- 1Sydney Medical School, University of Sydney, Sydney, NSW, Australia
- 2Northern Sydney Cancer Centre, Royal North Shore Hospital, Sydney, NSW, Australia
- 3Massachusetts General Hospital, Harvard Medical School, Boston, MA, United States
Background: Major depressive disorder (MDD) exhibits gender disparities, and emerging evidence suggests the involvement of the gut microbiome, necessitating exploration of sex-specific differences.
Methods: A review was conducted, encompassing a thorough examination of relevant studies available in Medline via Ovid, Embase via OvidSP, CINAHL, and PsycINFO databases from their inception to June 2023. The search strategy employed specific keywords and Medical Subject Headings (MeSH) terms tailored to major depressive disorder in women, encompassing unipolar depression, depressive symptoms, and dysbiosis.
Results: Five studies were included. Among the four studies, alterations in alpha (n=1) and beta diversity (n=3) in the gut microbiome of individuals with MDD were revealed compared to controls. Gender-specific differences were observed in four studies, demonstrating the abundance of specific bacterial taxa and highlighting potential sex-specific implications in MDD pathophysiology. Correlation analyses (n=4) indicated associations between certain bacterial taxa and the severity of depressive symptoms, with varying patterns between males and females. Studies (n=3) also highlighted promising findings regarding the potential utility of microbial markers in diagnosing MDD, emphasizing the crucial role of sex stratification in understanding the disease pathophysiology.
Conclusions: The findings underscore the importance of recognizing gender-specific differences in the composition of the gut microbiome and its relationship with MDD. Further comprehensive robust studies are required to unravel the intricate mechanisms underlying these disparities.
Introduction
Major Depression, also known as major depressive disorder (MDD), is a prevalent mental and emotional ailment affecting an estimated 185 million people globally (1). The World Health Organization classified depression as the fourth-leading burden of disease globally in 2008, with projections indicating it could become the second-leading cause by 2030 (2). Women are disproportionately affected, experiencing nearly double the prevalence compared to men (1), a trend observed across both developed and developing countries (3).
Various theories such as the biopsychosocial model, have attempted to elucidate the underlying reasons for this gender disparity, pointing to differences in hormones (4, 5), neurotransmitters (5, 6), and brain structure (7, 8). Recent research has also explored the intricate relationship between the gut microbiome and depression, uncovering potential links through the gut-brain axis (9–31). While significant advancements have been made, there remains a dearth of evidence to precisely elucidate the mechanisms driving these disparities or the potential for sex-specific biomarkers.
The concept of ‘gut dysbiosis’ - an abnormal alteration in the composition and function of the gut microbiome - has gained traction as a potential player in the pathogenesis of MDD and other psychiatric disorders (9–31). The intricate communication between the gut microbiome and the brain through various pathways, including neural, immune, and metabolic mechanisms, presents a promising avenue for further exploration. Recent studies have highlighted differences in the gut microbiota composition between individuals with MDD and control groups, pointing to potential sex-specific differences that warrant further investigation (19, 32–35).
This scoping review aims to explore the existing evidence on the relationship between major depression and the gut microbiome, particularly in the context of women, while also summarizing the sex-specific differences in the gut microbiome profiles of male and female subjects with major depression.
Methods
A comprehensive literature search was conducted from database inception to June 2023 in Medline via Ovid (1946-present), Embase via OvidSP (1947-present), Cinahl Complete, and PsycINFO via Ovid (1806-present). The search used specific keywords and MeSH terms related to major depression in women, including unipolar depression, depressive symptoms, and dysbiosis.
Inclusion criteria encompassed studies with adult human participants of both sexes, focusing on female-specific outcomes. Studies investigating the relationship between major depression and gastrointestinal microbiota in adult humans were included, while those exclusively concerning other psychiatric disorders (e.g., schizophrenia, chronic stress, PTSD, bipolar disorder), subtypes of depression (e.g., postpartum, late-life depression), or other medical conditions were excluded. Additionally, studies involving females under 18 years old were not considered.
Results
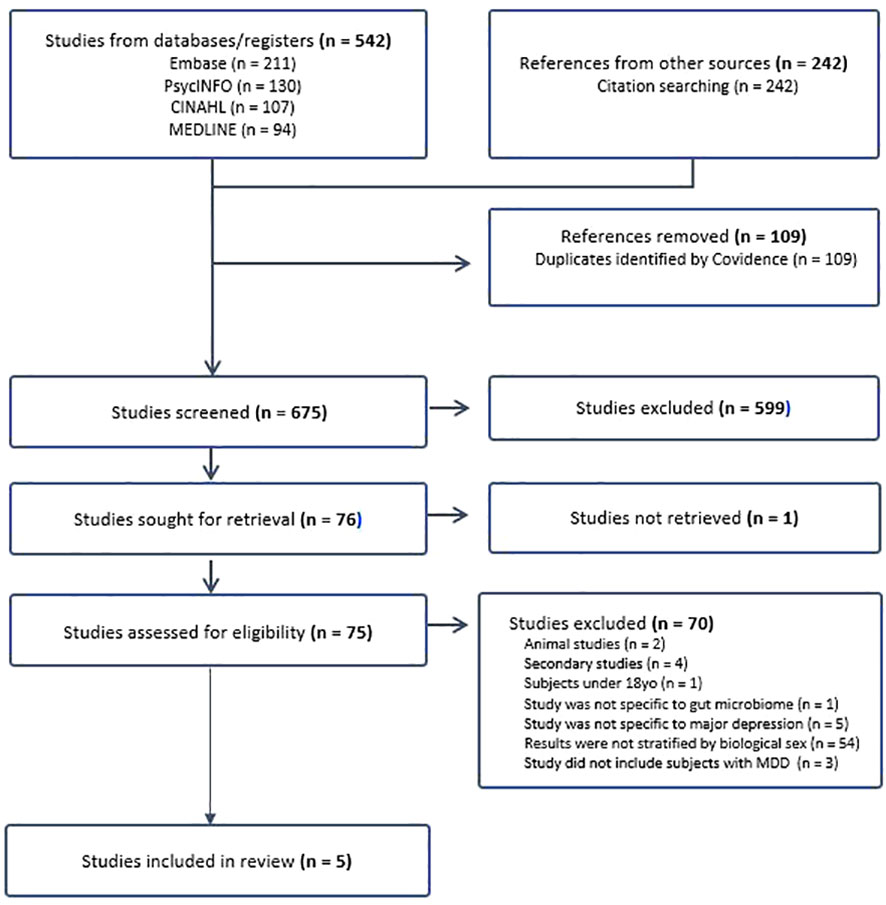
From the initial database search, 784 studies were identified, and after removing 109 duplicates, 675 studies underwent phase one screening. Following this, 76 studies were subjected to full-text retrieval, resulting in 75 fully assessed articles. Ultimately, five articles were included in the literature review (Figure 1 for the PRISMA flow chart).
Characteristics of studies
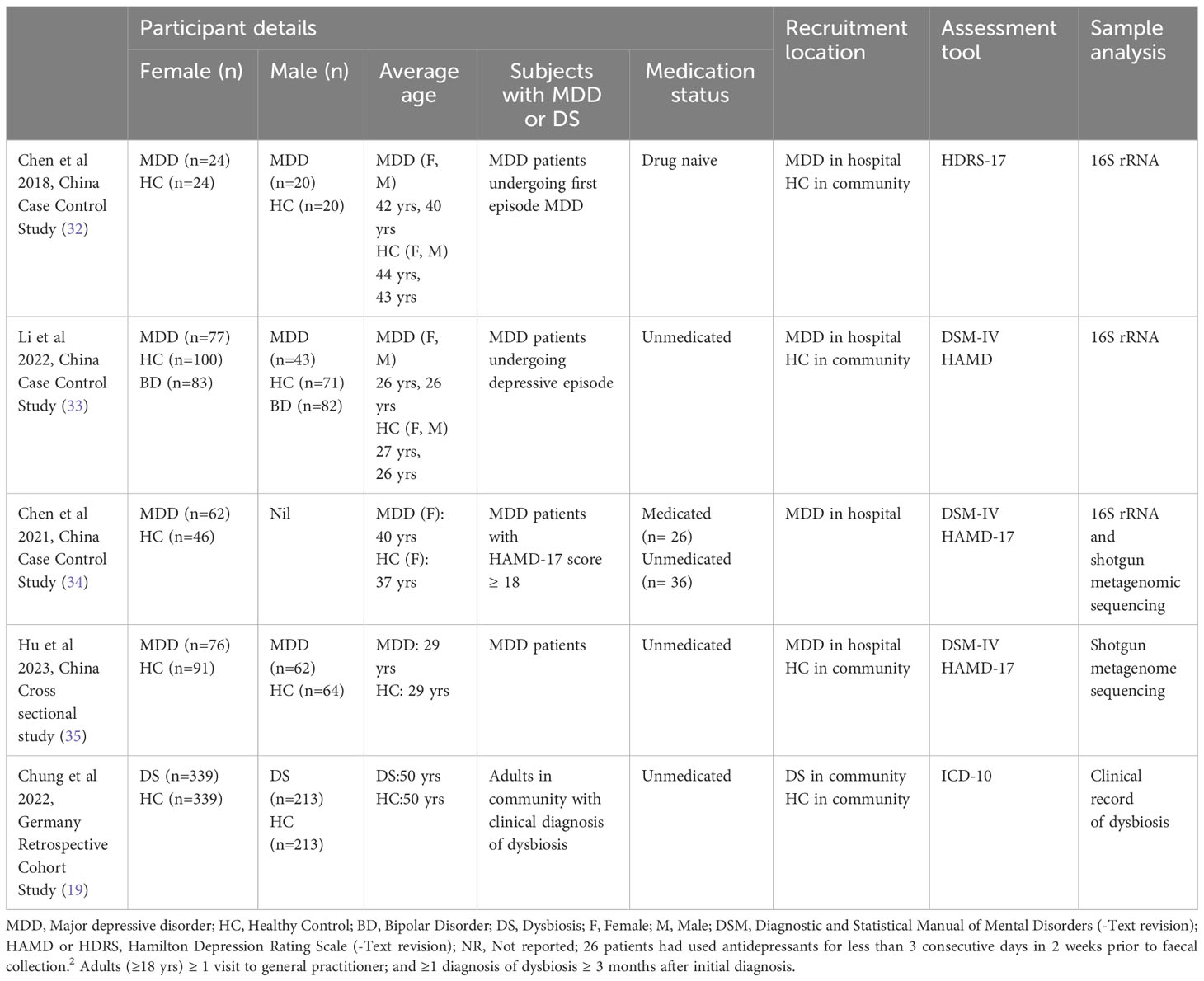
The review included a total of (n=780) subjects from case-controlled studies in China and (n=1104) subjects from a retrospective cohort study in Germany. Among the case-control studies, (n=239) female and (n=125) male subjects with MDD were compared to (n=261) female and (n=155) male healthy controls. Notably, one study by Li et al. (33) involved subjects with Bipolar disorder (BD) (n=166) experiencing a depressive episode, whose data were excluded from this review’s analysis (Table 1.1).
Gender-specific microbiome diversity alterations in subjects with major depression
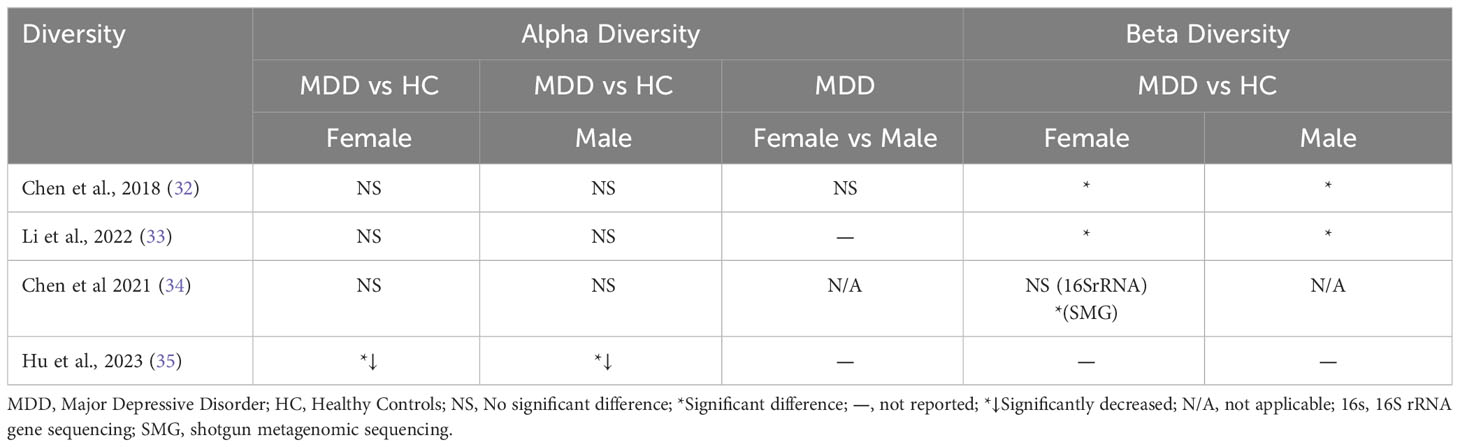
Alpha diversity remained unchanged in MDD subjects across three studies (32–34), while one study (35) reported a reduction. Beta diversity analysis revealed significant differences in both male and female MDD groups compared to matched healthy controls (HCs) in studies by Chen and Li (32, 33). In the female-only study by Chen et al. (34), alterations in beta diversity were observed only at the species level in female MDD subjects. Notably, Li et al. (33) found that while alpha diversity was significantly higher in female healthy controls compared to male healthy controls, this difference was not evident in the context of depression. Table 1.2 provides an overview of the key findings.
Gender-specific microbiome profile alterations in subjects with major depression
All case-control studies (32–34), highlighted notable differences in gut microbiota between individuals with major depressive disorder (MDD) and the respective control groups. These distinctions were particularly pronounced when comparing male and female cohorts. Further details can be found in Table 1.3. Upon examining studies encompassing both male and female subjects, females with MDD exhibited a higher relative abundance of Actinobacteria, Firmicutes, and Bacteroidetes compared to the control group (32, 33). In male MDD patients, an increase and decrease in Bacteroidetes clusters, along with an increase in Firmicutes clusters, was observed. In the study conducted by Chen et al. (34) focusing on female MDD patients, an increase in Bacteroidetes, Proteobacteria, Fusobacteria, and Verruomicrobia, and a decrease in Firmicutes and Actinobacteria was reported. Notably, only two studies (34, 35) investigated the microbiome at the species level, revealing significant changes at the family, genus, and species levels.

Table 1.3 Gender-Specific Microbiome Profile Alterations in Subjects with MDD compared to healthy controls.
Correlation of bacterial taxa with severity of depression symptoms
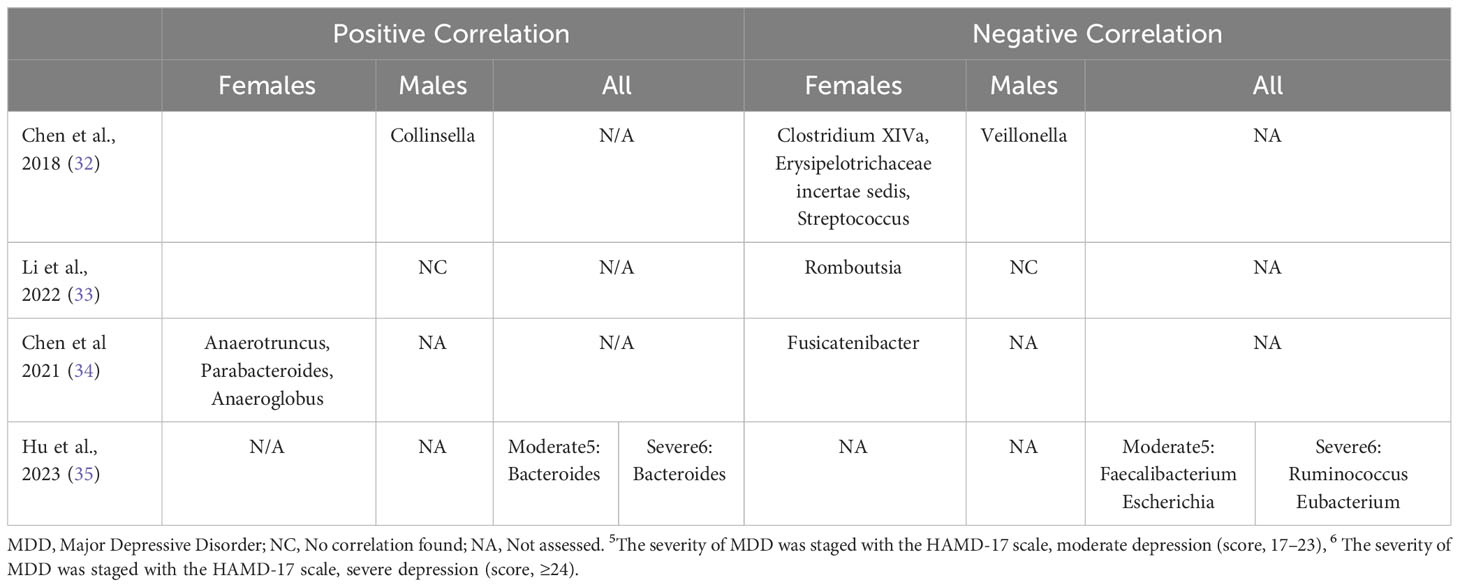
Four studies examined the relationship between the severity of depression symptoms and specific bacterial taxa at the genus level (32–35). Among female MDD subjects, three genera (Anaerotruncus, Parabacteroides, and Anaeroglobus) exhibited associations with increased depressive symptoms, whereas five genera (Clostridium XIVa, Erysipelotrichaceae incertae sedis, Streptococcus, Romboutsia, and Fusicatenibacter) were linked to reduced depressive symptoms. In male MDD subjects, two distinct genera (Collinsella, Veillonella) were found to be correlated with depression symptoms (refer to Table 1.4).
Potential diagnostic role of microbial markers and dysbiosis in major depression
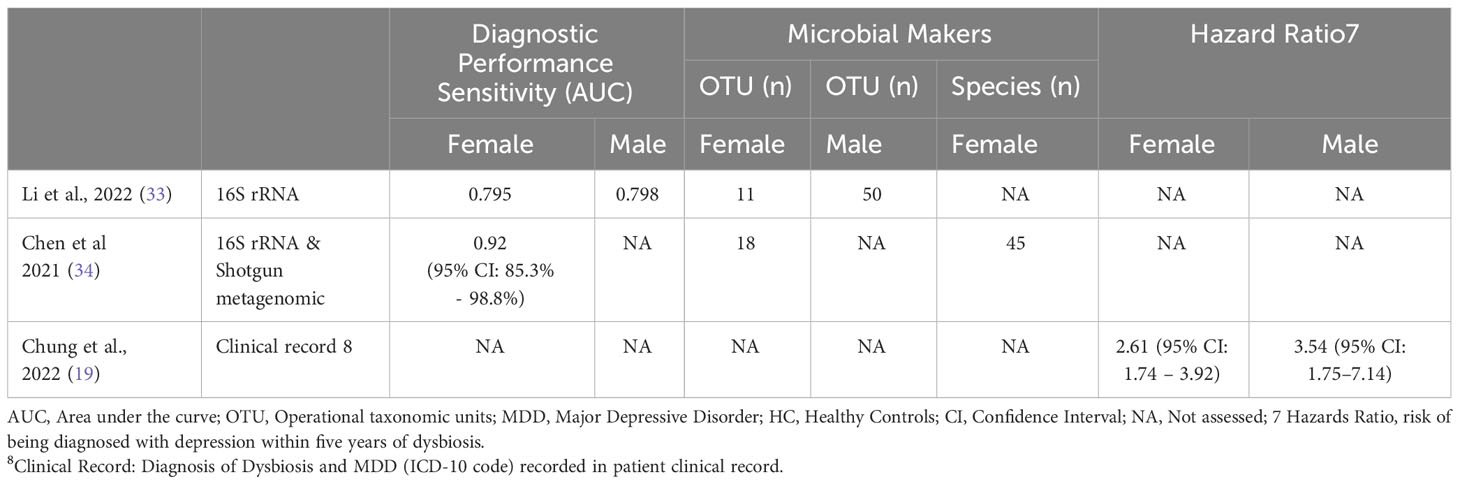
Two studies (33, 34) examined the accuracy of microbial markers in diagnosing MDD, identified sex-specific gut microbiota signatures, and evaluated diagnostic performance using the area under the receiver operating characteristic curve (AUC). Analysis of the diagnostic performance sensitivity of these microbial signatures showed area under the curve (AUC) values ranging from 0.79 to 0.92 for females and 0.79 for males with MDD. An additional study (19) investigated the risk of developing MDD within five years following an initial dysbiosis diagnosis and found a stronger association between dysbiosis and MDD diagnosis in males (HR:3.54, 95% CI: 1.75–7.14) compared to females HR:2.61 (95% CI: 1.74 – 3.92). (Refer to Table 1.5).
Discussion
Several recent studies have suggested that the gut microbiome profile is associated with Major Depressive Disorder (MDD), yet only a few have investigated the sex-specific link between MDD and the gut microbiome. This review represents the first comprehensive analysis examining the relationship between the gender-specific gut microbiome profile and MDD. To date, five primary studies have provided insights into the relationship between the gut microbiome and MDD in women (19, 32–35). These findings indicate a close association between the gut microbiome composition of females with MDD and the disorder itself, highlighting sex-specific differences in the gut microbiota of MDD patients. Certain genera were found to correlate with the severity of depression, and these correlations varied between males and females. Additionally, sex-specific differences were observed in the diagnostic performance of microbial markers and the risk of developing MDD following a dysbiosis diagnosis. While the underlying pathophysiological mechanism remains unclear, the distinct microbiome variability between sexes necessitates further investigation.
Regarding gender-specific microbiome diversity
Our review results are consistent with existing literature, emphasizing notable differences in the gut microbiota composition between individuals diagnosed with MDD and controls (9–18). These differences primarily involve microbial diversity and the prevalence of specific bacterial taxa. Four separate studies highlighted discernible variations in microbial diversity in both male and female MDD patients compared to their healthy counterparts (32–35). Notably, one study observed no significant difference in microbial diversity between male and female MDD patients (32). Most case-control studies found no alterations in alpha diversity among female MDD subjects compared to female healthy controls, while one study (35) reported reduced alpha diversity in female MDD subjects relative to healthy controls, mirroring a similar trend observed in male MDD subjects.
All studies examining beta diversity identified significant differences between female MDD patients and healthy controls (32–34), with two studies also noting distinct variations in beta diversity between male MDD patients and healthy controls (32, 33). One study focusing solely on females revealed alterations in beta diversity at the species level in female MDD subjects (34). Despite observing higher alpha diversity in healthy females compared to healthy males, this distinction was not observed in the depressed state (33).
These findings suggest gender-specific differences in the gut microbiome that may be influenced by various factors, such as the menstrual cycle stage, diet, age, and environmental factors. Overall, the results emphasize distinct beta diversity in both female and male MDD patients compared to healthy controls (32–34), with potential discrepancies in alpha diversity stemming from methodological variations in assessing microbiome diversity and the influence of confounding factors. Further clinical studies are warranted to comprehensively investigate the role of the gut microbiome in both male and female MDD patients, considering the potential implications for other diseases prevalent in females. The studies used various techniques, including 16S rRNA gene sequencing and shotgun metagenomic sequencing (SMG), to assess the microbiome. However, discrepancies in the methodologies employed suggest the need for standardized approaches in future research.
In terms of gender-specific microbiome profiles
The current study reveals notable differences in the gut microbiome profiles of females with MDD in comparison to both healthy controls (HCs) and males with MDD. Analyzing data from four cross-sectional studies (32–35), we identified several differential abundances in bacterial clusters in both female and male MDD groups relative to HCs. These alterations primarily involved Actinobacteria, Bacteroidetes, Firmicutes, Proteobacteria, Fusobacteria, and Verrucomicrobia, which represent the dominant bacterial phyla in the human gut (29) Notably, despite previous literature suggesting Bacteroides as a signature gut microbe of MDD (17), our review unveiled inconsistent directions of compositional changes, which may be partly attributed to variations in the severity of depression. Hu et al. (35) also highlighted the influence of depression severity on gut microbiome alterations. Furthermore, a recent review on MDD and the gut microbiome by Knuesel and Mohajeri (22) identified disparities across studies, suggesting potential variations arising from different underlying causes and manifestations of depression across different age groups. Notably, the influence of confounding factors, such as the stage of the menstrual cycle, dietary patterns, physical activity, and environmental factors (28, 36) may contribute to the discrepancies observed in the findings. The current body of literature, however, lacks a sufficient number of studies investigating sex-specific differences in the gut microbiome concerning MDD.
In the correlation of bacterial taxa with the severity of depressive symptoms
Several studies have indicated associations between specific bacterial taxa and the severity of depressive symptoms in individuals with MDD, as observed in the works of recent studies (19, 32–35). Notably, certain genera, including Anaerotruncus, Parabacteroides, and Anaeroglobus, were linked to increased depressive symptoms, whereas the presence of Clostridium XIVa, Erysipelotrichaceae incertae sedis, Streptococcus, Romboutsia, and Fusicatenibacter was associated with reduced symptoms. Despite Chen et al. (32) documenting correlations in males with MDD, the literature remains relatively limited and heterogeneous. A comprehensive review by Knuesel and Mohajeri (22) emphasized a negative correlation between Faecalibacterium and depressive symptoms, coupled with a positive correlation in cases of remission and improved quality of life. Similarly, Jiang et al. (9) demonstrated a negative association between Faecalibacterium prausnitzii (FP) and the severity of depressive symptoms. Likewise, Hu et al. (35) utilized shotgun sequencing, revealing a negative correlation between Faecalibacterium and depressive symptoms in a mixed-sex group of MDD patients with moderate depression. However, this correlation was not observed in the subgroup with severe depression, suggesting the potential confounding impact of depression severity. While the reviewed studies did not definitively establish the specific link between Faecalibacterium and the severity of depressive symptoms in females with MDD, they reported varying levels of Faecalibacterium in females with MDD compared to HCs. Despite existing disparities, Faecalibacterium remains a critical bacterial taxon of interest, previously associated with gut health and overall host well-being (37). Further exploration through improved methodological approaches, including controlling for sex as a biological factor and considering depression severity, is warranted to clarify the precise contribution of specific bacterial taxa to disease development or their status as a consequence of the disease.
As a potential diagnostic microbial marker in depression
The evaluation of the diagnostic efficacy of microbial markers in females with MDD is still in its preliminary stages. Two separate studies have identified sex-specific gut microbial markers capable of distinguishing between males with MDD, females with MDD, and HCs (33, 34). Examination of how well these microbial signatures perform diagnostically showed that the area under the curve (AUC) values ranged from 0.79 to 0.92 for females and 0.79 for males diagnosed with MDD. Although these findings are limited due to sparse data and disparate methodologies, the identification of sex-specific microbial panels with potential diagnostic capabilities highlights the significance of sex stratification in MDD case-control studies. Additionally, this discovery provides crucial insights into the divergent pathophysiological mechanisms and prognostic variances between male and female MDD patients. Moreover, a study by Chung et al. (19) observed sex-specific disparities in the risk of developing MDD within five years following an initial dysbiosis diagnosis, with a notably stronger association among males compared to females. While specific microbial markers were not identified, this observation, in conjunction with existing evidence indicating the presence of sex-specific gut microbial profiles in MDD, emphasizes the potential for comprehensive characterization of sex-specific risk factors and the formulation of non-invasive gut microbial-based screening or diagnostic tools for MDD.
The limitations of the present study
Include the heterogeneity in measurement and reporting methods, as well as the use of limited sample sizes and study designs, which impose certain restrictions on the interpretability of the results. However, these findings provide critical insights into the potential role of the gut microbiome in the context of MDD, especially concerning sex-specific differences. Future research should emphasize the inclusion of sex as a biological factor, conduct longitudinal studies to understand microbiome changes in response to clinical variations better, and carefully control for confounding factors to establish a more comprehensive understanding of the complex interplay between the gut microbiome and MDD.
Conclusion
Despite the existing knowledge gaps and limitations, the findings underscore the significance of sex-specific differences in the gut microbiome of MDD patients. These insights hold important implications for potential advancements in the diagnosis, treatment, and understanding of the pathophysiology of MDD, emphasizing the necessity for further comprehensive investigations into the role of the gut microbiome in the context of sex-specific differences.
Author contributions
LN: Conceptualization, Validation, Writing – review & editing, Data curation, Formal Analysis, Investigation, Methodology, Writing – original draft. GL: Validation, Writing – review & editing. SC: Validation, Writing – review & editing. MM: Validation, Writing – review & editing. AY: Validation, Writing – review & editing. BO: Conceptualization, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The publication fee for this article was supported by the Royal Northshore Public Hospital’s Radiation Oncology Department's Trust and Education Fund.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Global Burden of Disease Collaborative Network. Global health data exchange (GHDx), in: Global Burden of Disease Study 2019 (GBD 2019) (2019). Seattle, United States: Institute for Health Metrics and Evaluation (IHME. Available online at: https://vizhub.healthdata.org/gbd-results/ (Accessed 2023 Nov 19).
2. World Health Organization. Mental Health Gap Action Programme: Scaling up care for mental, neurological, and substance use disorders. Geneva: World Health Organization (2008). Available at: https://www.who.int/publications/i/item/9789241596206.
3. Kessler R, Bromet E. The epidemiology of depression across cultures. Annu Rev Public Health (2014) 34:119–38. doi: 10.1146/annurev-publhealth-031912-114409
5. Labaka A, Goñi-Balentziaga O, Lebeña A, Pérez-Tejada J. Biological sex differences in depression: A systematic review. Biol Res Nurs (2018) 20:383–92. doi: 10.1177/1099800418776082
6. Eid RS, Gobinath AR, Galea LAM. Sex differences in depression: Insights from clinical and preclinical studies. Prog Neurobiol (2019) 176:86–102. doi: 10.1016/j.pneurobio.2019.01.006
7. Hoekzema E, Barba-Müller E, Pozzobon C, Picado M, Lucco F, García-García D, et al. Pregnancy leads to long-lasting changes in human brain structure. Nat Neurosci (2017) 20:287–96. doi: 10.1038/nn.4458
8. Mohammadi S, Seyedmirzaei H, Salehi MA, Jahanshahi A, Zakavi SS, Dehghani Firouzabadi F, et al. Brain-based sex differences in depression: A systematic review of neuroimaging studies. Brain Imaging Behav (2023) 17:541–69. doi: 10.1007/s11682-023-00772-8
9. Jiang H, Ling Z, Zhang Y, Mao H, Ma Z, Yin Y, et al. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav Immun (2015) 48:186–94. doi: 10.1016/j.bbi.2015.03.016
10. Naseribafrouei A, Hestad K, Avershina E, Sekelja M, Linløkken A, Wilson R, et al. Correlation between the human fecal microbiota and depression. Neurogastroenterol Motility: Off J Eur Gastrointestinal Motil Soc (2014) 26:1155–62. doi: 10.1111/nmo.12378
11. Szczesniak O, Hestad KA, Hanssen JF, Rudi K. Isovaleric acid in stool correlates with human depression. Nutr Neurosci (2016) 19:279–83. doi: 10.1179/1476830515y.0000000007
12. Foster JA, McVey Neufeld KA. Gut-brain axis: How the microbiome influences anxiety and depression. Trends Neurosci (2013) 36:305–12. doi: 10.1016/j.tins.2013.01.005
13. Sonali S, Ray B, Tousif HA, Rathipriya AG, Sunanda T, Mahalakshmi AM, et al. Mechanistic insights into the link between gut dysbiosis and major depression: an extensive review. Cells (2022) 11:1362. doi: 10.3390/cells11081362
14. Sasso JM, Ammar RM, Tenchov R, Lemmel S, Kelber O, Grieswelle M, et al. Gut microbiome-brain alliance: A landscape view into mental and gastrointestinal health and disorders. ACS Chem Neurosci (2023) 14:1717–63. doi: 10.1021/acschemneuro.3c00127
15. Sanada K, Nakajima S, Kurokawa S, Barceló-Soler A, Ikuse D, Hirata A, et al. Gut microbiota and major depressive disorder: A systematic review and meta-analysis. J Affect Disordorders (2020) 266:1–13. doi: 10.1016/j.jad.2020.01.102
16. Barandouzi ZA, Starkweather AR, Henderson WA, Gyamfi A, Cong XS. Altered composition of gut microbiota in depression: A systematic review. Front Psychiatry (2020) 11:541. doi: 10.3389/fpsyt.2020.00541
17. Yang J, Zheng P, Li Y, Wu J, Tan X, Zhou J, et al. Landscapes of bacterial and metabolic signatures and their interaction in major depressive disorders. Sci Adv (2020) 6:20201202. doi: 10.1126/sciadv.aba8555
18. Dinan TG, Cryan JF. Melancholic microbes: A link between gut microbiota and depression? Neurogastroenterol Motil (2013) 25:713–9. doi: 10.1111/nmo.12198
19. Chung SY, Kostev K, Tanislav C. Dysbiosis: A potential precursor to the development of a depressive disorder. Healthcare (Basel) (2022) 10:20220810. doi: 10.3390/healthcare10081503
20. Yao H, Zhang D, Yu H, Shen H, Liu H, Meng F, et al. The microbiota-gut-brain axis in pathogenesis of depression: A narrative review. Physiol Behav (2023) 260:114056. doi: 10.1016/j.physbeh.2022.114056
21. Kelly JR, Borre Y, O' Brien C, Patterson E, El Aidy S, Deane J, et al. Transferring the blues: Depression-associated gut microbiota induces neurobehavioural changes in the rat. J Psychiatr Res (2016) 82:109–18. doi: 10.1016/j.jpsychires.2016.07.019
22. Knuesel T, Mohajeri MH. The role of the gut microbiota in the development and progression of major depressive and bipolar disorder. Nutrients (2021) 14:20211223. doi: 10.3390/nu14010037
23. Knudsen JK, Michaelsen TY, Bundgaard-Nielsen C, Nielsen RE, Hjerrild S, Leutscher P, et al. Faecal microbiota transplantation from patients with depression or healthy individuals into rats modulates mood-related behaviour. Sci Rep (2021) 11:21869. doi: 10.1038/s41598-021-01248-9
24. Zheng P, Zeng B, Zhou C, Liu M, Fang Z, Xu X, et al. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Mol Psychiatry (2016) 21:786–96. doi: 10.1038/mp.2016.44
25. Li N, Wang Q, Wang Y, Sun A, Lin Y, Jin Y, et al. Fecal microbiota transplantation from chronic unpredictable mild stress mice donors affects anxiety-like and depression-like behavior in recipient mice via the gut microbiota-inflammation-brain axis. Stress (Amsterdam Netherlands) (2019) 22:592–602. doi: 10.1080/10253890.2019.1617267
26. Łoniewski I, Misera A, Skonieczna-Żydecka K, Kaczmarczyk M, Kaźmierczak-Siedlecka K, Misiak B, et al. Major Depressive Disorder and gut microbiota – Association not causation. A scoping review. Prog Neuropsychopharmacol Biol Psychiatry (2021) 106:110111. doi: 10.1016/j.pnpbp.2020.110111
27. Audet MC. Stress-induced disturbances along the gut microbiota-immune-brain axis and implications for mental health: Does sex matter? Front Neuroendocrinol (2019) 54:100772. doi: 10.1016/j.yfrne.2019.100772
28. Manosso LM, Lin J, Carlessi AS, Recco KCC, Quevedo J, Gonçalves CL, et al. Sex-related patterns of the gut-microbiota-brain axis in the neuropsychiatric conditions. Brain Res Bull (2021) 171:196–208. doi: 10.1016/j.brainresbull.2021.04.001
29. Shobeiri P, Kalantari A, Teixeira AL, Rezaei N. Shedding light on biological sex differences and microbiota–gut–brain axis: a comprehensive review of its roles in neuropsychiatric disorders. Biol Sex Differ (2022) 13. doi: 10.1186/s13293-022-00422-6
30. Clarke G, Grenham S, Scully P, Fitzgerald P, Moloney RD, Shanahan F, et al. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol Psychiatry (2013) 18:666–73. doi: 10.1038/mp.2012.77
31. Jašarević E, Morrison KE, Bale TL. Sex differences in the gut microbiome - Brain axis across the lifespan. Philos Trans R Soc B: Biol Sci (2016) 371. doi: 10.1098/rstb.2015.0122
32. Chen JJ, Zheng P, Liu YY, Zhong XG, Wang HY, Guo YJ, et al. Sex differences in gut microbiota in patients with major depressive disorder. Neuropsychiatr Dis Treat (2018) 14:647–55. doi: 10.2147/NDT.S159322
33. Li Y, Zhang H, Zheng P, Yang J, Wu J, Huang Y, et al. Perturbed gut microbiota is gender-segregated in unipolar and bipolar depression. J Affect Disord (2022) 317:166–75. doi: 10.1016/j.jad.2022.08.027
34. Chen YH, Xue F, Yu SF, Li XS, Liu L, Jia YY, et al. Gut microbiota dysbiosis in depressed women: The association of symptom severity and microbiota function. J Affect Disord (2021) 282:391–400. doi: 10.1016/j.jad.2020.12.143
35. Hu X, Li Y, Wu J, Zhang H, Huang Y, Tan X, et al. Changes of gut microbiota reflect the severity of major depressive disorder: a cross sectional study. Trans Psychiatry (2023) 13:137. doi: 10.1038/s41398-023-02436-z
36. Valeri F, Endres K. How biological sex of the host shapes its gut microbiota. Front Neuroendocrinol (2021) 61:100912. doi: 10.1016/j.yfrne.2021.100912
Keywords: gut microbiome, depression, gender, biomarker, gut dysbiosis
Citation: Niemela L, Lamoury G, Carroll S, Morgia M, Yeung A and Oh B (2024) Exploring gender differences in the relationship between gut microbiome and depression - a scoping review. Front. Psychiatry 15:1361145. doi: 10.3389/fpsyt.2024.1361145
Received: 25 December 2023; Accepted: 02 February 2024;
Published: 19 February 2024.
Edited by:
Karen Tabb, University of Illinois at Urbana-Champaign, United StatesReviewed by:
Claudia Civai, London South Bank University, United KingdomCopyright © 2024 Niemela, Lamoury, Carroll, Morgia, Yeung and Oh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Byeongsang Oh, byeong.oh@sydney.edu.au
 Leila Niemela
Leila Niemela Gillian Lamoury1,2
Gillian Lamoury1,2 Albert Yeung
Albert Yeung Byeongsang Oh
Byeongsang Oh