- 1Department of Pharmacy, Shaanxi Provincial People’s Hospital, Xi’an, China
- 2Department of Pharmacy, Xijing Hospital, Fourth Military Medical University, Xi’an, China
- 3Department of Pharmacy, Gansu Provincial Hospital, Lanzhou, China
Purpose: To evaluate the cost-effectiveness of sotorasib versus docetaxel in non-small cell lung cancer (NSCLC) patients with KRASG12C mutation from the China and United States’social perspective.
Materials and Methods: A Markov model that included three states (progression-free survival, post-progression survival, and death) was developed. Incremental cost-effectiveness ratio (ICER), quality-adjusted life-year (QALY), and incremental QALY were calculated for the two treatment strategies. One-way sensitivity analysis was used to investigate the factors that had a greater impact on the model results, and tornado diagrams were used to present the results. Probabilistic sensitivity analysis was performed with 1,000 Monte Carlo simulations. Assume distributions based on parameter types and randomly sample all parameter distributions each time., The results were presented as cost-effectiveness acceptable curves.
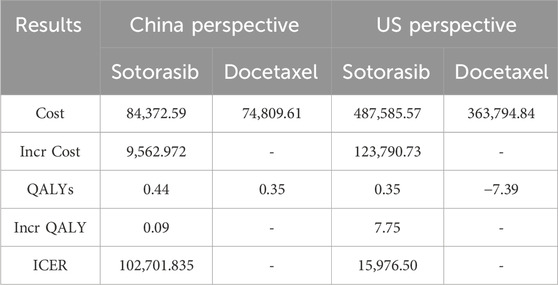
Results: This economic evaluation of data from the CodeBreak 200 randomized clinical trial. In China, sotorasib generated 0.44 QAYL with a total cost of $84372.59. Compared with docetaxel, the ICER value of sotorasib was $102701.84/QALY, which was higher than willingness to pay (WTP), so sotorasib had no economic advantage. In the US, sotorasib obtained 0.35 QALY more than docetaxel, ICER was $15,976.50/QALY, which was more than 1 WTP but less than 3 WTP, indicating that the increased cost of sotorasib was acceptable. One-way sensitivity analysis showed that the probability of sotorasib having economic benefits gradually increased when the cost of follow-up examination was reduced in China. And there was no influence on the conclusions within the range of changes in China. When the willingness to pay (WTP) exceeds $102,500, the probability of sotorasib having cost effect increases from 0% to 49%.
Conclusion: Sotorasib had a cost effect from the perspective in the United States. However, sotorasib had no cost effect from the perspective in China, and only when the WTP exceeds $102,500, the probability of sotorasib having cost effect increases from 0% to 49%.
Introduction
Lung cancer is the leading cause of cancer death worldwide, with an estimated 1.8 million deaths, accounting for 18.7% of all cancer deaths (Bray et al., 2024). Lung cancer was the cancer with the highest incidence and mortality rate in China, with 733,300 deaths in 2022 (Zheng et al., 2024). Non-small cell lung cancer (NSCLC) is the most common type of lung cancer, accounting for about 80%–85% (Zhou et al., 2017). In China, approximately 70% of patients are diagnosed with advanced stages and do not have the opportunity to undergo surgery (Zhou et al., 2017). In the past 10 years, the identification of specific driver mutant genes and the development of targeted therapy have changed the pattern of treatment of advanced NSCLC patients. KRAS gene mutation is the most common driver gene in NSCLC (Aredo et al., 2019). A study in western countries reported that 27% of lung adenocarcinoma patients carry KRAS mutations, with KRASG12C mutations reaching as high as 39% (El Osta et al., 2019). Meanwhile, a study reported that 10% of NSCLC patientshave KRAS mutations, and nearly 30% of them develop KRASG12C mutations in China (Liu et al., 2020). Due to the special structural type of KRAS, it had long been considered an undruggable target (Cox et al., 2014). In the past, the main treatment option for patients with KRAS mutations was chemotherapy, but previous studies have shown that chemotherapy had little effect on patients with KRAS mutations (Hames et al., 2016). Until the emergence of sotorasib broke the deadlock of KRAS mutation without targeted drugs.
Sotorasib is the first KRASG12C inhibitor successfully developed in the world after more than 40 years of KRAS mutant protein research. Its main mechanism of action is to irreversibly inhibit the small molecules of the KRASG12C target, block KRAS signal, inhibit cell growth, and promote cell apoptosis. In 2021, sotorasib was approved by the Food and Drug Administration (FDA), becoming the world’s first drug specifically for the treatment of locally advanced or metastatic NSCLC with KRASG12C mutation (Blair, 2021). In a single-arm, Phase II trial that included 124 patients with advanced NSCLC with KRASG12C mutation who had previously received standard were and received oral sotorasib (960 mg, qd), 100 (79.3%) patients developed disease control, complete response occurred in four patients (3.2%) and partial response occurred in 42 patients (33.9%) (Skoulidis et al., 2021). A Phase III study (de et al., 2023) showed that the median progression-free survival (PFS) of sotorasib and docetaxel was 5.6 months and 4.5 months, respectively. The 12-month PFS were 24.8% and 10.1%, respectively. Based on these clinical trial results, National Comprehensive Cancer Network (NCCN)guidelines and Chinese Society of Clinical Oncology (CSCO) guidelines recommend sotorasib as a second-line treatment for NSCLC with KRASG12C mutations (NCCN Clinical Practice Guidelines in Oncology NCCN Guidelines®, 2022; Organized by the Guideline Working Committee of the Chinese Society of Clinical Oncology, 2023).
Sotorasib undoubtedly provides a longer chance of survival for patients with KRASG12C NSCLC. However, sotorasib is costly and can increase the financial burden on patients. Pharmacoeconomics compares different drug administration regimens through cost analysis to select the most cost-effective regimen for patients (Schulman and Linas, 1997). There is no economic evaluation of sotorasib for the treatment of KRASG12C mutated NSCLC.
Therefore, this study evaluated the cost-effectiveness of sotorasib versus chemotherapy in patients with KRASG12C mutation NSCLC to support clinical and patient medication selection from the perspective of China and the United States (US).
Materials and methods
Model structure
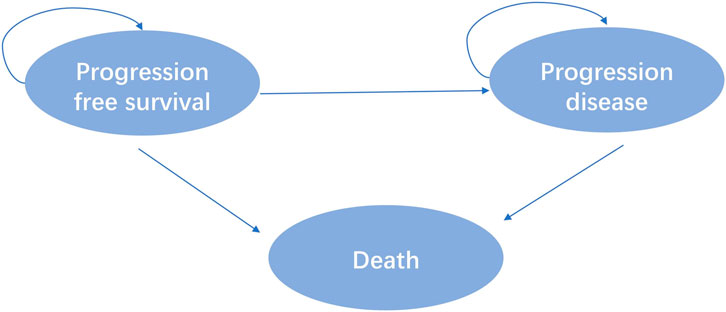
Eligible patients included were at least 18 years of age with advanced NSCLC with disease progression with a KRASG12C mutation following prior treatment with platinum-based chemotherapy and Programmed cell death protein 1 (PD-1) or Programmed cell death one ligand 1(PD-L1) inhibitors in the CodeBreak 200 clinic trial. A total of 345 patients were randomly assigned 1:1 to sotorasib or docetaxel. According to the outcome of advanced NSCLC disease, combined with the relevant literature of published clinical trials and economic evaluation (Cai et al., 2019). This study divided the disease model of advanced NSCLC into three independent and mutually exclusive health states: progression-free survival (PFS), progressive disease (PD) and death, as shown in Figure 1.
Model parameter
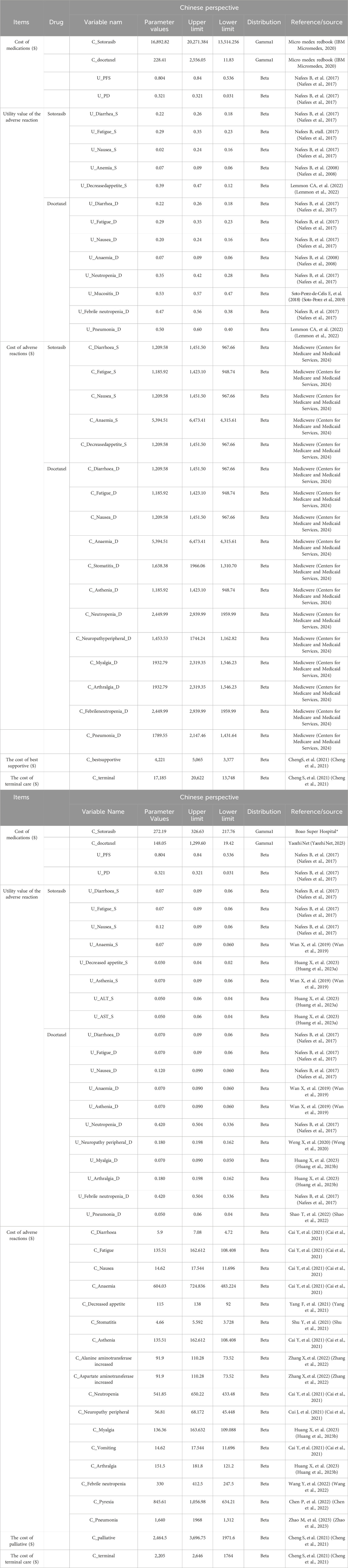
The PFS and OS curves of sotorasib and docetaxel were based on the results of the clinical trial (NCT04303780). We collected data points from PFS and OS curves by GetData Graph Digitizer (version 2.26), these data points were used to fit the following survival function, by using Exponential, Weibull, Gamma, Gengamma, Gompertz, Lognormal, and Loglogistic distributions to fit the data. According to the Akaike Information Criterion (AIC), R software was used to calculate the AIC value of each distribution, and the smallest distribution of AIC was the best fitting method for PFS and OS curves (Supplementary Figures S1, S2), and the parameter mean μ and standard variance σ were obtained. Our results showed that the lognormal model was the most reasonable function for extrapolating OS and PFS from the sotorasib and docetaxel groups. The key clinical parameter inputs were listed in Table 1 and Supplementary Table S1.
Utility
Health Utility refers to a patient’s preference for a specific health condition or treatment. Patients’ satisfaction with a state of health was assessed on an interval scale of 0–1, where 0 represented death and one represented complete health. Due to the lack of quality of life utility studies in patients with advanced NSCLC in China and the US, the utility values of PFS and PD states included were obtained from an international study of quality of life in patients with advanced NSCLC in this study (Nafees et al., 2017). The utility value of the PFS state was 0.804 (0.536–0.84) and the utility value of the PD state was 0.321 (0.031–0.321) in China. The utility value for the PFS state was 0.754 (0.536–0.84) and the utility value for the PD state was 0.095 (0.031–0.321) in the US.
Cost
Direct medical costs included the costs of drugs, management of adverse events (AEs), hospitalization cost, routine follow-up, best supportive, palliative and hospice. These costs were estimated from the published literature or institution. Dosage of docetaxel was calculated by body surface area 1.72 m2 for Chinese and 1.82 m2 for Americans, and the actual single dose was calculated by 140 mg. The incidence of AE was mainly from the clinical trial (NCT04303780) report in this study, mainly considering ≥3AEs, and the cost was mainly from previously published studies and professional websites. All cost values in China are based on the January 2023 exchange rate (1 USD = 6.92 RMB). The costs of adverse drug reactions, hospitalization costs, optimal support costs, palliative care costs, and hospice costs were derived from previously published literature, as shown in Table 1. The prices of drugs were also detailed in Table 1.
Effectiveness
Cost, QALY, and ICERs were the main output evaluation results. In this study, WTP threshold was set at 1-3 time the per capita gross domestic product (GDP) in 2022, which is $12,374.81-37,124.42 in China and $76348-229044 in the US.
Sensitivity analysis
In pharmacoeconomic evaluation, the result of cost-effectiveness is often biased due to the uncertainty of unreasonable data collection and evaluation methods. Therefore, sensitivity analysis is used to change the test conditions or change the parameter values within a certain range to evaluate its stability to the conclusion (Guanshen et al., 2015). One-way sensitivity analysis and probabilistic sensitivity analysis (PSA) were used to reflect the effects of uncertainty. The range and distribution of parameter variations were shown in Table 1. In the absence of upper and lower limit values, they were calculated as ±20% of the parameters. A second-order Monte Carlo simulation was used to sample 1,000 random simulations and the results are expressed as cost-effectiveness acceptable curves.
Results
Analysis of cost-effectiveness
The results of running the model for 120 cycles were shown in Table 2. In China, sotorasib generated 0.35 QAYL with a total cost of $84372.59. Compared with docetaxel, sotorasib increases the effectiveness by 0.09 QALY. ICER was $102701.84, which was higher than 3 times GDP, so sotorasib had no economic advantage. In the US, sotorasib obtained 0.35 QALYsmore than docetaxel, and ICER was $15,976.50/QALY, which was between 1 and 3 times GDP, indicating that the increased cost of sotorasib was acceptable.
One-way sensitivity analysis
The results of one-way sensitivity analysis showed that the three factors that have the greatest influence on the incremental cost-effectiveness ratio was the discount rate, follow-up cost, and the utility value of PFS, as shown in Figure 2. According to the monistic sensitivity analysis (Supplementary Figure S3), when the cost of follow-up examination was reduced, the probability of sotorasib having economic benefits gradually increased. When other parameters vary within the upper and lower limits, ICER had no effect.
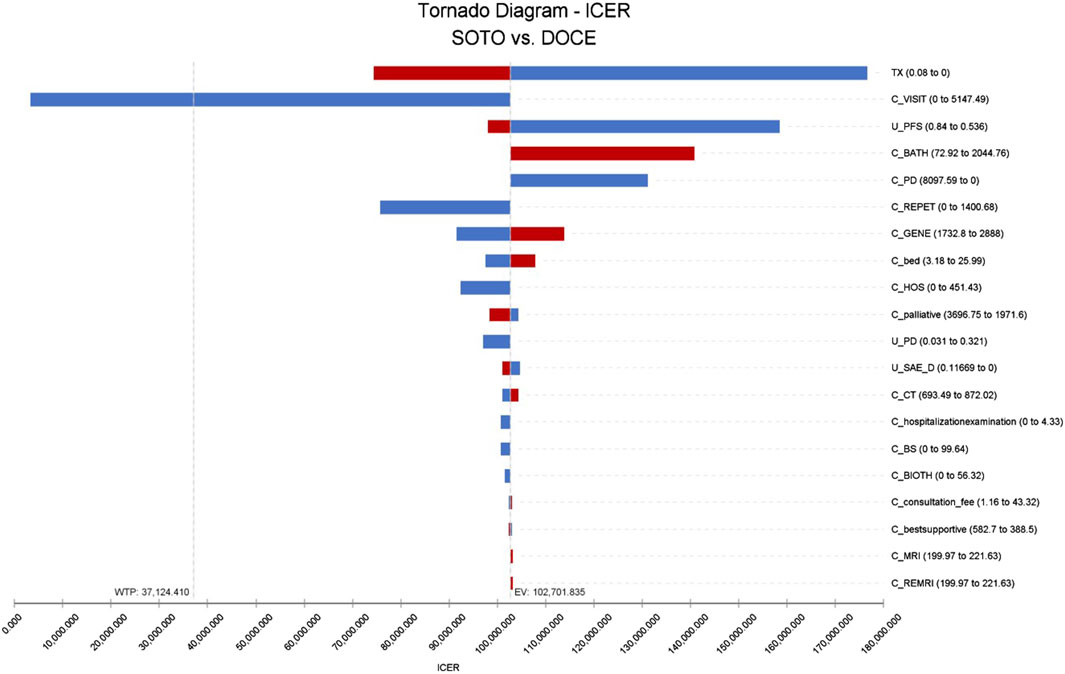
Figure 2. Tornado diagram of sotorasib vs. docetaxel in China perspective. Note: SOTO, sotorasib; DOCE, docetaxel.
The results of single factor sensitivity analysis showed that the three factors that had the greatest influence on the incremental cost-effectiveness ratio was discount rate, hospitalization cost, and the cost of treating diarrhea with docetaxel. Within the range of changes in the upper-lower limits of the set parameters, there was no influence on the conclusions, as shown in Figure 3.
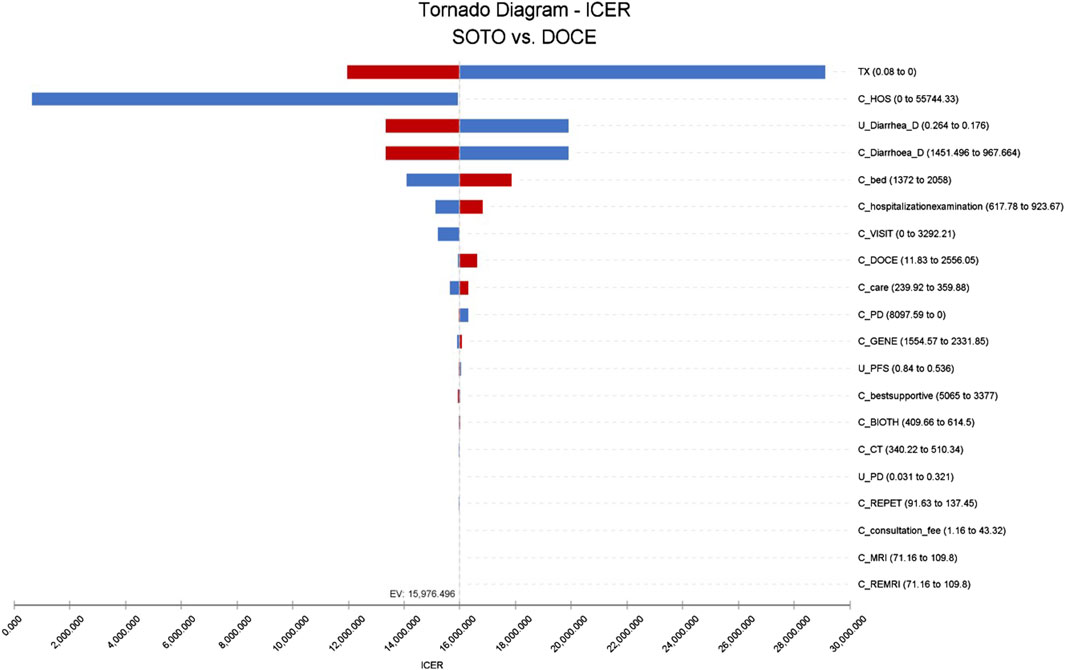
Figure 3. Tornado diagram of sotorasib vs. docetaxel in the US perspective. Note: SOTO, sotorasib; DOCE, docetaxel.
Cost-effectiveness acceptability curve
As shown in Figure 4, within the range of WTP, docetaxel had the highest acceptable probability. With the increase of the value of WTP, the probability that docetaxel had an economic advantage gradually decreases. When the WTP exceed $102,500, the probability of sotorasib being cost-effective increases from 0% to 49% and gradually becomes higher than docetaxel. It can be seen that docetaxel had economic advantages in the range of the WTP threshold from the perspective of China.
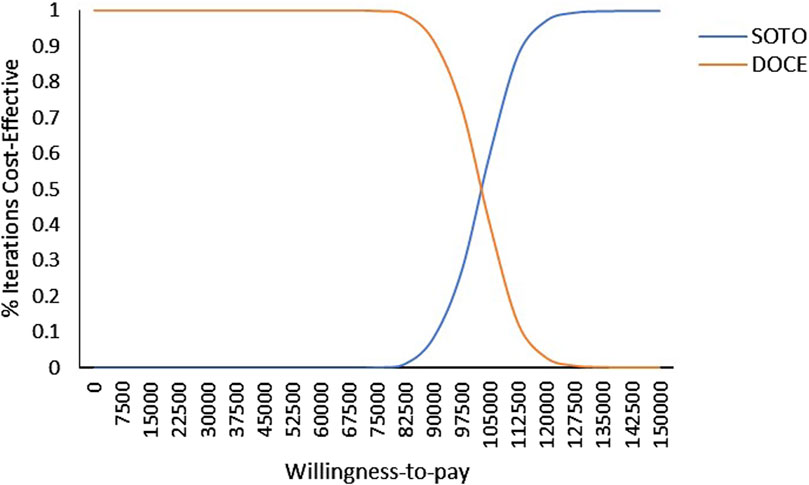
Figure 4. Cost-effectiveness acceptability curve of sotorasib and docetaxel taxel in Chinese perspective. Note: SOTO, sotorasib; DOCE, docetaxel.
As shown in Figure 5, in the US, the acceptability probability of docetaxel was highest when the WTP value was less than $15,650. With the increase of WTP, the probability that sotorasib had a cost effect gradually increases. It can be seen that when the WTP was less than $15,650, docetaxel had economic benefits, and when the WTP was more than $15,650, sotorasib was more cost-effective.
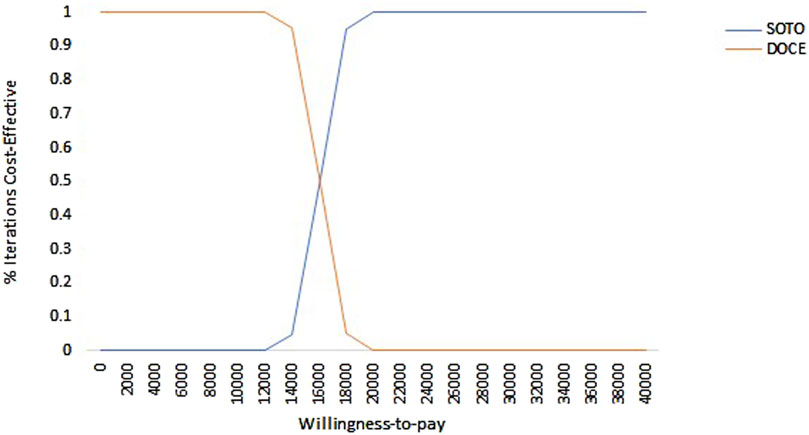
Figure 5. Cost-effectiveness acceptability curve of sotorasib and docetaxel in the US perspective. Note: SOTO, sotorasib; DOCE, docetaxel.
Discussion
Sotorasib was the first targeted drug approved by the FDA for the treatment of locally advanced or metastatic NSCLC with the KRASG12C mutation (Lu et al., 2023). However, sotorasib has not yet been listed in China, so it is urgent to evaluate the cost effectiveness of sotorasib. At the same time, it was necessary to comprehensively evaluate the cost effectiveness of sotorasib from multiple perspectives in China and the US. We hope that our research can provide reference for the listing of sotorasib in China.
The results of this study showed that sotorasib gained 0.09 QALY more than docetaxel, and ICER was $102701.84/QALY. In the US, sotorasib got 0.35 QALY more relative to docetaxel and ICER got $15,976.50/QALY. These results showed that sotorasib had not an economic advantage in China, but had an economic advantage in the US. The conclusion of univariate sensitivity analysis showed that the probability of sotorasib having economic benefits gradually increases when the cost of follow-up examination decreases in China. The probabilistic sensitivity analysis results showed that the acceptability of sotorasib gradually increases with the increase of WTP in China or the US, and sotorasib had cost effect when China’s WTP value increases to $102500, while the US only needs to increase to $15650 US to have cost effect.
One-way sensitivity analyses showed that the lower the cost of follow-up, the higher the economic benefit of sotorasib. The possible reason for this is that this study included the cost of examinations such as brain MRI, bone scan, and CT in the cost of follow-up. The PSA results suggest that an increase in WTP leads to a gradual increase in the acceptability of sotorasib, both in China and the United States. Nowadays, as the economic level of the world’s population continues to rise and the WTP increases each year, the acceptability of sotorasib will gradually rise above that of docetaxel to become an economically effective second-line treatment option.
A recent study evaluated the long-term efficacy of sotorasib in patients with advanced NSCLC with KRASG12C mutation showed that a quarter achieved long-term benefits and almost no late-onset treaty-related toxicity, and sotorasib showed a good safety profile (Dy et al., 2023). At present, clinical trials of sotorasib were also being carried out in China, and CodeBreak105 (NCT4380753) study was a Phase I study to explore the efficacy of sotorasib monotherapy in Chinese patients with KRAS G12C mutation. Therefore, if the price of sotorasib can be reduced, it was of great significance for Chinese lung cancer patients with KRASG12C mutation to choose sotorasib treatment. The establishment of the collection system in China had greatly reduced the price of drugs and greatly reduced the economic burden of cancer patients (State Council of China, 2021).
This study have some highlights should be noted. First, sotorasib had been approved in several countries (US Food and Drug Administration, 2021; BioSpace, 2022; European Medicines Agency, 2022), and there was little data on its economic evaluation. Although sotorasib had not yet been approved in China, clinical trials had already been conducted in China. Second, We used the Markov model to analyze the cost effect of sotorasib from the perspectives of China and the US, which was helpful for our decision to choose treatment options.
This study also had the following limitations. Firstly, this study did not consider the utility value decline caused by adverse reactions. Secondly, due to the lack of health utility evaluation studies in Chinese patients, the extraction of relevant health utility values from published literature may cause a certain degree of bias to the conclusions. In the single factor sensitivity analysis in China, the variation of the utility value of PFS will affect the final conclusion. Second, most of the data comes from RCT studies, which differ from real-world data, so decisions should be made when applied to the clinical practice. Finally, the incidence of AEs was mainly ≥3, and other AE with low incidence but high cost were not considered. Univariate sensitivity analysis in the US showed that the cost of adverse events for treating diarrhea had an impact on the model.
Conclusion
Our results showed that sotorasib had cost effect from the perspective of the US, and sotorasib had no cost effect from the perspective of China, and only when the WTP exceeds $102,500, the probability of sotorasib having cost effect increases from 0% to 49%.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
Y-NZ: Writing–original draft. MT: Writing–original draft. K-XS: Methodology, Writing–review and editing. BG: Data curation, Writing–review and editing. X-PS: Writing–original draft. PZ: Supervision, Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was funded by the National Institute of Hospital Administration (No.YLZLXZ23K003), Project of Bethune Seuso Pharmaceutical Research Capacity-Building (No. Z04JKM2021005), and Shaanxi Provincial People's Hospital Science and Technology Development Incubation Fund Project (2023YJY-54).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1348688/full#supplementary-material
References
Aredo, J. V., Padda, S. K., Kunder, C. A., Han, S. S., Neal, J. W., Shrager, J. B., et al. (2019). Impact of KRAS mutation subtype and concurrent pathogenic mutations on non-small cell lung cancer outcomes. Lung cancer Amsterdam, Neth. 133, 144–150. doi:10.1016/j.lungcan.2019.05.015
BioSpace (2022). LUMAKRAS® (sotorasib) receives approval in Japan for patients with KRAS G12C-mutated advanced non-small cell lung cancer. Available at: https://www.biospace.com/article/releases/lumakras-Sotorasib-receives-approval-in-japan-for-patients-with-kras-g12c-mutated-advanced-non-small-cell-lung-cancer/.
Blair, H. A. (2021). Sotorasib: first approval. Drugs 81 (13), 1573–1579. doi:10.1007/s40265-021-01574-2
Bray, F., Laversanne, M., Sung, H., Ferlay, J., Siegel, R. L., Soerjomataram, I., et al. (2024). Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA a cancer J. Clin. 74 (3), 229–263. doi:10.3322/caac.21834
Cai, H., Zhang, L., Li, N., Chen, S., Zheng, B., Yang, J., et al. (2019). Cost-effectiveness of osimertinib as first-line treatment and sequential therapy for EGFR mutation-positive non-small cell lung cancer in China. Clin. Ther. 41 (2), 280–290. doi:10.1016/j.clinthera.2018.12.007
Cai, Y., Hui, W., Zhu, M., Zhang, M., Gao, Z., and Wu, H. (2021). Cost-effectiveness analysis of pembrolizumab plus pemetrexed and platinum versus chemotherapy alone as first-line treatment in metastatic non-squamous non-small cell lung cancer: a reconstruction of partitioned survival model based on time dependent pricing mechanism of patient assistance program. Front. Oncol. 11, 768035. doi:10.3389/fonc.2021.768035
Centers for Medicare and Medicaid Services (2024). U.S. Centers for medicare and medicaid services. Available at: https://www.cms.gov/medicwere/payment/fee-for-service-providers.
Chen, P., Yang, Q., Li, Y., Jing, X., and Chen, J. (2022). Cost-effectiveness analysis of adjuvant therapy with atezolizumab in Chinese patients with stage IB-IIIA resectable NSCLC after adjuvant chemotherapy. Front. Oncol. 12, 894656. doi:10.3389/fonc.2022.894656
Cheng, S., Pei, R., Li, J., Li, B., Tang, L., Yin, T., et al. (2021). Atezolizumab compared to chemotherapy for first-line treatment in non-small cell lung cancer with high PD-L1 expression: a cost-effectiveness analysis from US and Chinese perspectives. Ann. Transl. Med. 9 (18), 1481. doi:10.21037/atm-21-4294
Cox, A. D., Fesik, S. W., Kimmelman, A. C., Luo, J., and Der, C. J. (2014). Drugging the undruggable RAS: mission possible? Nat. Rev. Drug Discov. 13 (11), 828–851. doi:10.1038/nrd4389
Cui, J., Zhang, X., Qu, S., and Wang, L. (2021). Cost-effectiveness analysis of nab-paclitaxel plus gemcitabine versus folfirinox in the treatment of metastatic pancreatic cancer in China. Expert Rev. pharmacoeconomics outcomes Res. 21 (4), 691–697. doi:10.1080/14737167.2020.1812386
de, L., Johannes, A., Mazieres, J., Dingemans, A. M. C., Mountzios, G., Pless, M., et al. (2023). Sotorasib versus docetaxel for previously treated non-small-cell lung cancer with KRASG12C mutation: a randomised, open-label, phase 3 trial. Lancet London, Engl. 401 (10378), 733–746. doi:10.1016/S0140-6736(23)00221-0
Dy, G. K., Govindan, R., Velcheti, V., Falchook, G. S., Italiano, A., Wolf, J., et al. (2023). Long-term outcomes and molecular correlates of sotorasib efficacy in patients with pretreated KRAS G12C-mutated non-small-cell lung cancer: 2-year analysis of CodeBreaK 100. J. Clin. Oncol. official J. Am. Soc. Clin. Oncol. 41 (18), 3311–3317. doi:10.1200/JCO.22.02524
El Osta, B., Behera, M., Kim, S., Berry, L. D., Sica, G., Pillai, R. N., et al. (2019). Characteristics and outcomes of patients with metastatic KRAS-mutant lung adenocarcinomas: the lung cancer mutation consortium experience. J. Thorac. Oncol. official Publ. Int. Assoc. Study Lung Cancer 14 (5), 876–889. doi:10.1016/j.jtho.2019.01.020
European Medicines Agency (2022). LUMYKRAS™ (Sotorasib): EU summary of product characteristics. Available at: https://www.ema.europa.eu/(Accessed October 5, 2022).
Guanshen, D. O. U., Jianlong, L. U., Qi, F., Wu, W., Sha, FENG, and Ying, X. (2015).The analysis of uncertainty in pharmacoeconomic evaluations[J].Shanghai Medical& Pharm.,36.(1),4.
Hames, M. L., Chen, H., Iams, W., Aston, J., Lovly, C. M., and Horn, L. (2016). Correlation between KRAS mutation status and response to chemotherapy in patients with advanced non-small cell lung cancer☆. Lung cancer Amsterdam, Neth., 92, 29–34. doi:10.1016/j.lungcan.2015.11.004
Huang, X., Lin, D., Lin, S., Luo, S., Huang, X., Deng, Y., et al. (2023a). Cost-effectiveness and value-based pricing of trastuzumab deruxtecan in metastatic breast cancer with low HER2 expression. Clin. breast cancer 23 (5), 508–518. doi:10.1016/j.clbc.2023.03.013
Huang, X., Liu, Y., Lin, S., Wang, H., Deng, Y., Rao, X., et al. (2023b). Adjuvant zoledronic acid therapy for postmenopausal women with early breast cancer in China: a cost-effectiveness analysis. Int. J. Qual. health care J. Int. Soc. Qual. Health Care 35 (2), mzad016. doi:10.1093/intqhc/mzad016
IBM Micromedex (2020). RED BOOK online. Available at: http://www.Micromedex.solutions.com (Accessed March 28, 2020).
Lemmon, C. A., Zabor, E. C., and Pennell, N. A. (2022). Modeling the cost-effectiveness of adjuvant osimertinib for patients with resected EGFR-mutant non-small cell lung cancer. Oncol. 27 (5), 407–413. doi:10.1093/oncolo/oyac021
Liu, S. Y., Sun, H., Zhou, J. Y., Jie, G. L., Xie, Z., Shao, Y., et al. (2020). Clinical characteristics and prognostic value of the KRAS G12C mutation in Chinese non-small cell lung cancer patients. Biomark. Res. 8, 22. doi:10.1186/s40364-020-00199-z
Lu, M., Penghui, D., Wang, Z., and Linrui, Li(2023). Advances in clinical research of sotorasib [J]. Guangdong Chem. Ind.,50.(8):86–87.
Nafees, B., Lloyd, A. J., Dewilde, S., Rajan, N., and Lorenzo, M. (2017). Health state utilities in non-small cell lung cancer: an international study. Asia-Pacific J. Clin. Oncol. 13 (5), e195–e203. doi:10.1111/ajco.12477
Nafees, B., Stafford, M., Gavriel, S., Bhalla, S., and Watkins, J. (2008). Health state utilities for non small cell lung cancer. Health Qual. life outcomes 6, 84. doi:10.1186/1477-7525-6-84
NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) (2022). Non-small cell lung cancer. Available at: https://www.cancer.gov/types/lung/patient/non-small-cell-lung-treatment-pdq.
Organized by the Guideline Working Committee of the Chinese Society of Clinical Oncology (2023) Guidelines for the diagnosis and treatment of non-small cell lung cancer by Chinese Society of Clinical Oncology. Beijing: People's Medical Publishing House.
Schulman, K. A., and Linas, B. P. (1997). Pharmacoeconomics: state of the art in 1997. Annu. Rev. public health 18, 529–548. doi:10.1146/annurev.publhealth.18.1.529
Shao, T., Ren, Y., Zhao, M., and Tang, W. (2022). Cost-effectiveness analysis of camrelizumab plus chemotherapy as first-line treatment for advanced squamous NSCLC in China. Front. public health 10, 912921. doi:10.3389/fpubh.2022.912921
Shu, Y., Zhang, Q., He, X., and Chen, L. (2021). Cost-effectiveness analysis of gefitinib plus chemotherapy versus gefitinib alone for advanced non-small-cell lung cancer with EGFR mutations in China. Cancer Manag. Res. 13, 8297–8306. doi:10.2147/CMAR.S334643
Skoulidis, F., Li, B. T., Dy, G. K., Price, T. J., Falchook, G. S., Wolf, J., et al. (2021). Sotorasib for lung cancers with KRAS p.G12C mutation. N. Engl. J. Med. 384 (25), 2371–2381. doi:10.1056/NEJMoa2103695
Soto-Perez, E., Aguiar, P. N., Cordón, M. L., Chavarri-Guerra, Y., and Lopes, G. L. (2019). Cost-effectiveness of cabozantinib in the second-line treatment of advanced hepatocellular carcinoma. J. Natl. Compr. Cancer Netw. JNCCN 17 (6), 669–675. doi:10.6004/jnccn.2018.7275
State Council of China (2021). China's centralized procurement leads to 50% drop in prices of over 100 drugs. Available at: http://english.www.gov.cn/statecouncil/ministries/202011/21/content_WS5fb86defc6d0f7257694042b.html (Accessed October 3, 2021).
US Food and Drug Administration (2021). FDA grants accelerated approval to Sotorasib for KRAS G12C mutated NSCLC. Available at: https://www.fda.gov/(Accessed October 5, 2022).
Wan, X., Luo, X., Tan, C., Zeng, X., Zhang, Y., and Peng, L. (2019). First-line atezolizumab in addition to bevacizumab plus chemotherapy for metastatic, nonsquamous non-small cell lung cancer: a United States-based cost-effectiveness analysis. Cancer 125 (20), 3526–3534. doi:10.1002/cncr.32368
Wang, Y., Huang, K., Sun, S., Deng, Y., and Xie, X. (2022). Cost-effectiveness analysis of gefitinib alone and combined with chemotherapy as first-line treatment for patients with advanced non-small-cell lung cancer. Risk Manag. Healthc. policy 15, 351–359. doi:10.2147/RMHP.S352827
Weng, X., Huang, X., Li, H., Lin, S., Rao, X., Guo, X., et al. (2020). First-line treatment with atezolizumab plus nab-paclitaxel for advanced triple-negative breast cancer: a cost-effectiveness analysis. Am. J. Clin. Oncol. 43 (5), 340–348. doi:10.1097/COC.0000000000000671
Yang, F., Fu, Y., Kumar, A., Chen, M., Si, L., and Rojanasarot, S. (2021). Cost-effectiveness analysis of camrelizumab in the second-line treatment for advanced or metastatic esophageal squamous cell carcinoma in China. Ann. Transl. Med. 9 (15), 1226. doi:10.21037/atm-21-1803
Yaozhi Net (2023). Drugdataexpy: marketing information, drug bid. Available at: https://db.yaozh.com (Accessed June 20, 2023).
Zhang, X., Fang, P., Su, G., Gui, S., and Shen, A. (2022). Cost-effectiveness of ensartinib for patients with anaplastic lymphoma kinase-positive non-small-cell lung cancer in China. J. Comp. Eff. Res. 11 (12), 871–878. doi:10.2217/cer-2022-0066
Zhao, M., Shao, T., Chi, Z., and Tang, W. (2023). Effectiveness and cost-effectiveness analysis of 11 treatment paths, seven first-line and three second-line treatments for Chinese patients with advanced wild-type squamous non-small cell lung cancer: a sequential model. Front. public health 11, 1051484. doi:10.3389/fpubh.2023.1051484
Zheng, R. S., Chen, R., Han, B. F., Wang, S. M., Li, L., Sun, K. X., et al. (2024). Cancer incidence and mortality in China, 2022. Zhonghua zhong liu za zhi Chin. J. Oncol. 46 (3), 47–53. doi:10.1016/j.jncc.2024.01.006
Keywords: cost-effectiveness, NSCLC, KRASG12C mutation, sotorasib, China
Citation: Zhu Y-N, Tang M, Sun K-X, Gao B, Shi X-P and Zhang P (2024) Cost-effectiveness of sotorasib as a second-line treatment for non-small cell lung cancer with KRASG12C mutation in China and the United States. Front. Pharmacol. 15:1348688. doi: 10.3389/fphar.2024.1348688
Received: 03 December 2023; Accepted: 24 May 2024;
Published: 14 June 2024.
Edited by:
Carlos Gil Ferreira, Instituto Oncoclínicas, BrazilReviewed by:
Denizar Vianna Araujo, Rio de Janeiro State University, BrazilGilson Gabriel Viana Veloso, Instituto Oncoclínicas, Brazil
Copyright © 2024 Zhu, Tang, Sun, Gao, Shi and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Peng Zhang, m13335383051@163.com
 Ya-Ning Zhu
Ya-Ning Zhu Meng Tang
Meng Tang Ke-Xin Sun
Ke-Xin Sun Bei Gao3
Bei Gao3