- 1Cancer Day-Care Unit, Division of Medical Oncology, Cancer Center, West China Hospital, Sichuan University/West China School of Nursing, Sichuan University, Chengdu, China
- 2Department of Critical Care Medicine, West China Hospital, Sichuan University/West China School of Nursing, Sichuan University, Chengdu, China
- 3Thoracic Oncology Ward, Cancer Center, West China Hospital, Sichuan University/West China School of Nursing, Sichuan University, Chengdu, China
- 4Department of Nursing, West China Hospital, Sichuan University/West China School of Nursing, Sichuan University, Chengdu, China
Introduction: Cancer-related distress can be described as a complex and unpleasant combination of psychological (such as cognitive, behavioral, and emotional), social, and spiritual challenges that may impact an individual’s ability to effectively cope with the physical symptoms of cancer and its treatment. Existing literature has confirmed psychological distress (PD) as an important sequela of breast cancer diagnosis and treatment. However, the incidence and risk factors for PD in adult female patients with breast cancer remain unclear; therefore, focusing on the PD of female breast cancer patients is meaningful, as they are at highest risk of contracting breast cancer, and might differ in their coping styles from men.
Objective: This review aimed to identify the incidence and risk factors for PD in adult woman patients with breast cancer, and to help guide targeted intervention to prevent distress.
Method: PubMed, Embase, Cochrane Library, CINAL, PsycINFO, China Knowledge Resource Integrated Database, Wanfang Database, the Chinese Biomedical Database, and Weipu Database were searched for data regarding the incidence and risk factors of PD in adult women with breast cancer.
Results: The prevalence of PD, assessed using the distress thermometer, ranged between 11.2%–86.7%, and a meta-analysis of 47 studies with 15,157 adult female breast cancer patients showed that the pooled prevalence was 52.0%. Further, this study identified 40 risk factors. However, owing to the inclusion of at least two studies for a certain risk factor, 10 risk factors were merged for the meta-analysis. Independent risk factors included higher education level, late-stage tumor, emotional concerns, no medical insurance, modified radical mastectomy, and history of depression; age and neuroticism were not associated with PD; and higher monthly income was revealed as a protective factor against it.
Conclusion: The incidence of PD in female patients with breast cancer is high and it involves 10 risk factors, though some are controversial owing to insufficient evidence. Further research is needed to explore the underlying mechanisms of PD and develop risk factor-based holistic intervention programs to reduce its incidence.
Systematic review registration: The protocol of this study has been registered in the database PROSPERO (registration ID: CRD42023433578).
1 Introduction
In 2020, breast cancer became the most common malignant tumor in the world, surpassing lung cancer and ranking first in female cancer (1). The development of multimodality diagnosis and treatment has greatly improved the long-term survival rate of patients with breast cancer and has resulted in an increasing number of breast cancer survivors. Currently, the 5- and 10-year survival rates of patients with breast cancer are 90% and 80%, respectively (2). Consequently, quality of life has become an important measure of patient outcomes in modern oncology (3). Mitchell et al. (4) showed that 30%–40% of patients with cancer had psychological problems, which led to a decrease in treatment compliance, medical satisfaction, and quality of life.
The International Psycho-Oncology Society (5) identified psychological distress (PD) as the sixth most important vital sign in 2010 and included PD assessment as a routine item in clinical care practice. The National Comprehensive Cancer Network (NCCN) (6) describes cancer-related distress as a complex and unpleasant combination of psychological (such as cognitive, behavioral, and emotional), social, and spiritual challenges that may impact an individual’s ability to effectively cope with the physical symptoms of cancer and its treatment. This suggests that PD should be rapidly identified, managed, recorded, and treated at any stage of cancer, especially when the condition changes. Furthermore, PD should be treated according to clinical guidelines. Unmanaged PD negatively affects cancer-related morbidity, mortality, and quality of life (7, 8). Marco and White (9) showed that patients with cancer have higher levels of PD than the normal population, with approximately 21% and 13% of patients suffering from anxiety and depression, respectively, causing a poorer quality of life.
In recent years, the literature on the incidence and risk factors of PD in patients with cancer has increased. In Denmark, 8% of women experienced severe distress throughout the first eight months following diagnosis (10). In Korea, 19.4% of patients with breast cancer were in a state of continuous high PD one year after diagnosis (11). The latest systematic review from 2020 found that the pooled prevalence of PD from 17 studies covering 3,870 patients with breast cancer was 50% (12). However, this systematic review does not distinguish between the sexes, yet male and female patients with breast cancer have different epidemiological patterns, risk factors, and diagnostic features. Further, their molecular and clinicopathological features, as well as personality traits and psychological characteristics are significantly different, and their PD may also be different (13, 14). Compared with male breast cancer, female breast cancer has a higher incidence rate (1). It is meaningful to carry out a systematic review based on female PD. In addition, the review mainly assesses English literature. Although this can reflect the incidence rate of PD in breast cancer patients to a large extent, considering that China’s breakthrough breast cancer population accounts for a considerable proportion of that of the world (1), the inclusion of Chinese literature could increase the persuasiveness of the results. Finally, although this systematic review confirms that the distress thermometer (DT) is a fast, self-reported distress screening tool for cancer patients, it does not it does not further explore the influencing factors of PD evaluated through this tool. Another systematic review from 2016 (15) explored the predictive factors of PD from the perspective of female patients with breast cancer; however, there is no consensus on the definition of PD in the included literature. Multiple measurement tools, such as the DT, Hamilton Anxiety and Depression Scale, Hospital Anxiety and Depression Scale, are used to measure PD, which may lead to heterogeneity in the final results.
The DT is a simple and convenient scale that has been widely used to screen for PD in patients with cancer. Its effectiveness and reliability have been tested in many countries and regions (16, 17). However, the optimal cutoff value of the scale remains disputed (18). In different studies, the optimal threshold for DT ranged from four to seven points (12). For breast cancer, studies in Denmark and the United States recommended a score of seven as the best cutoff to define PD, whereas studies in Indonesia recommended five as the best cutoff score (19–21). These differences are caused by variations in the cultural backgrounds, lifestyles, and expressions of different regions; for example, Western culture tends to be more extroverted, whereas Eastern culture is more introverted. Therefore, it is crucial to conduct a systematic review of studies on PD assessment among patients with breast cancer using DT diagnostic tools, which will help to clearly and more comprehensively understand the current situation of this population.
Some extant literature has reported many factors that increase the PD of patients with breast cancer. This includes demographic characteristics, such as age (22, 23) and education level (24); sociological characteristics, such as economic issues (25) and healthcare payment status (24); and disease status, such as oncological staging (23, 26), treatment type (27), and social support level (28). However, other studies have not found these associations (29, 30). Thus, elucidating the incidence and influencing factors of PD can help raise the awareness of PD among healthcare professionals and provide guidance and reference for developing targeted and optimal intervention measures. Through this study, we aimed to (1) determine the incidence of PD in women with breast cancer and (2) determine the predictive factors of PD.
2 Methods
2.1 Protocol and registration
This review was conducted in accordance with the Meta-Analysis of Observational Studies in Epidemiology guidelines. The detailed study protocol is available on the PROSPERO website under the registration number CRD42023433578.
2.2 Search strategy
We systematically searched the following databases for through April 2023: PubMed, Embase, Cochrane Library (CENTRAL), CINAL (via EBSCO), PsycINFO, China Knowledge Resource Integrated Database (CNKI), Wanfang Database, and the Chinese Biomedical Database (CBM), and Weipu Database (VIP). The search strategies were performed using a combination of mesh terms and free words. Search strings contained the terms “breast neoplasms,” “breast neoplas*,” “breast tumor*,” “breast cancer,” “breast carcinoma*,” “mammary cancer*,” “mammary carcin*,” “mammary neoplas*,” “breast metasta*,” “breast malig*,” “breast malignant neoplas*,” “malignant neoplasm of breast,” “breast malignant tumor*,” “malignant tumor of breast,” and “psychological distress,” “psychiatric distress,” “emotional distress,” emotional stress,” “mental distress,” “distress thermometer,” “distress symptom,” “distress;” and “risk factors,” “risk factor*,” “risk*,” “predictor,” “predictive factor,” “influence factor,” “correlat*,” “predict*,” “prevalence,” “incidence,” “incident,” “epidemiology,” “rate,” “frequency,” “occurrence,” “morbidity,” “proportion,” and “probability.” The precise search strategies for the English databases are presented in the Appendix (Supplementary Table 1). The reference lists included in the identified articles were manually searched for additional relevant publications.
2.3 Study selection
After removing duplicate studies, two investigators independently assessed eligible publications by screening titles and abstracts according to the inclusion and exclusion criteria. When at least one reviewer decided that an abstract was eligible for inclusion, full texts and articles were retrieved. Each publication was independently assessed by both investigators for inclusion in the final study. Disagreements were resolved through discussions.
The inclusion criteria were as follow: (1) cross-sectional studies and cohort studies; (2) study participants were adult (age ≥ 18 years) females diagnosed with breast cancer; (3) prevalence and/or risk factors of PD in patients with breast cancer; and (4) PD was evaluated using the DT, which was recommended by the NCCN Cancer Clinical Guidelines. The DT scoring system is similar to the classic visual distress scoring method, with 0 representing “no PD” and 10 representing “extreme PD.” The guidelines recommend considering PD of at least 4 points as clinically significant. However, they do not provide a clear definition of the scores for mild, moderate, severe, and extremely severe PD. Consequently, different countries have inconsistent classifications of DT. Most studies classify DT of at least 3 or 4 points as moderate distress, and at least 6 or 7 as severe. No language restrictions were applied to eligible studies. The exclusion criteria were as follows: (1) conference abstracts, reviews, and study protocols; (2) unavailable full text; (3) sample size below 50, which is deemed small by the British statistician Gosset (31). A study with a relatively small sample size is more likely to lack sufficient statistical power to detect the true positive association if the result is negative; when the result is positive, the finding could possibly be due to the smaller sample size; and (4) low research quality.
2.4 Data extraction
Two authors independently extracted the following data using a data extraction form developed a priori: (1) study characteristics, including author name, title, and year of publication; (2) population characteristics, including country, age, sample size, and the initial time of distress identified; and (3) DT cutoff score and prevalence of PD in breast cancer. The risk factors of PD, odds ratios (ORs), and 95% confidence intervals (CIs) of the independent risk factors for distress in breast cancer were extracted. If outcome data were unclear or not reported, we contacted the authors to obtain missing data. Disagreements were resolved through consensus or by a third author.
2.5 Quality appraisal
Two authors independently assessed the quality of the included studies, as previously described. The quality of cross-sectional studies was assessed using an 11-item checklist, which was recommended by the Agency for Healthcare Research and Quality (AHRQ) (32). Each item is scored as “1” if it was answered “YES” and “0” if it was answered “NO” or “UNCLEAR.” The highest score is 11, with the quality level being assessed as follows: low quality=0–3; moderate quality=4–7; and high quality=8–11. The AHRQ results showed that the included studies scored between 5 and 9.
The Newcastle–Ottawa Quality Scale (NOS) (33) was used to assess the quality of the cohort studies, including study participant selection, intergroup comparability, and exposure factors. The NOS includes three domains and eight items, of which 9 points are full. A score of 5 was considered high quality. Two reviewers independently evaluated the included studies. They discussed the results and arrived at a consensus on each item of the checklist for each study. The results of the NOS evaluation showed that all the included studies had a score of ≥5 and were of high quality. Four studies were rated full marks. The detailed scores are shown in Supplementary Tables 2, 3.
2.6 Statistical analysis
Statistical analyses were conducted using Stata 14 software and RevMan 5.3. The pooled prevalence and 95% CIs for PD were calculated using Stata 14, and the pooled risk factors and 95% CIs for PD were calculated using RevMan 5.3. Due to inconsistent cutoff values for DT included in the study, when combining the incidence of PD, when there was only one cutoff value, the incidence was directly included. When there were two cutoff values, the incidence of lower cutoff values was included. Statistical heterogeneity in the pooled results was assessed using the chi-square test, Cochran’s Q-test, and the inconsistency I2 test, with I2 values of 25%, 50%, and 75% indicating low, moderate, and high heterogeneity, respectively. Pooled prevalence, risk factors, and 95% CIs for PD were calculated using a random-effects model when Cochrane’s Q statistic detected significant heterogeneity, and they were shown in the forest plot. Subgroup analysis was performed to assess the incidence of PD in different DT cutoff scores, treatment phase at initial distress assessment, and countries, and the results were combined using the inverse variance method for ORs and 95% CIs. Descriptive analyses were performed for data that could not be combined. Publication bias was identified using a funnel plot, and asymmetry was tested using Egger’s linear regression method (p<0.1, considered significant).
3 Results
3.1 General results of the included studies
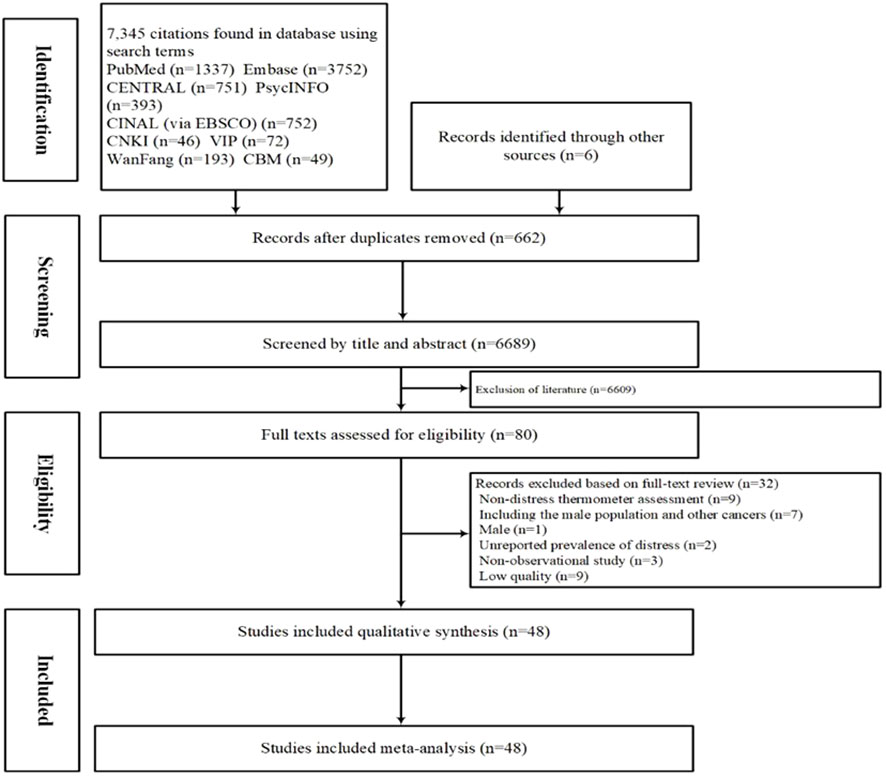
The initial search retrieved 7,351 articles, of which 662 were duplicates. After excluding 6,689 studies based on their titles and abstracts, the full texts of 80 publications were examined. Of these, 48 (13 in Chinese and 35 in English) met the inclusion criteria and were therefore included in the meta-analysis (Figure 1).
3.2 Characteristics of the included studies
The characteristics of the 48 analyzed studies are summarized in Table 1. The literature was published between 2006 and 2023, the sample sizes ranged from 77 to 1,400, with mean ages ranging from 45.1 to 77.0 years. Forty-seven studies with 15,157 adult female breast cancer in total reported the prevalence of PD. Thirteen studies reported risk factors for PD. In addition, regarding geographic regions, 19 studies were from Asia, 18 from Europe, 10 from North America, and 1 from Africa. Concerning the treatment phase at initial distress assessment, the studies included newly diagnosed breast cancer patients, breast cancer survivors, and those receiving surgical treatment, adjuvant therapy.
3.3 Prevalence of psychological distress
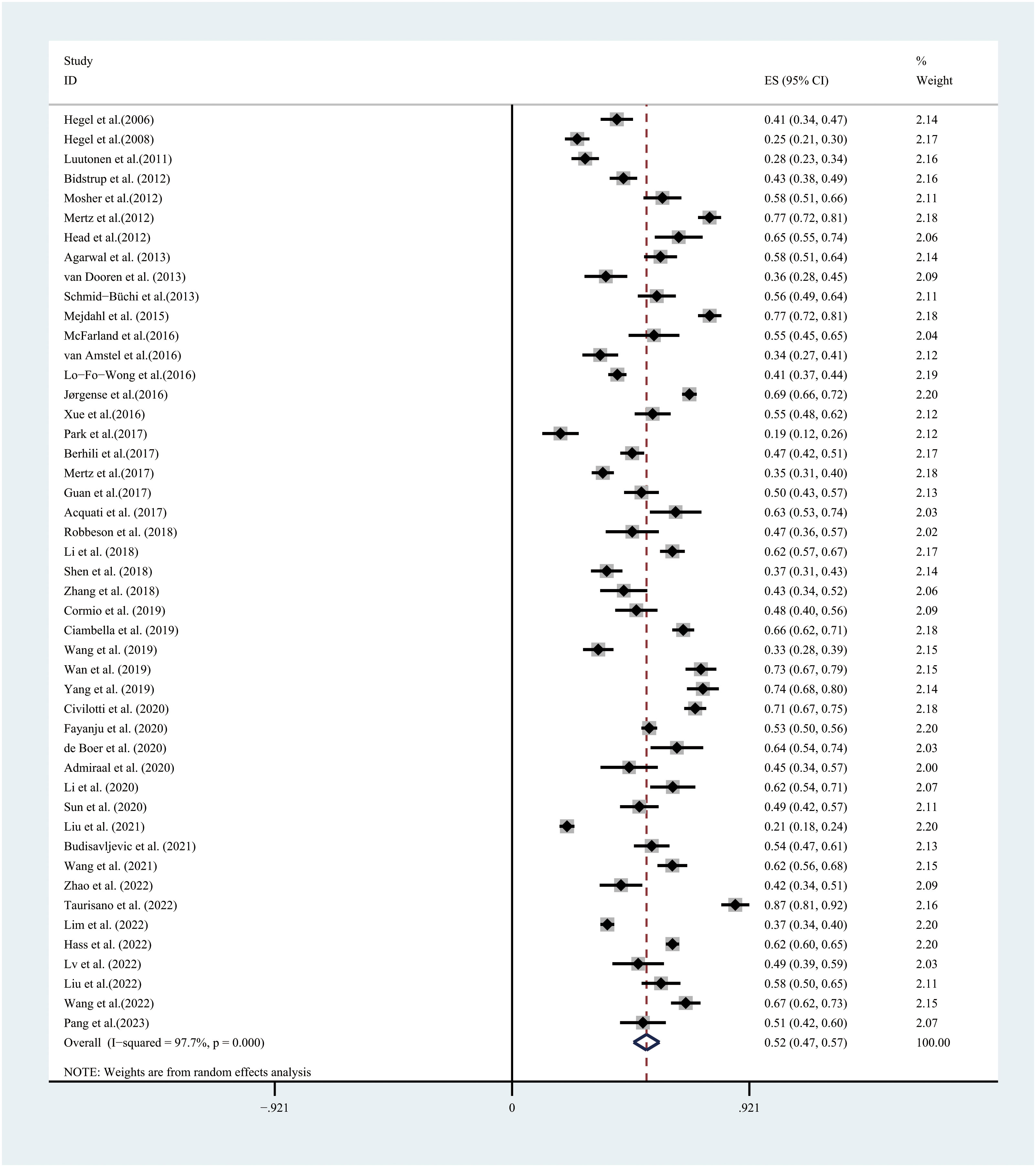
In the 47 studies available for the meta-analysis, the prevalence of PD, assessed using the DT, ranged between 11.2%–86.7%. PD, based on a random-effects model, showed that the overall PD prevalence was 52.0% (95% CI:47.0%–57.0%, I2 = 97.7%, P=0.000) (Figure 2).
3.4 Subgroup prevalence of psychological distress
A subgroup analysis was conducted to explore the heterogeneity between studies. Those with different DT cutoff scores (DT≥3, DT≥4, DT≥5, DT≥6, and DT≥7), countries (American, Asian, European, and African), and treatment phase at initial distress assessment (newly diagnosed, adjuvant treatment, mixed treatment phases, and survivorship) were grouped and analyzed separately; the pooled estimates showed that the pooled prevalence of PD was similar within subgroups. In the random-effects model, the estimates of pooled prevalence were calculated for different DT cutoff scores; DT≥3, DT≥4, DT≥5, DT≥6, and DT≥7 were 67.0%, 55.0%, 44.0%, 44.0%, and 32.0%, with high heterogeneity observed between studies (I2 = 98.2%, I2 = 96.7%, I2 = 98.7%, I2 = 51.7%, I2 = 95.8%). The estimated pooled prevalence rates of PD in American, Asian, European, and African countries were 50.0%, 51.0%, 54.0%, and 46.0%, respectively, with high heterogeneity observed between studies. The estimated pooled prevalence rates of PD were 53.0% for newly diagnosed participants, 45.0% for those undergoing adjuvant treatment, 55.0% for mixed treatment phase patients, and 49.0% for survivorship, but with significant heterogeneity. The estimated pooled results obtained from the subgroup analyses are shown in Table 2. A forest plot of the subgroup analysis results is presented in the Supplementary Materials (Supplementary Figures 1–3).
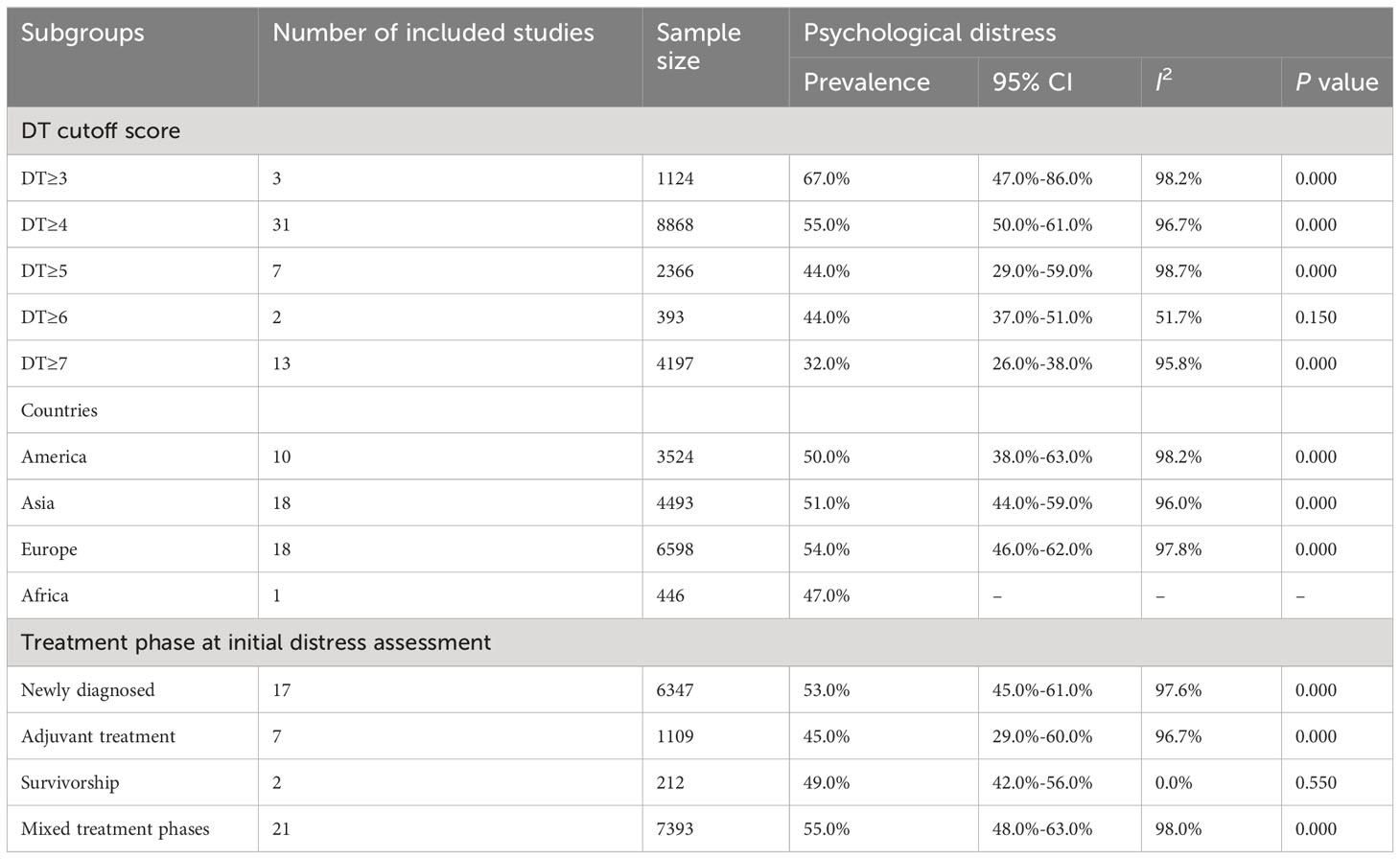
Table 2 Subgroup analyses based on different DT cutoff score, countries, and stage of breast cancer.
3.5 Risk factors
The pooled analysis revealed that higher education level, late stage of the tumor, emotional concerns, no medical insurance, modified radical mastectomy, and history of depression were independent risk factors. Age and neuroticism were not associated with PD, and higher monthly income was a protective factor against PD. Descriptive analyses were used for data that could not be combined. Detailed results of the risk factors are presented in Table 3.
3.6 Risk of bias in the included studies
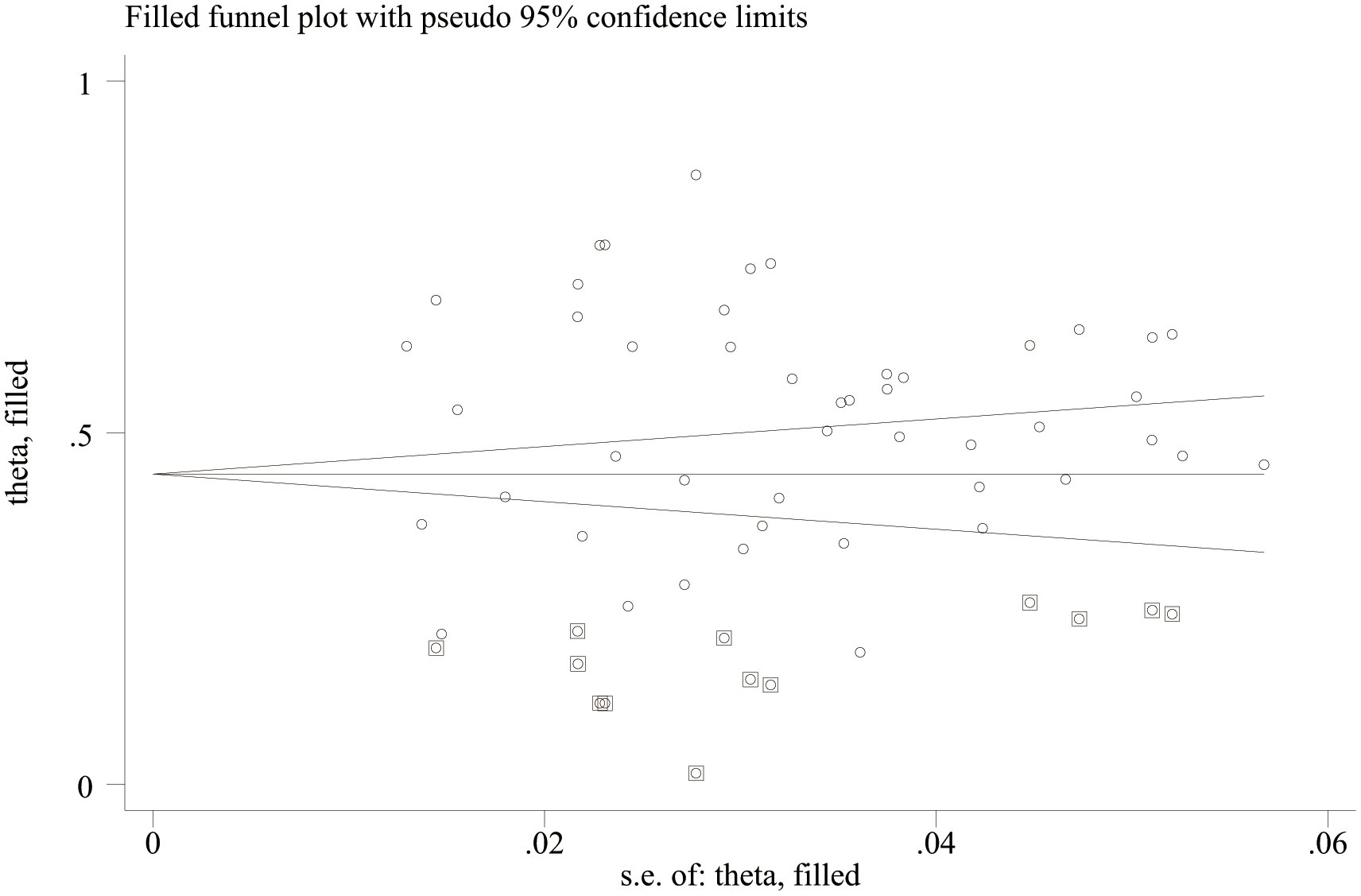
Publication bias analysis showed that there was no significant publication bias in the literature included in this study (Egger’s test, p = 0.763; Begg’s test, p = 0.673, Figure 3), indicating that, when compared with a single study, this study can more reliably reflect the PD of female patients with breast cancer and more objectively identify risk factors. There was no evidence of publication bias in the prevalence of PD.
4 Discussion
4.1 Resource identification initiative
This was a systematic review and meta-analysis of the incidence and risk factors of PD in patients with breast cancer. The DT revealed a prevalence of PD ranging between 11.2%–86.7%, while the meta-analysis revealed an overall estimated 52.0% prevalence of PD among female patients with breast cancer. Further, this study analyzed 10 potential risk factors and revealed that higher education level, late-stage tumor, emotional concerns, no medical insurance, modified radical mastectomy, and history of depression were independent risk factors. Age and neuroticism were not associated with PD, and higher monthly income was a protective factor for PD.
4.2 Prevalence of psychological distress
This study shows that female patients with breast cancer have a high prevalence rate of PD, and that the prevalence rate of PD varies across different regions. This indicates that the detection rate of PD in female patients with breast cancer may differ owing to the selection of samples, cultural differences, economic and social conditions, and other factors. The pooled prevalence rates of PD among newly diagnosed patients, patients undergoing adjuvant treatment, and survivorship were 53.0%, 45.0%, and 49.0%, respectively. A higher degree of PD in newly diagnosed patients was consistent with the findings of Ribnikar et al. (75). Most patients may be in denial at the time of diagnosis, which increases their negative emotions; the patients do not know much about cancer, malignancy, and aggressiveness, and the uncertainty of the disease is the main reason for PD (76, 77). However, the adjuvant phase is a period of high concern for current medical workers, and a range of care measures have been developed to alleviate the PD of patients with breast cancer who are in the radiotherapy and chemotherapy phases (78, 79). Relatively few interventions have been developed for the PD of newly diagnosed patients. Owing to limited medical resources, newly diagnosed patients often need to wait for admission to hospitals to receive treatment (80). Outside the hospital, it is difficult for nurses to intervene and mobilize family and community resources to manage the PD of newly diagnosed patients with breast cancer; thus, this should become the focus of future research.
4.3 Risk factors of psychological distress
4.3.1 Demographic variables
Age is not associated with PD. As such, PD management interventions targeting different age groups may not significantly reduce the PD of female patients with breast cancer. A higher education level is identified to be an independent risk factor for PD. People with higher education levels possess a better understanding of the occurrence, development, prognosis, and potential harm of diseases in various ways and are especially sensitive to disease prognoses, leading to heavier psychological burden (81). Medical insurance was not identified as a risk factor for PD. The treatment cycle of breast cancer is long, and as treatment becomes more extensive, the associated costs also increase, causing financial difficulties for patients (82). Patients with poor economic conditions may fear missing the optimal time for diagnosis and treatment, leading to heightened negative psychological emotions (83). By contrast, patients with good economic status can access more medical and social resources, resulting in lower levels of PD (84). Lack of medical insurance places greater economic pressure on patients and increases their psychological and spiritual burdens, further exacerbating their PD (85, 86). Higher monthly income was found to be a protective factor against PD. Furthermore, Tao et al. (87) revealed that 43.9% of patients with breast cancer were unemployed after diagnosis. Therefore, implementing various measures to promote the return of such patients to work and increase family income is crucial (88).
4.3.2 Clinical variables
Patients with late-stage tumors were found to be more prone to PD. Iwatani et al. (89) reported that tumor staging was an important predictor of PD, and patients with late-stage breast cancer were more likely to experience PD than those with early-stage cancer, consistent with the present study’s findings. There is a consensus that later stages have a worse prognosis (90). Terminal patients often experience poor treatment effects, leading to profound PD (91). Doctors and nurses can collaborate with psychotherapists and social groups for strengthening psychological intervention to address these negative experiences of PD. A history of depression is an independent risk factor for PD. Patients with a history of depression experienced more severe PD than patients with cancer. Another risk factor is modified radical mastectomy. In modified radical mastectomy, a procedure for breast cancer patients, the mammary glands, including the nipple areola complex, are removed (92). Moreover, during the procedure, there are different levels of lymph node dissection according to the particular stage of the disease (93). The surgical trauma is large, and adverse events related to wound healing are likely to occur after the surgery (94, 95). This may cause patients undergoing modified radical surgery to experience a higher level of physical pain. Additionally, post-operative visual defects of the breast may inevitably cause significant psychological trauma (96, 97). These outcomes may be important factors related to psychological distress in patients undergoing modified radical surgery for adenocarcinoma. Thus, healthcare professionals should provide psychological counseling and psychological support to patients undergoing modified radical surgery as early as possible.
4.3.3 Psychosocial variables
Neuroticism was not found to be associated with PD. This conclusion was drawn mainly after merging the results of two articles (i.e., 11, 46). Due to limitations in the quality and quantity of studies included, this conclusion still requires more high-quality literature to support it. Theoretically, neuroticism is a significant predictor of adverse psychological outcomes in patients with cancer (98). People with neurotic personalities experience intense negative emotions in the face of difficulties such as a breast cancer diagnosis and its treatment—a state that may contribute to PD (99, 100). Emotional concerns have been identified as risk factors for PD. Breast cancer patients will suffer the first major blow when diagnosed; after diagnosis, they usually receive comprehensive treatment such as surgery, radiotherapy and chemotherapy (11). The pain of treatment, changes in physical appearance, and a series of other adverse reactions (e.g., related to high costs, aesthetic problems, cognitive impairment, sexual dysfunction) lead to the continuous negative emotions of most breast cancer patients after diagnosis (91). Therefore, it is crucial to provide continuous emotional support to manage the PD of breast cancer patients. On the one hand, clinical medical staff should timely assess breast cancer patients’ emotional problems, dynamically screen their psychological pain risk, and promptly refer them to professional psychologists or psychological consultants when necessary (101); On the other hand, various psychological intervention measures can be adopted, such as cognitive training (102), mindfulness meditation training (103), music therapy (79), etc., to improve individual perceptual sensitivity, enhance emotional regulation ability, enhance focus, and accept oneself.
4.4 Implications
Considering the high prevalence rate of PD (52.0%) uncovered in this study, it is necessary to raise clinicians’ awareness of the PD of women with breast cancer and arouse their attention to the urgency with which it should be addressed. Further, this study uncovered the predictive factors of PD of women with breast cancer. Specifically, higher education level, late-stage tumor, emotional concerns, no medical insurance, modified medical were identified as risk factors of PD; higher monthly income was identified as a protective factor against PD. Therefore, in the future, management of these factors, especially those that are controllable, such as emotional concerns, should be integrated into efforts to manage the PD of women with breast cancer. Clinicians are further recommended to incorporate initial screening and daily dynamic assessment of PD into clinical pathway management. Additionally, measures such as cognitive training (91), mindfulness meditation training (101), and music therapy (78), which are increasingly being used to treat emotional concerns in cancer patients and have been proven effective, should be used to supplement their treatment. Moreover, as a protective factor, higher monthly income may be difficult to control, but clinicians could provide social fund support channels for patients.
4.5 Limitations
This study had some limitations. First, some of the 48 included studies were of average quality, as the availability of high-quality studies was limited. Second, some included studies were a cross-sectional design; therefore, we do not know precisely about the direction of the association for some of the risk factor. Third, although sensitivity analyses and subcomponents were performed in this study, a considerable amount of heterogeneity was present among the studies, and some studies’ underlying characteristics were unclear, leading to analysis limitations. Especially, although this study has confirmed that the incidence of PD of breast cancer patients differs across countries, considering that there are too few studies in individual countries, such as Africa, it did not further analyze the incidence of PD of breast cancer patients in different countries under different cutoff values. Future research can explore this area to strengthen the results. Forth, the population of men with breast cancer is significantly smaller than that of women with breast cancer; hence, this study excluded men with breast cancer, potentially missing important information. For example, men with breast cancer may experience higher levels of PD and may thus require more attention. Finally, this research included studies using only the DT as the PD assessment tool. Therefore, future research should explore more psychological problems identified using other evaluation tools.
5 Conclusions
The current analysis indicates an overall pooled prevalence of PD of 52.0%, highlighting the necessity of evaluating and managing the PD of female patients with breast cancer. This systematic review has also established a set of evidence-based predictors that can be used to identify females at higher risk of experiencing PD. For example, higher education level, late-stage tumor, emotional concerns, no medical insurance, and modified radical were identified as independent risk factors. Higher monthly income was revealed as a protective factor against PD, suggesting that it is meaningful to directly provide financial support to patients with breast cancer or encourage them to return to work. Understanding the risk and protective factors of PD can help healthcare personnel manage the PD and treatment of female patients with breast cancer. Furthermore, these results can provide useful information for the development of a risk stratification algorithm for female breast cancer patients’ PD. This algorithm could help identify women with a high risk of suffering PD, thus aiding in the accurate prediction and early intervention of PD.
Author contributions
LT: Writing – review & editing, Writing – original draft, Software, Methodology, Formal analysis, Data curation, Conceptualization. YX: Writing – review & editing, Writing – original draft, Software, Methodology, Formal analysis, Data curation, Conceptualization. XZ: Writing – original draft, Methodology. LF: Writing – original draft, Validation, Methodology. JL: Writing – original draft, Validation, Methodology. HC: Writing – original draft, Supervision, Conceptualization.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2024.1309702/full#supplementary-material
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. Nardin S, Mora E, Varughese FM, D’Avanzo F, Vachanaram AR, Rossi V, et al. Breast cancer survivorship, quality of life, and late toxicities. Front Oncol. (2020) 10:864. doi: 10.3389/fonc.2020.00864
3. Licu M, Ionescu CG, Paun S. Quality of Life in Cancer Patients: The modern psycho-oncologic approach for romania-a review. Curr Oncol. (2023) 30(7):6964–75. doi: 10.3390/curroncol30070504
4. Mitchell AJ, Chan M, Bhatti H, Halton M, Grassi L, Johansen C, et al. Prevalence of depression, anxiety, and adjustment disorder in oncological, haematological, and palliative-care settings: Aa meta-analysis of 94 interview-based studies. Lancet Oncol. (2011) 12:160–74. doi: 10.1016/S1470-2045(11)70002-X
5. Bultz BD, Groff SL, Fitch M, Blais MC, Howes J, Levy K, et al. Implementing screening for distress, the 6th vital sign: a Canadian strategy for changing practice. Psychooncology. (2011) 20:463–9. doi: 10.1002/pon.1932
6. Riba MB, Donovan KA, Andersen B, Braun I, Breitbart WS, Brewer BW, et al. Distress management, version 3.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. (2019) 17:1229–49. doi: 10.6004/jnccn.2019.0048
7. O’Donnell EK, Shapiro YN, Yee AJ, Nadeem O, Hu BY, Laubach JP, et al. Quality of life, psychological distress, and prognostic perceptions in patients with multiple myeloma. Cancer. (2022) 128(10):1996–2004. doi: 10.1002/cncr.34134
8. Randazzo D, Peters KB. Psychosocial distress and its effects on the health-related quality of life of primary brain tumor patients. CNS Oncol. (2016) 5:241–9. doi: 10.2217/cns-2016-0010
9. Marco DJT, White VM. The impact of cancer type, treatment, and distress on health-related quality of life: cross-sectional findings from a study of Australian cancer patients. Support Care Cancer. (2019) 27:3421–9. doi: 10.1007/s00520-018-4625-z
10. Bidstrup PE, Christensen J, Mertz BG, Rottmann N, Dalton SO, Johansen C. Trajectories of distress, anxiety, and depression among women with breast cancer: Looking beyond the mean. Acta Oncol (Stockholm Sweden). (2015) 54:789–96. doi: 10.3109/0284186X.2014.1002571
11. Park JH, Chun M, Jung YS, Bae SH. Predictors of psychological distress trajectories in the first year after a breast cancer diagnosis. Asian Nurs Res (Korean Soc Nurs Sci). (2017) 11:268–75. doi: 10.1016/j.anr.2017.10.003
12. Sun H, Lv H, Zeng H, Niu L, Yan M. Distress Thermometer in breast cancer: systematic review and meta-analysis. BMJ supportive palliative Care. (2022) 12:245–52. doi: 10.1136/bmjspcare-2021-002960
13. Gucalp A, Traina TA, Eisner JR, Parker JS, Selitsky SR, Park BH, et al. Male breast cancer: a disease distinct from female breast cancer. Breast Cancer Res Treat. (2019) 173:37–48. doi: 10.1007/s10549-018-4921-9
14. Hass HG, Herzberger A, Wöckel A, Stepien J. Male breast cancer: therapy-induced toxicities, psychological distress, and individual patient goals during oncological inpatient rehabilitation. Oncol Res Treat. (2022) 45:736–43. doi: 10.1159/000526704
15. Syrowatka A, Motulsky A, Kurteva S, Hanley JA, Dixon WG, Meguerditchian AN, et al. Predictors of distress in female breast cancer survivors: a systematic review. Breast Cancer Res Tr. (2017) 165:229–45. doi: 10.1007/s10549-017-4290-9
16. Momenimovahed Z, Salehiniya H. Epidemiological characteristics of and risk factors for breast cancer in the world. Breast Cancer (Dove Med Press). (2019) 11:151–64. doi: 10.2147/BCTT.S176070
17. Yang YL, Liu L, Wang Y, Wu H, Yang XS, Wang JN, et al. The prevalence of depression and anxiety among Chinese adults with cancer: a systematic review and meta-analysis. BMC Cancer. (2013) 13:393. doi: 10.1186/1471-2407-13-393
18. Sun H, Thapa S, Wang B, Fu X, Yu S. A systematic review and meta-analysis of the distress thermometer for screening distress in Asian patients with cancer. J Clin Psychol Med Settings. (2021) 28:212–20. doi: 10.1007/s10880-020-09705-9
19. Tang LL, Zhang YN, Pang Y, Zhang HW, Song LL. Validation and reliability of distress thermometer in Chinese cancer patients. Chin J Cancer Res. (2011) 23:54–8. doi: 10.1007/s11670-011-0054-y
20. Mitchell AJ. Short screening tools for cancer-related distress: a review and diagnostic validity meta-analysis. J Natl Compr Canc Netw. (2010) 8:487–94. doi: 10.6004/jnccn.2010.0035
21. Ahmad S, Fergus K, McCarthy M. Psychosocial issues experienced by young women with breast cancer: the minority group with the majority of need. Curr Opin Support Palliat Care. (2015) 9:271–8. doi: 10.1097/SPC.0000000000000162
22. Park SH, Han W, Yoo TK, Lee HB, Jin US, Chang H, et al. Oncologic safety of immediate breast reconstruction for invasive breast cancer patients: a matched case control study. J Breast Cancer. (2016) 19:68–75. doi: 10.4048/jbc.2016.19.1.68
23. Yang ML, Wan YP, Wang S, Hu XP. Psychological distress and its influencing factors in patients with breast cancer after surgery at the early stage. Mod Clin Nurs. (2019) 18:12–7. doi: 10.3969/j.issn.1671-8283.2019.02.003
24. Li AZ, Xia HN, Dong MF, Huang HZ. Analysis of psychological distress status and its influencing factors in newly diagnosed breast cancer patients. Hosp Manage Forum. (2018) 35:29–32. doi: 10.3969/j.issn.1671-9069.2018.06.010
25. Berhili S, Kadiri S, Bouziane A, Aissa A, Marnouche E, Ogandaga E, et al. Associated factors with psychological distress in Moroccan breast cancer patients: A cross-sectional study. Breast. (2017) 31:26–33. doi: 10.1016/j.breast.2016.10.015
26. Sun HH. The study of psychological distress status and its relevant factors among breast cancer patients. Wuhan: Hubei: Huazhong University of Science and Technology (2020).
27. Tu C, He Y, Ma X. Factors influencing psychological distress and effects of stepwise psychological care on quality of life in patients undergoing chemotherapy after breast cancer surgery. Am J Transl Res. (2022) 14:1923–33.
28. Boinon D, Sultan S, Charles C, Stulz A, Guillemeau C, Delaloge S, et al. Changes in psychological adjustment over the course of treatment for breast cancer: the predictive role of social sharing and social support. Psycho-oncology. (2014) 23:291–8. doi: 10.1002/pon.3420
29. Ploos van Amstel FK, van den Berg SW, van Laarhoven HW, Gielissen MF, Prins JB, Ottevanger PB. Distress screening remains important during follow-up after primary breast cancer treatment. Support Care Cancer. (2013) 21:2107–15. doi: 10.1007/s00520-013-1764-0
30. Park JH, Bae SH, Chun M, Jung YS, Jung YM. Factors influencing elevated distress scores at the end of primary treatment of breast cancer. Asian Oncol Nurs. (2015) 15:132–9. doi: 10.5388/aon.2015.15.3.132
31. Cheng XH, Yang HJ. Gosset and his small-sample theory. J Northwest Univ (Natural Sci Edition). (2015) 45:1017–9. doi: 10.16152/j.cnki.xdxbzr.2015-06-027
32. Rostom A, Dube C, Cranney A. Agency for healthcare research and quality (US) (2004). Available online at: https://www.ncbi.nlm.nih.gov/books/NBK35156.
33. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25:603–5. doi: 10.1007/s10654-010-9491-z
34. Hegel MT, Moore CP, Collins ED, Kearing S, Gillock KL, Riggs RL, et al. Distress, psychiatric syndromes, and impairment of function in women with newly diagnosed breast cancer. Cancer. (2006) 107:2924–31. doi: 10.1002/cncr.22335
35. Hegel MT, Collins ED, Kearing S, Gillock KL, Moore CP, Ahles TA. Sensitivity and specificity of the Distress Thermometer for depression in newly diagnosed breast cancer patients. Psychooncology. (2008) 17:556–60. doi: 10.1002/pon.1289
36. Luutonen S, Vahlberg T, Eloranta S, Hyväri H, Salminen E. Breast cancer patients receiving postoperative radiotherapy: distress, depressive symptoms and unmet needs of psychosocial support. Radiother Oncol. (2011) 100:299–303. doi: 10.1016/j.radonc.2011.01.014
37. Bidstrup PE, Mertz BG, Dalton SO, Deltour I, Kroman N, Kehlet H, et al. Accuracy of the Danish version of the 'distress thermometer'. Psychooncology. (2012) 21:436–43. doi: 10.1002/pon.1917
38. Mosher CE, Duhamel KN. An examination of distress, sleep, and fatigue in metastatic breast cancer patients. Psychooncology. (2012) 21:100–7. doi: 10.1002/pon.1873
39. Mertz BG, Bistrup PE, Johansen C, Dalton SO, Deltour I, Kehlet H, et al. Psychological distress among women with newly diagnosed breast cancer. Eur J Oncol Nurs. (2012) 16:439–43. doi: 10.1016/j.ejon.2011.10.001
40. Head BA, Schapmire TJ, Keeney CE, Deck SM, Studts JL, Hermann CP, et al. Use of the Distress Thermometer to discern clinically relevant quality of life differences in women with breast cancer. Qual Life Res. (2012) 21:215–23. doi: 10.1007/s11136-011-9934-3
41. Agarwal J, Powers K, Pappas L, Buchmann L, Anderson L, Gauchay L, et al. Correlates of elevated distress thermometer scores in breast cancer patients. Support Care Cancer. (2013) 21:2125–36. doi: 10.1007/s00520-013-1773-z
42. Schmid-Büchi S, Halfens RJ, Müller M, Dassen T, van den Borne B. Factors associated with supportive care needs of patients under treatment for breast cancer. Eur J Oncol Nurs. (2013) 17:22–9. doi: 10.1016/j.ejon.2012.02.003
43. Mejdahl MK, Mertz BG, Bidstrup PE, Andersen KG. Preoperative distress predicts persistent pain after breast cancer treatment: a prospective cohort study. J Natl Compr Canc Netw. (2015) 13:995–1003. doi: 10.6004/jnccn.2015.0120
44. McFarland DC, Andreotti C, Harris K, Mandeli J, Tiersten A, Holland J. Early childhood adversity and its associations with anxiety, depression, and distress in women with breast cancer. Psychosomatics. (2016) 57:174–84. doi: 10.1016/j.psym.2015.11.008
45. Ploos van Amstel FK, Tol J, Sessink KH, van der Graaf WTA, Prins JB, Ottevanger PB. A specific distress cutoff score shortly after breast cancer diagnosis. Cancer Nurs. (2017) 40:E35–40. doi: 10.1097/NCC.0000000000000380
46. Lo-Fo-Wong DN, de Haes HC, Aaronson NK, van Abbema DL, den Boer MD, van Hezewijk M, et al. Predictors of enduring clinical distress in women with breast cancer. Breast Cancer Res Treat. (2016) 158:563–72. doi: 10.1007/s10549-016-3896-7
47. Jørgensen L, Laursen BS, Garne JP, Sherman KA, Søgaard M. Prevalence and predictors of distress in women taking part in surgical continuity of care for breast cancer: A cohort study. Eur J Oncol Nurs. (2016) 22:30–6. doi: 10.1016/j.ejon.2016.01.004
48. Xue HY, Zhu N, Cao R, Liu YQ, Yin WR, Chen HY, et al. Investigation of psychological status of patients with breast cancer after adjuvant therapy. J Sichuan Univ (Med Sci Edi). (2016) 47:442–4. doi: 10.13464/j.scuxbyxb.2016.03.032
49. Mertz BG, Duriaud HM, Kroman N, Andersen KG. Pain, sensory disturbances, and psychological distress among Danish women treated for ductal carcinoma in situ: an exploratory study. Pain Manag Nurs. (2017) 18:309–17. doi: 10.1016/j.pmn.2017.03.004
50. Ng CG, Mohamed S, Kaur K, Sulaiman AH, Zainal NZ, Taib NA, et al. Perceived distress and its association with depression and anxiety in breast cancer patients. PloS One. (2017) 12:e0172975. doi: 10.1371/journal.pone.0172975
51. Acquati C, Kayser K. Predictors of psychological distress among cancer patients receiving care at a safety-net institution: the role of younger age and psychosocial problems. Support Care Cancer. (2017) 25:2305–12. doi: 10.1007/s00520-017-3641-8
52. Robbeson C, Hugenholtz-Wamsteker W, Meeus M, Devoogdt N, Nijs J, De Groef A. Screening of physical distress in breast cancer survivors: Concurrent validity of the Distress Thermometer and Problem List. Eur J Cancer Care (Engl). (2019) 28:e12880. doi: 10.1111/ecc.12880
53. Shen Y, Zhang J, Bu QY, Bai YL, Liu CX. A longitudinal study of psychological distress and its related factors in patients with breast cancer. Chin Nurs Manage. (2018) 18:617–22. doi: 10.3969/j.issn.1672-1756.2018.05.009
54. Zhang JL, Cai W, Wang XH, Hu AN, Yang XF, Lu YQ. Status and specific manifestations of persistent psychological distresses in patients with breast cancer post-operation. Chin J Prac Nurs. (2018) 34:2276–80. doi: 10.3760/cma.j.issn.1672-7088.2018.29.008
55. Cormio C, Caporale F, Spatuzzi R, Lagattolla F, Lisi A, Graziano G. Psychosocial distress in oncology: using the distress thermometer for assessing risk classes. Support Care Cancer. (2019) 27:4115–21. doi: 10.1007/s00520-019-04694-4
56. Ciambella CC, Taneja C, Dizon DS, Wiggins DL, Emmick CM, Leonard KL, et al. Distress: characterizing what causes the thermometer to shift in patients with newly diagnosed breast cancer attending a multidisciplinary clinic. Ann Surg Oncol. (2019) 26:3204–9. doi: 10.1245/s10434-019-07544-z
57. Wang Y, Qiang WM, Shen AM, Guo FL, Zhao SX. The analysis for psychological status of breast cancer patients in a period of time. Chin J Mod Nurs. (2019) 34:430–3. doi: 10.16821/J.CNKI.HSJX.2019.05.012
58. Wan XM, Wang X, Li YL, Zhang Y. Correlations of perceived benefit and psychological distress in patients with breast cancer after adjuvant chemotherapy. Mod Clin Nurs. (2019) 18:1–5. doi: 10.3969/j.issn.1671-8283.2019.07.001
59. Civilotti C, Acquadro Maran D, Santagata F, Varetto A, Stanizzo MR. The use of the Distress Thermometer and the Hospital Anxiety and Depression Scale for screening of anxiety and depression in Italian women newly diagnosed with breast cancer. Support Care Cancer. (2020) 28:4997–5004. doi: 10.1007/s00520-020-05343-x
60. Fayanju OM, Ren Y, Stashko I, Power S, Thornton MJ, Marcom PK, et al. Patient-reported causes of distress predict disparities in time to evaluation and time to treatment after breast cancer diagnosis. Cancer. (2021) 127:757–68. doi: 10.1002/cncr.33310
61. de Boer AZ, Derks MGM, de Glas NA, Bastiaannet E, Liefers GJ, Stiggelbout AM, et al. Metastatic breast cancer in older patients: A longitudinal assessment of geriatric outcomes. J Geriatr Oncol. (2020) 11:969–75. doi: 10.1016/j.jgo.2020.04.002
62. Admiraal JM, Hoekstra-Weebers JEHM, Schröder CP, Tuinier W, Hospers GAP, Reyners AKL. Distress, problems, referral wish, and supportive health care use in breast cancer survivors beyond the first year after chemotherapy completion. Support Care Cancer. (2020) 28:3023–32. doi: 10.1007/s00520-019-05030-6
63. Li LL, Li XM, Han DF, Li JY, Zhao WY. A longitudinal study of identification and predication of psychological distress trajectories among breast cancer patients. Chin J Nurs. (2020) 55:1140–6. doi: 10.3761/j.issn.0254-1769.2020.08.003
64. Liu JK, Kaji AH, Roth KG, Hari DM, Yeh JJ, Dauphine C, et al. Determinants of psychosocial distress in breast cancer patients at a safety net hospital. Clin Breast Cancer. (2022) 22:43–8. doi: 10.1016/j.clbc.2021.06.011
65. Budisavljevic A, Kelemenic-Drazin R, Dedic Plavetic N, Kardum Fucak I, Silovski T, Telesmanic Dobric V, et al. The first wave of the COVID-19 Pandemic and its impact on the level of distress in patients with breast cancer, a multicentric study. Psychiatr Danub. (2021) 33:341–9.
66. Wang J, Li FF, Li YQ, Zheng LL, Wang YY. Changes and influencing factors of psychological distress trajectory in patients diagnosed with breast cancer. Chin J Mod Nurs. (2021) 27:3723–8. doi: 10.3760/cma.j.cn115682-20210227-00889
67. Zhao H, Li X, Zhou C, Wu Y, Li W, Chen L. Psychological distress among Chinese patients with breast cancer undergoing chemotherapy: Concordance between patient and family caregiver reports. J Adv Nurs. (2022) 78:750–64. doi: 10.1111/jan.15004
68. Taurisano P, Abbatantuono C, Verri V, Pepe I, Stucci LS, Taurino A, et al. Pre-surgery supportive and goal-oriented strategies are associated with lower post-surgery perceived distress in women diagnosed with breast cancer. BMC Psychol. (2022) 10:2. doi: 10.1186/s40359-021-00714-3
69. Lim SY, Ke Y, Mok NK, Tan YY, Neo PSH, Chan A, et al. Factors associated with distress and the impact of distress on acute health-care service utilization among patients diagnosed with breast and gynecological cancers. Palliat Support Care. (2023), 1–8. doi: 10.1017/S1478951522001444
70. Hass HG, Seywald M, Wöckel A, Muco B, Tanriverdi M, Stepien J. Psychological distress in breast cancer patients during oncological inpatient rehabilitation: incidence, triggering factors and correlation with treatment-induced side effects. Arch Gynecol Obstet. (2023) 307:919–25. doi: 10.1007/s00404-022-06657-3
71. Lv D, Lan B, Sun XY, Yang M, Zhang L, Ma F. Relationship between dynamic changes of psychological distress and quality of life in Chinese early breast cancer patients. Chin J Oncol. (2022) 44:1119–24. doi: 10.3760/CMA.J.CN112152-20210412-00308
72. Liu Q. Screening tools and trajectory of psychological distress in breast cancer patients. Hebei: Hebei Medical University (2022).
73. Wang YQ, Zeng DY, Gong ZX, Wang YG. Investigation on psychological distressions of breastcancer patients in hospital and its influencing factors analysis. J ModMed Health. (2022) 38:2208–11. doi: 10.3969/J.ISSN.1009-5519.2022.13.011
74. Pang L, Li W, Yao S, Jing Y, Yin X, Cheng H. Psychological distress is involved in CRCI in breast cancer survivors via mediating cytokine levels. Cancer Med. (2023) 12:11806–15. doi: 10.1002/cam4.5847
75. Ribnikar D, Ribeiro JM, Pinto D, Sousa B, Pinto AC, Gomes E, et al. Breast cancer under age 40: a different approach. Curr Treat Options Oncol. (2015) 16:16. doi: 10.1007/s11864-015-0334-8
76. Dinapoli L, Colloca G, Di Capua B, Valentini V. Psychological aspects to consider in breast cancer diagnosis and treatment. Curr Oncol Rep. (2021) 23:38. doi: 10.1007/s11912-021-01049-3
77. Shen H, Masingboon K, Samartkit N. Factors related to preoperative uncertainty among patients with breast cancer in Wenzhou, China: A cross-sectional study. Belitung Nurs J. (2023) 9:236–43. doi: 10.33546/bnj.2648
78. Bani Mohammad E, Ahmad M. Virtual reality as a distraction technique for pain and anxiety among patients with breast cancer: a randomized control trial. Palliat Support Care. (2019) 17:29–34. doi: 10.1017/S1478951518000639
79. Chirico A, Maiorano P, Indovina P, Milanese C, Giordano GG, Alivernini F, et al. Virtual reality and music therapy as distraction interventions to alleviate anxiety and improve mood states in breast cancer patients during chemotherapy. J Cell Physiol. (2020) 235:5353–62. doi: 10.1002/jcp.29422/
80. Chen LW, Chou HH, Wang SY, Shih WM. Unmet care needs and uncertainty in patients newly diagnosed with breast cancer. Healthcare (Basel Switzerland). (2022) 10:2148. doi: 10.3390/healthcare10112148
81. Molarius A, Granström F. Educational differences in psychological distress? Results from a population-based sample of men and women in Sweden in 2012. BMJ Open. (2018) 8:e021007. doi: 10.1136/bmjopen-2017-021007
82. Ehsan AN, Wu CA, Minasian A, Singh T, Bass M, Pace L, et al. Financial toxicity among patients with breast cancer worldwide: A systematic review and meta-analysis. JAMA network Open. (2023) 6:e2255388. doi: 10.1001/jamanetworkopen.2022.55388
83. Semin JN, Palm D, Smith LM, Ruttle S. Understanding breast cancer survivors' financial burden and distress after financial assistance. Supportive Care Cancer Off J Multinational Assoc Supportive Care Cancer. (2020) 28:4241–8. doi: 10.1007/s00520-019-05271-5
84. Altice CK, Banegas MP, Tucker-Seeley RD, Yabroff KR. Financial hardships experienced by cancer survivors: a systematic review. J Natl Cancer Inst. (2016) 109:djw205. doi: 10.1093/jnci/djw205
85. Coughlin SS. Social determinants of breast cancer risk, stage, and survival. Breast Cancer Res Treat. (2019) 177:537–48. doi: 10.1007/s10549-019-05340-7
86. Coughlin SS, Ayyala DN, Tingen MS, Cortes JE. Financial distress among breast cancer survivors. Curr Cancer Rep. (2020) 2:48–53. doi: 10.25082/CCR.2020.01.004
87. Tao L, Hu X, Fu L, Zhang X, Chen H. Effects of family beliefs and family strength on individual resilience and quality of life among young breast cancer survivors: A cross-sectional study. J Clin Nurs. (2023) 32:2616–26. doi: 10.1111/jocn.16321
88. Schmidt ME, Scherer S, Wiskemann J, Steindorf K. Return to work after breast cancer: The role of treatment-related side effects and potential impact on quality of life. Eur J Cancer Care. (2019) 28:e13051. doi: 10.1111/ecc.13051
89. Iwatani T, Matsuda A, Kawabata H, Miura D, Matsushima E. Predictive factors for psychological distress related to diagnosis of breast cancer. Psychooncology. (2013) 22:523–9. doi: 10.1002/pon.3023
90. Youngblood VM, Nyirenda R, Nyasosela R, Zuze T, Yang Y, Kudowa E, et al. Outcomes and prognostic factors for women with breast cancer in Malawi. Cancer Causes Control CCC. (2020) 31:393–402. doi: 10.1007/s10552-020-01282-4
91. Travado L, Bastos L. Distress and psycho-oncological support for patients with advanced breast cancer. Semin Oncol Nurs. (2024) 40:151555. doi: 10.1016/j.soncn.2023.151555
92. Ozmen T, Ozmen V. Treatment changes in breast cancer management and de-escalation of breast surgery. Eur J Breast Health. (2023) 19:186–90. doi: 10.4274/ejbh.galenos.2023.2023-6-2
93. Zhang X, Oliveri JM, Paskett ED. Features, predictors, and treatment of breast cancer-related lymphedema. Curr Breast Cancer Rep. (2020) 12:244–54. doi: 10.1007/s12609-020-00381-0
94. Thalji SZ, Cortina CS, Guo MS, Kong AL. Postoperative complications from breast and axillary surgery. Surg Clinics North America. (2023) 103:121–39. doi: 10.1016/j.suc.2022.08.007
95. Al-Hilli Z, Wilkerson A. Breast surgery: management of postoperative complications following operations for breast cancer. Surg Clinics North America. (2021) 101:845–63. doi: 10.1016/j.suc.2021.06.014
96. Thakur M, Sharma R, Mishra AK, Singh K, Kar SK. Psychological distress and body image disturbances after modified radical mastectomy among breast cancer survivors: A cross-sectional study from a tertiary care centre in North India. Lancet Regional Health Southeast Asia. (2022) 7:100077. doi: 10.1016/j.lansea.2022.100077
97. Shekhar N, Jaiswal R, Joseph L, Jain S, Jain S, Kr A, et al. An Overview of Psychological Analysis of Breast Cancer Patients undergoing Modified Radical Mastectomy and Breast Conservation Surgery and its impact on Objectified Body Consciousness at a Tertiary Care Cancer Centre in South India. Clin Breast Cancer. (2023) 23:e394–400. doi: 10.1016/j.clbc.2023.05.017
98. Kredentser MS, Mackenzie CS, McClement SE, Enns MW, Hiebert-Murphy D, Murphy DJ, et al. Neuroticism as a moderator of symptom-related distress and depression in 4 noncancer end-of-life populations. Palliative Supportive Care. (2023), 1–9. doi: 10.1017/S147895152300127X
99. Dahl AA, Smedsland SK, Vandraas KF, Bøhn SK, Falk RS, Kiserud CE, et al. High neuroticism is associated with common late adverse effects in a nationwide sample of long-term breast cancer survivors. Breast Cancer Res Treat. (2023) 202:97–104. doi: 10.1007/s10549-023-07055-2
100. Segerstrom SC, Gloger EM, Hardy JK, Crofford LR. Exposure and reactivity to repetitive thought in the neuroticism-distress relationship. Cogn Ther Res. (2020) 44:659–67. doi: 10.1007/s10608-020-10078-4
101. Brandão T, Tavares R, Schulz MS, Matos PM. Measuring emotion regulation and emotional expression in breast cancer patients: A systematic review. Clin Psychol Rev. (2016) 43:114–27. doi: 10.1016/j.cpr.2015.10.002
102. Swainston J, Derakshan N. Training cognitive control to reduce emotional vulnerability in breast cancer. Psycho-oncology. (2018) 27:1780–6. doi: 10.1002/pon.4727
103. Bower JE, Partridge AH, Wolff AC, Thorner ED, Irwin MR, Joffe H, et al. Targeting depressive symptoms in younger breast cancer survivors: the pathways to wellness randomized controlled trial of mindfulness meditation and survivorship education. J Clin Oncol Off J Am Soc Clin Oncol. (2021) 39:3473–84. doi: 10.1200/JCO.21.0027
Keywords: breast cancer, psychological distress, incidence, risk factor, review
Citation: Tao L, Xiang Y, Zeng X, Fu L, Li J and Chen H (2024) Incidence and risk factors for psychological distress in adult female patients with breast cancer: a systematic review and meta-analysis. Front. Psychiatry 15:1309702. doi: 10.3389/fpsyt.2024.1309702
Received: 01 November 2023; Accepted: 23 February 2024;
Published: 13 March 2024.
Edited by:
Melissa Thong, German Cancer Research Center (DKFZ), GermanyReviewed by:
Daniela Doege, German Cancer Research Center (DKFZ), GermanyGillian Gillian Prue, Queen’s University Belfast, United Kingdom
Copyright © 2024 Tao, Xiang, Zeng, Fu, Li and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hong Chen, 1366109878@qq.com
†These authors have contributed equally to this work
 Lin Tao
Lin Tao Yuping Xiang
Yuping Xiang Xiaohong Zeng1
Xiaohong Zeng1 Lan Fu
Lan Fu Hong Chen
Hong Chen