- 1Department of Obstetrics and Gynecology, Shengjing Hospital of China Medical University, Shenyang, China
- 2School of Pharmacy, China Medical University, Shenyang, China
- 3Department of Pharmacy, The People’s Hospital of Liaoning Province, Shenyang, China
- 4The 1st Clinical Department, China Medical University, Shenyang, China
- 5Department of Pharmacy, Shengjing Hospital of China Medical University, Shenyang, China
Ethnopharmacological relevance: Murrayae Folium et Cacumen (MFC) is a plant considered to be a traditional Chinese medicine with culinary value as well. The dry leaves and twigs of Murraya paniculata and M. exotica are used to treat stomach aches, rheumatism, toothaches, swelling, and insect and snake bites. They are also used to prepare spicy chicken dishes.
Aim of the review: This review comprehensively summarizes the available information on the botanical characterization, phytochemistry, pharmacological activities, pharmacodynamics, pharmacokinetics, and toxicity of MFC.
Methods: Relevant scientific literature up to August 2023 was included in the study. Chinese and English studies on MFC were collected from databases, including PubMed, Elsevier, Web of Science, Springer, Science Direct, Wiley, ACS, and CNKI (Chinese). Doctoral and Master’s dissertations were also included.
Results: In total, 720 compounds have been identified and reported in the literature, including flavonoids, coumarins, alkaloids, sterols, phenylpropenols, organic acids, spirocyclopentenones, and volatile oils. Flavonoids and coumarins are the two most important bioactive compounds responsible for these pharmacological activities. MFC has anti-inflammatory, anti-bacterial, anti-microbial, anti-diabetic, anti-tumor, anti-oxidant, anti-depressant, potential anti-Alzheimer’s disease, chondroprotective, and analgesic properties. The pharmacological effects include interrupting the STAT3/NF-κB/COX-2 and EGFR signaling pathways, downregulating EpCAM expression, inhibiting NF-κB and ERK signals, inhibiting the EP/cAMP/PKA signaling pathway and miR-29a/Wnt/β-catenin signaling activity, and upregulating Foxo3a expression.
Conclusion: This review demonstrates that the chemical constituents, pharmacological activities, pharmacodynamics, pharmacokinetics, and toxicity of MFC support its use in traditional Chinese botanical medicines. MFC contains a wide range of chemical compounds. Flavonoids and coumarins promote strong pharmacological activity and, are low-toxicity natural phytomedicines that are widely used in medicine, food, ornamentation, and cosmetics, making MFC a promising compound for development and use in the treatment of several medical conditions.
1 Introduction
The genus Murraya comprises 21 accepted species (http://www.worldfloraonline.org). Murraya paniculata (L.) Jack and M. exotica L. (Rutaceae) are the most widely used Murraya species listed in the Chinese Pharmacopoeia (Chinese Pharmacopoeia Commission, 2020). Murrayae Folium et Cacumen (MFC), a traditional Chinese medicine, consists of dry leaves and twigs of M. paniculata and M. exotica (Lu et al., 2021a). It promotes qi, relieves pain, activates blood, and removes blood stasis and is used mainly to treat stomach pain, rheumatism, arthralgia, toothache, tumefaction, and snakebites (Chinese Pharmacopoeia Commission, 2020). MFC is the main ingredient in Sanjiu Weitai granules, a well-known Chinese-patented medicine used for the treatment of gastric conditions (Lu et al., 2021b). In addition, it is also used in the Baihu Dan in the Jingyue Complete Book to treat swelling of the head, face, limbs and eyes, and Zhitong Jing in the Compilation of Chinese Medicine to promote circulation and relieve pain.
The leaves of Murraya paniculata are used as a spice by the people of India, Southeast Asia, Pakistan, and Malaysia in a variety of food preparations. Malaysians typically use M. paniculata leaves to prepare soups, fish, and meat. M. paniculata has also been used to prepare spicy chicken dishes in popular fast-food restaurants (Ng et al., 2012; Saqib et al., 2015). In ancient China, India, and Indonesia, M. paniculata was used as a botanical medicine for numerous healthcare purposes. In the Chinese Pharmacopoeia, M. paniculata is reported to have analgesic effects and the potential to treat microbial infections and inflammatory diseases (Zhang et al., 2011). The ground stem bark of M. paniculata is used as an antidote for snake bites, whereas the ground roots are used to treat body pain. The leaves are irritating and astringent and are used by the Indonesian community to relieve diarrhea and dysentery (Saqib et al., 2015). M. paniculata has also been used to treat coughs, hysteria, and rheumatism (Gautam et al., 2012a). It is used to treat snake bites and as a detergent for other types of bites. The roots and bark are chewed and rubbed against the skin to treat pain. Crushed leaves are applied to fresh wounds and as a remedy for alcohol-related fluid retention. M. paniculata can be used to treat toothaches, stomach-aches, and gout. It is also used for abortion and to treat venereal diseases (Kinoshita and Firman, 1996a; Rahman et al., 2010). Terpenoid volatile oils extracted from the flowers of M. paniculata are used in the cosmetic industry (Rout et al., 2007; Rehman et al., 2013).
Murraya exotica is a dwarf tree or evergreen shrub commonly cultivated as an ornamental plant in many tropical and subtropical areas because of its glossy green leaves and clusters of fragrant white flowers (Sharma and Arora, 2015). It has also been used for the treatment of analgesia, anesthesia, abdominal pain, and rheumatism. The leaves of M. exotica are rich in coumarins, which exhibit anti-oxidant, anti-tumor, anti-mycobacterial, anti-fungal, anti-viral, and anti-inflammatory properties. The roots of M. exotica are rich in coumarins and alkaloids, such as paniculidines (A−F) (He et al., 2017).
Previous phytochemical studies on MFC (M. paniculata and M. exotica) have indicated the main bioactive compounds as coumarins, flavonoids, alkaloids, and volatile oils, of which the alkaloids are mainly present in the roots of plants. Pharmacological studies have demonstrated that MFC possesses anti-inflammatory, analgesic, anti-bacterial, anti-oxidant, and insecticidal properties (Lu et al., 2021a). To date, there have been no systematic reports on MFC. Therefore, it is necessary to summarize the phytochemistry, pharmacology, pharmacodynamics, pharmacokinetics, and toxicology of MFC to guide clinical use in the Chinese Pharmacopoeia.
2 Botanical characterization
2.1 Plant description
Murraya paniculata are 1.8–12 m tall shrubs or trees. Older branchlets are grayish-white to pale yellowish-gray. The leaves are 2–5 foliolate, and leaflet blades are mostly suborbicular to ovate to elliptical, margin entire or crenulate, and apex rounded to acuminate (http://www.worldfloraonline.org) (Figure 1A).
Murraya exotica are 2–8 m tall shrubs or trees. Older branchlets are grayish-white to pale yellowish-gray. The leaves are 3–7 foliolate, petiolules are rather short, leaflet blades are elliptic-obovate or obovate, and margins are entire, apex rounded, or obtuse (http://www.worldfloraonline.org) (Figure 1B).
2.2 Vernacular names
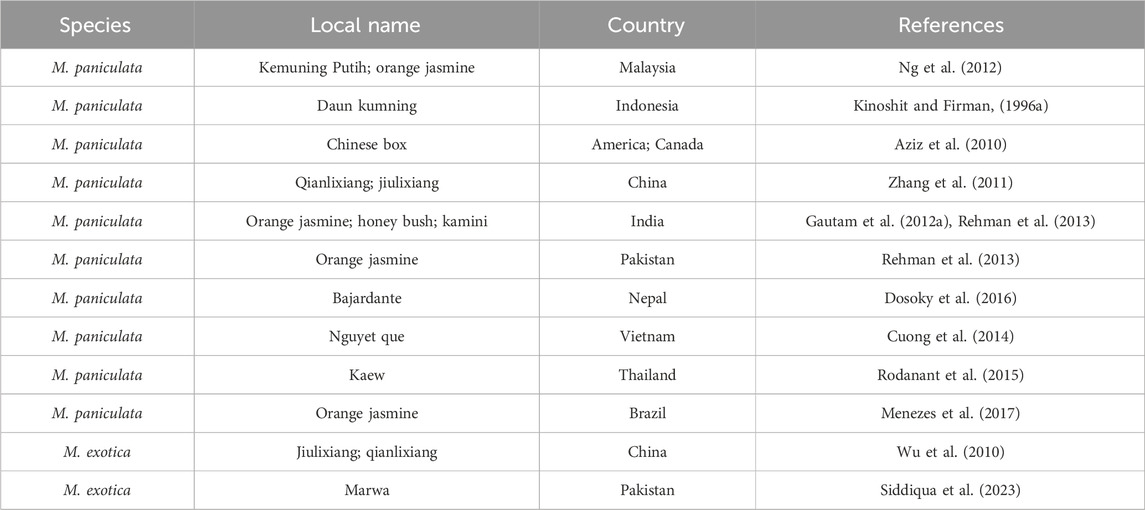
Vernacular names—in other words, local, common, or non-Latin names for a plant or animal—are derived from common native languages and are distinct from binomial nomenclature. The name is derived from the plant’s morphology, habits, habitats, organoleptic properties, and therapeutic uses (Hossain et al., 2021). The vernacular names of MFC are listed in Table 1.
3 Materials and methods
This review focuses on the research advances in the phytochemical constituents, pharmacological activities, pharmacodynamics, pharmacokinetics, and toxicology of MFC. Related scientific literature up to August 2023 was collected from the following databases: PubMed, Elsevier, Web of Science, Springer, Science Direct, Wiley, ACS, and CNKI (Chinese). Doctoral and Master′s dissertations were also included in the analysis. We used the terms (all fields) “Murrayae Folium et Cacumen,” “Murraya paniculata,” “Murraya exotica,” and “Murraya” and collated all published works from the China Medical University Library. Only data published in English or Chinese were included in the analysis. ChemDraw 20.0 was used to extract the chemical compounds. The PubChem database (https://pubchem.ncbi.nlm.nih.gov) was used to confirm the chemical classifications and structures. World Flora Online (http://www.worldfloraonline.org/) was used to verify the names of the plants.
3.1 Inclusion and exclusion criteria
Firstly, duplicate articles, review articles, conference abstracts; non-English and non-Chinese articles were excluded. Further exclusions were duplicate articles and articles unrelated to the topic. Finally, 125 eligible articles were included.
4 Phytochemistry
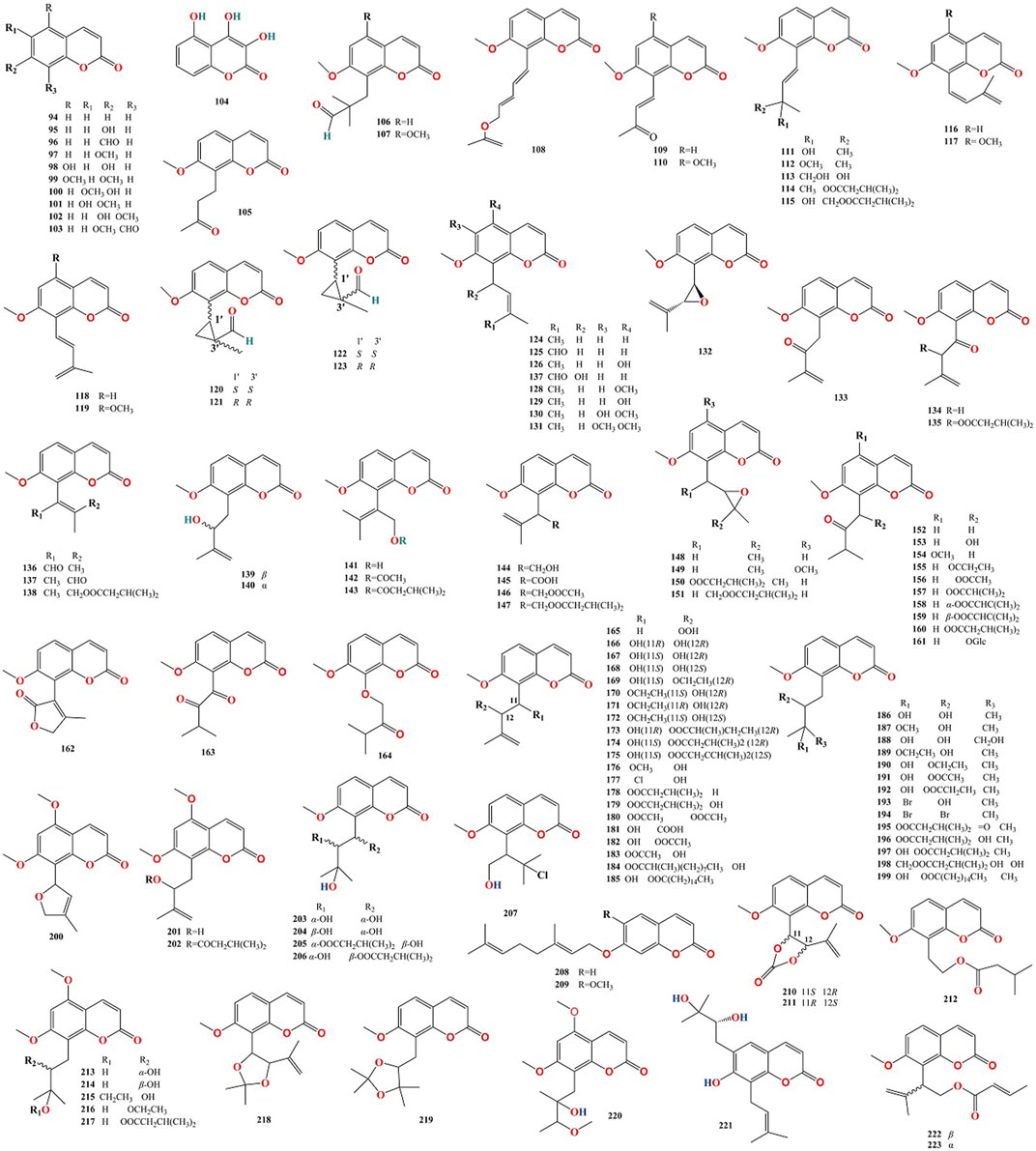
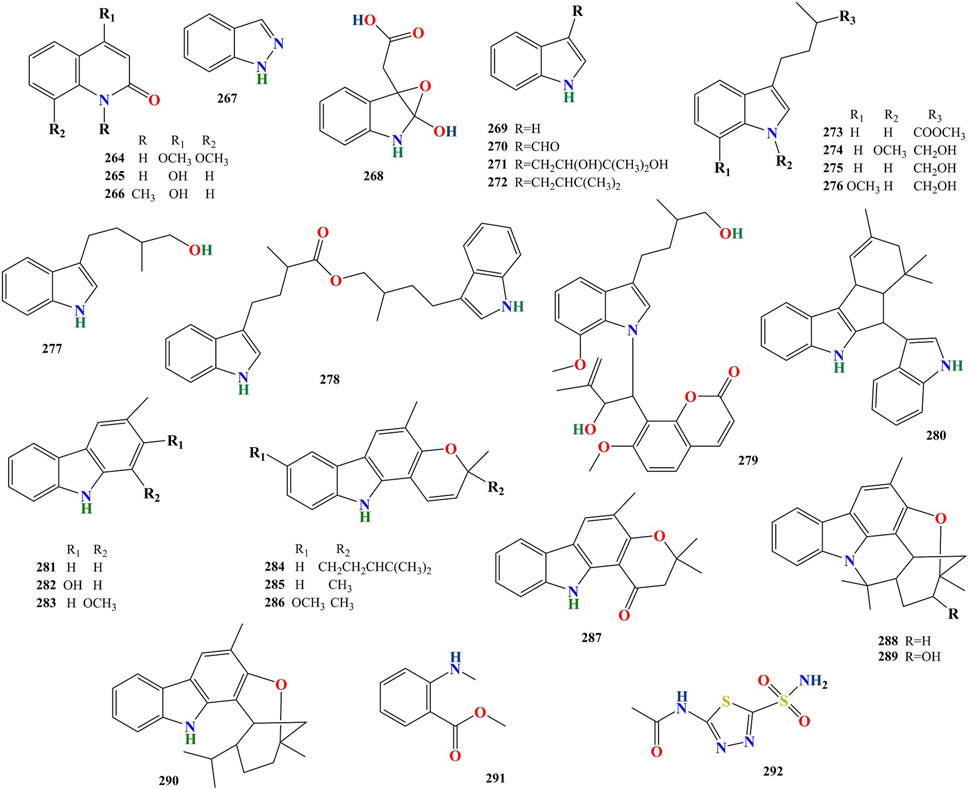
To date, 316 compounds have been identified in MFC, including flavonoids, coumarins, alkaloids, sterols, phenylpropanoids, organic acids, spirocyclopentenones, and 404 volatile oils. These have been identified using thin layer chromatography (TLC), high-performance liquid chromatography (HPLC), ultra- performance liquid chromatography (UPLC), nuclear magnetic resonance (NMR), ultraviolet (UV), mass spectrometry (MS), NMR-MS, ultra-performance liquid chromatography-electrospray ionization-mass spectrometry (UPLC-ESI-MS), HR-FAB-MS, heteronuclear multiple-bond correlation (HMBC), heteronuclear multiple-quantum correlation (HMQC), and gas chromatography-mass spectrometry (GC-MS) (Kong Y. et al., 1985; Liu et al., 2015a; Kaur et al., 2016; Liang et al., 2020c; Saikia et al., 2021). Flavonoids are the main compounds of M. paniculata, whereas coumarins are the main compounds of M. exotica. Alkaloids mainly exist in the roots of plants but rarely in the twigs and leaves (Lu et al., 2021a). A detailed list of these chemical compounds and their classes is presented in Figures 2–5, and Tables 2–5 and Supplementary Table S.
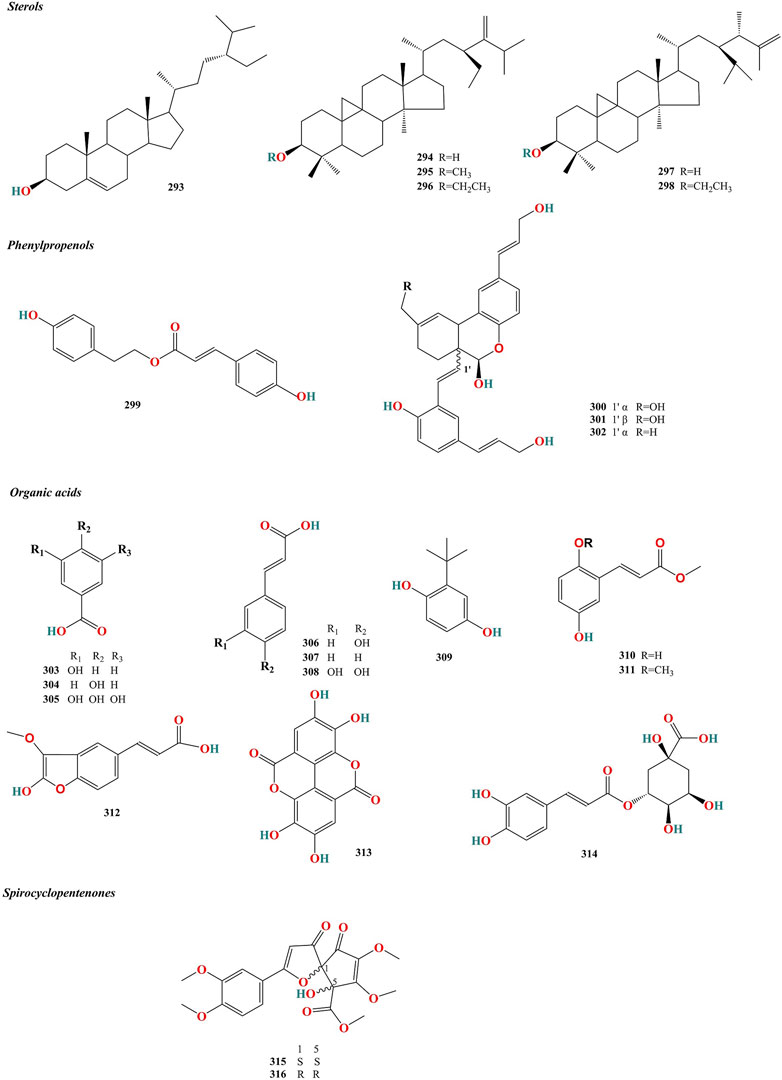
Figure 5. Chemical structures of sterols, pehylpropenols and other constituents isolated from Murrayae Folium et Cacumen.
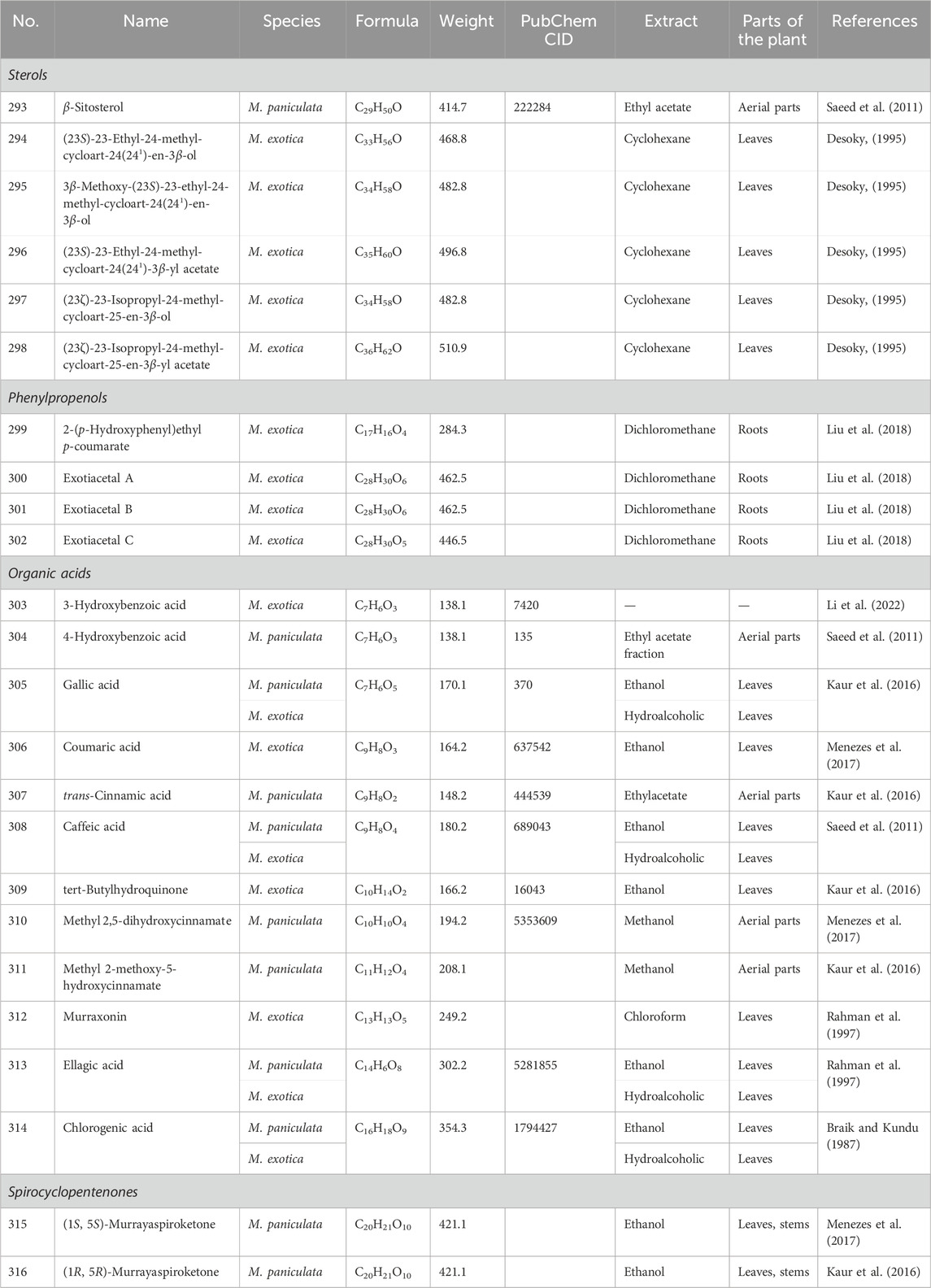
Table 5. Sterols, phenylpropenols, organic acids and spirocyclopentenones isolated from Murrayae Folium et Cacumen.
4.1 Flavonoids
Ninety-three flavonoids (1–93, Table 2) have been identified in MFC. Of these, 72 compounds belong to the polymethoxylated flavonoid family, 90 were isolated from M. paniculata, and 24 were isolated from M. exotica, confirming that flavonoids were isolated mainly from M. paniculata (Lu et al., 2021a). Twenty-one compounds were isolated from M. paniculata and M. exotica (Negi et al., 2015; Sangkaew et al., 2020). Depending on their structure, flavonoids can be divided into six types: flavones, flavonols, flavanones, flavan-3-ols, chalcones, and isoflavones; of these, flavones are the primary structures of flavonoids in MFC (Figure 3).
4.2 Coumarins
One hundred and seventy coumarins (94–263, Table 3) have been identified in MFC. Of these, 89 compounds were isolated from M. paniculata, 130 compounds were isolated from M. exotica, and 49 were isolated from both M. paniculata and M. exotica (Liu et al., 2018; Wang X. et al., 2019; Wang Y. et al., 2019). Depending on their structure, coumarins can be classified into six types: simple coumarins, prenylated coumarins, pyranocomarins, furanocoumarins, dimeric coumarins, and benzocoumarins (Figure 4).
4.3 Alkaloids
Twenty-nine alkaloids (264–292, Table 4) have been identified in MFC. Of these, 17 compounds were isolated from M. paniculata, and 12 compounds were isolated from M. exotica. No alkaloids have been isolated in both M. paniculata and M. exotica. Depending on their structure, alkaloids can be divided into six types: quinoline, indazole, indole, carbazole, organic amine, and other alkaloids (Figure 5).
4.4 Sterols
Six sterols (293–298, Table 5) have been isolated from MFC (Desoky, 1995; Saeed et al., 2011) (Figure 5).
4.5 Phenylpropenols
Four phenylpropenols (299–302, Table 5) have been isolated from the roots of M. exotica (Liu et al., 2018), compounds 300 and 301 of which are isomers (Figure 5).
4.6 Organic acids
Twelve organic acids (303–314, Table 5) have been isolated from MFC (Rahman et al., 1997; Saeed et al., 2011; Kaur et al., 2016; Menezes et al., 2017) (Figure 5).
4.7 Spirocyclopentenones
Two spirocyclopentenones (315 and 316, Table 5) have been isolated from M. paniculata (Liang et al., 2020a), including (1S, 5S)-murrayaspiroketone (315) and (1R, 5R)-murrayaspiroketone (316). Compounds 315 and 316 are enantiomers (Figure 5).
4.8 Volatile oils
Four-hundred and four volatile oils (317–711, Supplementary Table S) have been isolated from MFC (Pino et al., 2006; Raina et al., 2006; Krishnamoorthy et al., 2015; Silva et al., 2020; Saikia et al., 2021). The major volatile organic compounds dominated by benzenoids, sesquiterpenes, diterpenoids, triterpenoids, coumarins, and phenylethanoids in hydrodistillation and pentane, n-hexane, and dichloromethane extracts using GC-MS and gas chromatography-flame ionization detection (GC-FID). Of these, 287 compounds were isolated from M. paniculata, 244 compounds from M. exotica, and 127 from both M. paniculata and M. exotica. Sesquiterpenes are the predominant constituents of the essential oils from MFC. The main compounds are β-caryophyllene, spathulenol, α-zingiberene, α-copaene, germacrene D, and methyl palmitate (Lv et al., 2013; Dosoky et al., 2016; Silva et al., 2019).
5 Pharmacological activities
Modern pharmacological research has indicated that MFC has anti-inflammatory, anti-bacterial, anti-microbial, anti-diabetic, anti-tumor, and anti-oxidant properties (Lu et al., 2021a). The mechanisms of the compounds, extracts, and fractions from MFC are summarized in Table 6 and discussed in subsequent sections. Figure 6 summarizes the pharmacological mechanisms of MFC.
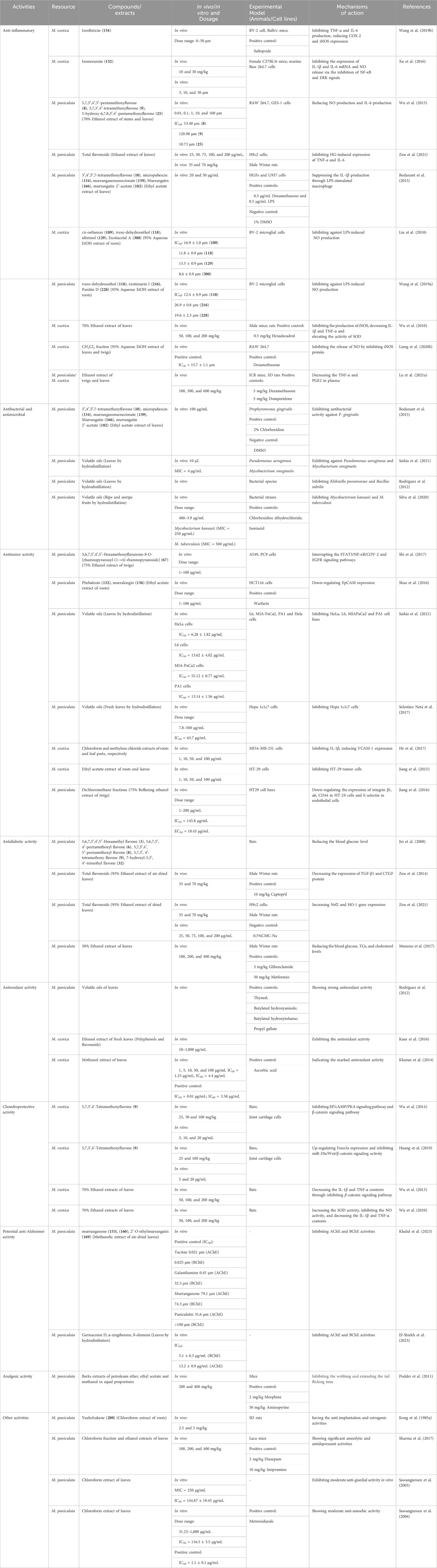
Table 6. Pharmacological Mechanism, models of compounds and various extracts in Murrayae Folium et Cacumen.
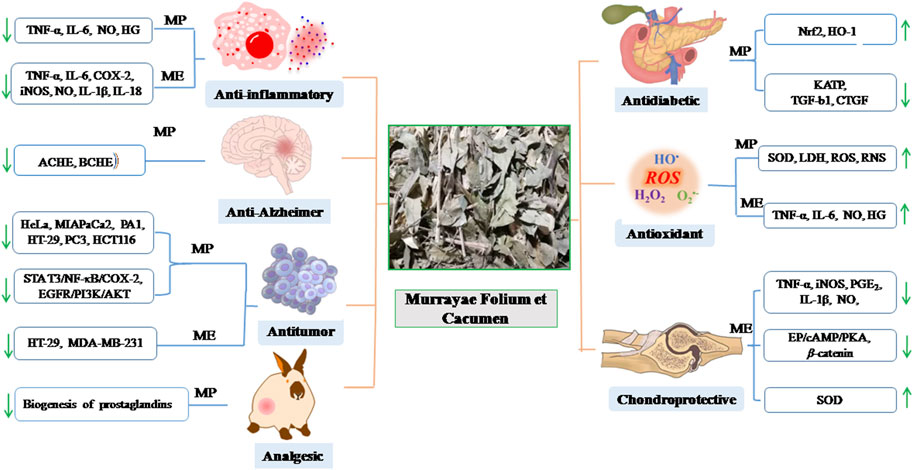
Figure 6. The pharmacological activity mechanism of Murrayae Folium et Cacumen (MP: Murraya. Paniculata; ME: M. exotica).
5.1 Anti-inflammatory activity
Gastric ulcers promote the release of inflammatory factors leading to acute and chronic gastric mucosal lesions (Wu et al., 2015). MFC is the main crude material of a patented Chinese drug compound “Sanjiu Weitai,” indicated for gastritis therapy (Lu et al., 2021a), indicating that MFC could exert anti-inflammatory effects.
A pharmacodynamical study demonstrated that the levels of interleukin (IL)-6, tumor necrosis factor-alpha (TNF-α), and prostaglandin E2 (PGE2) in the plasma of rats were significantly decreased at 300 and 600 mg/kg of M. paniculata and M. exotica, respectively, and there was no statistical difference in the inhibition of inflammatory cytokines between M. paniculata and M. exotica at the same dose, indicating a good anti-inflammatory effect (Lu et al., 2021a). Prenylated phenylpropenols and coumarin derivatives from MFC exhibit anti-inflammatory effects by inhibiting lipopolysaccharide (LPS)-induced nitric oxide (NO) production in BV-2 microglial cells (Liu et al., 2018; Wang X. et al., 2019). The total flavonoids of M. paniculata leaves efficiently exert anti-inflammatory effects by inhibiting high glucose-induced expression of TNF-α and IL-6 in H9c2 cells (Zou et al., 2021). Coumarin derivatives isolated from the extracts of M. exotica leaves and twigs show anti-inflammatory activity by inhibiting the release of NO via the nitric oxide synthase (iNOS) protein (Liang et al., 2020b). Wu et al. (2010) evaluated the anti-inflammatory activity models of ethanol extracts and coumarin compounds of M. exotica and reported that the mechanism of action may involve proinflammatory cytokines, such as IL-1β and TNF-α. Murracarpin (176) shows the most potential for anti-inflammatory activity.
Isosibiricin (154), a natural bioactive coumarin compound isolated from MFC, markedly inhibits the release of NO and production of TNF-α and IL-6 and reduces the expression of cyclooxygenase-2 (COX-2) and inducible iNOS in a concentration-dependent manner to exert anti-inflammatory effects (Kinoshita and Firman, 1996a; Wang Y. et al., 2019). Isomeranzin (7-methoxy-8-(3-methyl-2-oxobutyl) coumarin, 152) isolated from MFC produces the greatest inhibitory effect on proinflammatory factors, such as IL-1β and IL-6 mRNA expression and NO release. This suggests that compound 152 exerts anti-inflammatory effects primarily by selectively targeting macrophages via the inhibition of nuclear factor-kappa B (NF-κB) and extracellular signal-regulated kinase signals (Xu et al., 2016). The 2′-O-Ehylmurrangatin (169) isolated from M. paniculata leaves promotes moderate respiratory burst inhibition, which could have potential anti-inflammatory effects (Shaikh and Choudhary, 2011).
Murrangatin (166), murrangatin 2′-acetate (182), murranganonsenecionate (159), micropubescin (134), and 3′,4′,5′,7-tetramethoxyflavone (10) isolated from the leaves of the ethyl acetate extract of M. paniculata also show strong anti-inflammatory activity via LPS-stimulated macrophages to produce the inhibitory effect (Rodanant et al., 2015). Three flavonoids (8, 9, 25) isolated from the dried stems and leaves of M. paniculata mainly suppress LPS-activated production of NO and IL-6 with little cytotoxicity in a dose-dependent manner. Wu et al. (2015) suggested that the C-2, 3 double bond might have an important inhibitory effect on the production of NO and IL-6 by LPS-activated RAW 264.7 cells and mouse peritoneal macrophages.
5.2 Anti-bacterial and anti-microbial activities
To date, studies on anti-bacterial and anti-microbial activities have been focused on M. paniculata, not M. exotica.
Murrangatin (166), murrangatin 2′-acetate (182), murranganonsenecionate (159), micropubescin (134), and 3′,4′,5′,7-tetramethoxyflavone (10) isolated from the leaves of ethyl acetate extract of M. paniculata are active against Porphyromonas gingivalis. Compounds 166 and 182 show better anti-bacterial potency than the crude extract, indicating that coumarins are the main constituents responsible for the anti-bacterial effects (Rodanant et al., 2015). Different extracts of M. paniculata used against different strains of human pathogenic bacteria have exhibited a broad spectrum of anti-bacterial activities. The ethanol and hydroalcoholic extracts show mild-to-moderate activity against human pathogenic bacteria. The methanol extract of M. paniculata leaves shows obvious antibacterial activities against Gram-positive and Gram-negative bacteria. Anti-bacterial properties against human pathogens with high phenol and flavonoid content have been reported (Gautam et al., 2012a). One study reported that the ethanol extract of M. paniculata leaf showed broad-spectrum activity in inhibiting the growth of Staphylococcus aureus (half minimal inhibitory concentration [MIC50] = 406 μg/mL) (Panda et al., 2020).
Saikia et al. (2021) reported that volatile oils showed promising anti-bacterial activity against Mycobacterium smegmatis (MIC50 = 4 μg/mL) and Pseudomonas aeruginosa (MIC50 = 4 μg/mL). Twenty-nine compounds in the volatile oils of M. paniculata leaves were identified using GC-MS. Most of the volatile oils were sesquiterpene hydrocarbons (80%); the major compound was caryophyllene (20.93%). Silva et al. (2020) reported that volatile oils were also effective against Mycobacterium kansasii (MIC50 = 250 μg/mL) and moderately active against Mycobacterium tuberculosis, demonstrating anti-streptococcal and anti-mycobacterial activities (MIC50 = 500 μg/mL). GC-FID and GC-MS were used to analyze the volatile oils of M. paniculata leaves using hydrodistillation. Anti-fungal tests in vitro showed that essential oils had a 91.2% inhibitory effect on the growth of mycelia in Sclerotinia sclerotiorum. Eighteen compounds were identified using GC-MS in the volatile oils of M. paniculata leaves obtained using hydrodistillation. Rodríguez et al. (2012) reported that the volatile oils exerted moderate inhibitory effects against Klebsiella pneumoniae and Bacillus subtilis. GC-MS analysis of fresh leaf extract of M. paniculata by hydrodistillation identified 13 compounds using GC-MS analyses, including 99.3% sesquiterpenes and 0.3% monoterpenes. The major compounds were β-caryophyllene (320), α-zingiberene (325), and α-caryophyllene (336). Another study indicated that the volatile oils and β-caryophyllene (320) exhibited moderate anti-bacterial activity (MIC50 < 1.0 mg/mL) (Selestino Neta et al., 2017).
5.3 Anti-tumor (cytotoxic) activity
Polymethoxyflavones (PMFs) exhibit a wide range of biological activities, including anti-inflammatory, anti-carcinogenic, and anti-tumor activities. Owing to the hydrophobicity of the methoxyl groups relative to the hydroxyl groups, PMFs are more lipophilic and inhibit tumor cell growth compared with hydroxylated flavonoids (Pan et al., 2007). The roots of M. paniculata and M. exotica are rich in coumarins, which exhibit cytotoxic activity in tumor cell lines (Shao et al., 2016; He et al., 2017). The leaves of M. paniculata also exhibits cytotoxic activity (Selestino Neta et al., 2017; Saikia et al., 2021).
The 5, 6, 7, 3′, 4′, 5′-hexamethoxyflavanone-8-O-[rhamnopyranosyl-(1→4)-rhamnopyranoside] (67) isolated from 75% ethanol extract of twigs of M. paniculata inhibited the adhesion, migration and invasion of lung adenocarcinoma A549 cells in vitro. Compound 67 blocked the adhesion of A549 cells to human pulmonary microvascular endothelial cells by targeting COX-2, matrix metalloproteinase (MMP)-2, and MMP-9 and blocked the NF-κB/signal transducer and activator of transcription 3 and epidermal growth factor receptor/phosphatidylinositol 3-kinase/protein kinase B signaling pathways, thereby blocking the invasion and migration of targeted cancer cells and downregulating their epithelial–mesenchymal transition phenotype. Compound 67 inhibited the adhesion of cancer cells to abnormal endothelial cells by regulating the cellular microenvironment (Shi et al., 2017).
The raw methanol/dichloromethane extracted fraction (containing flavonoids and coumarins) of M. paniculata had a high adhesion inhibition rate, and the inhibition effect on human endothelial cells HT29 was concentration-dependent (1–30 μg/mL). In addition, this fraction inhibited the invasion and migration of HT29 cells. Oral administration of the fraction substantially inhibited lung metastasis in immunized mice inoculated with murine melanoma cells, without obvious side effects (Jiang et al., 2016).
Phebalosin (132) and murralongin (136), isolated from 80% acidic ethanol extracts of M. paniculata roots, inhibited the adhesion of cancer cells to the vascular intima because they specifically targeted cell–cell adhesion at low concentrations (Shao et al., 2016). Pharmacodynamic experiments showed that coumarin extracts from M. exotica roots had low cytotoxicity and high resistance to HT-29 tumor cells and could substantially inhibit the migration of tumor cells (Jiang et al., 2015). The root extracts of M. exotica were more efficient in restraining cell migration and had a slightly lower inhibition of cell adhesion in MDA-MB-231 cells than the leaf extracts in vitro. Compounds isolated from the roots of M. exotica show obvious inhibitory effects on the adhesion and migration of tumor cells (He et al., 2017).
The volatile oils from fresh leaves of M. paniculata exhibited a half maximal inhibitory concentration (IC50) value of 63.73 μg/mL for tumorous cells of hepatocytes in a study by Selestino Neta et al. (2017). Twenty-nine compounds in the essential oil of M. paniculata leaf were identified using GC-MS. The major compound was caryophyllene (20.93%), which had an obvious inhibitory effect on HeLa, MIAPaCa2, and PA1 cell lines (Saikia et al., 2021).
5.4 Anti-diabetic activity
The total flavonoids extracted from M. paniculata (TFMP) effectively alleviated kidney damage in diabetic rats. The effects of TFMP on diabetic nephropathy may involve the regulation of glucose, lipid metabolism, oxidative stress, and inflammatory cytokines (Zou et al., 2014). Zou et al. (2021) found that TFMP could decrease the occurrence of diabetic cardiomyopathy in type 2 diabetic rats, and the protective effect may upregulate the expression of the NF-E2-related factor 2 and heme oxygenase-1 genes, inhibiting oxidative stress, inflammation, and apoptosis (Zou et al., 2021). The extract of M. paniculata leaves decreases glucose levels in alloxan-induced diabetic rats, effectively treating diabetes-related complications, such as hypercholesterolemia and hypertriglyceridemia, and reducing the damage associated with diabetic status. The hypoglycemic effect is similar to that of glibenclamide and metformin, which are related to the inhibition of ATP-sensitive potassium channels (Menezes et al., 2017).
The flavones extracted from M. paniculata leaf mainly include 5, 6, 7, 3′, 4′, 5′-hexamethyl flavone (3), 5, 6, 7, 3′, 4′-pentamethoxyl flavone (6), 5, 7, 3′, 4′, 5′-pentamethoxyl flavone (8), 5, 7, 3′, 4′-tetramethoxy flavone (9), and 7-hydroxyl-5, 3′, 4′-trimethyl flavone (32). A patent disclosed that the flavonoids could remarkably reduce blood glucose; improve the disturbance of lipid metabolism; increase the C-peptide level and the content of insulin in the serum; improve the excretion index of β cells of insulin and insulin resistance; reduce the insulin resistance index, malondialdehyde content in the blood serum, and contents of IL-1β, IL-6, and TNF-α; and improve superoxidase dismutase (SOD) activity (Jin et al., 2008).
5.5 Anti-oxidant activity
Various in vitro studies have shown that 50% ethanol extract of M. paniculata leaves possessed strong anti-oxidant activity (Gautam et al., 2012b). GC-MS was used to identify 18 compounds in volatile oils of M. paniculata leaves obtained via hydrodistillation. These volatile oils showed strong anti-oxidant activity, and the main component is β-caryophyllene (320) (Rodríguez et al., 2012).
Research has shown that ethyl acetate fraction of M. exotica leaves, which is rich in polyphenols and flavonoids, exhibits the highest anti-oxidant activity of the different parts obtained using the sequential extraction method (Kaur et al., 2016). The methanol extract of M. exotica leaves has been reported to contain numerous flavonoids and polyphenols, and these compounds show marked anti-oxidant activity (Khatun et al., 2014).
5.6 Chondroprotective activity
Wu and coworkers (2010) found the 70% ethanol extract of M. exotica leaves significantly reduced iNOS activity and IL-1β and TNF-α contents, increaseed SOD activity, decreaseed NO production, protected cartilage and chondrocytes from destruction, and maintained the normal function of femoral condyle cartilage and the arrangement of different layers of chondrocytes. Another study demonstrated that 70% ethanol extracts of M. exotica decreased the contents of TNF-α and IL-1β in rat osteoarthritis synovial fluid by inhibiting the β-catenin signaling pathway, and reduced chondrocyte apoptosis in vitro (Wu et al., 2013). 5,7,3ʹ,4ʹ-Tetramethoxyflavone (9) has been demonstrated to improve chondrocyte apoptosis by inhibiting Wnt/β-catenin signaling in vivo and in vitro (Wu et al., 2014). Further studies show that compound 9 from M. exotica exhibits chondroprotective activity by upregulating Foxo3a expression and inhibiting miR-29a/Wnt/β-catenin signaling activity (Huang et al., 2019).
5.7 Potential anti-Alzheimer’s disease activity
Alzheimer’s disease (AD) is a neurodegenerative disease that causes progressive loss of neuronal structure and function, leading to cognitive decline and dementia (Kabir et al., 2021). Some natural products have promising anti-AD properties. Acetylcholinesterase/butyrylcholinesterase (AChE/BChE) inhibitors are desirable because they improve cognition with minimal side effects (Wojtunik-Kulesza et al., 2021). In one study, the essential oil of M. paniculata leaves was the most potent selective BChE inhibitor with an IC50 of 5.1 ± 0.3 μg/mL and showed strong inhibitory activity against AChE (IC50 = 13.2 ± 0.9 μg/mL). Germacrene D (324), α-zingiberene (325), and δ-elemene (333) had a high affinity for BChE. These volatiles, with their in vitro cholinesterase inhibitory potential, have demonstrated a novel and safe treatment for AD (El-Shiekh et al., 2023). Experimentally, paniculatin (160) is most potent against AChE (IC50 = 31.6 µM), whileas murranganone (153) is the most potent against BChE (IC50 = 74.3 µM); neither compound shows selectivity toward any of the two enzymes. Paniculatin (160) is a mixed-type inhibitor of both AChE and BChE, whereas murranganone (153) promotes pure noncompetitive inhibition of AChE and BChE. Compound 2′-O-ethylmurrangatin (169) has no inhibitory effect on AChE but has a very weak inhibitory effect on BChE. These three coumarins represent a new class of natural coumarins that are active against cholinesterases. Therefore, they can be considered potential candidates for AD treatment. These coumarins show non-selective, moderate-to-good in vitro activity against both AChE and BChE via a mixed-type inhibitory mechanism (Khalid et al., 2023).
5.8 Analgesic activity
The bark extract of M. paniculata (200 and 400 mg/kg) has an obvious inhibitory effect on the writhing bodies of mice, and the degree of the inhibitory effect increases with increasing dose. The results at both doses are comparable to those of the standard drug aminopyrine. A similar analgesic activity was observed using the radiant heat method (Podder et al., 2011).
5.9 Other activities
Yuehchukene (280) from M. paniculata has been shown to exhibit long-standing anti-implantation and estrogenic activity (Kong YC. et al., 1985).
M. paniculata is widely used to treat mental health disorders. The chloroform (200 mg/kg) and ethanol (400 mg/kg) extracts of M. paniculata leaves demonstrate marked anxiolytic and anti-depressant activities, respectively (Sharma et al., 2017). The chloroform extract of M. paniculata leaves showed a moderate level of anti-giardial and anti-amoebic activity in vitro in patients with AIDS in southern Thailand (Sawangjaroen et al., 2005; Sawangjaroen et al., 2006).
Cuong et al. (2014) reported that the chloroform fraction of the methanol extract of M. paniculata leaves had the most potent vasorelaxing effect on rat aortic rings contracted using 60 mM K+. Kimcuongin (225) and murracarpin (176) isolated from the chloroform fraction showed vasorelaxant activity with IC50 values of 37.7 µM and 139.3 µM, respectively, suggesting that M. paniculata has an anti-hypertensive effect (Cuong et al., 2014).
6 Pharmacodynamics and pharmacokinetics
6.1 Pharmacodynamics
One pharmacodynamic study of MFC indicated that both M. exotica and M. paniculata markedly inhibited the writhing reaction induced by acetic acid in mice and the paw swelling induced by carrageenan in rats; decreased IL-6, TNF-α, and PGE2 levels in the plasma of rats with swollen paws; and increased the gastric emptying rate and intestinal propulsive rate in a dose-dependent manner. M. exotica and M. paniculata did not indicate marked differences at the same dose and are therefore considered to have similar anti-inflammatory, analgesic, and gastrointestinal motion-promoting effects (Lu et al., 2021a). The authors compared the preventive effects of M. exotica and M. paniculata against alcohol-induced gastric lesions. MFC effectively attenuated ethanol-HCl-induced gastric diseases by reversing inflammatory development and preventing ethanol-HCl-induced necrosis and apoptosis (Lu et al., 2021b).
6.2 Pharmacokinetics
A novel, fast, and sensitive UPLC–tandem mass spectrometry method with a sample preparation procedure, low limit of quantitation, short run time, and good accuracy was used to easily detect murrayone (133) extracted from M. paniculata in rat plasma. This method has been successfully applied in pharmacokinetic studies. Zhai et al. (2020) studied Sprague-Dawley rats administered with 20, 50, or 125 mg/kg murrayone intra-gastrically or 20 mg/kg murrayone via intravenous bolus injection. The mean Tmax values of the relevant pharmacokinetic parameters ranged from 0.75 h to 1 h for all doses, indicating rapid murrayone absorption. The mean T1/2 was 3.52–5.97 h for all dose groups, indicating that the rate of murrayone elimination was also rapid. The relatively high Vd/F indicated that murrayone was widely distributed in the body and combined with tissues. The absorption rate of murrayone was high, and the absolute bioavailability of murrayone was 22.72%–37.81% in rats. The exposure level of murrayone was positively correlated with the dose administered, indicating that the in vivo pharmacokinetic behavior of murrayone is linear (Zhai et al., 2020).
7 Toxicology
The constituents and extracts of MFC have been studied for their pharmacological activity. However, studies on the potential toxicology of these compounds are limited.
In male and female Swiss mice, acute toxicity was assessed using a single oral dose of the hydroethanolic extract of M. paniculata leaves (2,000 and 5,000 mg/kg bw/day). No abnormal signs of toxicity (e.g., piloerection, diarrhea, or alteration in locomotor activity) or death were observed during the 14 days of observation (Menezes et al., 2015). The aqueous extracts of M. paniculata leaves against Artemia salina were presented as total phenolic compounds. The percentage mortality of brine shrimp increased with the concentration of the aqueous extract of M. paniculata, which has been shown to have a significant effect on brine shrimp (Bovornvattanangkul and Jiraungkoorskul, 2016). Liaqat et al. (2018) studied Wistar rats with free access to food and water that were administered with 400 mg/kg volatile oils of M. paniculata. The MIC and MBC results showed that the oils of M. paniculata were active against Gram-positive strains. Moreover, elevation in packed cell volume and depletion in mean corpuscular volume were observed; therefore, the volatile oils of M. paniculata are considered safe for internal use (Liaqat et al., 2018). The half lethal concentration (LC50) and 90% lethal concentration (LC90) values of petroleum ether extract and crude methanol extract from M. paniculata leaves were 0.471 ± 0.72 μg/mL and 0.773 ± 0.19 μg/mL, respectively, showing high cytotoxic activity in a brine shrimp lethal test (Mita et al., 2013). Another study showed that the ethanol extract (250 and 500 mg/kg dosages) of M. paniculata leaves produced strong anti-nociceptive activity and was toxic to brine shrimp (half lethal dose [LD50] = 32 μg/mL) (Sharker et al., 2009).
Khatun et al. (2014) identified the methanol extract from M. exotica leaves using a brine shrimp lethality assay. After 24 h, the LC50 and LC90 values of the extract were 1.27 μg/mLand 5.09 μg/mL, respectively, indicating cytotoxic effects (p < 0.01). The volatile oils of M. exotica possessed fumigant toxicity against Sitophilus zeamais and Tribolium castaneum adults, with LC50 values of 8.29 and 6.84 mg/L, respectively. The essential oils also showed contact toxicity against S. zeamais and T. castaneum adults with LD50 values of 11.41 and 20.94 μg/adult, respectively (Li et al., 2010).
8 Conclusion and future perspectives
MFC contains at least 720 components, 404 of which are volatile oils. Crude extracts and their chemical compounds have been shown to exert anti-inflammatory, anti-bacterial and anti-microbial, antitumor, anti-oxidant, anti-diabetic, anti-Alzheimer, and analgesic effects. Flavonoids, coumarins, and volatile oils are the most important bioactive compounds with pharmacological activity.
The compounds of M. paniculata and M. exotica differ in that flavonoids are the main compounds in M. paniculata, whereas coumarins are the main compounds in M. exotica. Flavonoid compounds, particularly polymethoxyflavones, exhibit anti-inflammatory, anti-bacterial, anti-microbial, antitumor, and chondroprotective effects. Coumarin compounds, especially prenylated coumarins, exhibit anti-inflammatory, anti-bacterial, anti-microbial, antitumor, potential anti-Alzheimer, chondroprotective, anti-implantation, estrogenic, and anti-hypertensive properties. Studies on volatile oil compounds have primarily focused on their anti-bacterial, anti-microbial and potential anti-Alzheimer effects.
Despite the remarkable outcomes of previous studies on MFC (M. paniculata and M. exotica), some questions remain, and further research is needed to bridge the current scientific gap. First, although MFC demonstrates considerable pharmacological activity, little is known about the active parts and components, and further research and exploration are warranted. Second, we focused more on the analysis of polymethoxyflavones and coumarins and less on the quality control of MFC. A quality control assessment of MFC is needed to ensure quality. Third, there are many studies on the pharmacological activities of M. paniculata and M. exotica; however, only two comparative studies on the pharmacodynamics have shown no statistical differences between M. paniculata and M. exotica.
Meanwhile, in pharmacological studies, most of the studies are limited to in vitro cell studies, while in vivo studies are rarely involved. So far, only two studies are related to clinical indications, which limits the further clinical use of MFC. In view of these gaps and challenges, we should focus on in vivo pharmacological research, the relationship between efficacy and mechanism of action, and activity research on the structure-activity relationship of compounds in the future. Fourth, the antitumor activity of MFC has been described as cytotoxic, but no in vivo model is currently available and further in vivo model and clinical tumor patient studies are needed. Fifth, there are no systematic reports on the mechanisms of MFC. MFC is mainly used in the treatment of stomach pain, rheumatism, arthralgia, toothache, and tumefaction; however, the underlying mechanisms remain unclear, except for the protective effects on gastric lesions. Volatile oils are the most abundant and promote important insecticidal activity; however, their composition varies markedly depending on their origin. Therefore, there is an urgent need to establish quality standards for MFC.
At present, the phenomenon of “heterogeneous equivalence” is widespread in many multisource traditional Chinese medicines. Therefore, several problems are associated with its clinical application, such as efficacy equivalence and quality control (Lu et al., 2021b). To ensure its efficacy and safety, it is necessary to study the rationality of two source plants, M. paniculata and M. exotica, used as the same kind of MFC. Therefore, we conducted a comprehensive review of the phytochemistry, pharmacology, pharmacodynamics, pharmacokinetics, and toxicity of M. paniculata and M. exotica. This systematic review of MFC provides a material and theoretical basis for rational and effective utilization and also provides direction for in-depth research.
In conclusion, although there have been many studies on the phytochemical and pharmacological effects of MFC, there are still many aspects that require further research and control to lay a theoretical foundation for their heterogeneous use.
Author contributions
YQ: Writing–review and editing. LW: Writing–review and editing. NW: Writing–review and editing. SW: Writing–review and editing. XZ: Writing–review and editing. TZ: Writing–review and editing. QJ: Funding acquisition, Writing–review and editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This current work was supported by Natural Science Foundation of Liaoning Province (No. 2015020746) and 345 Talent Project of Shengjing Hospital of China Medical University.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1337161/full#supplementary-material
Abbreviations
AChE, acetylcholinesterase; AD, Alzheimer’s disease; BChE, butyrylcholinesterase; COX-2, cyclooxygenase-2; GC-FID, gas chromatography-flameionization detection; Foxo3a, Forkhead box class O 3a; GC-MS, gas chromatography-mass spectrometry; IC50, half maximal inhibitory concentration; IL, interleukin; iNOS, nitric oxide synthase; LC50, lethal concentration 50; LPS, lipopolysaccharide; MFC, Murrayae Folium et Cacumen; MIC, minimum inhibitory concentration; MIC50, half minimal inhibitory concentration; MMP, matrix metalloproteinase; NF-κB, nuclear factor-kappa B; NO, nitric oxide; PGE2, and prostaglandin E2; PMFs, polymethoxyflavones; TFMP, total flavonoids extracted from Murraya paniculata; TNF-α, tumor necrosis factor alpha; SOD, superoxidase dismutase.
References
Alam, F., Mohammadin, K., Shafique, Z., Amjad, S. T., and Asad, M. H. H. b. (2021). Citrus flavonoids as potential therapeutic agents: a review. Phytother. Res. 36, 1417–1441. doi:10.1002/ptr.7261
Arya, N., Kaur, J., Verma, A., and Dhanik, J., (2017). Chemical composition of leaf essential oil of wild and domestic genotypes of Murraya paniculata L. J. Essent. Bear. Pl. 20, 468–473. doi:10.1080/0972060x.2017.1312552
Aziz, S., Sukari, M., Rahmani, M., Kitajima, M., and Ahpandi, N. (2010). Coumarins from Murraya paniculata rutaceae koumarin daripada Murraya paniculata rutaceae. Malay. J. Anal. Sci. 14, 1–5.
Barik, B., Dey, A., and Chatterjee, A. (1983a). Murrayatin, a coumarin from Murraya exotica. Phytochemistry 22, 2273–2275. doi:10.1016/S0031-9422(00)80160-0
Barik, B., Dey, A., Chatterjee, A., and Shoolery, J. (1983b). Coumarins of Murraya exotica-absolute configuration of auraptenol. Phytochemistry 22, 792–794. doi:10.1016/S0031-9422(00)86993-9
Barik, B., and Kundu, A. (1987). A cinnamic acid derivative and a coumarin from Murraya exotica. Phytochemistry 26, 3319–3321. doi:10.1016/S0031-9422(00)82496-6
Bhattacharyya, P., Roy, S., Biswas, A., Bhattacharyya, L., and Chakraborty, D. (1978). Mahanimbine and murrayazoline from Murraya exotica Linn. (syn. Murraya paniculata). J. Indian Chem. Soc. 308-309.
Bovornvattanangkul, T., and Jiraungkoorskul, W. (2016). Evaluation of total phenolic compound and cytotoxic activity of Murraya paniculata. Int. Food Res. J. 23, 1207–1211.
Chakraborty, D., Roy, S., Chakraborty, A., Mandal, A., and Chowdhury, B. (1980). Structure and synthesis of mexolide: a new antibiotic dicoumarin from murraya exotica linn. [syn. m. paniculata (l) jack.]. Tetrahedron 36, 3563–3564. [syn. m. paniculata (l) jack.]. doi:10.1016/0040-4020(80)88053-7
China Pharmacopoeia Committee (2020). Pharmacopoeia of the people’s Republic of China. Vol I. 11. Beijing: China Chemical Industry Press.
Choudhary, M., Azizuddin Khalid, A., and Sultani, S., (2002). A new coumarin from Murraya paniculata. Planta Med. 68, 81–83. doi:10.1055/s-2002-19874
Chowdhury, J., Bhuiyan, M., and Yusuf, M. (2008). Chemical composition of the leaf essential oils of Murraya koenigii (L.) Spreng and Murraya paniculata (L.) Jack. Bangl. J. Pharmacol. 3, 59–63. doi:10.3329/bjp.v3i2.841
Cuong, N. M., Khanh, P. N., Duc, H. V., Huong, T. T., Tai, B. H., Binh, N. Q., et al. (2014). Vasorelaxing activity of two coumarins from Murraya paniculata leaves. Biol. Pharm. Bull. 37, 694–697. doi:10.1248/bpb.b13-00905
Desoky, E. (1995). Phytosterols from murraya exotica. Phytochemistry 40, 1769–1772. doi:10.1016/0031-9422(95)00821-N
Dosoky, N., Satyal, P., Gautam, T., and Setzer, W. (2016). Composition and biological activities of Murraya paniculata (L.) Jack essential oil from Nepal. Medicines 3, 7. doi:10.3390/medicines3010007
dos Santos, D. A. P., de C. Braga, P. A., Fernandes, J. B., Vieira, P. C., Magalhães, A. F., Magalhães, E. G., et al. (2009). Anti-African trypanocidal and antimalarial activity of natural flavonoids, dibenzoylmethanes and synthetic analogues. J. Pharm. Pharmacol. 61, 257–266. doi:10.1211/jpp/61.02.0017
El-Sakhawy, F., El-Tantawy, M., and El-Sohly, M. (1998). Composition and antimicrobial activity of the essential oil of Murraya exotica L. Flavour Fragr. J. 13, 59–62. doi:10.1002/(sici)1099-1026(199801/02)13:1<59::aid-ffj693>3.3.co;2-c
El-Shiekh, R. A., Kassem, H. A. H., Khaleel, A. E., and Abd El-Mageed, M. M. A. (2023). Anticholinesterases activity of Murraya koenigii (L.) Spreng. and Murraya paniculata (L.) Jacq. essential oils with GC/MS analysis and molecular docking. Nat. Prod. Res. 1-5, 1–5. doi:10.1080/14786419.2023.2241150
Ferracin, R., da Silva, M., Fernandes, J., and Vieira, P. (1998). Flavonoids from the fruits of Murraya paniculata. Phytochemistry 47, 393–396. doi:10.1016/s0031-9422(97)00598-0
Ganguly, S., and Sarkar, A. (1978). Exozoline, a new carbazole alkaloid from the leaves of Murraya exotica. Phytochemistry 17, 1816–1817. doi:10.1016/S0031-9422(00)88715-4
Gautam, M. K., Gangwar, M., Nath, G., Rao, C. V., and Goel, R. K. (2012a). In–vitro antibacterial activity on human pathogens and total phenolic, flavonoid contents of Murraya paniculata Linn. leaves. Asian pac. J. Trop. bio. 2, S1660–S1663. doi:10.1016/S2221-1691(12)60472-9
Gautam, M. K., Gangwar, M., Singh, A., Rao, C. V., and Goel, R. K. (2012b). In vitro antioxidant properties of Murraya paniculata (L.) leaves extract. Inven. Rapid J. Ethnopharmacol.
He, S. D., Yang, X. T., Yan, C. C., Jiang, Z., Yu, S. H., Zhou, Y. Y., et al. (2017). Promising compounds from Murraya exotica for cancer metastasis chemoprevention. Integr. Cancer Ther. 16, 556–562. doi:10.1177/1534735416678981
Huang, X., Chen, Z., Shi, W., Zhang, R., Li, L., Liu, H., et al. (2019). TMF inhibits miR-29a/Wnt/β-catenin signaling through upregulating Foxo3a activity in osteoarthritis chondrocytes. Drug Des. devel. Ther. 13, 2009–2019. doi:10.2147/DDDT.S209694
Huang, Y. S., Wang, Y., Luo, X. P., and Yuan, K. (2013). Composition, antimicrobial and antioxidant activities of the essential oil of Murraya exotica from Hainan of China. Asian J. Chem. 25, 5055–5058. doi:10.14233/ajchem.2013.14387a
Imai, F., Itoh, K., Kishibuchi, N., Kinoshita, T., and Sankawa, U. (1989). Constituents of the root bark of Murrayapaniculata collected in Indonesia. Chem. Pharm. Bull. 37, 119–123. doi:10.1248/cpb.37.119
Ito, C., and Furukawa, H. (1987a). Three new coumarins from leaves of Murraya paniculata. Heterocycles 26, 2959–2962. doi:10.3987/r-1987-11-2959
Ito, C., and Furukawa, H. (1987b). Constituents of Murraya exotica L. structure elucidation of new coumarins. Chem. Pharm. Bull. 35, 4277–4285. doi:10.1248/cpb.35.4277
Ito, C., Furukawa, H., Ishii, H., Ishikawa, T., and Haginiwa, J. (1990). The chemical composition of Murraya panicdata. The structure of five new coumarins and one new alkaloid and the stereochemistry of murrangatin and related coumarins. J. Chem. Soc. Perkin Trans. 10, 2047–2055. doi:10.1002/chin.199043286
Jiang, Z., Pang, Y., Yu, X., Zhou, S., Qian, J., Zheng, N., et al. (2016). The paradigm-shifting idea and its practice: from traditional abortion Chinese medicine Murraya paniculata to safe and effective cancer metastatic chemopreventives. Oncotarget 7, 21699–21712. doi:10.18632/oncotarget.7932
Jiang, Z., Yang, J., Pang, Y., Yang, X., Yu, S., and Jia, L. (2015). Bioactivity-guided fast screen and identification of cancer metastasis chemopreventive components from raw extracts of Murraya exotica. J. Pharm. Biomed. Anal. 107, 341–345. doi:10.1016/j.jpba.2015.01.023
Jin, Y. R., Sui, D. Y., Li, X. W., Yu, X. F., Gui, M. Y., and Qu, S. C. (2008). Applications of murraya jasminorage leaf total flavones in preparation of medicament for curing diabetes. Patent application: China Unexamined APPLIC. open to Public inspection CN20081091362.
Jiwajinda, S., Santisopasri, V., and Ohigashi, H. (2000). Coumarin-related compounds as plant growth inhibitors from two rutaceous plants in Thailand. Biosci. Biotechnol. Biochem. 64, 420–423. doi:10.1271/bbb.64.420
Kabir, M. T., Uddin, M. S., Zaman, S., Begum, Y., Bin-Jumah, M. N., Ashraf, G. M., et al. (2021). Molecular mechanisms of metal toxicity in the pathogenesis of Alzheimer’s disease. Mol. Neurobiol. 58, 1–20. doi:10.1007/s12035-020-02096-w
Kaur, D., Kaur, A., and Arora, S. (2016). Delineation of attenuation of oxidative stress and mutagenic stress by Murraya exotica L. leaves. Springerplus 5, 1037. doi:10.1186/s40064-016-2709-0
Khalid, A., Khan, W., Zia, K., Azizuddin Ahsan, W., Alhazmi, H. A., Abdalla, A. N., et al. (2023). Natural coumarins from Murraya paniculata as mixed-type inhibitors of cholinesterases: in vitro and in silico investigations. Front. Pharmacol. 14, 1133809. doi:10.3389/fphar.2023.1133809
Khatun, A., Rahman, M., and Jahan, S. (2014). Preliminary phytochemical, cytotoxic, thrombolytic and antioxidant activities of the methanol extract of Murraya exotica Linn. leaves. Orient. Pharm. Exp. Med. 14, 223–229. doi:10.1007/s13596-014-0150-x
Kinoshita, T., and Firman, K. (1996a). Prenylcoumarin derivatives from the leaves of an Indonesian medicinal plant murraya paniculata(rutaceae). Chem. Pharm. Bull. 44, 1261–1262. doi:10.1248/cpb.44.1261
Kinoshita, T., and Firman, K. (1996b). Highly oxygenated flavonoids from Murraya paniculata. Phytochemistry 42, 1207–1210. doi:10.1016/0031-9422(96)00058-1
Kinoshita, T., and Firman, K. (1997). Myricetin 5,7,3',4',5'-pentamethyl ether and other methylated flavonoids from Murraya paniculata. Phytochemistry 45, 179–181. doi:10.1016/S0031-9422(96)00853-9
Kong, Y., Ng, K., Wat, K., Wong, A., Saxena, I., Cheng, K., et al. (1985a). Yuehchukene, a novel anti-implantation indole alkaloid from Murraya paniculata. Planta Med. 51, 304–307. doi:10.1055/s-2007-969497
Kong, Y. C., Ng, K. H., Wat, K. H., Wong, A., Saxena, I. F., Cheng, K. F., et al. (1985b). Yuehchukene, a novel anti-implantation indole alkaloid from Murraya paniculata. Planta Med. 51, 304–307. doi:10.1055/s-2007-969497
Krishnamoorthy, S., Chandrasekaran, M., Raj, G. A., Jayaraman, M., and Venkatesalu, V. (2015). Identification of chemical constituents and larvicidal activity of essential oil from Murraya exotica L. (Rutaceae) against Aedes aegypti, Anopheles stephensi and Culex quinquefasciatus (Diptera: Culicidae). Parasitol. Res. 114, 1839–1845. doi:10.1007/s00436-015-4370-x
Lapcik, O., Klejdus, B., Davidova, M., Kokoska, L., Kuban, V., and Moravcova, J. (2004). Isoflavonoids in the Rutaceae family:1. Fortunella obovata, Murraya paniculata and four Citrus species. Phytochem. Anal. 15, 293–299. doi:10.1002/pca.781
Li, N., Liu, X., Zhang, M., Zhang, Z., Zhang, B., Wang, X., et al. (2022). Characterization of a coumarin C-/O-prenyltransferase and a quinolone C-prenyltransferase from Murraya exotica. Org. Biomol. Chem. 20, 5535–5542. doi:10.1039/d2ob01054b
Li, P., Zhang, Y., Wang, Z., Chen, H., Li, X., and Jin, Y. (2018). A novel flavone from the leaves of Murraya paniculata. Chem. Nat. Comp. 54, 1061–1063. doi:10.1007/s10600-018-2555-0
Li, W. Q., Jiang, C. H., Chu, S. S., Zuo, M. X., and Liu, Z. L. (2010). Chemical composition and toxicity against Sitophilus zeamais and Tribolium castaneum of the essential oil of Murraya exotica aerial parts. Molecules 15, 5831–5839. doi:10.3390/molecules15085831
Li, X. M., Jiang, X. J., Yang, K., Wang, L. X., Wen, S. Z., and Wang, F. (2016). Prenylated coumarins from Heracleum stenopterum, Peucedanum praeruptorum, Clausena lansium, and Murraya paniculata. Nat. Prod. Bioprospect. 6, 233–237. doi:10.1007/s13659-016-0107-5
Li, Y. (2022). Studies on the chemical constituents of murraya paniculata and murraya exotica. (Master dissertation). Kunming, China: Yunnan Normal University.
Liang, H., Cao, N., Zeng, K., Zhao, M., Tu, P., and Jiang, Y. (2020a). Coumarin and spirocyclopentenone derivatives from the l eaves and stems of Murraya paniculata (L.) Jack. Phytochemistry 172, 112258. doi:10.1016/j.phytochem.2020.112258
Liang, H., Shi, Y., Zeng, K., Zhao, M., Tu, P., and Jiang, Y. (2020b). Coumarin derivatives from the leaves and twigs of Murraya exotica L. and their anti-inflammatory activities. Phytochemistry 177, 112416. doi:10.1016/j.phytochem.2020.112416
Liang, H., Zhao, M., Tu, P., and Jiang, Y. (2020c). Polymethoxylated flavonoids from murraya paniculata (L.) jack. . Biochem. Syst. Ecol. 93, 104162. doi:10.1016/j.bse.2020.104162
Liang, H. Z., Du, Z. Y., Yuan, S., Lu, M. Q., Xing, J. Y., Ma, Q., et al. (2021). Comparison of Murraya exotica and Murraya paniculata by fingerprint analysis coupled with chemometrics and network pharmacology methods. Chin. J. Nat. Med. 19, 713–720. doi:10.1016/S1875-5364(21)60087-0
Liaqat, I., Riaz, N., Saleem, Q. u.A., Tahir, H. M., Arshad, M., and Arshad, N. (2018). Toxicological evaluation of essential oils from some plants of Rutaceae family. Evid. Based Complement. Altern. Med. 2018, 4394687. doi:10.1155/2018/4394687
Liu, B., and Jiang, Y. (2015a). Simultaneous determination of three main analytes of Murraya exotica by HPLC. J. Chin. Pharm. Sci. 24, 88–94. doi:10.5246/jcps.2015.02.010
Liu, B. Y., Zhang, C., Zeng, K. W., Li, J., Guo, X. Y., Zhao, M. B., et al. (2015b). Exotines A and B, two heterodimers of isopentenyl-substituted indole and coumarin derivatives from Murraya exotica. Org. Lett. 17, 4380–4383. doi:10.1021/acs.orglett.5b02230
Liu, B. Y., Zhang, C., Zeng, K. W., Li, J., Guo, X. Y., Zhao, M. B., et al. (2018). Anti-inflammatory prenylated phenylpropenols and coumarin derivatives from Murraya exotica. J. Nat. Prod. 81, 22–33. doi:10.1021/acs.jnatprod.7b00518
Lu, M., Du, Z., Yuan, S., Ma, Q., Han, Z., Tu, P., et al. (2021b). Comparison of the preventive effects of Murraya exotica and Murraya paniculata on alcohol-induced gastric lesions by pharmacodynamics and metabolomics. J. Ethnopharmacol. 281, 114567. doi:10.1016/j.jep.2021.114567
Lu, M., Liang, H., Tu, P., and Jiang, Y. (2021a). Pharmacodynamic comparison of two different source plants of Murrayae Folium et Cacumen. J. Chin. Pharm. Sci. 30, 49–57. doi:10.5246/jcps.2021.01.005
Lv, H. N., Guo, X. Y., Tu, P. F., and Jiang, Y. (2013). Comparative analysis of the essential oil composition of Murraya paniculata and M. exotica. Nat. Prod. Commun. 8, 1934578X1300801–1475. doi:10.1177/1934578x1300801035
Mehmood, F., Manzoor, F., Khan, Z., Ali, M., Khan, I., and Rahim, S. (2012). Evaluation of toxicity and repellency of essential oils of family Rutaceae against black ants (Lasius Niger) in Pakistan. Asian J. Chem. 24, 3087–3090. doi:10.1016/j.arabjc.2010.09.012
Menezes, C. D. A., de Oliveira Garcia, F. A., de Barros Viana, S., Pinheiro, P. G., Felipe, C. F. B., de Albuquerque, T. R., et al. (2017). Murraya paniculata (L.) (Orange jasmine): potential nutraceuticals with ameliorative effect in alloxan-induced diabetic rats. Phytother. Res. 31, 1747–1756. doi:10.1002/ptr.5903
Menezes, I. R. A., Santana, T. I., Varela, V. J. C., Saraiva, R. A., Matias, E. F. F., Boligon, A. A., et al. (2015). Chemical composition and evaluation of acute toxicological, antimicrobial and modulatory resistance of the extract of Murraya paniculata. Pharm. Biol. 53, 185–191. doi:10.3109/13880209.2014.913068
Mita, T., Shihan, M., Rahman, M., Sharmin, T., Maleque, M., Alve, M., et al. (2013). In vitro antioxidant, cytotoxic, thrombolytic, antimicrobial and membrane stabilizing activities of Murraya paniculata. Amer. J. Res. Commun. 1, 226–237.
Naseem, M. K., Younis, A., Khan, M. A., and Ahmad, R. (2015). Gas chromatography-mass spectrometry of Murraya exotica essential oil extracted through different extraction techniques. J. Anim. Plant Sci. 25, 1730–1736.
Negi, N., Abou-Dough, A. M., Kurosawa, M., Kitaji, Y., Saito, K., Ochi, A., et al. (2015). Coumarins from Murraya exotica collected in Egypt. Nat. Prod. Commun. 10, 1934578X1501000–620. doi:10.1177/1934578x1501000420
Negi, N., Ochi, A., Kurosawa, M., Ushijima, K., Kitaguchi, Y., Kusakabe, E., et al. (2005). Two new dimeric coumarins isolated from Murraya exotica. Chem. Pharm. Bull. 53, 1180–1182. doi:10.1248/cpb.53.1180
Ng, M., Abdulhadi-Noaman, Y., Cheah, Y., Yeap, S., and Alitheen, N. (2012). Bioactivity studies and chemical constituents of Murraya paniculata (Linn) Jack. Int. Food Res. J. 19, 1307–1312.
Olawore, N. O., Ogunwande, I. A., Ekundayo, O., and Adeleke, K. A. (2005). Chemical composition of the leaf and fruit essential oils of Murraya paniculata (L.) Jack. (Syn.Murraya exotica Linn.). Flavour Fragr. J. 20, 54–56. doi:10.1002/ffj.1365
Pan, M., Lai, Y., Lai, C., Wang, Y., Li, S., Lo, C., et al. (2007). 5-Hydroxy-3,6,7,8,3',4'-hexamethoxyflavone induces apoptosis through reactive oxygen species production, growth arrest and DNA damage-inducible gene 153 expression, and caspase activation in human leukemia cells. J. Agric. Food Chem. 55, 5081–5091. doi:10.1021/jf070068z
Panda, S. K., Das, R., Lavigne, R., and Luyten, W. (2020). Indian medicinal plant extracts to control multidrug-resistant S. aureus, including in biofilms. S. Afr. J. Bot. 128, 283–291. doi:10.1016/j.sajb.2019.11.019
Paul, I., Bhadoria, P. B. S., and Mitra, A. (2020). Seasonal and diel variations in scent composition of ephemeral Murraya paniculata (Linn.) Jack flowers are contributed by separate volatile components. Biochem. Syst. Ecol. 89, 104004. doi:10.1016/j.bse.2020.104004
Peng, A., Qu, X., Li, H., Gao, L., Yu, B., and Yang, H. (2010). Isolation and purification of flavones from Murraya exotica L. by high-speed counter-current chromatography. Chin. J. Chromatogr. 28, 383–387. doi:10.3724/sp.j.1123.2010.00383
Pino, J. A., Marbot, R., and Fuentes, V. (2006). Aromatic plants from western Cuba. VI. Composition of the leaf oils of Murraya exotica L., Amyris balsamifera L., Severinia buxifolia (Poir.) Ten. and Triphasia trifolia (Burm. f.) P. Wilson. J. Essent. Oil Res. 18, 24–28. doi:10.1080/10412905.2006.9699376
Podder, M. K., Das, B. N., Saha, A., and Ahmed, M. (2011). Analgesic activity of bark of Murraya paniculata. Int. J. Med. Sci. 3, 105–108.
Rahman, A., Shabbir, M., Sultani, S., Jabbar, A., and Choudhary, M. (1997). Cinnamates and coumarins from the leaves of Murraya paniculata. Phytochemistry 44, 683–685. doi:10.1016/s0031-9422(96)00617-6
Rahman, M. A., Hasanuzzaman, M., Uddin, N., and Shahid, I. (2010). Antidiarrhoeal and anti-inflammatory activities of Murraya paniculata (L). Jack. Pharmacol. 3, 768–776.
Raina, V. K., Verma, S. C., Dhawan, S., Khan, M., Ramesh, S., Singh, S. C., et al. (2006). Essential oil composition of Murraya exotica from the plains of northern India. Flavour Fragr. J. 21, 140–142. doi:10.1002/ffj.1547
Rehman, F., Khan, M., and Khan, I., (2013). Molecular interactions of an alkaloid euchrestifoline as a new acetylcholinesterase inhibitor. Bangl. J. Pharmacol. 8, 361–364. doi:10.3329/bjp.v8i4.16417
Rodanant, P., Khetkam, P., Suksamrarn, A., and Kuvatanasuchati, J. (2015). Coumarins and flavonoid from Murraya paniculata (L.) Jack: antibacterial and anti-inflammation activity. Pak J. Pharm. Sci. 28, 1947–1951.
Rodríguez, E., Ramis-Ramos, G., Vander Heyden, Y., Simo-Alfonso, E., Lerma-Garcia, M., Saucedo-Hernandez, Y., et al. (2012). Chemical composition, antioxidant properties and antimicrobial activity of the essential oil of Murraya paniculata leaves from the mountains of central Cuba. Nat. Prod. Commun. 7, 1934578X1200701–1530. doi:10.1177/1934578x1200701129
Rout, P., Rao, Y., and Naik, S. (2010). Liquid CO2 extraction of Murraya paniculata Linn. flowers. Ind. Crop. Prod. 32, 338–342. doi:10.1016/j.indcrop.2010.05.011
Rout, P., Rao, Y., Sree, A., and Naik, S. (2007). Composition of essential oil, concrete, absolute, wax and headspace volatiles of Murrarya paniculata (Linn.) Jack flowers. Flavour Fragr. J. 22, 352–357. doi:10.1002/ffj.1804
Roy, S., and Bhattacharya, L. (1981). Girinimbine and koenimbine from murraya exotica linn. J. Indian Chem. Soc. 58, 1212.
Saeed, S., Shah, S., Mehmood, R., and Malik, A. (2011). Paniculacin, a new coumarin derivative from Murraya paniculata. J. Asian Nat. Prod. Res. 13, 724–727. doi:10.1080/10286020.2011.586343
Saied, S., Nizami, S. S., and Anis, I. (2008). Two new coumarins from Murraya paniculata. J. Asian Nat. Prod. Res. 10, 515–519. doi:10.1080/10286020801967292
Saikia, S., Tamuli, K. J., Narzary, B., Bordoloi, M., and Banik, D. (2021). Chemical composition, antimicrobial activity and cytotoxicity of Murraya paniculata (L.) Jack leaf essential oil from Assam, India: the effect of oil on cellular morphology of micro-organisms. Arch. Microbio. 204, 99. doi:10.1007/s00203-021-02665-0
Sangkaew, A., Samritsakulchai, N., Sanachai, K., Rungrotmongkol, T., Chavasiri, W., and Yompakdee, C. (2020). Two flavonoid-based compounds from Murraya paniculata as novel human carbonic anhydrase isozyme II inhibitors detected by a resazurin yeast-based assay. J. Microbio. Biotechnol. 30, 552–560. doi:10.4014/jmb.1910.10037
Saqib, F., Ahmed, M. G., Janbaz, K. H., Dewanjee, S., Jaafar, H. Z. E., and Zia-Ul-Haq, M. (2015). Validation of ethnopharmacological uses of Murraya paniculata in disorders of diarrhea, asthma and hypertension. BMC Complement. Altern. Med. 15, 319. doi:10.1186/s12906-015-0837-7
Sawangjaroen, N., Phongpaichit, S., Subhadhirasakul, S., Visutthi, M., Srisuwan, N., and Thammapalerd, N. (2006). The anti-amoebic activity of some medicinal plants used by AIDS patients in southern Thailand. Parasitol. Res. 98, 588–592. doi:10.1007/s00436-005-0119-2
Sawangjaroen, N., Subhadhirasakul, S., Phongpaichit, S., Siripanth, C., Jamjaroen, K., and Sawangjaroen, K. (2005). The in vitro anti-giardial activity of extracts from plants that are used for self-medication by AIDS patients in southern Thailand. Parasitol. Res. 95, 17–21. doi:10.1007/s00436-004-1264-8
Selestino Neta, M. C., Vittorazzi, C., Guimarães, A. C., Martins, J. D., Fronza, M., Endringer, D. C., et al. (2017). Effects of β-caryophyllene and Murraya paniculata essential oil in the murine hepatoma cells and in the bacteria and fungi 24-h time-kill curve studies. Pharm. Biol. 55, 190–197. doi:10.1080/13880209.2016.1254251
Shah, S., Saied, S., Mahmood, A., and Malik, A. (2014). Phytochemical screening of volatile constituents from aerial parts of Murraya paniculata. Pak. J. Bot. 46, 2051–2056.
Shaikh, A., and Choudhary, M. I. (2011). Bioassay studies of 2'-O-ethylmurrangatin isolated from a medicinal plant, Murraya paniculata. Turk. J. Biol. 35, 751–755. doi:10.3906/biy-1005-161
Shan, J., Wang, X., Ma, Y., Yang, R., Li, X., and Jin, Y. (2010). Studies on flavonoids from leaves of Murraya paniculata L. (I). Chin. Pharm. J. 45, 1910–1912.
Shao, J., Zhou, S., Jiang, Z., Chi, T., Ma, J., Kuo, M., et al. (2016). Warfarin and coumarin-like Murraya paniculata extract down-regulate EpCAM-mediated cell adhesion: individual components versus mixture for studying botanical metastatic chemopreventives. Sci. Rep. 6, 30549. doi:10.1038/srep30549
Sharker, S. M., Shahid, I. J., and Hasanuzzaman, M. (2009). Antinociceptive and bioactivity of leaves of murraya paniculata (L.) jack, rutaceae. Rutaceae. Rev. Bras. Farmacogn. 19, 746–748. doi:10.1590/S0102-695X2009000500016
Sharma, P., Batra, S., Kumar, A., and Sharma, A. (2017). In vivo antianxiety and antidepressant activity of Murraya paniculata leaf extracts. J. Integr. Med. 15, 320–325. doi:10.1016/S2095-4964(17)60352-2
Sharma, S., and Arora, S. (2015). A clinical evaluation of atrial fibrillation in rheumatic heart disease. Int. J. Pharm. Res. Rev. 4, 22–25.
Shi, Q., Jiang, Z., Yang, J., Cheng, Y., Pang, Y., Zheng, N., et al. (2017). A flavonoid glycoside compound from Murraya paniculata (L.) interrupts metastatic characteristics of A549 cells by regulating STAT3/NF-κB/COX-2 and EGFR signaling pathways. AAPS J. 19, 1779–1790. doi:10.1208/s12248-017-0134-0
Silva, F. F. A. d., Alves, C. C. F., Oliveira Filho, J. G. d., Vieira, T. M., Crotti, A. E. M., and Miranda, M. L. D. (2019). Chemical constituents of essential oil from Murraya paniculata leaves and its application to in vitro biological control of the fungus Sclerotinia sclerotiorum. Food Sci. Technol. 39, 413–417. doi:10.1590/fst.20218
Silva, F. F. A. d., Fernandes, C. C., Santiago, M. B., Martins, C. H. G., Vieira, T. M., Crotti, A. E. M., et al. (2020). Chemical composition and in vitro antibacterial activity of essential oils from Murraya paniculata (L.) Jack (Rutaceae) ripe and unripe fruits against bacterial genera Mycobacterium and Streptococcus. Braz. J. Pharm. Sci. 56. doi:10.1590/s2175-97902019000418371
Srivastava, S. D., Srivastava, S. K., and Shrivastava, S. (1996). New coumarins from Murraya paniculata. J. Indian Chern. Soc. 73, 666–668.
Sun, T., Li, X., Yang, J., Li, L., Jin, Y., and Shi, X. (2015). Graphene-encapsulated silica as matrix solid-phase dispersion extraction sorbents for the analysis of poly-methoxylated flavonoids in the leaves of Murraya panaculata (L.) Jack. . J. Sep. Sci. 38, 2132–2139. doi:10.1002/jssc.201500002
Uddin, S., Tewari, D., Sharma, G., Kabir, T., Bin-Jumah, M., Perveen, A., et al. (2021). Molecular mechanisms of metal toxicity in the pathogenesis of Alzheimer’s disease. Mol. Neurobiol. 58, 1–20. doi:10.1007/s12035-020-02096-w
Wang, J., Han, Z., Yang, L., Xu, Q., and Yang, H. (2010). Comparison of UPLC and HPLC for the determination of 6,8,3',4'-tetramethoxyl flavone in Murraya paniculata (L.) Jack. Chin. J. Pharm. Anal. 30, 1499–1501. doi:10.16155/j.0254-1793.2010.08.018
Wang, X., Liang, H., Zeng, K., Zhao, M., Tu, P., Li, J., et al. (2019a). Panitins A-G: coumarin derivatives from Murraya paniculata from Guangxi Province, China show variable NO inhibitory activity. Phytochemistry 162, 224–231. doi:10.1016/j.phytochem.2019.03.012
Wang, X. T., Zeng, K. W., Zhao, M. B., Tu, P. F., Li, J., and Jiang, Y. (2017). Three new indole alkaloid derivatives from the roots of Murraya paniculata. J. Asian Nat. Prod. Res. 20, 201–208. doi:10.1080/10286020.2017.1327950
Wang, Y., Lv, H., Cui, Q., Tu, P., Jiang, Y., and Zeng, K. (2019b). Isosibiricin inhibits microglial activation by targeting the dopamine D1/D2 receptor-dependent NLRP3/caspase-1 inflammasome pathway. Acta Pharmacol. Sin. 41, 173–180. doi:10.1038/s41401-019-0296-7
Wojtunik-Kulesza, K., Rudkowska, M., Kasprzak-drozd, K., Oniszczuk, A., and Borowicz-Reutt, K. (2021). Activity of selected group of monoterpenes in Alzheimer’s disease symptoms in experimental model studies-a non-systematic review. Int. J. Mol. Sci. 22, 7366. doi:10.3390/ijms22147366
Wu, J., Liu, K., and Shi, X. (2015). The anti-inflammatory activity of several flavonoids isolated from Murraya paniculata on murine macrophage cell line and gastric epithelial cell (GES-1). Pharm. Biol. 54, 868–881. doi:10.3109/13880209.2015.1089294
Wu, L., Li, P., Wang, X., Zhuang, Z., Farzaneh, F., and Xu, R. (2010). Evaluation of anti-inflammatory and antinociceptive activities of Murraya exotica. Pharm. Biol. 48, 1344–1353. doi:10.3109/13880201003793723
Wu, L., Liu, H., Li, L., Liu, H., Yang, K., Liu, Z., et al. (2014). 5,7,3',4'-Tetramethoxyflavone exhibits chondroprotective activity by targeting β-catenin signaling in vivo and in vitro. Biochem. Biophys. Res. Commun. 452, 682–688. doi:10.1016/j.bbrc.2014.08.129
Wu, L., Liu, H., Zhang, R., Li, L., Li, J., Hu, H., et al. (2013). Chondroprotective activity of murraya exotica through inhibiting β -catenin signaling pathway. Evid. Based Complement. Altern. Med. 2013, 752150. doi:10.1155/2013/752150
Wu, T., Chan, Y., Leu, Y., and Huang, S. (1994). A flavonoid and indole alkaloid from flowers of Murraya paniculata. Phytochemistry 37, 287–288. doi:10.1016/0031-9422(94)85045-3
Xia-Hou, Z. R., Feng, X. F., Mei, Y. F., Zhang, Y. Y., Yang, T., Pan, J., et al. (2022). 5-Demethoxy-10′-ethoxyexotimarin F, a new coumarin with MAO-B inhibitory potential from Murraya exotica L. Molecules 27, 4950. doi:10.3390/molecules27154950
Xu, G., Feng, L., Song, P., Xu, F., Li, A., Wang, Y., et al. (2016). Isomeranzin suppresses inflammation by inhibiting M1 macrophage polarization through the NF-κB and ERK pathway. Int. Immunopharmacol. 38, 175–185. doi:10.1016/j.intimp.2016.05.027
Yan, R., Shen, J., Liu, X., Zou, Y., and Xu, X. (2018). Preparative isolation and purification of hainanmurpanin, meranzin, and phebalosin from leaves of Murraya exotica L. using supercritical fluid extraction combined with consecutive high-speed countercurrent chromatography. J. Sep. Sci. 41, 2092–2101. doi:10.1002/jssc.201701423
Yang, J., and Su, Y. (1983). Studies on the constituents of murraya paniculata (L.) jack. Acta Pharm. Sin. 18, 760–765. doi:10.1021/bk-1984-0239.ch011
Yao, H., Jin, Y., Shan, J., Wang, X., Zhang, M., and Li, X. (2013). Two new natural methoxyflavonoids from leaves of Murraya paniculata (L.) Jack. . Chem. Res. Chin. Univ. 29, 884–887. doi:10.1007/s40242-013-3073-z
You, C. X., Zhang, W. J., Guo, S. S., Wang, C. F., Yang, K., Liang, J. Y., et al. (2015). Chemical composition of essential oils extracted from six Murraya species and their repellent activity against Tribolium castaneum. Ind. Crop. Prod. 76, 681–687. doi:10.1016/j.indcrop.2015.07.044
Zhai, S., Deng, X., Zhang, C., Zhou, Y., Xie, H., Jiang, Z., et al. (2020). A novel UPLC/MS/MS method for rapid determination of murrayone in rat plasma and its pharmacokinetics. J. Pharm. Biomed. Anal. 180, 113046. doi:10.1016/j.jpba.2019.113046
Zhang, J., Lu, J., Zhang, Q., Dai, L., Liu, Y., Tu, P., et al. (2013a). Simultaneous screening and identifying four categories of particular flavonoids in the leaves of Murraya exotica L. by HPLC-DAD-ESI-MS-MS. J. Chromatogr. Sci. 52, 103–114. doi:10.1093/chromsci/bms253
Zhang, J. Y., Li, N., Che, Y. Y., Zhang, Y., Liang, S. X., Zhao, M. B., et al. (2011). Characterization of seventy polymethoxylated flavonoids (PMFs) in the leaves of Murraya paniculata by on-line high-performance liquid chromatography coupled to photodiode array detection and electrospray tandem mass spectrometry. J. Pharm. Biomed. Anal. 56, 950–961. doi:10.1016/j.jpba.2011.08.019
Zhang, J. Y., Li, N., Zhou, Y., Jiang, Y., and Tu, P. F. (2012a). Simultaneous qualitative and quantitative determination of major polymethoxylated flavonoids in the leaves of Murraya paniculata by RRLC-DAD-ESI-MSn. Anal. Methods 4, 3399–3406. doi:10.1039/c2ay25242b
Zhang, J. Y., Lu, J. Q., Gao, X. Y., Zhang, Q., Li, N., Tu, P. F., et al. (2014). Characterization of thirty-nine polymethoxylated flavonoids (PMFs) in the branches of Murraya paniculata by HPLC-DAD-ESI-MS/MS. Chin. J. Nat. Med. 11, 63–70. doi:10.1016/S1875-5364(13)60009-6
Zhang, J. Y., Lu, J. Q., Wang, F., Cao, G. S., Tu, P. F., and Qiao, Y. J. (2013b). A strategy of EIC-MS coupled with diagnostic product ions analysis for efficient discovery of new hydroxylated polymethoxyflavonoid glycosides from the leaves of Murraya paniculata L. using HPLC-DAD-MS/MS. Anal. Methods 5, 2880. doi:10.1039/C3AY26475K
Zhang, Y., Li, J., Shi, S., Zan, K., and Tu, P. (2012b). Glycosides of flavone methyl ethers from Murraya paniculata. Biochem. Syst. Ecol. 43, 10–13. doi:10.1016/j.bse.2011.10.003
Zhang, Y., Li, J., Zhou, S., and Tu, P. (2010). Polymethoxylated flavonoids from the leaves of Murraya paniculata. Chin. Pharm. J. 45, 1139–1141.
Zhou, Q., Shi, Y., Qi, H., Liu, H., Wei, N., Jiang, Y., et al. (2020). Identification of two natural coumarin enantiomers for selective inhibition of TRPV2 channels. FASEB J. 34, 12338–12353. doi:10.1096/fj.201901541RRR
Zou, J., Sui, D., Fu, W., Li, Y., Yu, P., Yu, X., et al. (2021). Total flavonoids extracted from the leaves of Murraya paniculata (L.) Jack alleviate oxidative stress, inflammation and apoptosis in a rat model of diabetic cardiomyopathy. J. Funct. Foods 76, 104319. doi:10.1016/j.jff.2020.104319
Keywords: Murrayae Folium et Cacumen, Murraya paniculata, Murraya exotica, phytochemistry, pharmacology, toxicology
Citation: Qi Y, Wang L, Wang N, Wang S, Zhu X, Zhao T and Jiang Q (2024) A comprehensive review of the botany, phytochemistry, pharmacology, and toxicology of Murrayae Folium et Cacumen. Front. Pharmacol. 15:1337161. doi: 10.3389/fphar.2024.1337161
Received: 12 November 2023; Accepted: 06 March 2024;
Published: 28 March 2024.
Edited by:
Sonia Piacente, Università degli Studi di Salerno, ItalyReviewed by:
Lixia Chen, Shenyang Pharmaceutical University, ChinaDagmara Wróbel-Biedrawa, Jagiellonian University Medical College, Poland
Copyright © 2024 Qi, Wang, Wang, Wang, Zhu, Zhao and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tie Zhao, zhaotieaaa@163.com; Qinghua Jiang, lnzyjqh2002@163.com
†These authors have contributed equally to this work
 Yue Qi1†
Yue Qi1† Lin Wang
Lin Wang Qinghua Jiang
Qinghua Jiang