- 1Department of Cardiology, The Second Affiliated Hospital of Xi’an Jiaotong University, Xi’an, China
- 2Department of Respiratory and Critical Care Medicine, The Second Affiliated Hospital of Xi’an Jiaotong University, Xi’an, China
Background: Cardiovascular disease (CVD) poses a significant global health and economic challenge, with atherosclerosis being a primary cause. Over the past 40 years, substantial research has been conducted into the prevention and reversal of atherosclerosis, resulting in the development of lipid-lowering agents such as statins and fibrates. Despite the extensive literature and formulation of numerous therapeutic guidelines in this domain, a comprehensive bibliometric analysis of the current research landscape and trends has not been performed. This study aimed to elucidate the evolution and milestones of research into lipid-lowering treatments for coronary heart disease (CHD) in conjunction with hyperlipidemia through bibliometric analysis, offering insights into future directions for treatment strategies.
Methods: This study examined publications from 1986 to 2023 retrieved from the Web of Science database (Core Collection). Utilizing tools such as VOSviewer, Pajek, and CiteSpace, we analyzed publication and citation numbers, H-indexes, contributions by countries and institutions, authorship, journal sources, and keyword usage to uncover research trajectories and areas of focus.
Results: Our analysis of 587 publications revealed a recent surge in research output, particularly post-2003. The American Journal of Cardiology published the highest number of studies, with 40 articles, whereas Circulation received the highest number of citations (6,266). Key contributors included the United States, Japan, and China, with the United States leading in citation numbers and the H-index. Harvard University and Leiden University emerged as pivotal institutions, and Professors J. Wouter Jukema and Robert P. Giugliano were identified as leading experts. Keyword analysis disclosed five thematic clusters, indicating a shift in research towards new drug combinations and strategies, signaling future research directions.
Conclusion: The last 4 decades have seen a notable rise in publications on lipid-lowering therapies for CHD and hyperlipidemia, with the United States retaining world-leading status. The increase in international collaboration aids the shift towards research into innovative lipid-lowering agents and therapeutic approaches. PCSK9 inhibitors and innovative combination therapies, including antisense oligonucleotides and angiopoietin-like protein 3 inhibitors, provide avenues for future research, intending to maximize the safety and efficacy of treatment approaches.
1 Introduction
Cardiovascular disease (CVD) imposes a significant global health and economic burden, contributing to approximately one-third of all deaths; coronary artery disease (CAD) is the most common form of CVD (Nowbar et al., 2019; Stone et al., 2023; Tsao et al., 2023). As shown in Figure 1A, although the overall prevalence of CVD decreased from 1990 to 2019, there has been an upward trend since 2011. Over the same period, the proportion of deaths attributed to CVD, CAD, and abnormalities in high-density lipoprotein cholesterol (HDL-C) and low-density lipoprotein cholesterol (LDL-C) have increased consistently year over year, as depicted in Figures 1B–D (Cesare et al., 2022; Mensah et al., 2023; Martin et al., 2024; Naghavi et al., 2024). CAD is caused by atherosclerosis, with risk factors including hypertension, diabetes mellitus, and hyperlipidemia (Ference et al., 2017). Among them, a direct and positive correlation exists between blood lipid levels and cardiovascular risk (Joseph et al., 2017), which may be influenced by circadian disruption (Het Panhuis et al., 2023).
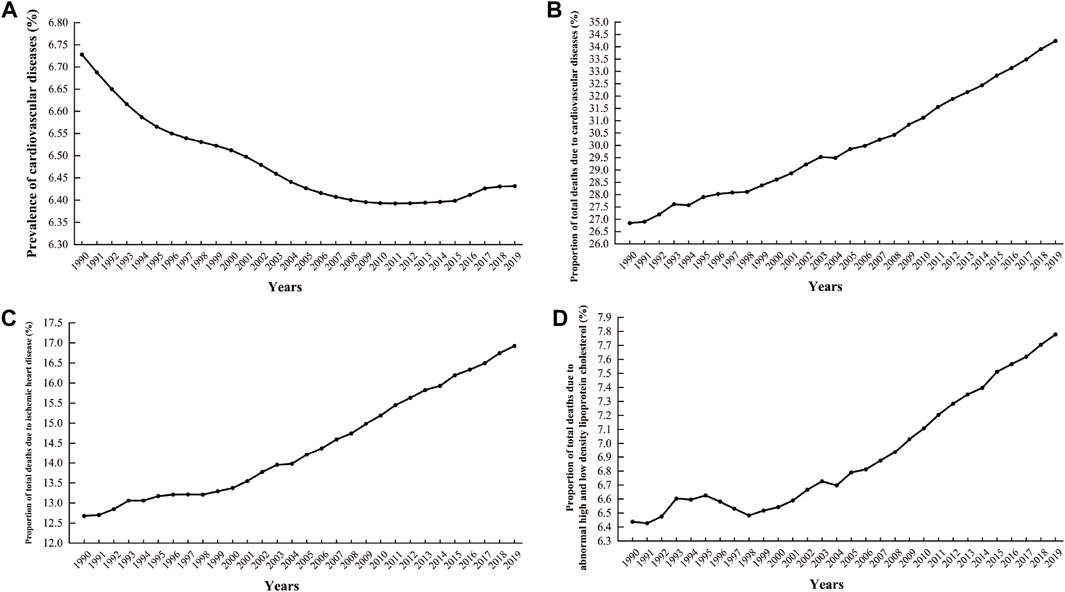
Figure 1. Global cardiovascular disease prevalence and associated deaths as a percentage from 1990 to 2019. (A) Prevalence rate of cardiovascular diseases; (B) The proportion of deaths attributable to cardiovascular diseases out of the total number of deaths; (C) The proportion of deaths attributable to ischemic heart disease out of the total number of deaths; (D) The proportion of deaths affected by abnormalities in high-density lipoprotein cholesterol and low-density lipoprotein cholesterol relative to the total number of deaths (Data sources: IHME, Global Burden of Disease Study (2019)—processed by Our World in Data).
Hyperlipidemia is widely recognized as a key contributor to coronary atherosclerosis, with cholesterol-rich lipoproteins accumulating in areas of the arterial intima prone to plaque formation (Skålén et al., 2002; Grundy et al., 2019). Lipids, including cholesterol and triglycerides (TG), form lipoproteins in the bloodstream because of their insolubility in aqueous environments. These lipoproteins, including very low-density lipoproteins, low-density lipoproteins (LDL), and high-density lipoproteins (HDL), constitute a crucial group of proteins (Soppert et al., 2020). Numerous studies have identified LDL-C as the most significant risk factor for the progression of atherosclerotic CVD (ASCVD), with other lipids, such as TG and triglyceride-rich lipoproteins (TRLs), also identified as risk factors for ASCVD. These findings are supported by observations made in patients with hyperlipidemia and ASCVD who maintain well-controlled levels of LDL-C (Kannel et al., 1961; Lewington et al., 2007; Miller et al., 2011; Nordestgaard, 2016a; Ganda et al., 2018; Toth et al., 2020). In patients with chronic kidney disease, the levels of plasma sphingolipids have been shown to impact the development and progression of CVD (Lidgard et al., 2023), possibly due to their role in promoting inflammatory responses in the vascular endothelium, accelerating the formation of atherosclerotic plaques, and diminishing their stability (Kugiyama et al., 1999; Karpe et al., 2001; Imke et al., 2005; Quispe et al., 2015). These effects are more pronounced in diabetic and insulin-resistant patients and might be responsible for the rapid progression of atherosclerosis in these individuals, making plasma sphingolipids potential prognostic indicators and therapeutic targets (Ginsberg et al., 2010; Toth et al., 2020). Kugiyama et al. suggested that fasting serum levels of residual lipoproteins could be used to predict coronary events in patients with CAD (Kugiyama et al., 1999). Di Angelantonio et al. concluded that risk assessments can be performed using factors such as total cholesterol, TG, and LDL-C (Di Angelantonio et al., 2012). Parish et al., 2012 demonstrated that the LDL-C particle size and content were strongly associated with major occlusive vascular events. Similarly, Mortensen et al. established a link between LDL-C levels and ASCVD events (Mortensen et al., 2023). Consequently, international guidelines universally recommend measuring LDL-C and TG levels to diagnose hyperlipidemia and predict CVD risk, indicating the preventive and therapeutic potential of reducing LDL-C and TG levels in the development and progression of coronary heart disease (CHD) and hyperlipidemia (Grundy et al., 2019; Visseren et al., 2021; Hong et al., 2023).
As research in this field intensified, the first statin lipid-lowering drug, lovastatin, became available in the United States in 1987. Subsequently, other drugs, such as fibrates and omega-3 fatty acids, emerged alongside a growing body of evidence of their critical role in lipid management for patients with CAD (Abifadel et al., 2009). However, only 23.9% of individuals at a very high risk of dyslipidemia achieved a reduction in LDL-C levels with statins alone (Yan et al., 2018). In addition, prolonged treatment with high doses of statins can cause tolerance issues and the development of secondary conditions (Al-Lamee et al., 2019; Almeida and Budoff, 2019; Dawson et al., 2022; Doenst et al., 2022). Statins increase the risk of new-onset diabetes, potentially due to a reduction in insulin sensitivity. The management of patients with diabetic dyslipidemia, therefore, requires careful consideration (Khankari et al., 2022). If pharmacotherapy is unsuccessful, surgical interventions are required, underscoring the urgent need for innovative drug discovery (Epstein et al., 2013; Al-Lamee et al., 2019). Consequently, researchers have investigated novel treatment strategies. In 2003, a collaborative team of researchers from Canada and France discovered proprotein convertase subtilisin/kexin 9 (PCSK9), leading to the development and approval of four drugs targeting this enzyme (Bao et al., 2024) and providing crucial insights into novel potential avenues for the treatment and prevention of CHD and hyperlipidemia.
Bibliometric analysis, a sophisticated statistical approach utilizing public literature databases, enables scrutinization and elucidation of evolving trends and research dynamics within specific fields (Tran et al., 2018). This method provides researchers with information on the volume and caliber of the existing literature at institutional and regional levels and forecasts future directions for scientific exploration. Bibliometric analysis is widely recognized as a robust tool for gauging the quality, influence, and authenticity of academic publications (Luukkonen, 1990; Ekinci et al., 2015; Zhao et al., 2018). Moreover, it has been used to evaluate literature in various fields of medical research. For instance, Wu et al., 2022 investigated the interplay between gut microbiota and colorectal cancer using tools such as CiteSpace and VOSviewer. Correspondingly, Yu et al., 2022 used these platforms to gain insights into metabolomics and its connection to CAD, whereas Zhang et al., 2023 mapped key themes and directions in 3D printing applications for cardiovascular ailments. Despite the burgeoning interest in CHD and hyperlipidemia, no bibliometric analysis focusing on lipid-lowering treatments for combined CHD and hyperlipidemia utilizing both VOSviewer and CiteSpace has been conducted yet. This study aimed to fill this gap by dissecting research hotspots and prospective research avenues within the field of lipid-lowering therapy for this combined condition. Through a comprehensive analysis of article formats, publications trends, journal prominence, keyword evolution, and collaborative networks of authors and institutions, this study sought to reveal the current state and future directions of the field, enhancing our understanding of lipid-lowering therapies for CHD and hyperlipidemia and actively contributing to the advancement of research in this area.
2 Materials and methods
2.1 Search strategy
After obtaining relevant title keywords and supplementing them with grid subject headings from PubMed, we conducted a search using the Web of Science database (Core Collection) as the data source, following the search format shown below: ((ALL=((coronary heart disease) OR (coronary artery disease) OR (coronary disease) OR (silent myocardial ischemia) OR (angina) OR (stenocardia) OR (myocardial infarction) OR (ischemic cardiomyopathy) OR (ischemic heart disease) OR (coronary death) OR (acute coronary syndrome) OR (CHD) OR (CAD) OR (ACS)) AND ALL=((total cholesterol) OR (triglyceride) OR (high-density lipoprotein cholesterol) OR (low-density lipoprotein cholesterol) OR (non-high-density lipoprotein cholesterol) OR (very low-density lipoprotein) OR (intermediate-density lipoprotein) OR (apolipoprotein A1) OR (apolipoprotein B) OR (lipids) OR (lipid metabolism) OR (fatty acid) OR (phospholipid) OR (lipoprotein))) AND ALL=((fibrates) OR (fibric acid) OR (statins) OR (ezetimibe) OR (niacin) OR (Omega-3) OR (proprotein convertase subtilisin-kexin type 9 inhibitor) OR (evolocumab) OR (alirocumab) OR (inclisiran)). The search covered the period from 1 January 1986, to 31 December 2023, and was performed on 31 December 2023.
2.2 Study selection
Figure 2 outlines the methodological screening process and the precise inclusion criteria used in this study. The search was initiated using the predefined terms described in the previous section. To mitigate potential bias resulting from database updates, two independent researchers reviewed the initially identified publications on the same day, ensuring the validity of the assessment process. During this thorough review, publications that did not meet our eligibility criteria were removed, resulting in a final total of 587 papers. The inclusion criteria were as follows: 1) publications on lipid-lowering therapy for treating CHD with hyperlipidemia, including studies of patients with CHD combined with hyperlipidemia and animal models of CHD with hyperlipidemia (hyperlipidemia included simple hypercholesterolemia and hypertriglyceridemia), that assessed the correlation between lipid-lowering therapies and disease; 2) studies published from 1 January 1986, to 31 December 2023, to ensure the inclusion of publications from 2023; 3) publications from the Web of Science (Core Collection) Citation Index Expanded (SCI-E) and Social Science Citation Index (SSCI) databases; 4) studies published as articles; and 5) studies published in the English language. The exclusion criteria were as follows: 1) reviews, meta-analyses, conference abstracts, letters, early access papers, conference proceedings, corrections, retractions, and other types of publications; 2) duplicate publications; and 3) publications with incomplete bibliographic information, such as title, country, authors, keywords, or source (Gao et al., 2023; Wang et al., 2023).
2.3 Data acquisition
We meticulously extracted comprehensive data from literature records, including the titles, authors, abstracts, keywords, and cited references of the publications. These records were then exported into various file formats to facilitate in-depth analysis. During this analytical phase, we evaluated several key bibliometric indicators, including the total number of publications, distribution of publications by country, citation counts, H-index, and detailed information on contributing countries, institutions, authors, journals, and prevalent keywords (Hirsch, 2007).
2.4 Data statistics
The imported data were further analyzed using VOSviewer (version 1.6.20) and CiteSpace (version 6.1.6) software. VOSviewer is particularly adept at mapping collaborations among authors, institutions, and countries/regions through co-citation networks. It generates collaborative network maps in which each node, represented as a dot, signifies a country, region, institution, or author. The publication count determines the node size, and the thickness of the connecting lines indicates the collaboration strength. This visualization facilitates a comprehensive understanding of the global research landscape (van Eck and Waltman, 2010). VOSviewer can also be used for keyword cluster analysis, in which frequently co-occurring keywords are grouped into distinct clusters, each marked with a unique color. The resulting graphic reveals prevailing research themes and trends (van Eck and Waltman, 2017). Use of the program Pajek enabled further refinement of cluster layouts to improve clarity and distinction. CiteSpace software specializes in literature co-citation analysis and tracks the evolution of keyword trends over time. Its graphical representation includes nodes symbolizing individual research articles, connected by lines that vary in color and width. These variations denote the strength of collaborations, with darker and thicker lines indicating stronger collaborations (Chaomei, 2005). CiteSpace also examines keyword frequency and identifies “keyword bursts,” crucial for pinpointing emerging research directions (Chen et al., 2012; Chen et al., 2014).
3 Results
3.1 Analysis of publication numbers, citation trends, and H-index by year
The database search yielded 12,918 records published between 1 January 1986, and 31 December 2023. From this pool, 4,423 were non-research papers and were therefore excluded. The screening process, involving both abstract and full-text reviews, refined the selection to a total of 587 papers. To mitigate potential bias from database updates, a final search was conducted on 31 December 2023, as illustrated in Figure 2.
Analysis of annual publication numbers revealed an initial phase (1991–2003) characterized by a modest and stable output. A publication surge occurred between 2003 and 2015, indicating a significant increase in research productivity. The period after 2015 showed a further increase in publications, laying a robust foundation for ongoing research (Figure 3). The trend analysis from 1991 to 2023 indicates a generally ascending trajectory in publication numbers, surpassing 20 in 2004 and 30 in 2016, with occasional dips, including in 2012 and 2018. The number of citations reached its peak in 2021, surpassing 4,400. The H-index of the field remained <10 until 2003 and then experienced a rapid ascent after 2004, peaking at 15–20 between 2005 and 2010. A gradual decline was observed from 2011 to 2018, with a notable decrease post-2019, potentially due to indexing delays. These patterns underscore the increasing interest in lipid-lowering therapies for CHD and hyperlipidemia and project a sustained positive trajectory in research output.
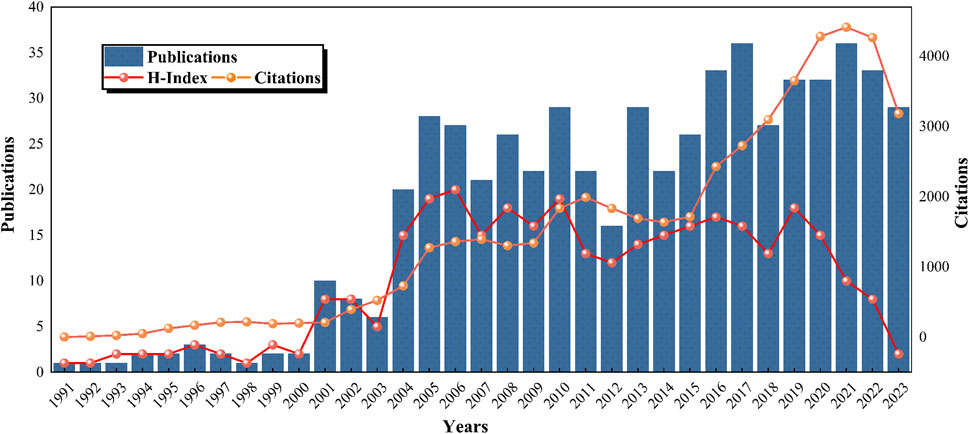
Figure 3. Global trends in publications, citations, and publication H-index in the field of lipid-lowering therapy for CHD combined with hyperlipidemia (1991–2023).
3.2 Analysis of journals
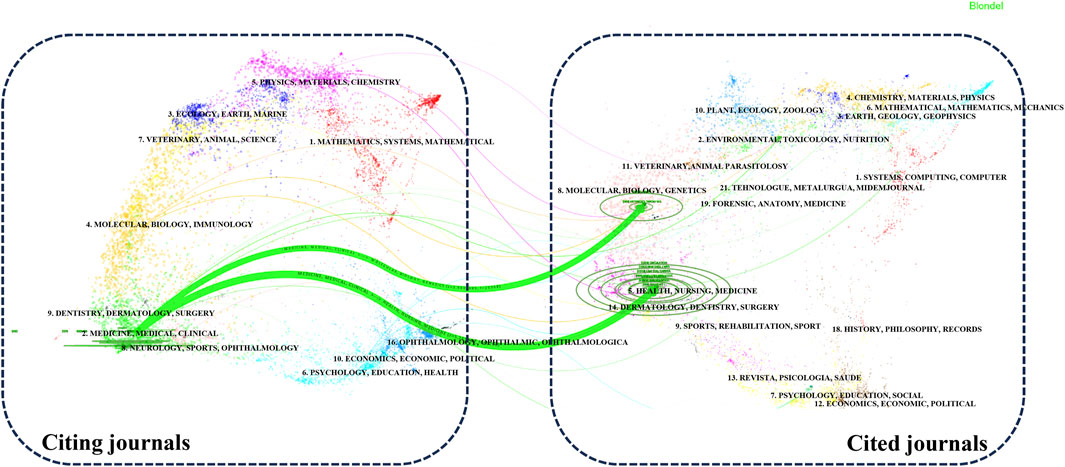
Our analysis of the publication landscape revealed that as of December 2023, 184 SCI-indexed journals featured articles on lipid-lowering therapy in the context of CHD co-occurring with hyperlipidemia. Table 1 displays the top 10 journals, accounting for 43.10% of the total publications in this area. The American Journal of Cardiology published the highest number of articles (40%, 6.81%), closely followed by Circulation. In terms of citations, Circulation had the highest number (6,266 citations), followed by the Journal of the American College of Cardiology. Circulation, although ranking second in publication volume, had the highest average citation count per article (179.03), an H-index of 33, and an impact factor (IF) of 39.92. These findings highlighted the significant influence of cardiovascular researchers in this field. Figure 4 illustrates the disciplinary focus of the journals involved, with citing journals on the left and cited journals on the right. The analysis revealed a prevailing publication trend within the “medicine, medical, clinical” discipline for research on lipid-lowering therapies in CHD with hyperlipidemia. Moreover, the citation impact of these publications is primarily attributed to journals within the “molecular, biology, genetics” and “health, nursing, medicine” disciplines, revealing a multidisciplinary interest and emphasizing the foundational role of these areas in advancing lipid-lowering therapeutic research.
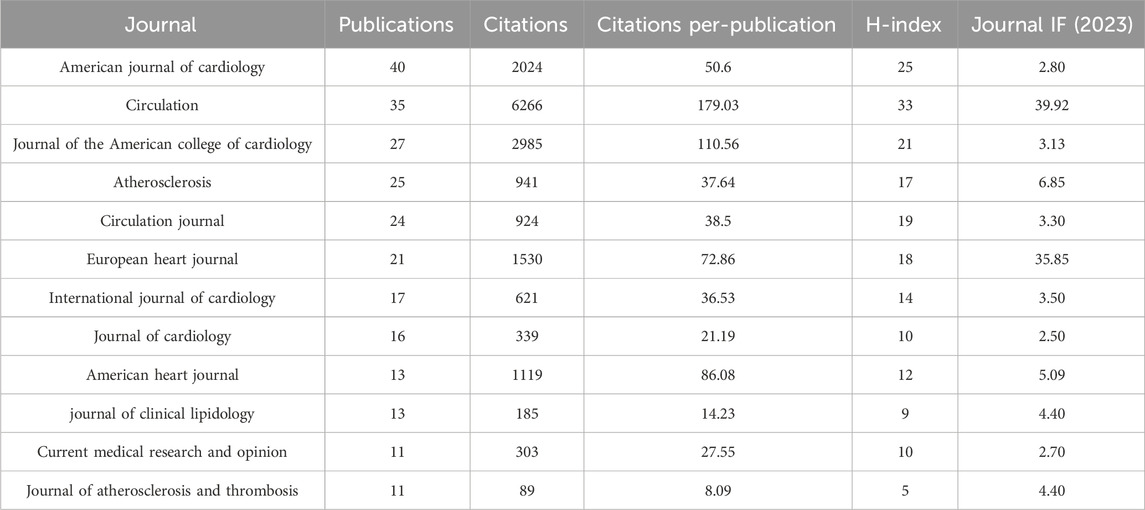
Table 1. The top 10 leading journals in the field of lipid-lowering therapies for coronary heart disease combined with hyperlipidemia from 1991 to 2023.
3.3 Analysis of countries/regions
A total of 68 countries have contributed to research on lipid-lowering therapies for CHD combined with hyperlipidemia. Figure 5A presents a map indicating the 10 countries with the highest number of publications, and Table 2 provides detailed information on publications from these countries. The highest number of publications originated from the United States (n = 217%, 26.72%), followed by Japan (n = 117%, 14.41%), and China (n = 87%, 10.71%). The United States also had the highest H-index (69), followed by Germany (38), and the highest number of total citations (n = 32,782), followed by Canada (n = 17,179), and the UK (n = 16,031). Cooperation between the 36 countries with ≥10 publications is depicted in Figure 5B. The United States, Japan, and China are the most central countries in the network, with the United States being the most prominent. China has fewer connections with other countries and should, therefore, intensify efforts to enhance communication and cooperation with the international community.
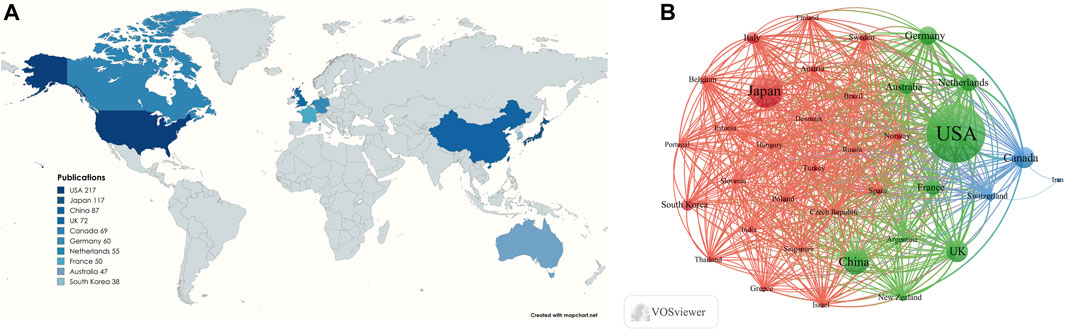
Figure 5. (A) Visualization of the number of the top 10 countries in terms of cumulative number of publications; (B) Cooperation networks in countries/regions all over the world.
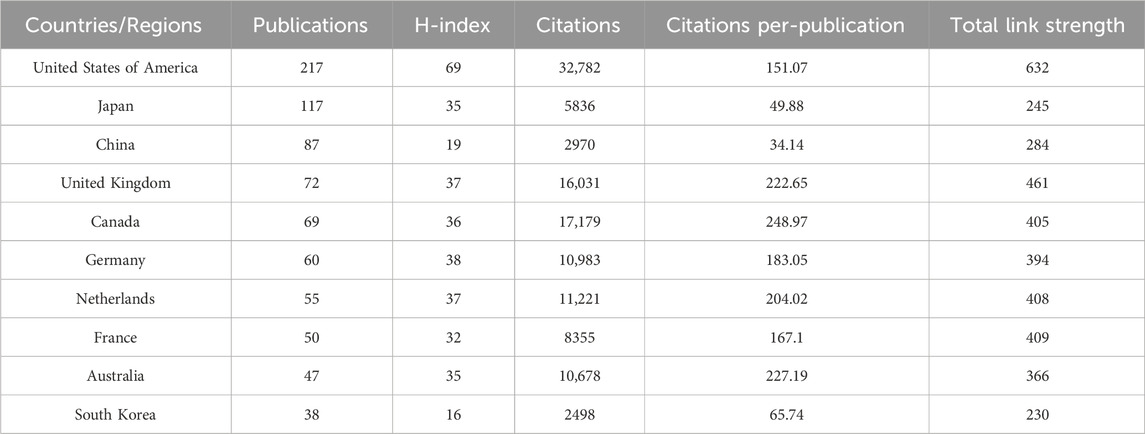
Table 2. The top 10 most productive countries/regions in the field of lipid-lowering therapies for coronary heart disease combined with hyperlipidemia from 1991 to 2023.
3.4 Analysis of institutions
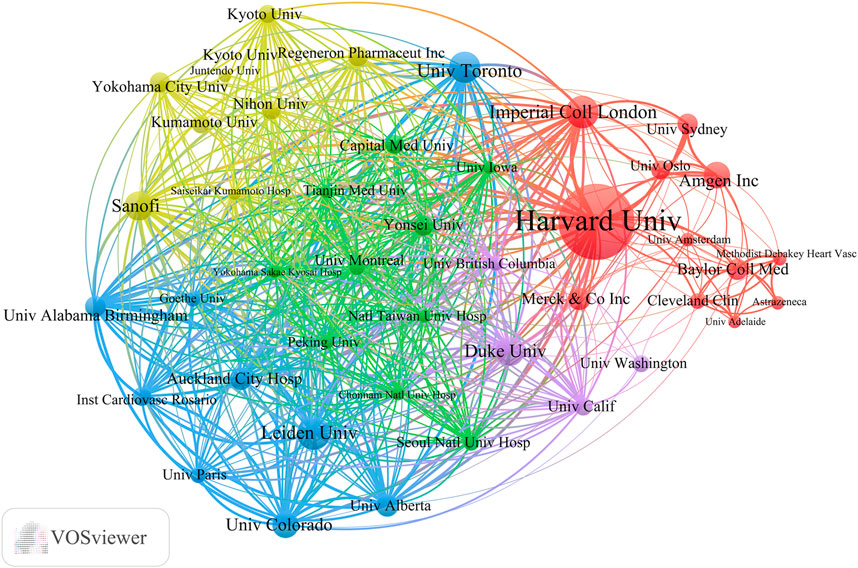
Globally, 2,805 universities and institutions have contributed to the advancement of lipid-lowering therapies for CHD in the context of hyperlipidemia. Table 3 outlines the top 10 contributors based on research output. Harvard University had the most publications (75), followed by Leiden University. Harvard University also possesses the highest H-index (43) and the highest total citation count (22,002). Its average citation rate per article was 293.36, which was second only to Imperial College London. Among these leading institutions, five are located in the United States. Figure 6 provides a visualization of the key institutions and their collaborative networks. Harvard University is a central node, indicating extensive collaboration, whereas Peking University is the most connected Chinese institution. This illustration accentuates the predominance of international collaborations over domestic ones, highlighting the global nature of research efforts in developing lipid-lowering therapies for patients with CHD and hyperlipidemia.
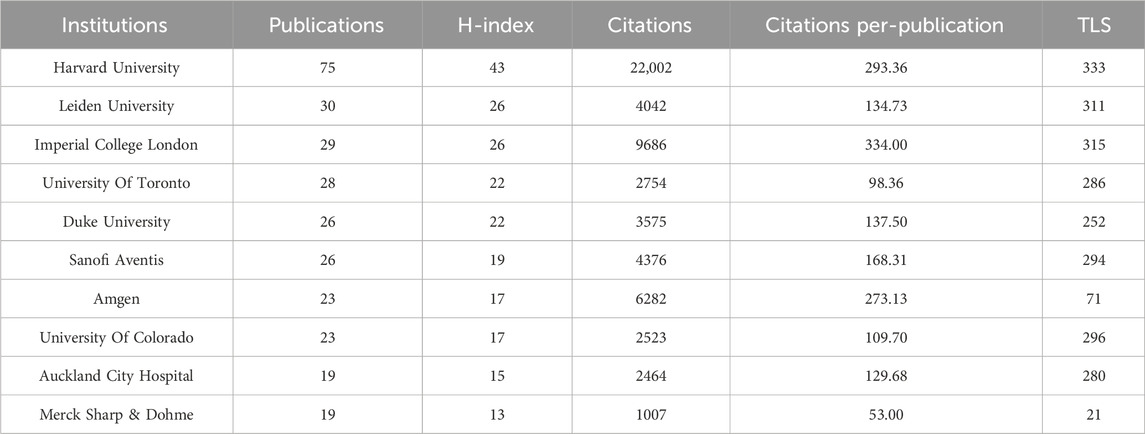
Table 3. The top 10 most productive universities/institutions in the field of lipid-lowering therapies for coronary heart disease combined with hyperlipidemia from 1991 to 2023.
3.5 Analysis of authors
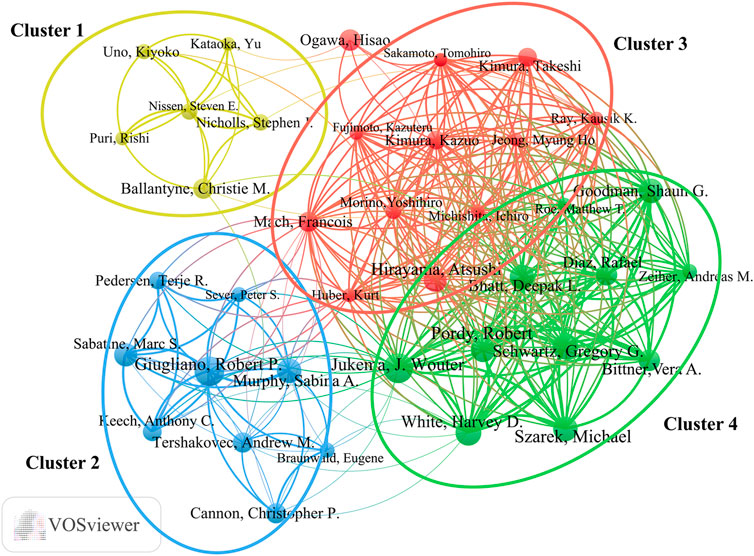
More than 5,900 researchers have contributed to publications in the field of lipid-lowering therapies for CHD and hyperlipidemia. As summarized in Table 4, noteworthy authors include J. Wouter Jukema from the Netherlands and Robert P. Giugliano from the United States, each with 22 publications. Jukema had the highest H-index at 21, whereas Giugliano had the most citations, at 8,825. The collaborative network graph created using VOSviewer in Figure 7 features 38 authors with ≥10 publications, presented in four clusters anchored by key figures such as Jukema, Hirayama, Giugliano, and Ballantyne. Clusters 3 and 4 exhibited strong connections, whereas clusters 1 and 2 displayed fewer interactions, highlighting the variation in collaboration within the research community.
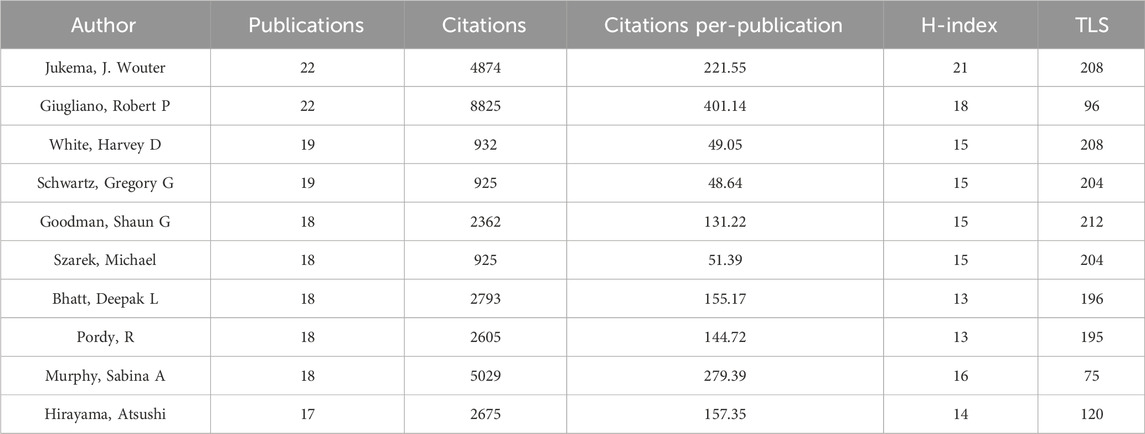
Table 4. The top 10 most productive scholars in the field of lipid-lowering therapies for coronary heart disease combined with hyperlipidemia from 1991 to 2023.
3.6 Analysis of co-cited references and reference bursts
Table 5 presents the 10 most influential papers in CHD and hyperlipidemia lipid-lowering therapy research based on Web of Science citations. The 2004 study by Cannon et al. in the New England Journal of Medicine leads with 3,617 citations; this study emphasized the advantages of early and substantial LDL-C reduction through statins in patients with ASCVD (Cannon et al., 2004). An earlier work in the same journal by Brown et al. underscored the combined efficacy of simvastatin and niacin in patients with low HDL levels (Brown et al., 2001). Nine out of these 10 pivotal articles originated from institutions in the United States, underscoring the pivotal role of the United States in this research domain, and they were predominantly published in the New England Journal of Medicine (Brown et al., 2001; Cannon et al., 2004; Nicholls et al., 2011; Robinson et al., 2015a; Cannon et al., 2015; Sabatine et al., 2017; Schwartz et al., 2018; Bhatt et al., 2019) or JAMA (Nissen et al., 2004; Nicholls et al., 2016).
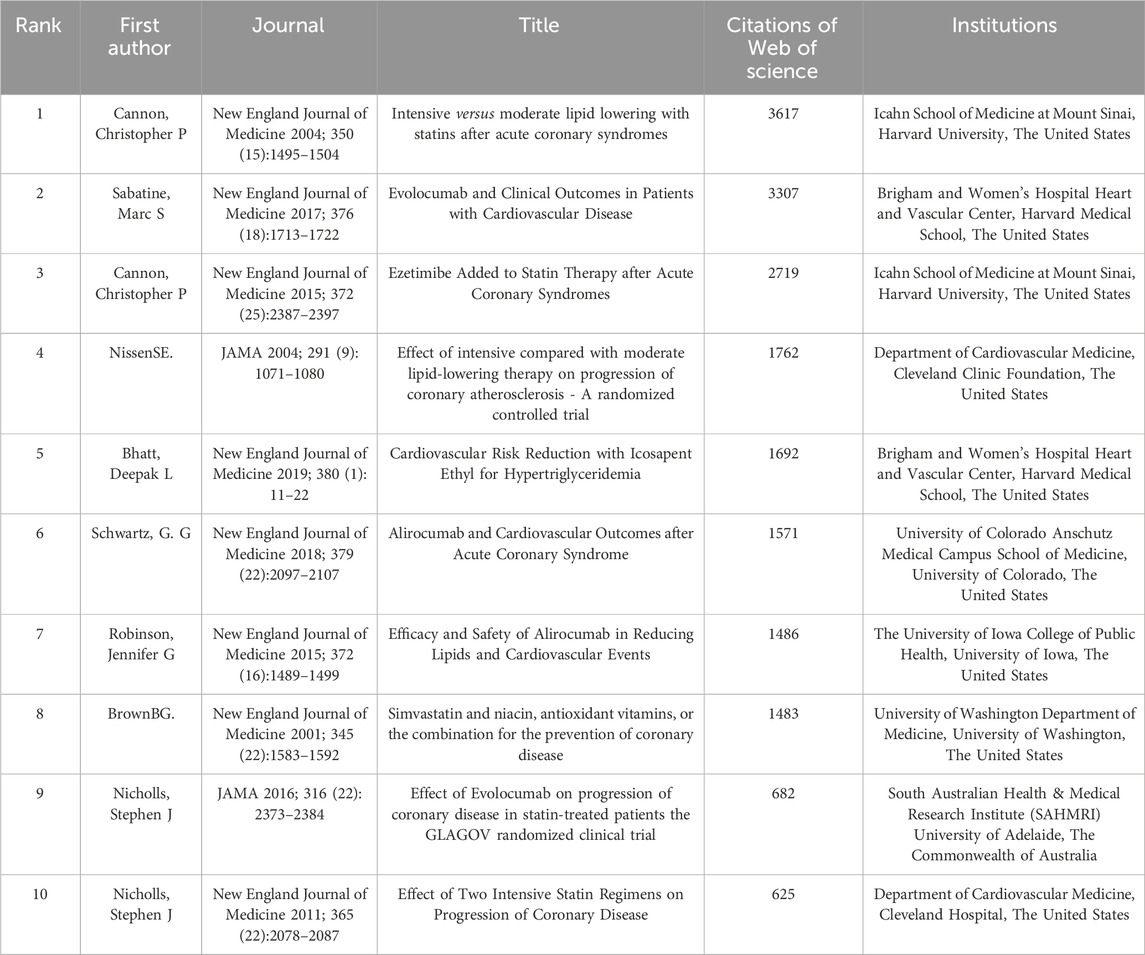
Table 5. The top 10 most cited articles in the field of lipid-lowering therapies for coronary heart disease combined with hyperlipidemia from 1991 to 2023.
We conducted a co-citation analysis of references using CiteSpace, with the distribution network of co-cited references spanning 1991–2023 presented in Figure 8A. Publications cited >14 times were included in the graphic, with 30 articles highlighted. The color intensity of bars and nodes indicates the publication date, with lighter colors representing more recent citations. Our log-likelihood ratio algorithm identified 14 clusters (Figure 8B), with the largest labeled “St-segment elevation” (# 0), followed by “coronary atheroma” (# 1). We utilized the modularization Q-value criterion to assess the significance of the cluster structure. Typically, when Q-max ≥0.3, the community structure is deemed significant (Wu et al., 2021). In our study, the modularity Q was 0.798, exceeding the threshold of 0.3, and the weighted average profile S was 0.9262, surpassing 0.7. These results indicate that our clustering is robust, convincing, and exhibits homogeneity. Figure 8C provides a timeline view of the reference co-citation clusters, where distinct colored nodes in the same row represent different years of citation in the cluster. Nodes closer to the right represent more recent citations. Figure 8D displays the 25 publications with the strongest citation bursts, with red bars indicating high citation frequency and blue bars indicating lower citation frequency (Zhiguo et al., 2023). The first citation burst in this field occurred in 2001 and lasted 3 years. The study with the strongest burst intensity (strength = 25.93) was published by Sabatine et al., 2017, in 2017 and highlighted the benefits of evolocumab (a PCSK9 inhibitor) in patients with ASCVD. The publications ranked second and third in burst intensity examined the effects of alirocumab (a PCSK9 inhibitor) in patients with ASCVD (strength = 24.45) (Schwartz et al., 2018) and the combination of ezetimibe with statins in patients with ASCVD (strength = 24.14) (Cannon et al., 2015). The greater the intensity of the burst, the more profound its impact on subsequent research.
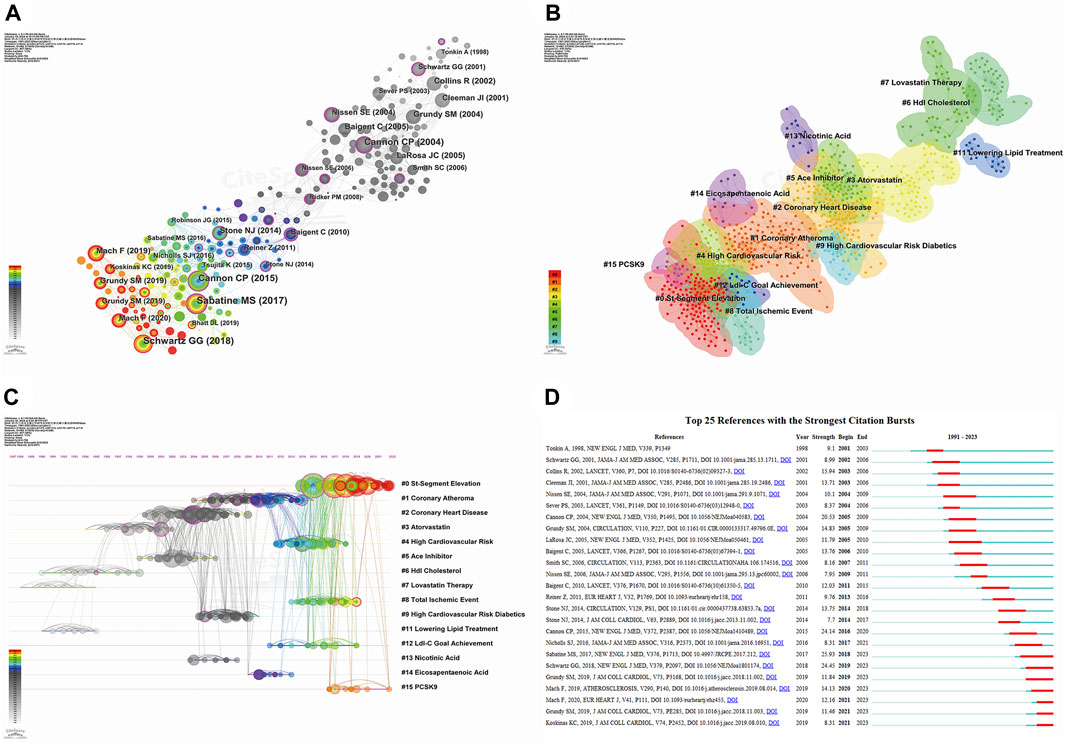
Figure 8. Analyzing reference co-citation through CiteSpace. (A) View of reference co-citations; (B) Cluster view of reference co-citation; (C) Timeline view of reference co-citation. Nodes closer to the right indicate a newer year of citation; (D) Top 25 references with the strongest citation bursts. Red bars indicate high citation frequency, blue bars indicate lower citation frequency.
3.7 Keyword visualization
Keywords reflect the core content of an article, and analysis of their co-occurrence can identify hot topics and active research areas. We conducted keyword analysis using VOSviewer with a threshold of 8. After combining recurring keywords and synonyms, we obtained 123 keywords. As illustrated in Figure 9A, the selected keywords can be broadly categorized into five clusters, with the most prominent keywords in each cluster being “statins” (Lee S. H. et al., 2023; Meng et al., 2023), “atorvastatin” (Cannon et al., 2004; Nissen et al., 2004), “fibrates” (Räber et al., 2022a; Lee et al., 2023b; Lee et al., 2023c; Kovach et al., 2023), “niacin” (Bonaca et al., 2018; O'Donoghue et al., 2019; Nakao et al., 2023), and “PCSK9” (Lou et al., 2022; Park et al., 2023; Schwartz et al., 2023). The five different clusters represent different lipid-lowering drugs and their combination, suggesting that these may be potential research hotspots. CiteSpace was used to perform cluster analysis on the generated keywords, as depicted in Figure 9B. A total of 13 clusters were formed, with the two most active being “statins” (# 0) and “atherosclerosis” (# 1). Most of the clusters and their synonyms can also be found in the co-occurrence analysis, such as “omega-3 fatty acids” (# 0), “atherosclerosis” (# 1), “simvastatin” (# 2), “pravastatin” (# 4), “PCSK9” (# 8), and “ezetimibe” (# 10). Figure 9C provides a timeline view of keyword clustering, indicating that research on lipid-lowering therapeutic drugs and strategies has persisted from 1991 to the present day, with a major focus on the period up to 2005. After 2005, novel drugs and therapeutic strategies using PCSK9 inhibitors and monoclonal antibodies gained popularity as a research topic. Figure 9D displays the top 25 keywords in terms of outbreak intensity, revealing that “pravastatin” had the strongest intensity (strength = 19.77) and was initiated first (1999). Current active keywords include “low-density lipoprotein cholesterol,” “acute coronary syndrome,” “safety,” and “PCSK9 inhibitor,” indicating emerging research avenues.
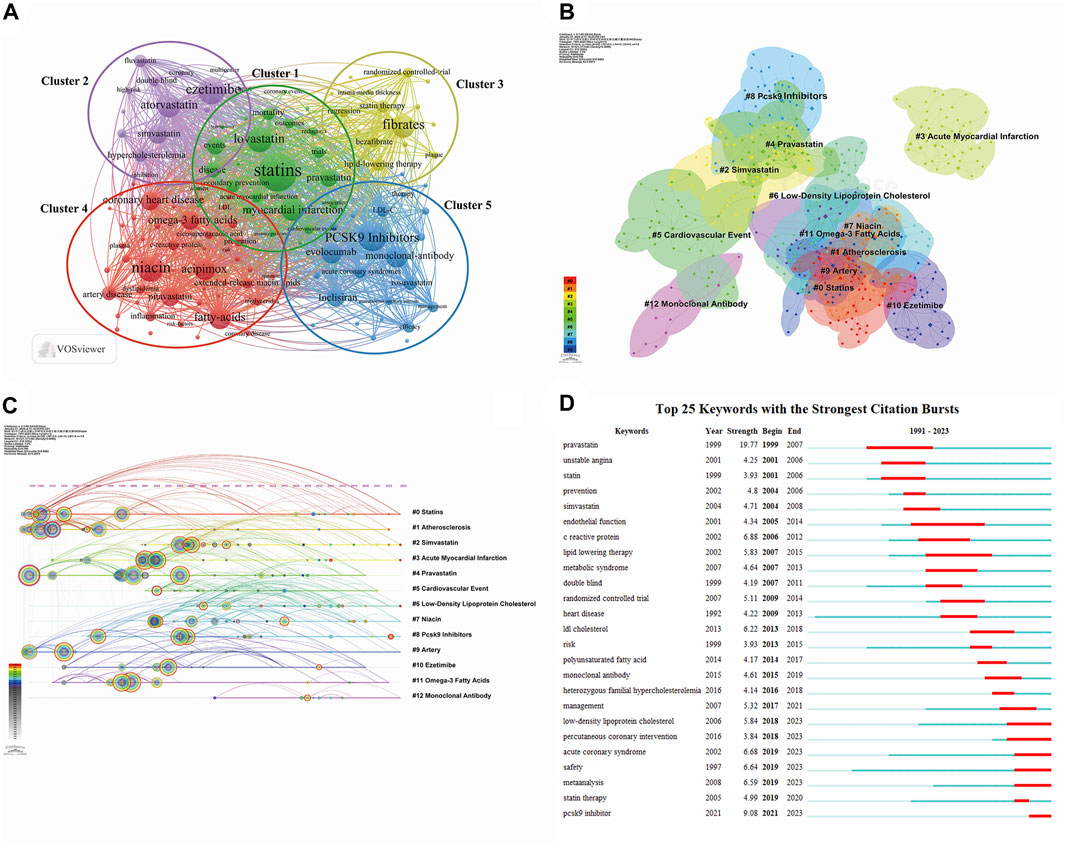
Figure 9. Visualization maps related to studies of lipid-lowering therapy for CHD combined with hyperlipidemia from 1991 to 2023. (A) The cooperation network of keywords in the field. The selected keywords can be broadly categorized into five clusters representing different types of lipid-lowering drugs and their combination applications; (B) Keyword clustering analysis in this field. Different colors represent different clusters, the smaller the count, the more keywords are in the cluster; (C) Timeline view of keywords in the field. The position of a node on the horizontal axis indicates the moment when it first appears, and its size is related to the number of references; (D) The top 25 keywords with the strongest citation bursts. The blue bar indicates the time period in which the keyword appeared, and the red bar indicates the start year, end year, and duration of the burst.
4 Discussion
4.1 Sources and distribution of publications
Before the dawn of the 21st century, the primary focus of lipid-lowering therapy for CHD in conjunction with hyperlipidemia was statins and fibrates. Research by Dairou highlighted the mechanism by which hydroxymethylglutaryl (HMG) CoA reductase inhibitors or statins curbed intracellular cholesterol production while promoting the expression of LDL-C receptors, effectively reducing LDL-C levels (Dairou, 1994). During this period, the United States emerged as a leader in the field, providing the first publications and maintaining the highest publication output to the present day. This leading position may be primarily attributed to the substantial investment in statins research and development by pharmaceutical giants such as Pfizer, Merk Sharp & Dohme (MSD), and Amgen. In particular, MSD’s development of Lovastatin, the first-generation drug for reducing LDL-C levels and the first to be approved for use in the United States, propelled the use and development of this class of drugs worldwide (Blankenhorn et al., 1991).
In the early 2000s, research on lipid-lowering therapies for CHD and hyperlipidemia began to have broader global participation. The field experienced a significant surge in publications starting in 2003, with key years such as 2004 and 2017 marked by substantial increases in publications, citations, and H-indexes, probably largely driven by the discovery of PCSK9 and the development of PCSK9 inhibitors. The American company Amgen significantly impacted this area by developing the first-generation PCSK9 inhibitor, evolocumab, which continues to be widely used. This increased the attention on innovative lipid-lowering agents and strategies. Despite broader international engagement, the top contributors remain primarily from Europe, North America, and Asia, with China as the leading developing country. Collaboration and output analysis highlighted strong ties among developed nations, with the United States, led by Harvard University, playing a pivotal role in shaping the research landscape. International cooperation is an important component of the development of advanced lipid-lowering treatments for CHD and hyperlipidemia.
Chinese scholars began publishing articles in this field in 2004 (Morales et al., 2004), and the number of publications from China has increased consistently each year to its current global ranking of third. Despite its substantial output, China lags behind other countries in terms of the average number of citations per publication and the H-index, indicating a weaker research impact. This discrepancy may be attributed to the numerous research institutions in China, the high volume of publications, and the dominance of large-scale research by developed countries. Key contributors from China include Prof. Tse Hung Fat from the University of Hong Kong, Prof. Zhou Yujie from Sichuan University, and Prof. Huo Yong from Peking University, who collaborate closely with each other and the international community (Goodman et al., 2019; Jukema et al., 2019; Ray et al., 2019; Steg et al., 2019; Szarek et al., 2019; White et al., 2019). As central figures in this field in China, they are expected to continue to foster cooperation and facilitate knowledge exchange.
Our analysis highlighted J. Wouter Jukema as a leading author in the field, publishing significant randomized controlled trials, epidemiological studies, and various research methods, with a focus on evaluation of the lipid-lowering efficacy of various lipid-lowering drugs in CHD with hyperlipidemia. Robert P. Giugliano, another leading author in the field, uses imaging techniques such as vascular ultrasound to examine arterial plaque composition and stability, assessing the effectiveness of new lipid-lowering drugs or therapies. The collaborative efforts of these two authors significantly influence the direction of the field (Cannon et al., 2015; Bonaca et al., 2018; O'Donoghue et al., 2019). Despite being the foremost publisher by article count, the American Journal of Cardiology did not have the highest IF or number of citations, suggesting a potential compromise between research quality and quantity. Circulation, with its high IF and leading citation metrics, may, therefore, be the more influential journal in this field.
Overall, the global research pattern on lipid-lowering therapies for CHD and hyperlipidemia displays a distinct geographical distribution, with developed nations, notably the United States, leading the field. However, as economic and healthcare standards improve, collaboration between developed and developing countries continues to rise. As a vital developing country, China has distinctly intensified its academic partnerships with developed nations, including the United States, particularly following its involvement in the ODYSSEY OUTCOMES study post-2017. This collaboration resulted in a significant increase in publications from Chinese institutions, which now account for 71.26% of the total research output in this domain, emphasizing the vast potential for cooperation with research institutions in developed countries.
4.2 Development and research trend of lipid-lowering therapeutic drugs and strategies
Our study identified five keyword clusters, as illustrated in Figure 9A. Each cluster represents a distinct drug development and therapeutic strategy for CVDs, with a shift in focus from clusters 1 to 5.
4.2.1 Cluster 1 (green): statins
Cluster 1 represents the pioneering role of statins, particularly lovastatin, the first statin drug developed and brought to market by MSD, in treating CHD and hyperlipidemia. This cluster marks the inception of CVD prevention and treatment through lipid-lowering strategies, laying the groundwork for the development of new lipid-lowering medications and therapies. The well-established link between cholesterol levels and atherosclerotic heart disease emphasizes the critical role of LDL-C elevation and HDL-C reduction (Kannel et al., 1979; Keys et al., 1984; Brown et al., 2006; Vergallo and Crea, 2020). Statistically, a 1 mg/dL reduction in LDL-C is associated with a 1% lower risk of cardiovascular events, whereas a 1 mg/dL increase in HDL-C levels corresponds to a 2%–3% risk reduction (Phan and Toth, 2013). Over 3 decades, studies have consistently validated the efficacy of statins, demonstrating not only their ability to slow coronary atherosclerosis progression but also their significant impact on reducing LDL-C levels and the risk of cardiovascular events (Pedersen et al., 1994; Sacks et al., 1996; Collins et al., 2002; Shepherd et al., 2002; Baigent et al., 2005). More recently, two studies have shown that statin use effectively reduces the prevalence of non-alcoholic steatohepatitis and fibrosis in patients with metabolic dysfunction, helping to prevent non-alcoholic fatty liver disease (Ayada et al., 2023; Li et al., 2023). This indicates that statins may also have hepatoprotective effects. The effects of them are likely linked to their ability to inhibit HMG-CoA reductase, reduce LDL-C and triglyceride levels, inhibit the proliferation of vascular smooth muscle, regulate platelet activity, reduce lipid synthesis, and suppress the production of pro-inflammatory cytokines (Paradela-Dobarro et al., 2013; Madasamy et al., 2016; van Rosendael et al., 2021; Ayada et al., 2023). Despite being the focus of early lipid-lowering therapy research, the limitations of statins become evident with disease progression. Low doses may not achieve therapeutic goals, and escalating doses often result in adverse effects such as muscle pain, cognitive issues, and liver and kidney toxicity, compromising treatment efficacy (Toth et al., 2008; Avis et al., 2010; Cohen et al., 2012). In addition, some research has indicated that pregnant women exposed to statins are at an elevated risk of preterm delivery, and this suggests an impact on the cardiovascular health of their offspring later in life (Chang et al., 2021; Wu et al., 2023). Therefore, developing more effective and safer lipid-lowering drugs and therapies may improve patient outcomes.
4.2.2 Cluster 2 (purple): combination of statins and ezetimibe
Cluster 2 centers on the synergistic use of ezetimibe, a cholesterol absorption inhibitor, and statins to address hyperlipidemia. Ezetimibe was jointly developed by MSD and Schering-Plough in the United States in 2002. It functions by selectively blocking the NPC1L1 protein in the intestinal tract, thereby inhibiting cholesterol absorption and reducing the transport of cholesterol to the liver. This effectively lowers blood LDL-C and total cholesterol levels. This pioneering selective cholesterol absorption inhibitor has been shown to achieve a 20% reduction in LDL-C levels (Bruckert et al., 2003).
The dual-action mechanism of ezetimibe hinders dietary cholesterol absorption and stimulates endogenous cholesterol production in the liver without negatively affecting triglyceride levels (Nutescu and Shapiro, 2003). When combined with statins, ezetimibe enhances the treatment of resistant hyperlipidemia or dyslipidemia, significantly decreasing LDL-C levels and boosting the effectiveness of lipid-lowering therapies (Stein et al., 2004). Gagné et al. demonstrated that combining 10 mg of ezetimibe with either 40 or 80 mg of a statin led to a 20.7% reduction in LDL-C levels. Due to its minimal drug interactions, ezetimibe is suitable for patients with low statin tolerance (Gagné et al., 2002). This breakthrough led to the development of other novel treatments for patients experiencing adverse effects from statin treatment.
4.2.3 Cluster 3 (yellow): combination of statins and fibrates
Cluster 3 focuses on the use of fibrates, either alone or in conjunction with statins, for lipid-lowering and plaque stabilization. Although statins have been the cornerstone of lipid-lowering treatment, particularly for patients with hyperlipidemia and CHD, their effectiveness in slowing atherosclerosis progression diminishes over time for high-risk patients. Research indicates that doubling the statin dose achieves only a 6% reduction in LDL-C levels and a 22% decrease in cardiovascular event risk, with high TG levels remaining a residual risk factor (Ray and Cannon, 2004; Ray and Cannon, 2006; Liew and Ray, 2008). As early as the late 1960s, the American pharmaceutical company Pfizer developed the first fibrate drug, gemfibrozil, intended for the treatment of pure hypertriglyceridemia. Fibrates, derived from phenoxyaromatic acid, effectively lower serum TG levels and elevate HDL-C levels by activating peroxisome proliferator-activated receptor alpha and lipoprotein lipase; they also confer anti-inflammatory and antithrombotic benefits (Perreault et al., 2011; Tenenbaum and Fisman, 2012; Gross et al., 2017; Kim and Kim, 2020). Clinical trials have demonstrated the significance of fibrates in treating atherosclerotic dyslipidemia, confirming their role as a potent option for patients with elevated TG levels (Brunner et al., 2000; Robins et al., 2001; Scott et al., 2009; Elam et al., 2017). Over the following 15 years, the Germany pharmaceutical company Bayer and the French pharmaceutical company Sanofi each developed their own fibrates, Fenofibrate and Bezafibrate, respectively. Fenofibrate continues to be a first-line medication for the treatment of patients with high TG and LDL-C levels.
4.2.4 Cluster 4 (red): combination of statins, niacin, and omega-3 fatty acids
Cluster 4 represents the combined use of niacin, omega-3 fatty acids, and statins for treating CHD with hyperlipidemia. Niacin, similar to fibrates, reduces TG levels and increases HDL-C levels. Kim et al. demonstrated that niacin activates sirtuin-1, enhancing mitochondrial fatty acid oxidation and reducing TG synthesis (Kim et al., 2010). Although combining niacin with statins impacts TG and HDL-C levels, a resultant significant reduction in the risk of cardiovascular events remains to be demonstrated (Bruckert et al., 2010; Boden et al., 2011; Haynes et al., 2013).
Omega-3 fatty acids, including eicosapentaenoic acid, docosahexaenoic acid, and docosapentaenoic acid, are recognized for their polyunsaturated nature and pharmacological significance (Calder, 2018). The earliest related medication was developed by Pronova BioPharma and approved for use in the United States in 2004. Omega-3 fatty acids lower the levels of TRLs and promote the production of anti-inflammatory and anticoagulant mediators, contributing to thrombosis resistance and atherosclerotic plaque stabilization (Larsson et al., 2004; Nordestgaard, 2016a). Although omega-3 fatty acids have been shown to decrease CHD mortality in high-risk patients (Mozaffarian and Wu, 2011), definitive evidence linking them to a significant reduction in major adverse cardiovascular events (MACE) in this population remains lacking (Aung et al., 2018). Thus, further research on the synergistic effects of niacin and omega-3 fatty acids on MACE, coupled with the optimization of dosing strategies, presents new avenues for investigation in this sector.
4.2.5 Cluster 5 (blue): combination of statins and PCSK9 inhibitors
Cluster 5 shows the synergistic use of statins with PCSK9 inhibitors to enhance ASCVD prevention efficacy.
PCSK9, a serine protease produced by liver cells, binds to LDL receptors, hindering their recycling and diminishing liver capacity to clear LDL-C. This, in turn, increases the risk of hyperlipidemia and the development of ASCVD (Abifadel et al., 2003; Piper et al., 2007; Urban et al., 2013; Lin et al., 2018). PCSK9 inhibitors have proven effective in significantly reducing LDL-C levels and cardiovascular risks (Hadjiphilippou and Ray, 2017). PCSK9 inhibitor monotherapy has been shown to achieve a >50% reduction in LDL-C levels compared with intensive statin treatments (Hadjiphilippou and Ray, 2017; Räber et al., 2022b), and more pronounced coronary plaque regression is observed when their use is combined with high-intensity statins (Schwartz et al., 2018).
In contrast, inclisiran, a small interfering RNA analog developed by Novartis in 2019, targets the PCSK9 protein in hepatocytes to disrupt RNA transcription and translation, reducing plasma LDL-C levels (Nair et al., 2014; Dyrbuś et al., 2020). Recent ORION trials have demonstrated the ability of inclisiran to substantially reduce LDL-C levels by 47.9% compared to placebo in patients with heterozygous familial hypercholesterolemia (Raal et al., 2020). Additionally, inclisiran demonstrated an even more pronounced reduction of 52.3% in patients unresponsive to other lipid-lowering medications (Ray et al., 2020). Fitzgerald et al. found that adverse reactions to inclisiran were primarily limited to coughing, headaches, and diarrhea, indicating an overall favorable safety profile (Fitzgerald et al., 2017). The findings from the ORION-7 study were consistent with these results, reporting no serious adverse reactions (Wright et al., 2020). Although inclisiran holds significant promise as a lipid-lowering therapy, direct evidence linking it to a reduction in MACE remains elusive. Future research should, therefore, prioritize investigating the impact of inclisiran on MACE while ensuring its ongoing safety.
4.3 Contribution of pharmaceutical companies to lipid-lowering drug research
Pharmaceutical companies play a crucial role in the research and development of lipid-lowering medications. Through their efforts, they have significantly advanced the treatment of hyperlipidemia and related CVDs. Supplementary Table S1 provides details of the 10 pharmaceutical companies with the highest number of publications. Since MSD pioneered the development of lovastatin in 1987, statins have revolutionized the management of high cholesterol levels and significantly reduced the risk of cardiovascular events for millions of patients worldwide. The success of lovastatin led to the development of a series of other statins. From 1991 to 2003, companies such as Sankyo, MSD, Novartis, Pfizer, and AstraZeneca introduced pravastatin, simvastatin, fluvastatin, atorvastatin, and rosuvastatin. These developments established the United States as a world leader in this field and also represented significant progress for Japan. As the demand for CVD treatments intensified at the beginning of the 21st century, the emergence of complex hyperlipidemia spurred the development of other lipid-lowering medications. Sanofi and Amgen introduced alirocumab and evolocumab, respectively, which were designed to be used in conjunction with statins to further reduce lipid levels (Kühnast et al., 2014; Robinson et al., 2015b). Companies such as MSD, Sanofi, Regeneron Pharmaceuticals, Amgen, AstraZeneca, and Pfizer have played pivotal roles in advancing the development and application of lipid-lowering drugs, which is consistent with the results shown in our table. However, despite being one of the top three pharmaceutical companies in the world, Novartis ranks at the bottom, which we suspect is due to the recent introduction and limited uptake of inclisiran. The development of new targets for lipid-lowering therapies is also accelerating. For example, clinical trials for drugs targeting antisense oligonucleotides (ASO) and angiopoietin-like protein 3 (ANGPTL3) are currently being conducted by companies such as Ionis Pharmaceuticals and Regeneron Pharmaceuticals. As research progresses, these companies will continue to play a crucial role in advancing lipid management to prevent CHD.
4.4 Hotspots and frontiers in lipid-lowering therapy
Keyword analysis highlighted the research hotspots and core content in this field; however, understanding the latest lipid-lowering treatments in hyperlipidemia and CHD is equally important. Recent research has proposed several unconventional treatment methods, such as cuproptosis therapy, endoplasmic reticulum stress inhibition therapy, and therapies involving H2S or SO2. A study published by Yang et al. noted an increased presence of copper ions within atherosclerotic plaques; however, an excessive deficiency of copper ions can elevate cholesterol levels (Yang et al., 2023). These seemingly contradictory findings may be attributed to the impact of copper ions on endothelial inflammatory factors. They underscore the need for a balanced concentration of copper ions to effectively reduce the risk of atherosclerosis and hyperlipidemia (Málek et al., 2000; Habas and Shang, 2018). Furthermore, research by Keylani et al. indicated that endoplasmic reticulum stress is closely linked to the progression of atherosclerosis and lipid metabolism disorders, a phenomenon attributed to its induction of specific inflammatory responses (Huang et al., 2018; Keylani et al., 2022). Atorvastatin improves atherosclerosis and reduces cellular damage by inhibiting inflammatory responses (Yang and Hu, 2015). Meanwhile, a study by Song et al. describes a different approach, finding that sirtuin-1 improves atherosclerosis by regulating the acetylation of certain functional proteins, and treatment with H2S enhances its expression, deacetylation function, and stability, ultimately inhibiting the development and progression of CHD (Du et al., 2019; Song et al., 2023).
Despite these innovative treatment methods, the use of lipid-lowering drugs remains the primary strategy for treating hyperlipidemia and CHD. Current research continues to seek the optimal combination therapy for managing these conditions. Figure 10 depicts several commonly used lipid-lowering medications: statins, ezetimibe, fibrates, omega-3 fatty acids, niacin, bile acid sequestrants, antioxidants, and PCSK9 inhibitors. Among these, statins continue to form the cornerstone of lipid-lowering therapy. Their continued popularity is due not only to their significant clinical efficacy but also to the extensive research underlying their use and their multi-system therapeutic effects.
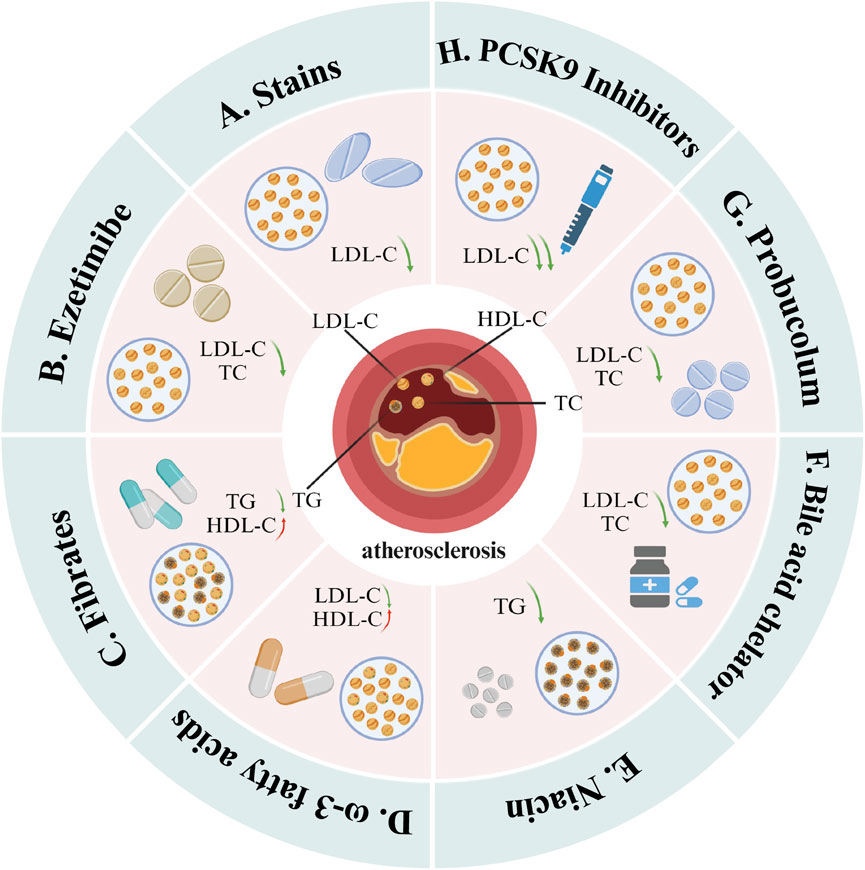
Figure 10. Mainstream drugs that can be used for lipid-lowering treatment. These include (A) statins, (B) ezetimibe, (C) fibrates, (D) ω-3 fatty acids, (E) niacin, (F) bile acid sequestrant, (G) antioxidant and (H) PCSK9 inhibitors. Among them, statins mainly reduce LDL-C levels; ezetimibe, bile acid sequestrants and antioxidants mainly reduce TC and LDL-C levels; fibrates and niacin mainly reduce TG levels, but fibrates also It has the effect of increasing HDL-C; ω-3 fatty acids can increase HDL-C levels and reduce LDL-C levels; PCSK9 inhibitors can significantly reduce LDL-C levels (Created with BioRender.com).
The advent of PCSK9 inhibitors marks a significant advancement in pharmacological lipid-lowering therapy, particularly for the treatment of primary hypercholesterolemia and the prevention of cardiovascular events. Evolocumab and alirocumab, as the first-generation PCSK9 inhibitors, are now widely used in patients with poor LDL-C control and acute coronary syndrome. The FOURIER trial conducted by O'Donoghue et al. demonstrated that long-term use of evolocumab can maintain LDL-C levels below 40 mg/dL, with good safety and tolerability profiles; furthermore, early treatment with evolocumab significantly reduced the risk of adverse cardiovascular events (O'Donoghue et al., 2022). Studies by Gencer et al. and Wiviott et al. confirmed this finding and indicated that evolocumab treatment results in a greater reduction in absolute risk in patients who recently experienced a myocardial infarction and are, therefore, at a higher risk of MACE (Gencer et al., 2020; Wiviott et al., 2020). For patients with CHD, the use of evolocumab significantly reduces the volume of atherosclerotic plaques and offers improved safety compared to the placebo group (Nicholls et al., 2016). Similarly, a post hoc analysis of the ODYSSEY OUTCOMES trial indicated that for patients who have recently been diagnosed with acute coronary syndrome, for whom the accumulation of metabolic risk factors is associated with an increased incidence of MACE, treatment with alirocumab reduces the incidence of MACE and demonstrates good tolerability across various subgroups (Ostadal et al., 2022).
The recently launched second-generation PCSK9 inhibitor, inclisiran, is still in the development phase. Although phase III clinical trials, ORION-10 and ORION-11, have further confirmed that it significantly lowers LDL-C levels, studies such as ORION-4, VICTORION-1 PREVENT, and VICTORION-2 PREVENT, which focus on the reduction of MACE rates, are yet to be completed. Additionally, due to its high treatment costs, inclisiran has not been widely adopted in current practice. However, with its unique advantage of requiring only biannual dosing, inclisiran should significantly improve patient compliance, and it may, therefore, have an important role to play in lipid management and CHD. Furthermore, it is worth noting that the research and development of third-generation PCSK9 inhibitors, such as MK-0616 and lerodalcibep, are actively underway. These are currently in phase III clinical trials and the post-research stage, respectively. The gradual publication of cardiovascular outcome studies and real-world research for these drugs should provide more comprehensive and detailed scientific evidence for their clinical use.
Finally, new-generation lipid-lowering drug targets, such as ASO and ANGPTL3, are gradually emerging. Drugs targeting these molecules are currently in phase II and III clinical trials, respectively, although further in-depth research is required. We expect that the publication of these trial results will further refine the treatment of dyslipidemia, ultimately reducing the risk of ASCVD events.
4.5 Advantages and limitations
This study represents a pioneering effort to chart the evolution, current trends, and emerging hotspots in lipid-lowering therapy for patients with CHD and hyperlipidemia. Utilizing tools such as VOSviewer, CiteSpace, and Pajek provides a comprehensive overview of the progression of the field, fosters a deeper understanding of research, and offers insights into notable scholars and their collaborative networks.
Nevertheless, this study has some limitations. First, it encompasses only literature from the SCI-E and SSCI indices in the Web of Science database, potentially overlooking studies in other databases. However, the Web of Science database is globally recognized for its extensive coverage and the high quality of its indexed journals, ensuring access to authoritative research (Zhang et al., 2020a; Zhang et al., 2020b). Furthermore, this study was confined to English language publications, potentially limiting its scope. Additionally, despite the search being conducted on 31 December 2023, newly published articles may not have been included. Finally, the selection of search terms might have introduced some bias; for example, the omission of certain lipid-lowering drugs or other terms for CHD and hyperlipidemia might have led to the exclusion of a small number of relevant studies. However, this bias is acceptable because our study aimed to elucidate the overall framework and development trajectory of research into lipid-lowering therapies for CHD in conjunction with hyperlipidemia. In future research, the comprehensiveness of our conclusions can be enhanced by expanding the list of search terms, extending the range of data sources, and incorporating a variety of analytical indicators (Wang et al., 2023).
5 Conclusion
Through bibliometric analysis, this study comprehensively mapped the landscape of lipid-lowering therapy research over the past 40 years, identifying influential countries, institutions, and authors. The notable surge in publications since 2003 underscores the widespread adoption of lipid-lowering therapy as the primary approach for treating and preventing CHD with hyperlipidemia. The evolution of therapeutic approaches reveals a distinct shift towards cutting-edge lipid-lowering agents, particularly PCSK9 inhibitors, and a strategic inclination towards combining these novel agents with statins or other drug classes. In the future, we anticipate a strong research emphasis on novel PCSK9 inhibitors and the development of safe and effective combination therapies. Future research endeavors should focus on pivotal areas such as “PCSK9 inhibitors,” “inclisiran,” “combination therapy,” “safety,” “ASO,” and “ANGPTL3”.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
QC: Conceptualization, Data curation, Formal Analysis, Methodology, Software, Validation, Visualization, Writing–original draft, Writing–review and editing. JS: Writing–original draft, Data curation, Formal Analysis, Investigation, Resources, Software. HZ: Data curation, Formal Analysis, Methodology, Software, Validation, Visualization, Writing–review and editing. ZW: Data curation, Investigation, Writing–original draft, Writing–review and editing. CL: Data curation, Methodology, Validation, Writing–review and editing. SZ: Formal Analysis, Investigation, Writing–review and editing. JD: Conceptualization, Funding acquisition, Methodology, Project administration, Resources, Supervision, Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research was funded by Shaanxi Social Development Funding (Grant No. 2017SF-134) and Shaanxi Science Funding (Grant No. 2020JQ-553) and Xi’an Jiaotong University Funding (Grant No. YXJLRH2022073).
Acknowledgments
We would like to thank Editage (www.editage.cn) for English language editing and BioRender (BioRender.com) for figures drawing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1393333/full#supplementary-material
References
Abifadel, M., Rabès, J. P., Devillers, M., Munnich, A., Erlich, D., Junien, C., et al. (2009). Mutations and polymorphisms in the proprotein convertase subtilisin kexin 9 (PCSK9) gene in cholesterol metabolism and disease. Hum. Mutat. 30 (4), 520–529. doi:10.1002/humu.20882
Abifadel, M., Varret, M., Rabès, J. P., Allard, D., Ouguerram, K., Devillers, M., et al. (2003). Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat. Genet. 34 (2), 154–156. doi:10.1038/ng1161
Al-Lamee, R. K., Nowbar, A. N., and Francis, D. P. (2019). Percutaneous coronary intervention for stable coronary artery disease. Heart 105 (1), 11–19. doi:10.1136/heartjnl-2017-312755
Almeida, S. O., and Budoff, M. (2019). Effect of statins on atherosclerotic plaque. Trends Cardiovasc. Med. 29 (8), 451–455. doi:10.1016/j.tcm.2019.01.001
Aung, T., Halsey, J., Kromhout, D., Gerstein, H. C., Marchioli, R., Tavazzi, L., et al. (2018). Associations of omega-3 fatty acid supplement use with cardiovascular disease risks: meta-analysis of 10 trials involving 77 917 individuals. JAMA Cardiol. 3 (3), 225–234. doi:10.1001/jamacardio.2017.5205
Avis, H. J., Hutten, B. A., Gagné, C., Langslet, G., McCrindle, B. W., Wiegman, A., et al. (2010). Efficacy and safety of rosuvastatin therapy for children with familial hypercholesterolemia. J. Am. Coll. Cardiol. 55 (11), 1121–1126. doi:10.1016/j.jacc.2009.10.042
Ayada, I., van Kleef, L. A., Zhang, H., Liu, K., Li, P., Abozaid, Y. J., et al. (2023). Dissecting the multifaceted impact of statin use on fatty liver disease: a multidimensional study. EBioMedicine 87, 104392. doi:10.1016/j.ebiom.2022.104392
Baigent, C., Keech, A., Kearney, P. M., Blackwell, L., Buck, G., Pollicino, C., et al. (2005). Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 366 (9493), 1267–1278. doi:10.1016/s0140-6736(05)67394-1
Bao, X., Liang, Y., Chang, H., Cai, T., Feng, B., Gordon, K., et al. (2024). Targeting proprotein convertase subtilisin/kexin type 9 (PCSK9): from bench to bedside. Signal Transduct. Target Ther. 9 (1), 13. doi:10.1038/s41392-023-01690-3
Bhatt, D. L., Steg, P. G., Miller, M., Brinton, E. A., Jacobson, T. A., Ketchum, S. B., et al. (2019). Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N. Engl. J. Med. 380 (1), 11–22. doi:10.1056/NEJMoa1812792
Blankenhorn, D. H., Azen, S. P., Crawford, D. W., Nessim, S. A., Sanmarco, M. E., Selzer, R. H., et al. (1991). EFFECTS OF COLESTIPOL-NIACIN THERAPY ON HUMAN FEMORAL ATHEROSCLEROSIS. Circulation 83 (2), 438–447. doi:10.1161/01.Cir.83.2.438
Boden, W. E., Probstfield, J. L., Anderson, T., Chaitman, B. R., Desvignes-Nickens, P., Koprowicz, K., et al. (2011). Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N. Engl. J. Med. 365 (24), 2255–2267. doi:10.1056/NEJMoa1107579
Bonaca, M. P., Nault, P., Giugliano, R. P., Keech, A. C., Pineda, A. L., Kanevsky, E., et al. (2018). Low-density lipoprotein cholesterol lowering with evolocumab and outcomes in patients with peripheral artery disease: insights from the FOURIER trial (further cardiovascular outcomes research with PCSK9 inhibition in subjects with elevated risk). Circulation 137 (4), 338–350. doi:10.1161/circulationaha.117.032235
Brown, B. G., Stukovsky, K. H., and Zhao, X. Q. (2006). Simultaneous low-density lipoprotein-C lowering and high-density lipoprotein-C elevation for optimum cardiovascular disease prevention with various drug classes, and their combinations: a meta-analysis of 23 randomized lipid trials. Curr. Opin. Lipidol. 17 (6), 631–636. doi:10.1097/MOL.0b013e32800ff750
Brown, B. G., Zhao, X. Q., Chait, A., Fisher, L. D., Cheung, M. C., Morse, J. S., et al. (2001). Simvastatin and niacin, antioxidant vitamins, or the combination for the prevention of coronary disease. N. Engl. J. Med. 345 (22), 1583–1592. doi:10.1056/NEJMoa011090
Bruckert, E., Giral, P., and Tellier, P. (2003). Perspectives in cholesterol-lowering therapy: the role of ezetimibe, a new selective inhibitor of intestinal cholesterol absorption. Circulation 107 (25), 3124–3128. doi:10.1161/01.Cir.0000072345.98581.24
Bruckert, E., Labreuche, J., and Amarenco, P. (2010). Meta-analysis of the effect of nicotinic acid alone or in combination on cardiovascular events and atherosclerosis. Atherosclerosis 210 (2), 353–361. doi:10.1016/j.atherosclerosis.2009.12.023
Brunner, D., Agmon, J., and Kaplinsky, E. (2000). Secondary prevention by raising HDL cholesterol and reducing triglycerides in patients with coronary artery disease. Circulation 102 (1), 21–27. doi:10.1161/01.cir.102.1.21
Calder, P. C. (2018). Very long-chain n-3 fatty acids and human health: fact, fiction and the future. Proc. Nutr. Soc. 77 (1), 52–72. doi:10.1017/s0029665117003950
Cannon, C. P., Blazing, M. A., Giugliano, R. P., McCagg, A., White, J. A., Theroux, P., et al. (2015). Ezetimibe added to statin therapy after acute coronary syndromes. N. Engl. J. Med. 372 (25), 2387–2397. doi:10.1056/NEJMoa1410489
Cannon, C. P., Braunwald, E., McCabe, C. H., Rader, D. J., Rouleau, J. L., Belder, R., et al. (2004). Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N. Engl. J. Med. 350 (15), 1495–1504. doi:10.1056/NEJMoa040583
Cesare, M. D., Bixby, H., Gaziano, T., Hadeed, L., Kabudula, C., McGhie, D. V., et al. (2022). The global burden of disease study at 30 years. Nat. Med. 28 (10), 2019–2026. doi:10.1038/s41591-022-01990-1
Chang, J. C., Chen, Y. J., Chen, I. C., Lin, W. S., Chen, Y. M., and Lin, C. H. (2021). Perinatal outcomes after statin exposure during pregnancy. JAMA Netw. Open 4 (12), e2141321. doi:10.1001/jamanetworkopen.2021.41321
Chaomei, C. (2005). CiteSpace II: detecting and visualizing emerging trends and transient patterns in scientific literature. J. Am. Soc. Inf. Sci. Technol. 57 (3), 359–377. doi:10.1002/asi.20317
Chen, C., Dubin, R., and Kim, M. C. (2014). Emerging trends and new developments in regenerative medicine: a scientometric update (2000 - 2014). Expert Opin. Biol. Ther. 14 (9), 1295–1317. doi:10.1517/14712598.2014.920813
Chen, C., Hu, Z., Liu, S., and Tseng, H. (2012). Emerging trends in regenerative medicine: a scientometric analysis in CiteSpace. Expert Opin. Biol. Ther. 12 (5), 593–608. doi:10.1517/14712598.2012.674507
Cohen, J. D., Brinton, E. A., Ito, M. K., and Jacobson, T. A. (2012). Understanding Statin Use in America and Gaps in Patient Education (USAGE): an internet-based survey of 10,138 current and former statin users. J. Clin. Lipidol. 6 (3), 208–215. doi:10.1016/j.jacl.2012.03.003
Collins, R., Armitage, J., Parish, S., Sleight, P., Peto, R., Meade, T., et al. (2002). MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet 360 (9326), 7–22. doi:10.1016/s0140-6736(02)09327-3
Dairou, F. (1994). Hypocholesterolemic statins. Evaluation and prospects. Presse Med. 23 (28), 1304–1310.
Dawson, L. P., Lum, M., Nerleker, N., Nicholls, S. J., and Layland, J. (2022). Coronary atherosclerotic plaque regression JACC state-of-the-art review. J. Am. Coll. Cardiol. 79 (1), 66–82. doi:10.1016/j.jacc.2021.10.035
Di Angelantonio, E., Gao, P., Pennells, L., Kaptoge, S., Caslake, M., Thompson, A., et al. (2012). Lipid-related markers and cardiovascular disease prediction. Jama 307 (23), 2499–2506. doi:10.1001/jama.2012.6571
Doenst, T., Thiele, H., Haasenritter, J., Wahlers, T., Massberg, S., and Haverich, A. (2022). The treatment of coronary artery disease. Dtsch. Arztebl Int. 119 (42), 716–723. doi:10.3238/arztebl.m2022.0277
Du, C., Lin, X., Xu, W., Zheng, F., Cai, J., Yang, J., et al. (2019). Sulfhydrated sirtuin-1 increasing its deacetylation activity is an essential epigenetics mechanism of anti-atherogenesis by hydrogen sulfide. Antioxid. Redox Signal 30 (2), 184–197. doi:10.1089/ars.2017.7195
Dyrbuś, K., Gąsior, M., Penson, P., Ray, K. K., and Banach, M. (2020). Inclisiran-New hope in the management of lipid disorders? J. Clin. Lipidol. 14 (1), 16–27. doi:10.1016/j.jacl.2019.11.001
Ekinci, S., Agilli, M., Ersen, O., and Ekinci, G. H. (2015). Letter to the editor regarding analysis of changing paradigms of management in 179 patients with spinal tuberculosis during a 12-year period and proposal of a new management algorithm. World Neurosurg. 84 (6), 2072. doi:10.1016/j.wneu.2014.12.003
Elam, M. B., Ginsberg, H. N., Lovato, L. C., Corson, M., Largay, J., Leiter, L. A., et al. (2017). Association of fenofibrate therapy with long-term cardiovascular risk in statin-treated patients with type 2 diabetes. JAMA Cardiol. 2 (4), 370–380. doi:10.1001/jamacardio.2016.4828
Epstein, S. E., Waksman, R., Pichard, A. D., Kent, K. M., and Panza, J. A. (2013). Percutaneous coronary intervention versus medical therapy in stable coronary artery disease: the unresolved conundrum. JACC Cardiovasc Interv. 6 (10), 993–998. doi:10.1016/j.jcin.2013.07.003
Ference, B. A., Ginsberg, H. N., Graham, I., Ray, K. K., Packard, C. J., Bruckert, E., et al. (2017). Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 38 (32), 2459–2472. doi:10.1093/eurheartj/ehx144
Fitzgerald, K., White, S., Borodovsky, A., Bettencourt, B. R., Strahs, A., Clausen, V., et al. (2017). A highly durable RNAi therapeutic inhibitor of PCSK9. N. Engl. J. Med. 376 (1), 41–51. doi:10.1056/NEJMoa1609243
Gagné, C., Gaudet, D., and Bruckert, E.Ezetimibe Study Group (2002). Efficacy and safety of ezetimibe coadministered with atorvastatin or simvastatin in patients with homozygous familial hypercholesterolemia. Circulation 105 (21), 2469–2475. doi:10.1161/01.cir.0000018744.58460.62
Ganda, O. P., Bhatt, D. L., Mason, R. P., Miller, M., and Boden, W. E. (2018). Unmet need for adjunctive dyslipidemia therapy in hypertriglyceridemia management. J. Am. Coll. Cardiol. 72 (3), 330–343. doi:10.1016/j.jacc.2018.04.061
Gao, Y., Wu, Z., Chen, Y., Shang, G., Zeng, Y., and Gao, Y. (2023). A global bibliometric and visualized analysis of the links between the autophagy and acute myeloid leukemia. Front. Pharmacol. 14, 1291195. doi:10.3389/fphar.2023.1291195
Gencer, B., Mach, F., Murphy, S. A., De Ferrari, G. M., Huber, K., Lewis, B. S., et al. (2020). Efficacy of evolocumab on cardiovascular outcomes in patients with recent myocardial infarction: a prespecified secondary analysis from the FOURIER trial. JAMA Cardiol. 5 (8), 952–957. doi:10.1001/jamacardio.2020.0882
Ginsberg, H. N., Elam, M. B., Lovato, L. C., Crouse, J. R., Leiter, L. A., Linz, P., et al. (2010). Effects of combination lipid therapy in type 2 diabetes mellitus. N. Engl. J. Med. 362 (17), 1563–1574. doi:10.1056/NEJMoa1001282
Goodman, S. G., Aylward, P. E., Szarek, M., Chumburidze, V., Bhatt, D. L., Bittner, V. A., et al. (2019). Effects of alirocumab on cardiovascular events after coronary bypass surgery. J. Am. Coll. Cardiol. 74 (9), 1177–1186. doi:10.1016/j.jacc.2019.07.015
Gross, B., Pawlak, M., Lefebvre, P., and Staels, B. (2017). PPARs in obesity-induced T2DM, dyslipidaemia and NAFLD. Nat. Rev. Endocrinol. 13 (1), 36–49. doi:10.1038/nrendo.2016.135
Grundy, S. M., Stone, N. J., Bailey, A. L., Beam, C., Birtcher, K. K., Blumenthal, R. S., et al. (2019). 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: executive summary: a report of the American College of Cardiology/American heart association task force on clinical practice guidelines. J. Am. Coll. Cardiol. 73 (24), 3168–3209. doi:10.1016/j.jacc.2018.11.002
Habas, K., and Shang, L. (2018). Alterations in intercellular adhesion molecule 1 (ICAM-1) and vascular cell adhesion molecule 1 (VCAM-1) in human endothelial cells. Tissue Cell. 54, 139–143. doi:10.1016/j.tice.2018.09.002
Hadjiphilippou, S., and Ray, K. K. (2017). Evolocumab and clinical outcomes in patients with cardiovascular disease. J. R. Coll. Physicians Edinb 47 (2), 153–155. doi:10.4997/jrcpe.2017.212
Haynes, R., Jiang, L., Hopewell, J. C., Li, J., Chen, F., Parish, S., et al. (2013). HPS2-THRIVE randomized placebo-controlled trial in 25 673 high-risk patients of ER niacin/laropiprant: trial design, pre-specified muscle and liver outcomes, and reasons for stopping study treatment. Eur. Heart J. 34 (17), 1279–1291. doi:10.1093/eurheartj/eht055
Het Panhuis, W., Schönke, M., Modder, M., Tom, H. E., Lalai, R. A., Pronk, A. C. M., et al. (2023). Time-restricted feeding attenuates hypercholesterolaemia and atherosclerosis development during circadian disturbance in APOE∗3-Leiden.CETP mice. EBioMedicine 93, 104680. doi:10.1016/j.ebiom.2023.104680
Hirsch, J. E. (2007). Does the H index have predictive power? Proc. Natl. Acad. Sci. U. S. A. 104 (49), 19193–19198. doi:10.1073/pnas.0707962104
Hong, S. J., Lee, Y. J., Lee, S. J., Hong, B. K., Kang, W. C., Lee, J. Y., et al. (2023). Treat-to-Target or high-intensity statin in patients with coronary artery disease: a randomized clinical trial. Jama 329 (13), 1078–1087. doi:10.1001/jama.2023.2487
Huang, A., Patel, S., McAlpine, C. S., and Werstuck, G. H. (2018). The role of endoplasmic reticulum stress-glycogen synthase kinase-3 signaling in atherogenesis. Int. J. Mol. Sci. 19 (6), 1607. doi:10.3390/ijms19061607
Imke, C., Rodriguez, B. L., Grove, J. S., McNamara, J. R., Waslien, C., Katz, A. R., et al. (2005). Are remnant-like particles independent predictors of coronary heart disease incidence? The Honolulu Heart study. Arterioscler. Thromb. Vasc. Biol. 25 (8), 1718–1722. doi:10.1161/01.ATV.0000173310.85845.7b
Joseph, P., Leong, D., McKee, M., Anand, S. S., Schwalm, J. D., Teo, K., et al. (2017). Reducing the global burden of cardiovascular disease, Part 1: the epidemiology and risk factors. Circ. Res. 121 (6), 677–694. doi:10.1161/circresaha.117.308903
Jukema, J. W., Zijlstra, L. E., Bhatt, D. L., Bittner, V. A., Diaz, R., Drexel, H., et al. (2019). Effect of alirocumab on stroke in ODYSSEY OUTCOMES. Circulation 140 (25), 2054–2062. doi:10.1161/circulationaha.119.043826
Kannel, W. B., Castelli, W. P., and Gordon, T. (1979). Cholesterol in the prediction of atherosclerotic disease. New perspectives based on the Framingham study. Ann. Intern Med. 90 (1), 85–91. doi:10.7326/0003-4819-90-1-85
Kannel, W. B., Dawber, T. R., Kagan, A., Revotskie, N., and Stokes, J. (1961). Factors of risk in the development of coronary heart disease--six year follow-up experience. The Framingham Study. Ann. Intern Med. 55, 33–50. doi:10.7326/0003-4819-55-1-33
Karpe, F., Boquist, S., Tang, R., Bond, G. M., de Faire, U., and Hamsten, A. (2001). Remnant lipoproteins are related to intima-media thickness of the carotid artery independently of LDL cholesterol and plasma triglycerides. J. Lipid Res. 42 (1), 17–21. doi:10.1016/s0022-2275(20)32331-2
Keylani, K., Arbab Mojeni, F., Khalaji, A., Rasouli, A., Aminzade, D., Karimi, M. A., et al. (2022). Endoplasmic reticulum as a target in cardiovascular diseases: is there a role for flavonoids? Front. Pharmacol. 13, 1027633. doi:10.3389/fphar.2022.1027633
Keys, A., Menotti, A., Aravanis, C., Blackburn, H., Djordevic, B. S., Buzina, R., et al. (1984). The seven countries study: 2,289 deaths in 15 years. Prev. Med. 13 (2), 141–154. doi:10.1016/0091-7435(84)90047-1
Khankari, N. K., Keaton, J. M., Walker, V. M., Lee, K. M., Shuey, M. M., Clarke, S. L., et al. (2022). Using Mendelian randomisation to identify opportunities for type 2 diabetes prevention by repurposing medications used for lipid management. EBioMedicine 80, 104038. doi:10.1016/j.ebiom.2022.104038
Kim, H. S., Xiao, C., Wang, R. H., Lahusen, T., Xu, X., Vassilopoulos, A., et al. (2010). Hepatic-specific disruption of SIRT6 in mice results in fatty liver formation due to enhanced glycolysis and triglyceride synthesis. Cell. Metab. 12 (3), 224–236. doi:10.1016/j.cmet.2010.06.009
Kim, N. H., and Kim, S. G. (2020). Fibrates revisited: potential role in cardiovascular risk reduction. Diabetes Metab. J. 44 (2), 213–221. doi:10.4093/dmj.2020.0001
Kovach, C. P., Mesenbring, E. C., Gupta, P., Glorioso, T. J., Ho, P. M., Waldo, S. W., et al. (2023). Projected outcomes of optimized statin and ezetimibe therapy in US military veterans with coronary artery disease. Jama Netw. Open 6 (8), e2329066. doi:10.1001/jamanetworkopen.2023.29066
Kugiyama, K., Doi, H., Takazoe, K., Kawano, H., Soejima, H., Mizuno, Y., et al. (1999). Remnant lipoprotein levels in fasting serum predict coronary events in patients with coronary artery disease. Circulation 99 (22), 2858–2860. doi:10.1161/01.cir.99.22.2858
Kühnast, S., van der Hoorn, J. W. A., Pieterman, E. J., van den Hoek, A. M., Sasiela, W. J., Gusarova, V., et al. (2014). Alirocumab inhibits atherosclerosis, improves the plaque morphology, and enhances the effects of a statin. J. Lipid Res. 55 (10), 2103–2112. doi:10.1194/jlr.M051326
Larsson, S. C., Kumlin, M., Ingelman-Sundberg, M., and Wolk, A. (2004). Dietary long-chain n-3 fatty acids for the prevention of cancer: a review of potential mechanisms. Am. J. Clin. Nutr. 79 (6), 935–945. doi:10.1093/ajcn/79.6.935
Lee, S. H., Lee, Y. J., Heo, J. H., Hur, S. H., Choi, H. H., Kim, K. J., et al. (2023a). Combination moderate-intensity statin and ezetimibe therapy for elderly patients with atherosclerosis. J. Am. Coll. Cardiol. 81 (14), 1339–1349. doi:10.1016/j.jacc.2023.02.007
Lee, S. J., Cha, J. J., Choi, W. G., Lee, W. S., Jeong, J. O., Choi, S., et al. (2023b). Moderate-intensity statin with ezetimibe combination therapy vs high-intensity statin monotherapy in patients at very high risk of atherosclerotic cardiovascular disease A post hoc analysis from the racing randomized clinical trial. Jama Cardiol. 8 (9), 853–858. doi:10.1001/jamacardio.2023.2222
Lee, S. J., Joo, J. H., Park, S., Kim, C., Choi, D. W., Hong, S. J., et al. (2023c). Combination lipid-lowering therapy in patients undergoing percutaneous coronary intervention. J. Am. Coll. Cardiol. 82 (5), 401–410. doi:10.1016/j.jacc.2023.05.042
Lewington, S., Whitlock, G., Clarke, R., Sherliker, P., Emberson, J., Halsey, J., et al. (2007). Blood cholesterol and vascular mortality by age, sex, and blood pressure: a meta-analysis of individual data from 61 prospective studies with 55,000 vascular deaths. Lancet 370 (9602), 1829–1839. doi:10.1016/s0140-6736(07)61778-4
Li, Z., Zhang, B., Liu, Q., Tao, Z., Ding, L., Guo, B., et al. (2023). Genetic association of lipids and lipid-lowering drug target genes with non-alcoholic fatty liver disease. EBioMedicine 90, 104543. doi:10.1016/j.ebiom.2023.104543
Lidgard, B., Bansal, N., Zelnick, L. R., Hoofnagle, A. N., Fretts, A. M., Longstreth, W. T., et al. (2023). Evaluation of plasma sphingolipids as mediators of the relationship between kidney disease and cardiovascular events. EBioMedicine 95, 104765. doi:10.1016/j.ebiom.2023.104765
Liew, T. V., and Ray, K. K. (2008). Intensive statin therapy in acute coronary syndromes. Curr. Atheroscler. Rep. 10 (2), 158–163. doi:10.1007/s11883-008-0023-1
Lin, X. L., Xiao, L. L., Tang, Z. H., Jiang, Z. S., and Liu, M. H. (2018). Role of PCSK9 in lipid metabolism and atherosclerosis. Biomed. Pharmacother. 104, 36–44. doi:10.1016/j.biopha.2018.05.024
Lou, B. W., Liu, H., Luo, Y. B., Jiang, G. T., Wu, H. Y., Wang, C., et al. (2022). In-hospital initiation of PCSK9 inhibitor and short-term lipid control in patients with acute myocardial infarction. Lipids Health Dis. 21 (1), 105. doi:10.1186/s12944-022-01724-9
Luukkonen, T. (1990). Bibliometrics and evaluation of research performance. Ann. Med. 22 (3), 145–150. doi:10.3109/07853899009147259
Madasamy, S., Shivasubramani, U., Villanueva, J., Bigos, M., Amento, E. P., and Wu, A. H. (2016). Nonenzymatic mechanism of statins in modulating cholesterol particles formation. Am. J. Cardiol. 118 (8), 1187–1193. doi:10.1016/j.amjcard.2016.07.035
Málek, F., Karel, I., Polásek, R., Spacek, R., Lisa, L., Dvorák, J., et al. (2000). Serum copper levels in patients with acute and chronic types of ischemic heart disease and its relation to lipoprotein levels and extent of coronary atherosclerosis. Vnitr Lek. 46 (10), 693–696.
Martin, S. S., Aday, A. W., Almarzooq, Z. I., Anderson, C. A. M., Arora, P., Avery, C. L., et al. (2024). 2024 heart disease and stroke statistics: a report of us and global data from the American heart association. Circulation 149 (8), e347–e913. doi:10.1161/cir.0000000000001209
Meng, P. N., Nong, J. C., Xu, Y., You, W., Xu, T., Wu, X. Q., et al. (2023). Morphologies and composition changes in nonculprit subclinical atherosclerosis in diabetic versus nondiabetic patients with acute coronary syndrome who underwent long-term statin therapy. Sci. Rep. 13 (1), 5338. doi:10.1038/s41598-023-32638-w
Mensah, G. A., Fuster, V., Murray, C. J. L., and Roth, G. A.Global Burden of Cardiovascular Diseases and Risks Collaborators (2023). Global burden of cardiovascular diseases and risks, 1990-2022. J. Am. Coll. Cardiol. 82 (25), 2350–2473. doi:10.1016/j.jacc.2023.11.007
Miller, M., Stone, N. J., Ballantyne, C., Bittner, V., Criqui, M. H., Ginsberg, H. N., et al. (2011). Triglycerides and cardiovascular disease: a scientific statement from the American Heart Association. Circulation 123 (20), 2292–2333. doi:10.1161/CIR.0b013e3182160726
Morales, D., Chung, N., Zhu, J. R., Sangwatanaroj, S., Yin, W. H., Lee, K., et al. (2004). Efficacy and safety of simvastatin in Asian and non-Asian coronary heart disease patients: a comparison of the GOALLS and STATT studies. Curr. Med. Res. Opin. 20 (8), 1235–1243. doi:10.1185/030079904125004367
Mortensen, M. B., Dzaye, O., Bøtker, H. E., Jensen, J. M., Maeng, M., Bentzon, J. F., et al. (2023). Low-density lipoprotein cholesterol is predominantly associated with atherosclerotic cardiovascular disease events in patients with evidence of coronary atherosclerosis: the western Denmark heart registry. Circulation 147 (14), 1053–1063. doi:10.1161/circulationaha.122.061010
Mozaffarian, D., and Wu, J. H. (2011). Omega-3 fatty acids and cardiovascular disease: effects on risk factors, molecular pathways, and clinical events. J. Am. Coll. Cardiol. 58 (20), 2047–2067. doi:10.1016/j.jacc.2011.06.063
Naghavi, M., Ong, K. L., Aali, A., Ababneh, H. S., Abate, Y. H., Abbafati, C., et al. (2024). Global burden of 288 causes of death and life expectancy decomposition in 204 countries and territories and 811 subnational locations, 1990-2021: a systematic analysis for the Global Burden of Disease Study 2021. Lancet. doi:10.1016/s0140-6736(24)00367-2
Nair, J. K., Willoughby, J. L., Chan, A., Charisse, K., Alam, M. R., Wang, Q., et al. (2014). Multivalent N-acetylgalactosamine-conjugated siRNA localizes in hepatocytes and elicits robust RNAi-mediated gene silencing. J. Am. Chem. Soc. 136 (49), 16958–16961. doi:10.1021/ja505986a
Nakao, K., Noguchi, T., Miura, H., Asaumi, Y., Morita, Y., Takeuchi, S., et al. (2023). Effect of eicosapentaenoic acid/docosahexaenoic acid on coronary high-intensity plaques detected using noncontrast T1-weighted imaging: the AQUAMARINE EPA/DHA randomized study. J. Atheroscler. Thrombosis 13, 122–134. doi:10.5551/jat.64063
Nicholls, S. J., Ballantyne, C. M., Barter, P. J., Chapman, M. J., Erbel, R. M., Libby, P., et al. (2011). Effect of two intensive statin regimens on progression of coronary disease. N. Engl. J. Med. 365 (22), 2078–2087. doi:10.1056/NEJMoa1110874
Nicholls, S. J., Puri, R., Anderson, T., Ballantyne, C. M., Cho, L., Kastelein, J. J., et al. (2016). Effect of evolocumab on progression of coronary disease in statin-treated patients: the GLAGOV randomized clinical trial. Jama 316 (22), 2373–2384. doi:10.1001/jama.2016.16951
Nissen, S. E., Tuzcu, E. M., Schoenhagen, P., Brown, B. G., Ganz, P., Vogel, R. A., et al. (2004). Effect of intensive compared with moderate lipid-lowering therapy on progression of coronary atherosclerosis: a randomized controlled trial. Jama 291 (9), 1071–1080. doi:10.1001/jama.291.9.1071
Nordestgaard, B. G. (2016a). Triglyceride-rich lipoproteins and atherosclerotic cardiovascular disease new insights from epidemiology, genetics, and biology. Circulation Res. 118 (4), 547–563. doi:10.1161/circresaha.115.306249
Nowbar, A. N., Gitto, M., Howard, J. P., Francis, D. P., and Al-Lamee, R. (2019). Mortality from ischemic heart disease analysis of data from the world health organization and coronary artery disease risk factors from NCD risk factor collaboration. Circulation-Cardiovascular Qual. Outcomes 12 (6), 11. doi:10.1161/circoutcomes.118.005375
Nutescu, E. A., and Shapiro, N. L. (2003). Ezetimibe: a selective cholesterol absorption inhibitor. Pharmacotherapy 23 (11), 1463–1474. doi:10.1592/phco.23.14.1463.31942
O'Donoghue, M. L., Fazio, S., Giugliano, R. P., Stroes, E. S. G., Kanevsky, E., Gouni-Berthold, I., et al. (2019). Lipoprotein(a), PCSK9 inhibition, and cardiovascular risk. Circulation 139 (12), 1483–1492. doi:10.1161/circulationaha.118.037184
O'Donoghue, M. L., Giugliano, R. P., Wiviott, S. D., Atar, D., Keech, A., Kuder, J. F., et al. (2022). Long-term evolocumab in patients with established atherosclerotic cardiovascular disease. Circulation 146 (15), 1109–1119. doi:10.1161/circulationaha.122.061620
Ostadal, P., Steg, P. G., Poulouin, Y., Bhatt, D. L., Bittner, V. A., Chua, T., et al. (2022). Metabolic risk factors and effect of alirocumab on cardiovascular events after acute coronary syndrome: a post-hoc analysis of the ODYSSEY OUTCOMES randomised controlled trial. Lancet Diabetes Endocrinol. 10 (5), 330–340. doi:10.1016/s2213-8587(22)00043-2
Paradela-Dobarro, B., Raposeiras-Roubín, S., Rodiño-Janeiro, B. K., Grigorian-Shamagian, L., García-Acuña, J. M., Aguiar-Souto, P., et al. (2013). Statins modulate feedback regulation mechanisms between advanced glycation end-products and C-reactive protein: evidence in patients with acute myocardial infarction. Eur. J. Pharm. Sci. 49 (4), 512–518. doi:10.1016/j.ejps.2013.05.001
Parish, S., Offer, A., Clarke, R., Hopewell, J. C., Hill, M. R., Otvos, J. D., et al. (2012). Lipids and lipoproteins and risk of different vascular events in the MRC/BHF Heart Protection Study. Circulation 125 (20), 2469–2478. doi:10.1161/circulationaha.111.073684
Park, J. I., Lee, S. J., Hong, B. K., Cho, Y. H., Shin, W. Y., Lim, S. W., et al. (2023). Efficacy and safety of moderate-intensity statin with ezetimibe combination therapy in patients after percutaneous coronary intervention: a post-hoc analysis of the RACING trial. Eclinicalmedicine 58, 101933. doi:10.1016/j.eclinm.2023.101933
Pedersen, T. R., Kjekshus, J., Berg, K., Haghfelt, T., Faergeman, O., Thorgeirsson, G., et al. (1994). Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 344 (8934), 1383–1389. doi:10.1016/s0140-6736(94)90566-5
Perreault, L., Bergman, B. C., Hunerdosse, D. M., Howard, D. J., and Eckel, R. H. (2011). Fenofibrate administration does not affect muscle triglyceride concentration or insulin sensitivity in humans. Metabolism 60 (8), 1107–1114. doi:10.1016/j.metabol.2010.12.003
Phan, B. A., and Toth, P. P. (2013). Is the future of statins aligned with new novel lipid modulation therapies? Curr. Atheroscler. Rep. 15 (2), 300. doi:10.1007/s11883-012-0300-x
Piper, D. E., Jackson, S., Liu, Q., Romanow, W. G., Shetterly, S., Thibault, S. T., et al. (2007). The crystal structure of PCSK9: a regulator of plasma LDL-cholesterol. Structure 15 (5), 545–552. doi:10.1016/j.str.2007.04.004
Quispe, R., Manalac, R. J., Faridi, K. F., Blaha, M. J., Toth, P. P., Kulkarni, K. R., et al. (2015). Relationship of the triglyceride to high-density lipoprotein cholesterol (TG/HDL-C) ratio to the remainder of the lipid profile: the Very Large Database of Lipids-4 (VLDL-4) study. Atherosclerosis 242 (1), 243–250. doi:10.1016/j.atherosclerosis.2015.06.057
Raal, F. J., Kallend, D., Ray, K. K., Turner, T., Koenig, W., Wright, R. S., et al. (2020). Inclisiran for the treatment of heterozygous familial hypercholesterolemia. N. Engl. J. Med. 382 (16), 1520–1530. doi:10.1056/NEJMoa1913805
Räber, L., Ueki, Y., Otsuka, T., Losdat, S., Häner, J. D., Lonborg, J., et al. (2022a). Effect of alirocumab added to high-intensity statin therapy on coronary atherosclerosis in patients with acute myocardial infarction the PACMAN-AMI randomized clinical trial. Jama-Journal Am. Med. Assoc. 327 (18), 1771–1781. doi:10.1001/jama.2022.5218
Räber, L., Ueki, Y., Otsuka, T., Losdat, S., Häner, J. D., Lonborg, J., et al. (2022b). Effect of alirocumab added to high-intensity statin therapy on coronary atherosclerosis in patients with acute myocardial infarction: the PACMAN-AMI randomized clinical trial. Jama 327 (18), 1771–1781. doi:10.1001/jama.2022.5218
Ray, K. K., and Cannon, C. P. (2004). Intensive statin therapy in acute coronary syndromes: clinical benefits and vascular biology. Curr. Opin. Lipidol. 15 (6), 637–643. doi:10.1097/00041433-200412000-00003
Ray, K. K., and Cannon, C. P. (2006). Intensive statin therapy for treating acute coronary syndromes. Expert Opin. Investig. Drugs 15 (10), 1151–1159. doi:10.1517/13543784.15.10.1151
Ray, K. K., Colhoun, H. M., Szarek, M., Baccara-Dinet, M., Bhatt, D. L., Bittner, V. A., et al. (2019). Effects of alirocumab on cardiovascular and metabolic outcomes after acute coronary syndrome in patients with or without diabetes: a prespecified analysis of the ODYSSEY OUTCOMES randomised controlled trial. Lancet Diabetes and Endocrinol. 7 (8), 618–628. doi:10.1016/s2213-8587(19)30158-5
Ray, K. K., Wright, R. S., Kallend, D., Koenig, W., Leiter, L. A., Raal, F. J., et al. (2020). Two phase 3 trials of inclisiran in patients with elevated LDL cholesterol. N. Engl. J. Med. 382 (16), 1507–1519. doi:10.1056/NEJMoa1912387
Robins, S. J., Collins, D., Wittes, J. T., Papademetriou, V., Deedwania, P. C., Schaefer, E. J., et al. (2001). Relation of gemfibrozil treatment and lipid levels with major coronary events: VA-HIT: a randomized controlled trial. Jama 285 (12), 1585–1591. doi:10.1001/jama.285.12.1585
Robinson, J. G., Farnier, M., Krempf, M., Bergeron, J., Luc, G., Averna, M., et al. (2015a). Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N. Engl. J. Med. 372 (16), 1489–1499. doi:10.1056/NEJMoa1501031
Robinson, J. G., Farnier, M., Krempf, M., Bergeron, J., Luc, G., Averna, M., et al. (2015b). Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N. Engl. J. Med. 372 (16), 1489–1499. doi:10.1056/NEJMoa1501031
Sabatine, M. S., Giugliano, R. P., Keech, A. C., Honarpour, N., Wiviott, S. D., Murphy, S. A., et al. (2017). Evolocumab and clinical outcomes in patients with cardiovascular disease. N. Engl. J. Med. 376 (18), 1713–1722. doi:10.1056/NEJMoa1615664
Sacks, F. M., Pfeffer, M. A., Moye, L. A., Rouleau, J. L., Rutherford, J. D., Cole, T. G., et al. (1996). The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. N. Engl. J. Med. 335 (14), 1001–1009. doi:10.1056/nejm199610033351401
Schwartz, G. G., Steg, P. G., Szarek, M., Bhatt, D. L., Bittner, V. A., Diaz, R., et al. (2018). Alirocumab and cardiovascular outcomes after acute coronary syndrome. N. Engl. J. Med. 379 (22), 2097–2107. doi:10.1056/NEJMoa1801174
Schwartz, G. G., Szarek, M., Bhatt, D. L., Bittner, V. A., Bujas-Bobanovic, M., Diaz, R., et al. (2023). Transiently achieved very low LDL-cholesterol levels by statin and alirocumab after acute coronary syndrome are associated with cardiovascular risk reduction: the ODYSSEY OUTCOMES trial. Eur. Heart J. 10. doi:10.1093/eurheartj/ehad144
Scott, R., O'Brien, R., Fulcher, G., Pardy, C., D'Emden, M., Tse, D., et al. (2009). Effects of fenofibrate treatment on cardiovascular disease risk in 9,795 individuals with type 2 diabetes and various components of the metabolic syndrome: the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study. Diabetes Care 32 (3), 493–498. doi:10.2337/dc08-1543
Shepherd, J., Blauw, G. J., Murphy, M. B., Bollen, E. L., Buckley, B. M., Cobbe, S. M., et al. (2002). Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet 360 (9346), 1623–1630. doi:10.1016/s0140-6736(02)11600-x
Skålén, K., Gustafsson, M., Rydberg, E. K., Hultén, L. M., Wiklund, O., Innerarity, T. L., et al. (2002). Subendothelial retention of atherogenic lipoproteins in early atherosclerosis. Nature 417 (6890), 750–754. doi:10.1038/nature00804
Song, Y., Xu, Z., Zhong, Q., Zhang, R., Sun, X., and Chen, G. (2023). Sulfur signaling pathway in cardiovascular disease. Front. Pharmacol. 14, 1303465. doi:10.3389/fphar.2023.1303465
Soppert, J., Lehrke, M., Marx, N., Jankowski, J., and Noels, H. (2020). Lipoproteins and lipids in cardiovascular disease: from mechanistic insights to therapeutic targeting. Adv. Drug Deliv. Rev. 159, 4–33. doi:10.1016/j.addr.2020.07.019
Steg, P. G., Szarek, M., Bhatt, D. L., Bittner, V. A., Brégeault, M. F., Dalby, A. J., et al. (2019). Effect of alirocumab on mortality after acute coronary syndromes. Circulation 140 (2), 103–112. doi:10.1161/circulationaha.118.038840
Stein, E., Stender, S., Mata, P., Sager, P., Ponsonnet, D., Melani, L., et al. (2004). Achieving lipoprotein goals in patients at high risk with severe hypercholesterolemia: efficacy and safety of ezetimibe co-administered with atorvastatin. Am. Heart J. 148 (3), 447–455. doi:10.1016/j.ahj.2004.03.052
Stone, P. H., Libby, P., and Boden, W. E. (2023). Fundamental pathobiology of coronary atherosclerosis and clinical implications for chronic ischemic heart disease management-the plaque hypothesis A narrative review. Jama Cardiol. 8 (2), 192–201. doi:10.1001/jamacardio.2022.3926
Szarek, M., White, H. D., Schwartz, G. G., Alings, M., Bhatt, D. L., Bittner, V. A., et al. (2019). Alirocumab reduces total nonfatal cardiovascular and fatal events the ODYSSEY OUTCOMES trial. J. Am. Coll. Cardiol. 73 (4), 387–396. doi:10.1016/j.jacc.2018.10.039
Tenenbaum, A., and Fisman, E. Z. (2012). Fibrates are an essential part of modern anti-dyslipidemic arsenal: spotlight on atherogenic dyslipidemia and residual risk reduction. Cardiovasc Diabetol. 11, 125. doi:10.1186/1475-2840-11-125
Toth, P. P., Fazio, S., Wong, N. D., Hull, M., and Nichols, G. A. (2020). Risk of cardiovascular events in patients with hypertriglyceridaemia: a review of real-world evidence. Diabetes Obes. Metab. 22 (3), 279–289. doi:10.1111/dom.13921
Toth, P. P., Harper, C. R., and Jacobson, T. A. (2008). Clinical characterization and molecular mechanisms of statin myopathy. Expert Rev. Cardiovasc Ther. 6 (7), 955–969. doi:10.1586/14779072.6.7.955
Tran, B. X., Pham, T. V., Ha, G. H., Ngo, A. T., Nguyen, L. H., Vu, T. T. M., et al. (2018). A bibliometric analysis of the global research trend in child maltreatment. Int. J. Environ. Res. Public Health 15 (7), 1456. doi:10.3390/ijerph15071456
Tsao, C. W., Aday, A. W., Almarzooq, Z. I., Anderson, C. A. M., Arora, P., Avery, C. L., et al. (2023). Heart disease and stroke statistics-2023 update: a report from the American heart association. Circulation 147 (8), e93–e621. doi:10.1161/cir.0000000000001123
Urban, D., Pöss, J., Böhm, M., and Laufs, U. (2013). Targeting the proprotein convertase subtilisin/kexin type 9 for the treatment of dyslipidemia and atherosclerosis. J. Am. Coll. Cardiol. 62 (16), 1401–1408. doi:10.1016/j.jacc.2013.07.056
van Eck, N. J., and Waltman, L. (2010). Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics 84 (2), 523–538. doi:10.1007/s11192-009-0146-3
van Eck, N. J., and Waltman, L. (2017). Citation-based clustering of publications using CitNetExplorer and VOSviewer. Scientometrics 111 (2), 1053–1070. doi:10.1007/s11192-017-2300-7
van Rosendael, A. R., van den Hoogen, I. J., Gianni, U., Ma, X., Tantawy, S. W., Bax, A. M., et al. (2021). Association of statin treatment with progression of coronary atherosclerotic plaque composition. JAMA Cardiol. 6 (11), 1257–1266. doi:10.1001/jamacardio.2021.3055
Vergallo, R., and Crea, F. (2020). Atherosclerotic plaque healing. N. Engl. J. Med. 383 (9), 846–857. doi:10.1056/NEJMra2000317
Visseren, F. L. J., Mach, F., Smulders, Y. M., Carballo, D., Koskinas, K. C., Bäck, M., et al. (2021). 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur. Heart J. 42 (34), 3227–3337. doi:10.1093/eurheartj/ehab484
Wang, J., Zhao, W., Zhang, Z., Liu, X., Xie, T., Wang, L., et al. (2023). A journey of challenges and victories: a bibliometric worldview of nanomedicine since the 21st century. Adv. Mater 36, e2308915. doi:10.1002/adma.202308915
White, H. D., Steg, P. G., Szarek, M., Bhatt, D. L., Bittner, V. A., Diaz, R., et al. (2019). Effects of alirocumab on types of myocardial infarction: insights from the ODYSSEY OUTCOMES trial. Eur. Heart J. 40 (33), 2801–2809. doi:10.1093/eurheartj/ehz299
Wiviott, S. D., Giugliano, R. P., Morrow, D. A., De Ferrari, G. M., Lewis, B. S., Huber, K., et al. (2020). Effect of evolocumab on type and size of subsequent myocardial infarction: a prespecified analysis of the FOURIER randomized clinical trial. JAMA Cardiol. 5 (7), 787–793. doi:10.1001/jamacardio.2020.0764
Wright, R. S., Collins, M. G., Stoekenbroek, R. M., Robson, R., Wijngaard, P. L. J., Landmesser, U., et al. (2020). Effects of renal impairment on the pharmacokinetics, efficacy, and safety of inclisiran: an analysis of the ORION-7 and ORION-1 studies. Mayo Clin. Proc. 95 (1), 77–89. doi:10.1016/j.mayocp.2019.08.021
Wu, H., Tong, L., Wang, Y., Yan, H., and Sun, Z. (2021). Bibliometric analysis of global research trends on ultrasound microbubble: a quickly developing field. Front. Pharmacol. 12, 646626. doi:10.3389/fphar.2021.646626
Wu, T., Zhou, K., Hua, Y., Zhang, W., and Li, Y. (2023). The molecular mechanisms in prenatal drug exposure-induced fetal programmed adult cardiovascular disease. Front. Pharmacol. 14, 1164487. doi:10.3389/fphar.2023.1164487
Wu, W., Ouyang, Y., Zheng, P., Xu, X., He, C., Xie, C., et al. (2022). Research trends on the relationship between gut microbiota and colorectal cancer: a bibliometric analysis. Front. Cell. Infect. Microbiol. 12, 1027448. doi:10.3389/fcimb.2022.1027448
Yan, B. P., Chiang, F. T., Ambegaonkar, B., Brudi, P., Horack, M., Lautsch, D., et al. (2018). Low-density lipoprotein cholesterol target achievement in patients surviving an acute coronary syndrome in Hong Kong and Taiwan - findings from the Dyslipidemia International Study II. Int. J. Cardiol. 265, 1–5. doi:10.1016/j.ijcard.2018.01.099
Yang, J. W., and Hu, Z. P. (2015). Neuroprotective effects of atorvastatin against cerebral ischemia/reperfusion injury through the inhibition of endoplasmic reticulum stress. Neural Regen. Res. 10 (8), 1239–1244. doi:10.4103/1673-5374.162755
Yang, Y., Feng, Q., Luan, Y., Liu, H., Jiao, Y., Hao, H., et al. (2023). Exploring cuproptosis as a mechanism and potential intervention target in cardiovascular diseases. Front. Pharmacol. 14, 1229297. doi:10.3389/fphar.2023.1229297
Yu, N., Wang, R., Liu, B., and Zhang, L. (2022). Bibliometric and visual analysis on metabolomics in coronary artery disease research. Front. Cardiovasc Med. 9, 804463. doi:10.3389/fcvm.2022.804463
Zhang, T., Yin, X., Yang, X., Man, J., He, Q., Wu, Q., et al. (2020a). Research trends on the relationship between microbiota and gastric cancer: a bibliometric analysis from 2000 to 2019. J. Cancer 11 (16), 4823–4831. doi:10.7150/jca.44126
Zhang, X., Yi, K., Xu, J. G., Wang, W. X., Liu, C. F., He, X. L., et al. (2023). Application of three-dimensional printing in cardiovascular diseases: a bibliometric analysis. Int. J. Surg. 110, 1068–1078. doi:10.1097/js9.0000000000000868
Zhang, X. L., Zheng, Y., Xia, M. L., Wu, Y. N., Liu, X. J., Xie, S. K., et al. (2020b). Knowledge domain and emerging trends in vinegar research: a bibliometric review of the literature from WoSCC. Foods 9 (2), 166. doi:10.3390/foods9020166
Zhao, J., Yu, G., Cai, M., Lei, X., Yang, Y., Wang, Q., et al. (2018). Bibliometric analysis of global scientific activity on umbilical cord mesenchymal stem cells: a swiftly expanding and shifting focus. Stem Cell. Res. Ther. 9 (1), 32. doi:10.1186/s13287-018-0785-5
Keywords: lipid-lowering therapy, coronary heart disease, hyperlipidemia, VOSviewer, CiteSpace, visualization analysis
Citation: Cheng Q, Sun J, Zhong H, Wang Z, Liu C, Zhou S and Deng J (2024) Research trends in lipid-lowering therapies for coronary heart disease combined with hyperlipidemia: a bibliometric study and visual analysis. Front. Pharmacol. 15:1393333. doi: 10.3389/fphar.2024.1393333
Received: 28 February 2024; Accepted: 03 May 2024;
Published: 17 May 2024.
Edited by:
Daniel Fernandes, Federal University of Santa Catarina, BrazilReviewed by:
Enchen Zhou, University of California, San Diego, United StatesZhen Wang, Huazhong University of Science and Technology, China
Copyright © 2024 Cheng, Sun, Zhong, Wang, Liu, Zhou and Deng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jie Deng, jie.deng@mail.xjtu.edu.cn
†These authors have contributed equally to this work and share first authorship
 Quankai Cheng
Quankai Cheng Jingjing Sun
Jingjing Sun Haicheng Zhong2
Haicheng Zhong2 Jie Deng
Jie Deng