- 1Department of Hospital Pharmacy, Meander Medisch Centrum, Amersfoort, Netherlands
- 2Department of Hospital Pharmacy, Ziekenhuis Rivierenland, Tiel, Netherlands
- 3Department of Clinical Decision Support, Health Base Foundation, Houten, Netherlands
- 4Medicines Information Centre, Royal Dutch Pharmacists Association (KNMP), The Hague, Netherlands
- 5Department of Hospital Pharmacy, Maasstad Hospital, Rotterdam, Netherlands
- 6Departments of Internal Medicine and Public Health Amsterdam Cardiovascular Sciences Amsterdam UMC, Amsterdam, Netherlands
Background: Hypertension, a significant risk factor for cardiovascular diseases, demands proactive management as cardiovascular diseases remain the leading cause of death worldwide. Reducing systolic and diastolic blood pressure levels below recommended reference values of <140/90 mmHg can lead to a significant reduction of the risk of CVD and all-cause mortality. However, treatment of hypertension can be difficult and the presence of comorbidities could further complicate this treatment. Drugs used to manage these comorbidities may inadvertently have an impact on blood pressure, resulting in a phenomenon known as drug-disease interaction. This study aims to assess the safety of medication that can affect blood pressure in patients with hypertension and provide practical recommendations for healthcare professionals.
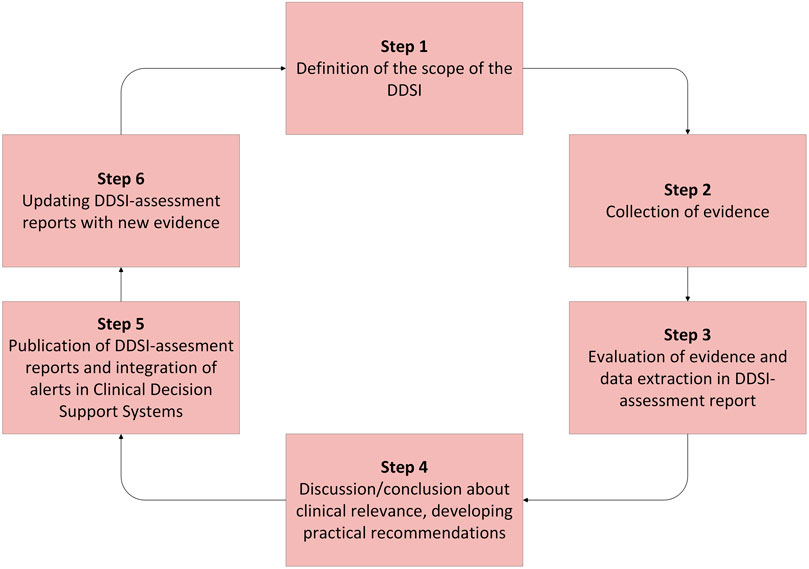
Methods: For the development of recommendations for the drug-disease interaction (DDSI) hypertension, a six-step plan that combined literature selection and multidisciplinary expert opinion was used. The process involved (1) defining the scope of the DDSI and selecting relevant drugs, (2) collecting evidence, (3) data-extraction, (4) reaching of expert consensus, (5) publication and implementation of the recommendations in healthcare systems and (6) updating the information.
Results: An increase of 10 mmHg in systolic blood pressure and 5 mmHg in diastolic blood pressure was defined as clinically relevant. Corticosteroids, danazol, and yohimbine caused a clinically relevant DDSI with hypertension. Several other drugs with warnings for hypertension in the official product information were assessed to have no clinically relevant DDSI due to minor influence or lack of data on blood pressure. Drugs with evidence for a relevant change in blood pressure which are prescribed under close monitoring of blood pressure according to clinical guidelines, were deemed to be not clinically relevant for signalling.
Conclusion: This study provides specific recommendations that can be implemented directly in clinical practice, for example, in clinical decision support systems, potentially resulting in safer drug use in patients with hypertension and better healthcare by reducing alert fatigue. Future research should focus on evaluating the effectiveness of implementation strategies and their impact on reducing unsafe use of medication in patients with hypertension.
1 Background
Cardiovascular diseases are the leading cause of deaths worldwide. In 2022, 22.9% of total deaths in the Netherlands could be attributed to cardiovascular diseases (Volksgezondheid en Zorg info, 2022). Hypertension is a key risk factor for cardiovascular diseases (Rapsomaniki et al., 2014; Flint et al., 2019; Centers for Disease Control and Prevention, National Center for Health Statistics, 2022; Volksgezondheid en Zorg info, 2022). According to the World Health Organization, hypertension affects 1.28 billion adults between 30 and 79 years worldwide (Mancia et al., 2023). From 1990 to 2019, the global prevalence of hypertension doubled among individuals aged 30–79 years leading to a prevalence of 34% in men and 32% in women in 2019. For children, the prevalence was estimated at 4.0% with an increasing prevalence in the last decades (Song et al., 2019). In the Netherlands, approximately 15% of the population reports high blood pressure in 2022 (Volksgezondheid en Zorg info, 2022).
Normally, systolic blood pressure (SBP) and diastolic blood pressure (DBP) are respectively below 120 and 80 mmHg in adults (Whelton et al., 2022; Mancia et al., 2023). Hypertension is defined as SBP values ≥ 140 mmHg and/or DBP ≥90 mmHg (Mancia et al., 2023). Uncontrolled blood pressure, defined by SBP of ≥130 mmHg or a DBP of ≥80 mmHg, requires proactive interventions (Whelton et al., 2022; Mancia et al., 2023). Lifestyle changes, complemented by drug therapy, are needed in managing this critical health concern in hypertensive patients. Studies have shown that reducing SBP and DBP to levels below recommended reference values of <140/90 mmHg can lead to a significant reduction of the risk of CVD and all-cause mortality (Ettehad et al., 2016; Bundy et al., 2017). For this reason, it is important to control hypertension to reduce the risk of cardiovascular death.
However, treatment of hypertension can be difficult as many factors such as stress, diet and non-adherence to antihypertensive drug treatment can influence blood pressure (Samadian et al., 2016). Moreover, most patients with hypertension have comorbidities, such as other cardiovascular diseases, diabetes or COPD. The drugs used to treat these other comorbidities - not related to blood pressure control - may have an effect on blood pressure. When pharmacotherapy used to treat a disease causes worsening of another disease, in this context hypertension, it is called a drug-disease interaction (DDSI) (van Tongeren et al., 2020).
DDSIs are often stated as a warning in the Summary of Product Characteristics (SmPC). However, these statements may not always be practical, since they are often poorly substantiated or not applicable in practice. For some comorbidities and conditions, such as renal impairment and cirrhosis, other sources can be consulted, but there are few guidelines for DDSIs available (van Tongeren et al., 2020). In the Netherlands, a best practice has been developed on how to systematically evaluate DDSIs and implement recommendations in clinical practice (van Tongeren et al., 2020; Diesveld et al., 2021). This best practice defines the patient population at risk, and evaluates specific drugs with respect to the clinical relevance of its influence on blood pressure.
According to the warnings in the SmPCs, healthcare professionals might want to avoid prescribing drugs with a DDSI to patients with hypertension, given the negative effects with respect to blood pressure. It can be difficult to recognize which drugs should be avoided in patients with hypertension. Official product information, handbooks, professional guidelines and original manuscripts may describe a change in blood pressure as an adverse reaction and consequently lists these drugs as a contra-indication or warn that the drug should be avoided in patients with hypertension. However, a shortcoming is that hypertension caused by drugs is often not further defined, neither is the extent of blood pressure change. Moreover, it is not always clear if adverse events and warnings shown in product information and other literature are clinically relevant. For physicians and pharmacists, this can be problematic because it is not clear what kind of action is needed to optimize medication safety for drugs prescribed to patients with hypertension. The aim of this study is to assess the safety of medication that can affect blood pressure in patients with hypertension and provide practical recommendations for healthcare professionals.
2 Materials and methods
For the development of recommendations for the DDSIs of hypertension, we used a previously developed six-step plan and made it specific for hypertension (Figure 1). (van Tongeren et al., 2020) This method combines literature and expert opinion to develop new practical recommendations. Our expert panel consisted of a fixed multidisciplinary panel of nine members: four representatives of specialists responsible for prescribing: two internist-clinical pharmacologist and two general practitioners, five pharmacists responsible for dispensing: two hospital pharmacists and three community pharmacists, and in addition to the fixed panel three experts in the field of hypertension research and treatment: two vascular internists and one hospital pharmacist.
The six steps of the development of practical DDSI-recommendations were as described below:
2.1 Scope of the DDSI
The scope of the DDSI described the minimal change (rise and fall) in SBP and DBP that was regarded as clinically relevant. Subsequently, the drugs to be evaluated were selected. All drugs that mentioned hypertension in the Summary of Product Characteristics (rubric 4.3 “Contra-indications” and 4.4 “Warnings and precautions for use”) were selected.
2.2 Collection of evidence
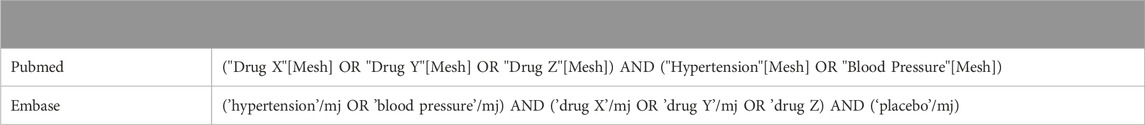
Several sources were consulted for data with respect to the drugs identified in step 1. The European Society of Hypertension Guidelines (2023) and the harmonization (2022) of the American College of Cardiology/American Heart Association and 2018 European Society of Cardiology/European Society of Hypertension were consulted as clinical practice guidelines for management of high blood pressure (Whelton et al., 2022; Mancia et al., 2023). For all drugs, PubMed and EMBASE were searched for literature concerning these drugs with potential risks for patients with hypertension (see Table 1 for search strategy). Studies were eligible if they fulfilled the criteria for the PICO (Patient, Intervention, Comparison and Outcomes) model or described outcomes in the general population with respect to the influence of the drug on blood pressure. The key-question for the PICO was: does drug X affect blood pressure in patients with hypertension in comparison to placebo or does drug X increase the risk of side effects in patients with hypertension compared to patients without hypertension? The quality of the studies that were compliant with the PICO was evaluated with criteria for drug-drug interactions adjusted for DDSIs: grade 4 for highest level of evidence, grade 1 for lowest (van Roon et al., 2005). Publications with the highest level of evidence were included, as were clinically relevant studies with lower levels of evidence that described the population at risk. Based on previously available evidence in clinical decision support systems (CDSS), studies published in a timeframe of 2011–2023 were included. Studies were excluded if they did not involve humans.
Moreover, the relevant rubrics in the European SmPCs and American FDA Prescribing Information describing contra-indications, warnings for special precaution, side effects and pharmacology were searched for information about the DDSI. Finally, national guidelines and handbooks describing hypertension were searched for information about the DDSI.
2.3 Data extraction
Data from the selected sources were extracted and presented in the DDSI assessment report. Essential information about the study type, number of patients with and without hypertension, drug dose, results and conclusions were summarized. Based on all the information collected, the assessment report included a preliminary conclusion on the clinical relevance of the DDSI and the proposed action in case of the DDSI.
2.4 Expert conclusion
The expert panel determined the scope of the DDSI, discussed the clinical relevance of the DDSIs, and made conclusions by consensus about the practical recommendations. If drugs met the criteria for clinical relevance, the expert panel decided to signal these DDSIs in CDSS according to clinically relevant literature and their expert opinion. The following reasons were distinguished for not signalling the DDSIs:
(1) Evidence that the specific drug had only a minor influence on blood pressure;
(2) No evidence (data) for an effect of the drug on blood pressure;
(3) Short term use with active monitoring of blood pressure;
(4) Drug is prescribed under close monitoring of blood pressure according to clinical guidelines.
The assessment reports were supplemented with the expert opinions and conclusions. The results of the assessment reports including the motivation of the expert panel for the reasons above and the underlying information in the SmPC and FDA Prescribing Information are presented in Tables 2–4. The references of the tables are included in Supplementary Material.
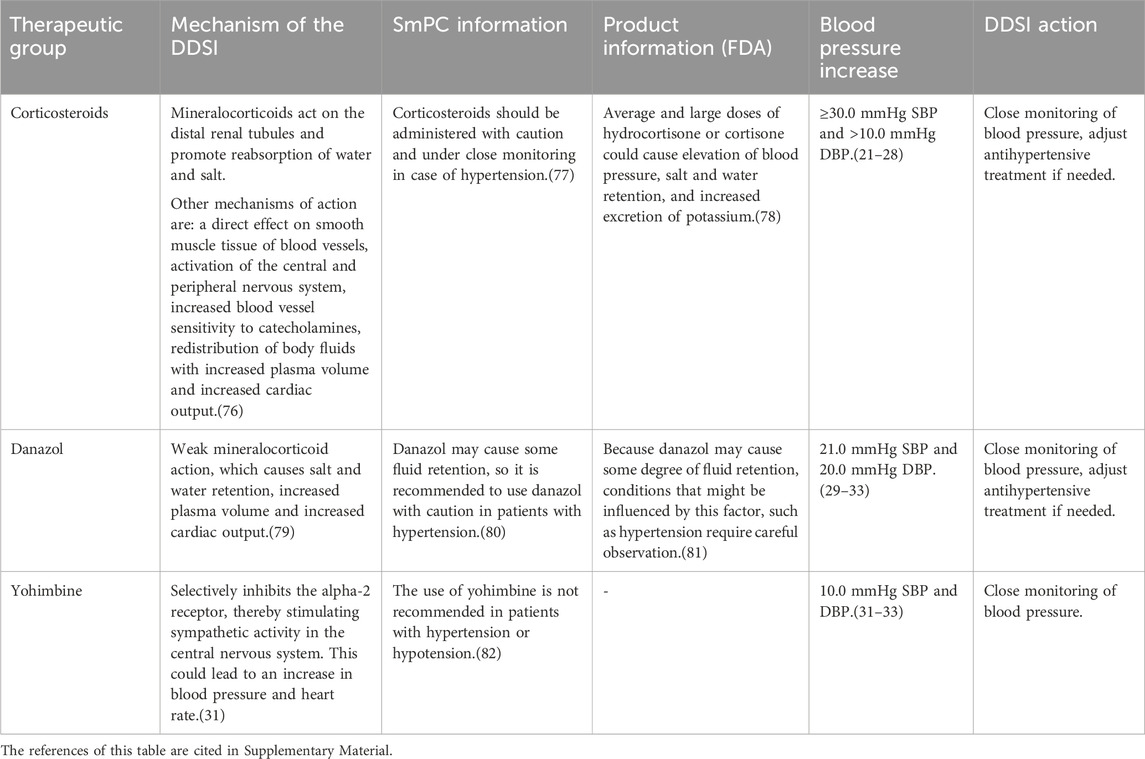
Table 2. Drugs evaluated as having a clinically relevant drug-disease interaction with hypertension, the underlying information in the SmPC and FDA product information, and the action recommended for healthcare professionals.
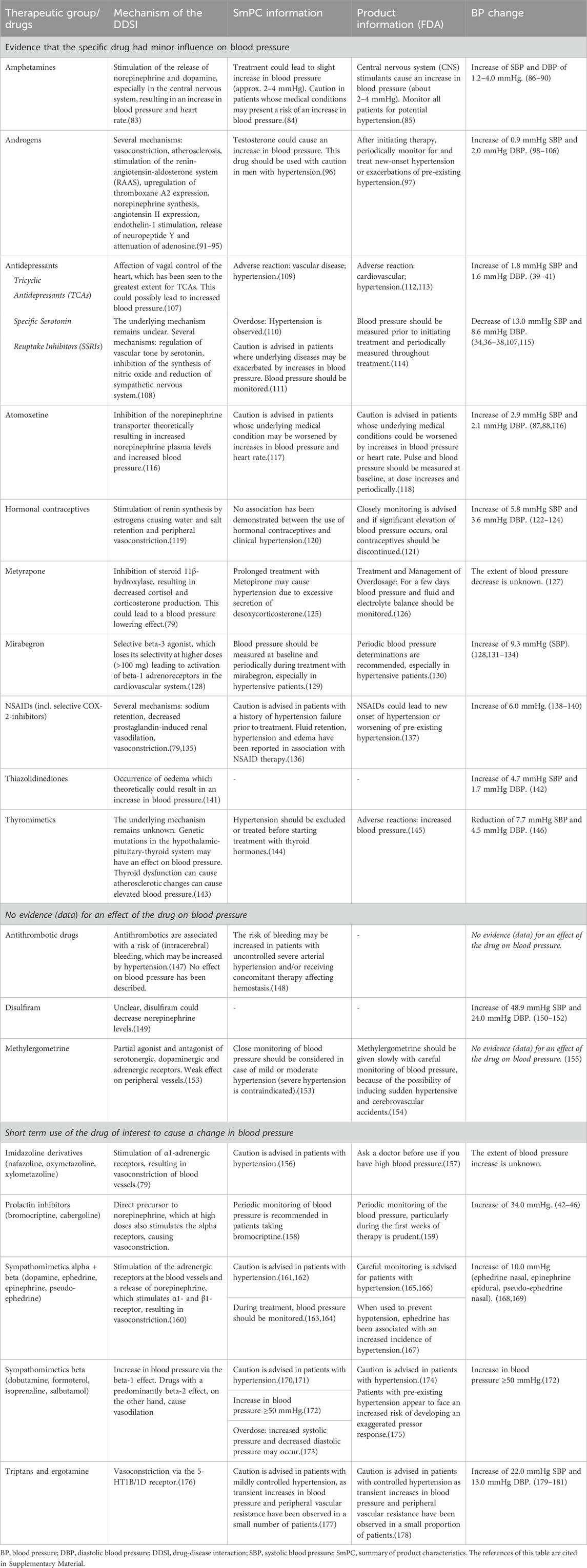
Table 3. Drugs evaluated as having no clinically relevant drug-disease interaction with hypertension, the underlying information in the SmPC and FDA product information, and the motivation of the expert panel for this conclusion.
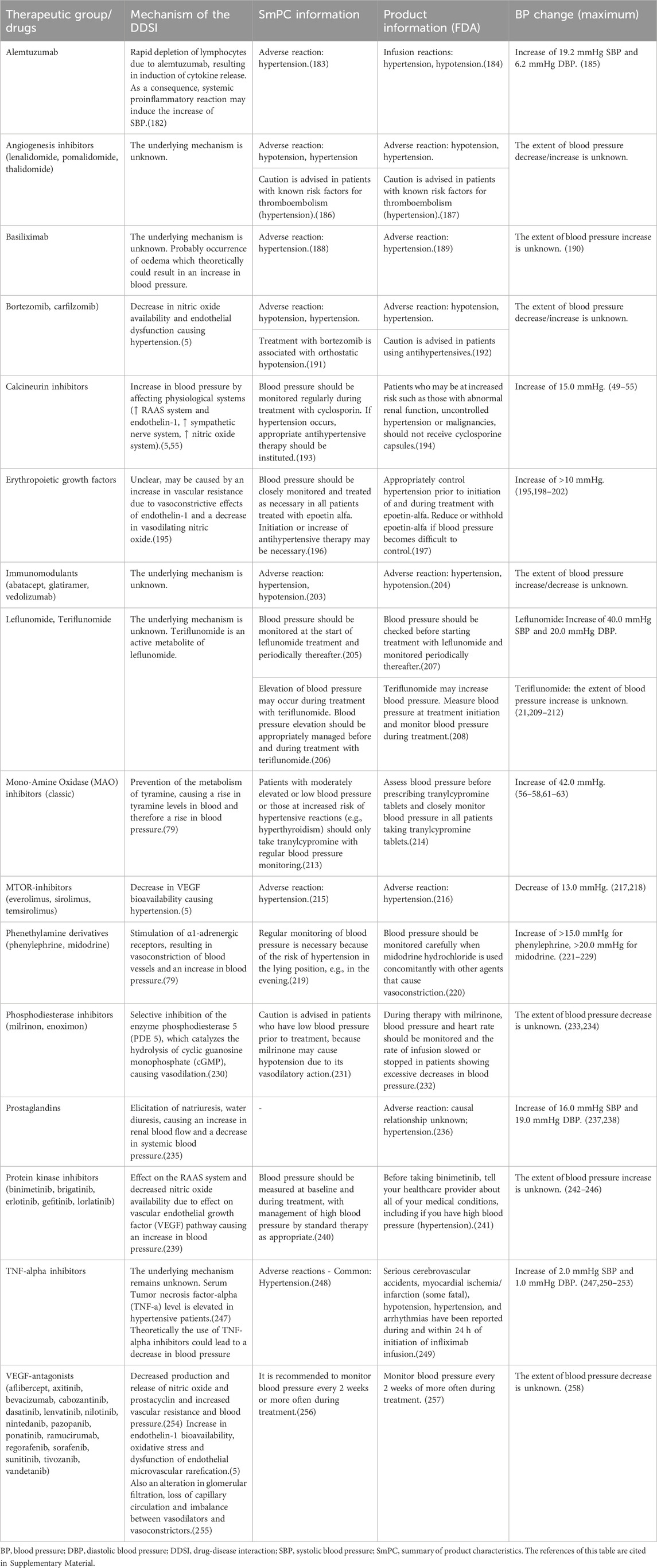
Table 4. Drugs evaluated as having a drug-disease interaction with hypertension, but are deemed to be not clinically relevant because of close monitoring of blood pressure according to clinical guidelines and the underlying information in the SmPC and FDA product information.
2.5 Publication and implementation
The conclusions and recommendations were published in the Dutch standards for Clinical Decision Support (Borgsteede et al., 2023; Royal Dutch Pharmacists Association and Koninklijke Nederlandse Maatschappij ter bevordering der Pharmacie, 2023) and were implemented nationwide in all Healthcare Information Systems in primary and secondary care. The assessment reports have been published (in Dutch) and can be consulted in the Dutch standards for Clinical Decision Support: The G-Standaard and Pharmabase. An example of a translated assessment report is included as Supplementary Material. For clinically relevant DDSIs, an alert is generated automatically in CDSS when the drug of interest is prescribed to a patient labelled with hypertension.
2.6 Updating
The last step is to keep the information up-to-date after implementation. New drugs will be evaluated once they have market authorization, and literature and risk information will be screened periodically for every single drug.
3 Results
3.1 Scope: hypertension as drug-disease interaction
The degree of changes in blood pressure is important for the DDSI hypertension. The meta-analysis by Whelton et al. reported that an increase of 20 mmHg for SBP and an increase of 10 mmHg for DBP was associated with an increased cardiovascular disease risk (Whelton et al., 2017). The expert panel defined an increase of >10 mmHg of SBP and >5 mmHg of DBP as clinically relevant, which could already increase the risk of cardiovascular diseases (Vishram et al., 2012; Whelton et al., 2017; Williams et al., 2018). For decreases in blood pressure, a reduction of at least 20 mmHg SBP and 10 mmHg DBP was defined as clinically relevant according to the European hypertension guideline with regard to orthostatic hypotension (Williams et al., 2018).
The databases were searched for the most recent evidence published until 2023. The drugs that were evaluated are listed in Table 2 (drugs with a clinically relevant DDSI), Table 3 (drugs with no clinically relevant DDSI) and Table 4 (drugs with close monitoring of blood pressure according to clinical guidelines). Table 2 also presents the conclusions and suggested recommendations on how to act when the DDSI occurs. Furthermore, motivations were given for not recommending an alert for a certain DDSI (Tables 3, 4). Below, the clinically relevant drugs for DDSI hypertension are described.
3.2 Clinically relevant drug-disease interactions
3.2.1 Corticosteroids
Corticosteroids can cause or exacerbate hypertension, especially when used for more than 2 weeks and in high doses (daily dose of prednisone equivalent >7.5 mg) (Distler et al., 1979; Panoulas et al., 2008; Sazliyana et al., 2011; Fardet et al., 2015; Miyabe et al., 2016; Baker et al., 2018; Bloechliger et al., 2018; Rice et al., 2018).
The advice for all high dosed corticosteroids that are used for more than 2 weeks is to monitor blood pressure more often. If necessary, the antihypertensive therapy has to be adjusted according to the blood pressure.
3.2.2 Danazol
Danazol has a weak mineralocorticoid activity, through which it can increase blood pressure via water and salt retention. A clinically relevant increase in blood pressure of approximately 20 mmHg has been observed after use of danazol in two case reports (Bretza et al., 1980; Pears and Sandercock, 1990). It is recommended to monitor blood pressure more often and to adjust the antihypertensive therapy if the blood pressure remains high during danazol treatment.
3.2.3 Yohimbine
Yohimbine is an α2-adrenergic receptor antagonist, which stimulate sympathetic activity in the central nervous system and is generally used for impotence. Studies have shown that in patients with pre-existing hypertension, blood pressure increased more than in patients who did not have hypertension prior to yohimbine administration (Damase-Michel et al., 1993; Grossman et al., 1993; Musso et al., 1995). For this reason, yohimbine has to be replaced by a phosphodiesterase-5 inhibitor in patients with hypertension.
3.3 Monitoring of blood pressure
Monitoring of blood pressure may be part of clinical follow-up by the prescriber, yet self-monitoring is becoming more common and recommended by hypertension guidelines. With a validated blood pressure measurement device and instructions, it is possible to correctly monitor blood pressure at home (Whelton et al., 2017). In case of a clinically relevant DDSI, blood pressure should be monitored at least once a month after initiating therapy, subsequently every 3 months until blood pressure stabilizes. After stabilization monitoring can take place every 6 months.
3.4 Drug-disease interactions evaluated as not clinically relevant
Many sources, mainly the official product information, stated that certain drugs should be avoided or used with caution in patients with hypertension. Yet, after evaluation of the available evidence by the expert panel it was concluded that most drugs had no clinically relevant DDSI. These drugs without a clinically relevant effect on blood pressure are listed in Table 3. Below, a summary of recommendations of drugs without a clinically relevant DDSI for hypertension are combined and presented according to the reason.
3.4.1 Evidence that the specific drug had minor influence on blood pressure
For a group of drugs, hypertension is described as a contra-indication in the SmPC. For some drugs (e.g., SSRI’s), no evidence was found in literature to support a clinically relevant DDSI for hypertension, nor was a plausible pharmacological mechanism described. For other drugs (e.g., amphetamines, hormonal contraceptives and thyromimetics), there was a plausible pharmacological mechanism, yet the magnitude of blood pressure change was unknown and there were no data to indicate that the changes in blood pressure did exceed the limits for clinical relevance. For these drugs, an alert for a DDSI was not considered relevant.
Antidepressants. Having depression is associated with increased blood pressure (Diminic-Lisica et al., 2014), and treating depression could lead to a reduction in blood pressure, despite the possible blood pressure-raising effect of antidepressants (Fu et al., 2015). In patients with hypertension, no risk of worsening hypertension was found for serotonin reuptake inhibitors (SSRIs) and venlafaxine (Thase, 1998; Diminic-Lisica et al., 2014; Fu et al., 2015; Razavi Ratki et al., 2016; Peixoto et al., 2019). In most patients using tricyclic antidepressants (TCAs), the increase in blood pressure did not reach the minimal clinically important difference (Licht et al., 2009; Breeden et al., 2018; Crookes et al., 2018).
3.4.2 No evidence (data) for an effect of the drug on blood pressure
Antithrombotics and methylergometrine were evaluated because of the warning about hypertension in SmPC’s/Product information. However, we did not find studies that indicated that these drugs altered blood pressure. These agents are therefore not signaled for the DDSI hypertension.
For other drugs such disulfiram no warnings were stated in the product information, although there were case-reports that published increase in blood pressure. According to the expert panel, these anecdotal case-reports provide insufficient evidence to limit the use of these drugs in patients with hypertension.
3.4.3 Short-term use
For the majority of blood pressure changes caused by ergotamine, imidazoline derivatives, prolactin inhibitors, sympathomimetics and triptans, the change in blood pressure is often transient. Due to the specific indication of drugs to cause transient high blood pressure or short-term use of some drugs, additional signaling through CDSSs was deemed not necessary for these drugs.
Prolactin inhibitors. Cases of intracranial hemorrhage and an increased risk of postpartum hypertension due to drug-induced hypertension have been reported for prolactin inhibitors (bromocriptine, cabergoline) (Watson et al., 1989; Kirsch et al., 2001). The manufacturers advise against the use of these drugs in hypertension, including gestational hypertension. However, based on available evidence and short-term use, the influence of prolactin inhibitors on blood pressure does not appear to be clinically relevant (Herings and Stricker, 1995; Gulleroglu et al., 2012; Bernard et al., 2015).
3.4.4 Close monitoring of blood pressure according to clinical guidelines
Table 4 summarizes drugs of which the DDSI with hypertension required no further action because of close monitoring of blood pressure according to clinical guidelines (Lyon et al., 2022). For these drugs monitoring of blood pressure for all patients is already recommended in protocols, due to known influences on blood pressure. Since use of these drugs is safe because of regular monitoring of the blood pressure, additional signaling for a DDSI via CDSS were not considered relevant, even though a clinically relevant effect on blood pressure was found in literature. Furthermore, additional alerts for these drugs could lead to alert fatigue (van Tongeren et al., 2020).
Calcineurin inhibitors. In a systematic review and clinical studies, a mean increase in blood pressure of 11 mmHg was seen for high doses of cyclosporin, which were above the lower limit for clinically relevant increase of blood pressure (Taylor et al., 1999; Klein et al., 2002; Higgins et al., 2004; Snanoudj et al., 2004; Robert et al., 2010; Marienhagen et al., 2019). However, there did not appear to be a group effect here, as tacrolimus had minimal effect on blood pressure (Woo et al., 1997; Taylor et al., 1999; Klein et al., 2002). Moreover, transplantation, for which calcineurin inhibitors are often used, could also lead to hypertension itself (Hošková et al., 2017).
MAO-inhibitors. Hypertension has been reported as side effect for the use of monoamine oxidase inhibitors (MAOIs) (Keck et al., 1989; Lavin et al., 1993; Taylor et al., 2005), and therefore patients need to avoid tyramine-containing foods that might provoke hypertensive crisis. (Stahl and Felker, 2008; Stahl, 2013). Another common side effect of MAOIs is orthostatic hypotension, hence blood pressure is monitored for classical MAOIs (Zandee et al., 2017). As selective MAOIs (such as moclobemide) are hardly associated with hypertension, monitoring of blood pressure is not needed (Bonnet, 2003; Yamada and Yasuhara, 2004).
4 Discussion
While hypertension is mentioned as contra-indication in many sources including the official product information, only a few drugs have a clinically relevant DDSI with hypertension. Interactions with corticosteroids, danazol, and yohimbine are associated with a significant increase in blood pressure (more than 10 mmHg SBP or 5 mmHg DBP) and determined as clinically relevant by our expert panel. The majority of hypertension contra-indications mentioned in the SmPCs of drugs were determined to be not clinically relevant. The most common reasons for this were a minor influence on blood pressure, lack of data indicating an effect on blood pressure, and short-term use of the drug. Moreover, alerts for drugs used under close monitoring of blood pressure according to clinical guidelines, e.g., oncology protocols, were considered to be of no additional value for drug safety.
Our study reveals that for many drugs the statements of hypertension as a DDSI in product information are often poorly supported by data. Healthcare professionals might withhold first-choice medication based on these statements, which may lead to suboptimal treatment. To ensure that healthcare professionals make good decisions, product information should quantify blood pressure changes, if possible with expected effect on systolic or and diastolic values. This level of detail is already mandatory for other pharmacological information, such as drug-drug interactions that mention the effects on plasma levels and drug exposure (European Medicines Agencey EMA, 2009). In addition, product information should provide guidance on appropriate actions when a DDSI occurs, such as dose adjustments or blood pressure monitoring (Weersink et al., 2019). This knowledge should be developed in clinical studies investigating medication safety in patients with hypertension, leading to recommendations concerning discontinuation of treatment, dose adaptation, or monitoring of blood pressure.
While evidence indicates blood pressure changes for some drugs used by patients with specific conditions such as oncology or transplantation, additional DDSI alerts are considered unnecessary. This is because blood pressure is already closely monitored during therapy for these conditions according to clinical guidelines. Additional alerts would lead to alert fatigue and unnecessary contact about drug therapy between healthcare professionals (Phansalkar et al., 2013). Clinical guidelines may differ between countries and settings, resulting in varying local guidelines for DDSI in different settings and regions.
Since there are only statements in product information for the DDSI hypertension, recommendations for uncontrolled hypertension fall outside the scope of this study. Uncontrolled hypertension occurs when blood pressure remains high despite treatment. Reasons for this condition can be (pseudo-)resistance to antihypertensive drug treatment due to specific conditions such as transplantation, consumption of specific dietary ingredients (e.g., salt) or non-adherence to antihypertensives (Spence, 2018). It is important to identify the root cause of uncontrolled hypertension and to avoid drugs that can change blood pressure even further. For example, in women with uncontrolled hypertension, oral contraceptives should be avoided (ACOG Practice Bulletin, 2019; Shufelt and LeVee, 2020), whereas oral contraceptives are not signaled for hypertensive patients due to an on average minor influence on blood pressure. As uncontrolled hypertension is generally monitored intensively, a change in blood pressure due to drugs could be noticed earlier in patients with uncontrolled hypertension than in patients with controlled hypertension.
One of the most important strengths of this study is its unique approach in evaluating the interaction between hypertension and medication, providing practical recommendations following a combination of literature review and expert opinion. With limited studies available in patients with hypertension, expert opinion adds relevant pharmacological and clinical perspectives to the practical recommendations. Moreover, we examined all the drugs marketed in the Netherlands, representing the majority of the drugs available in other countries. The multidisciplinary character of the expert panel guaranteed that different perspectives were included and that the recommendations were applicable in a wide range of healthcare settings.
An additional strength of this approach is that this study contributes to limiting alert fatigue. Alert fatigue is one of the main reasons why alerts are overridden (van der Sijs et al., 2006; Isaac et al., 2009; Nanji et al., 2014; Shah et al., 2021). In a systematic review, the various factors influencing the efficiency and appropriateness of alerts in CDSSs was investigated. The review concluded that most of the alerts were not properly designed, which explained high frequency of alert overrides in clinical care (Olakotan and Mohd, 2021). To combat alert fatigue, our study contributes by suggesting only clinically relevant DDSIs. However, defining clinically relevant blood pressure change poses a challenge, necessitating a multidisciplinary approach. With this systematic methodology, it is possible to balance clinical relevance and alert fatigue, hereby preventing alert overrides and possible unsafe situations.
A limitation to this study is the absence of a clearly defined boundary for relevant changes in blood pressure. It is not possible to define what the expected effects of drugs would be on systolic and diastolic blood pressure prior to the evaluation. For this reason, we defined these boundaries in our multidisciplinary setting. However, the limits we used may be arbitrary, as there are specific patient groups that may require stricter monitoring. In some patient groups with specific comorbidities, even a smaller increase (>5 mmHg) in SBP may be important (Whelton et al., 2017). Moreover, the increase in SBP is more important in patients >50 years, and the increase in DBP is important in patients <50 years (Williams et al., 2018). Presumably, the risk for hypertension could increase when more risk factors are present. As the current DDSI-alerts do not involve additional risk factors, a future development could be that clinically important differences should be determined in the context of multiple individual patient characteristics.
A final limitation lies in the challenge of adopting our recommendations in settings in other countries. In the Netherlands, healthcare providers have the professional freedom to deviate from official documents such as the SmPC, which may be different in international settings (Eickhoff et al., 2021). In addition, our recommendations are based on Dutch treatment guidelines and practice. With this study, we aimed to contribute to the international dissemination and implementation of knowledge about DSSIs for hypertension.
This study identified a considerable knowledge gap for drugs that might have an influence on blood pressure, yet without sufficient clinical data to support that claim. Future studies should focus on the clinical relevance of these potential DDSIs and quantify the extend of blood pressure change. In countries where healthcare professionals can use data about the patient’s comorbidities in their electronic prescribing system, it is possible to integrate alerts in a CDSS (Diesveld et al., 2021). Implementation of automatic alerts in prescribing systems and CDSSs, combined with multidisciplinary cooperation, could enhance patient care and potentially reduce alert fatigue (Weissenborn et al., 2017). Future research should investigate the effectiveness of such implementations on safe drug use in patients with hypertension and reducing alert fatigue, time and costs.
5 Conclusion
With a systematic approach, we evaluated the interactions between prescribed drugs and hypertension. We developed practical recommendations for healthcare professionals, based on the available evidence and expert opinion and implemented these in clinical decision support systems in the Netherlands. These recommendations can serve as a basis to be applied in daily practice in international settings as well, to improve medication safety and patient safety.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Author contributions
KÖ: Writing–original draft, Writing–review and editing, Data curation, Formal analysis. MD: Validation, Writing–review and editing, Data curation, Formal analysis. SG: Validation, Writing–review and editing, Data curation, Formal analysis. LP: Validation, Writing–review and editing. B-JV: Validation, Writing–review and editing. SB: Supervision, Writing–review and editing, Validation.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We would like to thank all healthcare professionals in the National Expert Panel of Drug-Disease Interactions in the Netherlands for their valuable input on these recommendations.
Conflict of interest
MD and SB are employed at Health Base Foundation (HBF), an independent, non-commercial foundation that maintains a drug information database (Pharmabase) and supports health care professionals with a clinical decision support system. SG is employed at the Medicines information centre of the Royal Dutch Pharmacists Association (KNMP), an independent, non-commercial organization that maintains information on medication safety for a drug information database (G-Standaard) and thereby provides information for clinical decision support systems. The drug-disease interactions studied in this manuscript are subject to medical information provided by HBF and G-Standaard.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1360146/full#supplementary-material
References
ACOG Practice Bulletin (2019). No. 206: Use of Hormonal Contraception in Women With Coexisting Medical Conditions. Obstetrics and Gynecology 133 (2), e128–50.
Baker, J. F., Sauer, B., Teng, C. C., George, M., Cannon, G. W., Ibrahim, S., et al. (2018). Initiation of disease-modifying therapies in rheumatoid arthritis is associated with changes in blood pressure. JCR J. Clin. Rheumatology 24 (4), 203–209. doi:10.1097/RHU.0000000000000736
Bernard, N., Jantzem, H., Becker, M., Pecriaux, C., Bénard-Laribière, A., Montastruc, J., et al. (2015). Severe adverse effects of bromocriptine in lactation inhibition: a pharmacovigilance survey. BJOG 122 (9), 1244–1251. doi:10.1111/1471-0528.13352
Bloechliger, M., Reinau, D., Spoendlin, J., Chang, S. C., Kuhlbusch, K., Heaney, L. G., et al. (2018). Adverse events profile of oral corticosteroids among asthma patients in the UK: cohort study with a nested case-control analysis. Respir. Res. 19 (1), 75. doi:10.1186/s12931-018-0742-y
Bonnet, U. (2003). Moclobemide: therapeutic use and clinical studies. CNS Drug Rev. 9 (1), 97–140. doi:10.1111/j.1527-3458.2003.tb00245.x
Borgsteede, S. D., Bogaard, L., Diesveld, M. M. E., van Donselaar - Pham, T. K. L., Eimermann, V. M., de Klerk, S., et al. (2023). Commentaren medicatiebewaking. Health Base Found.
Breeden, M., Brieler, J., Salas, J., and Scherrer, J. F. (2018). Antidepressants and incident hypertension in primary care patients. J. Am. Board Fam. Med. 31 (1), 22–28. doi:10.3122/jabfm.2018.01.170234
Bretza, J. A., Novey, H. S., Vaziri, N. D., and Warner, A. S. (1980). Hypertension: a complication of danazol therapy. Arch. Intern Med. 140 (10), 1379–1380. doi:10.1001/archinte.140.10.1379
Bundy, J. D., Li, C., Stuchlik, P., Bu, X., Kelly, T. N., Mills, K. T., et al. (2017). Systolic blood pressure reduction and risk of cardiovascular disease and mortality: a systematic review and network meta-analysis. JAMA Cardiol. 2 (7), 775–781. doi:10.1001/jamacardio.2017.1421
Centers for Disease Control and Prevention, National Center for Health Statistics (2022). About multiple cause of death, 1999–2020. CDC WONDER Online Database website. Atlanta, GA: Centers for Disease Control and Prevention.
Crookes, D. M., Demmer, R. T., Keyes, K. M., Koenen, K. C., and Suglia, S. F. (2018). Depressive symptoms, antidepressant use, and hypertension in young adulthood. Epidemiology 29 (4), 547–555. doi:10.1097/EDE.0000000000000840
Damase-Michel, C., Tran, M. A., Llau, M. E., Chollet, F., Senard, J. M., Guiraud-Chaumeil, B., et al. (1993). The effect of yohimbine on sympathetic responsiveness in essential hypertension. Eur. J. Clin. Pharmacol. 44 (2), 199–201. doi:10.1007/BF00315481
Diesveld, M. M. E., de Klerk, S., Cornu, P., Strobach, D., Taxis, K., and Borgsteede, S. D. (2021). Management of drug-disease interactions: a best practice from The Netherlands. Int. J. Clin. Pharm. 43 (6), 1437–1450. doi:10.1007/s11096-021-01308-0
Diminic-Lisica, I., Popovic, B., Rebic, J., Klaric, M., and Franciškovic, T. (2014). Outcome of treatment with antidepressants in patients with hypertension and undetected depression. Int. J. Psychiatry Med. 47 (2), 115–129. doi:10.2190/PM.47.2.c
Distler, A., Philipp, T., Lüth, B., and Wucherer, G. (1979). Studies on the mechanism of mineralocorticoid-induced blood pressure increase in man. Clin. Sci. 57 (s5), 303s–5s. doi:10.1042/cs057303s
Eickhoff, C., Griese-Mammen, N., Mueller, U., Said, A., and Schulz, M. (2021). Primary healthcare policy and vision for community pharmacy and pharmacists in Germany. Pharm. Pract. (Granada) 19 (1), 2248. doi:10.18549/PharmPract.2021.1.2248
Ettehad, D., Emdin, C. A., Kiran, A., Anderson, S. G., Callender, T., Emberson, J., et al. (2016). Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet 387 (10022), 957–967. doi:10.1016/S0140-6736(15)01225-8
European Medicines Agencey (EMA) (2009). A guideline on Summary of Product Characteristics (SmPC). European Commission, enterprise and industry directorate-general. Available at: https://health.ec.europa.eu/document/download/6a043dea-7d0f-4252-947b-cef58f53d37e_en.
Fardet, L., Nazareth, I., and Petersen, I. (2015). Synthetic glucocorticoids and early variations of blood pressure: a population-based cohort study. J. Clin. Endocrinol. Metab. 100 (7), 2777–2783. doi:10.1210/jc.2015-1127
Flint, A. C., Conell, C., Ren, X., Banki, N. M., Chan, S. L., Rao, V. A., et al. (2019). Effect of systolic and diastolic blood pressure on cardiovascular outcomes. N. Engl. J. Med. 381 (3), 243–251. doi:10.1056/NEJMoa1803180
Fu, W., Ma, L., Zhao, X., Li, Y., Zhu, H., Yang, W., et al. (2015). Antidepressant medication can improve hypertension in elderly patients with depression. J. Clin. Neurosci. 22 (12), 1911–1915. doi:10.1016/j.jocn.2015.03.067
Grossman, E., Rosenthal, T., Peleg, E., Holmes, C., and Goldstein, D. S. (1993). Oral yohimbine increases blood pressure and sympathetic nervous outflow in hypertensive patients. J. Cardiovasc Pharmacol. 22 (1), 22–26. doi:10.1097/00005344-199307000-00004
Gulleroglu, K., Olgac, A., Bayrakci, U., Erdogan, O., Kinik, S. T., and Baskin, E. (2012). Hyperprolactinemia as a rare cause of hypertension in chronic renal failure. Ren. Fail 34 (6), 792–794. doi:10.3109/0886022X.2012.672313
Herings, R. M. C., and Stricker, B. H. C. (1995). Bromocriptine and suppression of postpartum lactation. The incidence of adverse cardiovascular effects in women of child-bearing age. Pharm. World and Sci. 17 (4), 133–137. doi:10.1007/BF01872390
Higgins, R., Ramaiyan, K., Dasgupta, T., Kanji, H., Fletcher, S., Lam, F., et al. (2004). Hyponatraemia and hyperkalaemia are more frequent in renal transplant recipients treated with tacrolimus than with cyclosporin. Further evidence for differences between cyclosporin and tacrolimus nephrotoxicities. Nephrol. Dial. Transplant. 19 (2), 444–450. doi:10.1093/ndt/gfg515
Hošková, L., Málek, I., Kopkan, L., and Kautzner, J. (2017). Pathophysiological mechanisms of calcineurin inhibitor-induced nephrotoxicity and arterial hypertension. Physiological Res. 66, 167–180. doi:10.33549/physiolres.933332
Isaac, T., Weissman, J. S., Davis, R. B., Massagli, M., Cyrulik, A., Sands, D. Z., et al. (2009). Overrides of medication alerts in ambulatory care. Arch. Intern Med. 169 (3), 305–311. doi:10.1001/archinternmed.2008.551
Keck, P. E., Pope, H. G., and Nierenberg, A. A. (1989). Autoinduction of hypertensive reactions by tranylcypromine? J. Clin. Psychopharmacol. 9 (1), 48–51. doi:10.1097/00004714-198902000-00011
Kirsch, C., Iffy, L., Zito, G. E., and McArdle, J. J. (2001). The role of hypertension in bromocriptine-related puerperal intracranial hemorrhage. Neuroradiology 43 (4), 302–304. doi:10.1007/s002340000492
Klein, IHHT, Abrahams, A., van Ede, T., Hen??, R. J., Koomans, H. A., and Ligtenberg, G. (2002). Different effects of tacrolimus and cyclosporine on renal hemodynamics and blood pressure in healthy subjects. Transplantation 73 (5), 732–736. doi:10.1097/00007890-200203150-00012
Lavin, M. R., Mendelowitz, A., and Kronig, M. H. (1993). Spontaneous hypertensive reactions with monoamine oxidase inhibitors. Biol. Psychiatry 34 (3), 146–151. doi:10.1016/0006-3223(93)90384-p
Licht, C. M. M., de Geus, E. J. C., Seldenrijk, A., van Hout, H. P. J., Zitman, F. G., van Dyck, R., et al. (2009). Depression is associated with decreased blood pressure, but antidepressant use increases the risk for hypertension. Hypertension 53 (4), 631–638. doi:10.1161/HYPERTENSIONAHA.108.126698
Lyon, A. R., López-Fernández, T., Couch, L. S., Asteggiano, R., Aznar, M. C., Bergler-Klein, J., et al. (2022). 2022 ESC guidelines on cardio-oncology developed in collaboration with the European hematology association (EHA), the European society for therapeutic radiology and oncology (ESTRO) and the international cardio-oncology society (IC-OS). Eur. Heart J. 43 (41), 4229–4361. doi:10.1093/eurheartj/ehac244
Mancia, G., Kreutz, R., Brunström, M., Burnier, M., Grassi, G., Januszewicz, A., et al. (2023). 2023 ESH guidelines for the management of arterial hypertension the task force for the management of arterial hypertension of the European society of hypertension: endorsed by the international society of hypertension (ISH) and the European renal association (ERA). J. Hypertens. 41 (12), 1874–2071. doi:10.1097/HJH.0000000000003480
Marienhagen, K., Lehner, F., Klempnauer, J., Hecker, H., and Borlak, J. (2019). Treatment of cyclosporine induced hypertension: results from a long-term observational study using different antihypertensive medications. Vasc. Pharmacol. 115, 69–83. doi:10.1016/j.vph.2018.06.012
Miyabe, Y., Takei, T., Iwabuchi, Y., Moriyama, T., and Nitta, K. (2016). Amelioration of the adverse effects of prednisolone by rituximab treatment in adults with steroid-dependent minimal-change nephrotic syndrome. Clin. Exp. Nephrol. 20 (1), 103–110. doi:10.1007/s10157-015-1139-6
Musso, N. R., Vergassola, C., Pende, A., and Lotti, G. (1995). Yohimbine effects on blood pressure and plasma catecholamines in human hypertension. Am. J. Hypertens. 8 (6), 565–571. doi:10.1016/0895-7061(95)00037-P
Nanji, K. C., Slight, S. P., Seger, D. L., Cho, I., Fiskio, J. M., Redden, L. M., et al. (2014). Overrides of medication-related clinical decision support alerts in outpatients. J. Am. Med. Inf. Assoc. 21 (3), 487–491. doi:10.1136/amiajnl-2013-001813
Olakotan, O. O., and Mohd, Y. M. (2021). The appropriateness of clinical decision support systems alerts in supporting clinical workflows: a systematic review. Health Inf. J. 27 (2), 146045822110075.
Panoulas, V. F., Douglas, K. M. J., Stavropoulos-Kalinoglou, A., Metsios, G. S., Nightingale, P., Kita, M. D., et al. (2008). Long-term exposure to medium-dose glucocorticoid therapy associates with hypertension in patients with rheumatoid arthritis. Rheumatology. 47 (1), 72–75. doi:10.1093/rheumatology/kem311
Pears, J., and Sandercock, P. A. (1990). Benign intracranial hypertension associated with danazol. Scott Med. J. 35 (2), 49. doi:10.1177/003693309003500207
Peixoto, M., Cesaretti, M., Hood, S., and Tavares, A. (2019). Effects of SSRI medication on heart rate and blood pressure in individuals with hypertension and depression. Clin. Exp. Hypertens. 41 (5), 428–433. doi:10.1080/10641963.2018.1501058
Phansalkar, S., van der Sijs, H., Tucker, A. D., Desai, A. A., Bell, D. S., Teich, J. M., et al. (2013). Drug-drug interactions that should be non-interruptive in order to reduce alert fatigue in electronic health records. J. Am. Med. Inf. Assoc. 20 (3), 489–493. doi:10.1136/amiajnl-2012-001089
Rapsomaniki, E., Timmis, A., George, J., Pujades-Rodriguez, M., Shah, A. D., Denaxas, S., et al. (2014). Blood pressure and incidence of twelve cardiovascular diseases: lifetime risks, healthy life-years lost, and age-specific associations in 1·25 million people. Lancet 383 (9932), 1899–1911. doi:10.1016/S0140-6736(14)60685-1
Razavi Ratki, S. K., Seyedhosseini, S., Valizadeh, A., Rastgoo, T., Tavakkoli, R., Golabchi, A., et al. (2016). Can antidepressant drug impact on blood pressure level in patients with psychiatric disorder and hypertension? A randomized trial. Int. J. Prev. Med. 7 (1), 26. doi:10.4103/2008-7802.174891
Rice, J. B., White, A. G., Johnson, M., Wagh, A., Qin, Y., Bartels-Peculis, L., et al. (2018). Quantitative characterization of the relationship between levels of extended corticosteroid use and related adverse events in a US population. Curr. Med. Res. Opin. 34 (8), 1519–1527. doi:10.1080/03007995.2018.1474090
Robert, N., Wong, G. W., and Wright, J. M. (2010). Effect of cyclosporine on blood pressure. Cochrane Database Syst. Rev., CD007893. doi:10.1002/14651858.CD007893.pub2
Royal Dutch Pharmacists Association Koninklijke Nederlandse Maatschappij ter bevordering der Pharmacie (2023). Medicines information centre. Contra-Indicatie aandoeningen. Available at: https://kennisbank.knmp.nl.
Samadian, F., Dalili, N., and Jamalian, A. (2016). Lifestyle modifications to prevent and control hypertension. Iran. J. Kidney Dis. 10 (5), 237–263.
Sazliyana, S., Mohd Shahrir, M., Kong, C. N., Tan, H., Hamidon, B., and Azmi, M. (2011). Implications of immunosuppressive agents in cardiovascular risks and carotid intima media thickness among lupus nephritis patients. Lupus. 20 (12), 1260–1266. doi:10.1177/0961203311411347
Shah, S. N., Amato, M. G., Garlo, K. G., Seger, D. L., and Bates, D. W. (2021). Renal medication-related clinical decision support (CDS) alerts and overrides in the inpatient setting following implementation of a commercial electronic health record: implications for designing more effective alerts. J. Am. Med. Inf. Assoc. 28 (6), 1081–1087. doi:10.1093/jamia/ocaa222
Shufelt, C., and LeVee, A. (2020). Hormonal contraception in women with hypertension. JAMA 324 (14), 1451–1452. doi:10.1001/jama.2020.11935
Snanoudj, R., Kriaa, F., Arzouk, N., Beaudreuil, S., Hiesse, C., Durrbach, A., et al. (2004). Single-Center experience with cyclosporine therapy for kidney transplantation: analysis of a Twenty-Year period in 1200 patients. Transpl. Proc. 36 (2), 83S–8. doi:10.1016/j.transproceed.2004.01.089
Song, P., Zhang, Y., Yu, J., Zha, M., Zhu, Y., Rahimi, K., et al. (2019). Global prevalence of hypertension in children: a systematic review and meta-analysis. JAMA Pediatr. 173 (12), 1154–1163. doi:10.1001/jamapediatrics.2019.3310
Spence, J. D. (2018). Controlling resistant hypertension. Stroke Vasc. Neurol. 3 (2), 69–75. doi:10.1136/svn-2017-000138
Stahl, S. M. (2013). “Essential psychopharmacology,” in Neuroscientific basis and practical applications. 4 (Cambridge: Cambridge Medicine).
Stahl, S. M., and Felker, A. (2008). Monoamine oxidase inhibitors: a modern Guide to an unrequited Class of antidepressants. CNS Spectr. 13 (10), 855–870. doi:10.1017/s1092852900016965
Taylor, B. P., Quitkin, F. M., McGrath, P. J., and Stewart, J. W. (2005). Do antihypertensives make tranylcypromine safer? J. Clin. Psychiatry 66 (05), 657–658. doi:10.4088/jcp.v66n0519e
Taylor, D. O., Barr, M. L., Radovancevic, B., Renlund, D. G., Mentzer, Jr R. M., Smart, F. W., et al. (1999). A randomized, multicenter comparison of tacrolimus and cyclosporine immunosuppressive regimens in cardiac transplantation: decreased hyperlipidemia and hypertension with tacrolimus. J. Heart Lung Transplant. 18 (4), 336–345. doi:10.1016/s1053-2498(98)00060-6
Thase, M. E. (1998). Effects of venlafaxine on blood pressure: a meta-analysis of original data from 3744 depressed patients. J. Clin. Psychiatry 59 (10), 502–508. doi:10.4088/jcp.v59n1002
van der Sijs, H., Aarts, J., Vulto, A., and Berg, M. (2006). Overriding of drug safety alerts in computerized physician order entry. J. Am. Med. Inf. Assoc. 13 (2), 138–147. doi:10.1197/jamia.M1809
van Roon, E. N., Flikweert, S., le Comte, M., Langendijk, P. N. J., Kwee-Zuiderwijk, W. J. M., Smits, P., et al. (2005). Clinical relevance of drug-drug interactions: a structured assessment procedure. Drug Saf. 28 (12), 1131–1139. doi:10.2165/00002018-200528120-00007
van Tongeren, J. M. Z., Harkes-Idzinga, S. F., van der Sijs, H., Atiqi, R., van den Bemt, B. J. F., Draijer, L. W., et al. (2020). The development of practice recommendations for drug-disease interactions by literature review and expert opinion. Front. Pharmacol. 11, 707. doi:10.3389/fphar.2020.00707
Vishram, J. K. K., Borglykke, A., Andreasen, A. H., Jeppesen, J., Ibsen, H., Jørgensen, T., et al. (2012). Impact of age on the importance of systolic and diastolic blood pressures for stroke risk: the MOnica, Risk, Genetics, Archiving, and Monograph (MORGAM) Project. Hypertension 60 (5), 1117–1123. doi:10.1161/HYPERTENSIONAHA.112.201400
Volksgezondheid en Zorg info (2022). Ziekten van hart en vaatstelsel Sterftecijfers. Available at: https://www.vzinfo.nl/hart-en-vaatziekten/sterftecijfers.
Watson, D. L., Bhatia, R. K., Norman, G. S., Brindley, B. A., and Sokol, R. J. (1989). Bromocriptine mesylate for lactation suppression: a risk for postpartum hypertension? Obstetrics Gynecol. 74 (4), 573–576.
Weersink, R. A., Timmermans, L., Monster-Simons, M. H., Mol, P. G. M., Metselaar, H. J., Borgsteede, S. D., et al. (2019). Evaluation of information in summaries of product characteristics (SmPCs) on the use of a medicine in patients with hepatic impairment. Front. Pharmacol. 10, 1031. doi:10.3389/fphar.2019.01031
Weissenborn, M., Haefeli, W. E., Peters-Klimm, F., and Seidling, H. M. (2017). Interprofessional communication between community pharmacists and general practitioners: a qualitative study. Int. J. Clin. Pharm. 39 (3), 495–506. doi:10.1007/s11096-017-0450-6
Whelton, P. K., Carey, R. M., Aronow, W. S., Casey, D. E., Collins, K. J., Dennison Himmelfarb, C., et al. (2017). 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American heart association task force on clinical practice guidelines. Hypertension 71 (6), e13–e115. doi:10.1161/HYP.0000000000000065
Whelton, P. K., Carey, R. M., Mancia, G., Kreutz, R., Bundy, J. D., and Williams, B. (2022). Harmonization of the American College of Cardiology/American heart association and European society of Cardiology/European society of hypertension blood pressure/hypertension guidelines: comparisons, reflections, and recommendations. J. Am. Coll. Cardiol. 80 (12), 1192–1201. doi:10.1016/j.jacc.2022.07.005
Williams, B., Mancia, G., Spiering, W., Agabiti Rosei, E., Azizi, M., Burnier, M., et al. (2018). 2018 practice guidelines for the management of arterial hypertension of the European society of Cardiology and the European society of hypertension. Blood Press 27 (6), 314–340. doi:10.1080/08037051.2018.1527177
Woo, M., Przepiorka, D., Ippoliti, C., Warkentin, D., Khouri, I., Fritsche, H., et al. (1997). Toxicities of tacrolimus and cyclosporin A after allogeneic blood stem cell transplantation. Bone Marrow Transpl. 20 (12), 1095–1098. doi:10.1038/sj.bmt.1701027
Yamada, M., and Yasuhara, H. (2004). Clinical pharmacology of MAO inhibitors: safety and future. Neurotoxicology 25 (1–2), 215–221. doi:10.1016/S0161-813X(03)00097-4
Keywords: hypertension, drug-disease interaction, clinical decision support, adverse drug reaction, blood pressure, product information, drug information
Citation: Özokcu K, Diesveld MME, Gipmans SGH, Peeters LEJ, van den Born B-J and Borgsteede SD (2024) Developing practical recommendations for drug-disease interactions in patients with hypertension. Front. Pharmacol. 15:1360146. doi: 10.3389/fphar.2024.1360146
Received: 22 December 2023; Accepted: 26 March 2024;
Published: 17 April 2024.
Edited by:
Elise Peery Gomez-Sanchez, University of Mississippi Medical Center, United StatesReviewed by:
Rodrigo O. Maranon, CCT CONICET Tucuman, ArgentinaTitilayo Fakeye, University of Ibadan, Nigeria
Copyright © 2024 Özokcu, Diesveld, Gipmans, Peeters, van den Born and Borgsteede. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sander D. Borgsteede, sander.borgsteede@healthbase.nl
 Kübra Özokcu1,2
Kübra Özokcu1,2 Sander D. Borgsteede
Sander D. Borgsteede