- 1Medical College of Nantong University, Nantong, China
- 2Department of Anesthesiology, Hai’an Hospital of Traditional Chinese Medicine, Hai’an, China
- 3Department of Anesthesiology, Funing People’s Hospital of Jiangsu, Yancheng, China
Aim: There is no meta-analysis reporting the analgesic effect and safety of bupivacaine in patients undergoing hemorrhoidectomy. This meta-analysis provides quantitative evidence of the effect of bupivacaine in hemorrhoidectomy.
Methods: Studies were searched from PubMed, Embase, the Cochrane Library, and the Web of Science. Standardized mean difference (SMD), weighted mean difference (WMD), and odds ratios (ORs) with 95% confidence interval (CI) were used as effect indicators. Heterogeneity was assessed using the I2 index, and sensitivity analysis was conducted to determine the effect of the single study on the pooled results.
Results: A total of 18 studies were included in this meta-analysis. The pain level at 48 h was lower in the bupivacaine-combined other drug group than in the other drug group (WMD = −0.65, 95% CI: 1.18 to −0.11, and I2 = 37.50%). Compared to the bupivacaine group, the odds of pruritus (OR = 12.11, 95% CI: 1.49–98.59, and I2 = 0%) and urinary retention (OR = 4.45, 95% CI: 1.12–17.70, and I2 = 0%) were higher, and the pain level at 6 h (WMD = −2.13, 95% CI: 3.22 to −1.04, and I2 = 64.30%), at 12 h (WMD = −1.55, 95% CI: 2.19 to −0.90, and I2 = 56.10%), and at 24 h (SMD = −1.15, 95% CI: 1.89 to −0.42, and I2 = 82.5%) were lower in the bupivacaine-combined other drug group.
Conclusion: Bupivacaine-combined other drugs had a good analgesic effect after hemorrhoidectomy, but the adverse reactions should be considered.
Introduction
Hemorrhoids are the most common disease in and around the anus, with clinical symptoms including itching, bleeding, pain, and lumps near the anus (Sandler and Peery, 2019). Grade III or IV hemorrhoids require surgical treatments because they do not respond to pharmacotherapy (Zhang et al., 2020). Hemorrhoidectomy is a common surgery used to treat grade III and IV internal hemorrhoids and extensive external hemorrhoids; however, this surgery may lead to severe postoperative pain (Lohsiriwat and Jitmungngan, 2022). Pain and nausea are two common complications after hemorrhoidectomy, which lead to increased postoperative medication intake, delayed discharge, and frequent hospitalization (Medina-Gallardo et al., 2017). Therefore, pain prevention and management after hemorrhoidectomy is an emphasized problem for patients with hemorrhoids.
Some local anesthetics have been used, but the action duration of the anesthetics still needs to be prolonged to meet patient requirements (Farag and Esmat, 2016). Bupivacaine is a long-acting local anesthetic drug (Farag and Esmat, 2016). Like other local anesthetics, bupivacaine exerts its effect by inhibiting the initiation and conduction of nerve impulses, providing a non-opioid analgesic effect (Chitty et al., 2022). Previous studies have reported the analgesic effect of bupivacaine in patients undergoing hemorrhoidectomy (Hatami et al., 2022; Steen et al., 2022). Hatami et al. (2022) reported that the pain level was lower in the bupivacaine group than in the placebo group at 2 h, 4 h, 8 h, 12 h, and 24 h after hemorrhoidectomy in patients with grade III and IV hemorrhoids. Steen et al. (2022) reported that bupivacaine reduced the pain level of patients with symptomatic hemorrhoids after hemorrhoidectomy. However, there were some limitations in the individual original studies, including insufficient sample size or being limited to one region. A meta-analysis is a powerful tool that can combine the results of two or more individual studies, demonstrates a good evidence advantage, and contributes to healthcare decision making (Mohd-Ali and Chen, 2021; Ozek et al., 2021). Jiang et al. (2023) conducted a meta-analysis and reported the effect of bupivacaine on the postoperative analgesic, rehabilitation, and safety outcomes for surgical wound infiltration. However, there is no meta-analysis reporting the analgesic effect and safety of bupivacaine in patients undergoing hemorrhoidectomy.
Therefore, we performed a systematic review and meta-analysis based on previously published studies, which included a larger sample size on a worldwide basis, to comprehensively explore the effect of bupivacaine on analgesia and safety after hemorrhoidectomy.
Methods
Literature search strategy
This meta-analysis was performed according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Page et al., 2021). Literature search was performed in PubMed, Embase, the Cochrane Library, and the Web of Science up to June 2023. The literature search and screening were performed by two independent researchers (HXL and MC), and dispute was solved by discussion to reach consensus. The search terms are given in Supplementary File S1.
Inclusion and exclusion criteria
Studies meeting the following criteria were included: 1) patients undergoing hemorrhoidectomy; 2) intervention and control: bupivacaine-combined other drugs vs. other drugs, bupivacaine vs. other drugs, liposomal bupivacaine vs. other drugs, and bupivacaine combined other drugs vs. bupivacaine; 3) outcomes: analgesic effect and safety; and 4) study type: randomized controlled trials and cohort study.
Other drugs included hormones, antibiotics, non-steroidal drugs, and opioid drugs. The analgesic effect was assessed using the immediate pain level, pain level at 6 h, 12 h, 24 h, and 48 h and defecation, pain-free time, and cumulative pain intensity score. Safety was assessed by adverse reactions (nausea, urinary retention, bleeding, vomiting, dyschezia, fever, and pruritus), length of hospital stay, and time to restore daily life.
Studies meeting the following criteria were excluded: 1) animal studies; 2) not published in English; 3) case reports, conference abstracts, guidelines and expert consensus, editorial material, reviews, and meta-analysis; and 4) not relevant to the topic.
Data extraction and quality appraisal
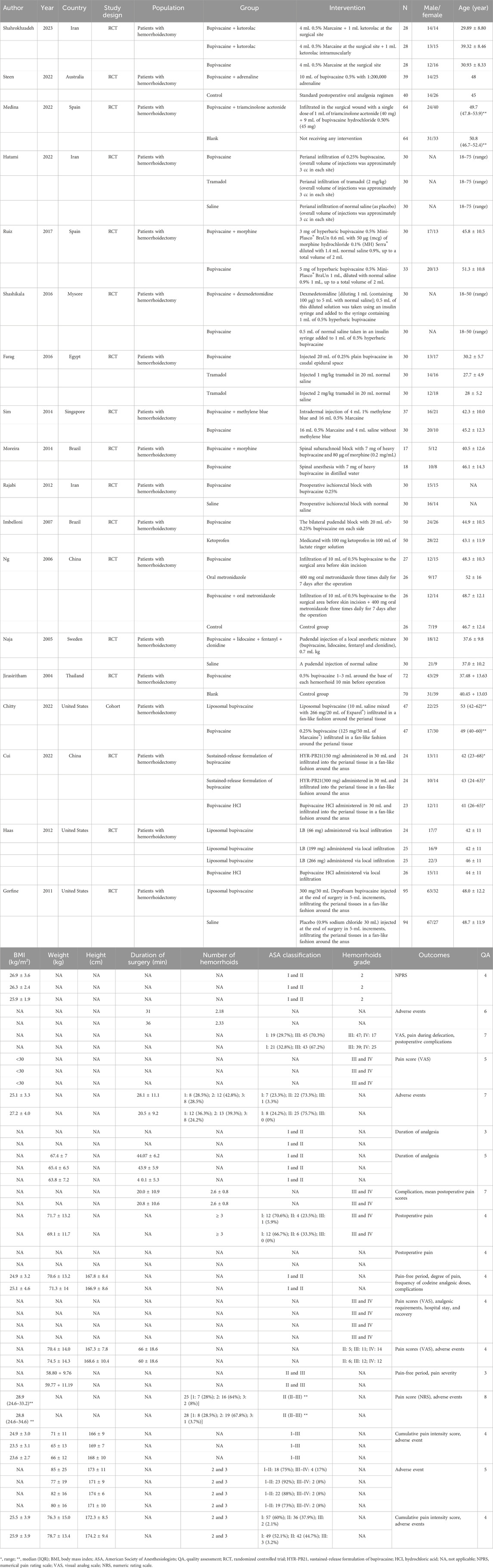
Two researchers (HXL and MC) independently extracted the following data: the first author, year of publication, country, study design, population, groups, intervention, sample size, gender, age, body mass index (BMI), duration of surgery, number of hemorrhoids, American Society of Anesthesiologists (ASA) classification, and hemorrhoid grade. A third researcher (HZC) provided consultation if conflicts existed.
The quality of cohort studies was assessed using the Newcastle–Ottawa Scale (NOS), which is a 9-point scale and divided the studies into poor (0–3 points), fair (4–6 points), and good quality (7–9 points) (Wells et al., 2011). The RCT quality was assessed using the modified Jadad scale, which is a 7-point scale and divided the studies into poor quality (1–3 points) and good quality (4–7 points) (Jadad et al., 1996; Chen et al., 2020).
Statistical analysis
For the pain level assessed using different pain scales, the standardized mean difference (SMD) and 95% confidence interval (CI) were estimated. For the pain level assessed using the same pain scale, the weighted mean difference (WMD) and 95% CI were estimated. The odds ratios (ORs) and 95% CI were estimated for the categorical data. Heterogeneity between studies was assessed using the I2 index. A random-effects model was used if I2 ≥ 50%, and a fixed-effects model was used if I2 < 50%. Sensitivity analysis was performed to determine the effect of the individual included study on the pooled results by eliminating the studies one by one. All statistical analyses were performed using STATA (15.1) (StataCorp, College Station, TX, United States).
Results
Study selection and characteristics of selected studies
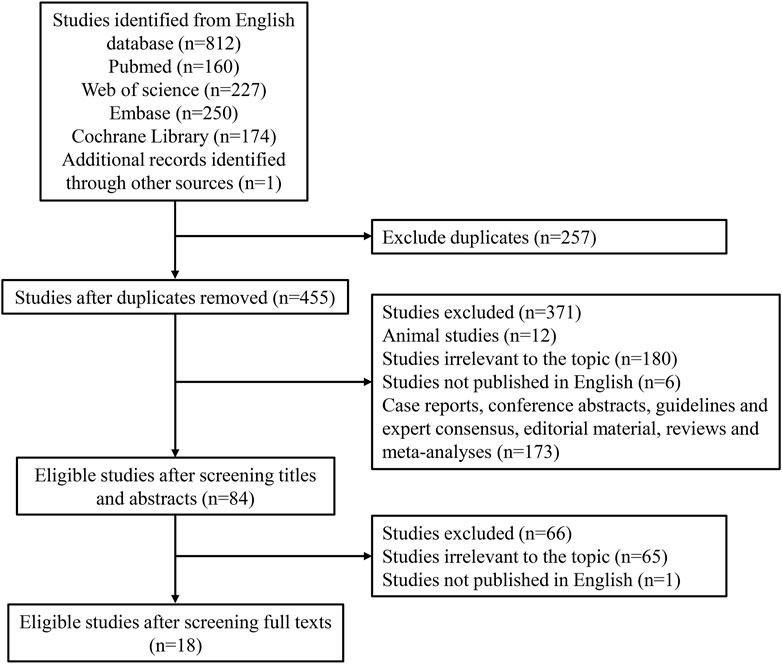
Figure 1 demonstrates the search process, which identified a total of 812 citations using the search strategy. Of these, 257 duplicates were excluded. After screening abstracts or titles, 12 animal studies, 180 irrelevant studies, 6 non-English studies, and 173 other study types (case reports, conference abstracts, guidelines and expert consensus, editorial material, reviews, and meta-analyses) were excluded. Furthermore, 65 irrelevant studies and 1 non-English study were excluded based on full-text reading. Finally, 18 eligible studies were included in our meta-analysis (Jirasiritham et al., 2004; Naja et al., 2005; Ng et al., 2006; Imbelloni et al., 2007; Gorfine et al., 2011; Haas et al., 2012; Rajabi et al., 2012; Moreira et al., 2014; Sim and Tan, 2014; Farag and Esmat, 2016; Shashikala and Prathibha, 2016; Ruiz-Castro et al., 2017; Chitty et al., 2022; Cui et al., 2022; Hatami et al., 2022; Medina-Gallardo et al., 2022; Steen et al., 2022; Shahrokhzadeh et al., 2023). The characteristics of the 18 eligible studies are shown in Table 1. There were 17 randomized controlled trials and 1 cohort study. Two studies were assessed to be of low quality, and 16 studies were assessed to be of high quality.
Effect of bupivacaine on analgesia and safety after hemorrhoidectomy
Comparing the bupivacaine-combined other drug group with the other drug group, the pain level at 48 h in the bupivacaine-combined other drug group was lower than in the other drug group, with the pooled WMD of −0.65 (95% CI: −1.18 to −0.11, I2 = 37.50%) (Figure 2). There was no significant difference in the pain level immediately, at 24 h, and at defecation, time to restore daily life, and urinary retention between the bupivacaine-combined other drug group and the other drug group (all p > 0.05).
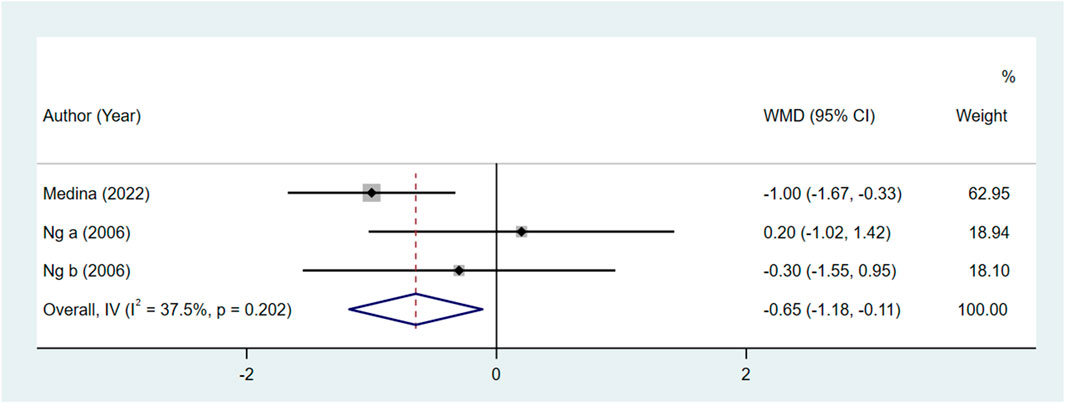
Figure 2. Forest plot of the pain level at 48 h between the bupivacaine-combined other drug group and other drug group.
Comparing the bupivacaine-combined other drug group with the bupivacaine group, we found that the odds of pruritus (OR = 12.11, 95% CI: 1.49–98.59, and I2 = 0%) (Figure 3A) and urinary retention (OR = 4.45, 95% CI: 1.12–17.70, and I2 = 0%) (Figure 3B) were higher in the bupivacaine-combined other drug group than in the bupivacaine group. The pain level at 6 h (WMD = −2.13, 95% CI: 3.22 to −1.04, and I2 = 64.30%) (Figure 3C) and 12 h (WMD = −1.55, 95% CI: 2.19 to −0.90, and I2 = 56.10%) (Figure 3D) was found to be lower in the combination group than in the bupivacaine group. The pooled results also showed that the pain level at 24 h was lower in the bupivacaine-combined other drug group than in the bupivacaine group (SMD = −1.15, 95% CI: −1.89 to −0.42, and I2 = 82.5%) (Figure 3E).
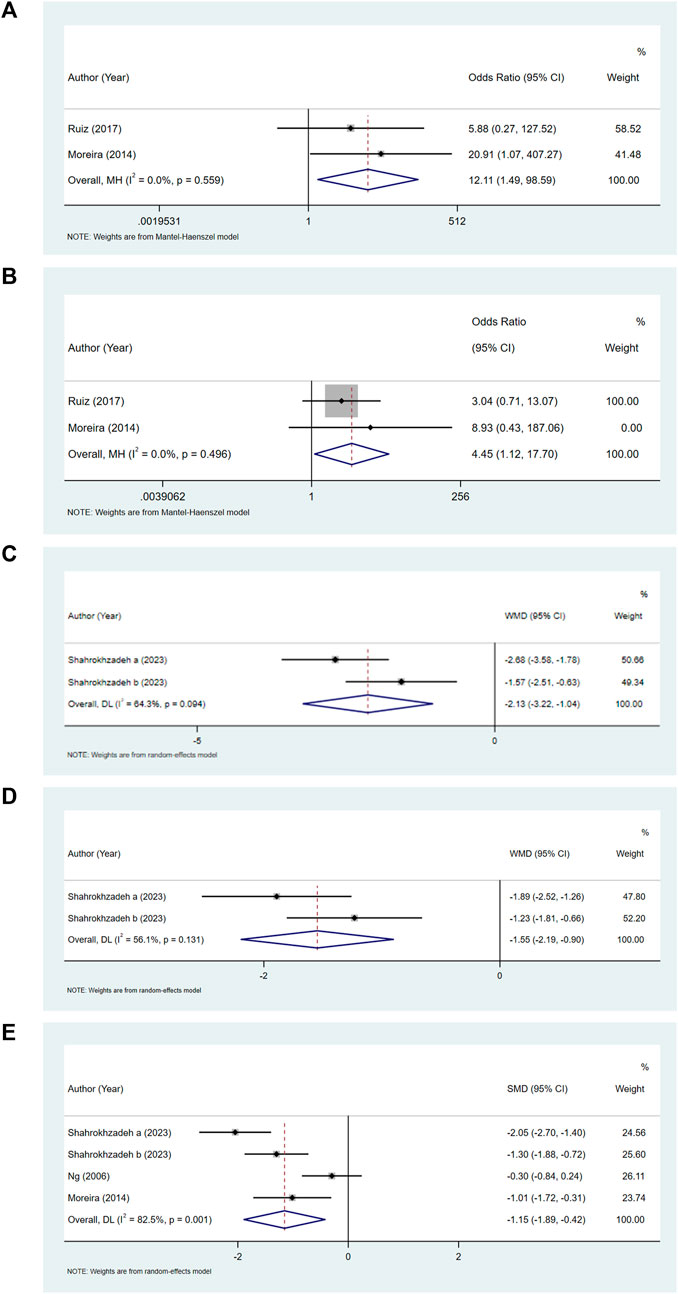
Figure 3. Forest plot of pruritus (A), urinary retention (B), and pain level at 6 h (C), 12 h (D), and 24 h (E).
Comparing the bupivacaine group with other drug group, no significance was observed in the pain level at 12 h, at 24 h, and at defecation and pain-free time between the two groups (all p > 0.05).
Comparing the liposomal bupivacaine group with other drugs, we also observed no significance in the cumulative pain intensity score, vomiting, dyschezia, and fever between the two groups (all p > 0.05).
Publication bias and sensitivity analysis
Sensitivity analysis was carried out via eliminating the studies one by one to determine the effect of the individual included study on the pooled results. The results displayed that the pooled results were not affected by omitting the individual study (Table 2). Publication bias was tested if more than nine studies were included for one outcome (Sterne et al., 2011). In this meta-analysis, there were no more than nine studies combined for analysis under one outcome; therefore, publication bias was not assessed.
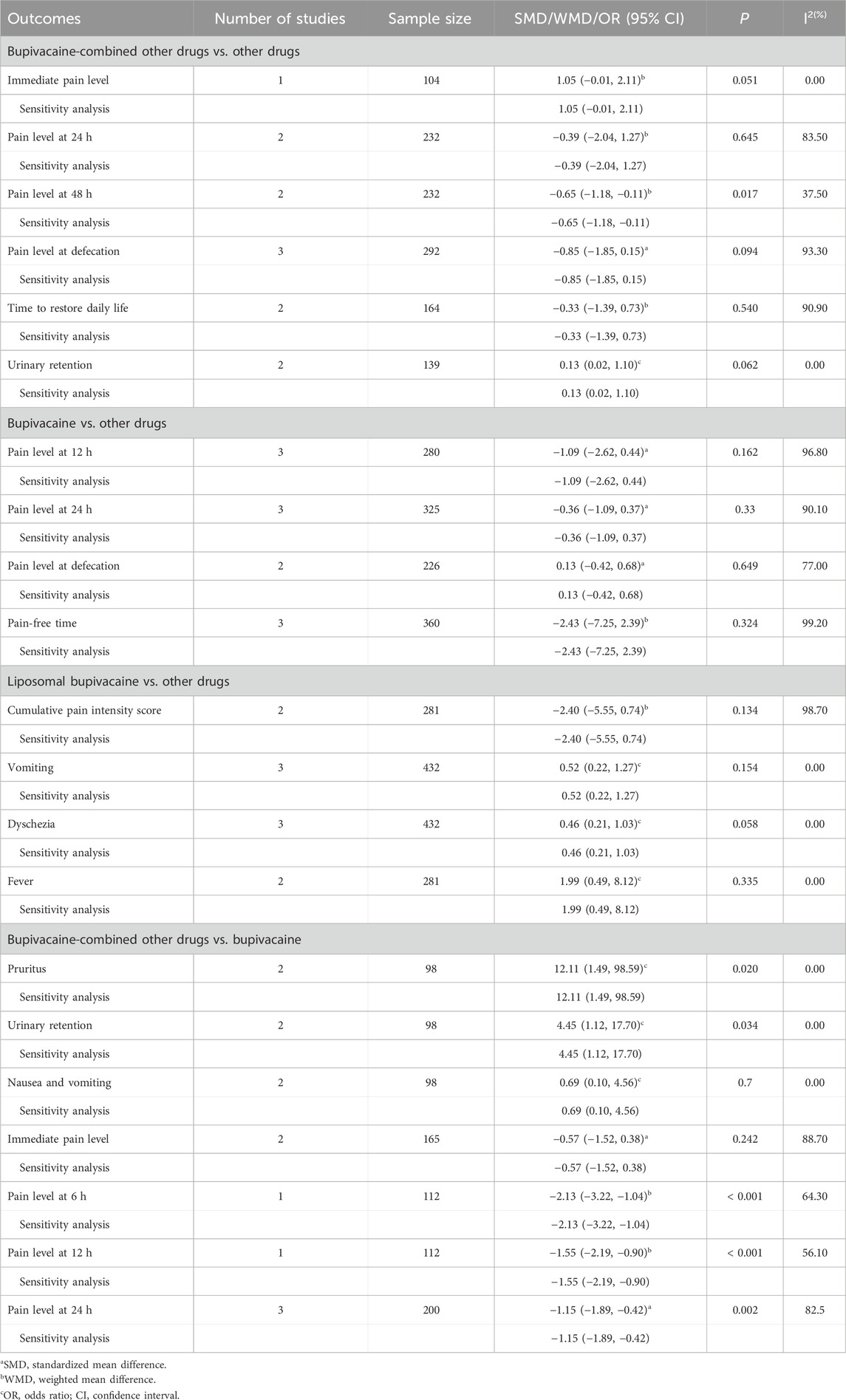
Table 2. Meta-analysis results of effect of bupivacaine on analgesia and safety after hemorrhoidectomy.
Discussion
Bupivacaine is a long-term local anesthetic drug that provides non-opioid analgesic effects by inhibiting the initiation and transmission of nerve impulses (Farag and Esmat, 2016; Chitty et al., 2022). Previous studies have reported the analgesic effect and safety of bupivacaine in patients undergoing hemorrhoidectomy (Hatami et al., 2022; Steen et al., 2022), while some limitations existed in the individual original studies, such as smaller sample size or performed in only one region. The meta-analysis could combine the results of two or more individual studies and demonstrate a good evidence advantage. This meta-analysis provided quantitative evidence of the effect of bupivacaine in hemorrhoidectomy. According to the analysis of our study, compared to the other drug group, the bupivacaine-combined other drug group induced a significant reduction in the postoperative pain level at 48 h. Compared to the bupivacaine group, the bupivacaine-combined other drug group decreased the postoperative pain level at 6 h, 12 h, and 24 h while increasing the odds of pruritus and urinary retention. In general, these findings confirmed the analgesic effects of bupivacaine-combined other drugs in hemorrhoidectomy, but the adverse reactions should be considered.
Postoperative pain remains a significant problem in hemorrhoidectomy because up to 40% of patients experience severe pain (Medina-Gallardo et al., 2022). Preemptive analgesia is a simple method to decrease the level and duration of postoperative pain (Van Backer et al., 2018). It is believed that preoperative blockade of the pain pathway can decrease the amount and duration of postoperative pain perception by preventing nociceptive input from afferent stimuli to the central nervous system during the surgical process (Van Backer et al., 2018). A study has shown that preemptive analgesia with perianal infiltration of 0.5% bupivacaine can better alleviate pain after hemorrhoidectomy (Jirasiritham et al., 2004). Medina et al. found that bupivacaine-combined triamcinolone acetonide achieved better post-hemorrhoidectomy pain relief at 48 h than the blank group (Medina-Gallardo et al., 2022). Accordingly, the results of our meta-analysis showed a significant pain reduction at 48 h in the bupivacaine-combined other drug group than in the other drug group. In addition, the present meta-analysis found that the bupivacaine-combined other drug group had a lower postoperative pain level at 6 h, 12 h, and 24 h compared to the bupivacaine group. In the included studies reporting these three outcomes, the other drugs were defined as ketorolac, morphine, and metronidazole (Ng et al., 2006; Moreira et al., 2014; Shahrokhzadeh et al., 2023). The reason for our finding may be that a combination with other drugs may strengthen the initial anesthetic effect of bupivacaine, thereby providing longer postoperative pain control (Ng et al., 2006; Moreira et al., 2014; Shahrokhzadeh et al., 2023).
The main objective of postoperative pain management is to provide sufficient pain relief while reducing adverse reactions (Wick et al., 2017). In this meta-analysis, we found that the bupivacaine-combined other drug group had a higher odds of pruritus and urinary retention. Due to different factors such as temporary detrusor muscle dysfunction, urethral spasm caused by anal pain, and excessive preoperative and postoperative intravenous infusion, urinary retention may occur after hemorrhoidectomy (Salvati and Kleckner, 1957; Cataldo and Senagore, 1991; Zaheer et al., 1998; Toyonaga et al., 2006). In the included studies reporting these two outcomes, the other drug used was morphine (Moreira et al., 2014; Ruiz-Castro et al., 2017). Morphine may cause several adverse reactions, such as pruritus and urinary retention (Moreira et al., 2014; Ruiz-Castro et al., 2017). Our findings suggested that adverse reactions should be considered when using bupivacaine-combined morphine, and whether the benefit of pain control is worth the risk of urinary retention and cutaneous pruritus should be discussed. In the future, more studies are needed to further explore the effect of bupivacaine-combined other drugs on adverse reactions.
This meta-analysis explores the effect of bupivacaine on the analgesia and safety in patients undergoing hemorrhoidectomy. Most of the included original studies are of high quality, and the pooled results show the good analgesic effects of bupivacaine-combined other drugs, which may provide evidence for the extra use of bupivacaine in hemorrhoidectomy for postoperative pain management. Furthermore, there are some limitations to this meta-analysis. First, we only included studies published in English, which may result in language bias. Second, the heterogeneity is high in some results. The dosage and use methods of bupivacaine, as well as the differences in control drugs, may be the source of heterogeneity. Due to the limitations in the included studies, we were unable to further conduct subgroup analysis to explore the sources of heterogeneity. Third, the number of studies is relatively small in some outcomes, which may affect the robustness of the results.
Conclusion
The results indicated that bupivacaine-combined other drugs had a good effect on pain relief after hemorrhoidectomy, but the adverse reactions should be further discussed.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
HL: conceptualization, project administration, supervision, writing–original draft, and writing–review and editing. MC: data curation, formal analysis, methodology, software, and writing–original draft. DZ: data curation, formal analysis, methodology, software, and writing–review and editing. WL: data curation, formal analysis, methodology, software, and writing–review and editing. HC: conceptualization, project administration, supervision, writing–original draft, and writing–review and editing.
Funding
The authors declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2023.1331965/full#supplementary-material
References
Cataldo, P. A., and Senagore, A. J. (1991). Does alpha sympathetic blockade prevent urinary retention following anorectal surgery? Dis. Colon Rectum 34 (12), 1113–1116. doi:10.1007/bf02050073
Chen, X., Lu, M., Xu, W., Wang, X., Xue, M., Dai, J., et al. (2020). Treatment of pediatric femoral shaft fractures with elastic stable intramedullary nails versus external fixation: a meta-analysis. Orthop. Traumatol. Surg. Res. 106 (7), 1305–1311. doi:10.1016/j.otsr.2020.06.012
Chitty, L., Ridley, B., Johnson, B., Ibrahim, M., Mongan, P. D., and Hoefnagel, A. L. (2022). Liposomal compared to 0.25% bupivacaine in patients undergoing hemorrhoidectomy: a pre- and post-implementation quality improvement evaluation. J. Clin. Anesth. 80, 110868. doi:10.1016/j.jclinane.2022.110868
Cui, J., Xu, Q., Yu, Z., Sun, J., Zheng, Y., Huang, W., et al. (2022). Local infiltration of HYR-PB21, a sustained-release formulation of bupivacaine, provides analgesia and reduces opioid requirement after haemorrhoidectomy: a randomised controlled trial. Br. J. Anaesth. 129 (6), 970–976. doi:10.1016/j.bja.2022.08.035
Farag, H. M., and Esmat, I. M. (2016). Efficacy of two doses of tramadol versus bupivacaine in perioperative caudal analgesia in adult hemorrhoidectomy. Saudi J. Anaesth. 10 (2), 138–142. doi:10.4103/1658-354x.168801
Gorfine, S. R., Onel, E., Patou, G., and Krivokapic, Z. V. (2011). Bupivacaine extended-release liposome injection for prolonged postsurgical analgesia in patients undergoing hemorrhoidectomy: a multicenter, randomized, double-blind, placebo-controlled trial. Dis. Colon Rectum 54 (12), 1552–1559. doi:10.1097/DCR.0b013e318232d4c1
Haas, E., Onel, E., Miller, H., Ragupathi, M., and White, P. F. (2012). A double-blind, randomized, active-controlled study for post-hemorrhoidectomy pain management with liposome bupivacaine, a novel local analgesic formulation. Am. Surg. 78 (5), 574–581. doi:10.1177/000313481207800540
Hatami, M., Talebi, M., Heiranizadeh, N., and Vaziribozorg, S. (2022). The effect of perianal tramadol infiltration on postoperative pain following hemorrhoidectomy. Am. Surg. 88 (1), 98–102. doi:10.1177/0003134820981683
Imbelloni, L. E., Vieira, E. M., Gouveia, M. A., Netinho, J. G., Spirandelli, L. D., and Cordeiro, J. A. (2007). Pudendal block with bupivacaine for postoperative pain relief. Dis. Colon Rectum 50 (10), 1656–1661. doi:10.1007/s10350-007-0216-7
Jadad, A. R., Moore, R. A., Carroll, D., Jenkinson, C., Reynolds, D. J., Gavaghan, D. J., et al. (1996). Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin. Trials 17 (1), 1–12. doi:10.1016/0197-2456(95)00134-4
Jiang, H., Ma, Q., Dong, J., and Ye, X. (2023). The effect of liposomal bupivacaine for surgical wound infiltration: a meta-analysis of randomised controlled trials. Int. Wound J. 20 (5), 1591–1608. doi:10.1111/iwj.14015
Jirasiritham, S., Tantivitayatan, K., and Jirasiritham, S. (2004). Perianal blockage with 0.5% bupivacaine for postoperative pain relief in hemorrhoidectomy. J. Med. Assoc. Thai 87 (6), 660–664.
Lohsiriwat, V., and Jitmungngan, R. (2022). Strategies to reduce post-hemorrhoidectomy pain: a systematic review. Med. Kaunas. 58 (3), 418. doi:10.3390/medicina58030418
Medina-Gallardo, A., Curbelo-Peña, Y., De Castro, X., Roura-Poch, P., Roca-Closa, J., and De Caralt-Mestres, E. (2017). Is the severe pain after Milligan-Morgan hemorrhoidectomy still currently remaining a major postoperative problem despite being one of the oldest surgical techniques described? A case series of 117 consecutive patients. Int. J. Surg. Case Rep. 30, 73–75. doi:10.1016/j.ijscr.2016.11.018
Medina-Gallardo, N. A., De Castro, X., De Caralt-Mestres, E., Curbelo-Peña, Y., Dardano-Berriel, A., Serrat Puyol, J., et al. (2022). Infiltration of bupivacaine and triamcinolone in surgical wounds of milligan-morgan hemorrhoidectomy for postoperative pain control: a double-blind randomized controlled trial. Dis. Colon Rectum 65 (8), 1034–1041. doi:10.1097/dcr.0000000000002250
Mohd-Ali, B., and Chen, L. Y. (2021). The morphology of corneal endothelial cells in long term soft contact lens wearers in Kuala Lumpur. Cont. Lens Anterior Eye 44 (1), 72–75. doi:10.1016/j.clae.2020.06.007
Moreira, H., Moreira, J. P., Isaac, R. R., Alves-Neto, O., Moreira, T. A., Vieira, T. H., et al. (2014). Morphine spinal block anesthesia in patients who undergo an open hemorrhoidectomy: a prospective analysis of pain control and postoperative complications. Ann. Coloproctol. 30 (3), 135–140. doi:10.3393/ac.2014.30.3.135
Naja, Z., Ziade, M. F., and Lönnqvist, P. A. (2005). Nerve stimulator guided pudendal nerve block decreases posthemorrhoidectomy pain. Can. J. Anaesth. 52 (1), 62–68. doi:10.1007/bf03018582
Ng, S.S.-M., Lee, J.F.-Y., Cheung, K.-Y., Leung, K.-L., Yiu, R.Y.-C., and Lau, W.-Y. (2006). Pre-emptive analgesia and metronidazole on post-haemorrhoidectomy pain control. Surg. Pract. 10, 102–105. doi:10.1111/j.1744-1633.2006.00308.x
Ozek, D., Karaca, E. E., Kazanci, B., and Evren Kemer, O. (2021). Evaluation of corneal densitometry and endothelial layer in soft contact lens users. Optom. Vis. Sci. 98 (6), 592–596. doi:10.1097/opx.0000000000001707
Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Bmj 372, n71. doi:10.1136/bmj.n71
Rajabi, M., Hosseinpour, M., Jalalvand, F., Afshar, M., Moosavi, G., and Behdad, S. (2012). Ischiorectal block with bupivacaine for post hemorrhoidectomy pain. Korean J. Pain 25 (2), 89–93. doi:10.3344/kjp.2012.25.2.89
Ruiz-Castro, M., San José Santos, M., Rodríguez-Miguel, A., and de Abajo Iglesias, F. J. (2017). Intraspinal administration of morphine hydrochloride combined with low doses of bupivacaine in hemorrhoidectomy: a clinical randomized trial. Minerva Anestesiol. 83 (9), 930–938. doi:10.23736/s0375-9393.17.11762-1
Salvati, E. P., and Kleckner, M. S. (1957). Urinary retention in anorectal and colonic surgery. Am. J. Surg. 94 (1), 114–117. doi:10.1016/0002-9610(57)90629-3
Sandler, R. S., and Peery, A. F. (2019). Rethinking what we know about hemorrhoids. Clin. Gastroenterol. Hepatol. 17 (1), 8–15. doi:10.1016/j.cgh.2018.03.020
Shahrokhzadeh, N., Khorramnia, S., Jafari, A., and Ahmadinia, H. (2023). Effectiveness of topical ketorolac in post-hemorrhoidectomy pain management: a clinical trial. Anesth. Pain Med. 13 (1), e130904. doi:10.5812/aapm-130904
Shashikala, T. K., and Prathibha, G. A. (2016). A clinical study to evaluate the effects of intrathecal dexmedetomidine 10 mcg on low dose hyperbaric 0.5% bupivacaine (5 mg) for saddle block anaesthesia in adult patients posted for elective perianal surgeries. J. Evol. Med. Dent. Sci. 5 (45), 2801–2804. doi:10.14260/jemds/2016/654
Sim, H. L., and Tan, K. Y. (2014). Randomized single-blind clinical trial of intradermal methylene blue on pain reduction after open diathermy haemorrhoidectomy. Colorectal Dis. 16 (8), O283–O287. doi:10.1111/codi.12587
Steen, C. J., Lam, D., Chandra, R., Chua, J. Y. J., An, V., and Keck, J. O. (2022). Pudendal nerve block for posthemorrhoidectomy pain: a prospective, single-blinded randomized control trial. Dis. Colon Rectum 65 (4), 546–551. doi:10.1097/dcr.0000000000002293
Sterne, J. A., Sutton, A. J., Ioannidis, J. P., Terrin, N., Jones, D. R., Lau, J., et al. (2011). Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. Bmj 343, d4002. doi:10.1136/bmj.d4002
Toyonaga, T., Matsushima, M., Sogawa, N., Jiang, S. F., Matsumura, N., Shimojima, Y., et al. (2006). Postoperative urinary retention after surgery for benign anorectal disease: potential risk factors and strategy for prevention. Int. J. Colorectal Dis. 21 (7), 676–682. doi:10.1007/s00384-005-0077-2
Van Backer, J. T., Jordan, M. R., Leahy, D. T., Moore, J. S., Callas, P., Dominick, T., et al. (2018). Preemptive analgesia decreases pain following anorectal surgery: a prospective, randomized, double-blinded, placebo-controlled trial. Dis. Colon Rectum 61 (7), 824–829. doi:10.1097/dcr.0000000000001069
Wells, G. A., Shea, B., O’Connell, D., Peterson, J., Welch, V., Losos, M., et al. (2011). The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses.
Wick, E. C., Grant, M. C., and Wu, C. L. (2017). Postoperative multimodal analgesia pain management with nonopioid analgesics and techniques: a review. JAMA Surg. 152 (7), 691–697. doi:10.1001/jamasurg.2017.0898
Zaheer, S., Reilly, W. T., Pemberton, J. H., and Ilstrup, D. (1998). Urinary retention after operations for benign anorectal diseases. Dis. Colon Rectum 41 (6), 696–704. doi:10.1007/bf02236255
Zhang, G., Liang, R., Wang, J., Ke, M., Chen, Z., Huang, J., et al. (2020). Network meta-analysis of randomized controlled trials comparing the procedure for prolapse and hemorrhoids, Milligan-Morgan hemorrhoidectomy and tissue-selecting therapy stapler in the treatment of grade III and IV internal hemorrhoids(Meta-analysis). Int. J. Surg. 74, 53–60. doi:10.1016/j.ijsu.2019.12.027
Keywords: bupivacaine, hemorrhoidectomy, adverse reactions, meta-analysis, effect and safety
Citation: Lu H, Cai M, Zhou D, Li W and Cao H (2024) The effect of bupivacaine on analgesia and safety in patients undergoing hemorrhoidectomy: a meta-analysis. Front. Pharmacol. 14:1331965. doi: 10.3389/fphar.2023.1331965
Received: 03 November 2023; Accepted: 26 December 2023;
Published: 01 May 2024.
Edited by:
Suren Soghomonyan, The Ohio State University, United StatesReviewed by:
Nune Soghomonian, Yerevan Medical Center, ArmeniaKimmy Bais, Ohio State University Hospital, United States
Copyright © 2024 Lu, Cai, Zhou, Li and Cao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hanzhong Cao, caohanzhongntu@outlook.com
†These authors share first authorship
 Haixia Lu1,2†
Haixia Lu1,2† Hanzhong Cao
Hanzhong Cao