- 1Chair of Crop Science and Plant Biology, Estonian University of Life Sciences, Tartu, Estonia
- 2Estonian Academy of Sciences, Tallinn, Estonia
Climate change is associated with higher atmospheric [CO2] and more frequent temperature extremes, with the strongest impact expected in the tropics where plants often operate close to their heat stress limit. How the resistance of foliage photosynthetic traits to heat stress varies with [CO2] elevation remains largely unknown, particularly in tropical species with continuously expanding canopies, where the heat resistance of leaves can vary with age. We studied the impact of heat shock stress resembling heatflecks due to fluctuating light (48 °C for 10 min) on foliage physiological traits and chemical contents in young-mature and old-mature foliage of the tropical species Persea americana Mill. plants grown under ambient (400 μmol mol-1) and elevated (800 μmol mol-1) [CO2]. Leaf characteristics were studied through a 48 h recovery period. Light-saturated net assimilation rate (A) decreased with leaf age in both ambient and elevated [CO2]. In young-mature leaves, A in plants grown under elevated [CO2] was greater than A in plants grown under ambient [CO2]. In old-mature leaves, A was similar under both [CO2] and this was associated with increased nutrient limitation under elevated [CO2]. Upon heat stress application, A decreased in all cases due to both reduction in stomatal conductance and inhibition of biochemical photosynthetic capacity (maximum Rubisco carboxylase activity). During recovery, A increased to pre-stress level in all but in young-mature plants grown under ambient [CO2] where A remained much lower (78% reduction) than in control plants. As young leaves have a longer remaining lifespan and higher future potential contribution to plant carbon gain, preservation of photosynthetic capacity in young leaves under elevated [CO2] suggests that elevated [CO2] can enhance long-term photosynthetic production in P. americana exposed to heat episodes.
1 Introduction
Since the industrial revolution, the global atmospheric CO2 concentration has risen from around 280 mol mol-1 to over 420 mol mol-1. Contingent on socio-economic trajectory taken, [CO2] is expected to reach 730-1000 μmol mol-1 by the end of this century (Strandsbjerg Tristan Pedersen et al., 2021). Whole plant responses to elevated [CO2] depend on modifications in plant photosynthesis and respiration rates (carbon balance), carbon allocation and nutrient availability, whereas the importance of individual factors depends on plant species and functional type (Drake et al., 1997; Ainsworth and Long, 2005; Dusenge et al., 2019). Alterations in leaf photosynthesis rates, in turn, are intimately linked to changes in leaf structure and chemical composition (Niinemets et al., 1999; Onoda et al., 2017). As the ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) is not [CO2]-saturated at current atmospheric [CO2], leaf photosynthesis is typically immediately enhanced upon the increase of [CO2] (Singh and Reddy, 2016; Cummins, 2021). Stimulated photosynthesis rate further leads to improved plant growth rate in short to medium term (Drake et al., 1997; Ainsworth and Long, 2005; Singh and Reddy, 2016; Reich et al., 2018). However, the stimulation of biomass accumulation under longer-term [CO2] increase may not be sustainable, and plants often reduce photosynthetic capacity through an acclimation process commonly referred to as ‘down-regulation’ due to factors such as limited nutrient and water availability and other sub- or supraoptimal environmental conditions (Sanz-Sáez et al., 2013; Atkin et al., 2015; Terrer et al., 2018; Zheng et al., 2019; Jiang et al., 2020). In addition, the impact of the increase in [CO2] on whole plant performance can be strongly affected by how [CO2] affects the rate of new leaf production and leaf senescence and nutrient resorption (Li et al., 2019; Abiola et al., 2025a), and thus, the share of young mature and old mature leaves. The impact of leaf age can be particularly important in species continuously forming new leaves such as common in many tropical long-living species, but there is little information of how elevated [CO2]-driven photosynthetic downregulation depends on leaf age.
The increase in [CO2], combined with rising levels of other greenhouse gases, has resulted in a 0.8 °C increase in mean annual global temperature (Hansen et al., 2010) and it is expected to rise further by 2-5 °C before the end of this century (Tian et al., 2020). Overall higher temperatures are associated with increased frequency of heatwaves (Breshears et al., 2021; Ahrens et al., 2021), and consequently, with more severe heat stress impacts on plants, curbing plant growth and survival in future climates. Both stomatal and biochemical factors can inhibit photosynthesis at temperatures slightly above the thermal optimum, and the contribution of these factors varies among species (Kask et al., 2016; Slot and Winter, 2017; Kumarathunge et al., 2018; Turan et al., 2019; Sulaiman et al., 2023). The thermal optimum itself, and accordingly the severity of stress at the given temperature is also species-dependent and might vary with the plant developmental stage (Ruelland and Zachowski, 2010; Okereke et al., 2021). In this study, the term “heatwave” denotes extreme temperatures that significantly exceed the optimal photosynthetic range of tropical species, typically 30-35 °C (Slot and Winter, 2017). Photosynthesis is highly susceptible to heat stress, especially photosystem II (PSII) and oxygen-evolving complex are the first components to sustain thermal damage (Falcioni et al., 2024; Paethaisong et al., 2025). Temperatures above 45 °C for just a few minutes can cause losses in membrane integrity, and lead to photoinhibition and reactive oxygen species (ROS) generation; ROS propagation and reduction of photosynthetic activity can continue even after returning to lower temperatures (Hüve et al., 2011; Bernacchi et al., 2025). Acclimatory responses to heat stress involve changes in the activities of photosynthetic enzymes, adjustments of stomatal openness for more effective transpiratory cooling, and increases in photoprotective energy dissipation, while redox signals from the photosynthetic electron transport activate heat shock proteins and antioxidant defenses which enhance both thermal protection and recovery capacity (Tarvainen et al., 2022; Diao et al., 2024).
The impact of heat stress varies among leaves of differential developmental stage with mature leaves generally exhibit higher heat tolerance compared to young leaves (Wahid et al., 2007; Marias et al., 2017). However, why heat responses of leaves of different developmental stages vary and how the contribution of stomatal and biochemical modifications to overall heat stress response varies among leaves of different ages are poorly understood (Gu et al., 2012; Zhu et al., 2021; Sun et al., 2024b). Higher heat resistance of older leaves might be attributed to their more developed structural characteristics such as greater leaf thickness, more robust cell walls, and higher mass per area, well-established antioxidant systems and rapid capacity for formation of heat shock proteins as well as non-specific protective compounds such as sugars (Hüve et al., 2011; Wahid et al., 2007; Marias et al., 2017; Yurina, 2023; Watson-Lazowski et al., 2024). Meanwhile, fully-developed photosynthesis apparatus is also essential in regulating plant heat responses by balancing carbon assimilation with protective mechanisms in mature leaves (Zahra et al., 2023; Bernacchi et al., 2025).
Apart from the direct effect of [CO2] on photosynthesis rate, higher [CO2] can potentially ameliorate the impact of heat stress (Rodrigues et al., 2016; Abo Gamar et al., 2019; Diao et al., 2024). Improvement of stress resistance by elevated [CO2] in stressed plants is evident in a reduced cellular oxidative damage (such as lipid peroxidation and protein oxidation), in a lower content of stress-generated reactive oxygen species (ROS) and in a lower degree of reduction in photosynthesis rate (Geissler et al., 2010; Sun et al., 2013a; Zinta et al., 2014; AbdElgawad et al., 2015; Ulfat et al., 2021). A greater stress resistance has been associated with reduced photorespiration and as a consequence, with lower production of active oxygen species and H2O2 (Foyer and Noctor, 2009; Munné-Bosch et al., 2013; Zinta et al., 2014), enhanced contents of lipophilic antioxidants and membrane-protecting enzymes through upregulation of antioxidant defense metabolism (Xu et al., 2014; Naudts et al., 2014) and higher content of sugars that stabilize membranes (Sun et al., 2013a; Ulfat et al., 2021). However, how elevated [CO2]-dependent improvement of heat stress resistance varies among leaves of different developmental stage has not been studied. Given that elevated [CO2] might alter the share of young and old leaves in the canopy, this is a relevant omission.
To the best of our knowledge, interactive effects of heat stress and elevated [CO2] on primary metabolism in leaves of different developmental stage have not been studied, especially in tropical plants with continuous leaf formation. We grew the saplings of tropical evergreen fruit tree avocado (Persea americana Mill., Lauraceae) under ambient [CO2] of 400 μmol mol-1 and elevated [CO2] of 800 μmol mol-1 and studied the interaction between photosynthetic acclimation to [CO2] and heat stress response in leaves of different age. We hypothesized that: (i) elevated [CO2] enhances photosynthesis rate, but leads to a down-regulation of biochemical photosynthetic capacity; (ii) the degree of photosynthetic downregulation is greater in older leaves; (iii) elevated [CO2] increases heat resistance of photosynthesis; (iv) older foliage sustains less physiological damage and exhibits faster recovery than young-mature foliage, and this difference is more pronounced under elevated [CO2]. Persea americana is an economically important crop species and there is an unprecedented increase in the production and consumption of its products worldwide (Schaffer et al., 2013). Thus, we expect that the results of this study contribute to predicting the avocado performance in future climates. Furthermore, we consider avocado as a representative tropical species with continuous leaf formation, and thus, we argue that this study provides general insight into how a large part of vegetation in humid tropics responds to global change.
2 Materials and methods
2.1 Plant material and growing conditions
We used seeds of Persea americana Mill. cv. Hass, a cultivar bred in California from Mexican-Guatemalan ancestry and currently cultivated worldwide. The fruits (origin Aconcagua basin, Chile) were bought from a local fruit shop. The seeds were planted in 2 L clay pots and grown at the plant growth room of the Estonian University of Life Sciences under the following conditions: light intensity of 500-700 μmol m-2 s-1 with day/night temperatures of 28/24 °C for a 12 h light period and 60% relative humidity. The growth substrate was a commercial organic garden soil and a mixture of peat and sand (0–2 mm, Bauhof, Tartu, Estonia). The plants were watered every other day to soil field capacity and fertilized with 10 g slow-release fertilizer with microelements (N:P:K = 14:11:25, AS Baltic Agro, Tartu, Estonia) once per month. The seedlings were grown under these conditions until they were 50–70 cm tall and had utilized all seed reserves. Before the start of CO2 treatments, the plants were transplanted into 5 L pots and the foliated parts of the plants were removed leaving ca. 5 cm long stems. Subsequently, the plants were transferred to the plant growth chamber (FITOCLIMA S600PLLH, Aralab, Lisbon, Portugal) to either ambient (400 μmol mol-1) or elevated [CO2] (800 μmol mol-1). Chamber conditions were consistent with those in the plant growth room, except that light intensity was maintained at 1000 μmol m-2 s-1. The plants were watered every other day to the soil field capacity and fertilized in the beginning and in the middle of the growth period (see Abiola et al., 2025a for details of the growth conditions). At the time of the experiment, the plants had approximately 30–40 leaves and were 80–100 cm tall, after a growth period of four months. In this experiment, new leaves were formed continuously, and the plastochron (interval between the formations of successive leaves) was 11–12 days. Fully expanded young-mature leaves were ca. 60–70 days old, and old-mature non-senescent leaves were ca. 110–120 days old. We estimated that the available C and N stored in the root system could have been responsible for less than 10% of new growth, and thus, most of the above-ground biomass developed after removal of above-ground plant parts and transfer of plants to the new growth environment resulted from de novo carbon fixation and nutrient uptake from soil.
2.2 Gas exchange measurements
A custom-made open gas-exchange system was used to measure the foliage gas-exchange rate (Copolovici and Niinemets, 2010 for the full description of the system). The measurement system was specifically designed for simultaneous measurement of photosynthesis, transpiration, and volatile organic compound (VOC) emission rates, with all components constructed from glass, stainless steel, and Teflon®. The 1.2 L measurement chamber was made with double glass walls. Water with preset temperature circulated between the chamber walls to regulate the chamber temperature (Copolovici and Niinemets, 2010). The CO2 and H2O vapor concentrations at the chamber inlets and outlets were measured with an infra-red dual-channel gas analyzer operated in differential mode (CIRAS III, PP-Systems, Amesbury, MA, USA).
After the measurement leaf was enclosed in the chamber, following standard conditions were established: light intensity at the leaf surface of 700 μmol m-2 s-1, chamber temperature of 24°C, CO2 concentration of 390-410 μmol mol-1 (ambient [CO2] measurements), relative air humidity 60%, and leaf-to-air vapor pressure deficit of 1.7 kPa. After the steady-state gas exchange rates were achieved, typically between 25–30 minutes after leaf enclosure, differences in CO2 and water vapor concentrations were recorded. Net assimilation rate (A), transpiration rate (E), stomatal conductance (gs) and intercellular CO2 concentration (Ci) were calculated according to von Caemmerer and Farquhar (1981). The apparent maximum Rubisco carboxylase activity (Vcmax) was computed according to Niinemets et al., 1999 as explained in detail in De Kauwe et al. (2016). In these calculations, average Rubisco kinetic characteristics for “warm” C3 species from Galmés et al. (2016) were used. As Ci was used as the substitute for chloroplastic CO2 concentration, the estimates of Vcmax provide an estimate of foliage photosynthetic activity without stomatal effects, but they do not consider possible variation in mesophyll conductance.
2.3 Heat stress treatments
Before the heat treatment, steady-state values of A and gs were recorded under standard conditions (24 °C). Heat stress was applied by immersing the sample leaves in distilled water at 48 °C for 10 min according to the procedure detailed in previous studies (Copolovici et al., 2012; Kask et al., 2016; Okereke et al., 2022). During immersion, the leaves were enclosed in a chemically inert polyester bag to avoid direct contact with water. The control treatment consisted of leaf immersion in distilled water at 25°C for 10 min. A controlled temperature water bath (VWR International, West Chester, Pennsylvania, USA) was used to maintain a highly stable temperature of the immersion medium. We choose this procedure as it does not depend on stomatal effects that can alter leaf temperature at given air temperature due to transpiratory cooling. We have demonstrated that the heat stress applied this way is highly repeatable and the severity of heat stress scales quantitatively with photosynthetic reduction, volatile emission and ion leakage (e.g., Okereke et al., 2022; Sulaiman et al., 2023).
In these experiments, four replicates of young-mature and old-mature leaves from different plants were used. Foliage gas-exchange measurements were conducted at 0.25, 1, 3, 24, and 48 h after the treatment under the standard conditions (chamber temperature of 24 °C).
2.4 Estimation of leaf dry mass per area, and carbon, nitrogen, and phosphorous contents per dry mass
Data of nitrogen (NM), carbon (CM), and phosphorus (PM) content per dry mass used in this study are those from (Abiola et al., 2025a). Fresh leaves were scanned at 300 dpi and leaf area was measured using ImageJ 1.8.0 (NIH, Bethesda, Maryland, USA). Leaf dry mass was estimated after oven-drying at 70°C for 48 h, and leaf dry mass per unit area (MA) was calculated.
2.5 Data analyses
The measurements of control and heat-treated plants were conducted in four replicate plants (n = 4), with one young-mature and one old-mature leaf measured per plant, using different plants grown under ambient and elevated [CO2]. The data were presented as averages ± SE. The degree of heat stress recovery of gas exchange characteristics for each plant age groups was calculated as:
where RC,i is the relative change of trait i and xi,before stress and xi,after stress the trait values assessed before and after stress application (Liu et al., 2022). Linear mixed models (LMM) with heat stress and [CO2] levels as fixed effects and time as a random effect were used to test the effects of individual and interactive effects of treatments and [CO2] on gas exchange characteristics through the stress recovery time. Tukey’s honestly significant difference (HSD) test following one-way ANOVA was used to compare averages at different heat stress recovery time points. Two-way analyses of variance with leaf age and [CO2] as main effects and leaf age x [CO2] interaction were used to estimate the global effects of growth [CO2], leaf age and their interaction. All statistical analyses were conducted with R statistical software ver. 4.2.0 (2021) and visualized with OriginPro 2018 (OriginLab Corporation, Northampton, USA). The data used for ANOVA and LMM were tested for normality of distribution (Kolmogorov-Smirnov test), and when necessary, the data were log-transformed to improve the normality of data. All statistical effects were considered significant at P < 0.05. All datasets analyzed during this study are available in the EMU DSpace repository (Abiola et al. 2025b).
3 Results
3.1 Effects of growth [CO2] and leaf age on photosynthetic characteristics
In Persea americana, the net assimilation rates ranged from 6.42 to 8.61 µmol m-² s-¹, with the lowest value observed in mature leaves under ambient [CO2] and the highest in young leaves under elevated [CO2] (Figure 1). The net assimilation rate (A) in unstressed young-mature leaves was 14% greater in elevated [CO2]-grown plants compared to ambient [CO2]-grown plants, indicated by a significant [CO2] effect on (P < 0.05), whereas A was similar between old-mature leaves, regardless of growth [CO2] (Table 1). A similar A measured suggest photosynthetic downregulation in high-[CO2]-grown plants, and this was supported by lower leaf nitrogen content per dry mass (NM) and leaf phosphorus content per dry mass (PM) under elevated [CO2] (Abiola et al., 2025a) for the effect of growth [CO2] on elemental contents). The apparent (Ci-based) maximum carboxylase activity of Rubisco (Vcmax) was higher in young-mature leaves under elevated [CO2] (mean ± SE = 51.95 ± 2.19) compared to young leaves under ambient [CO2] (42.38 ± 1.8, P < 0.001, Table 1). However, Vcmax was similar in old-mature leaves under both [CO2] conditions (P > 0.05, Table 1) despite reduced nutrient content (Abiola et al., 2025a) for the effect of growth [CO2] on elemental contents). For intercellular CO2 concentration (Ci), young-mature leaves under elevated CO2 exhibited significant main effects of growth [CO2] (mean ± SE = 175.02 ± 9.55) compared to ambient young-mature (205.15 ± 9.84, P < 0.001, Table 1). Again, old-mature leaves exhibited no Ci difference between [CO2] treatments (P > 0.05; Table 1).
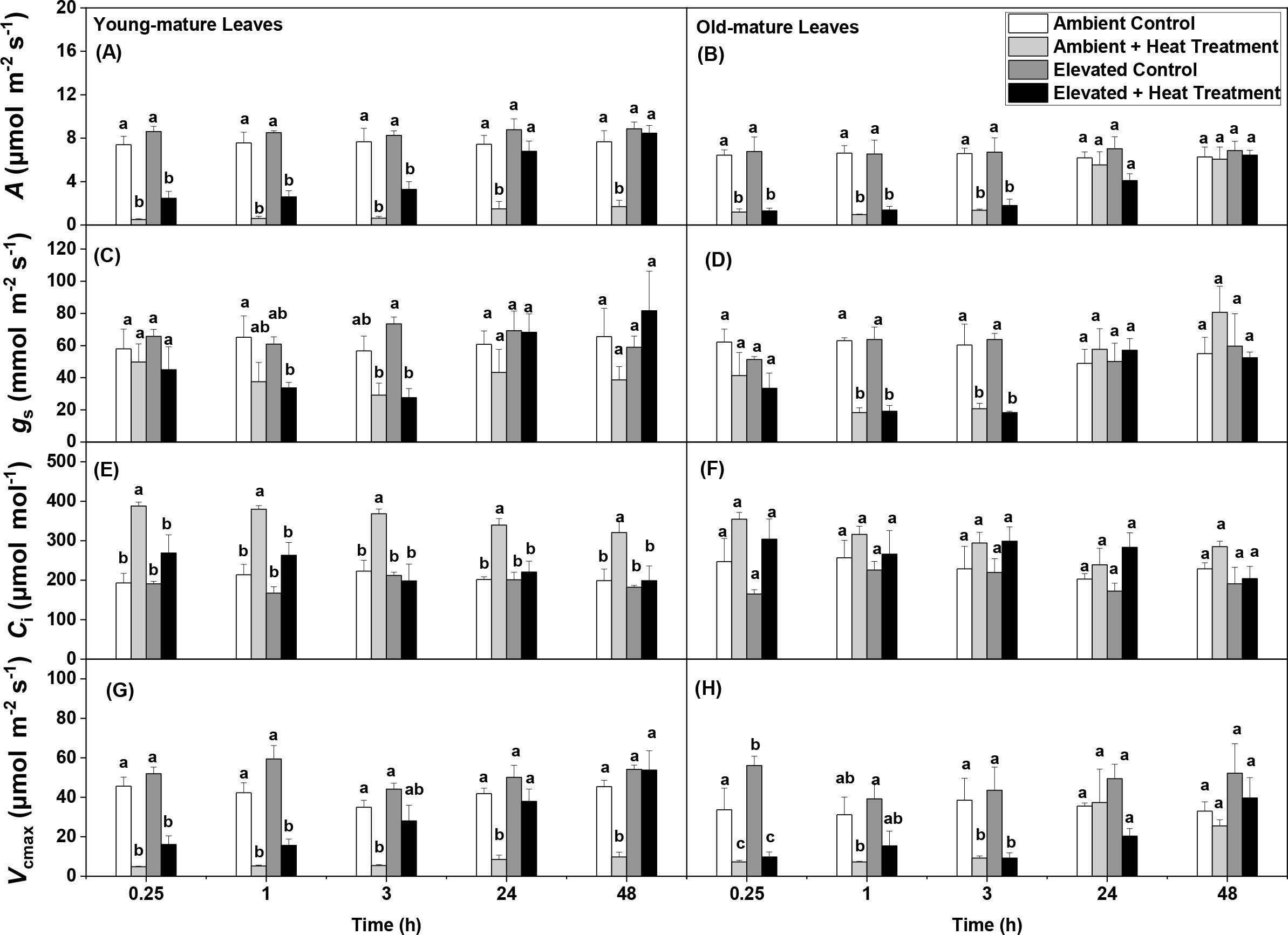
Figure 1. Light-saturated net assimilation rate (A) (A, B), stomatal conductance to water vapor (gs) (C, D), intercellular CO2 concentration (Ci) (E, F), and maximum carboxylase activity of Rubisco (Vcmax) (G, H) in control (25°C) and heat-treated (48°C) leaves of Persea americana measured at recovery time points of 0.25, 1, 3, 24 and 48 h. The heat treatment was applied by submerging the leaves in distilled water at 48°C for 10 min. For the control treatment, the water temperature was 25°C. All photosynthetic measurements were conducted at 24°C, at 700 mmol m-2 s-1 light, and at CO2 concentration of 390-410 mmol mol-1. Each bar represents the treatment average ± SE measured at different recovery times. Averages at each recovery time were compared by one-way ANOVA followed by a Tukey post-hoc test. Different lowercase letters denote significant differences (P < 0.05) among the treatment groups. n = 4.
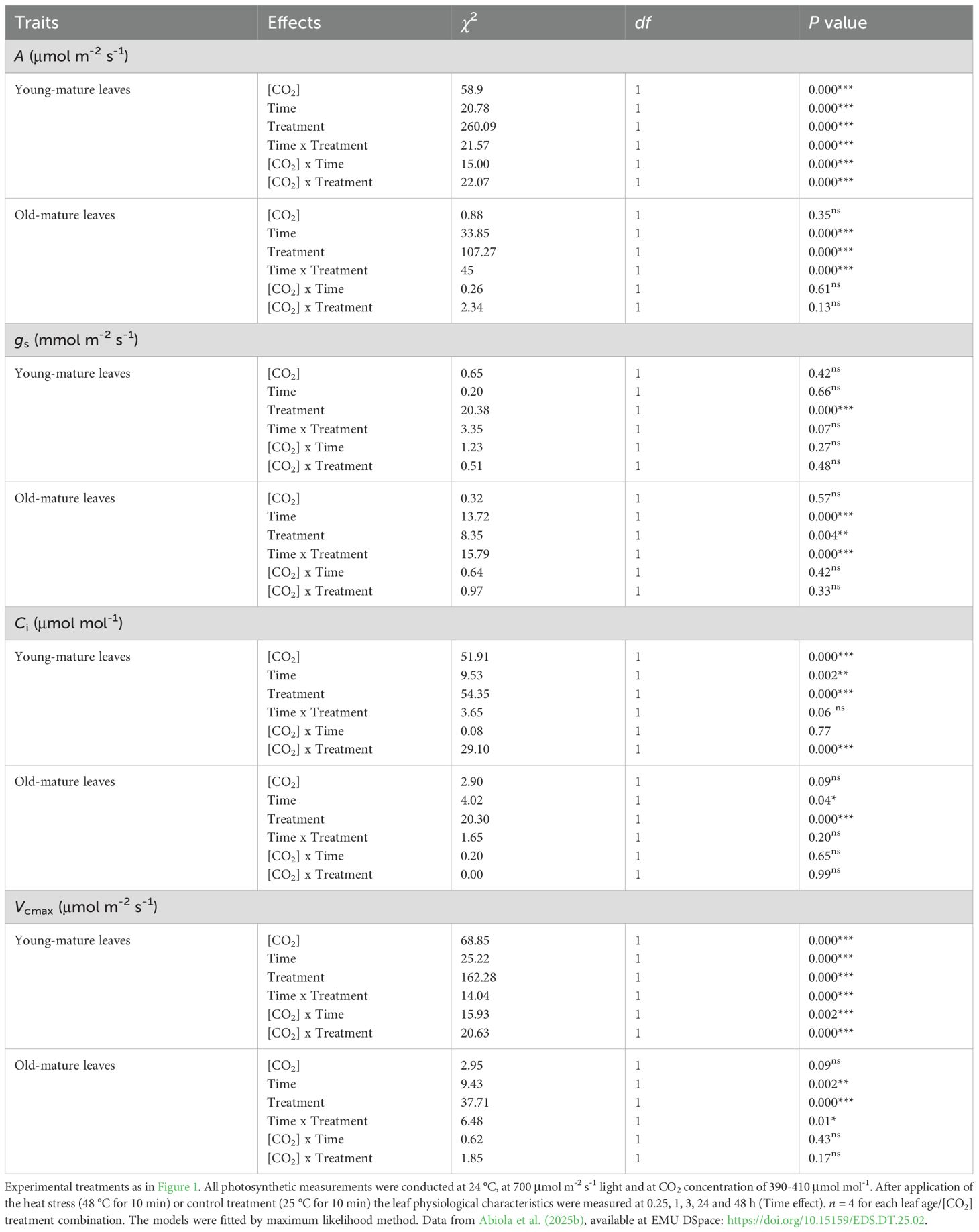
Table 1. Output of linear mixed model for individual and interactive effects of growth [CO2], ambient (400 μmol mol-1) vs. elevated (800 μmol mol-1) and treatment, control vs. heat stress, and recovery time on leaf gas exchange characteristics (light-saturated net assimilation rate, A; stomatal conductance to water vapor, gs; intercellular CO2 concentration (Ci) and maximum carboxylase activity of Rubisco; Vcmax) within the same leaf age groups (young- or old-mature leaves grown under ambient and elevated [CO2]) in Persea americana.
Meanwhile, in both growth [CO2], A decreased with leaf age (P < 0.05 in ambient [CO2] and P = 0.001 for elevated [CO2]), whereas the interactive effects of leaf age x [CO2] were not significant (P > 0.05; Table 2). There was no significant difference in Vcmax and stomatal conductance (gs) between leaf ages in both growth [CO2]. However, leaf dry mass per unit area (MA) increased with leaf age, and the interaction between leaf age x [CO2] was significant (P < 0.05, Table 2).
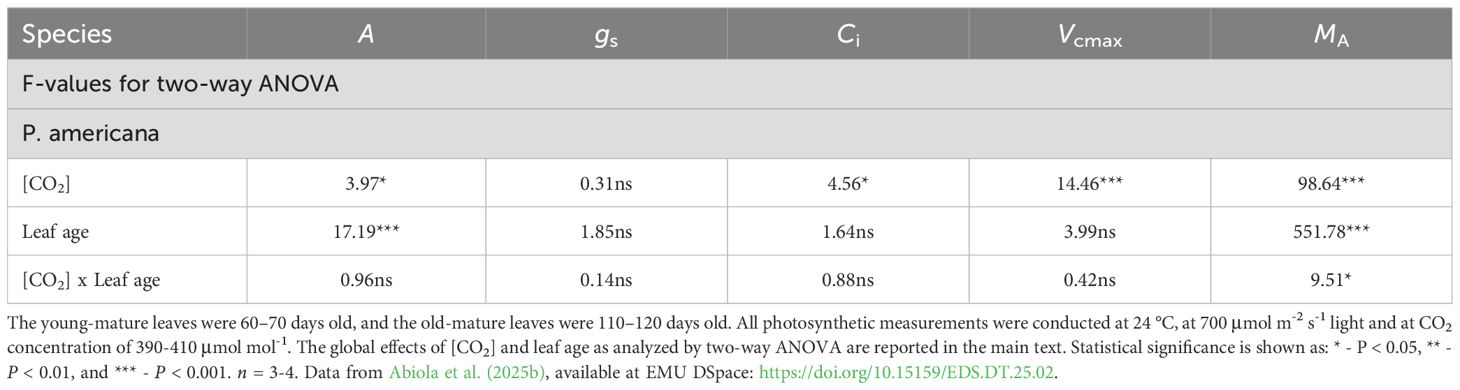
Table 2. Output of two-way ANOVA for individual and interactive effects of leaf age, young-mature vs. old-mature, and growth [CO2], ambient (400 μmol mol-1) vs. elevated (800 μmol mol-1), on light-saturated net assimilation rate (A, μmol m-2 s-1), stomatal conductance to water vapor (gs, mmol m-2 s-1), intercellular CO2 concentration (Ci, μmol mol-1), maximum carboxylase activity of Rubisco (Vcmax, μmol m-2 s-1), and leaf dry mass per unit area (MA) in P. americana.
3.2 Impacts of heat stress on foliage photosynthetic characteristics as affected by growth [CO2] and leaf age
Heat stress (48 °C) led to immediate decreases in foliage photosynthetic characteristics in all cases with full or partial recovery depending on growth [CO2] and leaf age. The degree of recovery varied between young- and old-mature leaves and dependence on the growth [CO2] as well (Figures 1, 2; Table 1). Heat shock resulted in decreases in light saturated photosynthesis (A) in both young and old-mature leaves grown under different [CO2] (Figures 1A, B, 2A, B, Table 1). The degree of reductions in A in all the heat-stressed plants was proportional to reductions in Vcmax (Figures 1A, B, G, H, 2A, B, E, F, Supplementary Figure S1, Table 1). The application of heat stress uncoupled the relationship between A and gs (Figures 1C, D, 2C, D, Table 1), and thus, the heat stress-dependent changes in net assimilation rate through recovery were primarily driven by changes in Vcmax (biochemical limitation) for different combinations of leaf age and growth [CO2] (Supplementary Figure S1).
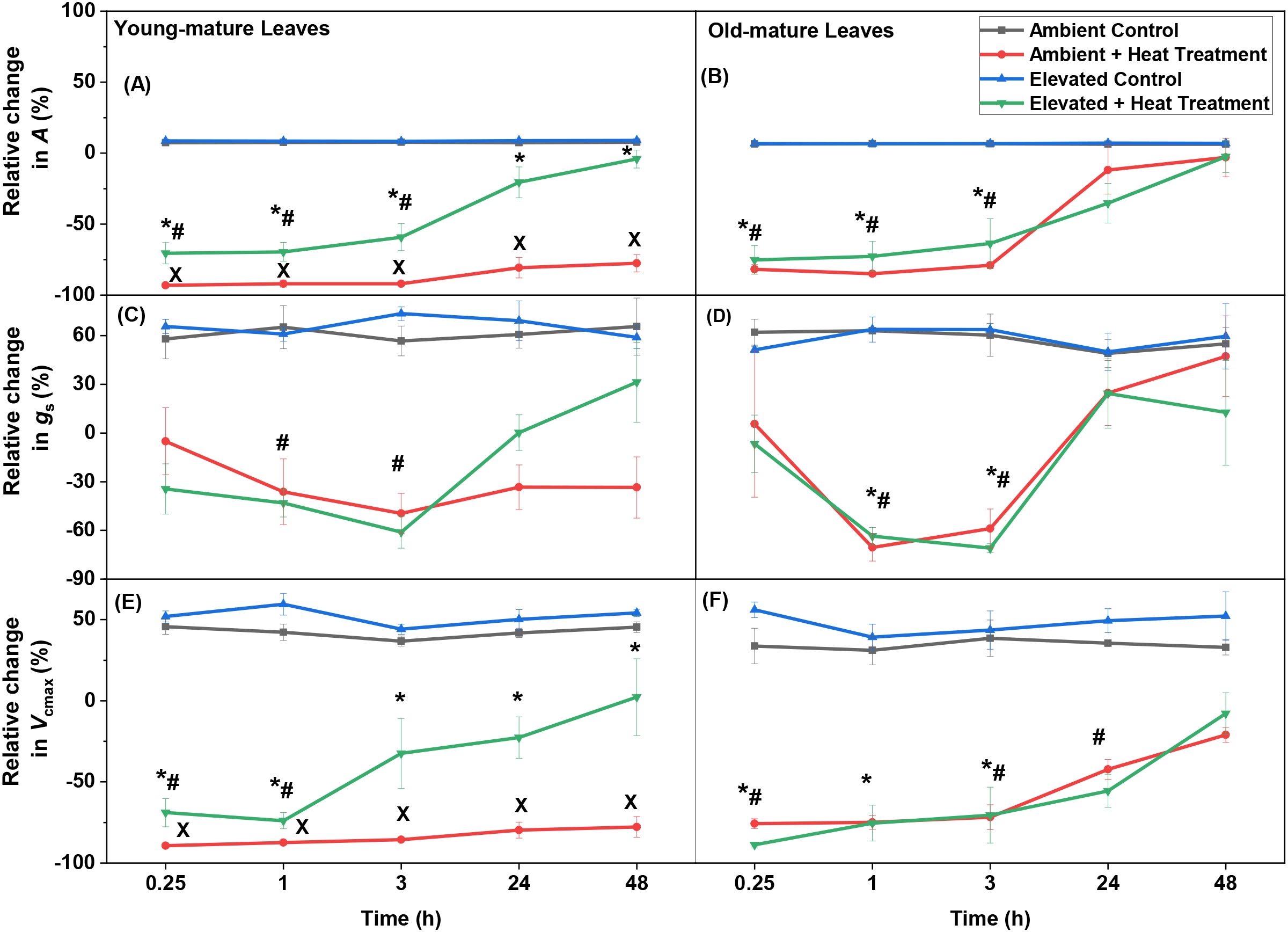
Figure 2. The relative change (Equation 1; degree of recovery) of net assimilation rate (A) (A, B), stomatal conductance to water vapor (gs) (C, D), and maximum carboxylase activity of Rubisco (Vcmax) (E, F) after heat stress application (48 °C applied for 10 min) in leaves of Persea americana with recovery time points of 0.25, 1, 3, 24 and 48 h (average ± SE). (A–F) show the degree of recovery of leaf age groups from plants grown at ambient (400 mmol mol-1) and elevated (800 mmol mol-1) [CO2], relative to each treatment/leaf age combination prior to heat stress application. The photosynthetic measurements were conducted at 24 °C, at 700 mmol m-2 s-1 light and at CO2 concentration of 390-410 mmol mol-1. Statistically significant differences (P < 0.05) of heat-treated leaf trait values relative to control treatment are shown by * for ambient [CO2]-grown plants and by # for elevated [CO2]-grown plants. Significant differences among heat-treated ambient and elevated-[CO2]-grown plants are shown by x (one-way ANOVA, P < 0.05; n = 4).
Reductions of A due to heat stress were the greatest in young-mature leaves of plants grown under ambient [CO2] (Figures 1A, 2A, Table 1). At the end of the 48 h heat stress recovery period, A, gs and Vcmax in all the stressed plants recovered fully, except for the young-mature leaves grown under ambient [CO2] (Figures 1A–D, G, H, 2A–F). In heat-stressed young-mature leaves grown under elevated [CO2], full recovery of A (P > 0.6 between control and at 48 h after heat shock) was accompanied by a greater recovery of gs and Vcmax and a decrease in intercellular CO2 concentration (Ci) than in young-mature leaves grown under ambient [CO2] (Figures 1A–F, 2A–F).
4 Discussion
We grew the saplings of tropical evergreen fruit tree avocado (Persea americana) under ambient (400 μmol mol-1) and elevated (800 μmol mol-1) growth [CO2] and determined the leaf photosynthetic responses to an heat shock treatment (10 min. exposure to 48 °C) in young-mature and old-mature leaves. The heat stress applied is close to the heat stress limit of vascular plants (Kask et al., 2016; Turan et al., 2019; Okereke et al., 2022) and allows gaining an insight into variation in heat stress resistance and recovery in dependence on leaf age and [CO2] treatment. In nature, it resembles the heat stress the plants might be exposed on hot days upon sunflecks when the leaves can rapidly heat up 5-10 °C above the ambient temperature (Leakey et al., 2005; Hüve et al., 2019).
So far, the effects of heat stress on primary and secondary metabolism have been investigated in numerous studies (Jardine et al., 2015; Teskey et al., 2015; Chatterjee et al., 2020; Okereke et al., 2021; 2022; Sulaiman et al., 2023), however, much less is known about the interaction between elevated [CO2] and heat stress, and no study has looked at heat stress and growth [CO2] x leaf age interaction. This is a significant omission as both aging and elevated [CO2] alter leaf physiological activity, structure and content of protective chemicals such as antioxidants and sugars that collectively can also affect heat resistance.
4.1 Leaf-age and growth [CO2]-dependent changes in foliage photosynthetic activity as related to leaf elemental contents and structure
Changes in foliage net assimilation rate (A) can occur due to alterations in stomatal conductance (gs), mesophyll conductance (gm) and maximum carboxylase activity of Rubisco (Vcmax) (Ethier et al., 2006; Niinemets, 2018). In our study we looked at changes in gs and apparent Vcmax that does not consider possible differences in gm. Generally, A decreases in non-senescent leaves with increasing leaf age, but the underlying physiological mechanisms can be species-specific (Ethier et al., 2006; Kositsup et al., 2010; Abiola et al., 2025a). In the current study, A was lower in old-mature leaves in comparison to young-mature leaves. Given the similar nutrient contents in the leaves with varying age in P. americana (Abiola et al., 2025a), the age-related reduction in photosynthetic capacity was not associated with limiting nutrient contents, but might indicate a decrease in mesophyll conductance due to thickening of cell walls (Niinemets et al., 2005, 2006; Onoda et al., 2017). This is plausible given the increase of leaf dry mass per unit area (MA) with increasing leaf age (Table 2).
Elevated [CO2] is expected to increase A as the result of enhanced CO2 availability for photosynthesis (Reich et al., 2018; Zheng et al., 2019; Jiang et al., 2020). However, previous studies have shown that A in plants under elevated [CO2] can increase, reduce or even remain unaffected (Urban et al., 2012; Abiola et al., 2025a). Unaffected or reduced A is indicative of photosynthetic downregulation (see Introduction). Typically, plants grown under elevated [CO2] have lower nutrient contents and higher MA (Sun et al., 2013a; Fleischer and Terrer, 2022; Abiola et al., 2025a). In our study, leaf nitrogen content per dry mass (NM) and leaf phosphorus content per dry mass (PM) were higher in both young-mature and old-mature leaves grown under ambient [CO2] compared to elevated [CO2]-grown plants (Abiola et al., 2025a). Previous studies have shown that decreases in foliage nutrient contents such as N and P contribute to decreases in photosynthesis under elevated [CO2]-grown plants (Jifon and Wolfe, 2002; Sanz-Sáez et al., 2013; Reich and Hobbie, 2013; Arrizabalaga-Arriazu et al., 2020). Lower NM is typically associated with lower photosynthetic capacity per dry mass (Amass), while the rate of photosynthesis per area (Aarea) also depends on MA, i.e., Aarea = MA x Amass (Onoda et al., 2017; Onoda and Wright, 2018). Thus, the enhancement of MA under elevated [CO2] might compensate for reductions in Amass. However, as discussed above, a higher MA can be associated with greater investment in cell walls, which reduces photosynthetic efficiency by lowering N allocation to photosynthetic proteins and decreasing CO2 diffusion due to low mesophyll conductance associated with thick cell walls (Tosens et al., 2016; Osnas et al., 2018). We argue that further studies should look at elevated [CO2] effects on mesophyll conductance in different aged leaves.
4.2 Leaf maturation and elevated [CO2] enhanced heat stress tolerance of photosynthesis in P. americana
We demonstrated that reductions of A in all heat-stressed P. americana were primarily due to non-stomatal factors, specifically, reductions in ribulose 1,5-bisphosphate carboxylase/oxygenase (Rubisco) activity (Figures 1, 2, Supplementary Figure S1). Rapid reductions in Rubisco maximum activity upon heat stress might be attributed to thermal inhibition of Rubisco enzymatic activity (Hüve et al., 2011; Kask et al., 2016; Djanaguiraman et al., 2018). However, photosynthesis recovered in all the heat shock-treated P. americana leaves except in young-mature leaves of P. americana grown under ambient [CO2] (Figures 1A, E), likely indicating direct thermal damage of photosynthetic components in these leaves (Hüve et al., 2019; Sulaiman et al., 2023).
Previous studies have associated severe heat stress (>47°C) with rapid reductions in A in several tropical species (Okereke et al., 2021; 2022). The current study showed that severe heat resulted in an immediate decrease in A by ca. 82% in old-mature leaves of P. americana (Figures 1B, 2B; Table 1), similar to the reductions observed previously in foliage of old-mature tropical herbaceous species including Amaranthus cruentus, A. hybridus, Solanum aethiopicum, Telfairia occidentalis and Vigna unguiculata (Okereke et al., 2021), but less than the reductions observed in the foliage of the tree species Carica papaya (Okereke et al., 2022). Also, unlike in C. papaya (Okereke et al., 2022), A in old-mature P. americana recovered completely after severe heat stress application (Figures 1B, 2B), suggesting greater heat stress tolerance of old-mature P. americana leaves. The recovery of old-mature leaves under ambient [CO2] indicates enhancement of photosynthetic apparatus tolerance of heat-stressed plants. As suggested in the Introduction, such an enhancement in heat resistance with increasing leaf age might have multiple causes, including greater antioxidative capacity, enhanced repair capacity and enhanced formation of stress-protective compounds, partly as the result of greater physiological activity (Albert et al., 2018; Krause et al., 2014; Drake et al., 2018; Tarvainen et al., 2022). However, in young-mature leaves of P. americana grown under ambient [CO2] (Figures 1A, 2A), photosynthesis did not recover (<78%) after severe heat stress application, suggesting the photosynthetic sensitivity of the young-mature leaves.
Both young and old-mature leaves grown under elevated [CO2] exhibit greater heat resistance than those grown under ambient [CO2], as evidenced by their enhanced recovery to control conditions following heat shock (Figures 1A, B, 2A, B). This demonstrate that increased leaf age and elevated [CO2] independently and synergistically improved heat shock tolerance and recovery of photosynthetic activities in P. americana. Typically, heat protection of photosynthesis by elevated [CO2] is associated with higher concentration of sugars in leaves under elevated [CO2] (Taub et al., 2000; Zhang et al., 2019). This is because of greater photosynthesis rate under elevated [CO2], and nutrient limitation of growth (lower sink activity) (Leakey et al., 2009; Habermann et al., 2019).
In conclusion, our study demonstrates that P. americana is a relatively heat tolerant species. Nevertheless, under transient heatwaves at current ambient [CO2], photosynthesis of young leaves is expected to be strongly reduced. Increases in ambient [CO2] are expected to improve the heat resistance of young leaves and thus, elevation in [CO2] might improve the whole canopy photosynthesis in heat-exposed avocado. Future research should examine effects of heat stress on a wider range of tropical woody species, including impacts on canopy-level carbon gain, reproductive development, and yield under future climate scenarios.
5 Conclusions
Our study suggests that the interactive effect of elevated [CO2] and heat stress might in nature contribute to improved heat resistance in plants with actively growing canopy. We demonstrated that leaf developmental stage and the growth [CO2] play a major role in how plant photosynthetic characteristics respond to heat stress conditions through a 48 h recovery period. The results showed a certain downregulation of photosynthesis in old-mature plants grown under elevated [CO2]. Upon heat stress application, photosynthetic reductions were mainly associated with Rubisco limitation with a minimal contribution of stomatal conductance. During 48 h recovery under ambient temperature, plants from all leaf age/treatment combinations recovered to the control condition at the end of the experiment, except young-mature leaves under ambient [CO2]. Thus, elevated [CO2] is expected to enhance the heat resistance of younger leaves. Given that these leaves have more time left to photosynthesize than older mature leaves, losing these leaves due to a heat stress episode would be highly devastating for the whole plant. Thus, the overall impact of elevated [CO2] in Persea americana would be the increased heat resistance of the long-term whole canopy photosynthetic production. Although elevated [CO2] improved the heat resistance of P. americana, heat waves during leaf development are expected to have a major impact on foliage photosynthetic activity under the current and future [CO2]. Future studies should examine the biochemical and physiological mechanisms responsible for greater heat resistance of young leaves in elevated [CO2]-grown plants and look at the generality of this finding across tropical species with continuously expanding canopies.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: EMU DSpace: https://dspace.emu.ee/bitstreams/3bc232b7-eddc-42fd-ad01-6adbfdc86248/download.
Author contributions
YA: Visualization, Methodology, Conceptualization, Writing – original draft, Writing – review & editing, Investigation, Formal analysis. HS: Writing – review & editing, Formal analysis. EK: Investigation, Conceptualization, Writing – review & editing, Methodology. ÜN: Funding acquisition, Conceptualization, Supervision, Methodology, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This research was funded by the European Research Council (advanced grant 322603, SIP-VOL+), Estonian Research Council (PRG2207, Centre of Excellence AgroCropFuture TK200 and “Plant Biology Infrastructure TAIM”, TT5). The equipment used in the study was partly purchased from funding by the EU Regional Development Fund (AnaEE Estonia, 2014-2020.4.01.20-0285, and the project “Plant Biology Infrastructure-TAIM”, 2014-2020.4.01.20-0282).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphgy.2025.1638556/full#supplementary-material
Supplementary Figure 1 | Correlations between the apparent (Ci-based) maximum carboxylation rate of Rubisco (Vcmax) and net CO2 assimilation rate (A) after heat treatment (48 °C applied for 10 min) in P. americana leaves grown at ambient (400 μmol mol-1 and elevated (800 μmol mol-1) [CO2]. Each data point represents an average of four independent replicates measured at 0.25, 1, 3, 24, and 48 h after heat stress treatments. Panel A shows young-mature leaves from ambient and elevated growth [CO2], while panel B shows old-mature leaves from ambient and elevated growth [CO2].
Abbreviations
A, light-saturated net assimilation rate; Ci, intercellular [CO2] concentrations; CM, leaf carbon content per unit dry mass; gs, stomatal conductance; MA, leaf dry mass per unit area; NM, leaf nitrogen content per unit dry mass; PM, leaf phosphorus content per unit dry mass; Rubisco, ribulose-1,5-bisphosphate carboxylase/oxygenase; Vcmax, maximum carboxylase activity of Rubisco.
References
AbdElgawad H., Farfan-Vignolo E. R., Vos D. D., and Asard H. (2015). Elevated CO2 mitigates drought and temperature-induced oxidative stress differently in grasses and legumes. Plant Sci 231, 1–10. doi: 10.1016/j.plantsci.2014.11.001
Abiola Y. O., Liu B., Sulaiman H. Y., Kaurilind E., Tosens T., and Niinemets Ü. (2025a). Contrasting leaf structural, photosynthetic and allocation responses to elevated [CO2] in different-aged leaves of tropical fruit trees Persea americana and Annona muricata. Plant Physiol. Biochem. 223, 109842. doi: 10.1016/j.plaphy.2025.109842
Abiola Y. O., Sulaiman H. Y., Kaurilind E., and Niinemets Ü. (2025b). Elevated growth [CO2] enhances heat stress resistance of photosynthesis in young leaves of avocado (Persea americana) [Data set. Estonian Univ. Life Sci. doi: 10.15159/EDS.DT.25.02
Abo Gamar M. I., Kisiala A., Emery R. J. N., Yeung E. C., Stone S. L., and Qaderi M. M. (2019). Elevated carbon dioxide decreases the adverse effects of higher temperature and drought stress by mitigating oxidative stress and improving water status in Arabidopsis thaliana. Planta 250, 1191–1214. doi: 10.1007/s00425-019-03213-3
Ahrens C. W., Challis A., Byrne M., Leigh A., Nicotra A. B., Tissue D., et al. (2021). Repeated extreme heatwaves result in higher leaf thermal tolerances and greater safety margins. New Phytol. 232, 1212–1225. doi: 10.1111/nph.17640
Ainsworth E. A. and Long S. P. (2005). What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytol. 165, 351–372. doi: 10.1111/j.1469-8137.2004.01224.x
Albert L. P., Wu J., Prohaska N., de Camargo P. B., Huxman T. E., Tribuzy E. S., et al. (2018). Age-dependent leaf physiology and consequences for crown-scale carbon uptake during the dry season in an Amazon evergreen forest. New Phytol. 219, 870–884. doi: 10.1111/nph.15056
Arrizabalaga-Arriazu M., Gomès E., Morales F., Irigoyen J. J., Pascual I., and Hilbert G. (2020). High Temperature and elevated carbon dioxide modify berry composition of different clones of grapevine (Vitis vinifera L.) cv. Tempranillo. Front. Plant Sci 11. doi: 10.3389/fpls.2020.603687
Atkin O. K., Bloomfield K. J., Reich P. B., Tjoelker M. G., Asner G. P., Bonal D., et al. (2015). Global variability in leaf respiration in relation to climate, plant functional types and leaf traits. New Phytol. 206, 614–636. doi: 10.1111/nph.13253
Bernacchi C. J., Long S. P., and Ort D. R. (2025). Safeguarding crop photosynthesis in a rapidly warming world. Science 388, 1153–1160. doi: 10.1126/science.adv5413
Breshears D. D., Fontaine J. B., Ruthrof K. X., Field J. P., Feng X., Burger J. R., et al. (2021). Underappreciated plant vulnerabilities to heat waves. New Phytol. 231, 32–39. doi: 10.1111/nph.17348
Chatterjee P., Kanagendran A., Samaddar S., Pazouki L., Sa T.-M., and Niinemets Ü. (2020). Influence of Brevibacterium linens RS16 on foliage photosynthetic and volatile emission characteristics upon heat stress in Eucalyptus grandis. Sci Total Environ. 700, 134453. doi: 10.1016/j.scitotenv.2019.134453
Copolovici L., Kännaste A., Pazouki L., and Niinemets Ü. (2012). Emissions of green leaf volatiles and terpenoids from Solanum lycopersicum are quantitatively related to the severity of cold and heat shock treatments. J. Plant Physiol. 169, 664–672. doi: 10.1016/j.jplph.2011.12.019
Copolovici L. and Niinemets Ü. (2010). Flooding induced emissions of volatile signalling compounds in three tree species with differing waterlogging tolerance. Plant Cell Environ. 33, 1582–1594. doi: 10.1111/j.1365-3040.2010.02166.x
Cummins P. L. (2021). The Coevolution of RuBisCO, photorespiration, and carbon concentrating mechanisms in higher plants. Front. Plant Sci 12. doi: 10.3389/fpls.2021.662425
De Kauwe M. G., Lin Y.-S., Wright I. J., Medlyn B. E., Crous K. Y., Ellsworth D. S., et al. (2016). A test of the ‘one-point method’ for estimating maximum carboxylation capacity from field-measured, light-saturated photosynthesis. New Phytol. 210, 1130–1144. doi: 10.1111/nph.13815
Diao H., Cernusak L. A., Saurer M., Gessler A., Siegwolf R. T. W., and Lehmann M. M. (2024). Uncoupling of stomatal conductance and photosynthesis at high temperatures: Mechanistic insights from online stable isotope techniques. New Phytol. 241, 2366–2378. doi: 10.1111/nph.19558
Djanaguiraman M., Boyle D. L., Welti R., Jagadish S. V. K., and Prasad P. V. V. (2018). Decreased photosynthetic rate under high temperature in wheat is due to lipid desaturation, oxidation, acylation, and damage of organelles. BMC Plant Biol. 18, 55. doi: 10.1186/s12870-018-1263-z
Drake B. G., Gonzàlez-Meler M. A., and Long S. P. (1997). More efficient plants: A Consequence of Rising Atmospheric CO2? Annu. Rev. Plant Biol. 48, 609–639. doi: 10.1146/annurev.arplant.48.1.609
Drake J. E., Tjoelker M. G., Vårhammar A., Medlyn B. E., Reich P. B., Leigh A., et al. (2018). Trees tolerate an extreme heatwave via sustained transpirational cooling and increased leaf thermal tolerance. Global Change Biol. 24, 2390–2402. doi: 10.1111/gcb.14037
Dusenge M. E., Duarte A. G., and Way D. A. (2019). Plant carbon metabolism and climate change: Elevated CO2 and temperature impacts on photosynthesis, photorespiration and respiration. New Phytol. 221, 32–49. doi: 10.1111/nph.15283
Ethier G. J., Livingston N. J., Harrison D. L., Black T. A., and Moran J. A. (2006). Low stomatal and internal conductance to CO2 versus Rubisco deactivation as determinants of the photosynthetic decline of ageing evergreen leaves. Plant Cell Environ. 29, 2168–2184. doi: 10.1111/j.1365-3040.2006.01590.x
Falcioni R., Chicati M. L., de Oliveira R. B., Antunes W. C., Hasanuzzaman M., Demattê J. A. M., et al. (2024). Decreased photosynthetic efficiency in Nicotiana tabacum L. under transient heat stress. Plants 13, 395. doi: 10.3390/plants13030395
Fleischer K. and Terrer C. (2022). Estimates of soil nutrient limitation on the CO2 fertilization effect for tropical vegetation. Global Change Biol. 28, 6366–6369. doi: 10.1111/gcb.16377
Foyer C. H. and Noctor G. (2009). Redox regulation in photosynthetic organisms: Signaling, acclimation, and practical implications. Antioxidants Redox Signaling 11, 861–905. doi: 10.1089/ars.2008.2177
Galmés J., Hermida-Carrera C., Laanisto L., and Niinemets Uuml;. (2016). A compendium of temperature responses of Rubisco kinetic traits: Variability among and within photosynthetic groups and impacts on photosynthesis modeling. J. Exp. Bot. 67, 5067–5091. doi: 10.1093/jxb/erw267
Geissler N., Hussin S., and Koyro H.-W. (2010). Elevated atmospheric CO2 concentration enhances salinity tolerance in Aster tripolium L. Planta 231, 583–594. doi: 10.1007/s00425-009-1064-6
Gu J., Weber K., Klemp E., Winters G., Franssen S. U., Wienpahl I., et al. (2012). Identifying core features of adaptive metabolic mechanisms for chronic heat stress attenuation contributing to systems robustness. Integr. Biol. 4, 480–493. doi: 10.1039/c2ib00109h
Habermann E., Dias de Oliveira E. A., Contin D. R., San Martin J. A. B., Curtarelli L., Gonzalez-Meler M. A., et al. (2019). Stomatal development and conductance of a tropical forage legume are regulated by elevated [CO2] under moderate warming. Front. Plant Sci 10. doi: 10.3389/fpls.2019.00609
Hansen J., Ruedy R., Sato M., and Lo K. (2010). Global surface temperature change. Rev. Geophysics 48, RG4004. doi: 10.1029/2010RG000345
Hüve K., Bichele I., Kaldmäe H., Rasulov B., Valladares F., and Niinemets Ü. (2019). Responses of Aspen leaves to heatflecks: Both damaging and non-damaging rapid temperature excursions reduce photosynthesis. Plants 8, 145. doi: 10.3390/plants8060145
Hüve K., Bichele I., Rasulov B., and Niinemets Ü. (2011). When it is too hot for photosynthesis: Heat-induced instability of photosynthesis in relation to respiratory burst, cell permeability changes and H2O2 formation. Plant Cell Environ. 34, 113–126. doi: 10.1111/j.1365-3040.2010.02229.x
Jardine K. J., Chambers J. Q., Holm J., Jardine A. B., Fontes C. G., Zorzanelli R. F., et al. (2015). Green leaf volatile emissions during high temperature and drought stress in a central amazon rainforest. Plants 4, 678–690. doi: 10.3390/plants4030678
Jiang M., Medlyn B. E., Drake J. E., Duursma R. A., Anderson I. C., Barton C. V. M., et al. (2020). The fate of carbon in a mature forest under carbon dioxide enrichment. Nature 580, 227–231. doi: 10.1038/s41586-020-2128-9
Jifon J. L. and Wolfe D. W. (2002). Photosynthetic acclimation to elevated CO2 in Phaseolus vulgaris L. @ is altered by growth response to nitrogen supply. Global Change Biol. 8, 1018–1027. doi: 10.1046/j.1365-2486.2002.00531.x
Kask K., Kännaste A., Talts E., Copolovici L., and Niinemets Ü. (2016). How specialized volatiles respond to chronic and short-term physiological and shock heat stress in Brassica nigra: Responses of Brassica nigra to heat stress. Plant Cell Environ. 39, 2027–2042. doi: 10.1111/pce.12775
Kositsup B., Kasemsap P., Thanisawanyangkura S., Chairungsee N., Satakhun D., Teerawatanasuk K., et al. (2010). Effect of leaf age and position on light-saturated CO2 assimilation rate, photosynthetic capacity, and stomatal conductance in rubber trees. Photosynthetica 48, 67–78. doi: 10.1007/s11099-010-0010-y
Krause G. H., Winter K., Krause B., and Virgo A. (2014). Light-stimulated heat tolerance in leaves of two neotropical tree species, Ficus insipida and Calophyllum longifolium. Funct. Plant Biol. 42, 42–51. doi: 10.1071/FP14095
Kumarathunge D. P., Medlyn B. E., Drake J. E., Tjoelker M. G., Aspinwall M. J., Battaglia M., et al. (2018). Acclimation and adaptation components of the temperature dependence of plant photosynthesis at the global scale. New Phytol. 222, 768–784. doi: 10.1111/nph.15668
Leakey A. D. B., Ainsworth E. A., Bernacchi C. J., Rogers A., Long S. P., and Ort D. R. (2009). Elevated CO2 effects on plant carbon, nitrogen, and water relations: Six important lessons from FACE. J. Exp. Bot. 60, 2859–2876. doi: 10.1093/jxb/erp096
Leakey A. D. B., Scholes J. D., and Press M. C. (2005). Physiological and ecological significance of sunflecks for dipterocarp seedlings. J. Exp. Bot. 56, 469–482. doi: 10.1093/jxb/eri055
Li L., Wang X., and Manning W. J. (2019). Effects of elevated CO2 on leaf senescence, leaf nitrogen resorption, and late-season photosynthesis in Tilia americana L. Front. Plant Sci 10. doi: 10.3389/fpls.2019.01217
Liu B., Kaurilind E., Zhang L., Okereke C. N., Remmel T., and Niinemets Ü. (2022). Improved plant heat shock resistance is introduced differently by heat and insect infestation: The role of volatile emission traits. Oecologia 199, 53–68. doi: 10.1007/s00442-022-05168-x
Marias D. E., Meinzer F. C., and Still C. (2017). Impacts of leaf age and heat stress duration on photosynthetic gas exchange and foliar nonstructural carbohydrates in Coffea arabica. Ecol. Evol. 7, 1297–1310. doi: 10.1002/ece3.2681
Munné-Bosch S., Queval G., and Foyer C. H. (2013). The impact of global change factors on redox signaling underpinning stress tolerance. Plant Physiol. 161, 5–19. doi: 10.1104/pp.112.205690
Naudts K., Van den Berge J., Farfan E., Rose P., AbdElgawad H., Ceulemans R., et al. (2014). Future climate alleviates stress impact on grassland productivity through altered antioxidant capacity. Environ. Exp. Bot. 99, 150–158. doi: 10.1016/j.envexpbot.2013.11.003
Niinemets Ü. (2018). When leaves go over the thermal edge. Plant Cell Environ. 41, 1247–1250. doi: 10.1111/pce.13184
Niinemets Ü., Cescatti A., Rodeghiero M., and Tosens T. (2005). Leaf internal diffusion conductance limits photosynthesis more strongly in older leaves of Mediterranean evergreen broad-leaved species. Plant Cell Environ. 28, 1552–1566. doi: 10.1111/j.1365-3040.2005.01392.x
Niinemets Ü., Cescatti A., Rodeghiero M., and Tosens T. (2006). ). Complex adjustments of photosynthetic potentials and internal diffusion conductance to current and previous light availabilities and leaf age in Mediterranean evergreen species Quercus ilex. Plant Cell Environ. 29, 1159–1178. doi: 10.1111/j.1365-3040.2006.01499.x
Niinemets Ü., Tenhunen J. D., Canta N. R., Chaves M. M., Faria T., Pereira J. S., et al. (1999). Interactive effects of nitrogen and phosphorus on the acclimation potential of foliage photosynthetic properties of cork oak, Quercus suber, to elevated atmospheric CO2 concentrations. Global Change Biol. 5, 455–470. doi: 10.1046/j.1365-2486.1999.00241.x
Okereke C. N., Kaurilind E., Liu B., Kanagendran A., Pazouki L., and Niinemets Ü. (2022). Impact of heat stress of varying severity on papaya (Carica papaya) leaves: Major changes in stress volatile signatures, but surprisingly small enhancements of total emissions. Environ. Exp. Bot. 195, 104777. doi: 10.1016/j.envexpbot.2021.104777
Okereke C. N., Liu B., Kaurilind E., and Niinemets Ü. (2021). Heat stress resistance drives coordination of emissions of suites of volatiles after severe heat stress and during recovery in five tropical crops. Environ. Exp. Bot. 184, 104375. doi: 10.1016/j.envexpbot.2021.104375
Onoda Y. and Wright I. J. (2018). “The leaf economics spectrum and its underlying physiological and anatomical principles,” in The Leaf: A platform for performing photosynthesis. Advances in Photosynthesis and Respiration, vol. 44 . Eds. Adams W. III and Terashima I. (Springer, Cham). doi: 10.1007/978-3-319-93594-2_16
Onoda Y., Wright I. J., Evans J. R., Hikosaka K., Kitajima K., Niinemets Ü., et al. (2017). Physiological and structural tradeoffs underlying the leaf economics spectrum. New Phytol. 214, 1447–1463. doi: 10.1111/nph.14496
Osnas J. L. D., Katabuchi M., Kitajima K., Wright S. J., Reich P. B., Van Bael S. A., et al. (2018). Divergent drivers of leaf trait variation within species, among species, and among functional groups. Proc. Natl. Acad. Sci. 115, 5480–5485. doi: 10.1073/pnas.1803989115
Paethaisong W., Suksawat M., Jirahiranpat A., Phetcharaburanin J., Wannapat S., Theerakulpisut P., et al. (2025). Short-term high temperature alters psbA gene expression and D1 protein related photosystem II function in rice seedlings. J. Agron. Crop Sci 211, e70043. doi: 10.1111/jac.70043
Reich P. and Hobbie S. (2013). Decade-long soil nitrogen constraint on the CO2 fertilization of plant biomass. Nat. Climate Change 3, 278–282. doi: 10.1038/nclimate1694
Reich P. B., Hobbie S. E., Lee T. D., and Pastore M. A. (2018). Unexpected reversal of C3 versus C4 grass response to elevated CO2 during a 20-year field experiment. Science 360, 317–320. doi: 10.1126/science.aas9313
Rodrigues W. P., Martins M. Q., Fortunato A. S., Rodrigues A. P., Semedo J. N., Simões, et al. (2016). Long-term elevated air [CO2] strengthens photosynthetic functioning and mitigates the impact of supra-optimal temperatures in tropical Coffea arabica and C. canephora species. Global Change Biol. 22, 415–431. doi: 10.1111/gcb.13088
Ruelland E. and Zachowski A. (2010). How plants sense temperature. Environ. Exp. Bot. 69, 225–232. doi: 10.1016/j.envexpbot.2010.05.011
Sanz-Sáez Á., Erice G., Aranjuelo I., Aroca R., RuÍz-Lozano J. M., Aguirreolea J., et al. (2013). Photosynthetic and molecular markers of CO2-mediated photosynthetic downregulation in nodulated Alfalfa. J. Integr. Plant Biol. 55, 721–734. doi: 10.1111/jipb.12047
Schaffer B., Wolstenholme B. N., and Whiley A. W. (Eds.) (2013). The avocado: Botany, production and uses. 2nd ed. (Wallingford, Oxfordshire, UK: CABI). doi: 10.1079/9781845937010.0000
Singh S. K. and Reddy V. R. (2016). Methods of mesophyll conductance estimation: Its impact on key biochemical parameters and photosynthetic limitations in phosphorus-stressed soybean across CO2. Physiologia Plantarum 157, 234–254. doi: 10.1111/ppl.12415
Slot M. and Winter K. (2017). Photosynthetic acclimation to warming in tropical forest tree seedlings. J. Exp. Bot. 68, 2275–2284. doi: 10.1093/jxb/erx071
Strandsbjerg Tristan Pedersen J., Duarte Santos F., van Vuuren D., Gupta J., Encarnação Coelho R., Aparício B. A., et al. (2021). An assessment of the performance of scenarios against historical global emissions for IPCC reports. Global Environ. Change 66, 102199. doi: 10.1016/j.gloenvcha.2020.102199
Sulaiman H. Y., Liu B., Abiola Y. O., Kaurilind E., and Niinemets Ü. (2023). Impact of heat priming on heat shock responses in Origanum vulgare: Enhanced foliage photosynthetic tolerance and biphasic emissions of volatiles. Plant Physiol. Biochem. 196, 567–579. doi: 10.1016/j.plaphy.2023.02.013
Sun W., Maseyk K., Lett C., and Seibt U. (2024b). Restricted internal diffusion weakens transpiration-photosynthesis coupling during heatwaves: Evidence from leaf carbonyl sulphide exchange. Plant Cell Environ. 47, 1813–1833. doi: 10.1111/pce.14840
Sun Z., Niinemets Ü., Hüve K., Rasulov B., and Noe S. M. (2013a). Elevated atmospheric CO2 concentration leads to increased whole-plant isoprene emission in hybrid aspen (Populus tremula × Populus tremuloides). New Phytol. 198, 788–800. doi: 10.1111/nph.12200
Tarvainen L., Wittemann M., Mujawamariya M., Manishimwe A., Zibera E., Ntirugulirwa B., et al. (2022). Handling the heat - photosynthetic thermal stress in tropical trees. New Phytol. 233, 236–250. doi: 10.1111/nph.17809
Taub D. R., Seemann J. R., and Coleman J. S. (2000). Growth in elevated CO2 protects photosynthesis against high-temperature damage. Plant Cell Environ. 23, 649–656. doi: 10.1046/j.1365-3040.2000.00574.x
Terrer C., Vicca S., Stocker B. D., Hungate B. A., Phillips R. P., Reich P. B., et al. (2018). Ecosystem responses to elevated CO2 governed by plant-soil interactions and the cost of nitrogen acquisition. New Phytol. 217, 507–522. doi: 10.1111/nph.14872
Teskey R., Wertin T., Bauweraerts I., Ameye M., Mcguire M. A., and Steppe K. (2015). Responses of tree species to heat waves and extreme heat events. Plant Cell Environ. 38, 1699–1712. doi: 10.1111/pce.12417
Tian Z., Luo Q., Li Y., and Zuo Z. (2020). Terpinene and β-pinene acting as signaling molecules to improve Cinnamomum camphora thermotolerance. . Ind. Crops Products 154, 112641. doi: 10.1016/j.indcrop.2020.112641
Tosens T., Nishida K., Gago J., Coopman R. E., Cabrera H. M., Carriquí M., et al. (2016). The photosynthetic capacity in 35 ferns and fern allies: Mesophyll CO2 diffusion as a key trait. New Phytol. 209, 1576–1590. doi: 10.1111/nph.13719
Turan S., Kask K., Kanagendran A., Li S., Anni R., Talts E., et al. (2019). Lethal heat stress-dependent volatile emissions from tobacco leaves: What happens beyond the thermal edge? J. Exp. Bot. 70, 5017–5030. doi: 10.1093/jxb/erz255
Ulfat A., Mehmood A., Ahmad K. S., and Ul-Allah S. (2021). Elevated carbon dioxide offers promise for wheat adaptation to heat stress by adjusting carbohydrate metabolism. Physiol. Mol. Biol. Plants 27, 2345–2355. doi: 10.1007/s12298-021-01080-5
Urban O., Hrstka M., Zitová M., Holišová P., Šprtová M., Klem K., et al. (2012). Effect of season, needle age and elevated CO2 concentration on photosynthesis and Rubisco acclimation in Picea abies. Plant Physiol. Biochem. 58, 135–141. doi: 10.1016/j.plaphy.2012.06.023
von Caemmerer S. and Farquhar G. D. (1981). Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta 153, 376–387. doi: 10.1007/BF00384257
Wahid A., Gelani S., Ashraf M., and Foolad M. (2007). Heat tolerance in plants: An overview. Environ. Exp. Bot. 61, 199–223. doi: 10.1016/j.envexpbot.2007.05.011
Watson-Lazowski A., Cano F. J., Kim M., Benning U., Koller F., George-Jaeggli B., et al. (2024). Multi-omic profiles of Sorghum genotypes with contrasting heat tolerance connect pathways related to thermotolerance. J. Exp. Bot., erae506. doi: 10.1093/jxb/erae506
Xu Z., Shimizu H., Ito S., Yagasaki Y., Zou C., Zhou G., et al. (2014). Effects of elevated CO2, warming and precipitation change on plant growth, photosynthesis and peroxidation in dominant species from North China grassland. Planta 239, 421–435. doi: 10.1007/s00425-013-1987-9
Yurina N. P. (2023). Heat shock proteins in plant protection from oxidative stress. Mol. Biol. 57, 951–964. doi: 10.1134/S0026893323060201
Zahra N., Hafeez M. B., Ghaffar A., Kausar A., Zeidi M. A., Siddique K. H. M., et al. (2023). Plant photosynthesis under heat stress: Effects and management. Environ. Exp. Bot. 206, 105178. doi: 10.1016/j.envexpbot.2022.105178
Zhang H., Pan C., Gu S., Ma Q., Zhang Y., Li X., et al. (2019). Stomatal movements are involved in elevated CO2-mitigated high temperature stress in tomato. Physiologia Plantarum 165, 569–583. doi: 10.1111/ppl.12752
Zheng Y., Li F., Hao L., Yu J., Guo L., Zhou H., et al. (2019). Elevated CO2 concentration induces photosynthetic down-regulation with changes in leaf structure, non-structural carbohydrates and nitrogen content of soybean. BMC Plant Biol. 19, 255. doi: 10.1186/s12870-019-1788-9
Zhu T., Fonseca De Lima C. F., and De Smet I. (2021). The heat is on: How crop growth, development, and yield respond to high temperature. J. Exp. Bot. 72, 7359–7373. doi: 10.1093/jxb/erab308
Zinta G., AbdElgawad H., Domagalska M. A., Vergauwen L., Knapen D., Nijs I., et al. (2014). Physiological, biochemical, and genome-wide transcriptional analysis reveals that elevated CO2 mitigates the impact of combined heat wave and drought stress in Arabidopsis thaliana at multiple organizational levels. Global Change Biol. 20, 3670–3685. doi: 10.1111/gcb.12626
Keywords: carbon dioxide enrichment, leaf developmental stage, nitrogen content, phosphorus content, gas exchange, Rubisco carboxylase activity, tropical fruit trees, thermal tolerance
Citation: Abiola YO, Sulaiman HY, Kaurilind E and Niinemets Ü (2025) Elevated growth [CO2] enhances heat stress resistance of photosynthesis in young leaves of avocado (Persea americana). Front. Plant Physiol. 3:1638556. doi: 10.3389/fphgy.2025.1638556
Received: 30 May 2025; Accepted: 22 October 2025;
Published: 05 November 2025.
Edited by:
Mina Momayyezi, University of California, Davis, United StatesReviewed by:
Carolina Elisa Sanhueza, University of Concepcion, ChileAbir Das, University of Kalyani, India
Copyright © 2025 Abiola, Sulaiman, Kaurilind and Niinemets. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yusuph Olawale Abiola, eXVzdXBoQGVtdS5lZQ==
 Yusuph Olawale Abiola
Yusuph Olawale Abiola Hassan Yusuf Sulaiman1
Hassan Yusuf Sulaiman1