoradiotherapy. Radiat. Oncol. 15 (1), 5. Epub 20200102. doi:10.1186/s13014-019-1453-3
Genova, C., Dellepiane, C., Carrega, P., Sommariva, S., Ferlazzo, G., Pronzato, P., et al. (2021). Therapeutic implications of tumor microenvironment in lung cancer: Focus on immune checkpoint blockade. Front. Immunol. 12, 799455. Epub 20220107. doi:10.3389/fimmu.2021.799455
Graves, E. E., Maity, A., and Le, Q. T. (2010). The tumor microenvironment in non-small-cell lung cancer. Semin. Radiat. Oncol. 20 (3), 156–163. doi:10.1016/j.semradonc.2010.01.003
Guo, P. (2010). The emerging field of RNA nanotechnology. Nat. Nanotechnol. 5 (12), 833–842. Epub 20101121. doi:10.1038/nnano.2010.231
Guo, Q., Liu, L., Chen, Z., Fan, Y., Zhou, Y., Yuan, Z., et al. (2022). Current treatments for non-small cell lung cancer. Front. Oncol. 12, 945102. Epub 20220811. doi:10.3389/fonc.2022.945102
Guo, S., Feng, J., Li, Z., Yang, S., Qiu, X., Xu, Y., et al. (2022). Improved cancer immunotherapy strategies by nanomedicine. Wiley Interdiscip Rev Nanomed Nanobiotechnol, e1873. Epub 20221228. doi:10.1002/wnan.1873
Hao, Y., Zhang, X., and Yu, L. (2022). Immune checkpoint inhibitor-related pneumonitis in non-small cell lung cancer: A review. Front. Oncol. 12, 911906. Epub 20220816. doi:10.3389/fonc.2022.911906
Hassanian, H., Asadzadeh, Z., Baghbanzadeh, A., Derakhshani, A., Dufour, A., Rostami Khosroshahi, N., et al. (2022). The expression pattern of Immune checkpoints after chemo/radiotherapy in the tumor microenvironment. Front. Immunol. 13, 938063. Epub 20220728. doi:10.3389/fimmu.2022.938063
Havel, J. J., Chowell, D., and Chan, T. A. (2019). The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy. Nat. Rev. Cancer 19 (3), 133–150. doi:10.1038/s41568-019-0116-x
He, W., Zhang, Z., Yang, W., Zheng, X., You, W., Yao, Y., et al. (2022). Turing milk into pro-apoptotic oral nanotherapeutic: De novo bionic chiral-peptide supramolecule for cancer targeted and immunological therapy. Theranostics 12 (5), 2322–2334. Epub 20220221. doi:10.7150/thno.70568
He, Y., Yu, H., Rozeboom, L., Rivard, C. J., Ellison, K., Dziadziuszko, R., et al. (2017). LAG-3 protein expression in non-small cell lung cancer and its relationship with PD-1/PD-L1 and tumor-infiltrating lymphocytes. J. Thorac. Oncol. 12 (5), 814–823. Epub 20170126. doi:10.1016/j.jtho.2017.01.019
Hou, B., Chen, T., Zhang, H., Li, J., Wang, P., and Shang, G. (2023). The E3 ubiquitin ligases regulate PD-1/PD-L1 protein levels in tumor microenvironment to improve immunotherapy. Front. Immunol. 14, 1123244. Epub 20230117. doi:10.3389/fimmu.2023.1123244
Hsieh, C. H., Hsieh, H. C., Shih, F. S., Wang, P. W., Yang, L. X., Shieh, D. B., et al. (2021). An innovative NRF2 nano-modulator induces lung cancer ferroptosis and elicits an immunostimulatory tumor microenvironment. Theranostics 11 (14), 7072–7091. Epub 20210513. doi:10.7150/thno.57803
Hu, X., Lin, Z., Wang, Z., and Zhou, Q. (2021). Emerging role of PD-L1 modification in cancer immunotherapy. Am. J. Cancer Res. 11 (8), 3832–3840.
Hu, X., Wang, J., Chu, M., Liu, Y., Wang, Z. W., and Zhu, X. (2021). Emerging role of ubiquitination in the regulation of PD-1/PD-L1 in cancer immunotherapy. Mol. Ther. 29 (3), 908–919. Epub 20210101. doi:10.1016/j.ymthe.2020.12.032
Hu, Y., Paris, S., Barsoumian, H., Abana, C. O., He, K., Sezen, D., et al. (2021). A radioenhancing nanoparticle mediated immunoradiation improves survival and generates long-term antitumor immune memory in an anti-PD1-resistant murine lung cancer model. J. Nanobiotechnology 19 (1), 416. Epub 20211211. doi:10.1186/s12951-021-01163-1
Hu, Y., Paris, S., Barsoumian, H., Abana, C. O., He, K., Wasley, M., et al. (2021). Radiation therapy enhanced by NBTXR3 nanoparticles overcomes anti-PD1 resistance and evokes abscopal effects. Int. J. Radiat. Oncol. Biol. Phys. 111 (3), 647–657. Epub 20210706. doi:10.1016/j.ijrobp.2021.06.041
Hu, Y., Paris, S., Bertolet, G., Barsoumian, H. B., He, K., Sezen, D., et al. (2022). Combining a nanoparticle-mediated immunoradiotherapy with dual blockade of LAG3 and TIGIT improves the treatment efficacy in anti-PD1 resistant lung cancer. J. Nanobiotechnology 20 (1), 417. Epub 20220919. doi:10.1186/s12951-022-01621-4
Huang, A. C., and Zappasodi, R. (2022). A decade of checkpoint blockade immunotherapy in melanoma: Understanding the molecular basis for immune sensitivity and resistance. Nat. Immunol. 23 (5), 660–670. Epub 20220303. doi:10.1038/s41590-022-01141-1
Huang, T., Peng, L., Han, Y., Wang, D., He, X., Wang, J., et al. (2022). Lipid nanoparticle-based mRNA vaccines in cancers: Current advances and future prospects. Front. Immunol. 13, 922301. Epub 20220826. doi:10.3389/fimmu.2022.922301
Huang, X., Kong, N., Zhang, X., Cao, Y., Langer, R., and Tao, W. (2022). The landscape of mRNA nanomedicine. Nat. Med. 28 (11), 2273–2287. Epub 20221110. doi:10.1038/s41591-022-02061-1
Irvine, D. J., and Dane, E. L. (2020). Enhancing cancer immunotherapy with nanomedicine. Nat. Rev. Immunol. 20 (5), 321–334. Epub 20200131. doi:10.1038/s41577-019-0269-6
Jiang, W., Pan, S., Chen, X., Wang, Z. W., and Zhu, X. (2021). The role of lncRNAs and circRNAs in the PD-1/PD-L1 pathway in cancer immunotherapy. Mol. Cancer 20 (1), 116. Epub 20210908. doi:10.1186/s12943-021-01406-7
Jin, R., Liu, C., Zheng, S., Wang, X., Feng, X., Li, H., et al. (2020). Molecular heterogeneity of anti-PD-1/PD-L1 immunotherapy efficacy is correlated with tumor immune microenvironment in East Asian patients with non-small cell lung cancer. Cancer Biol. Med. 17 (3), 768–781. doi:10.20892/j.issn.2095-3941.2020.0121
Johnson, D. B., Nebhan, C. A., Moslehi, J. J., and Balko, J. M. (2022). Immune-checkpoint inhibitors: Long-term implications of toxicity. Nat. Rev. Clin. Oncol. 19 (4), 254–267. Epub 20220126. doi:10.1038/s41571-022-00600-w
Kim, E., Kim, W., Lee, S., Chun, J., Kang, J., Park, G., et al. (2017). TRAF4 promotes lung cancer aggressiveness by modulating tumor microenvironment in normal fibroblasts. Sci. Rep. 7 (1), 8923. Epub 20170821. doi:10.1038/s41598-017-09447-z
Kim, J., Hong, J., Lee, J., Fakhraei Lahiji, S., and Kim, Y. H. (2021). Recent advances in tumor microenvironment-targeted nanomedicine delivery approaches to overcome limitations of immune checkpoint blockade-based immunotherapy. J. Control Release 332, 109–126. 10.1016/j.jconrel.2021.02.002.
Kim, S. S., Harford, J. B., Moghe, M., Rait, A., and Chang, E. H. (2018). Combination with SGT-53 overcomes tumor resistance to a checkpoint inhibitor. Oncoimmunology 7 (10), e1484982. Epub 20180801. doi:10.1080/2162402X.2018.1484982
Korman, A. J., Garrett-Thomson, S. C., and Lonberg, N. (2022). The foundations of immune checkpoint blockade and the ipilimumab approval decennial. Nat. Rev. Drug Discov. 21 (7), 509–528. Epub 20211222. doi:10.1038/s41573-021-00345-8
Lee, E., and Kazerooni, E. A. (2022). Lung cancer screening. Semin. Respir. Crit. Care Med. 43 (6), 839–850. Epub 20221128. doi:10.1055/s-0042-1757885
Li, H., Luo, Q., Zhang, H., Ma, X., Gu, Z., Gong, Q., et al. (2023). Nanomedicine embraces cancer radio-immunotherapy: Mechanism, design, recent advances, and clinical translation. Chem. Soc. Rev. 52 (1), 47–96. Epub 20230103. doi:10.1039/d2cs00437b
Li, L. L., Zhou, D. X., Lu, M., Zhou, D., Lin, X. F., Chen, Y., et al. (2022). An integrated biomarker of PD-L1 expression and intraepithelial CD8(+) T cell infiltration was associated with the prognosis of lung cancer patients after intracranial resection of brain metastases. Thorac. Cancer 13 (13), 1948–1960. Epub 20220520. doi:10.1111/1759-7714.14473
Li, T., and Qiao, T. (2022). Unraveling tumor microenvironment of small-cell lung cancer: Implications for immunotherapy. Semin. Cancer Biol. 86 (2), 117–125. doi:10.1016/j.semcancer.2022.09.005
Li, W., Jiang, J., Huang, L., and Long, F. (2022). Efficacy of PD-1/L1 inhibitors in brain metastases of non-small-cell lung cancer: Pooled analysis from seven randomized controlled trials. Future Oncol. 18 (3), 403–412. Epub 20211117. doi:10.2217/fon-2021-0795
Lin, B. Q., Zeng, Z. Y., Yang, S. S., and Zhuang, C. W. (2013). Dietary restriction suppresses tumor growth, reduces angiogenesis, and improves tumor microenvironment in human non-small-cell lung cancer xenografts. Lung Cancer 79 (2), 111–117. Epub 20121128. doi:10.1016/j.lungcan.2012.11.001
Ling, B., Zhang, Z., Xiang, Z., Cai, Y., Zhang, X., and Wu, J. (2022). Advances in the application of proteomics in lung cancer. Front. Oncol. 12, 993781. Epub 20220927. doi:10.3389/fonc.2022.993781
Liu, B., Cao, W., Qiao, G., Yao, S., Pan, S., Wang, L., et al. (2019). Effects of gold nanoprism-assisted human PD-L1 siRNA on both gene down-regulation and photothermal therapy on lung cancer. Acta Biomater. 99, 307–319. Epub 20190909. doi:10.1016/j.actbio.2019.08.046
Liu, J., Chen, T., Li, S., Liu, W., Wang, P., and Shang, G. (2022). Targeting matrix metalloproteinases by E3 ubiquitin ligases as a way to regulate the tumor microenvironment for cancer therapy. Semin. Cancer Biol. 86 (2), 259–268. 10.1016/j.semcancer.2022.06.004.
Liu, J., and Shang, G. (2022). The roles of noncoding RNAs in the development of osteosarcoma stem cells and potential therapeutic targets. Front. Cell Dev. Biol. 10, 773038. Epub 20220216. doi:10.3389/fcell.2022.773038
Liu, W., Powell, C. A., and Wang, Q. (2022). Tumor microenvironment in lung cancer-derived brain metastasis. Chin. Med. J. Engl. 135 (15), 1781–1791. Epub 20220805. doi:10.1097/CM9.0000000000002127
Ma, L. R., Li, J. X., Tang, L., Li, R. Z., Yang, J. S., Sun, A., et al. (2021). Immune checkpoints and immunotherapy in non-small cell lung cancer: Novel study progression, challenges and solutions. Oncol. Lett. 22 (5), 787. Epub 20210914. doi:10.3892/ol.2021.13048
Madeddu, C., Donisi, C., Liscia, N., Lai, E., Scartozzi, M., and Maccio, A. (2022). EGFR-mutated non-small cell lung cancer and resistance to immunotherapy: Role of the tumor microenvironment. Int. J. Mol. Sci. 23 (12), 6489. Epub 20220610. doi:10.3390/ijms23126489
Mansfield, A. S., Aubry, M. C., Moser, J. C., Harrington, S. M., Dronca, R. S., Park, S. S., et al. (2016). Temporal and spatial discordance of programmed cell death-ligand 1 expression and lymphocyte tumor infiltration between paired primary lesions and brain metastases in lung cancer. Ann. Oncol. 27 (10), 1953–1958. Epub 20160808. doi:10.1093/annonc/mdw289
Mansouri, S., Heylmann, D., Stiewe, T., Kracht, M., and Savai, R. (2022). Cancer genome and tumor microenvironment: Reciprocal crosstalk shapes lung cancer plasticity. Elife 11, e79895. Epub 20220908. doi:10.7554/eLife.79895
Mao, K., Tan, Q., Ma, Y., Wang, S., Zhong, H., Liao, Y., et al. (2021). Proteomics of extracellular vesicles in plasma reveals the characteristics and residual traces of COVID-19 patients without underlying diseases after 3 months of recovery. Cell Death Dis. 12 (6), 541. 10.1038/s41419-021-03816-3.
Martinez-Usatorre, A., Kadioglu, E., Boivin, G., Cianciaruso, C., Guichard, A., Torchia, B., et al. (2021). Overcoming microenvironmental resistance to PD-1 blockade in genetically engineered lung cancer models. Sci. Transl. Med. 13 (606), eabd1616. doi:10.1126/scitranslmed.abd1616
Mazzaschi, G., Madeddu, D., Falco, A., Bocchialini, G., Goldoni, M., Sogni, F., et al. (2018). Low PD-1 expression in cytotoxic CD8(+) tumor-infiltrating lymphocytes confers an immune-privileged tissue microenvironment in NSCLC with a prognostic and predictive value. Clin. Cancer Res. 24 (2), 407–419. Epub 20171026. doi:10.1158/1078-0432.CCR-17-2156
Meng, X., Wang, J., Zhou, J., Tian, Q., Qie, B., Zhou, G., et al. (2021). Tumor cell membrane-based peptide delivery system targeting the tumor microenvironment for cancer immunotherapy and diagnosis. Acta Biomater. 127, 266–275. Epub 20210402. doi:10.1016/j.actbio.2021.03.056
Miller, K. D., Nogueira, L., Devasia, T., Mariotto, A. B., Yabroff, K. R., Jemal, A., et al. (2022). Cancer treatment and survivorship statistics. CA Cancer J. Clin. 72 (5), 409–436. Epub 20220623. doi:10.3322/caac.21731
Munari, E., Marconi, M., Querzoli, G., Lunardi, G., Bertoglio, P., Ciompi, F., et al. (2021). Impact of PD-L1 and PD-1 expression on the prognostic significance of CD8(+) tumor-infiltrating lymphocytes in non-small cell lung cancer. Front. Immunol. 12, 680973. Epub 20210526. doi:10.3389/fimmu.2021.680973
Mussafi, O., Mei, J., Mao, W., and Wan, Y. (2022). Immune checkpoint inhibitors for PD-1/PD-L1 axis in combination with other immunotherapies and targeted therapies for non-small cell lung cancer. Front. Oncol. 12, 948405. Epub 20220817. doi:10.3389/fonc.2022.948405
Nakagawa, N., and Kawakami, M. (2022). Choosing the optimal immunotherapeutic strategies for non-small cell lung cancer based on clinical factors. Front. Oncol. 12, 952393. Epub 20220812. doi:10.3389/fonc.2022.952393
Nemeth, Z., Csizmadia, E., Vikstrom, L., Li, M., Bisht, K., Feizi, A., et al. (2016). Alterations of tumor microenvironment by carbon monoxide impedes lung cancer growth. Oncotarget 7 (17), 23919–23932. in: Pubmed; PMID 26993595. doi:10.18632/oncotarget.8081
Oldani, S., Petrelli, F., Dognini, G., Borgonovo, K., Parati, M. C., Ghilardi, M., et al. (2022). COVID-19 and lung cancer survival: An updated systematic review and meta-analysis. Cancers (Basel) 14 (22). 10.3390/cancers14225706.
Persano, S., Guevara, M. L., Li, Z., Mai, J., Ferrari, M., Pompa, P. P., et al. (2017). Lipopolyplex potentiates anti-tumor immunity of mRNA-based vaccination. Biomaterials 125, 81–89. Epub 20170221. doi:10.1016/j.biomaterials.2017.02.019
Peters, S., Paz-Ares, L., Herbst, R. S., and Reck, M. (2022). Addressing CPI resistance in NSCLC: Targeting TAM receptors to modulate the tumor microenvironment and future prospects. J. Immunother. Cancer 10 (7), e004863. doi:10.1136/jitc-2022-004863
Petroni, G., Buque, A., Coussens, L. M., and Galluzzi, L. (2022). Targeting oncogene and non-oncogene addiction to inflame the tumour microenvironment. Nat. Rev. Drug Discov. 21 (6), 440–462. Epub 20220315. doi:10.1038/s41573-022-00415-5
Pramanik, A., Patibandla, S., Gao, Y., Corby, L. R., Rhaman, M. M., Sinha, S. S., et al. (2022). Bio-conjugated magnetic-fluorescence nanoarchitectures for the capture and identification of lung-tumor-derived programmed cell death lighand 1-positive exosomes. ACS Omega 7 (18), 16035–16042. Epub 20220425. doi:10.1021/acsomega.2c01210
Pu, Z., Wei, Y., Sun, Y., Wang, Y., and Zhu, S. (2022). Carbon nanotubes as carriers in drug delivery for non-small cell lung cancer, mechanistic analysis of their carcinogenic potential, safety profiling and identification of biomarkers. Int. J. Nanomedicine 17, 6157–6180. 10.2147/IJN.S384592.
Punekar, S. R., Shum, E., Grello, C. M., Lau, S. C., and Velcheti, V. (2022). Immunotherapy in non-small cell lung cancer: Past, present, and future directions. Front. Oncol. 12, 877594. Epub 20220802. doi:10.3389/fonc.2022.877594
Rashed, H. E., Abdelrahman, A. E., Abdelgawad, M., Balata, S., and Shabrawy, M. E. (2017). Prognostic significance of programmed cell death ligand 1 (PD-L1), CD8+ tumor-infiltrating lymphocytes and p53 in non-small cell lung cancer: An immunohistochemical study. Turk Patoloji Derg. 1 (1), 211–222. doi:10.5146/tjpath.2017.01398
Reda, M., Ngamcherdtrakul, W., Nelson, M. A., Siriwon, N., Wang, R., Zaidan, H. Y., et al. (2022). Development of a nanoparticle-based immunotherapy targeting PD-L1 and PLK1 for lung cancer treatment. Nat. Commun. 13 (1), 4261. Epub 20220723. doi:10.1038/s41467-022-31926-9
Sanaei, M. J., Pourbagheri-Sigaroodi, A., Kaveh, V., Abolghasemi, H., Ghaffari, S. H., Momeny, M., et al. (2021). Recent advances in immune checkpoint therapy in non-small cell lung cancer and opportunities for nanoparticle-based therapy. Eur. J. Pharmacol. 909, 174404. 10.1016/j.ejphar.2021.174404.
Schafer, C. C., Wang, Y., Hough, K. P., Sawant, A., Grant, S. C., Thannickal, V. J., et al. (2016). Indoleamine 2,3-dioxygenase regulates anti-tumor immunity in lung cancer by metabolic reprogramming of immune cells in the tumor microenvironment. Oncotarget 7 (46), 75407–75424. in: Pubmed; PMID 27705910. doi:10.18632/oncotarget.12249
Sebban, S., Farago, M., Rabinovich, S., Lazer, G., Idelchuck, Y., Ilan, L., et al. (2014). Vav1 promotes lung cancer growth by instigating tumor-microenvironment cross-talk via growth factor secretion. Oncotarget 5 (19), 9214–9226. in: Pubmed; PMID 25313137. doi:10.18632/oncotarget.2400
Seshadri, D. R., and Ramamurthi, A. (2018). Nanotherapeutics to modulate the compromised micro-environment for lung cancers and chronic obstructive pulmonary disease. Front. Pharmacol. 9, 759. 10.3389/fphar.2018.00759.
Shalom, B., Farago, M., Salaymeh, Y., Sebban, S., Risling, M., Pikarsky, E., et al. (2022). Vav1 accelerates Ras-driven lung cancer and modulates its tumor microenvironment. Cell Signal 97, 110395. Epub 20220623. doi:10.1016/j.cellsig.2022.110395
Shen, N., Yang, C., Zhang, X., Tang, Z., and Chen, X. (2021). Cisplatin nanoparticles possess stronger anti-tumor synergy with PD1/PD-L1 inhibitors than the parental drug. Acta Biomater. 135, 543–555. Epub 20210814. doi:10.1016/j.actbio.2021.08.013
Shi, S., Ye, L., Yu, X., Jin, K., and Wu, W. (2022). Focus on mast cells in the tumor microenvironment: Current knowledge and future directions. Biochim. Biophys. Acta Rev. Cancer 1878 (1), 188845. 10.1016/j.bbcan.2022.188845.
Shirasawa, M., Yoshida, T., Matsumoto, Y., Shinno, Y., Okuma, Y., Goto, Y., et al. (2020). Impact of chemoradiotherapy on the immune-related tumour microenvironment and efficacy of anti-PD-(L)1 therapy for recurrences after chemoradiotherapy in patients with unresectable locally advanced non-small cell lung cancer. Eur. J. Cancer 140, 28–36. Epub 20201008. doi:10.1016/j.ejca.2020.08.028
Shirasawa, M., Yoshida, T., Shimoda, Y., Takayanagi, D., Shiraishi, K., Kubo, T., et al. (2021). Differential immune-related microenvironment determines programmed cell death protein-1/programmed death-ligand 1 blockade efficacy in patients with advanced NSCLC. J. Thorac. Oncol. 16 (12), 2078–2090. Epub 20210820. doi:10.1016/j.jtho.2021.07.027
Siegel, R. L., Miller, K. D., Fuchs, H. E., and Jemal, A. (2022). Cancer statistics, 2022. CA Cancer J. Clin. 72 (1), 7–33. Epub 20220112. doi:10.3322/caac.21708
Sittplangkoon, C., Alameh, M. G., Weissman, D., Lin, P. J. C., Tam, Y. K., Prompetchara, E., et al. (2022). mRNA vaccine with unmodified uridine induces robust type I interferon-dependent anti-tumor immunity in a melanoma model. Front. Immunol. 13, 983000. Epub 20221014. doi:10.3389/fimmu.2022.983000
Song, M., Ping, Y., Zhang, K., Yang, L., Li, F., Zhang, C., et al. (2019). Low-dose IFNγ induces tumor cell stemness in tumor microenvironment of non-small cell lung cancer. Cancer Res. 79 (14), 3737–3748. Epub 20190513. doi:10.1158/0008-5472.CAN-19-0596
Song, Z., Wang, X., Chen, F., Chen, Q., Liu, W., Yang, X., et al. (2022). LncRNA MALAT1 regulates METTL3-mediated PD-L1 expression and immune infiltrates in pancreatic cancer. Front. Oncol. 12, 1004212. Epub 20220921. doi:10.3389/fonc.2022.1004212
Stater, E. P., Sonay, A. Y., Hart, C., and Grimm, J. (2021). The ancillary effects of nanoparticles and their implications for nanomedicine. Nat. Nanotechnol. 16 (11), 1180–1194. Epub 20211110. doi:10.1038/s41565-021-01017-9
Su, W. P., Chang, L. C., Song, W. H., Yang, L. X., Wang, L. C., Chia, Z. C., et al. (2022). Polyaniline-based glyco-condensation on Au nanoparticles enhances immunotherapy in lung cancer. ACS Appl. Mater Interfaces 14 (21), 24144–24159. Epub 20220517. doi:10.1021/acsami.2c03839
Tison, A., Garaud, S., Chiche, L., Cornec, D., and Kostine, M. (2022). Immune-checkpoint inhibitor use in patients with cancer and pre-existing autoimmune diseases. Nat. Rev. Rheumatol. 18 (11), 641–656. Epub 20221005. doi:10.1038/s41584-022-00841-0
Tokito, T., Azuma, K., Kawahara, A., Ishii, H., Yamada, K., Matsuo, N., et al. (2016). Predictive relevance of PD-L1 expression combined with CD8+ TIL density in stage III non-small cell lung cancer patients receiving concurrent chemoradiotherapy. Eur. J. Cancer 55, 7–14. Epub 20160106. doi:10.1016/j.ejca.2015.11.020
Trivanovic, D., Persuric, Z., Agaj, A., Jakopovic, M., Samarzija, M., Bitar, L., et al. (2022). Int. J. Mol. Sci. 23 (23). 10.3390/ijms232315067.
Vilarino, N., Bruna, J., Bosch-Barrera, J., Valiente, M., and Nadal, E. (2020). Immunotherapy in NSCLC patients with brain metastases. Understanding brain tumor microenvironment and dissecting outcomes from immune checkpoint blockade in the clinic. Cancer Treat. Rev. 89, 102067. Epub 20200707. doi:10.1016/j.ctrv.2020.102067
Wang, L., Xu, H., Weng, L., Sun, J., Jin, Y., and Xiao, C. (2022). Activation of cancer immunotherapy by nanomedicine. Front. Pharmacol. 13, 1041073. Epub 20221222. doi:10.3389/fphar.2022.1041073
Wang, Y., Tan, H., Yu, T., Chen, X., Jing, F., and Shi, H. (2021). Potential immune biomarker candidates and immune subtypes of lung adenocarcinoma for developing mRNA vaccines. Front. Immunol. 12, 755401. Epub 20211130. doi:10.3389/fimmu.2021.755401
Wang, Z., Xing, Y., Li, B., Li, X., Liu, B., and Wang, Y. (2022). Molecular pathways, resistance mechanisms and targeted interventions in non-small-cell lung cancer. Mol. Biomed. 3 (1), 42. Epub 20221212. doi:10.1186/s43556-022-00107-x
Weiss, J. M., Pennell, N., Deal, A. M., Morgensztern, D., Bradford, D. S., Crane, J., et al. (2020). Nab-paclitaxel in older patients with non-small cell lung cancer who have developed disease progression after platinum-based doublet chemotherapy. Cancer 126 (5), 1060–1067. Epub 20200114. doi:10.1002/cncr.32573
Wu, J., and Lin, Z. (2022). Non-small cell lung cancer targeted therapy: Drugs and mechanisms of drug resistance. Int. J. Mol. Sci. 23 (23), 15056. Epub 20221201. doi:10.3390/ijms232315056
Wu, S. P., Liao, R. Q., Tu, H. Y., Wang, W. J., Dong, Z. Y., Huang, S. M., et al. (2018). Stromal PD-L1-positive regulatory T cells and PD-1-positive CD8-positive T cells define the response of different subsets of non-small cell lung cancer to PD-1/PD-L1 blockade immunotherapy. J. Thorac. Oncol. 13 (4), 521–532. Epub 20171218. doi:10.1016/j.jtho.2017.11.132
Xu, H. Z., Li, T. F., Wang, C., Ma, Y., Liu, Y., Zheng, M. Y., et al. (2021). Synergy of nanodiamond-doxorubicin conjugates and PD-L1 blockade effectively turns tumor-associated macrophages against tumor cells. J. Nanobiotechnology 19 (1), 268. Epub 20210906. doi:10.1186/s12951-021-01017-w
Xu, R., Lu, T., Zhao, J., Wang, J., Peng, B., and Zhang, L. (2022). Identification of tumor antigens and immune subtypes in lung adenocarcinoma for mRNA vaccine development. Front. Cell Dev. Biol. 10, 815596. Epub 20220221. doi:10.3389/fcell.2022.815596
Yang, H., Shi, J., Lin, D., Li, X., Zhao, C., Wang, Q., et al. (2018). Prognostic value of PD-L1 expression in combination with CD8(+) TILs density in patients with surgically resected non-small cell lung cancer. Cancer Med. 7 (1), 32–45. Epub 20171123. doi:10.1002/cam4.1243
Yang, Z., Gao, D., Zhao, J., Yang, G., Guo, M., Wang, Y., et al. (2023). Thermal immuno-nanomedicine in cancer. Nat. Rev. Clin. Oncol. 20 (2), 116–134. Epub 20230105. doi:10.1038/s41571-022-00717-y
Ye, H., He, X., and Feng, X. (2020). Developing neobavaisoflavone nanoemulsion suppresses lung cancer progression by regulating tumor microenvironment. Biomed. Pharmacother. 129, 110369. Epub 20200618. doi:10.1016/j.biopha.2020.110369
Yoneda, K., Kuwata, T., Kanayama, M., Mori, M., Kawanami, T., Yatera, K., et al. (2019). Alteration in tumoural PD-L1 expression and stromal CD8-positive tumour-infiltrating lymphocytes after concurrent chemo-radiotherapy for non-small cell lung cancer. Br. J. Cancer 121 (6), 490–496. Epub 20190807. doi:10.1038/s41416-019-0541-3
Yu, L., Sun, M., Zhang, Q., Zhou, Q., and Wang, Y. (2022). Harnessing the immune system by targeting immune checkpoints: Providing new hope for Oncotherapy. Front. Immunol. 13, 982026. Epub 20220908. doi:10.3389/fimmu.2022.982026
Yu, P., He, X., Lu, F., Li, L., Song, H., and Bian, X. (2022). Research progress regarding long-chain non-coding RNA in lung cancer: A narrative review. J. Thorac. Dis. 14 (8), 3016–3029. doi:10.21037/jtd-22-897
Zhang, L., Chen, Y., Wang, H., Xu, Z., Wang, Y., Li, S., et al. (2021). Massive PD-L1 and CD8 double positive TILs characterize an immunosuppressive microenvironment with high mutational burden in lung cancer. J. Immunother. Cancer 9 (6), e002356. doi:10.1136/jitc-2021-002356
Zhang, M., Yang, W., Wang, P., Deng, Y., Dong, Y. T., Liu, F. F., et al. (2020). CCL7 recruits cDC1 to promote antitumor immunity and facilitate checkpoint immunotherapy to non-small cell lung cancer. Nat. Commun. 11 (1), 6119. Epub 20201130. doi:10.1038/s41467-020-19973-6
Zhang, Q., Zhong, H., Fan, Y., Liu, Q., Song, J., Yao, S., et al. (2020). Immune and clinical features of CD96 expression in glioma by in silico analysis. Front. Bioeng. Biotechnol. 8, 592. Epub 20200630. doi:10.3389/fbioe.2020.00592
Zhao, D., Liu, X., Shan, Y., Li, J., Cui, W., Wang, J., et al. (2022). Recognition of immune-related tumor antigens and immune subtypes for mRNA vaccine development in lung adenocarcinoma. Comput. Struct. Biotechnol. J. 20, 5001–5013. Epub 20220905. doi:10.1016/j.csbj.2022.08.066
Zhao, J., Xu, R., Lu, T., Wang, J., and Zhang, L. (2022). Identification of tumor antigens and immune subtypes in lung squamous cell carcinoma for mRNA vaccine development. J. Thorac. Dis. 14 (9), 3517–3530. doi:10.21037/jtd-22-1113
Zhao, Y., Guo, S., Deng, J., Shen, J., Du, F., Wu, X., et al. (2022). VEGF/VEGFR-targeted therapy and immunotherapy in non-small cell lung cancer: Targeting the tumor microenvironment. Int. J. Biol. Sci. 18 (9), 3845–3858. Epub 20220529. doi:10.7150/ijbs.70958
Zhong, H., Lai, Y., Zhang, R., Daoud, A., Feng, Q., Zhou, J., et al. (2020). Low dose cyclophosphamide modulates tumor microenvironment by TGF-beta signaling pathway. Int. J. Mol. Sci. 21 (3), 957. Epub 20200131. doi:10.3390/ijms21030957
Zhong, H., Liu, S., Cao, F., Zhao, Y., Zhou, J., Tang, F., et al. (2021). Dissecting tumor antigens and immune subtypes of glioma to develop mRNA vaccine. Front. Immunol. 12, 709986. Epub 20210827. doi:10.3389/fimmu.2021.709986
Zhou, B., Zang, R., Zhang, M., Song, P., Liu, L., Bie, F., et al. (2022). Identifying novel tumor-related antigens and immune phenotypes for developing mRNA vaccines in lung adenocarcinoma. Int. Immunopharmacol. 109, 108816. Epub 20220430. doi:10.1016/j.intimp.2022.108816
Zhou, J., Gong, Z., Jia, Q., Wu, Y., Yang, Z. Z., and Zhu, B. (2018). Programmed death ligand 1 expression and CD8(+) tumor-infiltrating lymphocyte density differences between paired primary and brain metastatic lesions in non-small cell lung cancer. Biochem. Biophys. Res. Commun. 498 (4), 751–757. Epub 20180317. doi:10.1016/j.bbrc.2018.03.053
Zhou, J. G., Zhong, H., Zhang, J., Jin, S. H., Roudi, R., and Ma, H. (2019). Development and validation of a prognostic signature for malignant pleural mesothelioma. Front. Oncol. 9, 78. Epub 20190215. doi:10.3389/fonc.2019.00078
Zhuo, Y., Li, S., Hu, W., Zhang, Y., Shi, Y., Zhang, F., et al. (2022). Targeting SNORA38B attenuates tumorigenesis and sensitizes immune checkpoint blockade in non-small cell lung cancer by remodeling the tumor microenvironment via regulation of GAB2/AKT/mTOR signaling pathway. J. Immunother. Cancer 10 (5), e004113. doi:10.1136/jitc-2021-004113
Zulfiqar, B., Farooq, A., Kanwal, S., and Asghar, K. (2022). Immunotherapy and targeted therapy for lung cancer: Current status and future perspectives. Front. Pharmacol. 13, 1035171. 10.3389/fphar.2022.1035171.
Keywords: nanoparticles improve immunotherapy TME, PD-L1, PD-1, nanoparticles, immunotherapy, resistance
Citation: Zhang X, Wang X, Hou L, Xu Z, Liu Y and Wang X (2023) Nanoparticles overcome adaptive immune resistance and enhance immunotherapy via targeting tumor microenvironment in lung cancer. Front. Pharmacol. 14:1130937. doi: 10.3389/fphar.2023.1130937
Received: 23 December 2022; Accepted: 16 March 2023;
Published: 24 March 2023.
Edited by:
Hongzhou Cai, Nanjing Medical University, ChinaReviewed by:
Lizhi Zhang, Mayo Clinic, United StatesHong Shu, Guangxi Medical University Cancer Hospital, China
Copyright © 2023 Zhang, Wang, Hou, Xu, Liu and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xueju Wang, xueju@jlu.edu.cn
†These authors have contributed equally to this work
 Xin Zhang
Xin Zhang Xuemei Wang1†
Xuemei Wang1† Yu’e Liu
Yu’e Liu Xueju Wang
Xueju Wang