Corrigendum: Pharmacokinetics and bioequivalence study of esomeprazole magnesium enteric-coated tablets 20 mg in healthy Chinese subjects under fasting and fed conditions
- Drug Clinical Trial Institution, Wuxi People’s Hospital Affiliated with Nanjing Medical University, Wuxi, Jiangsu, China
Objective: The main purpose of this study was to evaluate the pharmacokinetics, bioequivalence, and safety properties between a new generic and a brand reference formulation of esomeprazole enteric-coated tablets 20 mg in healthy Chinese subjects under fasting and fed conditions.
Methods: The fasting study was an open-label, randomized, two-period crossover study conducted in 32 healthy Chinese volunteers, and the fed study was a four-period crossover study conducted in 40 healthy Chinese volunteers. Blood samples were collected at the specified time points and determined to obtain the plasma concentrations of esomeprazole. The primary pharmacokinetic parameters were calculated using the non-compartment method. Bioequivalence was analyzed by the geometric mean ratios (GMRs) of the two formulations and the corresponding 90% confidence intervals (CIs). The safety of the two formulations was assessed.
Results: The fasting and fed study showed that the pharmacokinetics of the two formulations was similar. Under the fasting condition, the 90% CIs of GMRs of the test-to-reference formulation were 87.92%–104.36% for Cmax, 87.82%–101.45% for AUC0-t, and 87.99%–101.54% for AUC0-∞; under the fed condition, the 90% CIs of GMRs of the test-to-reference formulation were 80.53%–94.95% for Cmax, 87.46%–97.26% for AUC0-t, and 87.46%–97.16% for AUC0-∞. The 90% CIs of GMRs fall within the bioequivalence range of 80.00%–125.00%. The two formulations had good safety and were well-tolerated, and no serious adverse events occurred.
Conclusion: According to relevant regulatory standards, esomeprazole enteric-coated generic and reference products exhibited bioequivalence and good safety in healthy Chinese subjects.
Clinical Trials Registration: http://www.chinadrugtrials.org.cn/index.html, identifier CTR20171347 and CTR20171484
Introduction
Gastroesophageal reflux disease (GERD) refers to the discomfort and/or complications caused by the flow of stomach contents back into the esophagus; heartburn and reflux are the typical symptoms (Vakil N et al., 2006). GERD has high morbidity, which is increasing gradually. The incidence rate of GERD in economically developed countries is about 15%–25%, which is higher than that in most economically underdeveloped countries (<10%) (El-Serag et al., 2014; Richter et al., 2018). GERD can lead to a decline in quality of life, and its recurrence and the requirement of long-term treatment can require a large number of medical resources and bring high costs to society (Kulig et al., 2003; Becher et al., 2011).
Effective control of the symptoms of GERD, prevention of the complications of GERD, and improvement of the patient’s quality of life are the main goals of GERD treatment (Dickman et al., 2015). At present, in addition to histamine type 2 receptor antagonists (H2RAs) commonly used in the clinic, proton pump inhibitors (PPIs) are the first-line drugs for GERD treatment. Research and development of PPIs have completely revolutionized the management of acid reflux. PPIs can significantly inhibit gastric acid secretion, improve the healing rate of the esophageal mucosa, and effectively control GERD symptoms (Gralnek et al., 2006).
Esomeprazole is the S-isomer of omeprazole and the first single optical isomer in the PPI family. Compared with other PPI drugs, esomeprazole has higher systemic bioavailability because it has hepatic first-pass metabolic effect and lower plasma clearance rate (Lindberg P et al., 2003). Many clinical studies have proved that esomeprazole can further improve the efficacy for GERD and other diseases related to gastric acid secretion, including gastric and duodenal ulcer, non-erosive reflux disease (NERD), and Zollinger–Ellison syndrome (Mckeage et al., 2008).
Because esomeprazole is unstable in an acidic environment, it is taken orally in the form of enteric-coated tablets. Currently, esomeprazole enteric-coated tablets at 20 mg and 40 mg, developed by AstraZeneca UK Limited, obtained marketing approval in over 125 countries. Some articles have reported the pharmacokinetic profile of esomeprazole (Hassan-Alin et al., 2000; Andersson et al., 2001). After administering 20 mg or 40 mg of enteric-coated tablets, the plasma concentrations of esomeprazole reach the peak value (Cmax) within 1–2 h (Hassan-Alin et al., 2005). The absolute bioavailability of 40 mg esomeprazole after single oral or repeated daily administration was 64% and 89%, respectively (Hassan-Alin et al., 2005). The absolute bioavailability of 20 mg of a single dose and multiple doses was 50% and 68%, respectively, which was less than that of 40 mg. The human plasma protein binding rate of esomeprazole was 97% in vitro. Although food intake can delay absorption and reduce the exposure of esomeprazole in the human body, it has no substantial effect on inhibiting the gastric acid secretion of esomeprazole (AstraZeneca UK Limited, 2022; AstraZeneca UK Limited, 2022). Esomeprazole is mainly metabolized by cytochrome
The two dosages (40 mg and 20 mg) of esomeprazole enteric-coated tablet (Nexium®, AstraZeneca UK Limited) were approved and listed in China in 2003. Until now, only one paper reported the pharmacokinetics of esomeprazole enteric-coated tablets in Chinese subjects, and the administration dosage in the study was 40 mg (Liu et al., 2020), which indicated that the pharmacokinetic data of esomeprazole enteric-coated tablets at 20 mg were lacking in the Chinese population. In addition, due to the difficulty of pharmaceutical preparations, there were no domestic generic drugs of 20 mg esomeprazole enteric-coated tablets marketed in China before this study. Recently, a generic esomeprazole enteric-coated tablet at 20 mg was researched by Jiangxi Shanxiang Pharmaceutical Co., Ltd. (Jiangxi, China). In order to meet the requirements of generic drug listing in China, we compared the bioavailability of the generic esomeprazole enteric-coated tablet at 20 mg with that of the original product (Nexium®, AstraZeneca UK Limited). According to the guidelines related to bioequivalence, two studies under fasting and feeding conditions need to be carried out in healthy Chinese subjects. The bioequivalence results obtained in the study will provide the listing support for the generic drug in China.
Methods
Study drugs
The 20 mg esomeprazole enteric-coated tablet used as the test product was produced and provided by Jiangxi Shanxiang Pharmaceutical Co., Ltd. (Batch No. 170701, valid until June 2019). Meanwhile, a 20 mg esomeprazole enteric-coated tablet manufactured by AstraZeneca UK Limited (Nexium®, Batch No. MW325, valid until June 2019) was chosen as the reference product, which was also provided by Jiangxi Shanxiang Pharmaceutical Co., Ltd. In each treatment period, each subject received the test preparation or reference preparation of esomeprazole enteric-coated tablet 20 mg.
Subjects
Eligible subjects needed to meet the following basic criteria: both men and women, aged over 18 years, weight of male subjects greater than 50 kg, weight of female subjects greater than 45 kg, and body mass index (BMI) within the range of 19.0–26.0 kg/m2. Clinical investigators introduced the study’s purpose, procedure, and potential risks to each subject in detail, and each one voluntarily signed an informed consent form after full consideration before participating in the study. After signing the informed consent forms, all enrolled subjects underwent a series of inquiries and examinations, including examination of demographic data, past medical history, physical examination, vital signs, clinical laboratory tests, serum immunovirological examination, a 12-lead electrocardiogram (ECG), X-ray of chest, and abdomen B-ultrasound evaluation. If these examination results were normal or abnormal but clinically insignificant, judged by research doctors, subjects were considered healthy and allowed to participate in the study.
Subjects with a history of severe digestive tract diseases or allergies to esomeprazole or its analogs were excluded because these abnormalities may potentially affect the absorption or disposition of esomeprazole. If subjects donated blood or had acute blood loss (blood volume of more than 450 mL) in the past 3 months, they were excluded. Subjects were excluded if they had a history of taking medicine within 30 days prior to screening, this covered all medicines including Chinese herbal medicine and or vitamin products, or if they took part in other clinical trials within 3 months prior to screening.
Meanwhile, lactating women or female subjects testing positive for pregnancy before enrollment were not allowed to participate in the study. All subjects had no fertility planning, and they and their partners were willing to take effective contraception and were not involved in egg or sperm donation programs.
Study design
This study was conducted in the Phase I Clinical Research Center of Wuxi People’s Hospital Affiliated with Nanjing Medical University (Chinese Clinical Trial Registry, Registration Nos CTR20171347 and CTR20171484; http://www.chinadrugtrials.org.cn/index.html) following the guidelines of Good Clinical Practice (GCP) and the ethical principles of the Declaration of Helsinki. The relative study documents, including study protocol, informed consent form, and other files, were reviewed and approved by the independent ethics committee of Wuxi People’s Hospital Affiliated with Nanjing Medical University with the approval number 2017LLPJ-I-17 (Wuxi, Jiangsu, China).
The study consisted of two mutually independent clinical trials, including a fasting bioequivalence study and a feeding bioequivalence study. Regarding study design, according to the within-subject variability of esomeprazole, we comprehensively evaluated how many periods of cross-over tests should be carried out. Some reported references (Cai et al., 2011; Ullah et al., 2010; Sostek et al., 2007) showed that the within-subject coefficient of variation for (CVw%) esomeprazole under fasting and fed conditions were about 20% and 40%, respectively. Hence, we adopted the study design of a two-period crossover in the fasting study and a four-period crossover in the fed study. In terms of sample size estimation, we set CVw% of Cmax and AUC0-∞ for esomeprazole to 20% and 40%, respectively; the significance level to 0.05; the power of the test to 0.8; the GMR of the two formulations to 0.95; and the equivalence threshold to 80.00%–125.00%. After calculation, we estimated the sample size to be 26 and 34 for the two trials, respectively. In addition, considering the possibility of early withdrawal of individual subjects and a drop-off rate of 20%, we finally enrolled 32 and 40 subjects to participate in the two trials.
Fasting bioequivalence study
The fasting study adopted an open-label, randomized, two-formulation, two-period crossover, single-dose study design. In total, 32 enrolled subjects were admitted to the Phase I ward the day before administration. After completing the relevant examination before check-in, according to the random number table generated by SAS software (version 9.4), 32 subjects were randomly divided into the TR or RT groups in a ratio of 1:1. After fasting for at least 10 h overnight, subjects in the TR group received the test preparation and the reference preparation, respectively, in two treatment periods, while subjects in RT group took the opposite. The washout period between the two treatment periods was 7 days. After each administration, the subjects underwent a thorough oral examination to confirm they had taken the drug. The subjects were forbidden to drink water within 1 h prior to and after administration and needed to fast for at least 4 h after administration before they accepted standard lunch. The safety assessment was conducted throughout the study.
Fed bioequivalence study
The fed study adopted an open-label, randomized, two-product, four-period crossover, single-dose study design. On the day before administration, 40 enrolled subjects were admitted to the Phase I ward. After completing the relevant examination before check-in, according to the random number table also generated by SAS software (version 9.4), 40 subjects were randomly divided into the TRTR group or RTRT group in a ratio of 1:1. The washout period between each treatment period was also 7 days. After fasting for at least 10 h overnight, the subjects started the standard high-fat and high-caloric breakfast 30 min before administration and completed it within 30 min. The calorific value of a standard high-fat breakfast was about 800–1000 kcal, including 150 kcal of protein, 250 kcal of carbohydrates, and 500–600 kcal of fat. Then, the subjects followed the same study procedure as in the fasting study.
Pharmacokinetic sample collection and analysis methods
Vacuum tubes (4 mL) with EDTA-K2 anticoagulant were used to collect blood samples for detecting plasma concentrations. The time points for blood sample collection for the two trials were slightly different. In the fasting study, the time points for blood sample collection were 0 h (predose) and 20 min, 40 min, 1, 1.25, 1.5, 1.75, 2, 2.25, 2.5, 3, 3.5, 4, 5, 6, 8, 10, 12, and 24 h after administration. In the fed study, the time points for blood sample collection were 0 h (predose) and 30 min, 1, 1.5, 2, 2.5, 3, 3.5, 4, 4.5, 5, 5.5, 6, 7, 8, 10, 12, and 24 h after administration. After blood collection, sample centrifugation needed to be completed within 1 h, and the centrifugation condition was 2,152 g 4°C for 10 min. Then, the plasma was added separately into two tubes containing 30-µL carbonate buffer (
The plasma samples were determined using HPLC-MS/MS methods. Before determining the plasma samples, the analytical method had been fully verified by linearity, sensitivity, specificity, intra- and inter-batch precision, accuracy, recovery, matrix effect, and stability. The standard curve range of esomeprazole was set to 3.0–3,000 ng/mL.
Pharmacokinetic and statistical analysis
We used the non-compartmental method to analyze pharmacokinetics, and the analysis software used was Phoenix WinNonlin software (version 8.2) from Pharsight Corporation (California). The primary pharmacokinetic parameters included Cmax, the time of reach to Cmax (Tmax), the area under the plasma concentration–time curve from time 0 to the time of the last measurable concentration (AUC0-t), the area under the plasma concentration–time curve from time 0 to infinity (AUC0-∞), and the terminal elimination half-life (t1/2). Cmax and Tmax were the actual observed measure values without analysis and calculation. AUC0-t was calculated using the linear/log trapezoidal method. AUC0-∞ was calculated as AUC0-∞ = AUC0-t + Ct/λz, where Ct was the last detectable concentration and λz was the elimination rate constant. λz was estimated using linear least-squares regression analysis for the concentration–time data obtained from the terminal log-linear phase. The results of t1/2 were obtained by dividing 0.693 by λz.
For the fasting study, an analysis of variance (ANOVA) was performed to compare the log-transformed pharmacokinetic parameters (Cmax, AUC0-t, and AUC0-∞) between the two formulations. The bioequivalence was evaluated by calculating the 90% CIs of the geometric least-squares mean (GLSM) ratio of the test preparation to reference preparation. If these 90% CIs were within the range of 80.00%–125.00%, the two formulations were considered bioequivalent.
For the fed study, the log-transformed pharmacokinetic parameters (Cmax, AUC0-t, and AUC0-∞) were also analyzed using ANOVA between the two formulations. Unlike the fasting study, in the fed study, we calculated the within-subject standard deviation (Swr) of Cmax, AUC0-t, and AUC0-∞. Swr was determined by the pharmacokinetic parameters of the reference formulation, and the value of Swr was determined for which the bioequivalence evaluation method was selected. For Cmax, AUC0-t, and AUC0-∞, (1) if Swr <0.294, average bioequivalence (ABE) was selected for analysis. Two one-sided t-tests with α = 0.05 were used to test the statistical hypothesis. Whether the 90% CIs of GLSM ratios of Cmax, AUC0-t, and AUC0-∞ of the test and reference formulation were within the range of 80.00%–125.00% determined if the two formulations were bioequivalent; and (2) if Swr ≥0.294, the reference-scale average bioequivalence (RSABE) was selected for analysis. The two formulations were considered bioequivalent if the statistical results met the following two criteria at the same time: (a) the upper bound of 95% CI of the test and reference formula (criteria bound) was less than or equal to 0, and (b) the point estimates of GMR of Cmax, AUC0-t, and AUC0-∞ of the test to reference formulation fall within the range of 80.00%–125.00%.
Safety assessment
During the study, safety was evaluated through clinical observation monitored by research doctors and adverse events (AEs) reported spontaneously by volunteers. At predefined time points, all subjects received vital signs, physical examinations, 12-lead ECG, and clinical laboratory tests. Vital signs were measured within 1 h before administration and 2, 8, and 24 h after administration in each treatment period. Clinical laboratory tests, 12-lead ECG, and physical examinations were conducted at the screening and end of the trial. For standardization of the AE report, we coded the name of all AEs in terms of the Medical Dictionary of Regulatory Activities (MedDRA®)and graded the severity of these AEs in accordance with the Common Terminology Criteria for Adverse Events (CTCAE, Version 5.0) published by the National Cancer Institute of the United States.
Results
Study population
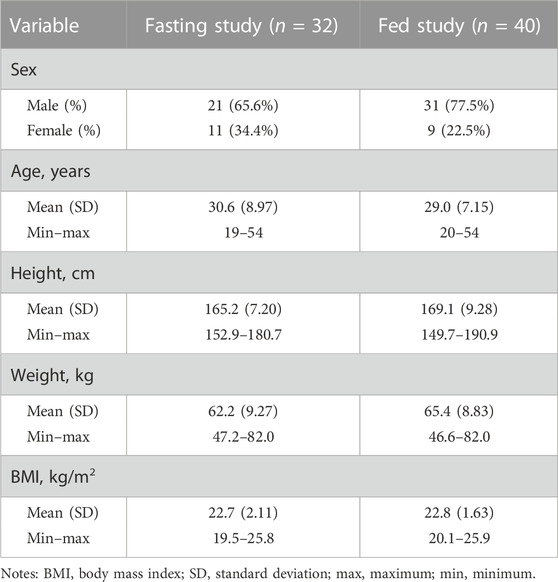
Figure 1 shows the study design and disposition of subjects in the two trials. In total, 92 and 108 healthy Chinese adult subjects in the two studies signed informed consent forms and participated in screening. In total, 32 and 40 healthy adult subjects met the enrollment criteria and were enrolled in the two studies. The fasting study included 21 male (65.6%) and 11 female subjects (34.4%), age 30.6 ± 8.97, and BMI 22.7 ± 2.11 kg/m2, and the fed study included 31 male (77.5%) and nine female subjects (22.5%), age 29.0 ± 7.15, and BMI 22.8 ± 1.63 kg/m2. The baseline demographic characteristics of the study population are shown in Table 1. A total of 32 subjects completed the whole study under fasting conditions, and 37 subjects completed four treatment periods under fed conditions, except for three subjects who withdrew from the study due to AEs in different treatment periods.
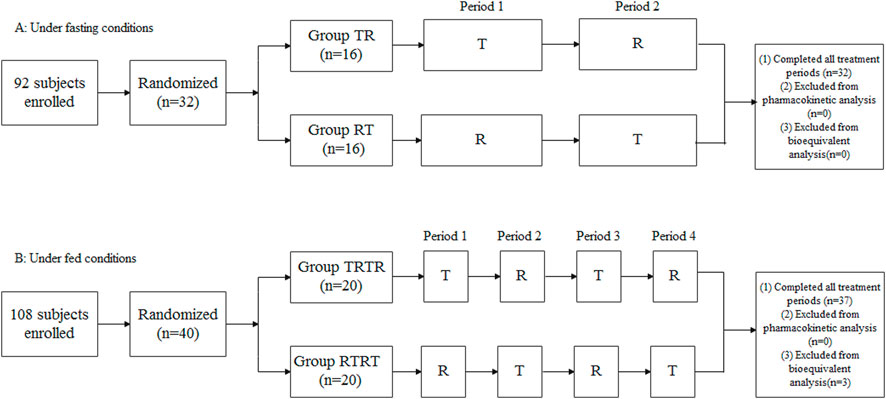
FIGURE 1. Study design and disposition of subjects. (A) Under fasting conditions, (B) Under fed conditions.
Pharmacokinetics and bioequivalence
In the fasting study, the data of 32 enrolled subjects were entered into the pharmacokinetic analysis. Figure 2 shows the mean plasma concentration–time curves of the two formulations under fasting conditions. The changing trend of the two plasma concentration–time curves is very similar. The primary pharmacokinetic parameters are listed in Table 2. For the test or reference formulation, Cmax were 769.13 ± 322.72 and 808.03 ± 354.40 ng/mL, AUC0-t were 1809.10 ± 1248.65 and 1934.00 ± 1412.94 ng h/m, AUC0-∞ were 1827.56 ± 1270.32 and 1946.32 ± 1421.02 ng h/m, and t1/2 were 1.27 ± 0.64 and 1.25 ± 0.64 h. The results of bioequivalence assessment of the fasting study are shown in Table 3. Cmax, AUC0-t, and AUC0-∞ had no period, sequence, or formulation effect. The 90% CIs of the GLSM ratios of the test to the reference formulation were 87.92%–104.36% for Cmax, 87.82%–101.45% for AUC0-t, and 87.99%–101.54% for AUC0-∞. The 90% CIs were within the accepted bioequivalence range of 80.00%–125.00%, which suggested that the two formulations of esomeprazole exhibited bioequivalence under fasting conditions. The CVw% for Cmax, AUC0-t, and AUC0-∞ were 20.41%, 17.13%, and 17.00%, respectively, indicating that esomeprazole has no high variability from the perspective of pharmacokinetics. The powers of Cmax, AUC0-t, and AUC0-∞ were >95%, proving that our expected sample size is enough and reasonable.
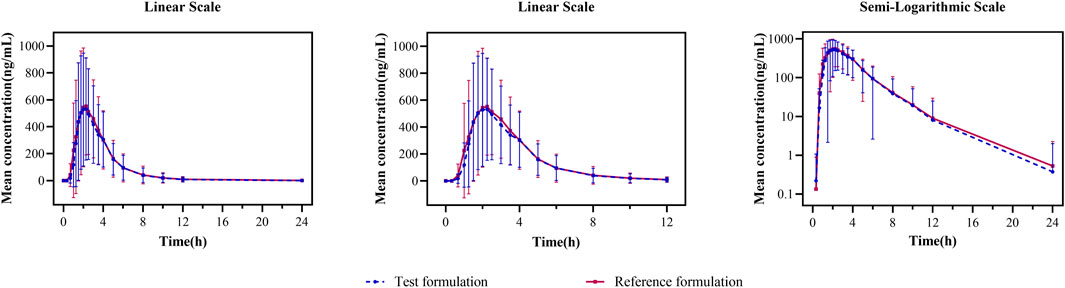
FIGURE 2. Mean plasma concentration–time curves for the test and reference formulations after a single dose of 20 mg of the esomeprazole enteric-coated tablet in healthy subjects under fasting conditions. N = 32. Bars represent SD. T: test formulation; R: reference formulation.
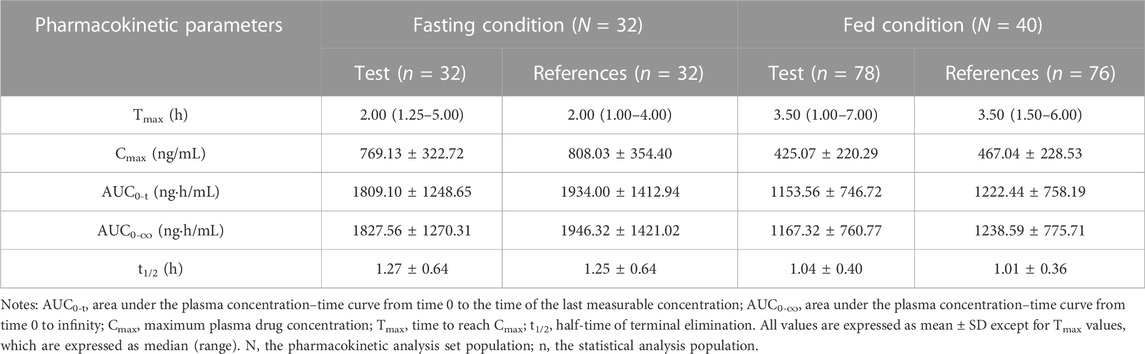
TABLE 2. Pharmacokinetic parameters of esomeprazole after a single dose 20 mg of test and reference esomeprazole magnesium enteric-coated tablet in healthy Chinese subjects under fasting and fed conditions.
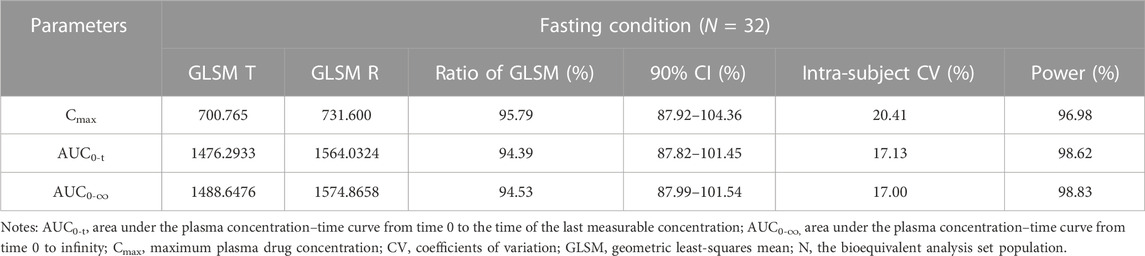
TABLE 3. Bioequivalence assessment of pharmacokinetic parameters of esomeprazole after a single dose 20 mg of test and reference esomeprazole magnesium enteric-coated tablet in healthy Chinese subjects under fasting conditions.
In the fed study, the data of 40 enrolled subjects were entered into the pharmacokinetic analysis. Figure 3 shows the mean plasma concentration–time curves of the two formulations under fed conditions. The changing trend of the four plasma concentration–time curves is also very similar. The primary pharmacokinetic analysis parameters are also listed in Table 2. For the test or reference formulation, Cmax were 425.07 ± 220.29 and 467.04 ± 228.53 ng/mL, AUC0-t were 1153.56 ± 746.72 and 1222.44 ± 758.19 ng h/m, AUC0-∞ were 1167.32 ± 760.77 and 1238.59 ± 775.71 ng h/m, and t1/2 were 1.04 ± 0.40 and 1.01 ± 0.36 h. The results of bioequivalence assessment of the fed study are shown in Table 4. The SWR for Cmax, AUC0-t, and AUC0-∞ of reference formulation were 0.2473, 0.1708, and 0.1686, respectively, which were less than 0.294. As a result, the ABE method was selected to evaluate the bioequivalence. The 90% CIs of the GLSM ratios were 80.53%–94.95% for Cmax, 87.46%–97.26% for AUC0-t, and 87.46%–97.17% for AUC0-∞, which were also within the accepted bioequivalence range of 80.00%–125.00%. These results suggested that the two formulations of esomeprazole exhibited bioequivalence under fed conditions. The CVw% for Cmax, AUC0-t, and AUC0-∞ were 25.12%, 17.20%, and 16.98%, respectively, which further showed that esomeprazole does not show high variability, regardless of fasting or fed conditions.
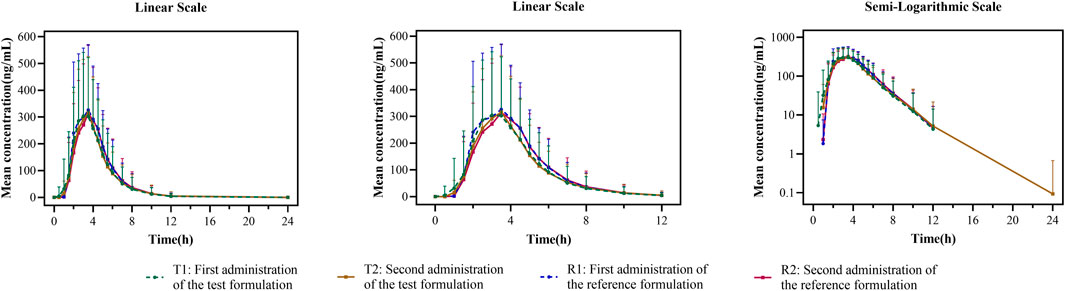
FIGURE 3. Mean plasma concentration–time curves for the test and reference formulations after a single dose of 20 mg of the esomeprazole enteric-coated tablet in healthy subjects under fed conditions. N = 40. Bars represent SD. T1: first administration of the test formulation; T2: second administration of the test formulation; R1: first administration of the reference formulation; R2: second administration of the reference formulation.
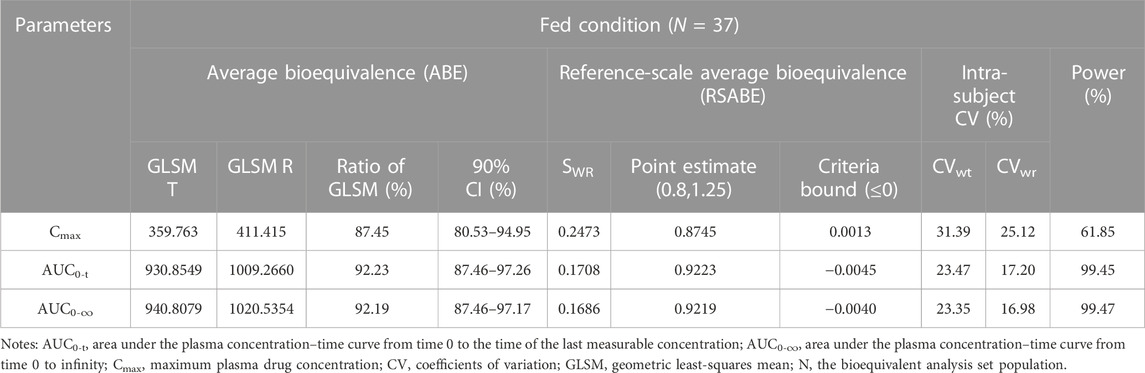
TABLE 4. Bioequivalence assessment of pharmacokinetic parameters of esomeprazole after a single dose 20 mg of test and reference esomeprazole magnesium enteric-coated tablet in healthy Chinese subjects under fed conditions.
Safety profile
In these two studies, the safety assessment set included a total of 72 subjects who received at least one dose of esomeprazole. In the fasting study, four AEs occurred in two subjects with an incidence of 6.3% (2/32). The four AEs were leukocyte count decrease, neutrophil count decrease, leukocyte count increase, and neutrophil count increase. The intensity of all the AEs was mild and graded as Grade 1. The outcome of all the AEs was recovered, and the subjects received no treatment. In the fed study, 17 AEs occurred in 11 subjects with an incidence of 27.5 (11/40). Among these AEs, eight AEs in five subjects were evaluated to be related to the study drug, including anemia, leukocyte count decrease, neutrophil count decrease, eosinophil count increase, fever, and blood bilirubin increase. In addition, three subjects did not complete the trial and withdrew early in the different treatment periods due to their respective AEs, including upper respiratory tract infection, fever, and chickenpox. No serious AEs occurred in both studies. These safety results showed that esomeprazole had good safety and was well-tolerated in healthy subjects under fasting and fed conditions.
Discussion
Although esomeprazole has been gradually used to treat GERD and other gastric acid-related diseases since 2003, there is relatively little public information on the pharmacokinetics of esomeprazole enteric-coated tablets 20 mg, especially among Chinese people. The present study fully compared and evaluated the pharmacokinetics and bioequivalence between a generic formulation and the original drug of the esomeprazole enteric-coated tablet in healthy Chinese subjects under fasting and feeding conditions.
We compared the primary pharmacokinetic parameters of esomeprazole enteric-coated tablets 20 mg with those previously reported under fasting and fed conditions. Under the fasting condition, Tmax and t1/2 of esomeprazole were about 2 and 1.2 h, respectively, similar to those in previous reports (Hassan-Alin et al., 2000; Hassan-Alin et al., 2005; AstraZeneca, 2022), which validated that the esomeprazole enteric-coated tablet is rapidly absorbed and eliminated after oral administration. However, in the present fasting study, the results of Cmax and AUC of esomeprazole were different from those previously reported (Andersson et al., 2001; Sostek et al., 2007; Ullah et al., 2010), but were consistent with those found in the literature on Chinese subjects (Liu et al., 2020). The research subjects in the previous studies were of Caucasian ethnicity, while our research subjects were of Asian ethnicity. In our study, Cmax was lower than that in the previous research results, but AUC was higher than that of the previous research results. The reason for the decrease in Cmax is unclear, and it may be related to the lower body weight or BMI of the Chinese subjects in this study than that of the Caucasian subjects in previous studies. The possible reason for the differences in systemic drug exposure (as indicated by AUC) relates to esomeprazole’s main metabolic enzyme CYP2C19. As known, the frequency distribution of CYP2C19 gene polymorphism has obvious ethnic and regional differences. It is reported that the incidence of poor metabolizer (PM) of CYP2C19 in Caucasians is 3%–5%, while the incidence of
Furthermore, we compared the pharmacokinetic profiles of esomeprazole under fasting and fed conditions in the two studies. Table 2 shows clearly that the absorption rate and absorption degree of esomeprazole were obviously affected by food, regardless of the use of test or reference products. When the subjects took esomeprazole enteric-coated tablets after high-fat breakfast, Cmax and AUC of esomeprazole decreased by about 40%; meanwhile, Tmax of esomeprazole was delayed from 2 to 3.5 h. Eating high-fat food may be the main factor causing these changes. As known, enteric-coated tablets are disintegrated and absorbed in the intestinal fluid. High-fat and high-calorie food can prominently reduce the rate of gastric emptying and prolong the time for the drug to reach the intestine tract (Deng et al., 2017), thus reducing the absorption rate and degree of the enteric-coated tablets. Therefore, compared with the fasting trial results, the Tmax values of esomeprazole were longer under fed conditions, while the Cmax and AUC values were lower. These results of the present study further supported the opinion that food can delay the absorption of esomeprazole and reduce its exposure in the human body (AstraZeneca, 2022).
Conclusion
The present study demonstrated that the test formulation and reference formulation of esomeprazole enteric-coated tablets exhibited bioequivalence in healthy Chinese subjects. In addition, the two preparations were well-tolerated, and there were no major safety problems. The test formulation became the first generic drug of esomeprazole enteric-coated tablets of 20 mg in China. The two formulations can replace each other in clinical use, reducing the economic burden on patients with GERD and other acid-related diseases in China to a certain extent. In addition, the current research results can provide an important reference for developing generic esomeprazole enteric-coated tablets.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved, before the study was carried out, by the independent ethics committee of Wuxi People’s Hospital Affiliated with Nanjing Medical University with the approval number of 2017LLPJ-I-17. The patients/participants provided their written informed consent to participate in this study.
Author contributions
QH and YD designed the study. YD, LQ, KH, YC, WQ, ZQ, YS, and ZX performed the clinical study. NC wrote and revised the manuscript.
Funding
This work was supported by the Wuxi Public Service Platform for Clinical Research and Evaluation of New Drugs and Medical Devices (GGFWPT 2019) and Jiangxi Shanxiang Pharmaceutical Co., Ltd.
Conflict of interest
Funder Jiangxi Shanxiang Pharmaceutical Co., Ltd. approved the publication of the study.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abelo, A., Andersson, T. B., Antonsson, M., Naudot, A. K., Skånberg, I., and Weidolf, L. (2000). Stereoselective metabolism of omeprazole by human cytochrome P450 enzymes. Drug Metab. Dispos. 28, 966–972.
Andersson, T., Hassan-Alin, M., Hasselgren, G., Röhss, K., and Weidolf, L. (2001). Pharmacokinetic studies with esomeprazole, the (S)-isomer of omeprazole. Clin. Pharmacokinet. 40, 411–426. doi:10.2165/00003088-200140060-00003
AstraZeneca (2022). Nexium (esomeprazole magnesium) prescribing information: Delayed-release capsules and delayed-release oral suspension. Available from: https://www.astrazeneca-us.com/medicines/astrazeneca-medications.html#! (Accessed Apr 14, 2022).
AstraZeneca UK Limited (2022). Nexium 20mg gastro-resistant tablets. Available from: https://www.medicines.org.uk/emc/product/4657/smpc#PHARMACOKINETIC_PROPS (Accessed Apr 14, 2022).
Becher, A., and El-Serag, H. (2011). Systematic review: The association between symptomatic response to proton pump inhibitors and health-related quality of life in patients with gastro-oesophageal reflux disease. Aliment. Pharmacol. Ther. 34, 618–627. doi:10.1111/j.1365-2036.2011.04774.x
Cai, W., wang, Z. T., Li, J., Hu, J. J., and Zhong, J. (2011). Bioequivalence study of two esomeprazole enteric coated formulations in healthy Chinese volunteers. Arzneimittelforschung 61, 502–505. doi:10.1055/s-0031-1296234
Deng, J. Y., Zhu, X., Chen, Z. M., Fan, C. H., Kwan, H. S., Wong, C. H., et al. (2017). A review of food-drug interactions on oral drug absorption. Drugs 77, 1833–1855. doi:10.1007/s40265-017-0832-z
Dickman, R., Maradey-Romero, C., Gingold-Belfer, R., and Fass, R. (2015). Unmet needs in the treatment of gastroesophageal reflux disease. J. Neurogastroenterol. Motil. 21, 309–319. doi:10.5056/jnm15105
El-Serag, H. B., Sweet, S., Winchester, C. C., and Dent, J. (2014). Update on the epidemiology of gastro-oesophageal reflux disease: A systematic review. Gut 63, 871–880. doi:10.1136/gutjnl-2012-304269
Gong, J., Hansen, L., and Iacono, L. (2018). Clinical pharmacokinetics and the impact of genetic polymorphism on a CYP2C19 substrate, BMS-823778, in healthy subjects. Healthy Subj. Drug Metab. Dispos. 46, 316–325. doi:10.1124/dmd.117.078824
Gralnek, I. M., Dulai, G. S., Fennerty, M. B., and Spiegel, B. M. R. (2006). Esomeprazole versus other proton pump inhibitors in erosive esophagitis: A meta-analysis of randomized clinical trials. Clin. Gastroenterol. Hepatol. 4, 1452–1458. doi:10.1016/j.cgh.2006.09.013
Hassan-Alin, M., Andersson, T., Bredberg, E., and Röhss, K. (2000). Pharmacokinetics of esomeprazole after oral and intravenous administration of single and repeated doses to healthy subjects. Eur. J. Clin. Pharmacol. 56, 665–670. doi:10.1007/s002280000206
Hassan-Alin, M., Andersson, T., Niazi, M., and Röhss, K. (2005). A pharmacokinetic study comparing single and repeated oral doses of 20 mg and 40 mg omeprazole and its two optical isomers, S-omeprazole(esomeprazole) and R-omeprazole, in healthy subjects. Eur. J. Clin. Pharmacol. 60, 779–784. doi:10.1007/s00228-004-0841-1
Hasselgren, G., Hassan-Alin, M., Andersson, T., Claar-Nilsson, C., and Röhss, K. (2001). Pharmacokinetic study of esomeprazole in the elderly. Clin. Pharmacokinet. 40, 145–150. doi:10.2165/00003088-200140020-00006
Hokimoto, S., Mizobe, M., Akasaka, T., Arima, Y., Kaikita, K., Nakagawa, K., et al. (2014). Impact of CYP2C19 polymorphism and proton pump inhibitors on platelet reactivity to clopidogrel and clinical outcomes following stent implantation. Thromb. Res. 133, 599–605. doi:10.1016/j.thromres.2014.01.003
Kulig, M., Leodolter, A., Vieth, M., Schulte, E., Jaspersen, D., Labenz, J., et al. (2003). Quality of life in relation to symptoms in patients with gastro-oesophageal reflux disease: An analysis based on the ProGERD initiative. Aliment. Pharmacol. Ther. 18, 767–776. doi:10.1046/j.1365-2036.2003.01770.x
Lindberg, P., Keeling, D., Fryklund, J., Andersson, T., Lundborg, P., and Carlsson, E. (2003). Review article: Esomeprazole-enhanced bio-availability, specificity for the proton pump and inhibition of acid secretion. Aliment. Pharmacol. Ther. 17, 481–488. doi:10.1046/j.1365-2036.2003.01481.x
Liu, Z. Z., Ren, Q., Zhou, Y. N., and Yang, H. M. (2020). Bioequivalence of two esomeprazole magnesium enteric-coated formulations in healthy Chinese subjects. World J. Clin. Cases 8, 5518–5528. doi:10.12998/wjcc.v8.i22.5518
Mckeage, K., Blich, S. K. A., Croxtall, J. D., Lyseng-Williamson, K. A., and Keating, G. M. (2008). Esomeprazole a review of its use in the management of gastric acid-related diseases in adults. Drugs 68, 1571–1607. doi:10.2165/00003495-200868110-00009
Naesdal, J., Andersson, T., Bodemar, G., Larsson, R., Regårdh, C. G., Skånberg, I., et al. (1986). Pharmacokinetics of [14C]omeprazole in patients with impaired renal function. Clin. Pharmacol. Ther. 40, 344–351. doi:10.1038/clpt.1986.186
Richter, J. E., and Rubenstein, J. H. (2018). Presentation and epidemiology of gastroesophageal reflux disease. Gastroenterology 154, 267–276. doi:10.1053/j.gastro.2017.07.045
Sjovall, H., Bjornsson, E., Holmberg, J., Hasselgren, G., Röhss, K., and Hassan-Alin, M. (2002). Pharmacokinetic study of esomeprazole in patients with hepatic impairment. Eur. J. Gastroenterol. Hepatol. 14, 491–496. doi:10.1097/00042737-200205000-00005
Sostek, M. B., Chen, Y., and Andersson, T. (2007). Effect of timing of dosing in relation to food intake on the pharmacokinetics of esomeprazole. Br. J. Clin. Pharmacol. 64, 386–390. doi:10.1111/j.1365-2125.2007.02889.x
Tanaka, T., Yamagami, H., Ihara, M., Miyata, T., Miyata, S., Hamasaki, T., et al. (2019). Association of CYP2C19 polymorphisms with clopidogrel reactivity and clinical outcomes in chronic ischemic stroke. Circ. J. 83, 1385–1393. doi:10.1253/circj.CJ-18-1386
Ullah, M. A., Dowla, S. U., Maruf, A. A., Azad, M. A. K., Shohag, M. H., Sultana, R., et al. (2010). Relative bioavailability and pharmacokinetic properties of two different enteric formulations of esomeprazole in healthy Bangladeshi male volunteers: An open-label, single-dose, randomized-sequence, two-way crossover study. Clin. Ther. 32, 1419–1426. doi:10.1016/j.clinthera.2010.07.007
Keywords: esomeprazole magnesium enteric-coated tablet, bioequivalence, pharmacokinetics, safety, healthy Chinese subjects
Citation: Ding Y, Chu N, Que L, Huang K, Chen Y, Qin W, Qian Z, Shi Y, Xu Z and He Q (2023) Pharmacokinetics and bioequivalence study of esomeprazole magnesium enteric-coated tablets 20 mg in healthy Chinese subjects under fasting and fed conditions. Front. Pharmacol. 14:1169103. doi: 10.3389/fphar.2023.1169103
Received: 18 February 2023; Accepted: 14 April 2023;
Published: 28 April 2023.
Edited by:
Yousuf Hussain Mohammed, The University of Queensland, AustraliaReviewed by:
Sojeong Yi, United States Food and Drug Administration, United StatesViera Lukacova, Simulations Plus, United States
Copyright © 2023 Ding, Chu, Que, Huang, Chen, Qin, Qian, Shi, Xu and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qing He, heqing0510@163.com
†These authors have contributed equally to this work and share first authorship
 Ying Ding†
Ying Ding† Nannan Chu
Nannan Chu Linling Que
Linling Que Kai Huang
Kai Huang Wei Qin
Wei Qin